Abstract
Lactobacillus rhamnosus GG is a well-established Gram-positive probiotic strain, whose health-benefiting properties are dependent in part on prolonged residence in the gastrointestinal tract and are likely dictated by adherence to the intestinal mucosa. Previously, we identified two pilus gene clusters (spaCBA and spaFED) in the genome of this probiotic bacterium, each of which contained the predicted genes for three pilin subunits and a single sortase. We also confirmed the presence of SpaCBA pili on the cell surface and attributed an intestinal mucus-binding capacity to one of the pilin subunits (SpaC). Here, we report cloning of the remaining pilin genes (spaA, spaB, spaD, spaE, and spaF) in Escherichia coli, production and purification of the recombinant proteins, and assessment of the adherence of these proteins to human intestinal mucus. Our findings indicate that the SpaB and SpaF pilin subunits also exhibit substantial binding to mucus, which can be inhibited competitively in a dose-related manner. Moreover, the binding between the SpaB pilin subunit and the mucosal substrate appears to operate through electrostatic contacts and is not related to a recognized mucus-binding domain. We conclude from these results that it is conceivable that two pilin subunits (SpaB and SpaC) in the SpaCBA pilus fiber play a role in binding to intestinal mucus, but for the uncharacterized and putative SpaFED pilus fiber only a single pilin subunit (SpaF) is potentially responsible for adhesion to mucus.
The human intestinal microbiota is comprised of more than 1,000 species of commensal and probiotic bacteria, including several members of the Gram-positive genus Lactobacillus (42, 52). Many strains of lactobacilli have a variety of health-promoting effects in humans and consequently have been used commercially as probiotics in foods and nutritional supplements (for a review, see reference 48). Often a necessary precondition for colonization of the human gastrointestinal (GI) tract by probiotic bacteria is preferential adherence to the intestinal mucosa, which in turn prolongs and stabilizes intestinal residence, possibly triggering a variety of defensive host cell immune responses and excluding pathogenic bacteria by competitive inhibition or steric hindrance (48). The outermost layer of the intestinal mucosa, which is a secreted and hydrated mucus gel that acts as a protective barrier and filter, consists primarily of a heterogeneous mixture of highly glycosylated membrane-associated and secreted glycoproteins called mucins (36). Although many studies have demonstrated that various probiotic Lactobacillus spp. adhere initially to the mucus gel layer, relatively few details about the overall molecular mechanism of mucosal adhesion are known (for a review, see reference 23). Nonetheless, several studies have reported that the adherence of Lactobacillus cells to the mucosal barrier is frequently due to a surface protein-mediated interaction. For example, Rojas et al. (44) determined that the ability of Lactobacillus fermentum 104R (reclassified as Lactobacillus reuteri 104R) to bind to porcine small intestinal mucus and gastric mucin was facilitated by a cell surface-localized mucus adhesion-promoting protein (MapA). Similarly, Macías-Rodríguez et al. (25) described two adhesion-associated proteins specific for porcine intestinal mucus-related substrates that are attached noncovalently to the cell surface of L. fermentum BCS87. Also, Roos and Jonsson (45) demonstrated adherence between the surface-associated Mub (mucus binding) protein from L. reuteri 1063 and intestinal mucus components derived from porcine and poultry sources. In addition, Pretzer et al. (38) identified a large multidomain surface protein in Lactobacillus plantarum WCFS1 with binding specificity for the mannose moieties in mucins. Interestingly, Kinoshita et al. (19) discovered that glyceraldehyde 3-phosphate dehydrogenase (GAPDH), an enzyme normally associated with glycolysis, is localized on the surface of L. plantarum LA318 cells and adheres tightly to human colonic mucin.
Until quite recently, only indirect or circumstantial evidence suggested that pilus-like structures extend from the surface of probiotic lactobacilli (28, 39). However, in a previous study (18) we demonstrated that Lactobacillus rhamnosus GG, a well-studied and widely used probiotic strain (48), is a piliated microbe. Pili are slender, elongated, heteromeric, proteinaceous surface appendages that are present in numerous other Gram-positive bacteria and often mediate adherence between pathogenic and nonpathogenic species and their host cell targets (for reviews, see references 20, 26, 40, and 49) but have now emerged as possible facilitators of adhesion for probiotic colonization of the GI tract (18). Prototypically, the pilus fiber is composed of one major pilin that forms the pilus backbone and two minor pilin subunits (26, 40, 49), one subunit that has a role in signaling the cessation of pilus polymerization (27, 30) and is deposited at the pilus base and at irregular intervals along the pilus backbone and another subunit with an adhesive property that is often localized at the pilus tip (1, 41). The current model of pilus assembly in Corynebacterium diphtheriae (27) suggests that these pilin subunits are connected covalently to one another through isopeptidyl bonds by a membrane-bound transpeptidase (pilin-specific sortase) to produce polymerized pili, which are then attached covalently to the cell wall by a different transpeptidase (the housekeeping sortase) that is capable of recognizing all C-terminal LPXTG-like substrates. The genes encoding these pilus proteins, as well as the pilin-specific sortase, are clustered at the same locus in the genome (54).
In a recent study (18), we discovered that in the L. rhamnosus GG genome the genes encoding two different pilus fibers are in the spaCBA and spaFED gene clusters and, based on a genomic comparison with another L. rhamnosus strain (LC705), that the spaCBA cluster is present in only L. rhamnosus GG. Moreover, in our previous work (18) the predicted genes for the major pilin subunit forming the pilus backbone (SpaA and SpaD), one ancillary minor pilin subunit (SpaB and SpaE) that (based on a model for pilus biogenesis) is likely located at the pilus base and decorates the pilus backbone (27), and another larger adherent minor pilin subunit (SpaC and SpaF) were identified in L. rhamnosus GG on the basis of amino acid identity with pilins from two enterococcal species. In addition, we also detected in the sequences of the predicted spaCBA and spaFED gene products the anticipated consensus motifs and domains characteristic of a pilin primary structure, including the Sec-dependent secretion signal, the sortase recognition site, the YPKN pilin-like motif, and the E box (18). Subsequently, expression and localization of intact SpaCBA pili on the cell surface of L. rhamnosus GG were confirmed by immunoblotting and immunogold-labeled electron microscopy using antiserum specific for the SpaC pilin (18). Adhesion interactions between the L. rhamnosus GG strain and intestinal mucosal surfaces have been reported and characterized in previous studies (15, 31, 33, 46, 55-57). However, in our recent study (18), SpaCBA pilus-mediated binding of L. rhamnosus GG cells to human intestinal mucus was revealed in adhesion experiments performed with both L. rhamnosus GG pretreated with SpaC antiserum and an L. rhamnosus GG spaC insertion mutant. More specifically, we demonstrated that there was significant binding between recombinant SpaC pilin protein and intestinal mucus and thus identified a mucus-binding capacity for one of the minor pilin components localized at the tip and along the backbone of the SpaCBA pilus (18). To expand on these findings, here we describe a study in which each of the remaining predicted pilin subunits (SpaA, SpaB, SpaD, SpaE, and SpaF) encoded by genes in the spaCBA and spaFED gene clusters was overproduced in a recombinant form, purified to apparent homogeneity, and characterized to determine its adherence to human intestinal mucus.
MATERIALS AND METHODS
Bacterial strains, growth conditions, plasmids, and genomic DNA.
