Abstract
A series of new squaraine rotaxanes have been synthesized with a tetralactam macrocycle and stopper groups of varying sizes and functionalities. In chloroform solvent, the relative size of the stopper group appears to have little influence on the high mechanical stability of the rotaxane structure. There is no evidence for unthreading (sometimes referred to as deslipping), even in the presence of competing chloride salts or elevated temperatures. A difference in rotaxane stability emerges as the polarity of the organic solvent is increased. Squaraine rotaxanes with small stopper groups undergo unthreading in the polar aprotic solvent DMSO. However, a water soluble tetracarboxylic acid derivative was found to be highly stable in aqueous solvents containing serum.
INTRODUCTION
Near infrared (NIR) organic fluorophores are increasingly used in modern bioimaging technologies, and are especially promising as probes for studies in living animals [1–4]. This latter application requires the fluorescent dyes to possess certain characteristics. First, they must emit in the region 650–900 nm (approximately the NIR) where background autofluorescence from biomolecules and undesired absorption by tissue is reduced [5–7]. Second, the dyes must be non-toxic and chemically inert in biological environments. Finally, the dyes must be amenable to conjugation with targeting ligands to produce imaging probes with high target selectivity [8, 9].
The squaraines are a well known class of NIR dyes with intense and narrow emission bands that are quite suited for bioimaging. However, there are some technical drawbacks; they are susceptible to nucleophilic attack and like many organic dyes they undergo self-quenching upon aggregation [10, 11]. We have discovered that both problems are eliminated by encapsulating the dye inside a macrocycle to form a squaraine rotaxane [12]. The macrocycle protects the C4O2 core of the squaraine thread from attack by nucleophiles and prevents interchromophore energy transfer upon aggregation. At the same time, the rotaxanes preserve the favorable photophysical properties of the precursor squaraines. The next step in our research is to determine how squaraine rotaxanes can be converted into high performance fluorescent probes for bioimaging [13].
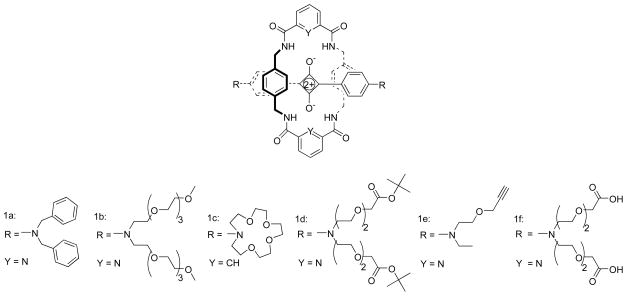
Our initial studies used rotaxanes such as 1a (Figure 1) with large N,N-bisbenzyl stopper groups on the squaraine to ensure that unthreading (sometimes referred to as deslipping) did not occur [12]. However, large hydrocarbon stopper groups are not desired for bioimaging probes, where the goal is to produce water soluble compounds with low molecular weight. Additionally, we need to develop stopper groups that can be conjugated to targeting ligands using standard coupling reactions. Here, we report that squaraine rotaxanes 1b–1f can be prepared with smaller and more flexible stopper groups. Spectroscopic studies in various solvents, in the presence of ions, and in biological solution, reveal exceptional mechanical stability even when the stopper groups are relatively small compared to the macrocyclic cavity.
FIGURE 1.
