Abstract
Escherichia coli and Serratia liquefaciens, two bacterial spacecraft contaminants known to replicate under low atmospheric pressures of 2.5 kPa, were tested for growth and survival under simulated Mars conditions. Environmental stresses of high salinity, low temperature, and low pressure were screened alone and in combination for effects on bacterial survival and replication, and then cells were tested in Mars analog soils under simulated Mars conditions. Survival and replication of E. coli and S. liquefaciens cells in liquid medium were evaluated for 7 days under low temperatures (5, 10, 20, or 30°C) with increasing concentrations (0, 5, 10, or 20%) of three salts (MgCl2, MgSO4, NaCl) reported to be present on the surface of Mars. Moderate to high growth rates were observed for E. coli and S. liquefaciens at 30 or 20°C and in solutions with 0 or 5% salts. In contrast, cell densities of both species generally did not increase above initial inoculum levels under the highest salt concentrations (10 and 20%) and the four temperatures tested, with the exception that moderately higher cell densities were observed for both species at 10% MgSO4 maintained at 20 or 30°C. Growth rates of E. coli and S. liquefaciens in low salt concentrations were robust under all pressures (2.5, 10, or 101.3 kPa), exhibiting a general increase of up to 2.5 orders of magnitude above the initial inoculum levels of the assays. Vegetative E. coli cells were maintained in a Mars analog soil for 7 days under simulated Mars conditions that included temperatures between 20 and −50°C for a day/night diurnal period, UVC irradiation (200 to 280 nm) at 3.6 W m−2 for daytime operations (8 h), pressures held at a constant 0.71 kPa, and a gas composition that included the top five gases found in the martian atmosphere. Cell densities of E. coli failed to increase under simulated Mars conditions, and survival was reduced 1 to 2 orders of magnitude by the interactive effects of desiccation, UV irradiation, high salinity, and low pressure (in decreasing order of importance). Results suggest that E. coli may be able to survive, but not grow, in surficial soils on Mars.
The search for extant life on Mars remains a stated goal of NASA's Mars Exploration Program and Astrobiology Institutes (13, 17). Intrinsic within such a life detection strategy is a requirement to understand how terrestrial life might survive, replicate, and proliferate on Mars. To mitigate the risks of the forward contamination of Mars, the bioloads on spacecrafts targeted for landing must be reduced to low density and diversity (4, 7). Planetary protection guidelines are designed to prevent both the forward contamination of the martian surface and to ensure the scientific integrity of any deployed life detection experiments. To date, 12 spacecraft have landed or crashed onto the Mars surface as a result of U.S., Russian, and European space program missions, but it is currently unknown if terrestrial microorganisms typically found on spacecraft surfaces can grow and replicate under conditions encountered on the surface (44, 45, 48).
Despite cleaning and sterilization measures taken to significantly reduce microbial bioloads on spacecraft (26, 56), diverse microbial communities remain at the time of launch (7, 31, 32, 44). The diversity of microorganisms found on spacecraft surfaces are generally characteristic of the clean rooms within which the spacecraft are processed. Spacecraft assembly facilities are oligotrophic extreme environments in which only the most resilient species survive the high-desiccation, low-nutrient conditions, controlled air circulation, and the rigors of bioburden reduction (56, 57). The biological inventory of microorganisms on spacecraft has mostly been limited to isolation and identification using standard culture-based microbiological assays (44, 48, 53). However, culture-based microbiological assays likely underestimate the biological diversity present on spacecraft, as traditional culture techniques fail to capture more than 99.9% of present phylotypes (7). Recently, the simultaneous use of culture-dependent and culture-independent techniques (e.g., Limulus amoebocyte lysate assay [LAL], ATP bioluminescence assay, lipopolysaccharide-based microbial detection, and DNA-based PCR) have identified many nonculturable species (31, 32, 57). Known culturable bacteria recovered from spacecraft surfaces include, but are not limited to, species of Acinetobacter, Bacillus, Corynebacterium, Escherichia, Flavobacterium, Micrococcus, Pseudomonas, Serratia, Staphylococcus, and Streptococcus (44, 53, 57).
After launch, spacecraft are exposed to interplanetary conditions of ultralow pressure (3 × 10−10 kPa), extreme desiccating conditions, fluctuating temperatures, solar UV irradiation, and ionizing radiation (22, 44). Furthermore, upon landing, the conditions on the surface of Mars are not much improved over interplanetary space. Diverse biocidal or inhibitory conditions on Mars have been identified in a number of recent publications (8, 21, 22, 35, 36, 38, 44, 48, 59) and include the following (not in order of priority): solar UVC irradiation, low pressure, extreme desiccating conditions, extreme diurnal temperature fluctuations, solar particle events, galactic cosmic rays, UV glow discharge from blowing dust, solar UV-induced volatile oxidants (e.g., O2−, O−, H2O2, NOx, O3), globally distributed oxidizing soils, extremely high salt levels (e.g., MgCl2, NaCl, FeSO4, and MgSO4) in surficial soils at some sites on Mars, high concentrations of heavy metals in martian soils, acidic conditions in martian regolith, high CO2 concentrations in the global atmosphere, and presence of perchlorates in some regoliths. UV irradiation, especially UVC photons (200 to 280 nm), may be the most biocidal of all factors to microbial survival on the martian surface (34, 37, 39, 47, 50, 52). Microorganisms found on sun-exposed surfaces of spacecraft are killed off within a few tens of minutes of exposure; but if covered by as little as a few hundred micrometers of martian soil, significant protection is provided (11, 34, 47). It is currently unknown if terrestrial microorganisms typically found on spacecraft surfaces can grow and replicate under conditions encountered on the surface of Mars (44, 48).
In the studies cited above, most research focused on the survival of dormant spores or vegetative cells under Mars conditions. In contrast, only a few papers have explored the possibility of growth and replication of terrestrial microorganisms under environmental conditions that approach those found in surficial soils of Mars (5, 25, 45, 48). Of these four, 2.5 kPa is the lowest pressure at which replication was observed for a few bacterial species (5, 45, 48).
The primary objective of the current study was to expose two non-spore-forming species to environmental stresses present on the surface of Mars to characterize the potential response of the bacteria to martian temperatures, salinities, and pressures. Two bacterial species, Escherichia coli and Serratia liquefaciens, were selected from over 30 prokaryotic species tested in preliminary experiments (5, 45). Their selection was based on their common association with humans, recovery from robotic spacecraft and space-based human life support systems (44, 53), and demonstrated replication at 2.5 kPa of total atmospheric pressure (5, 45). Experiments were conducted on cell suspensions in liquid medium at combinations of low pressure, high salt concentrations, and low temperatures, and then with cells mixed into soils and exposed to simulated Mars conditions. It was predicted for cell suspensions that (i) low temperatures would dramatically retard cell proliferation, (ii) high concentrations of salts would be biocidal on cell suspensions, and (iii) low pressure would have weak to moderate inhibitory effects on cell growth of both species. For cells in soils, growth was not expected under Mars simulations which exposed vegetative cells to low pressure, low temperatures, anaerobic gas composition, and high UVC irradiation similar to the martian surface. Although replication was not predicted, bacterial survival in analog Mars soils under simulated Mars conditions was anticipated.
MATERIALS AND METHODS
Bacterial growth and preparation.