Escherichia coli strains TOP10 [F− mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 recA1 araD139 Δ(ara-leu)7697 galU galK rpsL (Strr) endA1 nupG] and BL21(DE3)/pLysS [F− ompT hsdSB(rB− mB−) gal dcm (DE3)/pLysS (Camr)] were used for cloning and expression, respectively. E. coli was cultivated with agitation at 37°C in Luria-Bertani (LB) medium supplemented with 50 μg/ml kanamycin when necessary. The pET28b+ expression vector (Novagen) was used for cloning the L. rhamnosus GG spaA, spaB, spaD, spaE, and spaF genes. Genomic DNA was isolated from L. rhamnosus GG (= ATCC 53103) as described previously (18). Established protocols were employed for all DNA manipulations, including PCR amplification, restriction endonuclease digestion, ligation, and transformation, as described previously (47).
Cloning of the L. rhamnosus GG SpaCBA and SpaFED pilin genes.
The coding sequences of the SpaCBA and SpaFED pilin genes were obtained from the L. rhamnosus GG genome sequence (18). The spaA, spaB, spaD, spaE, and spaF genes, without the region encoding the N-terminal secretion signal and the C-terminal sortase recognition site, were each PCR amplified from L. rhamnosus GG genomic DNA using pairs of flanking 5′ and 3′ end oligonucleotide primers (Oligomer, Finland) with an EcoRI site (a SacI site for spaF) or an XhoI site (Table 1). The PCR products were digested with the EcoRI (or SacI for spaF) and XhoI restriction endonucleases, ligated into similarly digested expression vector pET28b+, and then transformed into E. coli TOP10. The resultant expression plasmids (pKTH5319 for spaA, pKTH5320 for spaB, pKTH5324 for spaD, pKTH5379 for spaE, and pKTH5341 for spaF) were isolated and propagated in E. coli BL21(DE3)/pLysS for intracellular production of C-terminal hexahistidine-tagged SpaCBA and SpaFED pilin proteins. For each of the recombinant pilins, seven residues (nine residues for SpaF) at the N terminus and two residues preceding the C-terminal hexahistidine tag originated from amino acids encoded by the expression vector. Cloning of the spaC gene has been described elsewhere (18).
TABLE 1.
Oligonucleotide primers used for PCR cloninga
| Gene | Open reading frame | Direction | Oligonucleotide primer sequenceb |
|---|---|---|---|
| spaA | LGG_00442 | Forward | 5′-TCGGGTTCAGAATTCTACGAATGATACGAC |
| Reverse | 5′-TGCCAGTACCACCCTCGAGTGGCAGAATAC | ||
| spaB | LGG_00443 | Forward | 5′-GCAGACACAGAATTCAACTGTGCCGACC |
| Reverse | 5′-CAACTGTATCACCCTCGAGTGGCAACAATTGACG | ||
| spaD | LGG_02370 | Forward | 5′-ACCCGTACAGAATTCGACAACGACTGTG |
| Reverse | 5′-GTCCGATTCCGCCCTCGAGCGGCAATAATTG | ||
| spaE | LGG_02371 | Forward | 5′-CCACATTGGGTTCAGAATTCTGATCAAACTG |
| Reverse | 5′-TGCGCCAATCGGACTCGAGCGGCAAATAAC | ||
| spaF | LGG_02372 | Forward | 5′-GCAAATTGGCAGGAGCTCGGTCCCGGTAG |
| Reverse | 5′-CCGCTACCACCCTCGAGCGGTAGGAGTG |
Oligonucleotide primers were designed using the L. rhamnosus GG genome sequence (18).
Restriction endonuclease sequences used to facilitate cloning (EcoRI [SacI for spaF] in the forward primers and XhoI in the reverse primers) are underlined.
Production and purification of recombinant SpaCBA and SpaFED pilin proteins.
The procedure used for production and purification of recombinant SpaA, SpaB, SpaD, SpaE, and SpaF pilins was essentially the procedure used previously for isolation of the SpaC minor pilin (18). In brief, E. coli cells were grown at 37°C to mid-log phase in LB medium containing 50 μg/ml kanamycin, protein expression was induced for 3 h with 1 mM isopropyl-β-d-1-thiogalactopyranoside (IPTG), and the cells were recovered by centrifugation. The pelleted cells were resuspended in lysis buffer (50 mM NaH2PO4 [pH 8.0], 300 mM NaCl, 10 mM imidazole), and the cell suspensions were then disrupted using sonication, which was followed by centrifugation and filtration (0.45 μm) to produce clarified cell-free lysates. The hexahistidine-tagged pilin proteins (SpaA, SpaB, SpaD, SpaE, and SpaF) were then purified by using Ni2+-chelating affinity chromatography. Each of the cell-free lysates was passed through an Ni-nitrilotriacetic acid (NTA) agarose (Qiagen) column, which was rinsed extensively with wash buffer (50 mM NaH2PO4 [pH 8.0], 300 mM NaCl, 20 mM imidazole), and the pilin proteins then were removed with elution buffer (50 mM NaH2PO4 [pH 8.0], 300 mM NaCl, 250 mM imidazole). Elution fractions containing purified proteins were pooled and buffer exchanged with 10 mM Tris-HCl (pH 8.0) for the SpaA, SpaD, SpaE, and SpaF pilins and with 50 mM sodium acetate (pH 5.1) for the SpaB pilin by using EconoPac 10 DG desalting columns (Bio-Rad). The recombinant pilins were concentrated using 10- or 30-kDa Microsep filters (Pall Life Sciences), the protein purity was assessed by SDS-PAGE, and the approximate protein concentrations were determined by measuring the A280.
Generation of SpaA and SpaB pilin-specific antibodies.
Antisera specific for the L. rhamnosus GG SpaA and SpaB pilin proteins were generated using the method used to produce SpaC antiserum described previously (18). Briefly, rabbits received 1-ml subcutaneous injections containing 400 μg purified recombinant SpaA or SpaB pilin mixed 1:1 with Freund's complete adjuvant and then, at 3-week intervals, a set of three subcutaneous booster injections (1 ml) of 200 μg pilin protein in Freund's incomplete adjuvant (1:1 mixture). Two weeks after the final booster injection, blood was collected for preparation of the SpaA and SpaB antisera.
Protein and whole-cell radiolabeling.
Radiolabeling of pilin proteins with 125I was carried out as described previously (18). Pierce iodination reagent was used to radiolabel purified SpaA, SpaB, SpaC, SpaD, SpaE, and SpaF pilin proteins and ovalbumin (Sigma) by following the manufacturer's protocol (Pierce), and the residual unbound radiolabel was removed by chromatography using D-Salt polyacrylamide desalting columns (Pierce). Metabolic radiolabeling of L. rhamnosus GG cells with tritiated thymidine was performed essentially as described previously (58). Briefly, L. rhamnosus GG was grown overnight in MRS broth containing 10 μl/ml [5′-3H]thymidine (16.7 Ci/nmol), the cells were recovered by centrifugation, washed, and resuspended in phosphate-buffered saline (PBS) (pH 7.2), and the optical density of the culture suspension was adjusted (A600, 0.25) to normalize the number of cells.
Isolation of human intestinal mucus.
Surgically removed tissue acquired from patients suffering from operable colorectal cancer and undergoing colonic resection was the source of intestinal mucus used for binding studies (32). The recovery and use of resected human intestinal tissue were sanctioned by the ethics committee of the Hospital District of Southwest Finland, and prior informed written consent was obtained from the participating patients. Intact mucosa-containing resected tissue from noncancerous segments was chosen, and the mucus layer was extracted using the method described previously (58).