Squaraine Rotaxanes
RESULT AND DISCUSSION
Synthesis and structure
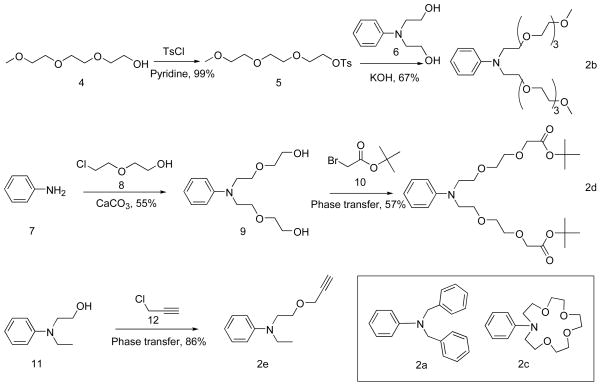
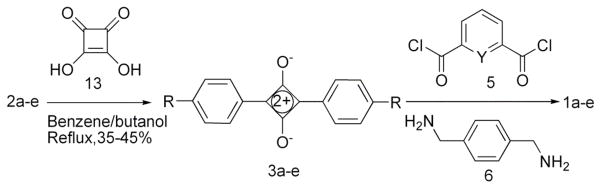
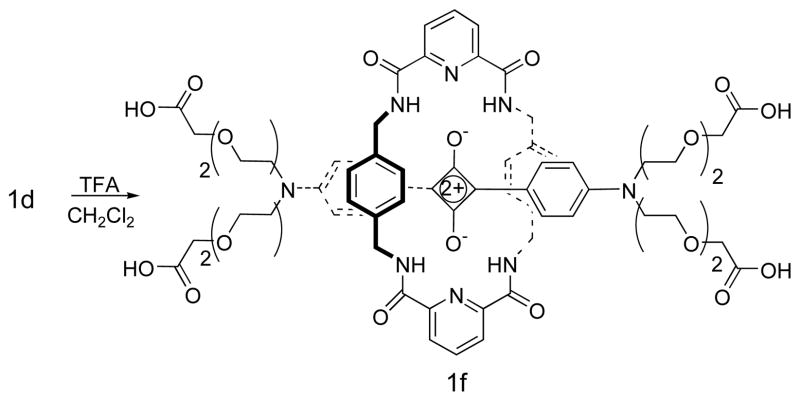
The precursor aniline derivatives, 2b, 2d, and 2f were obtained by the straightforward synthetic methods shown in Scheme 1; whereas 2a and 2c were commercially available. In each case, the aniline compound was added to a benzene/butanol solution of squaric acid and heated under azetropic distillation conditions to afford squaraine dyes 3a–3e in yields of 35–45%. The dyes were converted into squaraine rotaxanes with yields around 20% by conducting Leigh-type clipping reactions (Scheme 2) [12]. Rotaxane 1d was hydrolyzed by TFA to afford the water soluble tetracarboxylic acid rotaxane 1f (Scheme 3).
SCHEME 1.
Synthesis and structures of aniline precursors.
SCHEME 2.
Synthesis of squaraine rotaxanes.
SCHEME 3.
Synthesis of squaraine rotaxane 1f
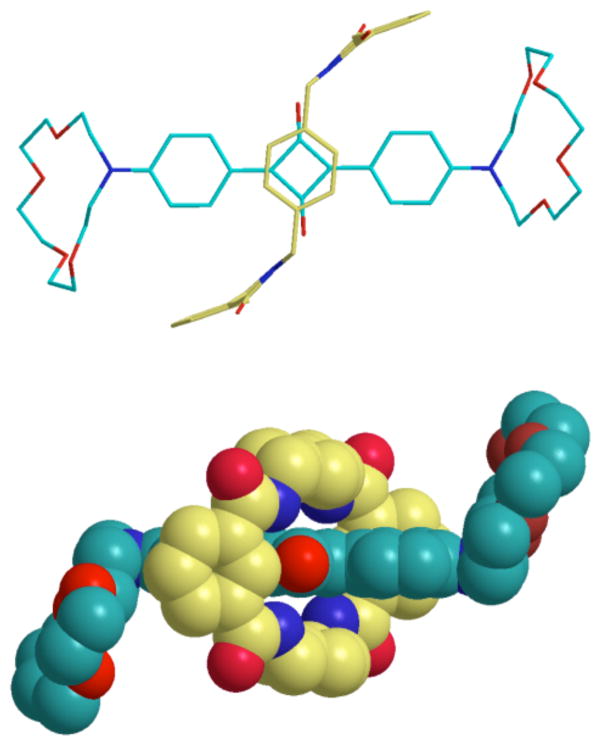
The rotaxane structures were characterized by standard spectrometric methods. The 1H NMR spectra exhibited the expected changes in chemical shift due to anisotropic shielding by the interlocked rotaxane components. Another signature of rotaxane formation is a 10–20 nm red-shift in absorption and emission maxima for the squaraine dye upon encapsulation inside the phenylene-containing tetralactam macrocycle. The bis(azacrown) squaraine rotaxane 1c was obtained as single crystals that were suitable for analysis by X-ray diffraction. As shown in Figure 2, the macrocycle adopts a chair-like conformation in the solid state. The four macrocyclic NH residues form bifurcated hydrogen bonds with the two squaraine oxygen atoms. The NH···O hydrogen-bond lengths and angles are 2.16 Å, 168.2° and 2.22 Å, 174.7° for the centrosymmetic structure. The parallel 1,4-xylylene units are stacked directly over both faces of the electron-deficient C4O2 core of the squaraine thread. The centroid to centroid distance between the two parallel aryl rings in the macrocycle is 7.20 Å. A comparison with previously elucidated squaraine rotaxane structures such as 1a [12] indicates that the more flexible crown ether stopper groups do not alter rotaxane co-conformation in the solid state.
