Abstract
Membrane inlet mass spectrometry was used to observe nitric oxide in the well-studied reaction of nitrite with hemoglobin. The membrane inlet was submerged in the reaction solutions and measured NO in solution via its flux across a semipermeable membrane leading to the mass spectrometer detecting the mass-to-charge ratio m/z 30. This method measures NO directly in solution and is an alternate approach compared with methods that purge solutions to measure NO. Addition to deoxy-Hb(FeII) (near 38 µM heme concentration) of nitrite in a range of 80 µM to 16 mM showed no accumulation of either NO or N2O3 on a physiologically relevant time scale with a sensitivity near 1 nM. The addition of nitrite to oxy-Hb(FeII) and met-Hb(FeIII) did not accumulate free NO to appreciable extents. These observations show that for several minutes after mixing nitrite with hemoglogin, free NO does not accumulate to levels exceeding the equilibrium level of NO. The presence of cyanide ions did not alter the appearance of the data; however, the presence of 2 mM mercuric ions at the beginning of the experiment with deoxy-Hb(FeII) shortened the initial phase of NO accumulation and increased the maximal level of free, unbound NO by about twofold. These experiments appear consistent with no role of met-Hb(FeIII) in the generation of NO and an increase in nitrite reductase activity caused by the presumed binding of mercuric to cysteine residues. These results raise questions about the ability of reduction of nitrite mediated by deoxy-Hb(FeII) to play a role in vasodilation.
Keywords: Nitric oxide, Nitrite, Nitrite reductase, Mass spectrometry, Membrane inlet, Hemoglobin
Introduction
Considerable research has focused on processes intrinsic to erythrocytes and hemoglobin that may play a role in vasoregulatory activity, processes such as the generation of NO from the anion nitrite and the oxylabile S-nitrosylation of cyteine residues [1,2]. There has been great interest in the reactions of nitrite with hemoglobin since initial reports of the nitrite reductase activity of deoxy-Hb(FeII) [3]. The possible association between nitrite and vasodilator activity has been considered for many years, a topic that is well reviewed [1,2,4,5]. This has stimulated studies to determine whether the interaction of nitrite with red cells produces vasodilation through mechanisms related to the nitrite reductase activity of deoxy-Hb(FeII). Profound issues have arisen over the mechanisms by which nitrite might produce vasodilation. One issue is whether NO is able to escape from the red cell since it is avidly bound up by deoxy-Hb(FeII). This has prompted some suggestions that there are processes near the cell membrane that promote efflux of NO and/or that the nitrite reductase activity of hemoglobin produces intermediates such as N2O3 that efflux the cell and decay to NO and NO2 in the plasma [1,2,4].
We demonstrate here the application of membrane inlet mass spectrometry to the reactions of nitrite with hemoglobin. This method is based on the use of semipermeable membranes that allow a flux of uncharged molecules from solution into the ionization chamber of the mass spectrometer. The use of membrane inlet mass spectrometry has been well described in the detection of volatile, uncharged organic solutes in aqueous solution and blood [6], and in the study of the reactions of CO2 and the catalysis by carbonic anhydrase [7]. Although the application of this technology to the measurement of dissolved NO has been reported [8], an application more practicable for biological reactions of NO is needed, one that could possibly also be used in physiological experiments. Accordingly, we report here the use of a membrane inlet system described earlier [9] applied to the study of the reactions of nitrite with hemoglobin.
In this work we demonstrate the type of data that can be obtained investigating the reactions of nitrite and hemoglobin while directly detecting nitric oxide in solution. Although the nitrite reductase activity of hemoglobin produces micromolar amounts of NO under our conditions, much of this NO is not detected since it is tightly bound to deoxy-Hb(FeII). Membrane inlet mass spectrometry detects nanomolar levels of NO on addition of 8 mM nitrite to 38 µM deoxy-Hb(FeII). Moreover, this is observed starting several minutes after the reaction of nitrite and hemoglobin begins. The addition of nitrite to oxy-Hb(FeII) and met-Hb(FeIII) did not generate free, unbound NO over the 25 min time course of these experiments. Although addition of cyanide to bind met-Hb(FeIII) did not alter the accumulation of free NO caused by reactions of nitrite with deoxy-Hb(FeII), addition of mercuric ions to bind thiol and possibly imidazole groups increased the rate and maximal level of the accumulation of NO. These reactions have been extensively studied by many methods over the years, and the membrane inlet mass spectrometric method provides a rather unique view.
Experimental methods
Materials
Human hemoglobin was purified from human red cells and also obtained from Sigma-Aldrich. That obtained from human red cells was purified by lysing cells with water and centrifuging to remove membranes. The resulting lysate was treated with an affinity gel (p-aminomethylbenzenesulfonamide-agarose, Sigma) to remove carbonic anhydrase. Hemoglobin from Sigma was nearly entirely in the form of met-Hb(FeIII) and was reduced to the ferrous form using dithionite. The resulting solution was passed through Sephadex G-25M under anerobic conditions and eluted using degassed buffer solutions. Hemoglobin from both sources gave identical results in the experiments reported here. Deoxy-Hb(FeII) was prepared from these samples by bubbling of helium, and oxy-Hb(FeII) by bubbling of oxygen. Met-Hb(FeIII) was prepared by oxidation using ferricyanide and subsequent passage of the sample through Sephadex G-25M. Visible spectra were taken to confirm the presence of the appropriate form of hemoglobin.
