Abstract
It is unclear whether adaptation to a new host typically broadens or compromises host range, yet the answer bears on the fate of emergent pathogens and symbionts. We investigated this dynamic using a soil isolate of Burkholderia cenocepacia, a species that normally inhabits the rhizosphere, is related to the onion pathogen B. cepacia, and can infect the lungs of cystic fibrosis patients. We hypothesized that adaptation of B. cenocepacia to a novel host would compromise fitness and virulence in alternative hosts. We modeled adaptation to a specific host by experimentally evolving 12 populations of B. cenocepacia in liquid medium composed of macerated onion tissue for 1,000 generations. The mean fitness of all populations increased by 78% relative to the ancestor, but significant variation among lines was observed. Populations also varied in several phenotypes related to host association, including motility, biofilm formation, and quorum-sensing function. Together, these results suggest that each population adapted by fixing different sets of adaptive mutations. However, this adaptation was consistently accompanied by a loss of pathogenicity to the nematode Caenorhabditis elegans; by 500 generations most populations became unable to kill nematodes. In conclusion, we observed a narrowing of host range as a consequence of prolonged adaptation to an environment simulating a specific host, and we suggest that emergent pathogens may face similar consequences if they become host-restricted.
Some emergent pathogens, such as Pseudomonas and Burkholderia species, persist in a wide range of plant and animal hosts, suggesting that the virulence factors needed to infect plants and animals are similar (5, 40). Yet whether adaptation to a new niche tends to compromise niche breadth or, in this case, host range is an open question. Adaptation to a novel host may restrict host range to various degrees, whether by diminishing host-specific virulence traits without affecting host colonization or by reducing the ability to initiate infection in alternative hosts. However, if factors needed to colonize plant and animal hosts are similar, then why are some bacterial populations restricted to a narrow host range while others are not? One explanation for a limited host range may be the result of genetic trade-offs associated with adaptation to a specific host (7, 18). Another explanation may be that prolonged adaptation to a specific host casts a “selective shadow” over unused functions that are relevant to colonizing other hosts but decay by genetic drift (7, 18). To address these possibilities, we quantified the direct and correlated effects of specific host adaptation by the opportunistic pathogen Burkholderia cenocepacia.
Members of the Burkholderia cepacia complex (Bcc), which are ubiquitous in the environment, were once used as biocontrol and bioremedial agents but now are banned from these applications because of the potential of some members to cause plant and human disease (39). The type species B. cepacia is well known as a pathogen of the common yellow onion, Allium cepa, in which it causes a characteristic yellow or brown rot. Another species, B. cenocepacia, can also infect onions as well as a range of plants and animals, including humans (2, 6, 26, 36). Bcc bacteria can cause serious infection in the lungs of cystic fibrosis (CF) patients (6, 26). These infections, called “cepacia syndrome,” are highly contagious among CF patients, and infections produce many negative effects on an already poor quality of life, including longer hospital stays, removal from lung transplant lists, blood poisoning, and eventual death (24). B. cenocepacia, one of the two Bcc species most commonly isolated from lung infections, is especially threatening and is associated with more severe cepacia syndrome (35). However, the mechanisms allowing B. cenocepacia to adapt to colonize both human and plant hosts are unclear. Several putative virulence mechanisms have been identified by random mutagenic screens or by knockouts of candidate genes (2, 12, 20, 25, 29, 35, 43, 46), but these mechanisms generally have not been shown to function in host adaptation. One way to directly study adaptation of bacterial populations to susceptible hosts is by experimental evolution, in which bacterial populations evolve in a controlled laboratory setting that enables study of the adaptive process over time (7).
We experimentally evolved populations of B. cenocepacia HI2424 to study the extent to which adaptation to the common yellow onion A. cepa affects host range. B. cenocepacia HI2424 is a soil isolate and is classified as part of the PHDC strain lineage, the strain first characterized as responsible for an outbreak of Bcc infections in large treatment centers located in the mid-Atlantic region of the United States (33). We found that adaptation of B. cenocepacia to the onion model was associated with reduced virulence but did not compromise the capacity to colonize (or be consumed by) the nematode Caenorhabditis elegans, and the coincidence of these events suggests that a genetic trade-off (antagonistic pleiotropy) between fitness in onion medium and nematode virulence exists. We also characterized several phenotypes potentially associated with adaptation to the onion or nematode virulence. Most phenotypes varied significantly among replicate populations, suggesting that adaptation to the onion model may follow several different pathways.
MATERIALS AND METHODS
Bacterial strains, media, culture conditions, and genetic marking.
All bacterial strains and origins are described in Table 1. All Escherichia coli strains harboring vectors were recovered from frozen stocks and streaked for isolation on T-soy agar, and single colonies were propagated in T-soy broth at 37°C with antibiotics. E. coli OP50 was recovered from frozen stocks and propagated in T-soy broth at 37°C without antibiotics.
TABLE 1.
Bacterial strains and plasmid vectors used in this study
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| E. coli strains | ||
| DH5α λpir | λ−φ80dlacZΔM15Δ(lacZYA-argF)U169 recA1 endA hsdR17(rK− mK−) supE44 thi-1 gyrA relA1with λ pir | Gibco-BRL, Inc. |
| OP50 | Control bacterium in C. elegans virulence assays; viable food source | C. Warren |
| B. cenocepacia strains | ||
| HI2424 | Natural soil isolate recovered from agricultural soil in upstate NY; polymyxin B resistant | 33 |
| P1 | Population evolved from HI2424 lacZ dhfr in galactose-minimal medium in shaking test tube environment for 1,500 generations | S. Poltak and V. Cooper, submitted |
| P2 | Population evolved from HI2424 lacZ dhfrin galactose-minimal medium in shaking test tube environment for 1,500 generations | S. Poltak and V. Cooper, submitted |
| Plasmids | ||
| pUC18 R6KT-mini-Tn7T | Apr; R6K replicon; oriT origin of transfer; contains Tn7 mini-transposable element | 4 |
| pFTP1 | Source of TMP antibiotic resistance cassette flanked by FRT recognition sites. | 4 |
| pTn7-FTP | Apr Tpr; derived from pUC18R6KT-mini-Tn7T | This study |
| pCELacZ | Apr Tpr; contains lacZ gene from pcrSMART lacZ; derived from pTn7-FTP | This study |
| pcrSMART | Kmr; pUC origin of replication; multiple cloning site | Lucigen |
| pcrSMART | Kmr; derived from pcrSMART; contains lacZ gene | This study |
| lacZ | With pLac promoter amplified from E. coli K12 | |
| pTNS2 | Apr; R6K replicon; encodes the TnsABC+D transposition pathway | 4 |
| pEVS104 | Kmr; F+ conjugal helper plasmid | C. Whistler |
| pAS-C8 | Tpr; fluorescent acyl-homoserine-lactone sensor plasmid harbored in Pseudomonas putida F117 | 25 |
| pSPRed | Cmr; constitutively-expressed DsRed fluorescent protein cloned into pBBR1MCS-1from pAKN132 | S. Poltak and V. Cooper, submitted |
Experimental evolution and competition experiments require competitively neutral markers to rule out cross-contamination and to enable competitors to be distinguished in mixed culture. Mini-Tn7 transposon vectors were constructed to insert E. coli lacZ into the chromosome using trimethoprim (TMP) for positive selection. Standard molecular methods were used throughout (34, 42). All plasmids were isolated using a Qiaprep Spin Miniprep kit (Qiagen) according to the manufacturer's protocols. All restriction enzymes were from New England Biolabs (NEB) and were used according to manufacturer's instructions (NEB). A TMP-resistant derivative of pUC18 R6KT-mini-Tn7T (4) was constructed by excising the TMP cassette with flanking Flp recognition target (FRT) sites (860 bp) from pFTP1 (4), using XmaI sites, and ligating it into the XmaI site of pUC18 R6KT-mini-Tn7T with T4 DNA ligase (NEB). The ligation mix was transformed into chemically competent E. coli DH5α λ pir, and TMP-resistant clones were cultured. E. coli lacZ and its native promoter were cloned into pcrSMART (Lucigen) by PCR using primers lacZF (5′-ATTTCGAAATGCTTCCGGCTCGTATGTTGTGT-3′) and lacZR (5′-ATTGTACAACATGGCCTGCCCGGTTATTATTA-3′). The final vector, pCELacZ, was assembled by cloning the lacZ fragment from pcrSMART-lacZ into pTn7-FTP using compatible EcoRI sites.