FIGURE 2.
X-ray crystal structure of 1c shown in sideview (top) and topview (bottom).
Stability in Non-Polar Solvent
Since the encapsulating phenylene-containing macrocycles are highly insoluble in most organic solvents it seemed to us that a moderate amount of unthreading might manifest itself as an irreversible process that is driven by macrocyclic precipitation [14]. However, we do not see this phenomenon in chloroform solution. We find that rotaxanes 1a–1e are soluble and completely stable with no spectroscopic evidence for unthreading. Even samples that were stored in CDCl3 at 50 °C for one week showed no changes in their 1H NMR spectra [13]. Furthermore, unthreading of 1c could not be induced by the addition of chloride anions that potentially could form competing hydrogen bonds with the NH residues [15,16]. The addition of excess lithium chloride in CDCl3 or tetrabutylammonium chloride in CD3CN did not induce any sign of unthreading as judged by 1H NMR or absorption spectroscopy. We conclude that in non-polar solvent, the surrounding macrocycle is held tightly to the squaraine thread by a combination of favorable aromatic stacking interactions between the electron poor C4O2 core of the squaraine and the electron rich 1,4-xylylene units in the macrocycle, and strong hydrogen bonds between the four macrocyclic NH residues and the two squaraine oxygen atoms.
Stability in polar aprotic DMSO
Polar aprotic organic solvents are known to greatly weaken the non-covalent association of host/guest complexes when the principle stabilizing forces are hydrogen bonding [17, 18], dispersion forces, or aromatic stacking [19]. Thus, it was not surprising to find that the highly polar DMSO promotes unthreading of squaraine rotaxanes. 1H NMR spectroscopy was used to monitor the structural integrity of 1a and 1e in three solvent systems; pure CDCl3, DMSO-d6/CDCl3 (1:9), and pure DMSO-d6. For rotaxane 1e, with its slender alkyne stopper groups, there was no NMR evidence after 50 days at 23 °C for unthreading in CDCl3; however, unthreading was observed when the solvent was DMSO-d6/CDCl3 (1:9) and pure DMSO–d6 with half-lives of 50 days and 12 hours, respectively (Table 1). The same NMR experiments with squaraine rotaxane 1a revealed the anticipated enhancement of mechanical stability afforded by the larger N,N-bisbenzyl stopper groups [20]. In the highly disruptive DMSO-d6, the half-life for squaraine-rotaxane 1a is approximately 40 days, which is eighty times longer than the half-life for squaraine-rotaxane 1e.
TABLE 1.
Half-lives for rotaxane unthreading at 23 °C.
| Compound (5 mM) | Solvent | t1/2 |
|---|---|---|
| 1a | CDCl3 | >50 days |
| DMSO-d6/CDCl3 (1:9) | >50 days | |
| DMSO-d6 | 40 days | |
| 1e | CDCl3 | >50 days |
| DMSO-d6/CDCl3 (1:9) | 50 days | |
| DMSO-d6 | 12 hours |
Stability in Water
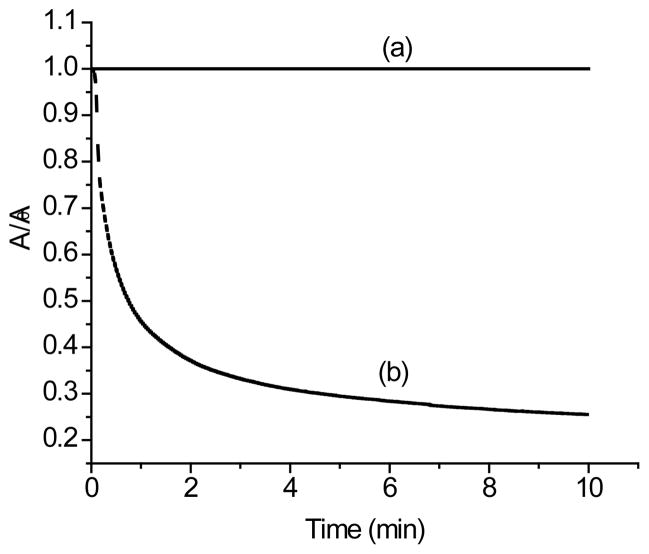
