Abstract
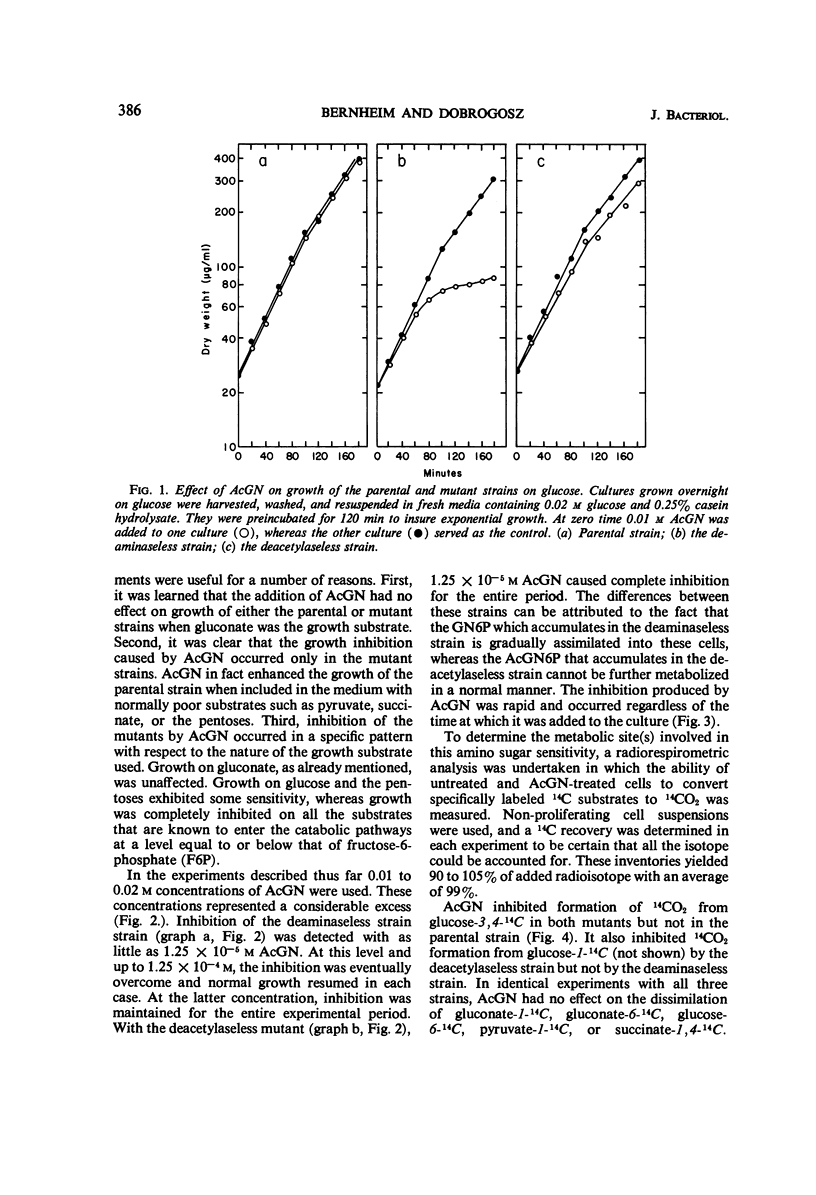
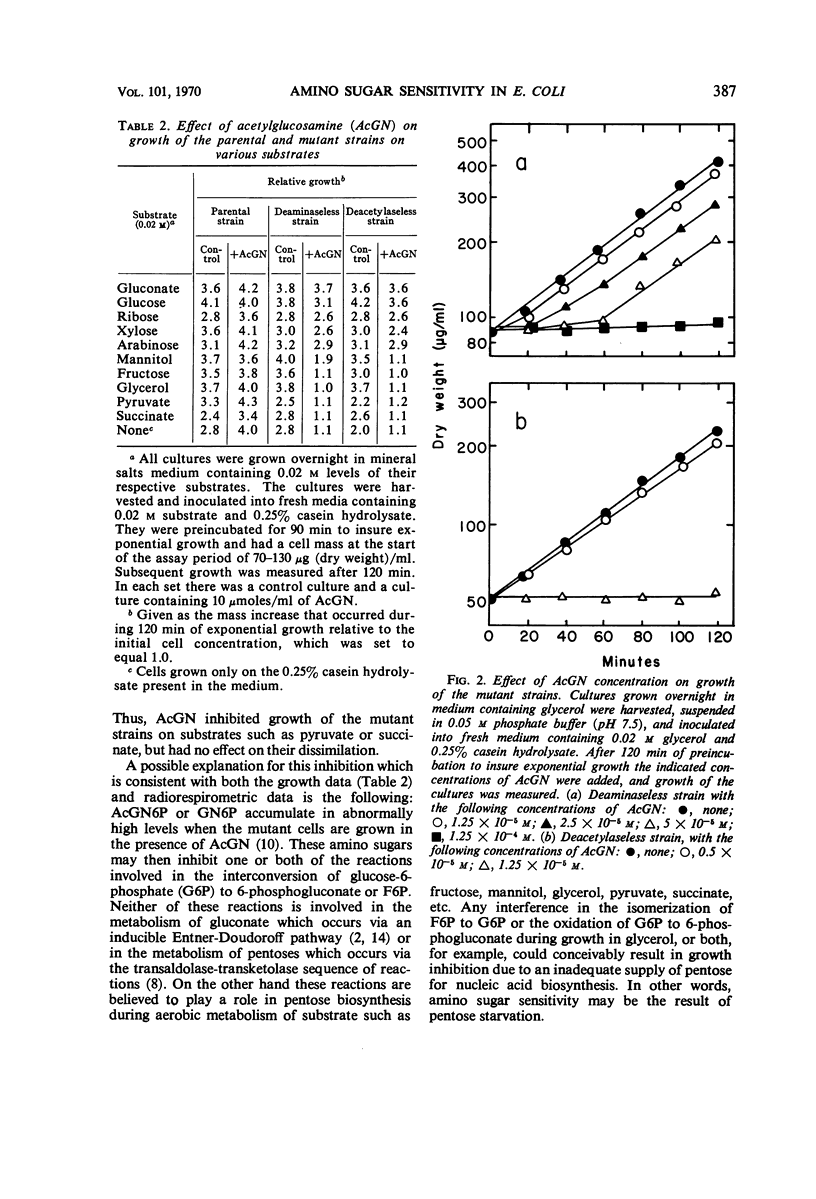
Studies were conducted on two mutants of Escherichia coli that lack either glucosamine-6-phosphate deaminase or N-acetylglucosamine-6-phosphate deacetylase and which accumulate glucosamine-6-phosphate or N-acetylglucosamine-6-phosphate, respectively, when grown in the presence of N-acetylglucosamine. The addition of 10−4 to 10−5mN-acetylglucosamine to these mutant strains caused a rapid and complete inhibition of growth on substrates that enter the catabolic pathways at or below the level of fructose-6-phosphate. Growth on glucose was inhibited to a lesser degree, whereas only minor inhibition occurred when the pentoses were used as substrates. Growth on gluconate was found to be totally unaffected by these levels of N-acetylglucosamine. The objective of this investigation was to determine the nature of this “amino sugar sensitivity” phenomenon and the conditions under which it could be overcome. It was found that this amino sugar sensitivity was abolished when an exogenous source of pentose such as uridine was included in the culture medium. Experiments are described indicating that the accumulated amino sugar phosphate metabolites interfere with an early step in hexose metabolism of both mutants, resulting in a pentose deficiency and consequent inhibition of growth on certain substrates.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dobrogosz W. J. Corepressor system for catabolite repression of the lac operon in Escherichia coli. J Bacteriol. 1969 Mar;97(3):1083–1092. doi: 10.1128/jb.97.3.1083-1092.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENGLESBERG E., BARON L. S. Mutation to L-rhamnose resistance and transduction to L-rhamnose utilization in Salmonella typhosa. J Bacteriol. 1959 Nov;78:675–686. doi: 10.1128/jb.78.5.675-686.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. C., Dobrogosz W. J. Gluconate metabolism in Escherichia coli. J Bacteriol. 1967 Mar;93(3):941–949. doi: 10.1128/jb.93.3.941-949.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUKASAWA T., NIKAIDO H. Galactose-sensitive mutants of Salmonella. II. Bacteriolysis induced by galactose. Biochim Biophys Acta. 1961 Apr 15;48:470–483. doi: 10.1016/0006-3002(61)90045-2. [DOI] [PubMed] [Google Scholar]