Vegetative cells of Escherichia coli (Migula) ATCC 35218 and Serratia liquefaciens (Grimes and Hennerty) ATCC 7592 were grown in a modified Luria Bertani (LB) broth that contained 10 g tryptone peptone and 5 g yeast extract per liter (henceforth referred to as mLB). The 0.5% NaCl salt typically found in LB medium was deleted from the mLB liquid medium in order to create a true 0% salt solution for all controls, and to prevent interference with other salts tested. Liquid mLB medium was dispersed into small 1.3- by 10-cm glass test tubes, capped, and autoclaved at 121°C and 1.1 kg/cm2 for 20 min. Sterilized test tubes of mLB liquid medium were removed from the autoclave and immediately placed in 7-liter polystyrene containers along with two AnaeroPack sachets and a CO2 indicator tablet (Mitsubishi Gas Chemical America, Inc., New York, NY). Van Horn et al. (54) reported that AnaeroPack sachets remove oxygen to a concentration of less than 0.1% within an hour. The polystyrene containers were flushed with a stream of ultrahigh purity (UHP) carbon dioxide (CO2) gas for approximately 25 s to reduce the time lag required to reach anaerobic conditions. Thus, the mLB liquid medium was allowed to cool under conditions that would minimize the dissolution of oxygen (O2) back into the medium. Liquid mLB medium was used for all assays in which E. coli or S. liquefaciens cells were incubated up to 7 days under various conditions that simulated Mars conditions.
Fresh cultures of E. coli and S. liquefaciens were prepared by growing bacteria in mLB broth at 30°C and 150 rpm for 24 h within a shaking microbial incubator (model Innova 4230; New Brunswick Scientific, Edison, NJ). Starting suspensions of vegetative cells were then diluted to densities just below the detection limit of the spectrophotometer (optical density [OD], 0.01), corresponding to approximately 8 × 105 or 1 × 105 CFU per inoculated tube for E. coli and S. liquefaciens, respectively. To confirm the desaturation of O2 within the mLB broth during the 7-day assays, the obligate aerobe, Deinococcus radiodurans (Brooks & Murray) R1, was inoculated into negative-control tubes and placed within the polystyrene containers. A set of three noninoculated tubes of mLB broth were used in the plastic containers as a second negative control to check for airborne contamination during the 7-day assays.
Interactive effects of temperature and salt on bacterial growth.
Bacteria were grown in all possible combinations of three salts (MgSO4, MgCl2, and NaCl), four salt concentrations (0, 5, 10, or 20%), and four temperatures (5, 10, 20, or 30°C) to study the interactive effects of salt and temperature on bacterial survival and growth. Salts were selected based on published literature identifying MgSO4, MgCl2, and NaCl on Mars (8, 55). Cultures were prepared in 5 ml sterile mLB broth containing each of the salts at each of the concentrations and incubated at each temperature. Cultures were maintained in shaking microbial incubators at temperature set points, under anaerobic conditions, and at 101.3 kPa pressures. Cell density was measured at 0, 1, 2, 4, and 7 days by OD at 400 nm using a UV-visible (UV-VIS) spectrophotometer (Spectronic Unicam, Rochester, NY). For each OD measurement, cultures were removed from the polystyrene containers long enough to agitate samples, record OD, replace anaerobic pouches and indicator tablets, and flush with CO2. Total time for reading a set of cultures was approximately 10 to 15 min. Each treatment combination was performed in triplicate, and the experiment was conducted twice (n = 6). Data were normalized to ODs at time zero to account for small differences in starting OD values, log transformed, and analyzed by analysis of variance (ANOVA) for a balanced factorial repeated measures design using version 11.5 of SPSS Windows (SPSS Inc., Chicago, IL). Statistical analyses were limited to lower salt concentrations because many cultures did not grow in salt concentrations above 5% for MgCl2 and NaCl. Therefore, data were analyzed across all salt concentrations for MgSO4 and between salts at 0 and 5% for MgCl2 and NaCl. Significant differences within treatment groups were estimated using an overlap rule for calculated 95% confidence intervals (12).
A number of salt treatments (see Results) failed to exhibit growth as measured by an increase in OD. This response might have been caused by (i) no growth of the test bacteria within the salt-temperature treatments or (ii) positive growth, but below the detection limit of the OD procedure. Thus, additional assays were conducted using serial dilutions and direct plating onto standard LB agar (Difco/Fisher, Pittsburg, PA) to obtain viable cell counts of CFU/ml of bacterial suspensions. The experiment was limited to those treatments which did not yield a growth response in the first OD procedure. After 7 days of incubation, cultures were serially diluted, with each dilution plated onto standard LB agar, and incubated overnight at 30°C. CFU were counted to determine the number of viable cells in the original suspension. Each treatment was performed in triplicate, and the experiment was conducted twice (n = 6). Data were log transformed and analyzed by ANOVA for a balanced two-way factorial design; 95% confidence intervals were used to test for significant differences among treatments.
Interactive effects of salt and low pressure on bacterial growth.
In order to measure interactive effects of hypobaric and hypersaline environments on the survival and growth of E. coli and S. liquefaciens, tests were conducted at combinations of salts and atmospheric pressures estimated to be only moderately restrictive to bacterial activity (5, 45, 48). Inoculum and mLB liquid medium were prepared, as described above, except that salts were limited to 0 or 5% concentrations due to weak or no-growth responses at higher concentrations in the interactive salt and temperature experiment (see Results). Cultures were incubated for 7 days under anaerobic conditions within polycarbonate vacuum desiccators (model 08-642-7; Fisher Scientific, Pittsburgh, PA) maintained at 2.5, 10.0, or 101.3 kPa. Four anaerobic pouches were placed inside each hypobaric chamber. Two hypobaric chambers were placed within separate shaking incubators set at 20°C. Chambers were outfitted with in-line sterile filters (0.2-μm PolyVent 16 filter; Whatman, Inc., Florham Park, NJ) to inhibit airborne contaminants flowing into the hypobaric compartments. At the start of each test, CO2 was flushed through the chambers, and initial anaerobic pouches were replaced after 4 days to maintain anaerobic conditions at low pressures. For experiments maintained at 2.5 kPa, it was necessary to add approximately 0.5 to 0.75 ml of sterile deionized water (SDIW) after 4 and 7 days to compensate for evaporation under low atmospheric pressure. Evaporation under 10.0 and 101.3 kPa was negligible. After 7 days, cultures were serially diluted, and each dilution was plated onto standard LB agar to obtain viable cell counts. Each treatment was performed in triplicate, and the experiment was conducted twice (n = 6). Data were log transformed and analyzed by ANOVA for a balanced two-way factorial design; 95% confidence intervals were used to test for significant differences among treatments.
Growth of bacteria in Mars analog soils.
Prior to conducting the Mars simulations, several benchtop experiments were conducted to gather preliminary data on bacterial survival and growth in Mars analog soils. Mars analog soils were composed of fine-grained volcanic palagonite from Hawaii (3, 47). To remove any biological contaminants, soil samples were autoclaved at 1.1 kg cm−1 and 121°C for 30 min and then stored at 130°C overnight. Five grams of sterilized Mars analog soil and 3.5 ml mLB broth were mixed in 60-mm-diameter petri dishes under aseptic conditions. The soil/medium samples were inoculated with 100 μl of 24-h-old cultures of E. coli or S. liquefaciens corresponding to approximately 2 × 106 and 5 × 106 CFU per sample, respectively. Treatments included (i) soil samples that were inoculated with E. coli or S. liquefaciens and then immediately assayed (i.e., not allowed to dry), (ii) soil samples inoculated and then air dried for 24 h or 7 days by placing open dishes in a laminar flow hood at 24°C, (iii) soil samples inoculated and then incubated at 20, 4, or −20°C for 24 h or 7 days, and (iv) blank samples without soil (i.e., inoculum added to SDIW and immediately assayed). Negative controls were composed of three uninoculated petri dishes with soil/medium mixtures desiccated in the laminar flow hood and assayed after 7 days. All treatments were maintained under saturated (i.e., field capacity or above) conditions, except those dried in the laminar flow hood. The moisture contents of the desiccated soil samples were <2% water for samples dried at 24°C for 24 h or 7 days.