The tetracarboxylic acid 1f was synthesized and tested as a fully water soluble squaraine rotaxane. In 10% serum solution, 1f absorbs strongly at 651 nm (log ε ~ 5.4) and emits at 679 nm with a quantum yield of 0.17 (Table 2). Not surprisingly the quantum yield increases when THF is added as an organic co-solvent. Previously, we have shown that biological nucleophiles can attack the electrophilic C4O2 core in squaraine dyes and bleach the dye’s color in a few minutes, but squaraine rotaxanes are highly resistant to this chemical attack. Thus, the loss of squaraine color in biological solution is a convenient indicator of rotaxane unthreading. In Figure 3 is a comparison of the color stability of water soluble rotaxane 1f, and the corresponding precursor squaraine dye 3d in the presence of serum. As expected, the bleaching half life for dye 3d is only 1 minute. Remarkably, the color for rotaxane 1f is unchanged over the duration of the experiment (indeed the color is essentially unchanged for samples of 1f left standing for many hours). This strongly suggests that rotaxane 1f does not undergo unthreading in water or in biological solutions even though its relatively small and flexible stopper groups can potentially slide through the macrocyclic cavity. It appears that the mechanical stability of squaraine rotaxanes is extremely high in aqueous biological solution. Although water is a very polar solvent that can disrupt hydrogen bonds, the hydrophobic aromatic stacking interactions are enhanced, which stabilizes the squaraine rotaxane structure and prevents unthreading. This is a pleasing result for us because good water solubility and high stability are essential features for high performance fluorescent bioimaging probes.
TABLE 2.
Absorption and emission properties in different solvent mixtures.a
| Solvent | Compound | λabs, nm | λem, nm | Φf |
|---|---|---|---|---|
| H2O | 1f | 651 | 679 | 0.15 |
| 10 % serum | 1f | 651 | 679 | 0.17 |
| 10 % serum/THF(4:1) | 1f | 646 | 673 | 0.34 |
| THF | 1d | 640 | 665 | 0.54 |
| 3d | 633 | 652 | 0.74 | |
| 10 % serum/THF(4:1) | 1d | 648 | 674 | 0.33 |
| 3d | 645 | 673 | - |
Samples were excited at 580 nm and emission monitored in the region 600–850 nm. The dye bis[4-(N,N-dimethylamino)phenyl]squaraine was used as a standard (Φf = 0.70 in CHCl3) to determine the relative quantum yield (error for Φf ± 5 %).
FIGURE 3.
Change in absorption maxima for: (a) 1f in 10% serum solution, and (b) 3d in 10% serum/THF (1:1).
CONCLUSION
The very high host/guest complementarity between squaraine dyes and the encapsulating tetralactam macrocycle provide excellent mechanical stability and little propensity for unthreading. In non-polar organic solvent the rotaxanes are stabilized by strong hydrogen bonds and in water by hydrophobic aromatic stacking interactions. Only in highly polar aprotic solvents like DMSO is there evidence for rotaxane unthreading which can be countered by employing sterically large stopper groups. The high rotaxane stability in aqueous solution means that size of the stopper group is not a major design constraint and that it should be possible to attach a wide range of targeting groups to these dyes and produce a diverse portfolio of bioimaging agents.
EXPERIMENTAL SECTION
Crystallography
Crystal data for 1c. C32H24N4O4, C36H48N2O10, Mr = 1558.22, T = 100 K, monoclinic, P21/c, a = 15.1808(3) Å, b = 13.1032(3) Å, c = 19.7252(4) Å, β = 106.479(1)°, V = 3762.51(14) Å3, Z = 2, R1 = 0.0856, wR2 = 0.1819. Crystals were grown by slow diffusion of hexanes into acetonitrile/chloroform solution. The structure was solved with three unique elements in the asymmetric unit, two components as the wheel and thread of the rotaxane and one solvent of crystallization which shows partial occupancy between a molecule of chloroform and a molecule of acetonitrile.