Binding of radiolabeled SpaCBA and SpaFED pilins to intestinal mucus.
Saturating amounts of intestinal mucus were added to Maxisorp microtiter plate wells (Nunc) and incubated overnight at 4°C; then the wells were washed extensively with PBS to remove unbound mucus, and the mucus was incubated in blocking solution (0.5% bovine serum albumin in PBS) for 1 h at room temperature. The blocking solution was aspirated away, and 50 pmol radiolabeled SpaA, SpaB, SpaD, SpaE, or SpaF pilin subunits (each dissolved in blocking solution) was then added to wells containing immobilized mucus and incubated for 1 h at 37°C. After the wells were washed three times with PBS, the amount of protein-bound 125I was determined using a Wallac 1480 WIZARD 3" automatic gamma counter (PerkinElmer). The mucus binding experiment was also performed with 50 pmol radiolabeled SpaC pilin (positive control) and ovalbumin (background control).
Competitive inhibition of mucus binding by the SpaB and SpaF pilins.
Experiments to examine the competitive binding of radiolabeled SpaB and SpaF pilins to mucus were carried using the procedure described previously (18). Using microtiter plate wells containing immobilized intestinal mucus (see above), 50 pmol radiolabeled SpaB or SpaF pilin (dissolved in blocking solution) was added to wells containing 0, 100, 300, and 900 pmol competing unlabeled SpaB or SpaF pilin and incubated for 1 h at 37°C. The wells were then washed three times with PBS, and the amount of bound radioactive (125I) protein was measured with an automatic gamma counter. As controls, 50 pmol radiolabeled SpaC pilin with competing protein (positive control) and 50 pmol radiolabeled ovalbumin without competing protein (background control) were also included in the competitive inhibition assay.
Inhibition of binding between SpaB minor pilin and mucus.
Radiolabeled (125I) SpaB pilin (50 pmol) was pretreated with either 2.5 μg/μl lysozyme (Sigma) or nearly saturating quantities (∼2.5 μg/μl) of unbound intestinal mucus and tested for binding to immobilized intestinal mucus using the method described previously (see above). The inhibition assay was also performed with 50 pmol radiolabeled SpaC pilin as a control.
Antibody-mediated inhibition of L. rhamnosus GG mucosal adhesion.
L. rhamnosus GG mucosal adhesion was inhibited by antisera specific for SpaA and SpaB pilins using the method described previously for SpaC antiserum-mediated inhibition of mucus binding (18). Briefly, a metabolically radiolabeled (3H) L. rhamnosus GG cell suspension was preincubated with each SpaCBA pilin-specific antiserum diluted 1:100, and 100 μl was added to microtiter plate wells coated with human colonic mucus (49) and incubated for 1 h at 37°C. Each of the wells was then washed twice with PBS to rinse away cells adhering weakly to the mucosal substrate. The remaining cells bound to the immobilized mucus were resuspended in a 1% SDS-0.1 N NaOH solution and incubated for 1 h at 60°C, and the radioactivity of the lysed cell suspension was then assessed by liquid scintillation counting. Mucosal adhesion was estimated by determining the ratio of the measured radioactivity of the lysed cell suspension to the measured radioactivity of the cell suspension added initially to the wells and was expressed as a percentage.
Statistical analysis.
The linear association between the amount of unlabeled pilin protein (0, 100, 300, and 900 pmol) and the measured amount (fmol) of radiolabeled (125I) pilin protein was tested to assess the dose-dependent pattern of inhibition of mucus binding by using the Pearson product-moment correlation coefficient (t = −4.05 and n = 16 for SpaB, t = −2.88 and n = 14 for SpaF, and t = −6.42 and n = 16 for SpaC). The unequal variance t test and the Bonferroni multiple-comparison test were used to evaluate the significance of differences in the amounts of bound radiolabeled pilin protein (125I) or L. rhamnosus GG cells (3H) between measured samples.
RESULTS
Production and purification of recombinant SpaCBA and SpaFED pilins.
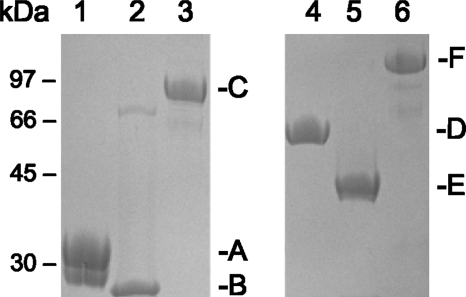
The spaA, spaB, spaD, spaE, and spaF genes were each amplified by PCR from L. rhamnosus GG using the cloning primers listed in Table 1, and the PCR products were cloned in a pET28b+ expression vector as described in Materials and Methods. Recombinant pilins with C-terminal hexahistidine tags were produced intracellularly in E. coli and purified by Ni2+-agarose affinity chromatography. Purified SpaA and SpaB pilins were used to produce SpaA- and SpaB-specific antibodies in rabbits (see Materials and Methods), and all purified SpaCBA and SpaFED pilin proteins were analyzed for adherence to intestinal mucus. Cloning, production, and purification of the SpaC minor pilin, as well as use of this pilin to generate SpaC antiserum, have been described previously (18). The molecular weights of the purified recombinant pilin proteins (SpaA, SpaB, SpaC, SpaD, SpaE, and SpaF) corresponding to bands detected by SDS-PAGE (Fig. 1) were in agreement with calculated molecular masses (Table 2). Examination of the additional properties of the pilin subunits (Table 2) also revealed that the highest isoelectric point (pI ∼8) was associated with the SpaB pilin. Interestingly, SDS-PAGE of purified recombinant SpaB pilin (Fig. 1, lane 2) revealed the presence of a weakly stained high-molecular-weight protein band of unknown origin, which may have represented an aggregate form of SpaB caused by SDS-induced precipitation. Since strongly alkaline proteins (e.g., pI ∼11) tend to precipitate in the presence of SDS at a pH below their isoelectric point, the extent of which is related to how positively charged the protein is at a particular pH (29) and which sometimes results in an anomalous SDS-PAGE migration pattern (7), a moderately alkaline SpaB protein might be prone to a similar behavior. In addition, the reason that a doublet band of purified SpaA pilin (Fig. 1, lane 1) appeared occasionally on SDS gels is not known, but it might be related to intramolecular isopeptide bonds identified previously that maintain rigidity in the pilin structure (6, 16, 17) and whose altered formation may cause pilin proteins to migrate aberrantly when they are analyzed by SDS-PAGE (41).
FIG. 1.
Purified recombinant SpaCBA and SpaFED pilin proteins. Purified recombinant SpaA (lane 1), SpaB (lane 2), SpaC (lane 3), SpaD (lane 4), SpaE (lane 5), and SpaF (lane 6) pilin proteins were separated using 12% SDS-PAGE, which was followed by staining with Coomassie brilliant blue R-250. The positions of molecular mass standards are indicated on the left, and the positions of the pilin protein bands are indicated on the right.
TABLE 2.
Properties of the recombinant SpaCBA and SpaFED pilin proteins
| Pilin | Molecular mass (kDa) | Length (amino acids) | Isoelectric point (pI) |
|---|---|---|---|
| SpaA | 31 | 283 | 5.1 |
| SpaB | 21 | 188 | 8.0 |
| SpaC | 91 | 836 | 5.0 |
| SpaD | 51 | 465 | 5.5 |
| SpaE | 45 | 400 | 5.9 |
| SpaF | 104 | 943 | 5.4 |
Mucus-binding properties of the SpaCBA and SpaFED pilins.
