Abstract
This study evaluated the effects of tannins on ruminal biohydrogenation (BH) due to shifts in the ruminal microbial environment in sheep. Thirteen lambs (45 days of age) were assigned to two dietary treatments: seven lambs were fed a barley-based concentrate (control group) while the other six lambs received the same concentrate with supplemental quebracho tannins (9.57% of dry matter). At 122 days of age, the lambs were slaughtered, and the ruminal contents were subjected to fatty acid analysis and sampled to quantify populations of Butyrivibrio fibrisolvens, which converts C18:2 c9-c12 (linoleic acid [LA]) to C18:2 c9-t11 (rumenic acid [RA]) and then RA to C18:1 t11 (vaccenic acid [VA]); we also sampled for Butyrivibrio proteoclasticus, which converts VA to C18:0 (stearic acid [SA]). Tannins increased (P < 0.005) VA in the rumen compared to the tannin-free diet. The concentration of SA was not affected by tannins. The SA/VA ratio was lower (P < 0.005) for the tannin-fed lambs than for the controls, suggesting that the last step of the BH process was inhibited by tannins. The B. proteoclasticus population was lower (−30.6%; P < 0.1), and B. fibrisolvens and protozoan populations were higher (+107% and +56.1%, respectively; P < 0.05) in the rumen of lambs fed the tannin-supplemented diet than in controls. These results suggest that quebracho tannins altered BH by changing ruminal microbial populations.
The fatty acid profile of the meat and milk of ruminants is strongly affected by diet (2, 15). When ingested, the dietary polyunsaturated fatty acids (PUFA) undergo a process known as biohydrogenation (BH) carried out by ruminal microorganisms (20). During the BH of C18:2(n-6) (linoleic acid [LA]) and C18:3(n-3) (linolenic acid [LNA]) a number of C18:1 and C18:2 isomers are formed (6). The last step in the BH process leads to the formation of C18:0 (stearic acid [SA]). Among the intermediate products formed during this process, the isomer C18:2 c9t11 (rumenic acid [RA]) is active in preventing cancer in mammals (17). Only a small amount of the RA found in meat and milk originates during BH. It is produced to a larger extent in muscle and mammary glands from the desaturation of C18:1 t11 (vaccenic acid [VA], another intermediate of ruminal BH) by the action of Δ9-desaturase enzyme (41, 43).
Ruminal BH is carried out mostly by bacteria belonging to the Butyrivibrio genus (38). Butyrivibrio fibrisolvens has the capacity to convert LA to RA and RA to VA, while Butyrivibrio proteoclasticus (previously classified as Clostridium proteoclasticum [35]) hydrogenates VA to SA (38, 39). According to Or-Rashid et al. (37), ruminal protozoa also play a role in BH by converting LA to RA. However, this issue is still controversial, as Devillard et al. (11) have reported that protozoa do not have the capability of hydrogenating LA. The proportion of BH intermediates in the rumen can vary depending on changes in ruminal microbial populations (7, 51). Changes in ruminal fatty acid profiles are also reflected in intramuscular fatty acid composition (48, 52).
Tannins are phenolic compounds that are widespread in plants. When ingested by ruminants in large amounts, tannins can reduce the activity and the proliferation of ruminal microorganisms (34). Tannins from Lotus corniculatus (33) or from Acacia spp. (12) reduce the proliferation of B. proteoclasticus B316T and B. proteoclasticus P18, respectively. Durmic et al. (12) reported that VA increased and SA decreased when extracts from Acacia iteaphylla, which contains condensed tannins (1), were incubated in vitro with sheep ruminal fluid inoculated with B. fibrisolvens JW11 and B. proteoclasticus P18 strains. In two recent in vitro studies, the inclusion of tannins in fermentor systems containing bovine ruminal fluid inhibited the conversion of VA to SA, while no effect was detected on RA production (21, 47). These results have been also confirmed in vivo in the rumen of sheep fed a diet with 4.0% dry matter (DM) quebracho tannin (48). However, to date there is no in vivo study focusing on the effects of dietary tannins on the proliferation of the microorganisms involved in ruminal BH.
We assessed whether dietary tannins may affect the BH pathway via changes in bacterial and protozoal ruminal populations. We gave particular emphasis to B. fibrisolvens and B. proteoclasticus. We also assayed the production of conjugated linoleic acids (CLAs) by linoleic acid isomerase (LA-I) enzyme.
MATERIALS AND METHODS
Animals and diets.
Thirteen male Comisana lambs, weaned at 45 days of age (mean weight ± standard deviation [SD], 14.4 ± 2.32 kg), were assigned to two dietary treatments and kept in singular pens. Seven lambs (control) received a concentrate containing (as-fed basis) the following: barley (55.1%), alfalfa hay (30.0%), soybean meal (13.0%), and vitamin and mineral premix (1.9%). The remaining six lambs received the same concentrate with supplemental quebracho tannins (from Schinopsis lorentzii; Figli di Guido Lapi S.pA., Castelfranco di Sotto, Pisa, Italy). Quebracho was 92.5% DM, whereas the concentrate was 90.5% DM. For each 1,000 g of DM of concentrate plus tannins, 95.7 g was constituted of quebracho powder, and the remaining 904.3 g was concentrate. The quebracho-supplemented diet was formulated to contain 6.4% (DM basis) tannins. Quebracho powder was added to the concentrate before the mixture was pelleted at a temperature of 40°C. The animals were adapted to the experimental diets over a period of 7 days. The lambs were weighed on the same day, once per week, before the morning feeding. Feed refusals were removed and weighed daily at 0800 h before fresh feed was supplied for calculation of dry matter intake (DMI). Animals always had access to water. After 70 days of experimental treatments, the animals were slaughtered. All the lambs had access to the experimental feed until 1 h before they were slaughtered.
Sampling.
Within 15 min after slaughtering, the rumen digesta were collected into clean plastic buckets and thoroughly mixed. Ruminal fluid pH was measured by a pH meter (Orion 9106; Orion Research Incorporated, Boston, MA). Within 20 min from slaughter, an aliquot of ruminal content (50 ml) was stored at −80°C pending freeze-drying. Another aliquot of ruminal content was filtered through two layers of cheesecloth: a portion (100 ml) of filtered ruminal fluid was stored at −30°C until fatty acid analysis; another portion (50 ml) was immediately handled for LA-I assay.
Rumen fatty acid analysis.