A previously published most-probable-number (MPN) microbial assay (47, 48, 50) was used to quantify viable bacteria bound to soil particles. In brief, soil samples were suspended in 50-ml sterile plastic centrifuge tubes containing 20 ml SDIW, sonicated for 4 min, vortexed for 2 min, and serially diluted, and 20 μl of each dilution was pipetted into each of 16 wells of a 96-well plate preloaded with 180 μl of mLB broth per well. The fully loaded 96-well dishes were incubated at 30°C for 24 h. The MPNs were estimated as described previously (47, 50). Each treatment was performed in triplicate, and the experiment was conducted three times (n = 9). Data were log transformed and analyzed by ANOVA for a balanced two-way factorial design; Tamhane's multiple comparisons test (SPSS, version 11.5) was used to determine differences between treatments.
Survival and growth of E. coli under Mars conditions.
A Mars Simulation Chamber (MSC) (described by Schuerger et al. [46]) was used to determine if E. coli could survive and possibly grow under simulated Mars conditions. Only E. coli was used for the Mars simulations because vegetative E. coli cells exhibited a stronger resistance to desiccation than did vegetative S. liquefaciens cells. The MSC is a stainless-steel low-pressure cylindrical chamber with internal dimensions measuring 70 cm long and 50 cm in diameter. The MSC system can accurately simulate five key components of the surface environment of Mars, including (i) pressures down to 0.01 kPa, (ii) UV irradiation from 190 to 400 nm, (iii) dust loading in the atmosphere from optical depths of 0.1 (dust-free sky) to 3.5 (global dust storm), (iv) temperatures from −100 to 30°C (based on Viking and Mars Pathfinder data [18, 28]), and (v) a Mars gas mix that included CO2 (95.3%), N2 (2.7%), Ar (1.6%), O2 (0.13%), and H2O (0.03%) (based on Viking data [40]). The Mars UV irradiation system has been described previously (46, 47, 50). The UVC (200 to 280 nm), UVB (280 to 320 nm), UVA (320 to 400 nm), and total UV fluence rates used in the current study were 3.6, 6.2, 25.9, and 35.6 W m−2, respectively. The VIS (400 to 700 nm) and near-infrared (NIR; 700 to 1,200 nm) fluence rates were 240 and 245 W m−2, respectively. Although not an experimental parameter, the relative humidity was measured by internal chamber sensors as fluctuating between 5 and 10% over the temperature range used in these experiments.
Soil samples were prepared as described above (5 g soil, 3.5 ml mLB liquid medium, 100 μl inoculum per sample). mLB broth mixed into soils contained one of the following: (i) no salt, (ii) 5% MgSO4, or (iii) a 15% salt mix composed of 5% MgCl2, 5% MgSO4, and 5% NaCl. Inoculated soil/mLB mixtures were allowed to desiccate for 24 h in an operating laminar flow hood with lids open at 24°C prior to placement in the MSC chamber. Three sets of treatments were prepared; one set was placed within an operating laminar flow hood at Earth-normal conditions of pressure and temperature, and two sets of samples were placed inside the MSC system. Of the samples maintained within the MSC, one set was exposed to UV irradiation during daytime hours (+UV), and one set was shielded from UV irradiation (−UV) by a sheet of aluminum foil placed between the +UV and −UV treatments. Conditions within the MSC were maintained at 0.71 kPa in a Mars atmosphere. Temperature was adjusted manually to create a diurnal fluctuation between 20°C for 8 h of day conditions and −50°C for 16 h of night conditions; ramp times between temperature shifts were 1 h in both directions. Soil samples inoculated with E. coli were maintained under the simulated Mars conditions for 7 days. The MPN assay was then used to estimate the number of viable E. coli cells present in all soils. Each treatment was performed in triplicate, and the experiment was conducted three times (n = 9). Data were transformed using a 0.25 power transformation and analyzed by ANOVA for a balanced two-way factorial design; 95% confidence intervals were used to test for significant differences among treatments.
RESULTS
Interactive effects of temperature and salt on bacterial growth.
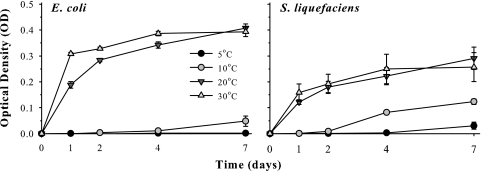
Cultures of both E. coli and S. liquefaciens grew faster at warmer temperatures and lower salt concentrations regardless of salt type, but all treatments (salt type and concentration, temperature, and interactive conditions) had significant effects on bacterial growth (Fig. 1, 2, and 3). No-salt controls for both E. coli and S. liquefaciens grew vigorously at 30 and 20°C, but growth slowed dramatically at 10°C (Fig. 1). In general, populations of both species increased from the minimum detection OD of 0.01 to between ODs of 0.2 and 0.4 within 24 to 48 h at 20 or 30°C, but achieved only ODs of between 0.01 and 0.1 for treatments incubated at 5 or 10°C, regardless of the salt tested. Cultures of Escherichia coli did not grow at 5°C in the no-salt control assays, but cultures of S. liquefaciens were able to grow weakly at 5°C after 7 days, achieving ODs of approximately 0.035.
FIG. 1.
Culture growth of Escherichia coli and Serratia liquefaciens in mLB broth maintained at 5, 10, 20, or 30°C. Cultures were incubated for 7 days in CO2 atmospheres. Error bars denote 95% confidence intervals (n = 6).
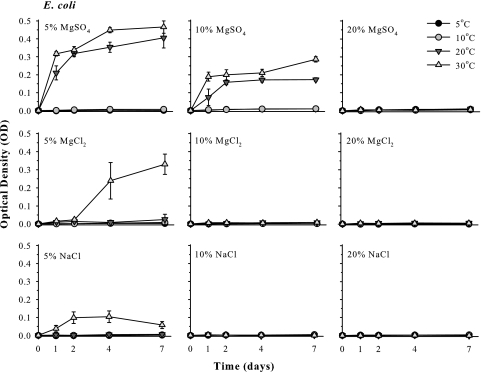
FIG. 2.
Culture growth of Escherichia coli in mLB broth doped with 5, 10, or 20% MgSO4, MgCl2, or NaCl. Cultures were incubated for 7 days in CO2 atmospheres and maintained at 5, 10, 20, or 30°C. Error bars denote 95% confidence intervals (n = 6).
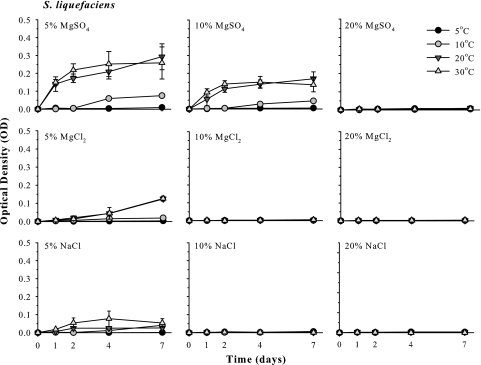
FIG. 3.
Culture growth of Serratia liquefaciens in mLB broth doped with 5, 10, or 20% MgSO4, MgCl2, or NaCl. Cultures were incubated for 7 days in CO2 atmospheres and maintained at 5, 10, 20, or 30°C. Error bars denote 95% confidence intervals (n = 6).
In the presence of 5% MgSO4, cultures of E. coli grew vigorously at 30 or 20°C, but not at lower temperatures (Fig. 2), achieving OD values between 0.3 and 0.4 after 2 days. The same general pattern occurred when E. coli was grown in 10% MgSO4, except growth rates at 30 and 20°C were significantly lower (P ≤ 0.05; OD values of 0.1 to 0.2) than those of E. coli cultures grown in 5% MgSO4. No increase in cell density for E. coli was observed in 20% MgSO4 at any incubation temperature. Cultures of E. coli grew in 5% MgCl2 at 30°C (OD values of 0.2 to 0.33), grew only weakly at 20°C (OD values of 0.02 to 0.03), and did not grow at 10 or 5°C. In contrast, cultures of E. coli failed to grow in 10 or 20% MgCl2. In sodium chloride solutions, cultures of E. coli grew weakly in 5% NaCl at 30°C (OD values of 0.05 to 0.1) but not at any other temperature or at any other concentration of NaCl.