B. cenocepacia HI2424 was marked using either pTn7-FTP to provide TMP resistance or pCELacZ to provide TMP and β-galactosidase activity. Both plasmids were delivered by four-parental conjugation (3). Briefly, the bacterial helper and the donors were cultured with the appropriate antibiotic (E. coli DH5α λpir/pTNS2 in 30 μg/ml ampicillin, E. coli DH5α λ pir/pEVS104 in 30 μg/ml kanamycin, and E. coli DH5α λ pir/Tn7-derived vectors in 50 μg/ml TMP). HI2424 was cultured in the absence of antibiotics. Overnight cultures were combined, spotted onto a 0.2-μm-pore-size nitrocellulose filter and incubated at 32°C for 12 h. Mixtures were plated, and TMP-resistant colonies were picked and subsequently screened for the lacZ insertion on 5-bromo-4-chloro-3-indolyl-beta-d-galactopyranoside (X-Gal). Tn7 insertions were confirmed by PCR using the primers lacZint-F (5′-TGTCGCTCCACAAGGTAA) and lacZint-R (5′-CACTTCAACATCAACGGTAATC).
Experimental evolution.
Twelve populations of B. cenocepacia were serially transferred for 152 days in a medium composed of macerated onions as follows. Common yellow onions (A. cepa) were obtained from Fiddlehead Farms Marketplace, Dover, NH. Six populations of B. cenocepacia HI2424 lacZ+ Tpr (designated populations D1 to D6) and six populations of B. cenocepacia HI2424 lacZ-null Tpr (populations L1 to L6) were founded from single clones and propagated daily by 1:100 dilution into 5 ml of 2% onion medium (2% macerated sterile A. cepa tissue, 1× M9 buffer) in 18- by 150-mm test tubes at 32°C. (The chemical composition of onion tissue is described in reference 48). The populations were maintained in this manner for 1,000 generations, and growth was equivalent to log2(100), or ∼6.6 generations occurring per 24 h; every 100 generations, 1-ml samples from each whole population were stored (−80°C).
Competition experiments.
Fitness of evolved B. cenocepacia populations relative to the ancestral clones was determined as outlined previously (30). Briefly, 50-μl samples of the evolved populations and the ancestral clones were recovered from a frozen state by overnight growth in T-soy broth at 32°C with orbital shaking at 130 rpm. Following incubation, each population was diluted 1:100 into 2% onion medium and grown for 24 h at 32°C with orbital shaking at 130 rpm. This allowed each population to acclimate to the onion medium. Following incubation, each evolved population was combined with its oppositely marked ancestor in a 1:1 ratio and grown for 24 h in the selective environment, with orbital shaking at 130 rpm. Initial and final densities (CFU/ml) of the two competitors were calculated by plating diluted samples on T-soy agar containing 0.04% X-Gal that allowed them to be distinguished by marker type. The net growth of each competitor was determined from plate counts, and the relative fitness of the evolved populations to that of the ancestor was expressed as the log ratio of their realized growth (30). Each experiment was performed with five replicates. Fitness values of less than 1.0 reflect reduced fitness relative to the ancestor; values greater than 1.0 reflect fitness increases (30).
A. cepa virulence experiments.
Onion scale virulence assays were performed as described previously (27). Briefly, whole onions were washed with 95% ethanol and cut in quarters with a sterile knife. Individual scales measuring approximately 60 mm in length and 35 mm in width were dipped in an onion wash solution (1× M9 buffer supplemented with 2 μg/ml nystatin, 2.5 μg/ml gentamicin, and 50 μg/ml tetracycline) and wounded on the inner surface with a sterile pipette tip. The scales were inoculated with 5 μl of bacterial culture that was previously grown overnight in 2% onion medium. The scales were individually incubated at 32°C for 48 h in sterile custard dishes containing sheets of sterile Whatman no. 1 filter paper premoistened with onion wash solution. Postincubation, zones of tissue maceration were measured in mm2. Virulence was calculated as area of tissue maceration.
C. elegans virulence experiments.
C. elegans strain N2 provided by the Caenorhabditis Genetic Center (Minneapolis, MN) was used throughout this study. Periodically, nematodes were thawed from −80°C and propagated on nematode growth medium (NGM) seeded with E. coli OP50 as a food source (45). Gravid adult nematodes (>107) were collected from the medium surface by washing with 1× M9 buffer and were then purified by sucrose floatation. Washed adults were suspended in bleach to harvest their eggs; the eggs were allowed to hatch, and synchronized L1 larvae were grown aseptically in C. elegans habitation and reproduction medium (CeHR) (45) for 48 h until they reached L4 stage.
Estimation of bacterial virulence to C. elegans was performed using a liquid model of C. elegans infection described elsewhere (9). Briefly, bacteria were recovered from a frozen state by culture in T-soy broth for 24 h at 32°C. Following incubation, each population was diluted 1:100 into filtered 2% onion medium and grown overnight. Samples (500 μl) of bacterial cultures were then standardized to an optical density at 600 nm (OD600) of 1.0 and added to a six-well plate with 5 ml of S medium (45). One hundred synchronized L4 nematodes were added to the bacteria in S medium and incubated at 24°C for 9 days. Percent nematode death was monitored by counting the number of dead nematodes per 50 total nematodes. The OD600 of each mixture was monitored using a Tecan Infinite M200 plate reader. For all statistical comparisons, we compared population virulence by calculating the area under the curve (AUC) of percent nematode mortality over time.
To visualize bacterial populations within nematodes, confocal laser scanning microscopy was performed on mounts of 10 C. elegans nematodes infected with single clones of bacteria at a magnification of ×200 and 543-nm emission with a band-pass (BP) 560- to 615-nm filter on a Zeiss LSM510 Meta. Bacteria were marked with pSPRed (S. Poltak and V. Cooper, submitted for publication) prior to infection of nematodes using mating procedures previously described (3). Nematodes were infected with bacteria for 120 h before imaging. Five nematodes cultured with the ancestor bacteria and five nematodes cultured with population L1 were washed twice with 1× M9 buffer before being mounted on glass slides containing an agar solution (2% agar, 0.1 M NaCl, 10 mM Na2HPO4, 1 mM KH2PO4).
Phenotypic assays.