Ruminal fluid fatty acids were methylated by direct trans-esterification (23). Two milliliters of acetyl chloride in methanol (5% vol/vol) was added to 5 g of ruminal fluid and incubated at 50°C overnight. The C9:0 and C23:0 compounds were added together before methylation as internal standards. The fatty acid methyl esters (FAME) were extracted with hexane, filtered over anhydrous sodium sulfate, and dried under nitrogen; the FAME were resuspended in 0.5 ml of hexane. The FAME were analyzed as described by Vasta et al. (47). The gas-liquid chromatograph (GLC) used was a GC 8000 Top (Thermo Fisher Scientific Inc., Milan, Italy) equipped with a 100-m wall-coated open tubular (WCOT) CP-Select capillary column (internal diameter, 0.25 mm; film thickness, 0.25 μm; Chrompack, Middelburg, Netherlands) and a flame ionization detector. Fatty acids from ruminal fluid were determined by means of the following GLC conditions: the oven temperature was maintained at 60°C for 5 min and was then increased (15°C min−1) to 170°C and maintained for 44 min. Then, the temperature was increased at a rate of 1°C min−1 up to 202°C, and this temperature was maintained for 1 min. Finally, the temperature was increased (3°C min−1) to 230°C and held for 9 min. Helium was used as the carrier gas at a constant inlet pressure (250 kPa). Inlet and detector temperatures were 270 and 300°C, respectively. For the first 2 min the instrument operated in splitless mode, and then the split ratio was 40:1. The detection limit of the analysis was 0.001% above that of the total fatty acid amount. Individual FAME were identified by comparison to a standard mixture of 52 Component FAME Mix (GLC 674; Nu-Chek Prep, Inc., Elysian, MN) and to 77 individual FAME standards (Larodan Fine Chemicals, Malmo, Sweden). The identification of C18:1 isomers was based on commercial standard mixtures (Supelco, Bellefonte PA) and published isomeric profiles (24). Using the chromatographic conditions described above, RA and C18:2 t7c9 coeluted. However, considering that RA is present in ruminants at much higher concentrations than the other conjugated C18:2, here and elsewhere we refer to the peak comprising both RA and C18:2 t7c9 as RA only.
Rumen microbial ecology.
Samples of rumen contents were freeze-dried before DNA extraction. Freeze-drying improves both the yield and quality of DNA extracted from gut contents (42). Freeze-dried samples were thoroughly mixed by physical disruption using a bead beater (Mini-Bead Beater; Biospec Products, Bartlesville, OK). DNA extraction and purification were performed from 50 mg of freeze-dried material using a QIAmp DNA Stool Mini Kit (Qiagen Ltd, West Sussex, United Kingdom) following the manufacturer's instructions except that we used a higher temperature (95°C) for lysis incubation. DNA samples were used as templates for terminal restriction fragment polymorphism (T-RFLP) analysis and quantitative real-time PCR (qPCR) amplification.
T-RFLP.
The diversity of total bacterial community in the rumen of the lambs was studied by T-RFLP.
PCR analysis of sample.
PCR was performed using a 16S rRNA bacteria-specific primer pair, cyanine-labeled 27F (5′-AGA GTT TGA TCC TGG CTG AG-3′) and unlabeled 1389R (5′-AGG GGG GGT GTG TAG AAG-3′) (16). PCR amplification was performed using a Bio-Rad MyCycler thermal cycler with the following program: an initial 4 min of denaturation at 94°C, followed by 25 cycles of 1 min of denaturation at 94°C, 1 min of annealing at 55°C, and 1 min of extension at 72°C. A final cycle of 1 min at 94°C, 1 min at 55°C, and elongation for 5 min at 72°C completed the PCR. The following reaction cocktail was used throughout: 0.05 U μl−1 of Taq DNA polymerase (Promega), 1× reaction buffer as supplied by manufacturer, 1.5 mM MgCl2, a 0.25 μM concentration of each primer, and a 0.2 mM concentration of each of the deoxynucleoside triphosphates (dNTPs) with 2 μl of template. All reactions were carried out in a final volume of 50 μl.
Restriction enzyme digestion.
The PCR product was purified using a Quick Clean PCR Kit (Dominion-MBL, Spain). The DNA concentration within each sample after purification was determined by spectrophotometry (Nanodrop ND-1000 spectrophotometer) and then diluted to 20 ng μl−1. Restriction enzyme digestion was performed using HhaI at 0.25 U μl−1 for 5 h. For digestion product analysis, 20 μl of purified restriction digest product was added to 1 μl of DNA size standard (GeneScan 600 LIZ) diluted 1:3 with sodium lauryl sulfate (SLS) and 20 μl of SLS (Applied Biosystems, AB). Terminal restriction fragments (TRFs) were determined using GeneMapper software, version 4.0 (Applied Biosystems), and aligned using the AFLP (amplified fragment length polymorphism) application in the software.
Analysis of TRF patterns and qPCR data.
Analysis of TRFs in samples was performed by using the Bray-Curtis distance between profiles to construct dendrograms. All analyses were carried out by CAP, version 4, software (Pisces Conservation Ltd., Lymintong, Hampshire, United Kingdom).
qPCR.
Quantitation of total bacteria and total protozoa and the relative abundance of B. fibrisolvens and B. proteoclasticus were measured by real-time qPCR. The 16S rRNA gene-targeted primer sets used in this study were the following: 5′-GTGSTGCAYGGYTGTCGTCA-3′ (forward) and 5′-ACGTCRTCCMCACCTTCCTC-3′ (reverse) for total eubacteria (26), 5′-GCTTTCGWTGGTAGTGTATT-3′ (forward) and 5′-CTTGCCCTCYAATCGTWCT-3′ (reverse) for total protozoa (45), 5′-ACCGCATAAGCGCACGGA-3′ (forward) and 5′-CGGGTCCATCTTGTACCGATAAAT-3′ (reverse) for B. fibrisolvens (44), and 5′-TCCGGTGGTATGAGATGGGC-3′ (forward) and 5′-GTCGCTGCATCAGAGTTTCCT-3′ (reverse) plus the molecular beacon 5′-FAM-CCGCTTGGCCGTCCGACCTCTCAGTCCGAGCGG-DABCYL-3′ [where FAM is 6-carboxyfluorescein and DABCYL is 4-(4-dimethylaminophenyl) diazenylbenzoic acid] for B. proteoclasticus (39).
Three replicates of each DNA extract were used. A no-template (sterile distilled water) negative control was loaded on each plate run to screen for possible contamination and dimer formation and to set the background fluorescence for plate normalization. Real-time PCR was performed using a Multicolor Real-Time PCR Detection system (Model iQ5; Bio-Rad Laboratories, Inc.). One microliter of DNA extract was added to amplification reaction mixtures (50 μl) containing 50 pmol of each primer and 25 μl of iQ SYBR green Supermix (Bio-Rad Laboratories). Cycling conditions were 94°C for 4 min; 45 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 1 min; and a final extension of 72°C for 6 min. Fluorescence readings were taken after each extension step, and a final melting analysis was obtained by slow heating with increments of 0.1°C s−1 from 65°C to 95°C, with fluorescence collection at intervals of 0.1°C. The threshold cycle (i.e., the amplification cycle in which product formation exceeds background fluorescence) of each sample was determined during the exponential phase of amplification. All postrun data analyses were performed using iQ5 Optical System software, version 2.0 (Bio-Rad Laboratories). Total numbers of copies of bacterial and protozoal rRNA genes were measured using a standard curve generated from plasmids including their respective rRNA gene fragments obtained as described by Ohene-Adjei et al. (36). The relative abundances of B. fibrisolvens and B. proteoclasticus were determined using total bacterial amplification as the reference gene (10). Dilutions of samples were used to check the PCR amplification efficiency for the relative quantification of specific DNAs in total rumen DNA preparations (40).
LA-I assay.