Inlet probe and kinetic measurements
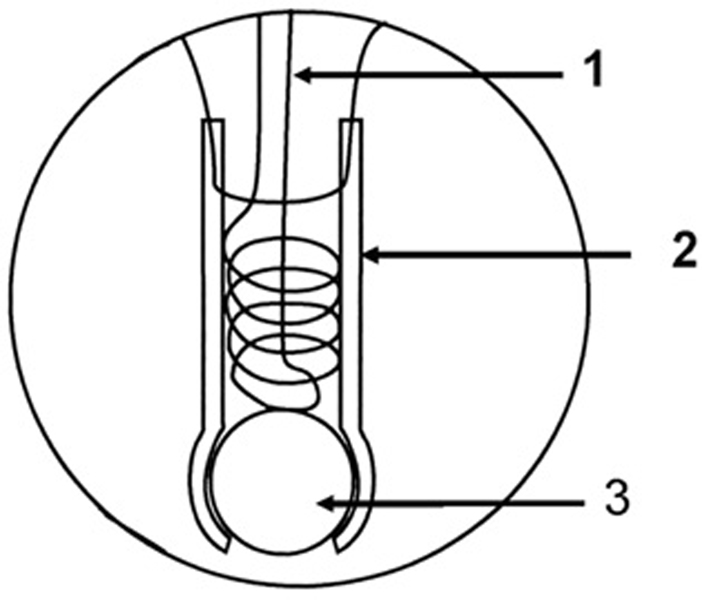
The inlet probe to the mass spectrometer consisted of a length of Silastic tubing (1.5 mm i.d. and 2.0 mm o.d., a Dow Corning product) which was sealed at one end by a glass bead and attached at the other end to a piece of glass tubing leading into a dry-ice acetone water trap and then into an Extrel EXM-200 mass spectrometer as described elsewhere (Fig. 1) [9]. This membrane inlet was immersed in solution contained in a 3-mL glass cuvette for optical spectroscopy (standard 1-cm pathlength cuvette) that was modified for introduction of samples and inert gas, and was sealable by injection septums and Teflon screw plugs [9]. Hence, references in the text to accumulation of NO in solution signify free, unbound NO. The vessel contained a small magnetic spinner. Mass spectra were obtained using electron impact ionization (70 eV) at an emission current of 1 mA. Source pressures were approximately 110−6 Torr. The resulting mass scans were well resolved with a return of ion current (detector response) to the baseline separating each mass unit. The reaction vessel containing the membrane inlet was inserted into a spectrophotometer (Hewlett-Packard 8453) for concomitant visible absorption measurements.
Fig. 1.
Diagram of the membrane inlet which is inserted in an anaerobic cell for mass spectrometric measurements: (1) Wire helix for support of the Silastic tubing; (2) Silastic tubing approximately 1 cm in length; (3) glass bead to seal the Silastic tubing. Taken from Ref. [9].
Solutions in this inlet vessel were purged of atmospheric gases by continuous bubbling of helium via a syringe placed through a septum, with outflow via a second syringe. Bubbling of helium was stopped and solutions containing reagents were injected through a syringe into the reaction vessel. The vessel itself was immersed in a water bath for temperature control, and experiments were carried out at 25° C. However, experiments in which we measured concomitant visible absorption spectra and NO generation were carried out at room temperature 23° C. In selected experiments KCN or HgCl2 was added and incubated up to 8 min with the hemoglobin solution in the reaction vessel prior to adding nitrite.
Calibration of the membrane inlet and mass spectrometer was achieved by injecting solutions containing known concentrations of NO into buffered solutions in the reaction vessel as described earlier [9]. The ion current was recorded when it reached a maximal plateau; this followed first-order kinetics with a half time of 5 s. The program to deconvolute visible spectra of mixtures of species of hemoglobin was generously provided by Dr. Mark T. Gladwin.
Results
Response of the membrane inlet mass spectrometer
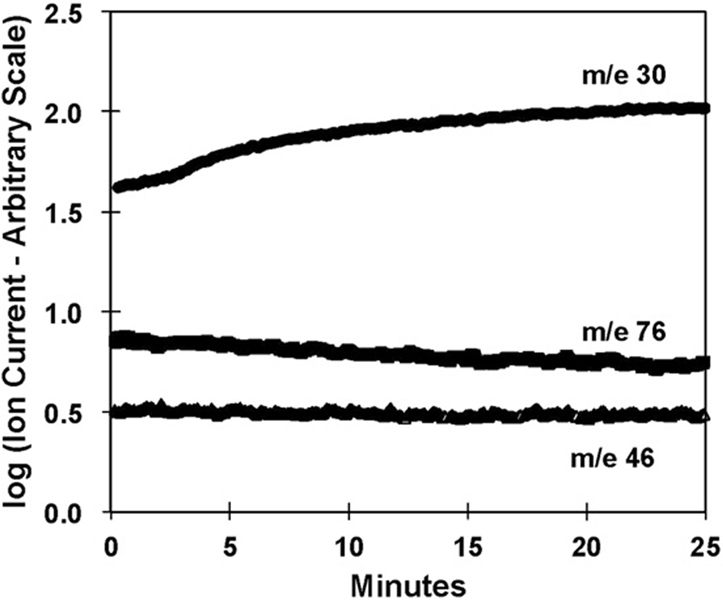
Fig. 2 shows typical ion currents obtained with the membrane inlet immersed in a solution containing 38 µM deoxy-Hb(FeII) at pH 6.8. At time zero, sodium nitrite was added to an initial concentration of 8 mM. The data show the ion currents at mass-to-charge ratios of m/z 30, 46, and 76 corresponding to NO, NO2, and N2O3, respectively. A substantial increase in the m/z 30 peak in the presence of deoxy-Hb(FeII), after an initial lag, corresponds to the generation of NO. No change was seen in the mass 46 or 76 peaks. The larger, initial ion current at time zero for m/z 30 compared with m/z 76 and 46 is due to background and is subtracted in subsequent figures.
Fig. 2.
Ion currents measured using the immersed membrane inlet to the mass spectrometer on addition of 8 mM NaNO2 (added at time zero) to a solution containing 38 µM deoxy-Hb(FeII) (heme concentration) showing the change at m/z 30, 46, and 76. Solutions also contained 50 mM phosphate buffer at pH 6.8, 110 mM NaCl, and 2 mM EDTA at 23 C.
