Abstract
It is well understood that protozoa play a major role in controlling bacterial biomass and regulating nutrient cycling in the environment. Little is known, however, about the movement of carbon from specific reduced substrates, through functional groups of bacteria, to particular clades of protozoa. In this study we first identified the active protozoan phylotypes present in activated sludge, via the construction of an rRNA-derived eukaryote clone library. Most of the sequences identified belonged to ciliates of the subclass Peritrichia and amoebae, confirming the dominance of surface-associated protozoa in the activated sludge environment. We then demonstrated that 13C-labeled protozoan RNA can be retrieved from activated sludge amended with 13C-labeled protozoa or 13C-labeled Escherichia coli cells by using an RNA stable isotope probing (RNA-SIP) approach. Finally, we used RNA-SIP to track carbon from bicarbonate and acetate into protozoa under ammonia-oxidizing and denitrifying conditions, respectively. RNA-SIP analysis revealed that the peritrich ciliate Epistylis galea dominated the acquisition of carbon from bacteria with access to CO2 under ammonia-oxidizing conditions, while there was no evidence of specific grazing on acetate consumers under denitrifying conditions.
Protozoa are the main consumers of bacteria in the environment, and as such they play a major role in controlling bacterial biomass (33) and regulating nutrient recycling (14). Therefore, being able to study the flow of carbon in food webs involving bacteria and protozoa can provide an insight into how protozoan grazing affects bacterial function in a wide range of systems.
Protozoan grazing has the potential to alter the genotypic and phenotypic composition of bacterial communities (15, 17, 18, 35). Perhaps as a result of this, protozoan grazing has also been found to have an effect on the function of activated sludge systems, including nitrogen removal processes. Studies using eukaryotic inhibitors to remove grazers from activated sludge have reported a wide range of effects following reduced grazing pressure. These effects include an increase in turbidity or planktonic cell densities, and either no effect (21, 32), higher nitrification rates (16, 20), or lower (29, 30) rates of nitrification in the absence of grazers. These conflicting reports on the effect of predation on nitrification might be due to protozoa displaying feeding preferences or to the indirect ways in which protozoan grazing can affect bacterial processes. For example, it has been shown that the release of substances, such as vitamins and nucleotides, secreted by protozoa as metabolic by-products, can act as growth factors, which enhance bacterial activity, including nitrification (31). In addition, the presence of some types of grazers, particularly ciliates, has been shown to be closely related to a decrease in biochemical oxygen demand (27), and it has been reported that ciliates can alter water flux and help redistribute nutrients in flocs (8), which in turn might have an impact on nitrification rates.
Although the data presented in the literature suggest that protozoan grazing is an important factor for activated sludge processes, there are still many questions as to whether its effects are directly linked to predation and possibly feeding preferences. In the present study we sought to identify protozoa assimilating carbon from autotrophic bacteria under ammonia-oxidizing conditions and acetate-consuming bacteria under denitrifying conditions using RNA-stable isotope probing (RNA-SIP).
Since it was first developed, RNA-SIP has been successfully used to identify functional groups of bacteria responsible for different processes, including denitrification and benzene and phenol degradation (9, 10, 19, 25, 26). In these studies, the flow of carbon was tracked from soluble labeled substrates into the bacteria consuming it. However, recent studies have shown that it is possible to use this technique to track the flow of carbon across more than one trophic level, unraveling some of the interactions observed in microbial food webs (11, 13, 23, 24). These studies exemplify the broad range of questions that can be addressed with SIP and have begun to define the boundaries beyond which SIP has limited utility.
MATERIALS AND METHODS
Clone library construction.
Activated sludge was collected from St. Marys Sewage Treatment Plant in Sydney, Australia, at the end of spring in November 2006. Sludge samples (0.5 ml) were collected and centrifuged for 10 min at 14,000 rpm and 4°C. RNA was extracted from pellets by using an RNeasy minikit (Qiagen Pty., Ltd., Australia) according to the manufacturer's instructions. An on-column DNase treatment was performed by using the Qiagen RNase-free DNase set (catalog no.79254). RNA was quantified by using a NanoDrop ND-1000 spectrophotometer (Biosciences Biolab, Australia) and reverse transcribed with the primer EK555sR (5′-GCTGCTGGCACCAGACT-3′), modified from EK555F (22), and the avian myeloblastosis virus reverse transcriptase (Promega Corp., Australia). The cDNA was used to amplify an ∼250-bp product from the 18S rRNA sequence from positions 300 to 555 using EK555sR and 300F (5′-AGGGTTCGATTCCGGAG-3′) (5) in a 100-μl PCR. The PCR contained 4 μl of cDNA, 10 μl of AmpliTaq 10× buffer (Applied Biosystems), 6 μl of a 25 mM MgCl2 solution (Applied Biosystems), 40 pmol each of the forward and reverse primers, 2 μl of a 10 mM mix of all four deoxynucleoside triphosphates, 0.4 μl of AmpliTaq DNA polymerase (Applied Biosystems, United Kingdom), and 5 μl of dimethyl sulfoxide and molecular-grade H2O (Eppendorf, Australia). The PCR conditions used were an initial denaturation at 95°C for 2 min; followed by 25 cycles of 95°C for 30 s, 61°C for 30 s, and 72°C for 30 s; and a final extension cycle of 10 min at 72°C. The products were purified by using a Qiagen PCR purification kit and cloned into pCR4-TOPO (Invitrogen, Australia). Positive clones were selected by using blue/white screening and checked for the insert by colony PCR using the M13F and M13R primer set. Plasmids were extracted from confirmed clones by using a PureLink HQ Mini-Plasmid purification kit (Invitrogen). Plasmid inserts were sequenced by using the M13F primer on an ABI3730 capillary sequencer (Applied Biosystems). Sequences were aligned by using Lasergene MegAlign software (version 6; DNAStar, Inc.). The phylogenetic affiliation of the sequences was obtained by using the basic local alignment search tool (BLAST) (2) function on the NCBI server (http://www.ncbi.nlm.nih.gov/blast). Clones from each phylogenetic hit were reamplified using the primers EK555R and GC300F for denaturing gradient gel electrophoresis (DGGE) analysis. Library coverage was calculated as described previously (12).
Bacterial and protozoan strains and culture conditions.
Escherichia coli strain BL-21 was grown overnight in 10% M9 minimal salts medium supplemented with 0.5% [13C]glucose (Isotec). The cells were harvested during stationary phase by a 10 min centrifugation step (14,000 rpm), where 100 ml of ∼107 cells/ml were concentrated into 1 ml to achieve a final concentration of ∼109 cells/ml. The ciliate, Tetrahymena rostrata, was routinely maintained in 10% M9 minimal salts medium without glucose and fed with heat-killed E. coli BL-21 that had been grown in 10% M9 minimal media with 13C-labeled glucose as described above. Ciliates were enumerated by live counts in known volumes by using a light microscope.