To test the effects of tannins on the production of CLAs by LA-I, the ruminal fluid collected at slaughter was handled as described in Vasta et al. (47), and the LA-I assay was performed by the method of Kepler and Tove (20) as modified by Vasta et al. (47). Briefly, 50 ml of strained ruminal content was centrifuged at 20,000 × g for 20 min at 4°C. The supernatant was discarded, and the pellet was washed with 0.05 M potassium phosphate buffer (pH 6.8) and centrifuged at 20,000 × g for 20 min at 4°C. Then, the supernatant was discarded, and the microbial pellet so obtained was suspended in 4 ml of 0.05 M potassium phosphate buffer (pH 6.8). To obtain a whole-cell extract (WCE), the microbial pellet was sonicated for three cycles of 60 s each, with 90-s intervals between each cycle. The power of the sonicator (Bandelin Sonoplus HD2070) was set at 240 W, and the vessel containing the suspended pellet was placed in an ice bath to keep the temperature of the medium below 4°C. An aliquot (2 ml) of the WCE was centrifuged at 20,000 × g for 15 min at 4°C, and the supernatant was used for microbial protein determination according to Lowry et al. (25). This procedure was used to eliminate the proteins and peptides derived from feed (32) and the free and bacteria membrane cell-bound tannins from the final supernatant. This procedure was previously adopted to measure microbial protein in the ruminal fluid in the presence of tannins (31, 47).
The remaining aliquot of WCE was stored at −80°C until the LA-I assay was performed as described by Vasta et al. (47) with minor modifications. Briefly, 1,580 μl of 0.1 M potassium phosphate buffer (pH 7.0) was added to 20 μl of the WCE (containing an amount of microbial protein ranging from 73.2 to 379.2 μg), followed by 300 μl of 1,3-propanediol. The reaction was started by the addition of 100 μl of 0.72 mM LA in 1,3-propanediol and stopped after 4 min of incubation at room temperature by the addition of 2.5 ml of a stopper solution of isopropanol, isooctane, and 2 N H2SO4 (40:10:1, vol/vol/vol). Then, 1.0 ml of isooctane and 1.0 ml of water were added, the reaction mixture was vortexed, and the isooctane layer containing the fatty acids was collected. For each lamb, the LA-I assay was run in duplicate. The absorbance of the isooctane layer was recorded at 233-nm wavelength by spectrophotometer against a blank (containing 20 μl of the WCE, 1,580 μl of 0.1 M potassium phosphate buffer, pH 7.0, and 300 μl of 1,3-propanediol) in which LA was added after the stopper solution. The absorbance recorded at a 233-nm wavelength (which is the λ max of the diene bonds [18]) refers to the concentration of all the CLA isomers (CLAs' unspecific absorbance). However, considering that in the rumen up to 90% of the conjugated linoleic acids produced from linoleic acid are represented by RA (8), we assume that the LA-I assay can be applied primarily to the production of RA (47).
Feedstuff analyses.
Lipids from feed were extracted according to Folch et al. (13) and then trans-esterified by cool-base-catalyzed trans-esterification using a 0.5 N methanolic solution of sodium methoxide according to Christie's procedure (9). FAME were analyzed by the same GC apparatus described above and determined by means of the following GLC conditions: the oven temperature was programmed at 150°C for 1 min and was then ramped up at 0.8°C min−1 to 175°C and maintained for 14 min. Then the temperature increased at a rate of 2°C min−1 up to 188°C, and this temperature was held for 18 min. Finally, the temperature was increased (2°C min−1) to 220°C and held for 12 min. Helium was used as a carrier gas at constant inlet pressure (250 kPa). Inlet and detector temperatures were 270 and 300°C, respectively.
For the tannin analysis, samples (200 mg) of feed offered (i.e., concentrate and concentrate plus tannins) were extracted in triplicate in aqueous acetone (70%, vol/vol) overnight at 4°C. After centrifugation (at 3,000 × g at 4°C for 15 min), total phenols (TP) were analyzed using the Folin-Ciocalteu reagent with tannic acid as a standard (30). Total phenols and total tannins were expressed as tannic acid equivalents. The chemical composition of the diets is shown in Table 1.
TABLE 1.
Chemical composition of diets
| Chemical composition |
Fatty acid composition |
||||
|---|---|---|---|---|---|
| Component | Value for indicated dietary treatmenta |
Component | Value for indicated dietary treatmentb |
||
| Concentrate | Concentrate plus tannins | Concentrate | Concentrate plus tannins | ||
| Total DMc | 905.00 | 893.60 | C12:0 | 0.54 | 0.25 |
| Crude protein | 168.00 | 171.20 | C14:0 | 1.63 | 1.61 |
| Soluble protein | 24.35 | 22.90 | C16:0 | 22.83 | 22.65 |
| Ether extract | 15.50 | 14.10 | C16:1 | 0.50 | 0.41 |
| Neutral detergent fiber | 287.30 | 254.30 | C18:0 | 4.72 | 4.78 |
| Acid detergent fiber | 160.70 | 139.45 | C18:1c9 | 15.46 | 15.34 |
| Acid detergent lignin | 17.65 | 10.95 | C18:1c11 | 0.94 | 0.95 |
| Ash | 70.60 | 80.60 | C18:2c9c12 | 43.92 | 44.79 |
| Total phenolsd | 0.57 | 7.86 | C18:3c9c12 c15 | 8.29 | 8.21 |
| Total tanninsd | 0.18 | 6.45 | |||
In g/kg DM unless otherwise noted.
In % of total extracted fatty acids.
In g/kg of feed.
In g of tannic acid equivalent/kg DM.
Statistical analyses.
Data were analyzed by one-way analysis of variance (ANOVA) with a model that included treatment effect and experimental error. Individual animals were considered experimental units. Differences between means were considered significant at a P value of <0.05. When the P value was ≤0.1 but >0.05, the differences between means were considered tendencies. Statistical analysis was performed using the Minitab 14 (Minitab Inc.) software.
RESULTS
Animal growth performances.
Dry matter intake (DMI) for lambs that received the tannin supplement was lower (P = 0.039) than that of the lambs that did not receive the supplement (750 versus 916 g day−1, respectively; standard error of the mean [SEM], ±41.4 g day−1) (data not shown). Lambs fed the tannin-containing concentrate had a lower average daily gain (ADG) than the animals that did not receive tannins (118 versus 200 g day−1, respectively; SEM, ±14 g day−1; P < 0.0005) (data not shown). The weight at slaughter was lower for the tannin-fed lambs than for the animals not supplemented with tannins (23.3 versus 29.1 kg, respectively; SEM, ±1.17 kg; P < 0.01) (data not shown).
Rumen pH and fatty acid composition.