Uncatalyzed generation of NO from nitrite
We observed the generation of NO due the uncatalyzed reactions of nitrite, as described in an earlier report of membrane inlet mass spectrometry [9]. The reactions of nitrous acid in aqueous solution, including the reactions that produce NO, have been extensively reported [10,11] and reviewed [12]. The overall dismutation of nitrous acid can be described by the reaction below, which includes more complex chemistry not shown:
The initial rates of NO formation from nitrous acid (nitrite) measured by EPR and chemiluminescence are described by second-order kinetics at concentrations of total nitrite below about 0.2 mM, and first order at higher concentrations [10,11]. The increase in NO resulting from an initial concentration of 8 mM nitrite in a buffered solution at pH 6.8 is shown in Fig. 3, and described in more detail in an earlier report for additional initial concentrations of added nitrite also measured by membrane inlet mass spectrometry [9]. Unlike the previous studies of this reaction by Samouilov et al. [10] and Park and Lee [11], the studies reported here did not purge NO with inert gases. Hence, the data of Fig. 3 show the approach to equilibrium that includes the full array of back reactions involving nitrogen oxides and nitrogen oxyacids. Using membrane inlet probes of different lengths of Silastic tubing, we estimate that the loss of NO into the mass spectrometer is negligible.
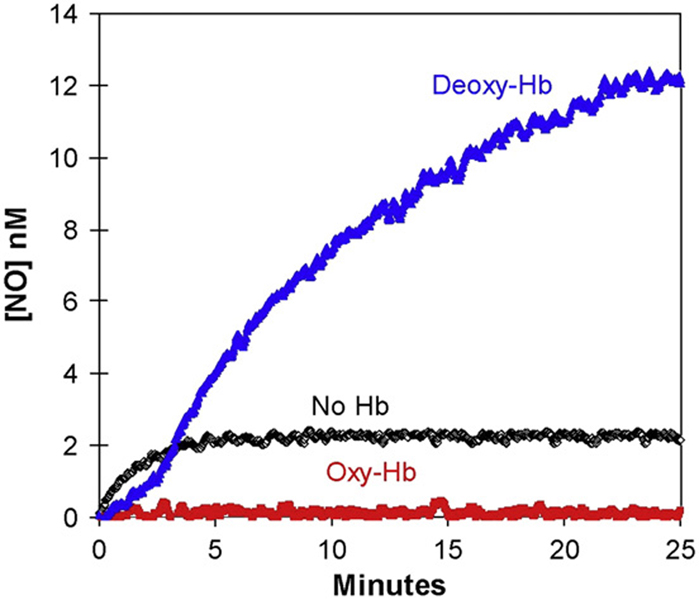
Fig. 3.
The time course of dissolved NO concentrations (obtained from m/z 30) on addition of nitrite to solutions containing (blue) 38 µM deoxy-Hb(FeII); or (red) 38 µM oxy-Hb(FeII); or (black) no hemoglobin. (These are heme concentrations.) At time zero, NaNO2 was added to attain a concentration of 8 mM. Solutions also contained 50 mM phosphate buffer at pH 6.8, 110 mM NaCl, 2 mM EDTA at 23° C. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Reaction of nitrite and hemoglobin
The addition of nitrite at 8 mM to a solution containing 38 µM deoxy-Hb(FeII) (heme concentration) caused a small lag of about 3 min in which the rate of free, unbound NO accumulation in solution as detected by the mass spectrometer was small, followed by a phase of greater rate of accumulation of NO (Fig. 3). The membrane inlet method is detecting free, unbound NO, not total NO in solution. The accumulation of NO proceeded to about 14 nM in the experiment using 38 µM deoxy-Hb(FeII) in Fig. 3, exceeding the equilibrium level of NO (2 nM) in the absence of deoxy-Hb(FeII). This level to which [NO] approaches is an exceedingly small fraction (~10−6) of the initial nitrite added. The initial phase of the experiment was accompanied by a change in the absorption spectra in Fig. 4 from that of deoxy-Hb(FeII) initally to spectra consistent with a mixture of NO-Hb(FeII) and forms of met-Hb(FeIII). To examine a possible role of met-Hb(FeIII) we added cyanide to reaction solutions. The addition of KCN at 0.2 mM either before the addition of nitrite or during the experiment had no effect on the generation of NO in the experiments of Fig. 3.
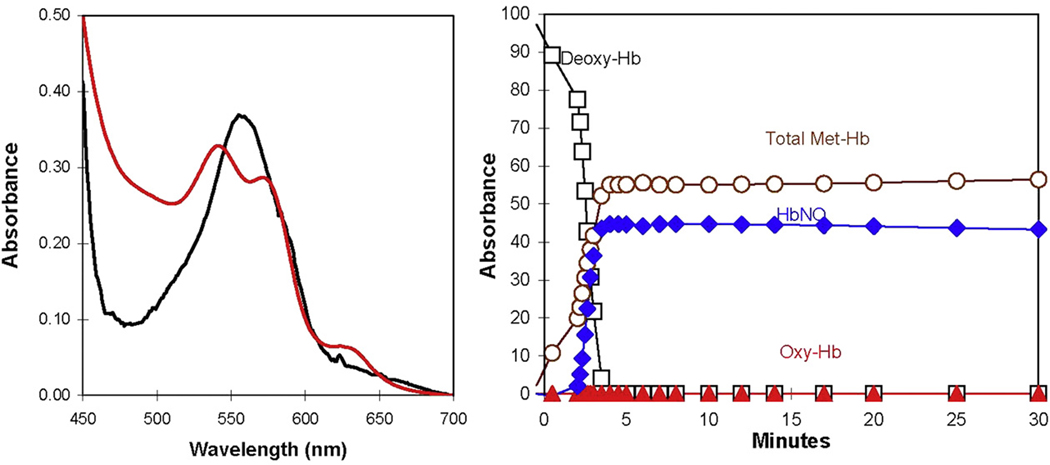
Fig. 4.