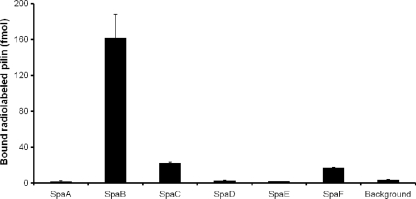
In our previous work, we established binding between the SpaC minor pilin and human intestinal mucus using radiolabeled protein (18). In the present study, we used the same approach to assess the mucus-binding properties of the predicted major pilin (SpaA and SpaD) and minor pilin (SpaB, SpaE, and SpaF) subunits associated with the two pilus gene clusters in L. rhamnosus GG. Recombinant forms of the pilin subunits were purified, radiolabeled with 125I, and screened for binding to human colonic mucus. The SpaC minor pilin was included as a positive control in the binding analysis. As shown by the mucus-binding profiles for the SpaCBA and SpaFED pilins (Fig. 2), only the SpaB, SpaC, and SpaF subunits exhibited significant binding to the mucosal substrate. Since the remaining three pilin subunits (SpaA, SpaD, and SpaE), all of which are predicted to contribute to the structural integrity of the pilus and lack discernible homology to recognized mucus-specific adhesins, demonstrated levels of mucus binding below the background level, the absence of appreciable adherence to mucus was not completely unexpected. However, the two minor pilin subunits (SpaC and SpaF), which were predicted to be localized at the tips of different pilus fibers and to be prototypically adhesive, exhibited an anticipated mucus-binding profile. Since the SpaB minor pilin subunit has predicted roles similar to those of the SpaA, SpaD, and SpaE pilins (see above), binding between the SpaB subunit and intestinal mucus was an unforeseen result. Moreover, the level of mucus binding measured for the SpaB subunit was approximately 7-fold greater than that measured for the SpaF and SpaC pilins. Since of the six SpaCBA and SpaFED pilin subunits only the SpaB minor pilin is a positively charged protein (Table 2), the binding between the SpaB pilin and negatively charged mucosal moieties might be mediated simply by electrostatic interactions and might be independent of a specific mucus-binding domain. Nonetheless, the two minor pilins (SpaB and SpaF) with measurable adherence to mucus were included in a competitive mucosal adhesion assay for additional characterization.
FIG. 2.
Adherence of SpaCBA and SpaFED pilin proteins to human intestinal mucus. The methods used to determine binding between 50-pmol portions of radiolabeled (125I) SpaCBA and SpaFED pilins and immobilized mucus and the methods used for the corresponding statistical analysis are described in Materials and Methods. The background level of mucus binding was based on the adherence of 50 pmol radiolabeled (125I) ovalbumin to mucus. The binding data for three or four measurements are expressed as means ± standard deviations, and the differences between data sets are considered significant (P ≤ 0.05).
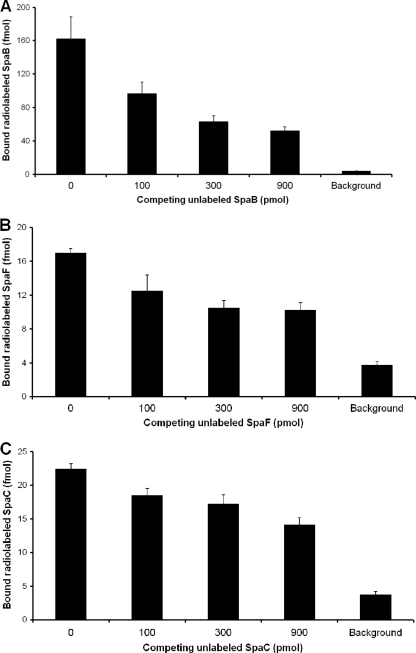
Competitive mucosal adhesion of the SpaB and SpaF minor pilins.
To assess whether the mucosal adhesion interaction for the SpaB and SpaF pilin subunits can be inhibited in a dose-related manner, as reported previously for the SpaC minor pilin (18), we performed in vitro competitive binding experiments (Fig. 3) using radiolabeled proteins and intestinal mucus as described in Materials and Methods. Both radiolabeled SpaC pilin (positive control) and ovalbumin (background control) were included in the competitive binding assay. Each of the radiolabeled pilin subunits (SpaB, SpaF, and SpaC) bound to the mucus substantially more than the background control (Fig. 3A, 3B, and 3C). Moreover, as supported by a statistical analysis of the binding data for all three pilin subunits, competing unlabeled proteins (100, 300, and 900 pmol) caused apparent dose-related inhibition of mucus binding, although the SpaB subunit exhibited the best dose-related reduction in adhesion (Fig. 3A). The results that we obtained here are in agreement with competitive adhesion results.
FIG. 3.
Competitive binding of the SpaB and SpaF minor pilins to mucus. The binding of 50-pmol portions of radiolabeled (125I) SpaB (A), SpaF (B), and SpaC (C) pilins to mucus was inhibited by competing amounts of the corresponding unlabeled pilin subunits (0, 100, 300, and 900 pmol) as described in Materials and Methods. The level of binding between 50 pmol radiolabeled (125I) ovalbumin and immobilized mucus was defined as the background level of mucus binding. The binding data are means ± standard deviations for three or four measurements. The differences for dose-related inhibition of mucus binding are considered significant (P ≤ 0.01) for each pilin subunit (SpaB, SpaF, and SpaC). For further details concerning the statistical analysis see Materials and Methods.
Influence of electrostatic interactions on the mucus-binding specificity of the SpaB minor pilin.
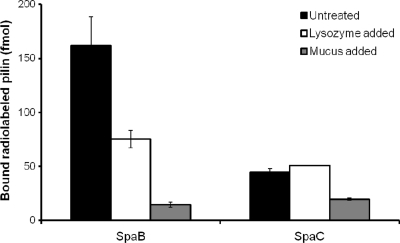
To determine whether electrostatic interactions influence the mucus-binding specificity of the basic SpaB pilin protein, we used positively charged lysozyme (pI ∼11) to inhibit the mucosal adhesion of SpaB pilin. In a previous study (14), it was reported that lysozyme can cross-link mucus by electrostatic means and, in doing so, contribute partially to the buildup of the macromolecular gel-like composition of mucus. As controls, we examined the effect of adding unbound mucus and performed the binding experiment with the SpaC minor pilin. The binding of SpaB pilin to intestinal mucus decreased approximately 50% in the presence of lysozyme (2.5 μg/μl), indicating that lysozyme competes with SpaB pilin for mucus and that electrostatic interactions may play some role in SpaB-mediated mucosal adhesion (Fig. 4). As expected, nearly saturating quantities of unbound mucus eliminated a significant portion of the binding between the SpaB pilin and bound mucus (Fig. 4). For the SpaC minor pilin, mucus binding was reduced about 2-fold in the presence of additional mucus and was not affected by large amounts of lysozyme.
FIG. 4.
Inhibition of binding between the SpaB minor pilin and mucus. The effect of 2.5 μg/ml lysozyme or unbound intestinal mucus on the adherence of 50 pmol radiolabeled (125I) SpaB and SpaC pilins to immobilized mucus was determined using the procedure described in Materials and Methods. The binding data obtained from three or four measurements are expressed as means ± standard deviations, and the differences between data are considered significant (P ≤ 0.05). For further information concerning the statistical analysis see Materials and Methods.