Rumen pH did not differ (P > 0.05) between the lambs in the two treatments (average ± SEM, 6.9 ± 0.170) (data not shown). Table 2 reports ruminal fluid fatty acid profiles. Supplemental tannins increased C12:0 and C13:0 (P < 0.01) and C14:0 and C16:0 (P < 0.05) in the ruminal fluid compared to lambs not receiving tannin supplement. Among the iso branched-chain fatty acids (BCFA), iso C12:0 and iso C15:0 were reduced (P < 0.05) while iso C13:0 was increased (P < 0.05) by tannin supplementation. The total concentration of the iso BCFA tended (P = 0.063) to be lower (−31.7%) in the ruminal fluid of the lambs that received the concentrate plus tannin diet than in lambs that did not receive tannins. The total concentration of anteiso BCFA was not affected (P > 0.05) by tannin supplementation. None of the detected C18:1 cis isomers were affected by tannin supplementation (P > 0.05). In contrast, among the C18:1 trans isomers, C18:1 t6 to t8, C18:1 t9, C18:1 t10, and C18:1 t12 were at lower (P < 0.1) concentrations in the ruminal fluid of the lambs that received tannins than in lambs not supplemented. The inclusion of tannins in the concentrate decreased the isomer C18:1 t4 (P < 0.01). The concentration of VA increased by 2-fold (P = 0.001), and the SA/VA ratio was strongly reduced (P = 0.002) by tannin supplementation. The concentration of C18:1 t16 increased (P < 0.001) with tannin supplementation. The concentrations of C18:2 t8t10, C18:2 c9c11, C18:2 c10c12, C18:2 t10c12, and C18:2 c11t13 were not affected by the treatment (P > 0.05). Among lambs in the control group, RA was detected in the ruminal fluid of only one animal while it was above the detection limit in the ruminal fluid of the other five of the six lambs that received the diet of concentrate plus tannins (Fig. 1). Tannin supplementation reduced (P < 0.05) the accumulation of C18:2 t11c15 and tended (P = 0.082) to reduce C18:3 c6c9c12. The concentration of total saturated fatty acids (SFA), total monounsaturated fatty acids (MUFA), and total PUFA did not differ between the two groups of lambs.
TABLE 2.
Effect of tannin supplementation on ruminal fatty acid composition
| Fatty acid (name, group, or ratio) | Value for indicated dietary treatmenta |
SEM | Significance | |
|---|---|---|---|---|
| Concentrate (n = 7) | Concentrate plus tannins (n = 6) | |||
| C12:0 | 0.24 | 0.93 | 0.150 | 0.014 |
| C13:0 | 0.16 | 0.33 | 0.035 | 0.005 |
| C14:0 | 1.82 | 2.55 | 0.181 | 0.036 |
| C15:0 | 2.25 | 2.38 | 0.187 | 0.746 |
| C16:0 | 16.43 | 22.86 | 1.426 | 0.016 |
| C17:0 | 1.25 | 1.17 | 0.114 | 0.735 |
| C18:0 | 50.45 | 43.92 | 2.074 | 0.120 |
| iso C12:0 | 0.09 | 0.05 | 0.011 | 0.082 |
| iso C13:0 | 0.30 | 0.52 | 0.056 | 0.040 |
| iso C14:0 | 0.67 | 0.44 | 0.071 | 0.115 |
| iso C15:0 | 2.58 | 1.32 | 0.308 | 0.035 |
| iso C16:0 | 1.06 | 0.75 | 0.192 | 0.448 |
| iso C17:0 | 0.75 | 0.67 | 0.115 | 0.745 |
| iso C18:0 | 0.26 | 0.14 | 0.055 | 0.282 |
| ∑iso BCFAb | 5.71 | 3.90 | 0.493 | 0.063 |
| anteiso C13:0 | 0.07 | 0.06 | 0.020 | 0.827 |
| anteiso C15:0 | 1.97 | 1.67 | 0.142 | 0.319 |
| anteiso C17:0 | 0.75 | 0.78 | 0.091 | 0.874 |
| ∑anteiso BCFAc | 3.62 | 2.95 | 0.181 | 0.431 |
| ∑OBCFAd | 9.75 | 8.06 | 0.636 | 0.197 |
| C16:1c9 | 0.13 | 0.12 | 0.045 | 0.843 |
| C17:1c9 | 0.23 | 0.12 | 0.028 | 0.036 |
| C18:1c9 | 2.24 | 3.02 | 0.458 | 0.417 |
| C18:1c11 | 0.49 | 0.49 | 0.111 | 0.990 |
| C18:1c12 | 0.26 | 0.25 | 0.036 | 0.886 |
| C18:1c15 | 0.88 | 0.57 | 0.122 | 0.214 |
| C18:1t4 | 0.07 | 0.02 | 0.009 | 0.009 |
| C18:1t6 to t8 | 0.67 | 0.28 | 0.115 | 0.091 |
| C18:1t9 | 0.40 | 0.16 | 0.066 | 0.068 |
| C18:1t10 | 3.52 | 1.21 | 0.679 | 0.089 |
| C18:1t11 | 1.47 | 3.06 | 0.287 | 0.001 |
| C18:1t12 | 0.50 | 0.31 | 0.053 | 0.077 |
| C18:1t13 | 0.79 | 0.54 | 0.087 | 0.153 |
| C18:1t15 | 0.67 | 0.61 | 0.085 | 0.773 |
| C18:1t16 | 0.11 | 0.51 | 0.069 | 0.000 |
| ∑TFAe | 8.19 | 6.71 | 0.853 | 0.412 |
| C18:2c10c12 | 0.012 | 0.006 | 0.007 | 0.671 |
| C18:2t10c12 | 0.06 | 0.01 | 0.020 | 0.288 |
| C18:2c11t13 | 0.007 | 0.008 | 0.003 | 0.950 |
| C18:2t11t13 | 0.03 | 0.03 | 0.006 | 0.918 |
| C18:2t8t10 | 0.09 | 0.17 | 0.040 | 0.353 |
| C18:2c9c11 | 0.01 | 0.03 | 0.010 | 0.213 |
| ∑CLAf | 0.23 | 0.66 | 0.188 | 0.265 |
| C18:2c9c12 | 1.17 | 1.83 | 0.296 | 0.285 |
| C18:2t11c15 | 0.40 | 0.17 | 0.057 | 0.044 |
| C18:2t9t12 | 0.05 | 0.02 | 0.015 | 0.424 |
| C18:3c6c9c12 | 0.26 | 0.07 | 0.056 | 0.082 |
| C18:3c9c12c15 | 0.21 | 0.28 | 0.046 | 0.491 |
| C18:0 /C18:1t11 | 36.39 | 15.74 | 3.850 | 0.002 |
| C15:0 /∑OBCFA | 0.22 | 0.30 | 0.017 | 0.024 |
| iso C15:0 /∑OBCFA | 0.26 | 0.15 | 0.028 | 0.044 |
| anteiso C15:0 /∑OBCFA | 0.20 | 0.21 | 0.012 | 0.777 |
| C17:0 /∑OBCFA | 0.13 | 0.15 | 0.007 | 0.322 |
| iso C17:0 /∑OBCFA | 0.077 | 0.073 | 0.010 | 0.949 |
| anteiso C17:0 /∑OBCFA | 0.077 | 0.10 | 0.028 | 0.307 |
| ∑SFAg | 83.10 | 82.29 | 1.472 | 0.797 |
| ∑MUFAh | 12.72 | 11.84 | 1.058 | 0.699 |
| ∑PUFAi | 2.30 | 3.01 | 0.367 | 0.359 |
In % of total fatty acids. n, number of sheep.