Introduction of 13C-labeled bacteria and protozoa into activated sludge.
An initial batch experiment was set up where 13C-labeled T. rostrata cells were added to 10 ml of freshly collected activated sludge and mixed thoroughly to a final concentration of 104 cells/ml. RNA from the amended sludge, as well as control RNA from unamended sludge, was extracted by using an RNeasy minikit (Qiagen). The cells were harvested by centrifugation and resuspended in 100 μl of Tris-EDTA (TE) buffer by vortexing, followed immediately by the addition of the RLT buffer plus β-mercaptoethanol as per the manufacturer's instructions. The RNA was quantified by using a Nanodrop ND-1000 Spectrophotometer (Biosciences Biolab, Australia).
A second batch experiment was set up where 13C-labeled E. coli BL21 cells were added to 10 ml of freshly collected activated sludge to a final concentration of 108 cells/ml in a 50-ml Falcon tube. The activated sludge was then incubated for 16 h at room temperature with shaking at 55 rpm. RNA from the amended sludge as well as control RNA from activated sludge without the addition of 13C-labeled E. coli, was extracted as described above.
[13C]bicarbonate pulse.
Activated sludge was collected from St. Marys Sewage Treatment Plant in Sydney, Australia, at the end of spring in November 2006. Four 200-ml subsamples were incubated aerobically in 1-liter Erlenmeyer flasks at room temperature and 150 rpm in the dark. Two samples were treated with ammonium sulfate (4 mM), while the other two acted as ammonium free controls. Control and treatment reactors received an unlabeled or 13C-labeled pulse of sodium bicarbonate (Sigma-Aldrich Pty., Ltd, Australia) to a final concentration of 2 mM. Pulses were added to sludge samples after 0, 2, and 4 h. To quantify CO2, 4-ml sludge samples were taken every 2 h for 10 h. Each sample was filtered through a 0.2-μm-pore-size membrane (Millipore), and the pH was adjusted to 10 and stored at 4°C until processing. The bicarbonate concentration in the sludge was determined by gas chromatography of headspace samples after acidification as previously described (1, 3). Activated sludge samples (0.5 ml) were collected after 0, 6, and 10 h and then centrifuged for 10 min at 25,000 × g and 4°C, and the RNA was extracted as described above.
[13C]acetate pulse.
Activated sludge was collected from St. Marys Sewage Treatment Plant in Sydney, Australia, at the end of spring in November 2006. Four 200-ml serum bottles were filled with the anoxic sludge and sealed with gray butyl stoppers and open-seal aluminum crimp caps, and the headspaces were flushed with N2 gas for 2 min. All four bottles were pretreated for 1 h with 500 μM [12C]sodium acetate, thereby enabling nitrate-reducing bacteria to consume any nitrate present in the sludge samples. After the pretreatment, two of the 200-ml samples were treated with [12C]sodium acetate, and two were treated with [13C]sodium acetate (Sigma-Aldrich Pty., Ltd, Australia) added to a final concentration of 1 mM (28), with or without sodium nitrate as an electron acceptor added to a final concentration of 5 mM. Pulses were repeatedly added to all four reactors every 30 min over a period of 2.5 h. In all, each reactor received six pulses of [12C]sodium acetate or [13C]sodium acetate with a cumulative concentration of 6 mM. Treatment reactors received five pulses of sodium nitrate with a cumulative concentration of 25 mM. Activated sludge samples (0.5 ml) were collected after 0, 3, and 8 h and then centrifuged for 10 min at 25,000 × g and 4°C, and the RNA was extracted as described above.
To quantify acetate concentration, 1.5-ml samples were collected and filtered as described above, and 900 μl of the filtrate was transferred to a gas chromatography sample vial. The samples were acidified with the addition of 100 μl of 10% (vol/vol) formic acid, and the vials were sealed and crimped. Samples were stored at 4°C until processing. Acetate concentration in the activated sludge samples was determined by using a Hewlett-Packard 5890 Series II gas chromatography/mass spectrometer (GC/MS) with an Agilent 6890 Series Injector at the Bioanalytical Mass Spectrometry Facility at the University of New South Wales, Sydney, Australia. The acidified samples were separated by using an 18-m Phenomenex ZB-FFAP column (0.25 mm, inner diameter), with helium as the carrier gas. Subsamples (10 μl) were injected into a prepacked deactivated glass wool liner with a split injection mode (split ratio of 50). The run conditions were as follows: injector temperature, 250°C; and oven temperature, 120°C ramped up at 10°C/min to 140°C and then ramped up in 40°C increments to 240°C. A 2-min solvent delay and single ion monitoring detection modes were used.
13C atom percentage quantification in activated sludge biomass.
Activated sludge samples (0.5 ml) collected in parallel with the samples used for RNA SIP analysis, were centrifuged for 10 min at 25,000 × g and 4°C, washed twice using chilled phosphate buffer solution, dried for 24 h in a drying oven, and analyzed for 13C atom% by continuous flow isotope ratio mass spectrometry (IRMS) using a Europa Roboprep CN elemental analyzer (EA) attached to a Finnigan MAT Conflo III and a 252 mass spectrometer at Environmental Isotopes Pty Ltd, North Ryde, Australia.
RNA-SIP analysis.
Extracted RNA (1 μg) was subject to equilibrium (isopycnic) CsTFA density gradient centrifugation as previously described (26). Gradients were harvested into 20 fractions from the bottom of the gradient (starting with fraction 1) by displacing the gradient media from the top using water and a syringe pump, and RNA was recovered from fractions by precipitation with isopropanol as described previously (26). Pellets were resuspended in 20 μl of H2O immediately before eight fractions (4 to 11) encompassing the expected distribution of RNA in the gradients were subjected to reverse transcription-PCR (RT-PCR). Fractions 4 to 11 corresponded to buoyant densities from 1.77 to 1.83 g/ml as determined by mass on an analytical balance. RNA recovered from the fractions was reverse transcribed as described above. The cDNA was used to amplify a ∼250-bp product from the 18S rRNA sequence from positions 300 to 555 using EK555sR and GC300F, which includes a 39-bp guanine and cytosine (GC) clamp on the 5′ end of the selected region (5′-CGCCCGCCGCGCCCCCGCCCCGGCCCGCCGCCCCCGCCCAGGGTTCGATTCCGGAG-3′) (5). The composition of the PCR was as described above for the clone library construction. The PCR conditions used were an initial denaturation at 95°C for 2 min, followed by 25 cycles of 95°C for 30 s, 61°C for 30 s, and 72°C for 30 s, and a final extension cycle of 10 min at 72°C.