Growth rates and trends for cultures of S. liquefaciens (Fig. 3) were similar to those observed for cultures of E. coli. Serratia liquefaciens cultures grew strongly at 5% MgSO4 at 30 and 20°C (OD values of up to 0.3), exhibited much slower growth at 10°C (OD values between 0.01 and 0.14), and did not grow at 5°C (Fig. 3). At 10% MgSO4, the same pattern was observed, but with lower growth rates than at 5% MgSO4. Growth was not observed for cultures of S. liquefaciens at all temperatures tested with 20% MgSO4. The same pattern occurred again in 5% MgCl2 but with even lower growth rates than those observed for MgSO4. No increase in bacterial growth was observed for higher concentrations of MgCl2. Serratia liquefaciens cultures grew weakly in 5% NaCl incubated at 30, 20, or 10°C (OD values between 0.01 and 0.10), but no increases in cell densities were observed above the background levels of the assay (i.e., OD of 0.01) at 5°C or in higher concentrations of NaCl.
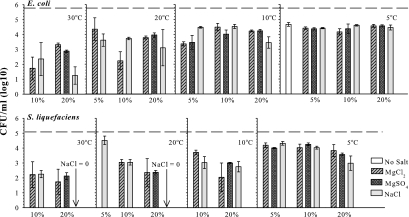
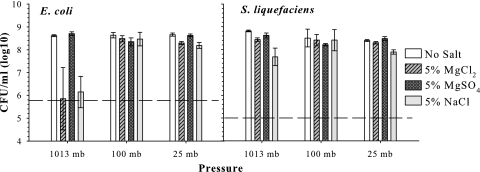
To confirm no-growth responses for the interactive salt and temperature experiment, a follow-up experiment was designed using a direct plating method to estimate the number of viable cells present in culture tubes after 7 days of treatment. Only treatments which resulted in no increase in OD in the first experiment (Fig. 2 and 3) were included in the new assay (Fig. 4). Salt and temperature treatments (Fig. 4) were prepared as described above. After 7 days, cultures were serially diluted and plated onto standard LB agar for direct enumeration of viable cells present in the high-salt solutions. In all cases, the number of recovered E. coli and S. liquefaciens cells was 1 to 5 orders of magnitude lower than the initial levels of inoculum (Fig. 4). Thus, the primary effect of higher concentrations of MgCl2, MgSO4, and NaCl salts on E. coli and S. liquefaciens was to kill a percentage of vegetative cells when stored for 7 days in mLB broth. Notably, no viable S. liquefaciens cells were recovered from 20% NaCl cultures maintained at 20 or 30°C but were recovered at 5 and 10°C. In general, the lower the temperature, the greater were the numbers of recovered cells for both E. coli and S. liquefaciens, regardless of the salt type or concentration.
FIG. 4.
Survival of Escherichia coli and Serratia liquefaciens after 7 days under low temperatures and high salinity in CO2 atmospheres. Only treatments which resulted in no increase in OD in the first experiment (Fig. 2 and 3) were included in the new assay. Cultures were harvested and counted after 7 days of incubation at 5, 10, 20, or 30°C. Dashed lines indicate starting levels of inoculum. Error bars denote 95% confidence intervals (n = 6).
Interactive effects of salt and low pressure on bacterial growth.
Cultures of E. coli generally grew vigorously in mLB at low pressures (Fig. 5), exhibiting approximately 2.5 orders of magnitude increases in cell densities over initial levels of inoculum for most treatments. Surprisingly, E. coli cultures failed to increase in number when incubated in 5% MgCl2 or NaCl solutions under an Earth-normal pressure of 101.3 kPa, but did increase >2 orders of magnitude when incubated at 10.0 or 2.5 kPa (P ≤ 0.05). There were no significant differences observed for bacterial growth of E. coli cultures among salts incubated at 10.0 or 2.5 kPa; all cultures increased between 2 and 3 orders of magnitude above initial inoculum levels. Serratia liquefaciens cultures grew robustly (>2 orders of magnitude above initial inoculum levels) under all pressures and in all salt concentrations tested; there were no significant differences in cell number among the three pressures (P > 0.05).
FIG. 5.
Viable cell densities of Escherichia coli and Serratia liquefaciens with and without salts under low pressure in CO2 atmospheres. Cultures were incubated in mLB at 20°C for 7 days under 2.5, 10.0, or 101.3 kPa. Dashed lines indicate starting levels of inoculum. Error bars denote 95% confidence intervals (n = 6).
Growth of bacteria in Mars analog soils.
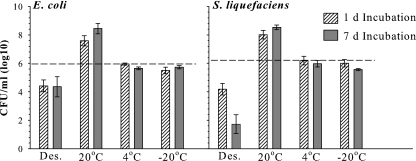
Incubation of E. coli and S. liquefaciens cells in nutrient-doped soils at 20°C for 1 and 7 days resulted in robust growth in both species with increases of approximately 2 orders of magnitude in cell densities, compared to other treatments (P ≤ 0.05; Fig. 6). However, when desiccated for 1 or 7 days in Mars analog soils, E. coli cell densities decreased approximately 2 orders of magnitude with no significant differences between 1- and 7-day treatments (P > 0.05). Survival was maintained at levels comparable to initial inocula when cells were incubated at 4 and −20°C (P > 0.05). Escherichia coli cells survived better at 4 and −20°C than those desiccated at room temperature and maintained at 24°C. Serratia liquefaciens mirrored the results of E. coli with one important exception; survival greatly decreased in 7-day desiccation assays from initial inoculum levels by about 4 orders of magnitude compared to only 2 orders of magnitude for E. coli. Based on this result, S. liquefaciens was eliminated from the Mars simulations conducted within the MSC system because E. coli appeared to have greater potential to withstand desiccation.
FIG. 6.
Viable cell densities of Escherichia coli and Serratia liquefaciens recovered from Mars analog soils after desiccation (Des.) or incubation at 20, 4, or −20°C for 1 or 7 days. Dashed lines indicate starting levels of inoculum. Error bars denote 95% confidence intervals (n = 9).
Survival and growth of E. coli in Mars simulations.
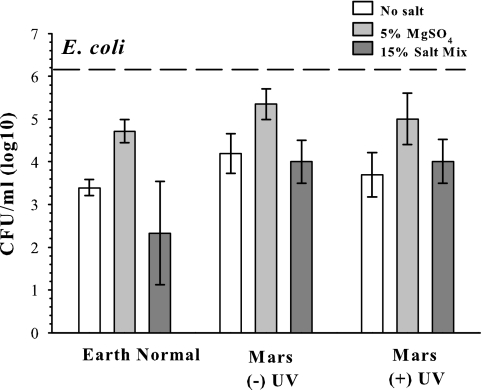
Escherichia coli failed to grow (i.e., increase cell densities above initial inoculum levels) under Mars conditions or in the Earth controls tested here; cell densities were 1 to 4 orders of magnitude below initial inoculum levels after 7 days (Fig. 7; P ≤ 0.05). Surprisingly, viable E. coli cells recovered from soils incubated under Mars conditions were significantly greater by 1 to 2 orders of magnitude than from Earth controls (P ≤ 0.01), even when soils were exposed to UV irradiation. However, cell densities from Mars simulations with either no salts or 5% MgSO4 and not exposed to UV irradiation were approximately ½ an order of magnitude greater than those from Mars assays with UV irradiation (P ≤ 0.05). Although differences due to UV irradiation for the no-salt and 5% MgSO4 treatments were close to the accuracy of the assay in determining titers (47, 50), the differences were consistently observed among the three repetitions of the experiment. The 15% salt mixture treatments were similar for the Mars −UV and Mars +UV conditions (P > 0.05). And finally, the 5% MgSO4 salt treatment enhanced survival of dormant E. coli cells by 1 to 2 orders of magnitude compared to either the no-salt or the 15% salt mixture treatments (P ≤ 0.05). The 5% MgSO4 response was observed for all three environmental treatments tested, including the Earth controls and the Mars −UV and Mars +UV conditions. Results were similar to those of benchtop desiccation experiments, in which recovered E. coli cells decreased approximately 2 orders of magnitude after 7 days of desiccation (Fig. 6).