Synthesis of tosylate 5
Triethylene glycol monomethyl ether (16.4 g, 0.1 mol) and pyridine (15 mL) were adding into a round bottom flask which was cooled in an ice bath. Tosyl chloride (22.8 g, 0.12 mol) was slowly added into the flask. The solution was allowed to warm to room temperature, and stired for 8 h. The solution was neutralizes with 1 M hydrochloric acid to pH 7. The solution was extracted with methylene chloride (3 × 100 mL), the combined organic layers were washed with water (3 × 100 mL), dried with anhydrous Na2SO4, and concentrated in vacuo to afford the product quantitatively as yellow oil. 1H NMR (300 MHz, CDCl3, TMS): δ 7.80 (d, J = 9.3, 2H), 7.33 (d, J = 9.3, 2H), 4.14 (m, 2H), 3.37–3.74 (m, 10H), 2.45 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 145.0, 133.1, 130.0, 128.1, 72.0, 70.8, 70.7, 69.5, 68.8, 59.1, 21.8; MS: (FAB), CHCl3, 319 [M+H]+.
Aniline 2b
N-phenyldiethanolamine (2.0 g, 0.011 mol), excess KOH (8.0 g) and compound 5 (8.1 g, 0.025 mol) in THF (100 mL) were refluxed for 24 h. Excess solvent was removed and the residue obtained was dissolved in water and extracted with dichloromethane (3 × 100 mL). The organic layer was separated and dried over anhydrous Na2SO4. The crude product was chromatographed over silica and eluted with a mixture of ethyl acetate/hexane (1/19) to afford a yellow oil as product (3.5 g, 67%). 1H NMR (300 MHz, CDCl3, TMS): δ 7.19 (m, 2H), 6.71(m, 3H), 3.65 (m, 32H), 3.38 (s, 6H); 13C NMR (75 MHz, CDCl3): δ 148.0, 129.5, 116.2, 111.9, 72.1, 70.8, 70.7, 68.7, 59.2, 51.1; MS: (FAB), CHCl3, 474 [M+H]+.
Aniline 9
Aniline (10.0 g, 0.1 mol), 2-(2-chloroethoxy)ethanol (37.2 g, 0.3 mol), and excess calcium carbonate (15.0 g) were heated in water under reflux with stirring until the mixture turned transparent after about 4 days. The liquid was cooled and extracted with ether (3 × 100 mL). The ether solution was collected and solvent was removed under vacuum to give crude product. The crude product was chromatographed over a silica and eluted with a mixture of methanol/chloroform (1/19) to afford the pure product as a yellow oil (14.8 g, 55%). 1H NMR (300 MHz, CDCl3, TMS): δ 7.20 (m, 2H), 6.73 (m, 3H), 3.68 (t, J = 6.0, 12H); 13C NMR (75 MHz, CDCl3): δ 148.3, 129.6, 117.1, 112.9, 72.8, 69.1, 61.8, 51.7; MS: (FAB), CHCl3, 270 [M+H]+.
Aniline 2d
Compound 9 (1.0 g, 3.7 mmol), and tert-butyl bromoacetate (4.3 g, 20 mmol) were dissolved in benzene (50 mL). Tetrabutylammonium bisulfate (0.37 mmol) was dissolved in 50% NaOH solution (50 mL) and the solutions were combined and stirred for 24 h at room temperature. The organic layer was isolated and washed with saturated NaCl solution (100 mL) and dried over anhydrous Na2SO4 and concentrated to give a yellow oil. The crude product was chromatographed over silica and eluted with a mixture of methanol/chloroform (1/49) to afford yellow oil as product (1.1 g, 57%). 1H NMR (300 MHz, CDCl3, TMS): δ 7.17 (m, 2H), 6.70(m, 3H), 6.68 (m, 2H), 4.00 (s, 4H), 3.60 (t, J = 6.0, 16H), 1.47 (s, 18H); 13C NMR (75 MHz, CDCl3): δ 169.8, 147.9, 129.5, 116.2, 113.3, 111.9, 81.8, 70.9, 69.3, 68.7, 51.1, 28.4; MS: (FAB), CHCl3, 498 [M+H]+.