Inhibition of L. rhamnosus GG mucosal adhesion by pilin-specific antibodies.
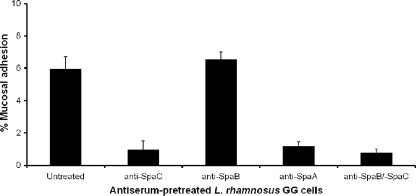
Previously, the presence of cell surface-localized intact pili encoded by the spaCBA gene cluster, the capacity of these pili for mucus binding, and the inhibition of binding have been demonstrated in L. rhamnosus GG using antiserum specific for the SpaC minor pilin (18). To characterize the mucosal adherence properties of SpaCBA pili further, we examined the effect of pretreating L. rhamnosus GG cells with SpaB antiserum. Untreated cells and cells pretreated with SpaA- and SpaC-specific antibodies were also included as controls. Mucosal adhesion by the untreated cells was detected as anticipated, and the results obtained after pretreatment of cells with SpaC antiserum confirmed our previous findings (18).
Surprisingly, pretreatment with SpaB antiserum did not block mucosal adhesion (Fig. 5) despite the demonstration that there was significant mucus binding by the recombinant SpaB pilin (Fig. 2) and despite the functionality of the SpaB-specific antibodies, as indicated by the results obtained when they were used to identify surface-localized SpaCBA pilus fibers by immunogold-labeled electron microscopy (J. Reunanen, A. P. A. Hendrickx, I. von Ossowski, A. Palva, and W. M. de Vos, unpublished data). Previously, the spaB gene in the spaCBA pilus gene cluster has been predicted to encode an ancillary minor pilin (18) that, according to a current model of pilus assembly (27), likely corresponds to the same type of pilin subunit deposited at the pilus base and sparingly along the pilus backbone and implicated in signaling the cessation of pilus polymerization. Consequently, the binding of SpaB- specific antibodies to SpaB localized similarly in the SpaCBA pilus structure may prevent only SpaB-mediated adhesion to mucus and thus result in little steric hindrance that affects the mucus-binding capacity of the SpaC pilin. This conclusion was supported by the finding that cells pretreated with both SpaB and SpaC antiserum could not adhere to mucus (Fig. 5). Mucus binding appears to have been blocked for the most part by the SpaC-specific antibody, indicating the possibility that the contribution of the SpaB pilin in assembled pilus fibers to L. rhamnosus GG mucosal adhesion is less than the contribution deduced from the high level of mucus binding for the individual SpaB pilin subunit (Fig. 2). Moreover, since each type of pilin is primarily at a different location in the pilus structure, an SpaC pilin at the tip of a pilus fiber (18) may be more accessible to mucosal substrates than an SpaB pilin at its predicted position at the pilus base.
FIG. 5.
SpaCBA pilin antibody-mediated inhibition of L. rhamnosus GG mucosal adhesion. Radiolabeled (3H) L. rhamnosus GG cells were pretreated with SpaA-, SpaB-, and SpaC-specific antisera, and mucus binding was examined as described in Materials and Methods. The binding data from six measurements are expressed as means ± standard deviations, and the differences between data are considered significant (P ≤ 0.05). For additional information concerning the statistical analysis see Materials and Methods.
Another unexpected result was the 5-fold reduction in mucus binding by the cells pretreated with SpaA antiserum (Fig. 5), even though the SpaA pilin subunit did not adhere appreciably to intestinal mucus (Fig. 2). However, because the SpaA major pilin is the predicted predominant subunit comprising the pilus backbone (18), the pilus-bound SpaA-specific antibodies may simply hinder sterically the SpaC pilin subunits shown to be deposited at the pilus tip and intermittently throughout the SpaCBA pilus (18) and therefore cause obstruction of SpaC-mediated mucosal adhesion.
DISCUSSION
The continuous shedding of intestinal epithelial cells and the peristaltic movement of food and water through the intestine create a dynamic environment with considerable challenges for stable bacterial colonization of the gastrointestinal tract (52). Since a sizeable portion of the intestinal microbiota coexists symbiotically with the host, it is an advantage that the intestine has an environmental niche favoring localized commensal growth. The mucus gel layer, which spans the epithelial cell lining of the inner intestinal walls, is naturally the first region for possible microbial contact and attachment, can provide an environment comprised of a conserved glycan-based matrix for localizing microbes and binding sites to prevent microbial washout from the intestine, and can be a carbohydrate-rich source of nutrients (34, 52). In the intestinal microbiota, a diverse range of commensal, probiotic, and pathogenic species have adapted to a mucosal milieu by developing unique surface-associated features with mucus-related specificity (2).
The prevailing consensus about what constitutes an effective probiotic includes the general rule that these bacteria must have an inherent ability to maintain persistent growth in the GI tract (9, 59). However, several studies using various probiotic strains have reported that rather than becoming fortified species in the intestinal microbiota, probiotic organisms are allochthonous and require constant replenishing to persist for extended periods of time in the intestine (3, 5, 10, 59). Moreover, studies have also revealed differences in the levels of intestinal persistence between many probiotic bacteria (13, 18). Although there is still some debate about whether persistent colonization is essential for the beneficial immunomodulating effects of probiotics (9), lactobacilli must still rely on specific adherence mechanisms for displacing pathogens and preventing their own immediate washout from the intestinal tract. Nonetheless, a plethora of studies have demonstrated binding between a variety of probiotic Lactobacillus spp. and mucus-related substrates (23), but very few studies have pinpointed by either genomic or proteomic means the cell surface components directly responsible for mucosal adhesion. In a recent study (18) we reported that elongated pilus fibers are present on the cell surface of the probiotic L. rhamnosus GG strain. Moreover, we attributed a mucus-binding capacity to these pili (encoded by the spaCBA gene cluster) and, in particular, to one of the individual pilin subunits (SpaC) constituting the pilus fiber. Such pili, by virtue of their long- and slender-appendage architecture, their observed propensity to tether around the cell exterior, and their binding specificity for mucus are ideal surface structures for ensuring that microbes remain attached and embedded within a mucosal matrix. Consequently, the L. rhamnosus GG strain, which has been characterized as an effective probiotic, is expected to have a distinct advantage over nonpiliated probiotic lactobacilli for maintaining extended or stable residence in the GI tract.
The structural constituents of the two pilus fibers (SpaCBA and SpaFED) encoded in the genome of L. rhamnosus GG include one major pilin and two minor pilin subunits, all of which resemble prototypically the structural components of pili found in many other Gram-positive bacteria (26, 40, 49). So far, only the SpaC minor pilin subunit of the SpaCBA pilus has been assessed for binding specificity (18), leaving five predicted pilus components, the SpaA and SpaD major pilin subunits and the SpaB, SpaE, and SpaF minor pilin subunits, uncharacterized. In this study, we cloned the genes encoding these five components (spaA, spaB, spaD, spaE, and spaF), produced and purified recombinant forms of pilin proteins, and performed a systematic assessment of the binding specificities of these proteins for human intestinal mucus. Following the initial screen for mucus-binding properties, we observed no appreciable mucosal adhesion with the SpaA, SpaD, and SpaE pilin subunits, a result that in hindsight was expected when the predicted roles of these components in an assembled pilus fiber and the lack of mucus-related binding domain homologs in each of the primary structures were taken into account (18). Although pilin subunits from some Gram-positive bacteria show adherence to the extracellular matrix (ECM) proteins underlying the intestinal epithelium (11, 21, 51), our attempts to screen for similar binding specificities with the three pilin subunits unable to adhere to mucus were largely unsuccessful (data not shown). So far, major pilin subunits comprising the pilus backbone and minor pilin subunits located at the pilus base of other Gram-positive pili have not exhibited specific adhesion properties (20, 26, 40, 49). However, the SpaB and SpaF minor pilins each showed significant adherence to the mucosal substrate, and in a competitive adhesion assay the inhibition of mucus binding was dose related, with the best dose-dependent reduction in adhesion exhibited by the SpaB pilin. Considering that the SpaF pilin is one of the two large pilus components (Table 2) and is a predicted adherent tip pilin, a mucus-binding capacity could conceivably be anticipated. Previously, we described the presence of a von Willebrand factor-like domain with possible lectin-type binding in the primary structure of the SpaC pilin (18), but for the SpaF pilin similar or related domain homologs were not observed. Although pilus fibers encoded by the spaFED gene cluster have not been identified on the surface of L. rhamnosus GG cells yet, our results suggest that only one of the three pilin subunits (SpaF) in an assembled pilus structure possesses mucus-binding specificity.