FIG. 7.
Recovery of viable Escherichia coli cells in Mars simulations and Earth controls. Escherichia coli cells were incubated for 7 days in Mars analog soils in Earth-normal conditions and in the MSC with and without UV exposure. Diurnal conditions within the MSC were changed from 20°C and UV irradiation (daytime) to −50°C without UV (nighttime). A Mars atmosphere was maintained at 0.71 kPa. The dashed line represents the average initial inoculum. Error bars denote 95% confidence intervals (n = 9).
DISCUSSION
The risk that Mars may be contaminated by microorganisms transported from Earth on spacecraft will depend on four key factors: (i) survival of viable microorganisms during transit from Earth launch to Mars landing, (ii) dispersal of viable microorganisms away from landed or crashed vehicles, (iii) long-term survival of dispersed microbes on Mars, and (iv) the ability of dispersed microorganisms to undergo replicative growth in the Mars surface environment (50). Significant literature supports the conclusion that viable microorganisms have survived launch and transport to Mars (44, 47, 53). Dispersal mechanisms of viable microbes away from landed or crashed spacecraft on Mars have not been adequately studied and remain significant black boxes in any Mars microbial survival and proliferation model. Long-term survival on Mars is unlikely if microbes are directly exposed to solar UV irradiation (11, 37, 47, 49, 50) but likely if the microbes are protected from UV irradiation by thin dust layers or embedded within UV-protected niches in spacecraft (33, 39, 47). In contrast, the greatest unknown in any Mars microbial survival and proliferation model is whether terrestrial microorganisms found on spacecraft are capable of growth and replication under Mars conditions. The current study provides evidence that Escherichia coli, a potential spacecraft contaminant (44, 53), may survive but not grow on the surface of Mars. Results suggest that desiccation, UV irradiation, high salinity, and low pressure (in decreasing order of importance) were factors in reducing the number of viable E. coli cells over the course of the 7-day Mars simulations, consistent with related studies on microbial survival under Mars conditions (37, 39, 47, 49, 51, 52).
Recovery of viable E. coli cells from nutrient-doped analog soils exposed to Mars conditions were lower than initial starting populations in soils, suggesting that E. coli cells were partially killed during the course of the Mars simulations. No evidence was found that E. coli cells were able to replicate under the simulated Mars conditions tested, consistent with other studies (16, 51) that examined the potential for microbial growth and replication on Mars. Desiccation at the time of soil preparation, and subsequently within the Mars chamber maintained at 0.71 kPa, was concluded to be the primary environmental factor responsible for lowering the recovery of viable E. coli cells from soils.
In a recent study (4), a NASA working group studying how terrestrial microorganisms might grow and replicate in special regions on the martian surface (defined as those regions where there is a reasonable chance of finding life on Mars, or where terrestrial life might proliferate) concluded that in almost all cases considered, the water activity (aw) of the surficial regolith would be significantly lower than the aw required for either bacterial (0.88), hypersaline archaea (0.75), or fungal (0.62) growth and replication. Although not directly measured, the aw of the desiccated Mars analog soils incubated under Mars conditions was likely <0.1, based on a relative humidity of approximately 10% found within the MSC system during all experiments. Thus, the results confirm that the highly desiccated analog soils within the Mars chamber would be unlikely to support microbial activity under the conditions tested. In several preliminary experiments (A. C. Schuerger, unpublished data), hydrated Mars analog soils similar to those used in the experiments depicted in Fig. 6 and 7 would transition from field capacity to <5% of free water within 3 to 4 h at 20°C at 0.71 kPa. Even at lower temperatures and pressures near the triple point of water for Mars (0.01°C at 0.61 kPa), liquid water was quickly removed from the soil matrix by rapid evaporation. The experiments presented here represent a realistic simulation of what is likely to occur on Mars if viable vegetative E. coli or S. liquefaciens cells are dispersed into soils surrounding landed or crashed spacecrafts. Microbial cells would encounter a highly desiccated soil matrix and, even if dispersed in a liquid, would quickly reach aw levels significantly lower than are required to support growth and replication of terrestrial microorganisms.
Although replication was not observed, a majority of E. coli cells did survive 7 days under simulated Mars conditions, even when UV irradiation was present at the surface of the Mars analog soils. Survival of E. coli was actually higher under Mars simulations than under Earth conditions, which was likely due to the preserving effects of low temperatures on cell viability. This conclusion is supported by results from the experiment on survival of E. coli and S. liquefaciens in saline solutions at diverse temperatures; survival rates were highest at 5 and 10°C (Fig. 4). On average, the presence of UV irradiation either had no effect (15% salt mixtures) (Fig. 7) or lowered the number of recoverable cells by less than half an order of magnitude compared to soils not irradiated by UV (Fig. 7). UV irradiation does not penetrate below the top 200 to 500 μm of soil particles (10, 34, 47), so only microbial cells on UV-exposed soil particles within the first few hundred micrometers of the tops of analog soils were potentially inactivated by UV photons.
The presence of UV irradiation on the surface of Mars may form volatile oxidants in the martian atmosphere (e.g., H2O2−, OH−, O2−) that may diffuse into surficial soils to depths of several centimeters (14, 59). However, results of the current Mars simulations do not support the hypothesis that volatile oxidants contributed to the biocidal nature of the martian conditions. In fact, the samples exposed to UV irradiation were not statistically different from those without UV (P > 0.05). If UV irradiation formed volatile oxidants during the 7-day simulations reported here, either the concentrations of the oxidants were too low to affect E. coli or the oxidants failed to diffuse significantly into the soils.
Hypobaria during Mars simulations (0.71 kPa) was likely one other environmental factor to have contributed to the slight reduction in recovered E. coli cells. Hypobaric pressures similar to those of the surface of Mars (0.69 kPa global average) have been shown to reduce viability of both spore-forming species like Bacillus subtilis (19, 47) and non-spore-forming species like Deinococcus radiodurans, E. coli, Halobacterium halobium, and Staphylococcus epidermidis (20, 29, 43). In contrast, freeze-thaw cycles appear unable to reduce the survival of dormant spores or cells maintained under simulated Mars conditions (16, 41, 60) and, thus, likely had no role in reducing the number of viable E. coli cells during the current Mars simulations.
One intriguing result from the current study was the observation that the inhibition of growth observed for E. coli maintained in 5% MgCl2 or NaCl solutions at 101.3 kPa was relaxed at 10.0 and 2.5 kPa (Fig. 5). This response was not observed for similarly exposed S. liquefaciens cells. One plausible explanation is the presence of ClC Cl− channels in E. coli, but lacking in S. liquefaciens, which have been implicated in ameliorating the inhibitory effects of acid stress (1, 23, 24, 42). Chloride channels exhibit a wide range of cellular functions in bacterial and archaeal cells, including regulation of cellular pH, cell homeostasis, organic solute transport, cell migration, cell proliferation and differentiation, and stress response. Recently, the ClC Cl− channels have been shown to enhance the recovery of growth for E. coli under mild to moderate acid conditions (1, 24). During the low-pressure culture-based assays used here (Fig. 5), liquid mLB cultures were placed in CO2-enriched anaerobic atmospheres immediately upon removal from the autoclave to inhibit the reoxygenation of the medium. Solutions within high-CO2 atmospheres can increase in acidity (i.e., lower pH) due to the formation of carbonic acid in solution. Although not tested here, the ClC Cl− channels also may be involved in the relaxation of cellular stress encountered under low pressures. It is clear from three previous reports (5, 45, 48) that some species of bacteria are inhibited by hypobaric conditions. Perhaps some of the inhibition of microbial growth and replication at low pressure might be relaxed in bacterial and archaeal species with ClC Cl− channels when cells are in the presence of Cl− salts. Chloride salts have been confirmed for Mars regolith in several locations, including Meridiani Planum (Opportunity rover), Gusev Crater (Spirit rover), and the northern polar plains (Phoenix lander) (8, 21, 35). Current results suggest that bacterial species with ClC Cl− channels may be more capable of survival, and possibly growth, on Mars.