Growth rates were determined from measurements of the OD600 in 96-well plates; maximum growth rates occurred between hours 12 and 13 of growth. Biofilm production was measured by crystal violet staining of stationary phase cultures grown in T-soy broth in 96-well plates as described previously (37). T-soy broth was used in place of 2% onion medium because measuring biofilm production in the presence of onion particulate complicated our quantification, and filtered onion medium did not support enough growth for robust biofilm production. Production of C8 acyl-homoserine-lactone molecules that mediate quorum sensing in Bcc (32) was performed in 96-well plates and detected using methods previously described using the plasmid pAS-C8 as a fluorescence reporter (53). Swimming and swarming motility were measured as diameters of spread on 0.3% T-soy agar following 24 h of incubation at 37°C.
To visualize bacterial populations in the selective environment, stationary phase cultures were grown in Luria-Bertani (LB) broth, and 3-μl samples were smeared onto microscope slides containing an agar solution (2% agar, 0.1 M NaCl, 10 mM Na2HPO4, 1 mM KH2PO4). Mounts were visualized using standard light microscopy at magnifications of ×400 and ×1,000.
Determination of diet breadth.
The total catabolic diet breadth of the lacZ+ evolved populations and the lacZ+ ancestor clone was determined as described previously (10). Briefly, bacteria were recovered from −80°C by growth in T-soy broth for 24 h at 32°C. Each culture was then diluted 1:100 into fresh T-soy broth and grown overnight. The cultures were washed in 1× phosphate-buffered saline (PBS) and standardized to an OD600 of 1.0. Assays were run in three replicates for each sample in Biolog (Hayward, California) GN2 plates by taking OD600 readings every 2 h for 12 h, once at 18 h, and once at 24 h using a Tecan Infinite M200 plate reader. The growth curve for each well was standardized by the water control and was integrated into one AUC value of catabolic function. To assess whether the evolved populations systematically gained or lost functions, the means of each evolved population were compared with the means of the ancestor replicates by a two-tailed t test. This was performed for each carbon source, and thus the critical P value was corrected for multiple comparisons.
RESULTS
Chromosomal marking by lacZ is competitively neutral.
We tested whether the Tn7 lacZ-dhfr insertion in B. cenocepacia HI2424 affected relative fitness by comparing it to an isogenic strain harboring only a Tn7 dhfr insertion. We also quantified the yield (CFU/ml) of both competitors in the selective environment after 24 h. Based on five replicates, the average fitness ratio of lacZ-dhfr-marked to dhfr-marked B. cenocepacia in 2% onion medium was 1.008 (± 0.053 standard error), and the yields of both competitors did not significantly differ when grown separately (t = 0.278; P = 0.80).
Direct effects of experimental evolution.
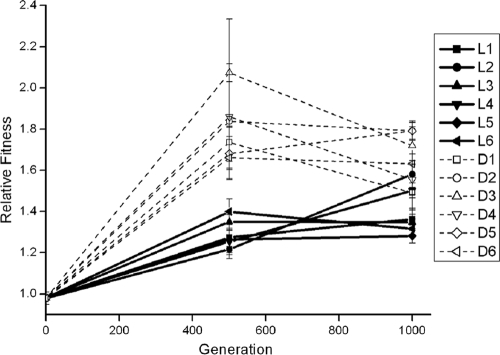
Adaptation of all 12 B. cenocepacia populations to onion medium was quantified following 500 and 1,000 generations of selection (Fig. 1). After 500 generations, mean fitness of all populations increased by an average of 55% relative to the ancestor grand mean based on five replicates per population of 1.55 ± 0.17 (95% confidence interval [CI]). After 1,000 generations, even greater adaptation was observed (t = 3.35; P = 0.003), reaching a mean of 1.97 ± 0.25. However, significant variation among experimental blocks and block-by-population interactions were observed (Table 2), likely as a function of different batches of onions. This effect may obscure significant differences among populations but was minimized when the same batch of onion medium was used in different experiments (data not shown). Nevertheless, the significant interaction term in the analysis of variance (ANOVA) and the change in fitness rank over time (Fig. 1) suggest that different populations may have adapted at different rates and/or by the substitution of functionally distinct mutations.
FIG. 1.
Adaptation by 12 B. cenocepacia populations during 1,000 generations in onion medium. Fitness was quantified by direct competition with the ancestor of the opposite marker; Lac+ populations (D1 to D6) and Lac− populations (L1 to L6) are represented as indicated on the figure. Error bars are ± standard error (df = 4).
TABLE 2.
Analysis of variance of fitness relative to the ancestor in onion medium following 1,000 generations of evolution
| Group | Type III sum of squares | df | MSa | F | Significance |
|---|---|---|---|---|---|
| Population | 9.629 | 11 | 0.875 | 3.019 | 0.116 |
| Experimental block | 11.799 | 1 | 11.799 | 40.691 | 0.001 |
| Block-by-population interaction | 1.450 | 5 | 0.290 | 7.583 | 0.000 |
MS, mean sum of squares.
The vast increases in competitive fitness can be explained by improvements in several traits, including exponential growth rate, the total cell yield of each population, and adaptation to general aspects of the laboratory environment. We quantified maximum growth rate over 24 h of the evolved (1,000 generation) populations and ancestors in 2% onion medium and found that 11 of 12 populations grew significantly faster than the ancestor (Table 3). The mean rate increase was 72.7%, from an ancestral value of 0.0078 ± 0.0018 to 0.0135 ± 0.0009. Cell yield (CFU/ml) of these populations in onion medium following 24 h of growth declined slightly but significantly (t = 2.15; P = 0.04).
TABLE 3.
Components of fitness in macerated onion medium
| Population | Maximum growth ratea | Yield (CFU/ml × 108)b |
|---|---|---|
| L1 | 0.0138 ± 0.0023 | 2.04 ± 0.21 |
| L2 | 0.0130 ± 0.0024 | 2.28 ± 0.26 |
| L3 | 0.0153 ± 0.0059 | 2.30 ± 0.34 |
| L4 | 0.0134 ± 0.0019 | 2.17 ± 0.08 |
| L5 | 0.0148 ± 0.0043 | 2.42 ± 0.20 |
| L6 | 0.0095 ± 0.0019 | 2.12 ± 0.14 |
| D1 | 0.0117 ± 0.0031 | 2.52 ± 0.46 |
| D2 | 0.0138 ± 0.0010 | 2.18 ± 0.23 |
| D3 | 0.0147 ± 0.0030 | 2.07 ± 0.16 |
| D4 | 0.0144 ± 0.0013 | 1.90 ± 0.14 |
| D5 | 0.0126 ± 0.0023 | 2.18 ± 0.20 |
| D6 | 0.0147 ± 0.0018 | 2.19 ± 0.18 |
| Wild type | 0.0078 ± 0.0018 | 2.38 ± 0.14 |
Change in OD600 per hour ±95% CI.
Values are ±95% CI.
To test whether populations adapted to the general laboratory environment rather than specifically to the onion medium, we measured the relative fitness of six populations in an environment identical to the selective environment, except that 1% galactose was substituted for macerated onions as the carbon source. Mean fitness of the six populations in galactose medium was 0.99 ± 0.05 (95% CI), which does not differ from neutrality (t = 0.45; P = 0.67). In addition to this test, we assayed the fitness in onion medium of two laboratory-evolved populations (P1 and P2) that are also derived from the same ancestor as the onion-evolved populations (see Fig. S1A in the supplemental material). Populations P1 and P2 were evolved similarly to the onion-evolved populations, except that 1% galactose minimal medium was substituted for 2% onion medium throughout serial passage in the laboratory for 1,500 generations. Mean fitness of the galactose-evolved populations in onion medium was 1.05 ± 0.06 (95% CI), which does not differ from neutrality (t = 1.478; P = 0.163). In summary, adaptation in onion medium was specific to that environment, not a response to general laboratory conditions, and can be explained primarily by an increase in growth rate.