(Left) The visible absorption spectra at (black) 0.5 min and (red) 30 min after the addition of 8 mM NaNO2 to a solution containing 38 µM deoxy-Hb(FeII) in an experiment similar to Fig. 3. Conditions were as described in the legend to Fig. 3. (Right) The change with time of the percentage of species of hemoglobin estimated by deconvolution of the absorption data such as shown in the left figure. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
We reversed the addition of nitrite and deoxy-Hb(FeII), adding 38 µM deoxy-Hb(FeII) to a solution containing a large concentration of nitrite (8 mM) that had been allowed to establish equilibrium with NO at pH 6.8. The results demonstrated that on addition of deoxy-Hb(FeII) there was a rapid drop in NO concentration to deplete the solution of NO (data not shown).
An experiment was performed adding sufficient diethylamine NONOate, an NO donor [13], to a buffered solution containing no hemoglobin to achieve 1 µM NO in solution under conditions of Fig. 3. When this concentration of diethylamine NONOate was added under identical conditions except that solutions contained 38 µM deoxy-Hb(FeII), no accumulation of free NO was observed (data not shown). These observations are presumed to be caused by the tight and rapid binding of NO to deoxy-Hb(FeII).
Experiments were carried out in which the concentration of nitrite was more similar to that of deoxy-Hb(FeII). However, addition of nitrite at 80 µM to a solution containing 30 µM deoxy-Hb(FeII) did not produce sufficient NO for detection with the membrane inlet. Our lower limit of detection with current instrumentation is near 1 nM[9].
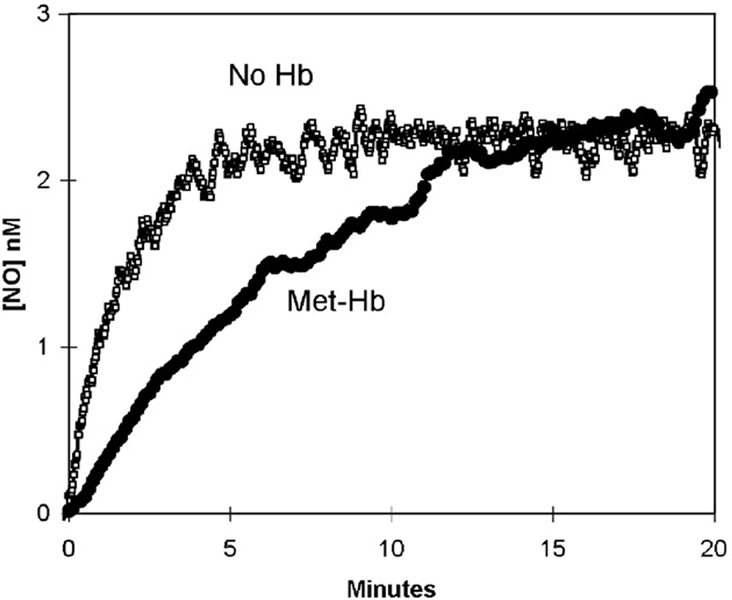
Fig. 3 shows that addition of nitrite at 8 mM to a solution of 38 µM oxy-Hb(FeII) resulted in no detectable NO, because reactions with oxy-Hb(FeII) have depleted NO from solution. After addition of nitrite, the visible spectra for oxy-Hb(FeII) decreased steadily while spectra for forms of met-Hb(FeIII) began to appear (data not shown).
The addition of nitrite at 8 mM to a solution containing 40 µM met-Hb(FeIII) was only very slightly different from that of the uncatalyzed dismutation (Fig. 5). The data showed a slightly slower accumulation of NO compared with the uncatalyzed appearance of NO up to about 10 min followed by a level of NO that was close to that of the uncatalyzed dismutation of nitrite. The visible spectra observed during this experiment showed species of met-Hb(FeIII); species of Hb(FeII) were not observed (data not shown). Addition of KCN during the course of such experiments did not alter the appearance of the data.
Fig. 5.
The generation of nitric oxide measured by membrane inlet mass spectrometry after the addition of 8 mM NaNO2 to solutions containing 40 µM met-Hb(FeIII) or no hemoglobin. Solution conditions were as described in Fig. 3.
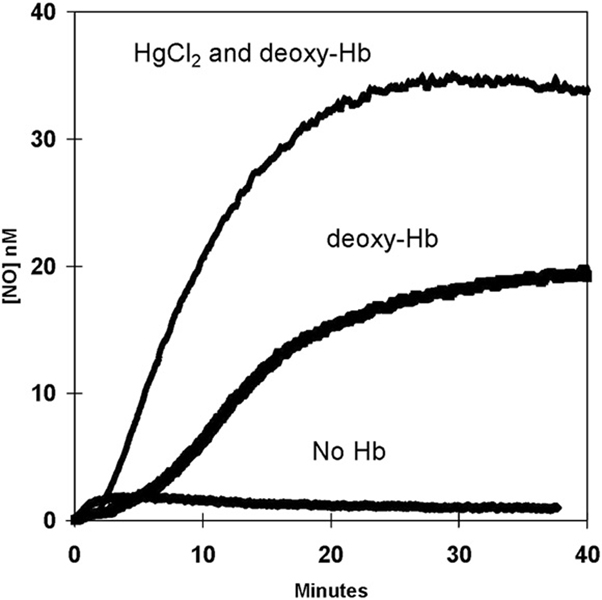
The incubation for 8 min of 2.0 mM HgCl2 and 36 µM deoxy-Hb (FeII) followed by the addition of nitrite (8 mM at time zero of Fig. 6) appeared to shorten the initial phase of free NO accumulation and to enhance the rate of approach of NO to a plateau level greater than that for the absence of mercuric ions (Fig. 6). In a separate experiment, the addition of HgCl2 during the course of the appearance of the NO signal caused a more rapid accumulation of free NO immediately on addition of HgCl2 (data not shown). The presence of HgCl2 at 2.0 mM caused no change in the uncatalyzed generation of NO from nitrite.
Fig. 6.