The GC-clamped PCR products were separated by using DGGE on 10% (wt/vol) polyacrylamide gels with a 30 to 50% urea-formamide denaturing gradient using the DGGE 2401 system (C.B.S. Scientific Co.) as previously described (26). The gels were run at 60°C, 55 V, and 300 A for 17 h; stained with SYBR gold nucleic acid gel stain (Invitrogen, Australia) for 15 min; and visualized by using a Bio-Rad Gel Doc imaging system (Bio-Rad Laboratories Pty., Ltd., Australia). The gels were evaluated manually to determine the presence or absence of bands in each fraction. Bands of interest were excised by using a 1-ml pipette tip and resuspended in 20 μl of H2O. One microliter of this was used to reamplify the bands before running them in another DGGE gel to assess purity.
Sequencing of DGGE bands.
Once the purity of the excised bands was established, they were analyzed by reamplifying for sequencing using the primers EK555sR and the non-GC-clamped 300F (5). The PCR products were cleaned by using the QIAquick PCR purification kit (Qiagen) and quantified by using a Beckman U.V. spectrophotometer DU 640 (Beckman). PCR products were sequenced by using an ABI3730 capillary sequencer (Applied Biosystems). Contigs and alignments of the recovered sequences were assembled by using DNAStar, Lasergene, SeqMan, and MegAlign software (version 6). The phylogenetic affiliation of the contigs was obtained by using BLAST (2) on the NCBI server.
Nucleotide sequence accession numbers.
Sequence data were deposited with GenBank under accession numbers GQ167353 to GQ167386 and GQ918140.
RESULTS
Composition of the protozoan community in activated sludge.
A clone library was constructed with RNA from activated sludge using primers targeting the 18S rRNA gene of protozoa. To the best of our knowledge, this is the first protozoan clone library to be constructed from activated sludge. Sequencing results from the clone library revealed a diverse community of active protozoa including four phylotypes affiliated with Ciliophora (ciliates) (representing 24.1% of the total clones) and five different phylotypes of Amoebozoa (amoeba) (representing 58.6% of the total clones) (Table 1). These two dominant groups found in the library are composed of organisms that live attached to surfaces. There were also two clones found to belong to the order Cercomonadida, two to the family Eimeriidae, and one match belonging to an uncultured eukaryote clone. One sequence (Arcella hemisphaerica) dominated the clone library, representing ca. 37.9% of the sequenced clones. Clone library coverage was calculated at 62% after screening 29 clones, indicating that more than half of the diversity of active protozoa present in activated sludge was represented in the clone library.
TABLE 1.
Sequencing best-match BLAST results for bands cut from DGGE gels and clones retrieved from library
| BLAST best match | % Similarity | No. of clones | Classification | Accession no. |
|---|---|---|---|---|
| Bands | ||||
| Tetrahymena sp. strain NI SSU rRNA gene, partial sequence (band of interest in Fig. 1) | 98 | NAa | Ciliophora, Hymenostomatida | GQ918140 |
| Epicarchesium abrae SSU rRNA gene, complete sequence (band 1, Fig. 2) | 97 | NA | Ciliophora, Peritrichia | GQ167353 |
| Spumella sp. strain 9-1-D1 SSU rRNA gene, partial sequence (band 2, Fig. 2) | 98 | NA | Heterokontophyta, Ochromonadales | GQ167354 |
| Zoothamnium duplicatum SSU rRNA gene, partial sequence (band 3, Fig. 2) | 96 | NA | Ciliophora, Peritrichia | GQ167355 |
| Hartmannella sp. strain 2 4/3Da/10 SSU rRNA gene, partial sequence (band 4, Fig. 2) | 92 | NA | Amoebozoa, Lobosea | GQ167356 |
| Epistylis galea SSU rRNA gene, complete sequence (band of interest in Fig. 5) | 95 | NA | Ciliophora, Peritrichia | GQ167357 |
| Clones | ||||
| Arcella hemisphaerica SSU rRNA gene, partial sequence | 93-99 | 11 | Amoebozoa, Lobosea | GQ167358-60, GQ167362, GQ167365-66, GQ167370-71, GQ167376-78 |
| Zoothamnium duplicatum SSU rRNA gene, partial sequence | 98-99 | 4 | Ciliophora, Peritrichia | GQ167364, GQ167374, GQ167384, GQ167386 |
| Chaos nobile CCAP 1511/2 SSU rRNA, partial sequence | 92 | 3 | Amoebozoa, Lobosea | GQ167363, GQ167372, GQ167375 |
| Cercomonadida environmental sample clone Elev_18S_776 SSU rRNA, partial sequence | 96 | 2 | Cercozoa, Cercomonadida | GQ167381, GQ167383 |
| Eimeriidae environmental sample clone Elev_18S_1405 SSU rRNA gene, partial sequence | 94-99 | 2 | Apicomplexa, Coccidia | GQ167373, GQ167382 |
| Epistylis urceolata SSU rRNA gene, complete sequence | 98 | 2 | Ciliophora, Peritrichia | GQ167369, GQ167385 |
| Euglypha acanthophora strain Millstream SSU rRNA gene, partial sequence | 100 | 1 | Amoebozoa, Lobosea | GQ167379 |
| Balamuthia mandrillaris isolate V451 SSU rRNA gene, partial sequence | 93 | 1 | Amoebozoa, Lobosea | GQ167367 |
| Stenamoeba sp. strain CRIB68 SSU rRNA gene, partial sequence | 94 | 1 | Amoebozoa, Thecamoebidae | GQ167380 |
| Acineta sp. strain OSW-2003-3 SSU rRNA gene, partial sequence | 90 | 1 | Ciliophora, Phyllopharyngea | GQ167368 |
| Uncultured eukaryote clone D4P08E02 SSU rRNA gene, partial sequence | 94 | 1 | NA | GQ167361 |
NA, not applicable.
Tracking carbon flow in food webs involving bacteria and protozoa.
Initially, we tested whether 13C-labeled protozoan RNA can be detected in activated sludge from within a pool of predominantly bacterial 16S rRNA. To do this, we added 104 cells/ml of 13C-labeled Tetrahymena rostrata and successfully recovered and matched the 13C-labeled RNA after density gradient centrifugation. Figure 1 shows the band corresponding to T. rostrata in gradient fractions heavier than those containing the rest of the protozoan community (Fig. 1B). This band did not appear in the gradient loaded with RNA that had not been amended with labeled T. rostrata (Fig. 1A). This demonstrates that labeled 18S rRNA sequences can be retrieved from within a pool of predominantly 16S rRNA for cell densities above 104 cells/ml.
FIG. 1.