We assayed virulence on intact onion scales to determine if adaptation to 2% onion medium was associated with increased onion tissue maceration. Evolved and ancestral populations were grown to stationary phase and used to inoculate onion scales. Though tissue maceration and severe odor were observed for all evolved populations and the ancestors, there was no quantitative difference detected in the zone of tissue maceration (t = −1.22; P = 0.23), nor was there a qualitative difference in odor between evolved populations and the ancestor clones (data not shown). Therefore, passage in 2% onion medium did not correlate with an increase in tissue maceration ability.
Effects of onion adaptation on nematode virulence.
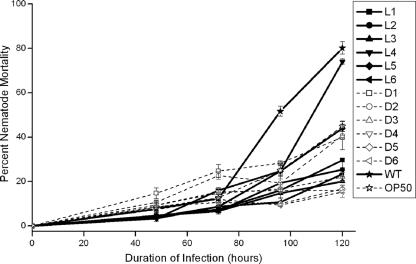
Pathogenicity to the nematode C. elegans was quantified using a liquid model of infection, using the ancestor clone and all evolved populations after 500 generations of onion adaptation (Fig. 2). We also assayed nematode virulence of the evolved populations after 1,000 generations of onion adaptation, but since pathogenicity did not differ significantly in three populations assayed after 500 generations (t = 0.439; P = 0.67), we do not discuss these findings further. Generally, evolved populations showed a significant decrease in nematode killing ability compared to the ability of the ancestral clone (t = 7.11; P = 1.32 × 10−8). Infection by the ancestral clone resulted in a mean nematode mortality of 81%, whereas infection by the evolved populations after 500 generations resulted in a mean mortality of 30.6%. Optical density of the evolved populations did not differ significantly from that of the ancestor (t = −0.062; P = 0.951) (see Fig. S2 in the supplemental material); yet nematodes infected with ancestor populations were sluggish and slow to respond to mechanical stimulus, and nematodes infected with evolved populations displayed vigorous movement and immediate response to touch. Populations varied significantly in their ability to kill nematodes (ANOVA, F = 38.337; P = 1.95 × 10−13), which suggests that varied adaptations to the onion medium also affected nematode virulence differently. To ensure that reduced nematode killing compared to the ancestor was not due to differences in starting inocula, we quantified the cell yield of ancestor and evolved populations after 24 h of growth in filtered onion medium and found no significant differences between ancestor and evolved populations (t = −1.303; P = 0.234).
FIG. 2.
Nematode pathogenicity declines during adaptation to onion medium. Bacterial virulence was measured in monoxenic liquid culture as percent mortality over time. Lac+ populations (D1 to D6) and Lac− populations (L1 to L6) evolved for 500 generations in 2% liquid onion medium were introduced to axenically raised nematodes and observed over 7 days. E. coli OP50 (open star) was used as a negative control, and the wild-type (WT) HI2424 Lac− ancestor (filled star) was used as a positive control. Error bars are standard error (df = 2).
To test if the evolved populations experienced reduced nematode killing as the result of adaptation to a general laboratory environment and not the onion model, we assayed nematode killing for the galactose-evolved population P1 (see Fig. S1B in the supplemental material). This population did not differ in nematode killing ability compared to the ancestor (t = 1.55; P = 0.197), and the nematodes infected with P1 appeared sluggish. Therefore, we conclude that the reduction in nematode killing by the onion-evolved populations was a correlated effect of adaptation to the onion model and not simply to the laboratory environment.
To determine if decreased nematode virulence was the result of overall catabolic decay, we measured the ability of the lacZ+ populations and their ancestor to use 95 different carbon sources (see Table S1 in the supplemental material). Of these, 81 substrates produced measurable growth. For each substrate, we compared the evolved population mean values with the ancestor replicate values using two-tailed t tests and corrected the P criterion for 81 comparisons. This analysis revealed no significant increases or decreases in growth on any carbon source although some gains and losses specific to individual populations may have occurred. Further, the sums of these growth functions were generally equivalent between evolved and ancestral replicates; five of six evolved populations demonstrated slightly (∼3.7%) increased growth, but this difference was not significant (t = 0.78; P = 0.28). Therefore, selection in the onion model apparently neither favored specialization on a particular carbon source nor broadened metabolism. These results appear to rule out decreased metabolic capacity as a cause of reduced nematode pathogenicity.
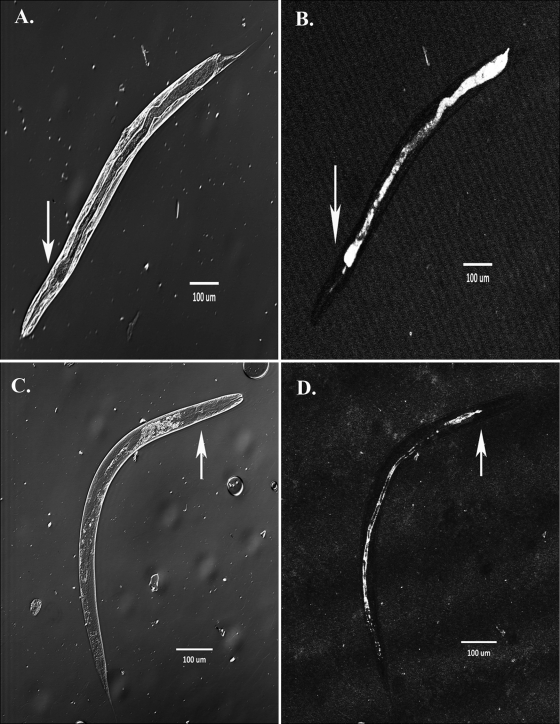
To determine whether the reduced nematode killing by the evolved bacteria was associated with an inability to initiate infection, we used confocal microscopy to visualize 10 individual nematodes that were cultured with a red fluorescent protein (RFP)-marked clone from one evolved population (L1) or an RFP-marked ancestor clone (Fig. 3). All nematodes cultured with the virulent ancestor were lethargic and sluggish, behavior consistent with infection. Furthermore, the ingested ancestor bacteria were highly concentrated behind the pharynx in the grinder region of all five nematodes (Fig. 3B), and the intestinal lumen was distended (Fig. 3A), which correlates with constipation and starvation of nematodes (23). In contrast, all nematodes cultured by the evolved attenuated population L1 were active and moved rapidly, consistent with ingestion of a nonpathogen or a food source. In all but one nematode, evolved bacteria were uniformly distributed throughout the gut (Fig. 3D), which is suggestive of normal digestion, and the intestine appeared nondistended (Fig. 3C).
FIG. 3.
Differential colonization and pathogenicity of evolved population L1 and the ancestral strain. (A and B) A light microscopy image at a magnification of ×200 of the ancestor marked with red fluorescent protein colonizing C. elegans (A) and confocal fluorescent microscopy of the same nematode (B) are shown. We modified the confocal image to exclude air bubbles (upper right and lower left corners). Ingested bacteria are highly concentrated behind the grinder region of the worm (white arrows) and cause distension throughout the intestine. (C and D) A light microscopy image at a magnification of ×200 of evolved population L1 marked with red fluorescent protein colonizing C. elegans (C) and confocal fluorescent microscopy of the same nematode (D) are shown. Ingested bacteria are distributed throughout the nondistended intestine of the nematode at lower density.