The effect of HgCl2 on the accumulation of NO in solutions of 36 µM deoxy-Hb(FeII). Sodium nitrite was added at 8.0 mM at zero time. (Top) The accumulation of NO in the presence of 2.0 mM HgCl2 with solutions incubated 8 min before addition of nitrite; (middle) the accumulation of NO under identical conditions but with no HgCl2; (bottom) the uncatalyzed dismutation of nitrite in solutions containing no hemoglobin and 2.0 mM HgCl2. Other conditions are in the legend to Fig. 3 except that 2 mM diethylenetriaminepentaacetate was used as chelator.
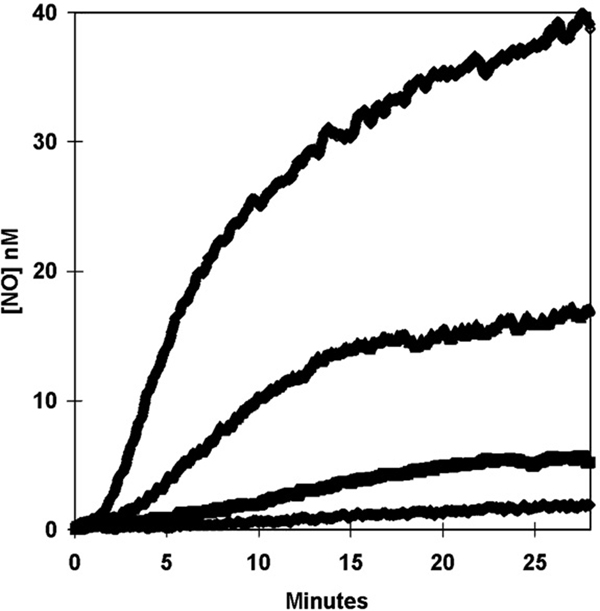
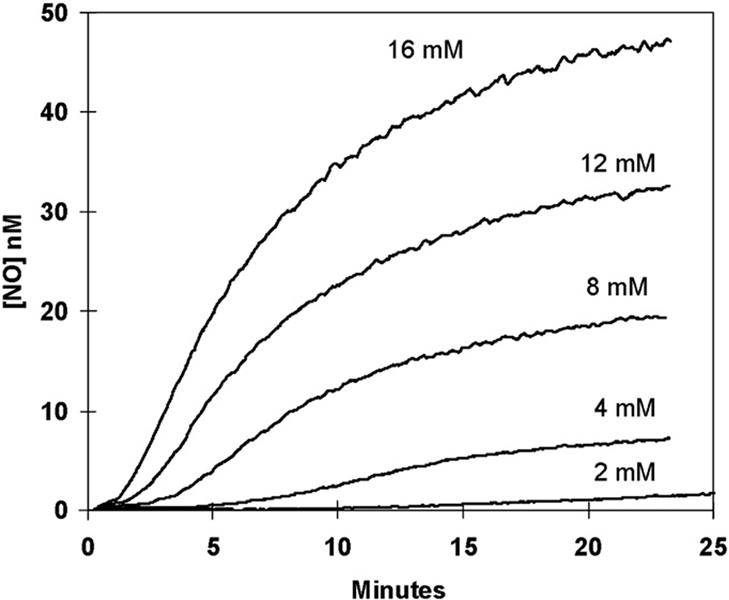
On addition of nitrite to deoxy-Hb(FeII), both the initial velocity of the appearance of the NO signal and the plateau level at long times increased with decreasing pH (Fig. 7). The increase in the initial velocity of NO formation is consistent with dependence on an ionization with pKa less than 6.5. This is possibly the pKa of HNO2 which is near 3.3, although it could represent a pKa on hemoglobin itself. The accumulation of free NO as a function of the initial concentration of nitrite is shown in Fig. 8. With increasing initial nitrite, the maximal level of NO at times near 20 min increased monotonically. In addition, with increasing initial nitrite there was a shortening of the duration of the plateau from over 10 min at 2 mM nitrite to less than 2 min at 16 mM nitrite (Fig. 8).
Fig. 7.
The variation with pH of the generation of NO measured by membrane inlet mass spectrometry after the addition at time zero of NaNO2 to achieve a concentration of 8 mM to solutions containing 20 µM deoxy-Hb(FeII). The pH of solutions was (from the top down) pH 6.5; pH 6.7; pH 6.9; and pH 7.2. Otherwise, solution conditions were as described in Fig. 3.
Fig. 8.
The variation with concentration of nitrite of the generation of NO measured by membrane inlet mass spectrometry. Sodium nitrite was added at time zero to achieve the final concentrations indicated. Solutions also contained 14 µM deoxy-Hb(FeII). Solution conditions were as described in Fig. 3 except pH was 6.7 at 25° C and 2 mM diethylenetriaminepentaacetate was used as chelator.
Discussion
The advantages of membrane inlet mass spectrometry have not previously been applied to studies of the generation of NO from nitrite reactivity with hemoglobin. These advantages include a sensitive, direct, and real-time measure of the accumulation in solution of free, unbound NO. Moreover, this technology is quite adaptable to the goals of many in vivo and physiological experiments. The method has the capability of detecting related nitrogen oxides concurrently, if they accumulate sufficiently in solution and are sufficiently stable, as well as oxygen and carbon dioxide. This study demonstrates some properties of the appearance of NO in solution resulting from the reactivity of nitrite with hemoglobin observed using membrane inlet mass spectrometry. In all of these experiments, NO is detected in the solution phase.
The data of Fig. 3 show the accumulation of free, unbound NO in well-buffered solutions containing an initial concentration of 38 µM deoxy-Hb(FeII) (heme concentration) on addition of nitrite at 8 mM and pH 6.8. These concentrations are clearly not in the range of physiological levels, but were selected on the basis of the substrate profiles of Fig. 7 to give a measurable level of NO. There appear two phases in the increase of the m/z 30 peak during the accumulation of free, unbound NO in solutions containing nitrite and deoxy-Hb(FeII) (Fig. 3), an initial phase of slower generation of NO (up to about 2.5 min) followed by a phase of more rapid generation of NO.