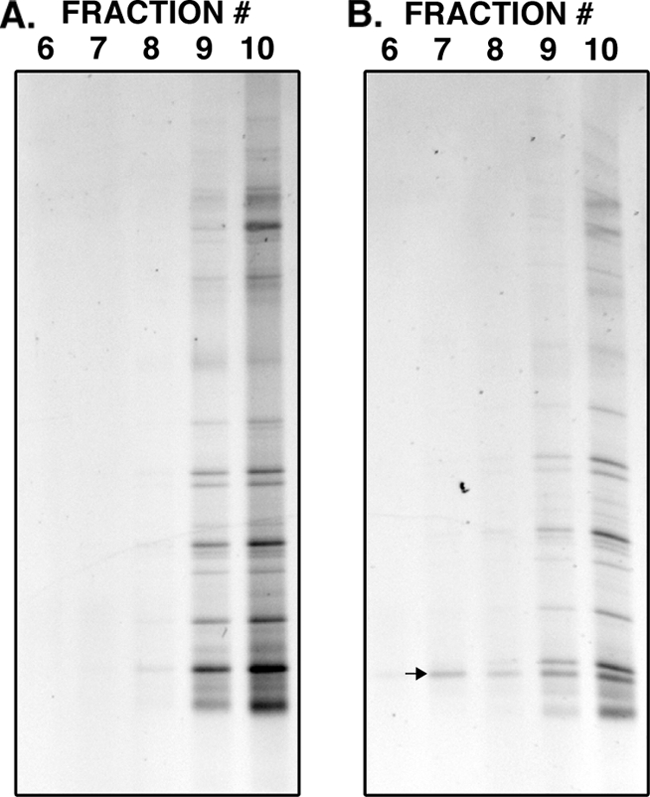
DGGE profiles generated from gradient fractions (6 to 10) derived from RNA from activated sludge without amendment with 13C-labeled Tetrahymena rostrata (A) and with amendment with 13C-labeled T. rostrata (B). Fraction 6 has the highest buoyant density, while fraction 10 has the lowest. One template (see arrow) was only found in the treatment amended in with 13C-labeled T. rostrata, and was present through the gradient, including in the fractions with higher buoyant density. Sequencing confirmed this band as belonging to the ciliate Tetrahymena sp. (Ciliophora, Hymenostomatida, 98%).
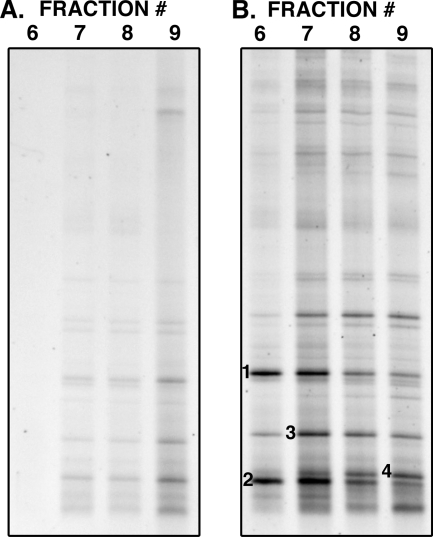
To confirm that 13C-labeled protozoan RNA can be retrieved from activated sludge amended with labeled bacterial cells, E. coli BL21 grown on [13C6]glucose was added to activated sludge to a final concentration of 108 cells/ml and incubated for 16 h at room temperature. RNA was then extracted and subject to density centrifugation. Figure 2 shows the protozoan community fingerprints of density gradient fractions derived from activated sludge with or without amendment with 13C-labeled E. coli. In the fractions derived from unamended sludge (Fig. 2A), all bands were most intense in the lower density fractions (8 and 9) with weaker profiles observed in the higher density fractions (6 and 7). The protozoan community profile after incubation with 13C-labeled E. coli (Fig. 2B) showed that the templates from which all bands in the profile were derived had increased in density with three bands showing outstanding movement into higher density fractions (bands 1 to 3). These data suggest that while three protozoan phylotypes dominated the acquisition of carbon from E. coli, all members of the protozoan community had access to the labeled carbon. This confirms that 13C-labeled protozoan RNA can be retrieved from activated sludge amended with labeled bacterial cells.
FIG. 2.
DGGE profiles generated from gradient fractions (6-9) derived from RNA from activated sludge without amendment with 13C-labeled E. coli (A) and with amendment with 13C-labeled E. coli (B). Fractions 6 have the highest buoyant density, while fractions 9 have the lowest. Although the entire protozoan community appears to have acquired carbon from E. coli, the bands annotated 1 (Epicarchesium abrae, Ciliophora, Peritrichia, 97%), 2 (Spumella sp., Heterokontophyta, Ochromonadales, 98%), and 3 (Zoothamnium duplicatum, Ciliophora, Peritrichia, 96%) represent protozoa that have dominated the acquisition of carbon from E. coli. Band 4 (Hartmannella sp., Amoebozoa, Lobosea, 92%) represents an abundant community member that was not labeled.
The bands annotated 1 to 4 in Fig. 2 were cut out, reamplified, checked for purity and sequenced. Bands 1, 2, and 3, which demonstrated clear increases in buoyant density, had the closest match to an uncultured ciliate clone closely related to Epicarchesium abrae (97%, Ciliophora, Peritrichia) (band 1), a Spumella sp. heterotrophic nanoflagellate from the order Chrysomonadida (98%) (band 2), and the peritrich Zoothamnium duplicatum (96%, Ciliophora, Peritrichia) (band 3). We conclude that these organisms, which are all known to be strict suspension feeders, consumed more of the planktonic 13C-labeled E. coli cells than other members of the protozoan community. Band 4, representing an abundant community member that was not labeled with 13C more than the rest of the community, was identified as belonging to an amoebae Hartmannella sp. (92%, Amoebozoa, Lobosea), known to be a surface feeder.
CO2 consumption in the presence or absence of ammonium.
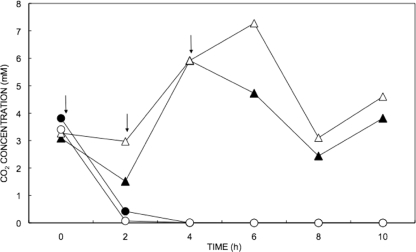
To determine whether specific protozoan phylotypes dominate the acquisition of carbon from CO2-assimilating microbes in ammonia-oxidizing activated sludge, 200-ml sludge samples were pulsed three times with 2 mM unlabeled or 13C-labeled sodium bicarbonate in the presence or absence of 4 mM ammonium sulfate. The concentration of CO2 in these four treatments over 10 h is shown in Fig. 3.
FIG. 3.
CO2 concentration in activated sludge in the presence (circles) or absence (triangles) of ammonium sulfate over 10 h. Closed symbols show the CO2 concentration in samples pulsed with [12C]bicarbonate, while the open symbols show the CO2 concentration in the treatments with [13C]bicarbonate. Arrows show the three time points at which the labeled pulses were added. The data were gathered from a single reactor per treatment.