Correlated effects of experimental evolution.
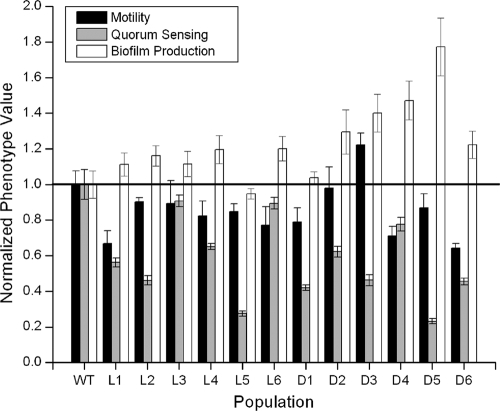
We examined three separate phenotypes related to virulence in plant and animal models to determine whether the evolved populations diverged from the ancestor. These were (i) motility, (ii) biofilm production, and (iii) C8 acyl-homoserine-lactone production, a key attribute of quorum sensing in Bcc (Fig. 4). Though the motility of three populations increased (D2, D3, and L3), the mean motility of the remaining populations declined relative to the ancestor (t = 3.32; P = 0.002). Evolved populations varied significantly in their ability to swim (F = 4.126; P = 3.6 × 10−4) but did not systematically increase or decrease. Most populations increased in biofilm production (t = −2.00; P = 0.04) but also varied significantly (F = 6.040; P = 2.0 × 10−6). In light of this finding, we also examined attachment to onion particulate matter using phase-contrast microscopy of ancestor and evolved bacteria growing in onion medium (data not shown). Both the ancestor and evolved bacteria adhered to onion particulate, which could explain why increased biofilm production may have been favored. Lastly, all evolved populations produced significantly less C8 acyl-homoserine-lactone (t = 6.976; P = 7.15 × 10−10), which is indicative of reduced quorum-sensing function, but varied significantly in their levels of production (F = 50.788; P < 0.001). Although significant variation was observed in all three traits, some populations may have undergone coordinated change. To determine whether certain populations may have adapted along similar pathways, we developed a trait matrix to estimate the minimum number of different adaptive routes by the 12 evolved populations (see Table S2 in the supplemental material). Taken together, every population evolved a unique combination of adaptive and correlated traits, which implies unique genetic causes or correlates of adaptation.
FIG. 4.
Motility, acyl-homoserine lactone production (quorum sensing), and biofilm production of wild-type (WT) B. cenocepacia and 12 populations evolved in 2% onion medium for 500 generations. Swimming motility was measured using 0.3% swim agar (in mm). Biofilm production and C8 acyl-homoserine lactone production were measured as previously described (25, 37). All values were standardized by the ancestral values for each phenotype (the horizontal line represents the ancestor clone; values greater than 1.0 indicate increases and values less than 1.0 indicate decreases). Genotype designations are as follows: L, Lac− evolved populations; D, Lac+ evolved populations; WT, Lac+ ancestor clone. Error bars indicate standard error (motility, df = 3; biofilm, df = 5; quorum sensing, df = 5).
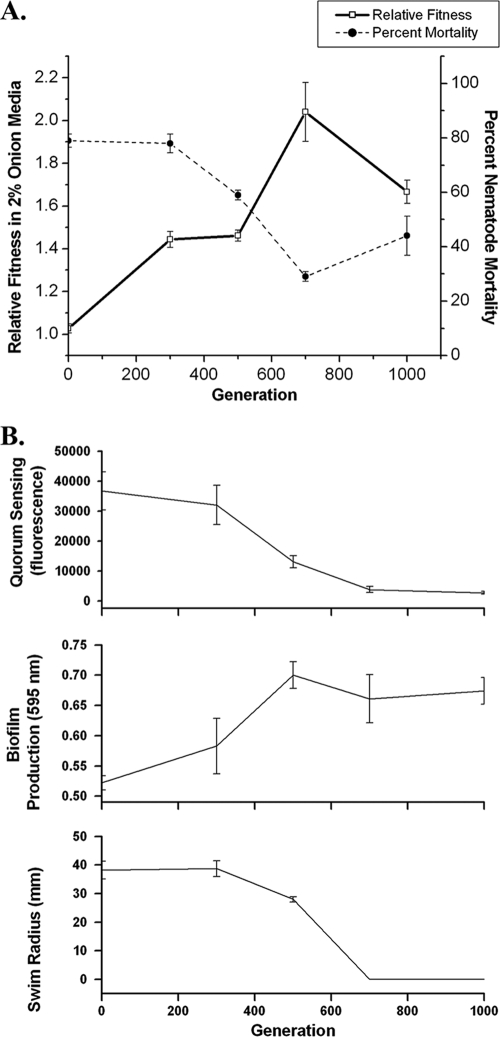
Adaptation to onion medium was therefore associated with significant alterations in nematode pathogenicity, motility, biofilm production, and quorum-sensing function. We studied changes in these traits more closely over time in one population (Fig. 5). The increased fitness in onion medium between 300 and 700 generations correlated directly with decreased nematode virulence (Fig. 5A). At the same time, biofilm production increased and motility decreased (Fig. 5B). In this population, relative fitness actually decreased between 700 and 1,000 generations, which correlated with a slight restoration in nematode virulence. Together these results demonstrate the coordinated response of several traits to adaptation in onion medium.
FIG. 5.
(A) Relative fitness and percent nematode mortality over time for a single population (L1) of B. cenocepacia evolved in 2% onion medium for 1,000 generations. Error bars are standard error (fitness, df = 4; nematode virulence, df = 2). Increased fitness is temporally correlated with decreased nematode pathogenicity. (B) Dynamics of three phenotypes associated with B. cenocepacia virulence in population L1 (error bars indicate standard errors): top, C8 acyl-homoserine-lactone production (25) (df = 5); center, biofilm production (37) (df = 5); bottom, motility quantified as radii of movement through 0.3% agar (df = 3).
DISCUSSION
Adaptation to onion medium was rapid and varied among populations.
After 500 generations of evolution in onion medium, nine evolved populations significantly out-competed the ancestor, and after 1,000 generations of evolution, all 12 populations were significantly more fit (Fig. 1). This adaptation was indeed specific to onion medium because two populations evolved in minimal galactose medium in a separate experiment (Poltak and Cooper, submitted) did not differ from the ancestor during competition in the selective environment. Rather, increased fitness was likely primarily the result of increased maximum growth rate, which increased by a magnitude (73%) that would explain much of the mean 97% fitness increase (Table 3). Maximum growth rate has also been shown to be the major component of fitness in E. coli populations that were experimentally evolved by serial transfer (50). Similarly, in both these Burkholderia populations and in other E. coli populations, the apparent yield based on the number of CFU declined over time, which could reflect a shift toward production of fewer, larger cells (51) though we have not yet quantified mean cell volume. Yet the apparent variability among these populations is unusual in comparison to other serial transfer experiments (1, 31, 52), ranging in one complete block from a minimum of 1.49 to a maximum of 2.78. This variation was significantly associated with experimental blocks and not with populations alone, but the interaction term of experimental block and population was highly significant (Table 2). The effects of experimental blocks likely reflect the natural complexity and variability of onions as susceptible hosts; even tissue grinding and sterilization did not eliminate all environmental structure or properties inherent to onion batches. The population-by-environment interaction suggested that different adaptations to onion medium may have been selected in different populations.