∑iso BCFA = iso C12:0 + iso C13:0 + iso C14:0 + iso C15:0 + iso C16:0 + iso C17:0 + iso C18.:0.
∑anteiso BCFA = anteiso C13:0 + anteiso C15:0 + anteiso C17:0.
∑OBCFA = C15:0 + iso C15:0 + anteiso C15:0 + C17:0 + iso C17:0 + anteiso C17:0.
∑TFA = C18:1 t4 + C18:1 t6 to t8 + C18:1 t9 + C18:1 t10 + C18:1 t11 + C18:1 t12 + C18:1 t13 + C18:1 t15 + C18:1 t16.
∑CLA = C18:2 c10c12 + C18:2 t10c12 + C18:2 c11t13 + C18:2 t11t13 + C18:2 t8t10 + C18:2 c9t11 + C18:2 c9c11. The average values for C18:2 c9t11 are not reported in the table but are reported in Fig. 1.
∑SFA = C10:0 + C11:0 + C12:0 + iso C12:0 + C13:0 + iso C13:0 + anteiso C13:0 + C14:0 + iso C14:0 + C15:0 + iso C15:0 + anteiso C15:0 + C16:0 + iso C16:0 + C17:0 + iso C17:0 + anteiso C17:0 + C18:0 + iso C18:0 + C20:0 + C21:0 + C22:0 + C24:0.
∑MUFA = C16:1 c9 + C17:1 c9 + C18:1 c9 + C18:1 c11 + C18:1 c12 + C18:1 c15 + C18:1 t4 + C18:1 t6 to t8 + C18:1 t9 + C18:1 t10 + C18:1 t11 + C18:1 t12 + C18:1 t13 + C18:1 t15 + C18:1 t16 + C18:1 c9, 12-OH.
∑PUFA = C18:2 c10c12 + C18:2 t10c12 + C18:2 c11t13 + C18:2 t11t13 + C18:2 t8t10 + C18:2 c9t11 + C18:2 c9c11 + C18:2 t11c15 + C18:2 t9t12 + C18:3 c6c9c12 + C18:3 c9c12c15.
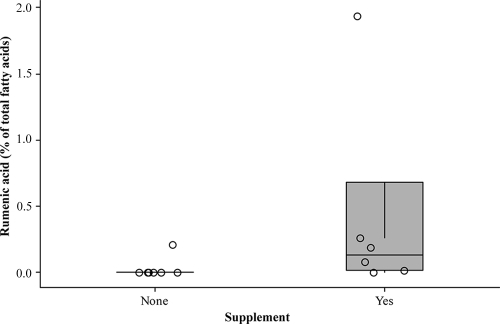
FIG. 1.
Box plot of the distribution of RA in the ruminal fluid of the lambs fed concentrate with or without tannin supplementation. Circles represent individual animals.
Rumen microbial ecosystem and CLA production by LA-I.
The ruminal content of the lambs from the two dietary treatments differed in bacterial species distribution. The dendrogram obtained from the T-RFLP profiles showed that the bacteria from the lambs fed the concentrate with or without tannins tended to be clustered separately (data not shown). The number of total protozoa was higher (P = 0.001) in the rumen of the lambs supplemented with tannins than in the animals not supplemented (Table 3). The total number of bacteria did not differ between the two groups of animals. Among the total ruminal bacterial community, tannin supplementation strongly increased (P = 0.022) the relative abundance of B. fibrisolvens. Conversely, supplemental tannins decreased (P = 0.094) the relative abundance of B. proteoclasticus compared to lambs not fed the tannin supplement.
TABLE 3.
Effect of tannin supplementation on rumen content microbial population and on LA-I activity
| Ruminal ecosystem parameter | Value for indicated dietary treatment |
SEM | Significance | |
|---|---|---|---|---|
| Concentrate (n = 7)a | Concentrate plus tannins (n = 6)a | |||
| Microbial population | ||||
| No. of protozoa (log10 copies/g FMc) | 5.01 | 7.82 | 0.506 | 0.001 |
| No. of bacteria (log10 copies/g of FM) | 9.55 | 9.64 | 0.134 | 0.766 |
| B. fibrisolvens (% of total bacteria) | 4.22 | 8.76 | 1.044 | 0.022 |
| B. proteoclasticus (% total bacteria) | 3.99 | 2.77 | 0.363 | 0.094 |
| Amount of microbial protein (mg/ml) | 6.33 | 16.23 | 1.5 | <0.0005 |
| CLA production (nmol/ml/min)b | 2.47 × 105 | 2.13 × 105 | 3.27 × 104 | 0.693 |
n, number of sheep.
LA-I assay.
FM, fresh matter.
The CLA produced (nmol ml−1 min−1) by LA-I during the assay was not affected by tannin supplementation (P = 0.693) (Table 3). The supplementation of tannins resulted in a higher (P < 0.0005) concentration of microbial proteins in the WCE than in the WCE from the lambs not supplemented with tannins. In the WCE from the lambs fed tannins, the LA-I specific activity was lower (P = 0.010) than that in the WCE from the lambs not supplemented with tannins (6.63 ×105 versus 1.94 × 106 nmol CLA mg of microbial protein−1 min−1, respectively) (data not shown).
DISCUSSION
To date, little information has been published on the effect of tannins on ruminal BH. Two in vitro studies (21, 47) have shown that, in the presence of tannins, fermented ruminal fluid contains greater levels of VA and lower concentrations of SA than fluid fermented in the absence of tannin. Vasta et al. (48) recently found that dietary tannins impair the conversion of VA to SA in vivo. The authors hypothesized that tannins could have inhibited the bacteria that convert VA to SA to a larger extent than the bacteria which biohydrogenate LA to RA and to VA.
In the present study, tannin supplementation strongly impacted the whole microbial community. The increase in the total number of protozoa in the rumen of the tannin-fed lambs was not expected, as tannins generally depress protozoal populations (34). Quebracho tannins can have diverse effects on ruminal protozoa: results obtained in vitro with bovine ruminal fluid (28) and in vivo with heifers (3) showed that they reduce ruminal protozoa. In other instances, they did not affect the total counts of protozoa in vitro (21) or in vivo (4). There is an open debate about the role played by ruminal protozoa in BH: on one hand, protozoa incorporate both RA and VA of bacterial origin (11) and contribute up to the 40% of RA and VA flow from the rumen to the duodenum (51); on the other hand, controversial results are reported regarding an active role of protozoa in the BH pathway. A study conducted by Devillard et al. (11) failed to detect the ability of protozoa from sheep to convert LA to RA or VA. In contrast, Or-Rashid et al. (37) reported that mixed rumen protozoa can convert LA to RA but cannot undertake the successive steps of the BH pathway.