Aniline 2f
2-(N-Ethylanilino)ethanol (3.0 g, 0.018 mol) and propargyl chloride (4.9 g, 0.066 mol) were dissolved in benzene (60 mL). Tetrabutylammonium bisulfate (0.6 g, 1.77 mmol) was dissolved in 50% NaOH solution (60 mL) and the solutions were combined and stirred for 48 h at room temperature. The organic layer was isolated and washed with saturated NaCl solution (100 mL) and dried over anhydrous Na2SO4 and concentrated to give a light yellow oil. The crude product was chromatographed over silica and eluted with a mixture of ethyl acetate/hexane (1/49) to afford a light yellow oil (3.18 g, 86%); 1H NMR (500 MHz, CDCl3, TMS): δ 7.21 (app t, 2H, J = 7.0), 6.70 (d, 2H, J = 8.0), 6.66 (t, 1H, J = 7.0), 4.17 (d, 2H, J = 2.0), 3.69 (t, 2H, J = 6.5), 3.53 (t, 2H, J = 6.5), 3.42 (q, 2H, J = 7.0), 2.43 (t, 1H, J = 2.0), 1.16 (t, 3H, J = 7.0); 13C NMR (125 MHz, CDCl3): δ 147.5, 129.1, 115.7, 111.7, 79.5, 74.4, 67.5, 58.2, 49.7, 45.2, 12.0; MS: (FAB), CHCl3, 204 [M+H]+.
General Procedure to Synthesize Squaraine Dye
Aniline derivative 2a–e (1.83 mmol) was added to a solution of squaric acid 13 (0.92 mmol) in a mixture of n-butanol (15 mL) and benzene (30 mL) in a 100 mL round bottom flask equipped with a Dean-Stark apparatus. After refluxing for 12 h at 95 °C, the solvent was removed under vacuum and the crude product was obtained. The crude product was purified by column chromatography using a silica column that was eluted with a mixture of methanol/chloroform (1/49).
Squaraine dye 3b: (0.33 g, 35% yield)
1H NMR (300 MHz, CDCl3, TMS): δ 8.35 (d, J = 9.0, 4H), 6.80 (d, J = 9.0, 4H), 3.54 – 3.76 (m, 64H), 3.37 (s, 12H); 13C (75 MHz, CDCl3): δ 188.9, 183.4, 154.2, 133.5, 120.3, 112.9, 72.2, 71.1, 70.9, 70.8, 68.7, 59.2, 51.6, 29.9 MS: (FAB), CHCl3, 1025 [M+H]+.
Squaraine dye 3c: (0.25 g, 40% yield)
1H NMR (300 MHz, CDCl3, TMS): δ 8.38 (d, J = 9.0, 4H), 6.77 (d, J = 9.0, 4H), 3.63–3.82 (m, 40H); 13C (75 MHz, CDCl3): δ 188.9, 183.5, 153.9, 133.5, 120.4, 112.9, 71.5, 70.7, 70.3, 68.5, 53.6 MS: (ESI), CHCl3, 669 [M+H]+.
Squaraine dye 3d: (0.45 g, 45% yield)
1H NMR (300 MHz, CDCl3, TMS): δ 8.36 (d, J = 9.0, 4H), 6.81 (d, J = 9.0, 4H), 3.99(s, 8H), 3.66–3.77 (m, 32H), 1.47 (s, 36H); 13C (75 MHz, CDCl3): δ 188.8, 183.4, 169.7, 154.2, 133.4, 120.4, 112.9, 81.9, 71.1, 71.0, 69.2, 68.7, 51.8, 28.4 MS: (ESI), CHCl3, 1074 [M+H]+.
Squaraine dye 3e: (0.2 g, 44% yield)
1H NMR (500 MHz, CDCl3, TMS): δ 8.37 (d, 4H, J = 9.3), 6.77 (d, 4H, J = 9.3), 4.16 (d, 4H, J = 2.3), 3.75 (t, 4H, J = 5.5), 3.69 (t, 4H, J = 5.5), 3.59 (q, 4H, J = 7.0), 2.43 (t, 2H, J = 2.3), 1.25 (t, 6H J = 7.0); 13C (125 MHz, CDCl3): δ 188.8, 183.3, 153.4, 133.3, 119.9, 112.3, 79.1, 75.0, 67.3, 58.6, 50.3, 46.4, 12.3; MS: (FAB), CHCl3, 485 [M+H]+.
General Procedure to Synthesize Rotaxanes
Clear solutions of the corresponding 2,6-pyridinedicarbonyl dichloride (2.56 mmol) and p-xylylenediamine (2.56 mmol) in anhydrous chloroform (20 mL) were simultaneously added dropwise using a mechanical syringe pump apparatus over five hours to a stirred solution of squaraine dye 3a–e (0.32 mmol) and triethylamine (6.4 mmol) in anhydrous CHCl3 (40 mL). After stirring overnight, the reaction mixture was filtered through a pad of celite to remove any precipitation. The crude product was purified by column chromatography using a silica column that was eluted with a mixture of methanol/chloroform (1/49).