The reduction of nitrite by deoxy-Hb(FeII) is a complex, autocatalytic, and allosteric reaction that ultimately results in concentrations of NO-Hb(FeII) and met-Hb(FeIII) that are nearly equal (Eqs. (1) and (2)) [1,14]. This was evident in our experiments from the emergence, within 3 min, of an absorption spectrum consistent with the sum of spectra of NO-Hb(FeII) and species of met-Hb(FeIII) (Fig. 4). Three minutes after addition of nitrite, there was no significant change in the visible spectra that we could detect.
| (1) |
| (2) |
This initial phase of accumulation of free, unbound NO is understood to be caused by the concomitant uncatalyzed and the hemoglobin enhanced generation of NO from nitrite accompanied by the rapid and tight binding of NO to deoxy-Hb(FeII). This is consistent with the visible spectra of Fig. 4 showing the rapid decrease in deoxy-Hb(FeII) and concomitant increase in NO-Hb(FeII). It is also consistent with simulations based on numerical solutions to the kinetic equations of Eqs. (1) and (2).
The plateau near 14 nM at times greater than 20 min in Fig. 3 is a steady-state level for the accumulation of free NO, a level which represents a complex contribution of several processes including the uncatalyzed reactions of NO and the nitrite reductase activity of deoxy-Hb(FeII). The logarithm of the rates of NO accumulation (after the initial phase) is a linear function of pH (R2=0.998) with the rate increasing as pH decreases (data after 2 min in Fig. 7). This linear dependence is consistent with previous observations on the pH dependence of the generation of NO caused by the nitrite reductase activity of hemoglobin [15]. Hence, the accumulation of free, unbound NO observed in these experiments is likely due to the same reactions of nitrite with hemoglobin as described previously [3,15].
Our results are consistent with the presence of a small amount of unreacted deoxy-Hb(FeII) remaining at long times in the overall reaction with nitrite. That is, we may be observing in Fig. 3 the small remnant of the overall reaction of deoxy-Hb(FeII) with nitrite in which concentrations of deoxy-Hb(FeII) have become very low. One possibility for a contribution to the steady-state level of deoxy-Hb (FeII) is the very slow dissociation of NO from nitrosyl-hemoglobin. This off-rate is estimated at 2 10−5 s−1 for NO-Hb(FeII) in the R-state [16]. Although this is an exceptionally slow rate constant, it is consistent with the nanomolar levels of NO at the plateau of Fig. 3 according to straightforward simulations based on Eqs. (1) and (2).
Addition of nitrite to a solution containing oxy-Hb(FeII) showed no accumulation of NO (Fig. 3). That is, the generation of NO by the uncatalyzed dismutation of nitrite appears to have reacted with oxy-Hb(FeII). The reaction of NO with oxy-Hb(FeII) is again complex but the mechanism leading to the production of met-Hb(FeIII) and nitrate is well studied (Eq. (3)) [1,17–20]. In addition, in these experiments the data for oxy-Hb(FeII) of Fig. 3 provide a useful control for the specific nature of the reactions of nitrite and NO at the metal of hemoglobin.
| (3) |
Although NO is a product of the reaction of nitrite with deoxy-Hb(FeII) (Eq. (1)), it is acknowledged that autocapture of NO by deoxy-Hb(FeII) (Eq. (2)) in the interior of red blood cells precludes the export of NO directly to plasma levels needed for vasodilation (1–10 nM) [21]. The data of the current report are consistent with that view. Even at the very large levels of nitrite used and the very large ratio of concentrations of nitrite to hemoglobin, the levels of NO achieved by membrane inlet mass spectrometry do not exceed 1 nM for several minutes after mixing (Fig. 3, Fig. 7, and Fig. 8). However, several limiting qualifications of these experiments need to be noted. It should be noted that the response time of our instrumentation has a half-time for detection of NO that is about 5 s. Hence, we would likely not be able to observe at the mass spectrometer a quick pulse or transitory initial increase in NO concentration of duration less than or about equal to a second. Moreover, the data of this study involve nonphysiological concentrations of nitrite (mM) and nonphysiological concentrations hemoglobin (mM); under such conditions we do not observe concentrations of NO (1–10 nM) on the time scale of seconds relevant for vascular regulation. It is recognized that the conditions of these experiments diverge greatly from the physiological conditions in the red cell (Hb 5 mM, nitrite ~ 1 µM, and hemoglobin saturation ~ 50–100%).
A number of other mediators of NO generation have been proposed, prominent among which is hemoglobin as an S-nitrosothiol synthase with the resulting modified thiol of Cys β-93 acting as a signaling agent in respiratory control and other systems, a topic which is extensively reviewed both in mechanism and as a cause of certain vascular disease states [2,5,22,23]. In this view, experiments show that the nitrosylated thiol in hemoglobin regulates bioactivity such as vasodilation, with controls in which this activity is suppressed when the nitrosylation of hemoglobin is decreased [22]. These examples of the influence of nitrosylation extend to several physiological systems such as vasodilation in rat aortic rings [24] and hypoxic pulmonary vasoconstriction in isolated rat lungs [25]. The current report appears consistent with these ideas, with the qualifications stated above, to the extent that on a relevant time scale we observed no release of NO in the nitrite reductase reactions of deoxy-Hb(FeII).
Another possible mediator is the formation of N2O3 which does not have a strong binding affinity to hemoglobin and could diffuse out of the red cell [26]. The lifetime of N2O3 in solution is near 1 ms determined by its reaction with nucleophiles and its dissociation to NO and NO2 [27]. It is of interest then that under the conditions of these experiments with current instrumentation there was no evidence of a mass peak at m/z 76 that would indicate the formation of N2O3 with sufficient stability to pass across the membrane inlet and enter the mass spectrometer. There is rapid dissociation of N2O3 in the gas phase, so perhaps the current method is not capable of detecting this species. Also of interest is the suggestion that there is more than one isomer of N2O3 [28] and that the isomer made in the reactions with hemoglobin could have different properties compared with those studied with chemical generation of N2O3.