In the current study, high salt concentrations (>10%) generally inhibited the survival and replication of both E. coli and S. liquefaciens under a diversity of conditions found at the surface of Mars. In contrast, lower concentrations of salts (<10%) had either a neutral or positive effect on survival and growth. Thus, the effects of salts on microbial survival, metabolism, and replication on Mars are likely to be both complex and species dependent. For example, salts may aid the survival and growth of microorganisms on the surface of Mars by depressing the freezing point of water, making liquid water available over a broader range of temperatures (6, 9), or by providing protection from desiccation by encasing microbes in fluid inclusions, a phenomenon present in most salt crusts or crystals (2). Concerning the first mechanism, E. coli and S. liquefaciens were unlikely to have benefited from salt-depressed freezing points under Mars conditions, as both species failed to grow at 5°C in all salt types and concentrations (Fig. 2 and 3). In contrast, liquid briny waters may improve the potential for growth of cold-tolerant halophilic species if they are delivered to Mars, and one recent study (30) has identified both halophilic and psychrophilic species recovered from spacecraft surfaces and spacecraft processing facilities. Relative to the second mechanism, salt crusts were observed on analog soils during Mars simulations, and it is plausible that the salts may have provided some protection from the otherwise harsh desiccating conditions during the experiments. Although controversial, halotolerant bacteria have been isolated from inclusions within 250-million-year-old salt crystals (58). While this is an extreme example of bacterial survival within inclusions, microbes commonly become entrapped within salt crystals (2, 33). Though salts may aid cells in survival against desiccation or growth at subzero temperatures, there is a balance between these benefits and the cost of high osmolarity on cell survival, metabolism, and replication.
Also, the current study confirms the work of Schuerger and Nicholson (48), whose results show that diverse environmental factors can interact to adversely affect bacterial survival more than can single stressing agents. For example, combinations of high-temperature and high-salt conditions were more inhibitory to both E. coli and S. liquefaciens (Fig. 1, 2, and 3) than was each parameter alone. The average global temperature on Mars is −61°C, significantly below the average global temperature on Earth (15°C). Although typical daytime highs experienced by the Viking landers on Mars never exceeded −10°C (27), daytime highs near 30oS latitude can achieve 20°C during austral summer (28). Thus, although in general low temperatures on Mars might not permit the growth and replication of most mesophilic species found on spacecraft surfaces (44), low temperatures may act to enhance survival when on the surface of Mars. Conversely, a landing zone near 30oS latitude during the austral summer for Mars may combine higher temperatures and salt concentrations to increase the biocidal nature of the martian terrain. Additional research is required for interactive studies on microbial survival, metabolism, replication, and adaptation under Mars conditions in order to accurately predict the risks to Mars from terrestrial microorganisms that are successfully transferred from Earth.
In summary, results from the current study do not support the conclusion that E. coli cells can grow under Mars conditions but do indicate that E. coli cells can persist for at least short periods of time on Mars. In comparison, S. liquefaciens cells were more sensitive to desiccation than were E. coli cells and would likely have fared even worse than E. coli under Mars conditions. Furthermore, we confirm earlier work (5, 45, 48) that at least some bacteria can undergo metabolic activity and replication in CO2-enriched anaerobic atmospheres down to 2.5 kPa. Results suggest that spacecraft microorganisms may not pose a serious risk for near-term Mars science missions because it appears that at least some spacecraft microorganisms cannot replicate in the desiccated, cold, salty, and hypobaric martian environment. However, if transported microorganisms can maintain their viability over long periods of time, more conducive conditions might occur during periods of high obliquity. For example, Fanale et al. (15) modeled climate change on Mars at high obliquity (near 60o off-nadir on a 104- to 105-year cycle) and predicted that atmospheric surface pressures may rise to 2.5 kPa with a concurrent rise in temperature and moisture content in the atmosphere. Under such a scenario, surviving bacteria may encounter a more benign martian environment. If long-term microbial survival is possible on Mars, then past and future explorations of Mars may provide the microbial inoculum for seeding Mars with terrestrial life. However, two key processes must be studied further in order to constrain and test this hypothesis. First, a wide range of microbial species have been recovered from spacecrafts, but little is known about how each species might survive the diversity of potential biocidal factors found on Mars. Most work has been conducted on members of the genus Bacillus, and very little research has been published on non-spore-forming species. Thus, a diversity of microbial species should be studied to characterize their potential for long-term survival on Mars. Second, most work to date has examined survival of dormant spores or cells under simulated Mars conditions. However, it is essential to conduct studies on whether terrestrial microorganisms can undergo active metabolism and replication under Mars conditions. Until more comprehensive understandings of these two processes are achieved, accurately predicting the risks of the forward contamination of Mars will remain elusive.
Acknowledgments
We acknowledge the Florida Space Grant Consortium, NASA's Planetary Protection Office (grant NNX08AQ81A), the Department of Biology at the University of Central Florida, and the James and Annie Ying Eminent Scholarship for their financial support of the research described herein.
We also thank Wayne L. Nicholson for his helpful advice and Jaydeep Mukherjee for his gracious support.
Footnotes
Published ahead of print on 12 February 2010.
REFERENCES
- 1.Accardi, A., and C. Miller. 2004. Secondary active transport mediated by a prokaryotic homologue of ClC Cl− channels. Nature 427:803-807. [DOI] [PubMed] [Google Scholar]
- 2.Adamski, J. C., J. A. Roberts, and A. H. Goldstein. 2006. Entrapment of bacteria in fluid inclusions in laboratory-grown halite. Astrobiology 6:552-562. [DOI] [PubMed] [Google Scholar]
- 3.Allen, C. C., J. L. Gooding, M. Jercinovic, and K. Klaus. 1981. Altered basaltic glass: a terrestrial analog to the soil of Mars. Icarus 45:347-369. [Google Scholar]
- 4.Beaty, D. W., K. L. Buxbaum, M. A. Meyer, N. G. Barlow, W. V. Boynton, B. C. Clark, J. W. Deming, P. T. Doran, K. S. Edgett, S. L. Hancock, J. W. Head, M. H. Hecht, V. Hipkin, T. L. Kieft, R. L. Mancinelli, E. V. McDonald, C. P. McKay, M. T. Mellon, H. Newsom, G. G. Ori, D. A. Paige, A. C. Schuerger, M. L. Sogin, J. A. Spry, A. Steele, K. L. Tanaka, and M. A. Voytek. 2006. Findings of the Special Regions Science Analysis Group. Astrobiology 6:677-732.17067257 [Google Scholar]
- 5.Berry, B. J., W. L. Nicholson, and A. C. Schuerger. 2006. Proliferation of common spacecraft contaminants appears limited under simulated martian conditions. Astrobiology 6:254. [Google Scholar]
- 6.Brass, G. W. 1980. Stability of brines on Mars. Icarus 43:20-28. [Google Scholar]
- 7.Chyba, C. F., S. Clifford, A. Delemere, M. S. Favero, E. J. Mathur, J. C. Niehoff, G. G. Ori, D. A. Paige, A. Pearson, J. C. Priscu, M. S. Race, M. L. Sogin, and C. Takacs-Vesback. 2006. Preventing the forward contamination of Mars. The National Academies Press, Washington, DC.