Squaraine rotaxane 1b: (99.0 mg, 20% yield)
1H NMR (300 MHz, CDCl3, TMS): δ 10.03 (t, J = 6.0, 4H), 8.50 (d, J = 7.5), 8.16 (t, J = 8.5, 2H), 8.06 (d, J = 9.0, 4H), 6.60 (s, 8H), 6.20 (d, J = 9.0, 4H), 4.51 (d, J = 6.0, 8H), 3.53–3.66 (m, 64H), 3.37 (s, 12H); 13C (75 MHz, CDCl3): δ 194.4, 184.9, 163.4, 153.9, 149.6, 138.8, 136.4, 133.6, 129.0, 125.0, 119.5, 111.5, 71.9, 70.8, 70.7, 70.6, 70.5, 68.3, 59.0, 51.3, 43.5. MS: (ESI), CHCl3, 1560 [M+H]+.
Squaraine rotaxane 1c: (111.0 mg, 29% yield)
1H NMR (300 MHz, CDCl3, TMS): δ 9.39 (s, 2H), 8.31 (d, J = 9.0 Hz, 4H), 8.23 (t, J = 6.0Hz, 4H), 7.65 (m, 6H), 6.70 (s, 8H), 6.40 (d, J = 9.0 Hz, 4H), 4.43 (d, J = 9.0Hz, 8H), 3.72 (m, 40H); 13C (75 MHz, CDCl3): δ 185.5, 181.8, 166.4, 154.4, 136.6, 134.3, 133.1, 131.9, 129.5, 125.1, 118.4, 113.0, 71.4, 70.7, 69.9, 68.3, 53.9, 50.4, 44.6; MS: (FAB), CHCl3, 1201 [M+H]+.
Squaraine rotaxane 1d: (77.0 mg, 15% yield)
1H NMR (300 MHz, CDCl3, TMS): δ 10.03 (t, J = 5.6, 4H), 8.52 (d, J = 8.5, 4H), 8.17 (d, J = 8.5, 2H), 8.09 (d, J = 8.5, 4H), 6.62 (s, 8H), 6.25(d, J = 8.5, 4H), 4.53(d, J = 8.5, 8H), 4.00 (s, 8H), 3.69 (m, 32H), 1.48 (s, 36H); 13C (75 MHz, CDCl3): δ 186.3, 185.1, 169.5, 163.6, 153.9, 149.6, 138.9, 136.7, 133.3, 128.7, 125.3, 119.5, 111.8, 81.7, 70.8, 69.0, 68.4, 51.3, 43.4, 28.1; MS: (FAB), CHCl3, 1607 [M+H]+.
Squaraine rotaxane 1e: o (71.0 mg, 22% yield)
1H NMR (500 MHz, CDCl3, TMS): δ 10.02 (t, 4H, J = 6.0), 8.51 (d, 4H, J = 8.0), 8.14 (t, 2H, J = 8.0), 8.08 (d, 4H, J = 9.0), 6.60 (s, 8H), 6.20 (d, 4H, J = 9.0), 4.52 (d, 8H, J = 6.0), 4.17 (d, 4H, J = 2.0), 3.67 (t, 4H, J = 6.0), 3.57 (t, 4H, J = 6.0), 3.46 (q, 4H, J = 7.0), 2.51 (t, 2H, J = 2.0), 1.18 (t, 6H, J = 7.0); 13C (125 MHz, CDCl3): δ 185.2, 184.5, 163.6, 153.5, 149.5, 138.7, 136.6, 133.6, 128.9, 125.2, 119.0, 111.6, 79.1, 75.2, 67.1, 58.6, 50.1, 46.3, 43.4, 12.2; MS: (ESI), CHCl3, 1019 [M+H]+.
Squaraine rotaxane 1f
The precursor 1e (20.0 mg) was dissolved in methylene dichloride (3 mL) and TFA (1 mL) was added. The solution was stirred at room temperature for 6 h, then the solvent removed. The residue was taken up in chloroform (100 mL) and washed with water (2 × 50 mL), dried with anhydrous Na2SO4 and concentrated to give 1f quantitatively as a blue solid that was dried in vacuo. 1H NMR (300 MHz, DMSO): δ 9.89 (t, 4H, J = 6.0), 8.30–8.42 (m, 5H), 7.89 (d, 4H, J = 9.0), 6.46 (s, 8H), 6.26 (d, 4H, J = 9.0), 4.38 (d, 8H, J = 6.0), 4.02 (s, 8H), 3.39–4.02 (m, 32H); 13C (75 MHz, DMSO): δ 184.6, 182.7, 171.7, 153.9, 148.9, 139.9, 136.4, 132.6, 128.2, 124.9, 118.3, 111.8, 69.9, 67.6, 67.5, 50.4, 42.3, 40.4; MS: (FAB), H2O, 1383 [M+H]+.