The effect of β-Cys93 and SNO-Hb formation on the accumulation of NO is extensively studied [2,29,30] and considered here. The data of Fig. 6 demonstrate the effect of HgCl2 on the accumulation of free, unbound NO in the presence of nitrite and deoxy-Hb(FeII). Mercuric ions in these experiments are expected to bind thiolates, and possibly exposed imidazole rings of histidines. It is apparent in Fig. 6 that the presence of mercuric ions at the beginning of the experiment shortens the initial phase of NO accumulation and substantially increases the rate of NO accumulation and the maximal level of NO accumulation in the second phase. The presumed binding of the thiolate of β-Cys93 by mercuric ions alters all aspects of the accumulation of NO in solutions of deoxy-Hb(FeII). Further work on this effect is in progress. We can comment that straightforward simulations of the generation of NO based on numerical solutions of the kinetic equations (Eqs. (1) and (2)) are consistent with mercuric ions causing an increase in the rate of Eq. (1).We note that this is likely a complex effect since altered transfer of NO to Cys93 will likely perturb other equilibria of the system such as the allosteric transition between T and R states of hemoglobin [30].
In summary, the value of this study is to explore the use of membrane inlet mass spectrometry in the generation of NO fromnitrite and deoxy-Hb(FeII). The method is based on direct measurement of free, unbound NO in solution; there is no purging of NO by inert, carrier gas. These observations of the reactions of nitrite with deoxy-Hb(FeII) in solution show that for several minutes after mixing, free NO does not accumulate to levels significant for vasoregulatory control (levels exceeding the equilibrium level of NO in the absence of hemoglobin (2 nM)) even under our exaggerated conditions of nitrite concentrations much larger than physiological. The data suggest that under these conditions, the nitrite reductase reactions are dominated by the autocapture of NO by deoxy-Hb(FeII) preventing the accumulation of free, unbound NO.
Acknowledgments
We thank Dr. Mark T. Gladwin for the program to deconvolute hemoglobin spectra and Dr. Daniel Kim-Shapiro for helpful discussions. This work was supported in part by a grant from the Thomas H. Maren Foundation, from the NIH (GM25154), and from funds made available by the University of Florida.
Abbreviations
- Hb
hemoglobin
- NO
nitric oxide
References
- 1.Kim-Shapiro DB, Gladwin MT, Patel RP, Hogg N. The reaction between nitrite and hemoglobin: the role of nitrite in hemoglobin-mediated hypoxic vasodilation. J. Inorg. Biochem. 2005;99:237–246. doi: 10.1016/j.jinorgbio.2004.10.034. [DOI] [PubMed] [Google Scholar]
- 2.Singel DJ, Stamler JS. Chemical physiology of blood flow regulation by red blood cells: the role of nitric oxide and S-nitrosohemoglobin. Annu. Rev. Physiol. 2005;67:99–145. doi: 10.1146/annurev.physiol.67.060603.090918. [DOI] [PubMed] [Google Scholar]
- 3.Doyle MP, Pickering RA, DeWeert TM, Hoekstra JW, Pater D. Kinetics and mechanism of the oxidation of human deoxyhemoglobin by nitrites. J. Biol. Chem. 1981;256:12393–12398. [PubMed] [Google Scholar]
- 4.Gladwin MT, Crawford JH, Patel RP. The biochemistry of nitric oxide, nitrite, and hemoglobin: role in blood flow regulation. Free Radic. Biol. Med. 2004;36:707–717. doi: 10.1016/j.freeradbiomed.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 5.Gaston B, Singel D, Doctor A, Stamler JS. S-Nitrosothiol signaling in respiratory biology. Am. J. Respir. Crit. Care Med. 2006;173:1186–1193. doi: 10.1164/rccm.200510-1584PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brodbelt JS, Cooks RG, Tou JC, Kallos GJ, Dryzga MD. In vivo mass-spectrometric determination of organic-compounds in blood with a membrane probe. Anal. Chem. 1987;59:454–458. doi: 10.1021/ac00130a017. [DOI] [PubMed] [Google Scholar]
- 7.Silverman DN. Carbonic anhydrase: oxygen-18 exchange catalyzed by an enzyme with rate-contributing proton-transfer steps. Methods Enzymol. 1982;87:732–752. doi: 10.1016/s0076-6879(82)87037-7. [DOI] [PubMed] [Google Scholar]
- 8.Lewis RS, Deen WM, Tannenbaum SR, Wishnook JS. Membrane mass spectrometer inlet for quantitation of nitric oxide. Biol. Mass Spectrometry. 1993;22:45–52. doi: 10.1002/bms.1200220106. [DOI] [PubMed] [Google Scholar]
- 9.Tu CK, Swenson ER, Silverman DN. Membrane inlet for mass spectrometric measurement of nitric oxide. Free Radic. Biol. Med. 2007;43:1453–1457. doi: 10.1016/j.freeradbiomed.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 10.Samouilov A, Kuppusamy P, Zweier JL. Evaluation of the magnitude and rate of nitric oxide production from nitrite in biological systems. Arch. Biochem. Biophys. 1998;357:1–7. doi: 10.1006/abbi.1998.0785. [DOI] [PubMed] [Google Scholar]
- 11.Park J-Y, Lee Y-N. Solubility and decomposition kinetics of nitrous acid in aqueous solution. J. Phys. Chem. 1988;92:6294–6302. [Google Scholar]