The extent of adaptation over the first 500 generations was positively associated with the presence of the lacZ marker, but this difference disappeared by generation 1,000 (Fig. 1). This may be an effect of evolutionary history since the presence of lacZ seemed to influence the evolutionary outcomes of evolving populations. Such a pattern related to genetic marking has been reported in at least one other experimental evolution (41). Unfortunately, we cannot determine if the fitness of the lacZ+ populations increased due to natural selection alone or as the interaction of genetic history and natural selection (41).
Adaptation to onion medium reduced nematode pathogenicity and influenced a range of physiological traits.
Variability among populations undergoing adaptation to a common environment could result from two concurrent processes: selection of different adaptive alleles or from random fixation of different alleles either by genetic drift or by genetic hitchhiking (8, 10, 11). Because each population was founded by a single clone and all new variation in each population arose de novo by mutation, selection of different alleles among different populations is plausible. However, the typically low mutation rates of these bacteria (estimated to be on the order of 10−2/genome/generation S. Poltak and V. Cooper, unpublished data) combined with the large effective population sizes of this experiment (the harmonic mean of the daily bottleneck and final populations, ∼4 × 106 [21]) strongly hamper effects of genetic drift. Thus, variation among populations is unlikely due to drift alone. Nevertheless, it is possible that a common set of favorable alleles became physically linked to different, secondary mutations in different populations. If this were the case, we would expect strong convergence in adaptive traits but little convergence among correlated, nonadaptive traits because different mutations would hitchhike in different populations. We explored this alternative by measuring several heritable phenotypes of the adapted populations and their extent of convergence (Fig. 4).
Many phenotypes may change during selection on what may seem, a priori, to be a narrowly defined trait (e.g., more rapid growth in onion medium). These correlated effects may be direct or indirect consequences of adaptation (30) because selection acted directly on a broader range of traits than predicted, selection acted on traits that were pleiotropic, or variation accumulated by nonselective means. If correlated effects change in parallel among replicate populations, then adaptation may have occurred by similar genetic bases (15). During this study, we observed several patterns of correlated effects during adaptation to onion medium: (i) losses of function or trade-offs common to all of the evolved populations (nematode pathogenicity; quorum-sensing ability); (ii) common adaptations specific to the selective environment (increased fitness, increased growth rate, and reduced yield in CFU/ml); (iii) both gains and losses of function among replicate populations (biofilm production, motility, and diet breadth); and (iv) changes that depended on the initial genetic condition or on evolutionary history (effects of the lacZ marker).
To further explore the linkages among the evolved responses, we analyzed all of these correlated effects in the context of a trait matrix (see Table S2 in the supplemental material). We found that no two populations were statistically inseparable; thus, each population appears to have adapted to onion medium by distinct underlying mechanisms. These differences between populations might erode if selection continued over a longer period and favored greater convergence of beneficial alleles, but they need not. For example, relatively subtle differences in fitness among serially transferred E. coli populations after 2,000 generations of evolution have persisted for more than 20,000 generations (10, 31). On balance, the distinct adaptive responses of these evolved Burkholderia populations combined with their variable indirect responses are better explained by unique combinations of adaptive and pleiotropic alleles rather than by rampant genetic hitchhiking of secondary, neutral mutations. However, such variability may ultimately stem from adaptation of a maladapted, natural isolate in a foreign laboratory environment, in which many different mutations are adaptive.
Perhaps the most remarkable correlated effect was the parallel and consistent loss of nematode pathogenicity, which can be viewed as a restriction of host range for B. cenocepacia (Fig. 2). This outcome can be explained as a product of antagonistic pleiotropy: a beneficial mutation in the onion model is a detrimental one in the worm model. Furthermore, variability of worm virulence within a given population (Fig. 5A) suggests that it is genetically heterogeneous; therefore the fall and rise of nematode virulence may reflect competition between different clones with distinct pleiotropic effects. In addition, because selection occurred over a relatively short period and because changes in fitness, nematode pathogenicity, biofilm production, motility, and quorum-sensing function (Fig. 5B) were precisely temporally correlated, it seems likely that few mutations (and perhaps only one) affected the complete suite of measured phenotypes (10). It is important to note that our findings show trade-offs associated with virulence factors and not necessarily with the initiation of infection. Adaptation to the onion model did not limit colonization of an alternate host, yet the loss of virulence after only 500 generations has implications for reduction of host range during long-term adaptation. More generally, this implies that adaptation to a specific environment may reduce host or niche breadth by antagonistic pleiotropy, which has been observed in studies of viral adaptation to single hosts (14, 16).
The mechanisms of onion disease are well described in B. cepacia ATCC 25416. These bacteria macerate onion tissue by secreting a pectate hydrolase enzyme (peh) that degrades pectin molecules and compromises plant cell walls (13, 22, 47) and also by producing a plant tissue watersoaking (PTW) phenotype associated with onion tissue maceration (22). B. cenocepacia does not contain the peh gene but is capable of the PTW phenotype, which is attributed to plant-cell cytotoxic effector molecules delivered by a type IV secretion system found in both B. cepacia and B. cenocepacia (19). As yet, the effector molecules used by both species in plant virulence remain uncharacterized. However, we did not observe a significant increase in onion disease symptoms following selection in onion medium. Tissue maceration by evolved populations did not increase over that caused by the ancestor, but this phenotype did not decrease either. This result may simply reflect the mass-action, homogenized environment of onion medium that did not select for improved tissue invasion but rather only tissue catabolism. In addition, the ability to adapt to grow in onions is apparently tied to an initial capacity for tissue invasion: pilot selection experiments involving two avirulent strains of B. cenocepacia failed to grow well in onion medium or on onions themselves (not shown).
The suite of other phenotypic changes that may be linked to pathogenicity in onions or nematodes illustrates that selection in specific hosts or host analogs may influence host range (Fig. 4). For example, motility is a trait required for pathogenicity by a range of bacterial species (17, 28, 40), but its reduction in some populations associated with adaptation to onion medium may be responsible for reduced nematode virulence (38). In addition, the overall increase in biofilm production and adherence to onion tissue among adapted populations could suggest greater pathogenic potential in other hosts: biofilm production is strongly associated with the ability to establish respiratory infections (49), including by B. cenocepacia (25, 35). Yet high-biofilm-producing mutants of B. cenocepacia HI2424 are less virulent in C. elegans (S. Poltak and V. Cooper, unpublished data), which suggests that altered biofilm production as a correlated effect of host selection is ambiguous. Similarly, the ability to conduct quorum sensing by the production and sensing of C8 homoserine lactones declined in these populations; this function underlies pathogenicity in several hosts by B. cenocepacia (25, 44).
Convergence toward increased biofilm production and decreased motility among the evolved populations suggests that increased biofilm could be an adaptive response to growth in the onion model, which is supported by phase-contrast images of bacteria adhering to onion particulate. However, selection for biofilm formation was not particularly strong since adherence was not required for transfer to fresh medium in our model. This could explain the variation seen in biofilm formation and motility of the evolved populations. Also, there is no correlation between increased biofilm formation and decreased motility, as would be expected if these populations were adapting to a biofilm lifestyle. Furthermore, the galactose-evolved populations P1 and P2 (Poltak and Cooper, submitted) also showed modest increases in biofilm production due to adherence to the walls of the test tube, which demonstrates adaptation to the general lab environment. Thus, we do not suggest that the predominant form of selection was for biofilm adaptation but that the variability in biofilm production and motility were likely due to a combination of lab adaptation and adaptation to our onion model.