Acknowledgments
We are grateful for funding support from the NIH (USA).
References
- 1.Massoud TF, Gambhir SS. Genes Dev. 2003;17:545. doi: 10.1101/gad.1047403. [DOI] [PubMed] [Google Scholar]
- 2.Becker A, Hessenius C, Licha K, Ebert B, Sukowski U, Semmler W, Wiedenmann B, Grotzinger C. Nat Biotechnol. 2001;19:327. doi: 10.1038/86707. [DOI] [PubMed] [Google Scholar]
- 3.Chen XY, Conti PS, Moats RA. Cancer Res. 2004;64:8009. doi: 10.1158/0008-5472.CAN-04-1956. [DOI] [PubMed] [Google Scholar]
- 4.Leevy WM, Gammon ST, Jiang H, Johnson JR, Maxwell DJ, Marquez M, Piwnica-Worms D, Smith BD. J Am Chem Soc. 2006;128:16476. doi: 10.1021/ja0665592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frangioni JV. Curr Opin Chem Biol. 2003;7:626. doi: 10.1016/j.cbpa.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Mahmood U, Weissleder R. Mol Cancer Ther. 2003;2:489. [PubMed] [Google Scholar]
- 7.Ntziachristos V, Ripoll J, Weissleder R. Opt Lett. 2002;27:1652. doi: 10.1364/ol.27.001652. [DOI] [PubMed] [Google Scholar]
- 8.Pham W, Choi YD, Weissleder R, Tung CH. Bioconj Chem. 2004;15:1403. doi: 10.1021/bc049924s. [DOI] [PubMed] [Google Scholar]
- 9.Cheng Z, Levi J, Xiong ZM, Gheysens O, Keren S, Chen XY, Gambhir SS. Bioconj Chem. 2006;17:662. doi: 10.1021/bc050345c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen HJ, Law KY, Whitten DG. J Phys Chem. 1996;100:5949. [Google Scholar]
- 11.Ros-Lis JV, Garcia B, Jimenez D, Martinez-Manez R, Sancenon F, Soto J, Gonzalvo F, Valldecabres MC. J Am Chem Soc. 2004;126:4064. doi: 10.1021/ja031987i. [DOI] [PubMed] [Google Scholar]
- 12.Arunkumar E, Forbes CC, Noll BC, Smith BD. J Am Chem Soc. 2005;127:3288. doi: 10.1021/ja042404n. [DOI] [PubMed] [Google Scholar]
- 13.Johnson JR, Fu N, Arunkumar E, Leevy WM, Gammon ST, Piwnica-Worms D, Smith BD. Angew Chem Int Ed Engl. 2007;46:5528. doi: 10.1002/anie.200701491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnston AG, Leigh DA, Murphy A, Smart JP, Deegan MD. J Am Chem Soc. 1996;118:10662. [Google Scholar]
- 15.Lin CF, Lai CC, Liu YH, Peng SM, Chiu SH. Chem Eur J. 2007;13:4350. doi: 10.1002/chem.200601432. [DOI] [PubMed] [Google Scholar]
- 16.Sambrook MR, Beer PD, Wisner JA, Paul RL, Cowley AR, Szemes F, Drew MGB. J Am Chem Soc. 2005;127:2292. doi: 10.1021/ja046278z. [DOI] [PubMed] [Google Scholar]
- 17.Lane AS, Leigh DA, Murphy A. J Am Chem Soc. 1997;119:11092. [Google Scholar]
- 18.Gassensmith JJ, Arunkumar E, Barr L, Baumes JM, DiVittorio KM, Johnson JR, Noll BC, Smith BD. J Am Chem Soc. 2007;129:15054. doi: 10.1021/ja075567v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu AX, Mukhopadhyay P, Chakraborty A, Fettinger JC, Isaacs L. J Am Chem Soc. 2004;126:10035. doi: 10.1021/ja0486972. [DOI] [PubMed] [Google Scholar]
- 20.Affeld A, Hübner GM, Seel C, Schalley CA. Eur J Org Chem. 2001:2877. [Google Scholar]