The level of binding between the SpaB minor pilin and intestinal mucus, which was considerably greater than the level measured for the SpaF and SpaC pilins, was a rather surprising outcome in the absence of any homology to established mucus-specific adhesins (18). When the physical and chemical properties of the six pilin subunits were compared, we found that the isoelectric point for the SpaB pilin (pI ∼8) was higher than those for the five other pilins (pI ∼5), and as a result, we investigated whether electrostatic interactions have a role in SpaB-mediated mucosal adhesion. Several reports have described the use of high ionic strength (1 M NaCl) to disrupt charge-dependent bonds and thereby establish charge mechanisms for an array of protein-mediated interactions (4, 35, 50). However, we instead used large amounts of a positively charged protein to inhibit binding between the SpaB pilin and mucus. Lysozyme, a highly positively charged enzyme (pI ∼11) that is normally found at basal levels throughout the intestinal tract (37) and possibly forms cross-links with mucus by electrostatic bonding (14), was able to compete with the SpaB pilin for the negatively charged mucosal substrate. Since SpaB pilin-mediated mucosal adhesion was inhibited effectively by lysozyme, the determinants of binding between SpaB pilin and mucus are likely governed by electrostatic contacts, and therefore, as proposed for lysozyme, the SpaB pilin may have the same capacity to cross-link with mucus. Moreover, given that the SpaC tip pilin is also deposited occasionally throughout the pilus fiber (18), as predicted for SpaB, the frequency of incorporation in the SpaCBA pilus fiber has a direct influence on the overall strength of L. rhamnosus GG mucosal adhesion. Previously, we established the contribution of the SpaC component to SpaCBA pilus-mediated mucosal adhesion by pretreating L. rhamnosus GG cells with SpaC-specific antiserum (18). In the present study, the results of a similar experiment using SpaB antiserum were inconclusive and did not confirm directly involvement of the SpaB pilin mucus-binding capacity in the assembled SpaCBA pilus fibers.
Our results provide further insight into the binding specificities of the major and minor pilin subunits of the pilus fibers encoded by the L. rhamnosus GG spaCBA and spaFED gene clusters. Continuing our earlier work, we showed that two pilin components binding to mucus, one component possibly in a lectin-type manner (SpaC) (18) and the other component through electrostatic contacts (SpaB), may be involved in SpaCBA pilus-mediated adherence to intestinal mucus. Moreover, we established that the SpaF minor pilin is the only mucus-binding component in the putative SpaFED pilus fiber. Several studies have reported that various Gram-positive pathogens use mucosal adhesion to facilitate invasive colonization of the GI tract (for a review, see reference 53). Our report of pilus fibers in the L. rhamnosus GG strain with an evident mucus-binding predisposition supports the well-recognized mechanism by which probiotics displace pathogenic bacteria using competitive elimination or steric hindrance (8, 12, 24, 43). Since the intestinal mucosal barrier is a nonhomogeneous collection of various protein- and carbohydrate-rich components, some of the components might be potential receptor-binding sites to which the pilus fibers of L. rhamnosus GG adhere specifically. By using the three recombinant mucus-binding pilin subunits (SpaB, SpaC, and SpaF), we plan to identify, isolate, and characterize the equivalent host cell receptors located in the intestinal mucosa. At present, the three-dimensional structure of the major and minor pilin subunits has been described for four piliated Gram-positive species (6, 16, 17, 22), so the availability of soluble and functional L. rhamnosus GG pilin proteins should facilitate obtaining additional structural insight into the assembly and function of pilus fibers of a probiotic strain.
Acknowledgments
This study was supported by the Academy of Finland (research grants 118165 and 117877) and was part of the Research Program on Nutrition, Foods, and Health (ELVIRA) and the Center of Excellence in Microbial Food Safety Research (MiFoSa). Willem de Vos is funded as a Finland Distinguished Professor by the Finnish Funding Agency for Technology and Innovation (TEKES).
Valio Ltd., Finland, has registered L. rhamnosus GG as LGG.
We thank Ilkka Palva for his invaluable guidance and discussions during this study. We also thank Katariina Kojo and Marko Sutinen of the University of Helsinki for their skillful technical support during purification of proteins.
Footnotes
Published ahead of print on 29 January 2010.