All four treatments had an initial CO2 concentration between 3 and 4 mM, before the addition of the first pulse. Five hours after the initial pulse the samples treated with ammonium sulfate had consumed both the 0.7 to 0.8 mmol of CO2 present initially and the 1.2 mmol of CO2 added. In samples not treated with ammonium sulfate, only 1 mmol of the total CO2 present had been consumed after 10 h. The presence of ammonium sulfate therefore increased the rate of CO2 consumption by ∼4-fold.
Acetate consumption in the presence or absence of nitrate.
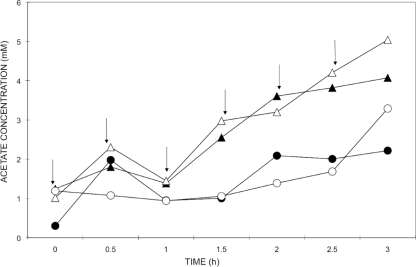
To determine whether specific protozoan phylotypes dominate the acquisition of carbon from acetate assimilating microbes in nitrate reducing activated sludge, 200-ml sludge samples were pulsed six times with 1 mM unlabeled or 13C-labeled sodium acetate in the presence or absence of 5 mM sodium nitrate. The concentration of acetate in these four treatments over 3 h is shown in Fig. 4.
FIG. 4.
Acetate concentration in activated sludge in the presence (circles) or absence (triangles) of sodium nitrate over 3 h. Closed symbols show the acetate concentration in samples pulsed with [12C]acetate, while the open symbols show the acetate concentration in the treatments with [13C]acetate. Arrows show the six time points at which the labeled pulses were added. The data were gathered from a single reactor per treatment.
The concentration of acetate found in the treatments with or without the addition of nitrate as an electron acceptor, increased in both cases as the pulses were added to the sludge, indicating that acetate was added at a faster rate than it was being consumed and was not limiting. After 1 h both curves showed a decrease in acetate, before a continued increase. Between 1 and 3 h, when the other four pulses were added, the treatment without nitrate accumulated acetate at a higher rate than the treatment with nitrate, to reach a final acetate concentration of 4.55 mM out of the total of 6 mM, which had been added to the sludge. In contrast, the treatment with nitrate reached a maximum acetate concentration of 2.75 mM. In the absence of nitrate 24% of the acetate was consumed, compared to 54% in the presence of nitrate, representing a difference between treatments of 0.36 mmol of acetate consumed.
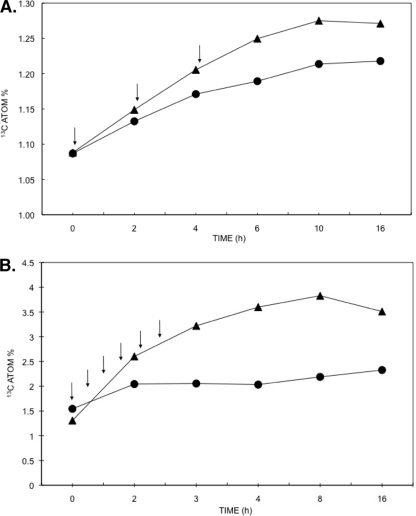
13C incorporation into activated sludge biomass.
The 13C atom% of activated sludge biomass was determined by isotope ratio mass spectrometry in order to confirm the incorporation of 13C into the activated sludge biomass (Fig. 5). In all ammonia oxidation treatments (Fig. 5A), the initial 13C atom% was 1.08%, which is consistent with the natural abundance of this stable isotope in biological systems (34). In both treatments where the [13C]bicarbonate was pulsed into the system, the 13C atom% value increased. However, the level of 13C enrichment was higher in the presence of ammonium sulfate, a finding consistent with the increase in CO2 consumption, suggesting that the presence of ammonium sulfate increased the incorporation of 13C into sludge biomass. Increases in 13C atom% ceased 4 h after the third and final [13C]bicarbonate pulse.
FIG. 5.
13C atom% of activated sludge biomass pulsed with [13C]bicarbonate in the presence (triangles) or absence (circles) of ammonium sulfate (A) and with [13C]acetate in the presence (triangles) or absence (circles) of sodium nitrate (B). Arrows show the time points at which the pulses were added to each treatment. The data were gathered from a single reactor per treatment.
In the nitrate reduction treatments (Fig. 5B) the initial 13C atom% values were 1.31 and 1.54% for the treatments with and without nitrate, respectively. This increase in 13C compared to the natural abundance was consistent with the fact that the first biomass sample was taken after the addition of the first pulse. In both treatments with or without sodium nitrate 13C atom% increased. However, at its peak (8 h) the level of 13C enrichment was 1.7 times higher in the presence of the electron acceptor, suggesting that the presence of sodium nitrate increased the incorporation of 13C into sludge biomass. Increases in 13C atom% in the treatment without nitrate leveled off after the 2-h time point, which coincided with the second last addition of the pulse. In contrast in the treatment with nitrate, the 13C atom% continued to increase steadily peaking after 8 h of incubation at 3.83%. For both experiments, the 13C atom% in the two treatments pulsed with [12C]bicarbonate or [12C]acetate remained at 1.08% after 16 h (data not shown), confirming that the enrichment observed in the other two treatments was due to incorporation of the 13C in the labeled substrates.
Protozoan grazing on CO2-assimilating microbes during ammonia oxidation.
To identify protozoa acquiring carbon from CO2 assimilating microbes during ammonia oxidation, activated sludge samples from [12C]- and [13C]bicarbonate fed treatments were analyzed by using RNA-SIP and DGGE. Community fingerprints were generated from 18S rRNA templates in gradient fractions with buoyant densities ranging from 1.77 to 1.83 g/ml (fractions 4 to 11), from six gradients derived from the 12C or 13C pulse after 0, 6, and 10 h. This analysis revealed that only one 18S rRNA template increased in relative abundance between 0 and 6 h of the 13C-labeled pulse, suggesting that it had increased in buoyant density. To illustrate the finding, the results are summarized in Fig. 6, which shows the fingerprints from a gradient fraction of moderate density (1.8 g/ml) from each of the six different gradients. It can be seen that only one band was observed to increase in intensity in this high density fraction between 0 and 6 h of the 13C-labeled pulse. This was not observed in the same fractions from the gradients derived from the 12C pulse confirming that this increase in band intensity in this fraction was not associated with an increase in the abundance of the template throughout the gradient. BLAST results from sequences of bands cut from the gel revealed that the closest match for the SSU rRNA sequence that increased in density was the suspension-feeding ciliate Epistylis galea (Ciliophora, Peritrichia) with 95% identity, suggesting high consumption rates on planktonic CO2-assimilating microbes in ammonia-oxidizing activated sludge.
FIG. 6.