- GLASER L., BROWN D. H. Purification and properties of d-glucose-6-phosphate dehydrogenase. J Biol Chem. 1955 Sep;216(1):67–79. [PubMed] [Google Scholar]
- NAKADA H. I., WOLFE J. B. Glucosamine degradation by Escherichia coli. II. The isomeric conversion of glucosamine 6-PO4 to fructose 6-PO4 and ammonia. Arch Biochem Biophys. 1956 Oct;64(2):489–497. doi: 10.1016/0003-9861(56)90291-0. [DOI] [PubMed] [Google Scholar]
- Okinaka R. T., Dobrogosz W. J. Catabolite repression and pyruvate metabolism in Escherichia coli. J Bacteriol. 1967 May;93(5):1644–1650. doi: 10.1128/jb.93.5.1644-1650.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG C. H., STERN I., GILMOUR C. M., KLUNGSOYR S., REED D. J., BIALY J. J., CHRISTENSEN B. E., CHELDELIN V. H. Comparative study of glucose catabolism by the radiorespirometric method. J Bacteriol. 1958 Aug;76(2):207–216. doi: 10.1128/jb.76.2.207-216.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. J. Control of amino sugar metabolism in Escherichia coli and isolation of mutants unable to degrade amino sugars. Biochem J. 1968 Feb;106(4):847–858. doi: 10.1042/bj1060847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. J., Pasternak C. A. The purification and properties of N-acetylglucosamine 6-phosphate deacetylase from Escherichia coli. Biochem J. 1967 Oct;105(1):121–125. doi: 10.1042/bj1050121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarmolinsky M. B., Wiesmeyer H., Kalckar H. M., Jordan E. HEREDITARY DEFECTS IN GALACTOSE METABOLISM IN ESCHERICHIA COLI MUTANTS, II. GALACTOSE-INDUCED SENSITIVITY. Proc Natl Acad Sci U S A. 1959 Dec;45(12):1786–1791. doi: 10.1073/pnas.45.12.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zablotny R., Fraenkel D. G. Glucose and gluconate metabolism in a mutant of Escherichia coli lacking gluconate-6-phosphate dehydrase. J Bacteriol. 1967 May;93(5):1579–1581. doi: 10.1128/jb.93.5.1579-1581.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]