- 12.Turney TA, Wright GA. Nitrous acid and nitrosation. Chem. Rev. 1959;59:497–513. [Google Scholar]
- 13.Maragos CM, Morley D, Wink DA, Dunams TM, Saavedra JE, Hoffman A, Bove AA, Isaac L, Hrabie JA, Keefer LK. Complexes of.NO with nucleophiles as agents for the controlled biological release of nitric oxide: vasorelaxant effects. J. Med. Chem. 1991;34:3242–3247. doi: 10.1021/jm00115a013. [DOI] [PubMed] [Google Scholar]
- 14.Huang Z, Shiva S, Kim-Shapiro DB, Patel RP, Ringwood LA, Irby CE, Huang KT, Ho C, Hogg N, Schechter AN, Gladwin MT. Enzymatic function of hemoglobin as a nitrite reductase that produces NO under allosteric control. J. Clin. Invest. 2005;115:2099–2107. doi: 10.1172/JCI24650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang Z, Shiva S, Kim-Shapiro D, Patel R, Ringwood L, Irby C, Huang K, Ho C, Hogg N, Schechter A, Gladwin M. Enzymatic function of hemoglobin as a nitrite reductase that produces NO under allosteric control. J. Clin. Invest. 2005;115:2099–2107. doi: 10.1172/JCI24650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooper CE. Nitric oxide and iron proteins. Biochim. Biophys. Acta-Bioenergetics. 1999;1411:290–309. doi: 10.1016/s0005-2728(99)00021-3. [DOI] [PubMed] [Google Scholar]
- 17.Herold S, Exner M, Nauser T. Kinetic and mechanistic studies of the NO center dot-mediated oxidation of oxymyoglobin and oxyhemoglobin. Biochemistry. 2001;40:3385–3395. doi: 10.1021/bi002407m. [DOI] [PubMed] [Google Scholar]
- 18.Doyle MP, Hoekstra JW. Oxidation of nitrogen-oxides by bound dioxygen in hemoproteins. J. Inorg. Biochem. 1981;14:351–358. doi: 10.1016/s0162-0134(00)80291-3. [DOI] [PubMed] [Google Scholar]
- 19.Grubina R, Huang Z, Shiva S, Joshi M, Azarov I, Basu S, Ringwood L, Jiang A, Hogg N, Kim-Shapiro D, Gladwin M. Concerted nitric oxide formation and release from the simultaneous reactions of nitrite with deoxy- and oxyhemoglobin. J. Biol. Chem. 2007;282:12916. doi: 10.1074/jbc.M700546200. [DOI] [PubMed] [Google Scholar]
- 20.Herold S, Rock G. Mechanistic studies of the oxygen-mediated oxidation of nitrosylhemoglobin. Biochemistry. 2005;44:6223–6231. doi: 10.1021/bi0475929. [DOI] [PubMed] [Google Scholar]
- 21.Jeffers A, Xu XL, Huang KT, Cho M, Hogg N, Patel RP, Kim-Shapiro DB. Hemoglobin mediated nitrite activation of soluble guanylyl cyclase. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2005;142:130–135. doi: 10.1016/j.cbpb.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 22.Luchsinger BP, Rich EN, Yan Y, Williams EM, Stamler JS, Singel DJ. Assessments of the chemistry and vasodilatory activity of nitrite with hemoglobin under physiologically relevant conditions. J. Inorg. Biochem. 2005;99:912–921. doi: 10.1016/j.jinorgbio.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 23.Allen BW, Piantadosi CA. How do red blood cells cause hypoxic vasodilation? The SNO-hemoglobin paradigm. Am. J. Physiol. Heart Circ. Physiol. 2006;291:H1507–H1512. doi: 10.1152/ajpheart.00310.2006. [DOI] [PubMed] [Google Scholar]
- 24.Dalsgaard T, Simonsen U, Fago A. Nitrite-dependent vasodilation is facilitated by hypoxia and is independent of known NO-generating nitrite reductase activities. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H3072. doi: 10.1152/ajpheart.01298.2006. [DOI] [PubMed] [Google Scholar]
- 25.Deem S, Min JH, Moulding JD, Eveland R, Swenson ER. Red blood cells prevent inhibition of hypoxic pulmonary vasoconstriction by nitrite in isolated, perfused rat lungs. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H963–H970. doi: 10.1152/ajpheart.00812.2006. [DOI] [PubMed] [Google Scholar]
- 26.Basu S, Grubina R, Huang J, Conradie J, Huang Z, Jeffers A, Jiang A, He X, Azarov I, Seibert R, Mehta A, Patel R, King SB, Hogg N, Ghosh A, Gladwin MT, Kim-Shapiro DB. Catalytic generation of N2O3 by the concerted nitrite reductase and anhydrase activity of hemoglobin. Nat. Chem. Biol. 2007;3:785–794. doi: 10.1038/nchembio.2007.46. [DOI] [PubMed] [Google Scholar]
- 27.Ford E, Hughes MN, Wardman P. Kinetics of the reactions of nitrogen dioxide with glutathione, cysteine, and uric acid at physiological pH. Free Radic. Biol. Med. 2002;32:1314–1323. doi: 10.1016/s0891-5849(02)00850-x. [DOI] [PubMed] [Google Scholar]
- 28.Challis BC, Kyrtopoulos SA. Chemistry of nitroso-compounds. 12. Mechanism of nitrosation and nitration of aqueous piperidine by gaseous dinitrogen tetraoxide and dinitrogen trioxide in aqueous alkaline-solutions—evidence for the existence of molecular isomers of dinitrogen tetraoxide and dinitrogen trioxide. J. Chem. Soc. Perkin Trans. 1978;2:1296–1302. [Google Scholar]
- 29.Pawloski JR, Hess DT, Stamler JS. Export by red blood cells of nitric oxide bioactivity. Nature. 2001;409:622–626. doi: 10.1038/35054560. [DOI] [PubMed] [Google Scholar]
- 30.Angelo M, Singel DJ, Stamler JS. An S-nitrosothiol (SNO) synthase function of hemoglobin that utilizes nitrite as a substrate. Proc. Natl. Acad. Sci. USA. 2006;103:8366–8371. doi: 10.1073/pnas.0600942103. [DOI] [PMC free article] [PubMed] [Google Scholar]