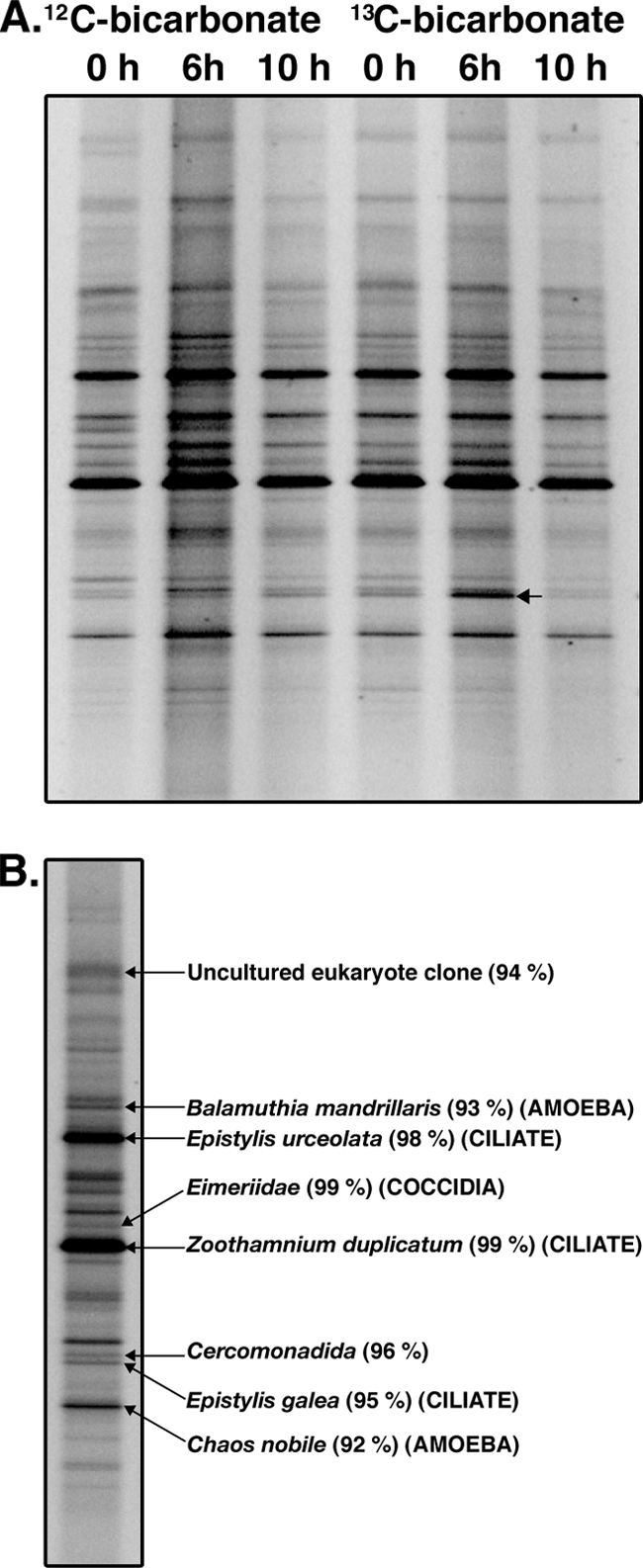
(A) Protozoan DGGE profiles of high density (>1.8 g/ml) fractions from [12C]bicarbonate and [13C]bicarbonate treatments in the presence of ammonium sulfate over time. One template (see arrow) displayed an increase in density 6 h after addition of the initial [13C]bicarbonate pulse. The band was derived from an 18S rRNA sequence similar to Epistylis galea (Ciliophora, Peritrichia, 95%). (B) DGGE profile of untreated sludge showing the identity of a selection of community members.
Protozoan grazing on acetate-assimilating microbes during nitrate reduction.
Protozoan community profiles were generated using 18S rRNA and analyzed by DGGE. No specific labeling was observed throughout the duration of the sampling regime. A summary gel of the profiles for 0, 3, and 8 h, focusing on the fractions in the “heavy” (1.8 g/ml) part of the gradient, confirms that there were no changes in the relative intensities of any band, either between treatments or time points (Fig. 7).
FIG. 7.
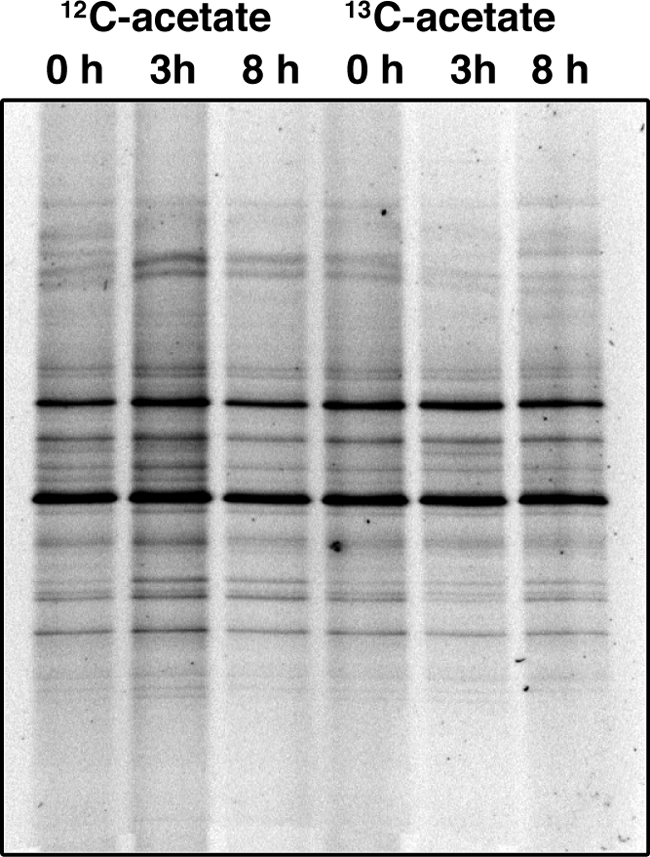
Protozoan DGGE profiles of high density (>1.8 g/ml) fractions from [12C]acetate and [13C]acetate treatments in the presence of sodium nitrate over time (0, 3, and 8 h). No changes in the relative intensities of any band were observed either between treatments or time points.
DISCUSSION
Very little is known about the movement of carbon from bacteria to protozoa in complex microbial communities such as activated sludge. Although it is clear that protozoa have feeding preferences based on a variety of factors, it is not known whether specific protozoan lineages graze on specific functional groups of bacteria. In the present study we assessed the utility of RNA-SIP in the identification of protozoa grazing on autotrophic bacteria under ammonia-oxidizing conditions and acetate consumers under denitrifying conditions in activated sludge.