In this study, we evaluated the degree to which adaptation to one specific host model affected host range. We concluded that not only does adaptation to a specific host restrict host range but also the favored mutations that increase fitness in one host themselves likely decrease fitness in another host. In addition, we concluded that mutations affecting the pathogenicity of B. cenocepacia are also variable and pleiotropic; variation in these correlated traits suggests that different populations adapted by distinct genetic pathways but with generally antagonistic, pleiotropic effects on host range. These findings are significant in the context of disease control and the use of prolonged passage on a single host to attenuate the virulence and restrict the host range of opportunistic pathogens. We anticipate that future studies may identify the mutations that increased fitness in the onion model and elucidate their specific contributions to host range restriction.
Supplementary Material
Acknowledgments
We are grateful to S. Poltak, S. Wrocklage, L. Benton, C. Whistler, S. Perros, and A. Kuehn for helpful discussions.
This work was supported by New Hampshire Agricultural Experiment Station grant H-496 to V.S.C.
Footnotes
Published ahead of print on 12 February 2010.
Supplemental material for this article may be found at http://aem.asm.org/.
Scientific contribution 2424 from the New Hampshire Agricultural Experiment Station.
REFERENCES
- 1.Bennett, A. F., R. E. Lenski, and J. E. Mittler. 1992. Evolutionary adaptation to temperature. I. Fitness responses of Escherichia coli to changes in its thermal environment. Evolution 46:16-30. [DOI] [PubMed] [Google Scholar]
- 2.Carvalho, A. P. D., G. M. C. Ventura, C. B. Pereira, R. S. Leao, T. W. Folescu, L. Higa, L. M. Teixeira, M. C. M. Plotkowski, V. L. C. Merquior, R. M. Albano, and E. A. Marques. 2007. Burkholderia cenocepacia, B. multivorans, B. ambifaria and B. vietnamiensis isolates from cystic fibrosis patients have different profiles of exoenzyme production. APMIS 115:311-318. [DOI] [PubMed] [Google Scholar]
- 3.Choi, K.-H., D. DeShazer, and H. P. Schweizer. 2006. Mini-Tn7 insertion in bacteria with multiple attTn7 sites: example Burkholderia mallei ATCC 23344. Nat. Protoc. 1:162-169. [DOI] [PubMed] [Google Scholar]
- 4.Choi, K.-H., J. B. Gaynor, K. G. White, C. Lopez, C. M. Bosio, R. R. Karkhoff-Schweizer, and H. P. Schweizer. 2005. A Tn7-based broad host-trange bacterial cloning and expression system. Nat. Methods 2:443-450. [DOI] [PubMed] [Google Scholar]
- 5.Coenye, T., and P. Vandamme. 2003. Diversity and significance of Burkholderia species occupying diverse ecological niches. Environ. Microbiol. 5:719-729. [DOI] [PubMed] [Google Scholar]
- 6.Coenye, T., P. Vandamme, J. R. Govan, and J. J. LiPuma. 2001. Taxonomy and identification of the Burkholderia cepacia complex. J. Clin. Microbiol. 39:3427-3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper, V. S. 2007. Experimental evolution of pathogens, p. 215-224. In M. Tibayrenc (ed.), Encyclopedia of infectious diseases: modern methodologies. John Wiley and Sons, Hoboken, NJ.
- 8.Cooper, V. S., A. F. Bennett, and R. E. Lenski. 2001. Evolution of thermal dependence of growth rate of Escherichia coli populations during 20,000 generations in a constant environment. Evolution 55:889-896. [DOI] [PubMed] [Google Scholar]
- 9.Cooper, V. S., W. A. Carlson, and J. J. LiPuma. 2009. Susceptibility of Caenorhabditis elegans to Burkholderia infection depends on prior diet and secreted bacterial attractants. PLoS One 4:e7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper, V. S., and R. E. Lenski. 2000. The population genetics of ecological specialization in evolving Escherichia coli populations. Nature 407:736-739. [DOI] [PubMed] [Google Scholar]
- 11.Cooper, V. S., D. Schneider, M. Blot, and R. E. Lenski. 2001. Mechanisms causing rapid and parallel losses of ribose catabolism in evolving populations of Escherichia coli B. J. Bacteriol. 183:2834-2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corbett, C. R., M. N. Burtnick, C. Kooi, D. E. Woods, and P. A. Sokol. 2003. An extracellular zinc metalloprotease gene of Burkholderia cepacia. Microbiology 149:2263-2271. [DOI] [PubMed] [Google Scholar]
- 13.Cother, E. J., and V. Dowling. 1985. Association of Pseudomonas cepacia with internal breakdown of onion—a new record for Austrailia. Aust. Plant Pathol. 14:10-12. [Google Scholar]
- 14.Crill, W. D., H. A. Wichman, and J. J. Bull. 2000. Evolutionary reversals during viral adaptation to alternating hosts. Genetics 154:27-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crozat, E., N. Phillippe, R. E. Lenski, J. Geiselmann, and D. Schneider. 2005. Long-term experimental evolution in Escherichia coli. XII. DNA topology as a key target of selection. Genetics 169:523-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duffy, S., P. E. Turner, and C. L. Burch. 2006. Pleiotropic costs of niche expansion in the RNA bacteriophage Φ6. Genetics 172:751-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Easom, C. A., and D. J. Clarke. 2008. Motility is required for the competitive fitness of entomopathogenic Photorhabdus luminescens during insect infection. BMC Microbiol. 8:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ebert, D. 1998. Evolution—experimental evolution of parasites. Science 282:1432-1435. [DOI] [PubMed] [Google Scholar]
- 19.Engledow, A. S., E. G. Medrano, E. Mahenthiralingam, J. J. LiPuma, and C. F. Gonzalez. 2004. Involvement of a plasmid-encoded type IV secretion system in the plant tissue watersoaking phenotype of Burkholderia cenocepacia. J. Bacteriol. 186:6015-6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fehlner-Gardiner, C. C., T. M. H. Hopkins, and M. A. Valvano. 2002. Identification of a general secretory pathway in a human isolate of Burkholderia vietnamiensis (formerly B-cepacia complex genomovar V) that is required for the secretion of hemolysin and phospholipase C activities. Microb. Pathog. 32:249-254. [DOI] [PubMed] [Google Scholar]
- 21.Gerrish, P. J., and R. E. Lenski. 1998. The fate of competing beneficial mutations in an asexual population. Genetica 103:127-144. [PubMed] [Google Scholar]
- 22.Gonzalez, C. F., E. A. Pettit, V. A. Valadez, and E. M. Provin. 1997. Mobilization, cloning, and sequence determination of a plasmid-encoded polygalacturonase from a phytopathogenic Burkholderia (Pseudomonas) cepacia. Mol. Plant Microbe Interact. 10:840-851. [DOI] [PubMed] [Google Scholar]
- 23.Hope, I. A. (ed.) 1999. C. elegans, a practical approach. Oxford University Press, New York, NY.