- 8.Clark, B. C., R. V. Morris, S. M. McLennan, R. Gellart, B. Joliff, A. H. Knoll, S. W. Squyres, T. K. Lowenstein, D. W. Ming, N. J. Tosca, A. Yen, P. R. Christensen, S. Gorevan, J. Brückner, W. Calvin, G. Dreibus, W. Farrand, G. Klingelhoefer, H. Waenke, J. Zipfel, J. F. Bell III, J. Grotzinger, H. Y. McSween, and R. Rieder. 2005. Chemistry and mineralogy of outcrops at Meridiani Planum. Earth Planet. Sci. Lett. 240:73-94. [Google Scholar]
- 9.Clark, B. C., and D. C. Van Hart. 1981. The salts of Mars. Icarus 45:370-378. [Google Scholar]
- 10.Cockell, C. S., D. C. Catling, W. L. Davis, K. Snook, R. L. Kepner, P. Lee, and C. McKay. 2000. The ultraviolet environment of Mars: biological implications past, present, and future. Icarus 146:343-359. [DOI] [PubMed] [Google Scholar]
- 11.Cockell, C. S., A. C. Schuerger, D. Billi, E. I. Friedmann, and C. Panitz. 2005. Effects of a simulated martian UV flux on the cyanobacterium, Chroococcidiopsis sp. 029. Astrobiology 5:127-140. [DOI] [PubMed] [Google Scholar]
- 12.Cumming, G., F. Fidler, and D. L. Vaux. 2007. Error bars in experimental biology. J. Cell Biol. 177:7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Des Marais, D. J., J. A. Nuth III, L. J. Allamandola, A. P. Boss, J. D. Farmer, T. M. Hoehler, B. M. Jakosky, V. S. Meadows, A. Pohorille, B. Runnegar, and A. M. Sporman. 2008. The NASA astrobiology roadmap. Astrobiology 8:715-730. [DOI] [PubMed] [Google Scholar]
- 14.Encrenaz, T., B. Bezard, T. K. Greathouse, M. J. Richter, J. H. Lacy, S. K. Atreya, A.-S. Wong, S. Lebonnois, F. Lefevre, and F. Forget. 2004. Hydrogen peroxide on Mars: evidence for spatial and seasonal variations. Icarus 170:424-429. [Google Scholar]
- 15.Fanale, F. P., J. R. Salvail, W. B. Banerdt, and R. S. Saunders. 1982. Mars: the regolith-atmosphere-cap system and climate change. Icarus 50:381-407. [Google Scholar]
- 16.Foster, T. L., L. Winans, R. C. Casey, and L. E. Kirschner. 1978. Response of terrestrial microorganisms to a simulated martian environment. Appl. Environ. Microbiol. 35:730-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garvin, J. B., O. Figueroa, and F. M. Naderi. 2001. NASA's new Mars Exploration Program: the trajectory of knowledge. Astrobiology 1:439-446. [DOI] [PubMed] [Google Scholar]
- 18.Golombek, M. P., R. A. Cook, T. Economou, W. M. Folkner, A. F. C. Haldemann, P. H. Kallemeyn, J. M. Knudsen, R. M. Manning, H. J. Moore, T. J. Parker, R. Rieder, J. T. Schofield, P. H. Smith, and R. M. Vaughn. 1999. Overview of the Mars Pathfinder mission: launch through landing, surface operations, data sets, and science results. J. Geophys. Res. 104(E4):8523-8553. [Google Scholar]
- 19.Green, R. H., D. M. Taylor, E. A. Gustan, S. J. Fraser, and R. L. Olson. 1971. Survival of microorganisms in a simulated martian environment. Space Life Sci. 3:12-24. [DOI] [PubMed] [Google Scholar]
- 20.Hagen, C. A., J. F. Godfrey, and R. H. Green. 1971. The effect of temperature on the survival of microorganisms in a deep space vacuum. Space Life Sci. 3:108-117. [DOI] [PubMed] [Google Scholar]
- 21.Hecht, M. H., S. P. Kounaves, R. C. Quinn, S. J. West, M. M. Young, D. W. Ming, D. C. Catling, B. C. Clark, W. V. Boynton, J. Hoffman, L. P. DeFlores, K. Gospodinova, J. Kapit, and P. H. Smith. 2009. Detection of perchlorate and the soluble chemistry of martian soil at the Phoenix lander site. Science 325:64-67. [DOI] [PubMed] [Google Scholar]
- 22.Horneck, G., G. Reitz, P. Rettberg, C. Baumstark-Khan, and R. Gerzer. 2003. Critical issues in connection with human missions to Mars: protection of and from the martian environment. Adv. Space Res. 31:87-95. [DOI] [PubMed] [Google Scholar]
- 23.Iyer, R., T. M. Iverson, A. Accardi, and C. Miller. 2002. A biological role for prokaryotic ClC chloride channels. Nature 419:715-718. [DOI] [PubMed] [Google Scholar]
- 24.Jordan, K. N., and K. W. Davies. 2001. Sodium chloride enhances recovery and growth of acid-stressed E. coli O157:H7. Lett. Appl. Microbiol. 32:312-315. [DOI] [PubMed] [Google Scholar]
- 25.Kanervo, E., K. Lehto, K. Stahle, H. Lehto, and P. Maenpaa. 2005. Characterization of growth and photosynthesis of Synechocystis sp. PCC 6803 cultures under reduced atmospheric pressures and enhanced CO2 levels. Intern. J. Astrobiol. 4:97-100. [Google Scholar]
- 26.Kempf, M. J., F. Chen, R. Kern, and K. Venkateswaran. 2005. Recurrent isolation of hydrogen peroxide-resistant spores of Bacillus pumilus from a spacecraft assembly facility. Astrobiology 5:391-405. [DOI] [PubMed] [Google Scholar]
- 27.Kieffer, H. H., B. M. Jakosky, C. W. Snyder, and M. S. Matthews. 1992. Mars, p. 1498. University of Arizona Press, Tucson, AZ.
- 28.Kieffer, H. H., T. Z. Martin, A. R. Peterfreund, B. M. Jakosky, E. D. Miner, and F. D. Palluconi. 1977. Thermal and albedo mapping of Mars during the Viking primary mission. J. Geophys. Res. 82:4249-4291. [Google Scholar]
- 29.Koike, J., and T. Oshima. 1993. Planetary quarantine in the solar system. Survival rates of some terrestrial organisms under simulated space conditions by proton irradiation. Acta Astronaut. 29:629-632. [DOI] [PubMed] [Google Scholar]
- 30.La Duc, M. T., A. Dekas, S. Osman, C. Moissl, D. Newcombe, and K. Venkateswaran. 2007. Isolation and characterization of bacteria capable of tolerating the extreme conditions of clean room environments. Appl. Environ. Microbiol. 73:2600-2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.La Duc, M. T., R. Kern, and K. Venkateswaran. 2004. Microbial monitoring of spacecraft and associated environments. Microb. Ecol. 47:150-158. [DOI] [PubMed] [Google Scholar]
- 32.La Duc, M., W. Nicholson, R. Kern, and K. Venkateswaran. 2003. Microbial characterization of the Mars Odyssey spacecraft and its encapsulation facility. Environ. Microbiol. 5:977-985. [DOI] [PubMed] [Google Scholar]
- 33.Mancinelli, R. L. 2005. Halophiles: a terrestrial analog for life in brines on Mars, p. 139-147. In N. Gunde-Cimerman, A. Oren, and A. Plemenitas (ed.), Adaptation to life at high salt concentrations in archaea, bacteria, and eukarya. Springer, Dordrecht, The Netherlands.