REFERENCES
- 1.Abbot, E. L., W. D. Smith, G. P. Siou, C. Chiriboga, R. J. Smith, J. A. Wilson, B. H. Hirst, and M. A. Kehoe. 2007. Pili mediate specific adhesion of Streptococcus pyogenes to human tonsil and skin. Cell. Microbiol. 9:1822-1833. [DOI] [PubMed] [Google Scholar]
- 2.Adlerberth, I., M. Cerquetti, I. Poilane, A. Wold, and A. Collington. 2000. Mechanisms of colonization and colonization resistance of the digestive tract. Microb. Ecol. Health Dis. Suppl. 2:223-239. [Google Scholar]
- 3.Alander, M., R. Satokari, R. Korpela, M. Saxelin, T. Vilpponen-Salmela, T. Mattila-Sandholm, and A. von Wright. 1999. Persistence of colonization of human colonic mucosa by a probiotic strain, Lactobacillus rhamnosus GG, after oral consumption. Appl. Environ. Microbiol. 65:351-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnberg, N., A. H. Kidd, K. Edlund, J. Nilsson, P. Pring-Åkerblom, and G. Wadell. 2002. Adenovirus type 37 binds to cell surface sialic acid through a charge-dependent interaction. Virology 302:33-43. [DOI] [PubMed] [Google Scholar]
- 5.Bonetti, A., L. Morelli, and E. Campominosi. 2002. Assessment of the persistence in the human intestinal tract of two probiotic lactobacilli, Lactobacillus salivarius I 1794 and Lactobacillus paracasei I 1688. Microb. Ecol. Health Dis. 14:228-232. [Google Scholar]
- 6.Budzik, J. M., C. B. Poor, K. F. Faull, J. P. Whitelegge, C. He, and O. Schneewind. 2009. Intramolecular amide bonds stabilize pili on the surface of bacilli. Proc. Natl. Acad. Sci. U. S. A. 106:19992-19997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campagnoni, A. T., and C. S. Magno. 1974. Molecular weight estimation of mouse and guinea-pig myelin basic proteins by polyacrylamide gel electrophoresis in the presence of sodium dodecyl sulphate: influence of ionic strength. J. Neurochem. 23:887-890. [DOI] [PubMed] [Google Scholar]
- 8.Chan, R. C., G. Reid, R. T. Irvin, A. W. Bruce, and J. W. Costerton. 1985. Competitive exclusion of uropathogens from human uroepithelial cells by Lactobacillus whole cells and cell wall fragments. Infect. Immun. 47:84-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corthésy, B., H. R. Gaskins, and A. Mercenier. 2007. Cross-talk between probiotic bacteria and the host immune system. J. Nutr. 137:781S-790S. [DOI] [PubMed] [Google Scholar]
- 10.De Champs, C., N. Maroncle, D. Balestrino, C. Rich, and C. Forestier. 2003. Persistence of colonization of intestinal mucosa by a probiotic strain, Lactobacillus casei subsp. rhamnosus Lcr35, after oral consumption. J. Clin. Microbiol. 41:1270-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hilleringmann, M., F. Giusti, B. C. Baudner, V. Masignani, A. Covacci, R. Rappuoli, M. A. Barocchi, and I. Ferlenghi. 2008. Pneumococcal pili are composed of protofilaments exposing adhesive clusters of Rrg A. PLoS Pathog. 4:e1000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isolauri, E., P. V. Kirjavainen, and S. Salminen. 2002. Probiotics: a role in the treatment of intestinal infection and inflammation? Gut 50:III54-III59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobsen, C. N., V. Rosenfeldt Nielsen, A. E. Hayford, P. L. Møller, K. F. Michaelsen, A. Pærregaard, B. Sandström, M. Tvede, and M. Jakobsen. 1999. Screening of probiotic activities of forty-seven strains of Lactobacillus spp. by in vitro techniques and evaluation of the colonization ability of five selected strains in humans. Appl. Environ. Microbiol. 65:4949-4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jenssen, A. O., O. Smidsrød, and O. Harbitz. 1980. The importance of lysozyme for the viscosity of sputum from patients with chronic obstructive lung disease. Scand. J. Clin. Lab. Invest. 40:727-731. [DOI] [PubMed] [Google Scholar]
- 15.Juntunen, M., P. V. Kirjavainen, A. C. Ouwehand, S. J. Salminen, and E. Isolauri. 2001. Adherence of probiotic bacteria to human intestinal mucus in healthy infants and during rotavirus infection. Clin. Diagn. Lab. Immunol. 8:293-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang, H. J., F. Coulibaly, F. Clow, T. Proft, and E. N. Baker. 2007. Stabilizing isopeptide bonds revealed in Gram-positive bacterial pilus structure. Science 318:1625-1628. [DOI] [PubMed] [Google Scholar]
- 17.Kang, H. J., N. G. Paterson, A. H. Gaspar, H. Ton-That, and E. N. Baker. 2009. The Corynebacterium diphtheriae shaft pilin SpaA is built of tandem Ig-like modules with stabilizing isopeptide and disulfide bonds. Proc. Natl. Acad. Sci. U. S. A. 106:16967-16971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kankainen, M., L. Paulin, S. Tynkkynen, I. von Ossowski, J. Reunanen, P. Partanen, R. Satokari, S. Vesterlund, A. P. A. Hendrickx, S. Lebeer, S. J. C. De Keersmaecker, J. Vanderleyden, T. Hämäläinen, S. Laukkanen, N. Salovuori, J. Ritari, E. Alatalo, R. Korpela, T. Mattila-Sandholm, A. Lassig, K. Hatakka, K. T. Kinnunen, H. Karjalainen, M. Saxelin, K. Laakso, A. Surakka, A. Palva, T. Salusjärvi, P. Auvinen, and W. M. de Vos. 2009. Comparative genomic analysis of Lactobacillus rhamnosus GG reveals pili containing a human-mucus binding protein. Proc. Natl. Acad. Sci. U. S. A. 106:17193-17198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kinoshita, H., H. Uchida, Y. Kawai, T. Kawasaki, N. Wakahara, H. Matuo, M. Watanabe, H. Kitazawa, S. Ohnuma, K. Miura, A. Horii, and T. Saito. 2008. Cell surface Lactobacillus plantarum LA 318 glyceraldehyde 3-phosphate dehydrogenase (GAPDH) adheres to human colonic mucin. J. Appl. Microbiol. 104:1667-1674. [DOI] [PubMed] [Google Scholar]
- 20.Kline, K. A., S. Fälker, S. Dahlberg, S. Normark, and B. Henriques-Normark. 2009. Bacterial adhesins in host-microbe interactions. Cell Host Microbe 5:580-592. [DOI] [PubMed] [Google Scholar]
- 21.Konto-Ghiorghi, Y., E. Mairey, A. Mallet, G. Duménil, E. Caliot, P. Trieu-Cuot, and S. Dramsi. 2009. Dual role for pilus in adherence to epithelial cells and biofilm formation in Streptococcus agalactiae. PLoS Pathog. 5:e1000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krishnan, V., A. H. Gaspar, N. Ye, A. Mandlik, H. Ton-That, and S. V. Narayana. 2007. An IgG-like domain in the minor pilin GBS52 of Streptococcus agalactiae mediates lung epithelial cell adhesion. Structure 15:893-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lebeer, S., J. Vanderleyden, and S. C. J. De Keersmaecker. 2008. Genes and molecules of Lactobacillus supporting probiotic action. Microbiol. Mol. Biol. Rev. 72:728-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee, Y. K., K. Y. Puong, A. C. Ouwehand, and S. Salminen. 2003. Displacement of bacterial pathogens from mucus and Caco-2 cell surface by lactobacilli. J. Med. Microbiol. 52:925-930. [DOI] [PubMed] [Google Scholar]
- 25.Macías-Rodríguez, M. E., M. Zagorec, F. Ascencio, R. Vázquez-Juárez, and M. Rojas. 2009. Lactobacillus fermentum BCS87 expresses mucus- and mucin-binding proteins on the cell surface. J. Appl. Microbiol. 107:1866-1874. [DOI] [PubMed] [Google Scholar]
- 26.Mandlik, A., A. Swierczynski, A. Das, and H. Ton-That. 2008. Pili in Gram-positive bacteria: assembly, involvement in colonization and biofilm development. Trends Microbiol. 16:33-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mandlik, A., A. Das, and H. Ton-That. 2008. The molecular switch that activates the cell wall anchoring step of pilus assembly in gram-positive bacteria. Proc. Natl. Acad. Sci. U. S. A. 105:14147-14152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGroarty, J. A. 1994. Cell surface appendages of lactobacilli. FEMS Microbiol. Lett. 124:405-410. [DOI] [PubMed] [Google Scholar]