- 24.Huang, C. H., T. N. Jang, C. Y. Liu, C. P. Fung, K. W. Yu, and W. W. Wong. 2001. Characteristics of patients with Burkholderia cepacia bacteremia. J. Microbiol. Immunol. Infect. 34:215-219. [PubMed] [Google Scholar]
- 25.Huber, B., F. Feldmann, M. Kothe, P. Vandamme, J. Wopperer, K. Riedel, and L. Eberl. 2004. Identification of a novel virulence factor in Burkholderia cenocepacia H111 required for efficient slow killing of Caenorhabditis elegans. Infect. Immun. 72:7220-7230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Isles, A., I. Maclusky, M. Corey, R. Gold, C. Prober, P. Fleming, and H. Levison. 1984. Pseudomonas cepacia infection in cystic fibrosis: an emerging problem. J. Pediatr. 104:206-210. [DOI] [PubMed] [Google Scholar]
- 27.Jacobs, J. L., A. C. Fasi, A. Ramette, J. J. Smith, R. Hammerschmidt, and G. W. Sundin. 2008. Identification and onion pathogenicity of Burkholderia cepacia complex isolates from the onion rhizosphere and onion field soil. Appl. Environ. Microbiol. 74:3121-3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Josenhans, C., and S. Suerbaum. 2002. The role of motility as a virulence factor in bacteria. Int. J. Med. Microbiol. 291:605-614. [DOI] [PubMed] [Google Scholar]
- 29.Kothe, M., M. Antl, B. Huber, K. Stoecker, D. Ebrecht, I. Steinmetz, and L. Eberl. 2003. Killing of Caenorhabditis elegans by Burkholderia cepacia is controlled by the cep quorum-sensing system. Cell. Microbiol. 5:343-351. [DOI] [PubMed] [Google Scholar]
- 30.Lenski, R. E. 1991. Quantifying fitness and gene stability in microorganisms, p. 173-192. In L. R. Ginzburg (ed.), Assessing ecological risks of biotechnology. Butterworth-Heinemann, Boston, MA. [DOI] [PubMed]
- 31.Lenski, R. E., M. R. Rose, S. C. Simpson, and S. C. Tadler. 1991. Long-term experimental evolution in Escherichia coli. I. Adaptation and divergence during 2,000 generations. Am. Nat. 138:1315-1341. [Google Scholar]
- 32.Lewenza, S., B. Conway, E. P. Greenberg, and P. A. Sokol. 1999. Quorum sensing in Burkholderia cepacia: identification of the LuxRI homologs CepRI. J. Bacteriol. 181:748-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.LiPuma, J. J., T. Spiker, T. Coenye, and C. F. Gonzalez. 2002. An epidemic Burkholderia cepacia complex strain identified in soil. Lancet 359:2002-2003. [DOI] [PubMed] [Google Scholar]
- 34.Lund, A. H., M. Duch, and F. S. X. Pedersen. 1996. Increased cloning efficiency by temperature-cycle ligation. Nucleic Acids Res. 24:800-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mahenthiralingam, E., T. A. Urban, and J. B. Goldberg. 2005. The multifarious, multireplicon Burkholderia cepacia complex. Nat. Rev. Microbiol. 3:144-158. [DOI] [PubMed] [Google Scholar]
- 36.Marolda, C. L., B. Hauroder, M. A. John, R. Michel, and M. A. Valvano. 1999. Intracellular survival and saprophytic growth of isolates from the Burkholderia cepacia complex in free-living amoebae. Microbiology 145:1509-1517. [DOI] [PubMed] [Google Scholar]
- 37.O'Toole, G. A., L. A. Pratt, P. I. Watnick, D. K. Newman, V. B. Weaver, and R. Kolter. 1999. Genetic approaches to study of biofilms. Methods Enzymol. 310:91-109. [DOI] [PubMed] [Google Scholar]
- 38.O'Quinn, A. L., E. M. Wiegand, and J. A. Jeddeloh. 2001. Burkholderia pseudomallei kills the nematode Caenorhabditis elegans using an endotoxin-mediated paralysis. Cell. Microbiol. 3:381-393. [DOI] [PubMed] [Google Scholar]
- 39.Parke, J. L., and D. Gurian-Sherman. 2001. Diversity of the Burkholderia cepacia complex and implications for risk assessment of biological control strains. Annu. Rev. Phytopathol. 39:225-258. [DOI] [PubMed] [Google Scholar]
- 40.Rahme, L. G., E. J. Stevens, S. F. Wolfort, J. Shao, R. G. Tompkins, and F. M. Ausubel. 1995. Common virulence factors for bacterial pathogenicity in plants and animals. Science 268:1899-1902. [DOI] [PubMed] [Google Scholar]
- 41.Riley, M. S., V. S. Cooper, R. E. Lenski, L. J. Forney, and T. L. Marsh. 2001. Rapid phenotypic change and diversification of a soil bacterium during 1,000 generations of experimental evolution. Microbiology 147:995-1006. [DOI] [PubMed] [Google Scholar]
- 42.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 43.Sokol, P. A., P. Darling, D. E. Woods, E. Mahenthiralingam, and C. Kooi. 1999. Role of ornibactin biosynthesis in the virulence of Burkholderia cepacia: characterization of pvdA, the gene encoding l-ornithine N5-oxygenase. Infect. Immun. 67:4443-4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sokol, P. A., U. Sajjan, M. B. Visser, S. Gingues, J. Forstner, and C. Kooi. 2003. The CepIR quorum-sensing system contributes to the virulence of Burkholderia cenocepacia respiratory infections. Microbiology 149:3649-3658. [DOI] [PubMed] [Google Scholar]
- 45.Sulston, J., and J. Hodgkin. 1988. Methods, p. 587-606. In W. B. Wood (ed.), The nematode Caenorhabditis elegans. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 46.Tomich, M., A. Griffith, C. A. Herfst, J. L. Burns, and C. D. Mohr. 2003. Attenuated virulence of a Burkholderia cepacia type III secretion mutant in a murine model of infection. Infect. Immun. 71:1405-1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ulrich, J. M. 1975. Pectic enzymes of Pseudomonas cepacea and penetration of polygalacturonase into cells. Physiol. Plant Pathol. 5:37-44. [Google Scholar]
- 48.U.S. Department of Agriculture 2009. USDA National Nutrient Database for Standard Reference, release 22. Agricultural Research Service, USDA, Beltsville, MD. http://www.ars.usda.gov/ba/bhnrc/ndl.
- 49.Van Alst, N. E., K. F. Picardo, B. H. Iglewski, and C. G. Haidaris. 2007. Nitrate sensing and metabolism modulate motility, biofilm formation, and virulence in Pseudomonas aeruginosa. Infect. Immun. 75:3780-3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vasi, F., M. Travisano, and R. E. Lenski. 1994. Long-term experimental evolution in Escherichia coli. II. Changes in life-history traits during adaptation to a seasonal environment. Am. Nat. 144:432-456. [Google Scholar]
- 51.Vasi, F. K., and R. E. Lenski. 1999. Ecological strategies and fitness tradeoffs in Escherichia coli mutants adapted to prolonged starvation. J. Genet. 78:43-49. [Google Scholar]
- 52.Velicer, G. J., L. Kroos, and R. E. Lenski. 1998. Loss of social behaviors by Myxococcus xanthus during evolution in an unstructured habitat. Proc. Natl. Acad. Sci. U. S. A. 95:12376-12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wopperer, J., S. T. Cardona, B. Huber, C. A. Jacobi, M. A. Valvano, and L. Eberl. 2006. A quorum-quenching approach to investigate the conservation of quorum-sensing-regulated functions within the Burkholderia cepacia complex. Appl. Environ. Microbiol. 72:1579-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.