We demonstrated that it is possible to retrieve labeled 18S rRNA sequences from activated sludge using RNA-SIP after direct addition of labeled protozoa (Fig. 1). Additionally, we showed that labeled 18S rRNA sequences could be retrieved from activated sludge after feeding with 13C-labeled bacterial cells (Fig. 2). This experiment revealed that peritrichs closely related to Epicarchesium abrae and Zoothamnium duplicatum and a Spumella sp. heterotrophic nanoflagellate from the order Chrysomonadida dominate the acquisition of carbon from planktonic E. coli cells in activated sludge. These results are consistent with a previous study, where it was shown that between 65 and 75% of the decrease of the E. coli population in activated sludge can be attributed to predation by ciliates (4). However, the present study also shows that other protozoa, such as flagellates, are capable of grazing on planktonic E. coli populations. The utility of this method for identifying grazers of bacterial populations was also recently shown in a marine system, where labeled picocyanobacteria were added to seawater, and their predators were identified using RNA-SIP (7).
Once the utility of RNA-SIP to track carbon from bacteria to protozoa in activated sludge was established, we showed that it is also possible to use this method to track carbon flow through more than one trophic level: from labeled substrate, to bacteria, to protozoa. The RNA-SIP analysis of activated sludge samples pulsed with 13C-labeled bicarbonate in the presence of ammonium sulfate, identified a single protozoan phylotype that increased in density after the pulse was consumed, the peritrich ciliate Epistylis galea (Fig. 6).
Members of this genus are known to be abundant in the protozoan community of wastewater treatment plants (8, 27). The subclass Peritrichia is composed of sessile stalked organisms that live attached to surfaces. In activated sludge, this is usually the surface of flocs. Like most peritrichs, E. galea feeds by filtering bacteria from the plankton. Recently, culture independent methods, showed that in nitrifying wastewater treatment plants, ammonia is oxidized to nitrite by bacteria belonging to a wide variety of different Betaproteobacteria, including to Nitrosomonas europaea, Nitrosomonas eutropha, Nitrosococcus mobilis, members of the Nitrosomonas marina cluster, and four phylogenetic lineages with no cultured representatives (36). E. galea is likely to have become labeled in our experiments through predation on members of these genera in the planktonic phase.
Given that aggregated biomass, rather than its planktonic counterpart, is mostly responsible for the processing of carbon, nitrogen and phosphorous in wastewater treatment, we speculate that E. galea fed from planktonic cells generated from the growth and subsequent dispersal of autotrophs from flocs. Besides E. galea, no other protozoan phylotype was labeled sufficiently to generate the necessary increase in buoyant density required for reliable identification by stable isotope probing. This may indicate that E. galea dominates the acquisition of carbon from autotrophs in activated sludge or that other predators consuming autotrophs have a more varied diet (i.e., less prey selectivity), consuming enough unlabeled carbon to evade detection by RNA-based SIP.
In addition to the [13C]bicarbonate pulse, we conducted a 13C-labeled acetate pulse under nitrate reducing conditions. While [13C]acetate and nitrate were consumed and the 13C atom% of the biomass increased more in the presence of nitrate than in its absence, we could not detect increases in the density of the 18S rRNA of any particular protozoa. This suggests that no protozoan phylotype was labeled sufficiently to generate the necessary increase in buoyant density required for identification by stable isotope probing. This may be a consequence of a reduction in protozoan grazing activity in activated sludge under anoxic conditions (6) or may reflect dilution of 13C throughout the community.
In summary, analysis of a clone library of the protozoan community in activated sludge revealed that most of the identified sequences belonged to the ciliates of the subclass Peritrichia and to amoebae. These results confirm the dominance of surface associated protozoa in the activated sludge environment. In accordance with this, RNA-SIP identified the peritrich E. galea as a consumer of CO2-assimilating microbes in activated sludge. RNA-SIP was unable to identify any other protozoa involved in grazing on CO2-assimilating microbes under ammonia-oxidizing conditions or acetate-consuming bacteria under denitrifying conditions, suggesting that protozoa do not feed specifically on these functional groups.
This apparent dilution of 13C throughout the activated sludge community, with many species possibly receiving insufficient enrichment for identification in density gradients, represents a limitation to nucleic acid-based stable isotope probing methods in the examination of carbon flow across trophic levels in this ecosystem. Although this may not be the case for substrates other than CO2 or acetate, the approach shows greatest potential in the identification of keystone predators of labeled cultivable bacteria in activated sludge.
Acknowledgments
A.M.M. was supported by a scholarship from the Environmental Biotechnology Cooperative Research Centre. M.M. was supported by the Environmental Biotechnology Cooperative Research Centre and Orica Australia Pty, Ltd. This study was funded by the Centre for Marine Bio-innovation and the Environmental Biotechnology Cooperative Research Centre.
Footnotes
Published ahead of print on 5 February 2010.
REFERENCES
- 1.Adamczyk, J., M. Hesselsoe, N. Iversen, M. Horn, A. Lehner, P. H. Nielsen, M. Schloter, P. Roslev, and M. Wagner. 2003. The isotope array, a new tool that employs substrate-mediated labeling of rRNA for determination of microbial community structure and function. Appl. Environ. Microbiol. 69:6875-6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 3.Chai, X. S., Q. Luo, and J. Y. Zhu. 2001. Analysis of nonvolatile species in a complex matrix by headspace gas chromatography. J. Chromatogr. A 909:249-257. [DOI] [PubMed] [Google Scholar]
- 4.Curds, C. R., and G. J. Fey. 1969. The effect of ciliated protozoa on the fate of Escherichia coli in the activated-sludge process. Water Res. 3:853-867. [Google Scholar]
- 5.Elwood, H. J., G. J. Olsen, and M. L. Sogin. 1985. The small-subunit rRNA gene sequences from the hypotrichous ciliates Oxytricha nova and Stylonychia pustulata. Mol. Biol. Evol. 2:399-410. [DOI] [PubMed] [Google Scholar]
- 6.Fenchel, T., and B. J. Finlay. 1990. Anaerobic free-living protozoa: growth efficiencies and the structure of anaerobic communities. FEMS Microbiol. Ecol. 74:269-275. [Google Scholar]