Populations of B. fibrisolvens were higher, and populations of B. proteoclasticus tended to be lower in the rumen of the tannin-fed lambs than in the lambs not supplemented. It is likely that while some bacterial strains were reduced by tannins, others could benefit, which would explain the increase in the population of B. fibrisolvens in the rumen of the tannin-fed lambs. Durmic et al. (12) found that B. proteoclasticus strain P18 was more sensitive to a tannin-rich plant (Acacia mearnsii) extract than B. fibrisolvens JW11. The effects of tannins on B. fibrisolvens have also been investigated by Jones et al. (19), who found in vitro that tannins from sainfoin leaves inhibit the growth and activity of B. fibrisolvens A38 by causing changes in the bacteria's morphology. However, the comparison of results obtained from different studies on the effects of tannins on ruminal microorganisms should be made with caution as they are all likely to be dependent on specific conditions. The in vivo or in vitro experimental conditions, the tannin source, the level of inclusion of tannins into the medium or into the diet, the animal species donor of the ruminal fluid, and the different complexities of rumen microbial environment vis-à-vis individual bacterial strains can bring about different interactions between tannins and the growth and activity of ruminal microorganisms.
In some cases, ruminal microbial protein, measured with labeled nitrogen (5) or with Lowry's method (31, 47), was reduced in the presence of tannins. In other instances, tannins did not affect ruminal microbial protein (33). Makkar et al. (27, 29) report that ruminal microbial protein synthesis is higher in the presence of tannins than in diets not containing tannins. The increase in microbial protein that we have observed in the present experiment in the presence of tannins is consistent with the increased protozoal population in the rumen of the tannin-fed animals, especially considering that protozoa constitute up to the 40% of net microbial nitrogen in the rumen (46).
In the present study SA concentration in the rumen was not affected by tannin supplementation while VA was higher in the rumen fluid of the tannin-fed lambs than in lambs fed only concentrate (Table 2). The SA/VA ratio, which can be considered a possible indicator of the rate of the last step of the BH process (48), was much lower for the tannin-fed lambs than for the lambs not supplemented (Table 2). This result could be because the conversion of VA to SA was inhibited by tannins. It is likely that this result could be due to the decrease in the relative abundance of B. proteoclasticus bacteria in the presence of tannins. Wallace et al. (50) showed that SA is formed by B. proteoclasticus only during the growing phase of this bacteria, and therefore, according to Kim et al. (22), the activity of B. proteoclasticus (and therefore SA production) may not be proportional to concentrations of 16S rRNA gene copies.
We did not detect RA in the ruminal fluid of six of the seven lambs fed the control diet while the ruminal content of all the tannin-fed lambs, with the exception of one animal, had levels of RA above the detection limit of the analysis (Fig. 1). To try to explain the possible mechanism responsible for this result, we have formulated two hypotheses: (i) in the rumen of the tannin-fed lambs, more RA was produced than in the rumen of the animals fed the tannin-free diet, or (ii) less RA was converted to VA in the rumen of the tannin-fed lambs than in the rumen of the lambs not fed the supplement. The first of the two hypotheses is unlikely, as suggested by the results of the LA-I assay; in fact, the production of CLAs in the ruminal fluids during the assay did not differ between the sheep fed tannins and those animals not fed tannins (Table 3). More likely, the greater accumulation of RA in the rumen of the tannin-fed lambs might have been due to a lower conversion of RA to VA. As suggested by the SA/VA ratio, the greater concentration of VA in the ruminal fluid of the tannin-fed lambs could be due to a lower conversion of SA to VA. Therefore, it is likely that the enzymatic conversion of RA to VA was inhibited through a feedback mechanism (14), resulting in an accumulation of RA. Conversely, in the rumen of the lambs not fed tannins biohydrogenation might have proceeded at a higher rate and to a greater extent than in the rumen of tannin-fed lambs, and therefore only traces of RA were found in the rumen of the lambs not fed tannins. RA is produced from LA mainly by B. fibrisolvens (20, 50), and protozoa also seem to convert LA to RA (37). Although in the rumen of the tannin-supplemented lambs both B. fibrisolvens and total protozoa were higher than in lambs not supplemented, CLA production during the assay did not differ between the two groups of animals. This result can be explained by the activity of LA-I enzyme, which was lower for the tannin-fed lambs than for the controls (6.63 × 105 versus 1.94 × 106 nmol of CLA mg of microbial protein−1 min−1, respectively) (data not shown). Therefore, despite the lower LA-I activity in the ruminal fluid of the lambs supplemented with tannins, the production of RA might have been equalized by the greater amount of protein (Table 3). The lower value of the ratio between iso C15:0 and the sum of the odd and branched-chain fatty acids (∑OBCFA) in the rumen of the tannin-supplemented lambs supports the hypothesis that the activity of strains of cellulolytic bacteria—which are enriched in iso C15:0 (49)—was depressed compared to activity in the rumen of the animals not receiving tannins. Vasta et al. (47) reported that tannins reduced BCFA synthesis in vitro. Dietary tannins reduced iso BCFA in sheep rumen in vivo (48).
Conclusion.
This study shows for the first time in vivo that dietary tannins affect ruminal biohydrogenation through changes in the ruminal microbial community. The inclusion of tannins in the diet increased the relative abundance of B. fibrisolvens and decreased the abundance of B. proteoclasticus. The conversion of VA to SA was reduced by tannin supplementation, probably because of a lower proportion of B. proteoclasticus bacteria, which are responsible for the last step of biohydrogenation. Rumenic acid production by LA-I enzyme was not affected by tannins while LA-I activity was depressed by tannin supplementation. To conclude that tannins could be a useful tool to increase the accumulation of VA in the rumen and favor the endogenous synthesis of RA, further research is needed using diets containing tannins at a level below 4.5% DM. This would not compromise animal growth performance (34).
Acknowledgments
This work was supported by the Italian Ministry of Research, PRIN 2007, protocol 200778K3KJ_003, entitled Effects of Tannins on Sheep and Goat Meat Quality (main investigator, A.P.), and by MIUR, Ricerca di Ateneo 2006 (main investigator, L.B.).
We are grateful to Patricia Lopez Andres for technical help and to Fred Provenza for revising the manuscript.
Footnotes
Published ahead of print on 19 February 2010.