- 29.Moskaitis, J. E., and A. T. Campagnoni. 1986. A comparison of the dodecyl sulfate-induced precipitation of the myelin basic protein with other water-soluble proteins. Neurochem. Res. 11:299-315. [DOI] [PubMed] [Google Scholar]
- 30.Nobbs, A. H., R. Rosini, C. D. Rinaudo, D. Maione, G. Grandi, and J. L. Telford. 2008. Sortase A utilizes an ancillary protein anchor for efficient cell wall anchoring of pili in Streptococcus agalactiae. Infect. Immun. 76:3550-3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ouwehand, A. C., E. M. Tuomola, S. Tölkkö, and S. Salminen. 2001. Assessment of adhesion properties of novel probiotic strains to human intestinal mucus. Int. J. Food Microbiol. 64:119-126. [DOI] [PubMed] [Google Scholar]
- 32.Ouwehand, A. C., S. Salminen, S. Tölkkö, P. Roberts, J. Ovaska, and E. Salminen. 2002. Resected human colonic tissue: new model for characterizing adhesion of lactic acid bacteria. Clin. Diagn. Lab. Immunol. 9:184-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ouwehand, A. C., S. Salminen, P. J. Roberts, J. Ovaska, and E. Salminen. 2003. Disease-dependent adhesion of lactic acid bacteria to the human intestinal mucosa. Clin. Diagn. Lab. Immunol. 10:643-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ouwehand, A. C., M. Derrien, W. de Vos, K. Tiihonen, and N. Rautonen. 2005. Prebiotics and other microbial substrates for gut functionality. Curr. Opin. Biotechnol. 16:212-217. [DOI] [PubMed] [Google Scholar]
- 35.Parker, M. H., and P. E. Prevelige, Jr. 1998. Electrostatic interactions drive scaffolding/coat protein binding and procapsid maturation in bacteriophage P22. Virology 250:337-349. [DOI] [PubMed] [Google Scholar]
- 36.Patsos, G., and A. Corfield. 2009. Management of the human mucosal defensive barrier: evidence for glycan legislation. Biol. Chem. 390:581-590. [DOI] [PubMed] [Google Scholar]
- 37.Peeters, T., and G. Vantrappen. 1975. The Paneth cell: a source of intestinal lysozyme. Gut 16:553-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pretzer, G., J. Snel, D. Molenaar, A. Wiersma, P. A. Bron, J. Lambert, W. M. de Vos, R. van der Meer, M. A. Smits, and M. Kleerebezem. 2005. Biodiversity-based identification and functional characterization of the mannose-specific adhesin of Lactobacillus plantarum. J. Bacteriol. 187:6128-6136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pridmore, R. D., B. Berger, F. Desiere, D. Vilanova, C. Barretto, A. C. Pittet, M. C. Zwahlen, M. Rouvet, E. Altermann, R. Barrangou, B. Mollet, A. Mercenier, T. Klaenhammer, F. Arigoni, and M. A. Schell. 2004. The genome sequence of the probiotic intestinal bacterium Lactobacillus johnsonii NCC 533. Proc. Natl. Acad. Sci. U. S. A. 101:2512-2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Proft, T., and E. N. Baker. 2009. Pili in Gram-negative and Gram-positive bacteria—structure, assembly and their role in disease. Cell. Mol. Life Sci. 66:613-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quigley, B. R., D. Zähner, M. Hatkoff, D. G. Thanassi, and J. R. Scott. 2009. Linkage of T3 and Cpa pilins in the Streptococcus pyogenes M3 pilus. Mol. Microbiol. 72:1379-1394. [DOI] [PubMed] [Google Scholar]
- 42.Rajilić-Stojanović, M., H. Smidt, and W. M. de Vos. 2007. Diversity of the human gastrointestinal tract microbiota revisited. Environ. Microbiol. 9:2125-2136. [DOI] [PubMed] [Google Scholar]
- 43.Reid, G., R. C. Chan, A. W. Bruce, and J. W. Costerton. 1985. Prevention of urinary tract infection in rats with an indigenous Lactobacillus casei strain. Infect. Immun. 49:320-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rojas, M., F. Ascencio, and P. L. Conway. 2002. Purification and characterization of a surface protein from Lactobacillus fermentum 104R that binds to porcine small intestinal mucus and gastric mucin. Appl. Environ. Microbiol. 68:2330-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roos, S., and H. Jonsson. 2002. A high-molecular-mass cell-surface protein from Lactobacillus reuteri 1063 adheres to mucus components. Microbiology 148:433-442. [DOI] [PubMed] [Google Scholar]
- 46.Ruas-Madiedo, P., M. Gueimonde, A. Margolles, C. G. D. L. Reyes-Gavilan, and S. Salminen. 2006. Exopolysaccharides produced by probiotic strains modify the adhesion of problotics and enteropathogens to human intestinal mucus. J. Food Prot. 69:2011-2015. [DOI] [PubMed] [Google Scholar]
- 47.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 48.Saxelin, M., S. Tynkkynen, T. Mattila-Sandholm, and W. M. de Vos. 2005. Probiotic and other functional microbes: from markets to mechanisms. Curr. Opin. Microbiol. 16:204-211. [DOI] [PubMed] [Google Scholar]
- 49.Scott, J. R., and D. Zähner. 2006. Pili with strong attachments: Gram-positive bacteria do it differently. Mol. Microbiol. 62:320-330. [DOI] [PubMed] [Google Scholar]
- 50.Seiradake, E., D. Henaff, H. Wodrich, O. Billet, M. Perreau, C. Hippert, F. Mennechet, G. Schoehn, H. Lortat-Jacob, H. Dreja, S. Ibanes, V. Kalatzis, J. P. Wang, R. W. Finberg, S. Cusack, and E. J. Kremer. 2009. The cell adhesion molecule “CAR” and sialic acid on human erythrocytes influence adenovirus in vivo biodistribution. PLoS Pathog. 5:e1000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sillanpää, J., S. R. Nallapareddy, V. P. Prakash, X. Qin, M. Höök, G. M. Weinstock, and B. E. Murray. 2008. Identification and phenotypic characterization of a second collagen adhesin, Scm, and genome-based identification and analysis of 13 other predicted MSCRAMMs, including four distinct pilus loci, in Enterococcus faecium. Microbiology 154:3199-3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sonnenburg, J. L., L. T. Angenent, and J. I. Gordon. 2004. Getting a grip on things: how do communities of bacterial symbionts become established in our intestine? Nat. Immunol. 5:569-573. [DOI] [PubMed] [Google Scholar]
- 53.Talay, S. R. 2005. Gram-positive adhesins. Contrib. Microbiol. 12:90-113. [DOI] [PubMed] [Google Scholar]
- 54.Telford, J. L., M. A. Barocchi, I. Margarit, R. Rappuoli, and G. Grandi. 2006. Pili in Gram-positive pathogens. Nat. Rev. Microbiol. 4:509-519. [DOI] [PubMed] [Google Scholar]
- 55.Tuomola, E. M., and S. J. Salminen. 1998. Adhesion of some probiotic and dairy Lactobacillus strains to Caco-2 cell cultures. Int. J. Food Microbiol. 41:45-51. [DOI] [PubMed] [Google Scholar]
- 56.Tuomola, E. M., A. C. Ouwehand, and S. J. Salminen. 1999. The effect of probiotic bacteria on the adhesion of pathogens to human intestinal mucus. FEMS Immunol. Med. Microbiol. 26:137-142. [DOI] [PubMed] [Google Scholar]
- 57.Tuomola, E. M., A. C. Ouwehand, and S. J. Salminen. 2000. Chemical, physical and enzymatic pre-treatments of probiotic lactobacilli alter their adhesion to human intestinal mucus glycoproteins. Int. J. Food Microbiol. 60:75-81. [DOI] [PubMed] [Google Scholar]
- 58.Vesterlund, S., J. Paltta, M. Karp, and A. C. Ouwehand. 2005. Measurement of bacterial adhesion—in vitro evaluation of different methods. J. Microbiol. Methods 60:225-233. [DOI] [PubMed] [Google Scholar]
- 59.Walter, J. 2008. Ecological role of lactobacilli in the gastrointestinal tract: implications for fundamental and biomedical research. Appl. Environ. Microbiol. 74:4985-4996. [DOI] [PMC free article] [PubMed] [Google Scholar]