- 34.Mancinelli, R. L., and M. Klovstad. 2000. Martian soil and UV radiation: microbial viability assessment on spacecraft surfaces. Planet. Space Sci. 48:1093-1097. [Google Scholar]
- 35.Ming, D. W., D. W. Mittlefehldt, R. V. Morris, D. C. Golden, R. Gelbert, A. Yen, B. C. Clark, S. W. Squyres, W. H. Farrand, S. W. Ruff, R. E. Arvisdon, G. Klingelhöfer, H. Y. McSwee, D. S. Rodionov, C. Schröder, P. A. de Souza, Jr., and A. Wang. 2006. Geochemical and mineralogical indicators for aqueous processes in the Columbia Hills of Gusev Crater, Mars. J. Geophys. Res. 111(E02S12):1-23. [Google Scholar]
- 36.Morris, R. V., D. W. Ming, T. G. Graff, R. V. Arvidson, G. F. Bell III, S. W. Squyres, S. A. Mertzman, J. E. Gruener, D. C. Golden, L. Le, and G. A. Robinson. 2000. Mineralogy, composition, and alteration of Mars Pathfinder rocks and soils: evidence from multispectral, elemental, and magnetic data on terrestrial analogue, SNC meteorite, and Pathfinder samples. J. Geophys. Res. 105(E1):1757-1817. [Google Scholar]
- 37.Newcombe, D. A., A. C. Schuerger, J. N. Benardini, D. Dickinson, R. Tanner, and K. Venkateswaran. 2005. Survival of spacecraft associated microbes under simulated martian UV irradiation. Appl. Environ. Microbiol. 71:8147-8156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Newsom, H. E., and J. J. Hagerty. 1997. Chemical components of the martian soil: soil degassing, hydrothermal alteration, and chondritic debris. J. Geophys. Res. 102(E8):19345-19355. [Google Scholar]
- 39.Osman, S., Z. Peeters, M. T. La Duc, R. L. Mancinelli, P. Ehrenfreund, and K. Venkateswaran. 2008. Effect of shadowing on survival of bacteria under conditions simulating the martian atmosphere and UV radiation. Appl. Environ. Microbiol. 74:959-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Owen, T. 1992. The composition and early history of the atmosphere of Mars, p. 818-834. In H. H. Kieffer, B. M. Jakosky, C. W. Snyder, and M. S. Mathews (ed.), The University of Arizona Press, Tucson, AZ.
- 41.Packer, E., S. Scher, and C. Sagen. 1963. Biological contamination of Mars II. Cold and aridity as constraints on the survival of terrestrial microorganisms in simulated martian environments. Icarus 2:293-316. [PubMed] [Google Scholar]
- 42.Roeßler, M., X. Sewald, and V. Müller. 2003. Chloride dependence of growth in bacteria. FEMS Microbiol. Lett. 225:161-165. [DOI] [PubMed] [Google Scholar]
- 43.Saffary, R., R. Nandakumar, D. Spencer, F. T. Robb, J. M. Davila, M. Swartz, L. Ofman, R. J. Thomas, and J. DiRuggiero. 2000. Microbial survival of space vacuum and extreme ultraviolet irradiation: strain isolation and analysis during a rocket flight. FEMS Microbiol. Lett. 215:163-168. [DOI] [PubMed] [Google Scholar]
- 44.Schuerger, A. C. 2004. Microbial ecology of the surface exploration of Mars with human-operated vehicles, p. 363-386. In C. S. Cockell (ed.), Martian expedition planning. Univelt Publishers, Escondido, CA.
- 45.Schuerger, A. C., B. J. Berry, and W. L. Nicholson. Terrestrial bacteria typically recovered from Mars spacecraft do not appear able to grow under simulated martian conditions, abstr. 1397. 37th Annu. Lunar Planet. Sci. Conf., March 2006.
- 46.Schuerger, A. C., P. Fajardo-Cavazos, C. A. Clausen, J. E. Moores, P. H. Smith, and W. L. Nicholson. 2008. Slow degradation of ATP in simulated martian environments suggests long residence times for the biosignature molecule on spacecraft surfaces. Icarus 194:86-100. [Google Scholar]
- 47.Schuerger, A. C., R. L. Mancinelli, R. G. Kern, L. J. Rothschild, and C. P. McKay. 2003. Survival of endospores of Bacillus subtilis on spacecraft surfaces under simulated martian environments: implications for the forward contamination of Mars. Icarus 165:253-276. [DOI] [PubMed] [Google Scholar]
- 48.Schuerger, A. C., and W. L. Nicholson. 2006. Interactive effects of low pressure, low temperature, and CO2 atmospheres inhibit the growth of Bacillus spp. under simulated martian conditions. Icarus 185:143-152. [Google Scholar]
- 49.Schuerger, A. C., J. T. Richards, P. E. Hintze, and R. Kern. 2005. Surface characteristics of spacecraft components affect the aggregation of microorganisms and may lead to different survival rates of bacteria on Mars landers. Astrobiology 5:545-559. [DOI] [PubMed] [Google Scholar]
- 50.Schuerger, A. C., J. T. Richards, D. A. Newcombe, and K. J. Venkateswaran. 2006. Rapid inactivation of seven Bacillus spp. under simulated Mars UV irradiation suggests minimum forward contamination around landing sites. Icarus 181:52-62. [Google Scholar]
- 51.Smith, D. J., A. C. Schuerger, M. M. Davidson, T. C. Onstott, S. W. Pacala, and C. Bakermans. 2008. Survivability of Psychrobacter cryohalolentis K5 under simulated martian surface conditions. Astrobiology 9:221-228. [DOI] [PubMed] [Google Scholar]
- 52.Tauscher, C., A. C. Schuerger, and W. L. Nicholson. 2006. Survival and germinability of Bacillus subtilis spores exposed to simulated Mars solar radiation: implications for planetary protection and lithopanspermia. Astrobiology 6:592-605. [DOI] [PubMed] [Google Scholar]
- 53.Taylor, G. R. 1974. Space microbiology. Annu. Rev. Microbiol. 28:121-137. [DOI] [PubMed] [Google Scholar]
- 54.Van Horn, K. G., K. Warren, and E. J. Baccaglini. 1997. Evaluation of the AnaeroPack System for growth of anaerobic bacteria. J. Clin. Microbiol. 35:2170-2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vaniman, D. T., D. L. Bish, S. J. Chipera, C. I. Fialips, J. W. Carey, and W. C. Feldman. 2004. Magnesium sulfate salts and the history of water on Mars. Nature 431:663-665. [DOI] [PubMed] [Google Scholar]
- 56.Venkateswaran, K., S. Chung, J. H. Allton, and R. G. Kern. 2004. Evaluation of various cleaning methods to remove Bacillus spores from spacecraft hardware materials. Astrobiology 4:377-390. [DOI] [PubMed] [Google Scholar]
- 57.Venkateswaran, K., M. Satomi, S. Chung, R. Kern, R. Koukol, C. Basic, and D. White. 2001. Molecular microbial diversity of a spacecraft assembly facility. Syst. Appl. Microbiol. 24:311-320. [DOI] [PubMed] [Google Scholar]
- 58.Vreeland, R. H., W. D. Rosenzweig, and D. W. Powers. 2000. Isolation of a 250-million-year-old halotolerant bacterium from a primary salt crystal. Nature 407:897-900. [DOI] [PubMed] [Google Scholar]
- 59.Yen, A. S., S. S. Kim, M. H. Hecht, M. S. Frant, and B. Murray. 2000. Evidence that the reactivity of the martian soil is due to superoxide ions. Science 289:1909-1912. [DOI] [PubMed] [Google Scholar]
- 60.Young, R. S., P. H. Deal, J. F. Bell III, and J. L. Allen. 1964. Bacteria under simulated martian conditions. Life Sci. Space Res. 2:105-111. [PubMed] [Google Scholar]