REFERENCES
- 1.Al-Soqeer, A. A. 2008. Nutritive value assessment of Acacia species using their chemical analyses and in vitro gas production technique. Res. J. Agric. Biol. Sci. 4:688-694. [Google Scholar]
- 2.Aurousseau, B., D. Bauchart, E. Calichon, D. Micol, and A. Priolo. 2004. Effect of grass or concentrate feeding systems and rate of growth on triglyceride and phospholipid and their fatty acids in the M. longissimus thoracis of lambs. Meat Sci. 66:531-541. [DOI] [PubMed] [Google Scholar]
- 3.Baah, J., M. Ivan, A. N. Hristov, K. M. Koenig, L. M. Rode, and T. A. McAllister. 2007. Effects of potential dietary antiprotozoal supplements on rumen fermentation and digestibility in heifers. Anim. Feed Sci. Technol. 137:126-137. [Google Scholar]
- 4.Benchaar, C., T. A. McAllister, and P. Y. Chouinard. 2008. Digestion, ruminal fermentation, ciliate protozoal populations, and milk production from dairy cows fed cinnamaldehyde, quebracho condensed tannin, or Yucca schigadera saponin extracts. J. Dairy Sci. 91:4765-4777. [DOI] [PubMed] [Google Scholar]
- 5.Bento, M. H. L., H. P. S. Makkar, and T. Acamovic. 2005. Effect of mimosa tannin and pectin on microbial protein synthesis and gas production during in vitro fermentation of 15N-labelled maize shoots. Anim. Feed. Sci. Technol. 123-124:365-377. [Google Scholar]
- 6.Bessa, R. J. B., S. P. Alves, E. Jerònimo, C. M. Alfaia, J. A. M. Prates, and J. Santos-Silva. 2007. Effect of lipid supplements on ruminal biohydrogenation intermediates and muscle fatty acids in lamb. Eur. J. Lipid Sci. Technol. 109:868-883. [Google Scholar]
- 7.Boeckaert, C., B. Vlaeminck, V. Fievez, L. Maignien, J. Dijkstra, and N. Boon. 2008. Accumulation of trans C18:1 fatty acids in the rumen after dietary algal supplementation associated with changes in the Butyrivibrio community. Appl. Environ. Microbiol. 74:6923-6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chin, S. F., W. Liu, J. M. Storkson, Y. L. Ha, and M. W. Pariza. 1992. Dietary sources of conjugated dienoic isomers of linoleic acid, a newly recognized class of anticarcinogens. J. Food Comp. Anal. 5:185-197. [Google Scholar]
- 9.Christie, W. W. 1982. A simple procedure of rapid transmethylation of glycerolipids and cholesteryl esters. J. Lipid Res. 23:1072-1075. [PubMed] [Google Scholar]
- 10.Denman, S. E., and C. S. McSweeny. 2005. Quantitative (real-time) PCR, p. 105-118. In H. P. S. Makkar and C. S. McSweeney (ed.), Methods in gut microbial ecology for ruminants. Springer, Dordrecht, Netherlands.
- 11.Devillard, E., F. M. McIntosh, C. J. Newbold, and R. J. Wallace. 2006. Rumen ciliate protozoa contain high concentrations of conjugated linoleic acid and vaccenic acid, yet do not hydrogenate linoleic acid or desaturate stearic acid. Br. J. Nutr. 96:697-704. [PubMed] [Google Scholar]
- 12.Durmic, Z., C. S. McSweeney, G. W. Kemp, P. P. Hutton, R. J. Wallace, and P. E. Vercoe. 2008. Australian plants with potential to inhibit bacteria and processes involved in ruminal biohydrogenation of fatty acids. Anim. Feed Sci. Technol. 145:271-284. [Google Scholar]
- 13.Folch, J., M. Lees, and G. H. Sloane-Stanley. 1957. A simple method for isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226:497-509. [PubMed] [Google Scholar]
- 14.Gerhart, J. C., and A. B. Pardee. 1962. The enzymology of control by feedback inhibition. J. Biol. Chem. 237:891-896. [PubMed] [Google Scholar]
- 15.Griinari, J. M., D. A. Dwyer, M. A. McGuire, D. E. Bauman, D. L. Palmquist, and K. V. V. Nurmela. 1998. trans-Octadecenoic acids and milk fat depression in lactating dairy cows. J. Dairy Sci. 81:1251-1261. [DOI] [PubMed] [Google Scholar]
- 16.Hongoh, Y., P. Deevong, T. Inoue, S. Moriya, S. Trakulnaleamsai, M. Ohkuma, C. Vongkaluang, N. Noparatnaraporn, and T. Kudo. 2005. Intra- and interspecific comparisons of bacterial diversity and community structure support coevolution of gut microbiota and termite host. Appl. Environ. Microbiol. 71:6590-6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ip, C., S. F. Chin, J. A. Scimeca, and M. W. Pariza. 1991. Mammary cancer prevention by conjugated dienoic derivative of linoleic acid. Cancer Res. 51:6118-6124. [PubMed] [Google Scholar]
- 18.Jackson, J. E., R. F. Paschke, E. Tolberg, H. M. Boyd, and D. H. Wheeler. 1952. Isomers of linoleic acid. Infrared and ultraviolet properties of methyl esters. Am. Oil Chem. Soc. 29:229-234. [Google Scholar]
- 19.Jones, G. A., T. A. McAllister, A. D. Muir, and K.-J. Cheng. 1994. Growth and proteolysis by four strains of ruminal bacteria. Appl. Environ. Microbiol. 60:1374-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kepler, C. R., and S. B. Tove. 1967. Biohydrogenation of unsaturated fatty acids. III. Purification and properties of a linoleate Δ12-cis,Δ11-trans-isomerase from Butyrivibrio fibrisolvens. J. Biol. Chem. 242:5686-5692. [PubMed] [Google Scholar]
- 21.Khiaosa-Ard, R., S. F. Bryner, M. R. L. Scheeder, H.-R. Wettstein, F. Leiber, M. Kreuzer, and C. R. Soliva. 2009. Evidence for the inhibition of the terminal step of ruminal α-linolenic acid biohydrogenation by condensed tannins. J. Dairy Sci. 92:177-188. [DOI] [PubMed] [Google Scholar]
- 22.Kim, J. N., S. A. Huws, M. R. F. Lee, J. D. Wood, S. M. Muetzel, R. J. Wallace, and N. S. Scollan. 2008. Fish oil increases the duodenal flow of long chain polyunsaturated fatty acids and trans-11 18:1 and decreases 18:0 in steers via changes in the rumen bacterial community. J. Nutr. 138:889-896. [DOI] [PubMed] [Google Scholar]
- 23.Kramer, J. K., V. Fellner, M. E. Dugan, F. D. Sauer, M. M. Mossoba, and M. P. Yurawecz. 1997. Evaluating acid and base catalyst in the methylation of milk and rumen fatty acids with special emphasis on conjugated dienes and total trans fatty acids. Lipids 32:1219-1228. [DOI] [PubMed] [Google Scholar]
- 24.Kramer, K. G., M. Hernandez, C. Cruz-Hernabdez, J. Kraft, and M. E. R. Dugan. 2008. Combining results of two GC separations partly achieves determination of all cis and trans 16:1, 18:1, 18:2, and 18:3 except CLA isomers of milk fat as demonstrated using Ag-ion SPE fractionation. Lipids 43:259-273. [DOI] [PubMed] [Google Scholar]
- 25.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin-phenol reagents. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 26.Maeda, H., C. Fujimoto, Y. Haruki, T. Maeda, S. Kokeguchi, M. Petelin, H. Arai, I. Tanimoto, F. Nishimura, and S. Takashiba. 2003. Quantitative real-time PCR using TaqMan and SYBR green for Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis, Prevotella intermedia, tetQ gene and total bacteria. FEMS Immunol. Med. Microbiol. 39:81-86. [DOI] [PubMed] [Google Scholar]