- 7.Frias-Lopez, J., A. Thompson, J. Waldbauer, and S. W. Chisholm. 2009. Use of stable isotope-labeled cells to identify active grazers of picocyanobacteria in ocean surface waters. Environ. Microbiol. 11:512-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fried, J., and H. Lemmer. 2003. On the dynamics and function of ciliates in sequencing batch biofilm reactors. Water Sci. Technol. 47:189-196. [PubMed] [Google Scholar]
- 9.Ginige, M. P., P. Hugenholtz, H. Daims, M. Wagner, J. Keller, and L. L. Blackall. 2004. Use of stable-isotope probing, full-cycle rRNA analysis, and fluorescence in situ hybridization-microautoradiography to study a methanol-fed denitrifying microbial community. Appl. Environ. Microbiol. 70:588-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ginige, M. P., J. Keller, and L. L. Blackall. 2005. Invest. of an acetate-fed denitrifying microbial community by stable isotope probing, full-cycle rRNA analysis, and fluorescent in situ hybridization-microautoradiography. Appl. Environ. Microbiol. 71:8683-8691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glaubitz, S., T. Lueders, W. R. Abraham, G. Jost, K. Jurgens, and M. Labrenz. 2009. 13C-isotope analyses reveal that chemolithoautotrophic gamma- and epsilonproteobacteria feed a microbial food web in a pelagic redoxcline of the central Baltic Sea. Environ. Microbiol. 11:326-337. [DOI] [PubMed] [Google Scholar]
- 12.Good, I. J. 1953. The population frequencies of species and the estimation of the population parameters. Biometrika 40:237-264. [Google Scholar]
- 13.Griffiths, R. I., M. Manefield, N. Ostle, N. McNamara, A. G. O'Donnell, M. J. Bailey, and A. S. Whiteley. 2004. 13CO2 pulse-labeling of plants in tandem with stable isotope probing: methodological considerations for examining microbial function in the rhizosphere. J. Microbiol. Methods 58:119-129. [DOI] [PubMed] [Google Scholar]
- 14.Güde, H. 1985. Influence of phagotrophic processes on the regeneration of nutrients in two stage continuous culture systems. Microb. Ecol. 11:193-204. [DOI] [PubMed] [Google Scholar]
- 15.Hahn, M. W., and M. G. Hofle. 1998. grazing pressure by a bacterivorous flagellate reverses the relative abundance of Comamonas acidovorans PX54 and Vibrio strain CB5 in chemostat cocultures. Appl. Environ. Microbiol. 64:1910-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeppsson, U., N. M. Lee, and H. Aspergen. 1995. Modelling microfauna influence on nitrification in aerobic biofilm processes. Int. IAWQ Conf. Workshop Biofilm Struct. Growth Dynamics 1995:77-85. [Google Scholar]
- 17.Jezbera, J., K. Hornak, and K. Simek. 2005. Food selection by bacterivorous protists: insight from the analysis of the food vacuole content by means of fluorescence in situ hybridization. FEMS Microbiol. Ecol. 52:351-363. [DOI] [PubMed] [Google Scholar]
- 18.Jürgens, K., and C. Matz. 2002. Predation as a shaping force for the phenotypic and genotypic composition of planktonic bacteria. Antonie Van Leeuwenhoek 81:413-434. [DOI] [PubMed] [Google Scholar]
- 19.Kasai, Y., Y. Takahata, M. Manefield, and K. Watanabe. 2006. RNA-based stable isotope probing and isolation of anaerobic benzene-degrading bacteria from gasoline-contaminated groundwater. Appl. Environ. Microbiol. 72:3586-3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee, N. M., and T. Welander. 1994. Influence of predators on nitrification in aerobic biofilm processes. Water Sci. Technol. 29:355-363. [Google Scholar]
- 21.Lee, Y., and J. A. Oleszkiewicz. 2003. Effects of predation and ORP conditions on the performance of nitrifiers in activated sludge systems. Water Res. 37:4202-4210. [DOI] [PubMed] [Google Scholar]
- 22.Lopez-Garcia, P., F. Rodriguez-Valera, C. Pedros-Alio, and D. Moreira. 2001. Unexpected diversity of small eukaryotes in deep-sea Antarctic plankton. Nature 409:603-607. [DOI] [PubMed] [Google Scholar]
- 23.Lueders, T., R. Kindler, A. Miltner, M. W. Friedrich, and M. Kaestner. 2006. Identification of bacterial micropredators distinctively active in a soil microbial food web. Appl. Environ. Microbiol. 72:5342-5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lueders, T., B. Wagner, P. Claus, and M. W. Friedrich. 2004. Stable isotope probing of rRNA and DNA reveals a dynamic methylotroph community and trophic interactions with fungi and protozoa in oxic rice field soil. Environ. Microbiol. 6:60-72. [DOI] [PubMed] [Google Scholar]
- 25.Manefield, M., R. Griffiths, N. P. McNamara, D. Sleep, N. Ostle, and A. Whiteley. 2007. Insights into the fate of a 13C-labeled phenol pulse for stable isotope probing (SIP) experiments. J. Microbiol. Methods 69:340-344. [DOI] [PubMed] [Google Scholar]
- 26.Manefield, M., A. S. Whiteley, R. I. Griffiths, and M. J. Bailey. 2002. RNA stable isotope probing, a novel means of linking microbial community function to phylogeny. Appl. Environ. Microbiol. 68:5367-5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin-Cereceda, M., S. Serrano, and A. Guinea. 2001. Biofilm communities and operational monitoring of a rotating biological contactor system. SO Water Air Soil Pollut. 126:193-206. [Google Scholar]
- 28.Nielsen, J. L., and P. H. Nielsen. 2002. Enumeration of acetate-consuming bacteria by microautoradiography under oxygen and nitrate respiring conditions in activated sludge. Water Res. 36:421-428. [DOI] [PubMed] [Google Scholar]
- 29.Petropoulos, P., and K. A. Gilbride. 2005. Nitrification in activated sludge batch reactors is linked to protozoan grazing of the bacterial population. Can. J. Microbiol. 51:791-799. [DOI] [PubMed] [Google Scholar]
- 30.Pogue, A. J., and K. A. Gilbride. 2007. Impact of protozoan grazing on nitrification and the ammonia- and nitrite-oxidizing bacterial communities in activated sludge. Can. J. Microbiol. 53:559-571. [DOI] [PubMed] [Google Scholar]
- 31.Ratsak, C. H., K. A. Maarsen, and S. A. L. M. Kooijman. 1996. Effects of protozoa on carbon mineralization in activated sludge. Water Res. 30:10-12. [Google Scholar]
- 32.Rensink, J. H., and W. H. Rulkens. 1997. Using metazoa to reduce sludge production. Water Sci. Technol. 36:171-179. [Google Scholar]
- 33.Sherr, E. B., and B. F. Sherr. 2002. Significance of predation by protists in aquatic microbial food webs. Antonie Van Leeuwenhoek 81:293-308. [DOI] [PubMed] [Google Scholar]
- 34.Smith, B. N. 1972. Natural abundance of the stable isotopes of carbon in biological systems. Bioscience 22:226-231. [Google Scholar]
- 35.Vazquez-Dominguez, E., E. O. Casamayor, P. Catala, and P. Lebaron. 2005. Different marine heterotrophic nanoflagellates affect differentially the composition of enriched bacterial communities. Microb. Ecol. 49:474-485. [DOI] [PubMed] [Google Scholar]
- 36.Wagner, M., and A. Loy. 2002. Bacterial community composition and function in sewage treatment systems. Curr. Opin. Biotechnol. 13:218-227. [DOI] [PubMed] [Google Scholar]