- 27.Makkar, H. P. S., G. Francis, and K. Becker. 2007. Bioactivity of phytochemicals in some lesser-known plants and their effects and potential applications in livestock and aquaculture production systems. Animal 1:1371-1391. [DOI] [PubMed] [Google Scholar]
- 28.Makkar, H. P. S., and K. Becker. 1995. Degradation of condensed tannins by rumen microbes exposed to quebracho tannins (QT) in rumen simulation technique (RISITEC) and effects of QT on fermentative processes in the RUSITEC. J. Sci. Food Agric. 69:495-500. [Google Scholar]
- 29.Makkar, H. P. S., M. Blümmel, and K. Becker. 1995. In vitro effects and interactions of tannins and saponins and fate of tannins in rumen. J. Sci. Food Agric. 69:481-493. [Google Scholar]
- 30.Makkar, H. P. S., M. Blümmel, N. K. Borowy, and K. Becker. 1993. Gravimetric determination of tannins and their correlations with chemical and protein precipitation methods. J. Sci. Food Agric. 61:161-165. [Google Scholar]
- 31.Makkar, H. P. S., B. Singh, and R. K. Dawra. 1988. Effect of tannin-rich leaves of oak (Quercus incana) on various microbial enzyme activities of the bovine rumen. Br. J. Nutr. 60:287-296. [DOI] [PubMed] [Google Scholar]
- 32.Makkar, H. P. S., O. P. Sharma, R. K. Dawra, and S. S. Negi. 1982. Simple determination of microbial protein in rumen liquor. J. Dairy Sci. 65:2170-2173. [DOI] [PubMed] [Google Scholar]
- 33.Min, B. R., G. T. Attwood, K. Reilly, W. Sun, J. S. Peters, T. N. Barry, and W. C. McNabb. 2002. Lotus corniculatus condensed tannins decrease in vivo populations of proteolytic bacteria and affect nitrogen metabolism in the rumen of sheep. Can. J. Microbiol. 48:911-921. [DOI] [PubMed] [Google Scholar]
- 34.Min, B. R., T. N. Barry, G. T. Attwood, and W. C. McNabb. 2003. The effect of condensed tannins on the nutrition and health of ruminants fed fresh temperate forages: a review. Anim. Feed Sci. Technol. 106:3-19. [Google Scholar]
- 35.Moon, C. D., D. M. Pacheco, W. J. Kelly, S. C. Leahy, D. Li, J. Kopečný, and G. Attwood. 2008. Reclassification of Clostridium proteoclasticum as Butyrivibrio proteoclasticus comb. nov., a butyrate producing ruminal bacterium. Int. J. Syst. Evol. Microbiol. 58:2041:2045. [DOI] [PubMed] [Google Scholar]
- 36.Ohene-Adjei, S., R. M. Teather, M. Ivan, and R. Forster. 2007. Postinoculation protozoan establishment and association patterns of methanogenic archaea in the ovine rumen. Appl. Environ. Microbiol. 73:4609-4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Or-Rashid, M. M., O. Al Zahal, and B. W. McBride. 2008. Studies on the production of conjugated linoleic acid from linoleic and vaccenic acids by mixed rumen protozoa. Appl. Microbiol. Biotechnol. 81:533-541. [DOI] [PubMed] [Google Scholar]
- 38.Paillard, D., N. McKain, L. C. Cheaudhary, N. D. Walker, F. Pizette, I. Koppova, N. R. McEwan, J. Kopecny, P. E. Vercoe, P. Louis, and R. J. Wallace. 2007. Relation between phylogenetic position, lipid metabolism and butyrate production by different Butyrivibrio-like bacteria from the rumen. Antonie van Leeuwenhoek 91:417-422. [DOI] [PubMed] [Google Scholar]
- 39.Paillard, D., N. McKain, M. T. Rincon, K. J. Shingfield, D. I. Givens, and R. J. Wallace. 2007. Quantification of ruminal Clostridium proteoclasticum by real-time PCR using a molecular beacon approach. J. Appl. Microbiol. 103:1251-1261. [DOI] [PubMed] [Google Scholar]
- 40.Pfaffl, M. W. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29:2002-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Piperova, L. S., J. Sampugna, B. B. Teter, K. F. Kalscheur, M. P. Yurawecz, Y. Ku, K. M. Morehouse, and R. A. Erdman. 2002. Duodenal and milk trans octadecenoic acid and conjugated linoleic acid (CLA) isomers indicate that postabsorptive synthesis is the predominant source of cis-9-containing CLA in lactating dairy cows. J. Nutr. 132:1235-1241. [DOI] [PubMed] [Google Scholar]
- 42.Ruiz, R., and L. A. Rubio. 2009. Lyophilisation improves the extraction of PCR-quality community DNA from pig faecal samples. J. Sci. Food Agric. 89:723-727. [Google Scholar]
- 43.Santora, J. E., D. L. Palmquist, and K. L. Roehrig. 2000. trans-Vaccenic acid is desaturated to conjugated linoleic acid in mice. J. Nutr. 130:208-215. [DOI] [PubMed] [Google Scholar]
- 44.Stevenson, D. M., and P. J. Weimer. 2007. Dominance of Prevotella and low abundance of classical ruminal bacterial species in the bovine rumen revealed by relative quantification real-time PCR. Appl. Microbiol. Biotechnol. 75:165-174. [DOI] [PubMed] [Google Scholar]
- 45.Sylvester, J. T., S. K. Karnati, Z. Yu, M. Morrison, and J. L. Firkins. 2004. Development of an assay to quantify ciliate protozoal biomass in cows using real-time PCR. J. Nutr. 134:3378-3384. [DOI] [PubMed] [Google Scholar]
- 46.Van Soest, P. J. 1982. Nutritional ecology of the ruminant, 2nd ed. Cornell University Press, Ithaca, NY.
- 47.Vasta, V., H. P. S. Makkar, M. Mele, and A. Priolo. 2009. Ruminal biohydrogenation as affected by tannins in vitro. Br. J. Nutr. 102:82-92. [DOI] [PubMed] [Google Scholar]
- 48.Vasta, V., M. Mele, A. Serra, M. Scerra, G. Luciano, M. Lanza, and A. Priolo. 2009. Metabolic fate of fatty acids involved in ruminal biohydrogenation in sheep fed concentrate or herbage with or without tannins. J. Anim. Sci. 87:2674-2684. [DOI] [PubMed] [Google Scholar]
- 49.Vlaeminck, B., V. Fievez, D. Demeyer, and R. J. Dewhurst. 2006. Effect of forage:concentrate ratio on fatty acid composition of rumen bacteria isolated from ruminal and duodenal digesta. J. Dairy Sci. 89:2668-2678. [DOI] [PubMed] [Google Scholar]
- 50.Wallace, R. J., L. C. Cheaudhary, N. McKain, N. R. McEwan, A. J. Richardson, P. E. Vercoe, N. D. Walker, and D. Paillard. 2006. Clostridium proteoclasticum: a ruminal bacterium that forms stearic acid from linoleic acid. FEMS Microbiol. Lett. 265:195-201. [DOI] [PubMed] [Google Scholar]
- 51.Yáñez-Ruiz, D. R., N. D. Scollan, R. J. Merry, and C. J. Newbold. 2006. Contribution of rumen protozoa to duodenal flow of nitrogen, conjugated linoleic acid and vaccenic acid in steers fed silages differing in their soluble carbohydrate content. Br. J. Nutr. 96:861-869. [DOI] [PubMed] [Google Scholar]
- 52.Yáñez-Ruiz, D. R., S. Williams, and C. J. Newbold. 2007. Effect of the absence of protozoa on rumen biohydrogenation and the fatty acid composition of lamb muscle. Br. J. Nutr. 97:938-948. [DOI] [PubMed] [Google Scholar]