Abstract
Psychrobacter arcticus strain 273-4, which grows at temperatures as low as −10°C, is the first cold-adapted bacterium from a terrestrial environment whose genome was sequenced. Analysis of the 2.65-Mb genome suggested that some of the strategies employed by P. arcticus 273-4 for survival under cold and stress conditions are changes in membrane composition, synthesis of cold shock proteins, and the use of acetate as an energy source. Comparative genome analysis indicated that in a significant portion of the P. arcticus proteome there is reduced use of the acidic amino acids and proline and arginine, which is consistent with increased protein flexibility at low temperatures. Differential amino acid usage occurred in all gene categories, but it was more common in gene categories essential for cell growth and reproduction, suggesting that P. arcticus evolved to grow at low temperatures. Amino acid adaptations and the gene content likely evolved in response to the long-term freezing temperatures (−10°C to −12°C) of the Kolyma (Siberia) permafrost soil from which this strain was isolated. Intracellular water likely does not freeze at these in situ temperatures, which allows P. arcticus to live at subzero temperatures.
Temperature is one of the most important parameters that determine the distribution and extent of life on earth, and it does this by affecting cell structure and function. High temperatures break covalent bonds and ionic interactions between molecules, inactivating proteins and disrupting cell structures. Low temperatures reduce biochemical reaction rates and substrate transport and induce the formation of ice that damages cell structures. Not surprisingly, an organism's compatibility with the temperature of its habitat is ultimately determined by its underlying genetic architecture.
The strong emphasis in research on mesophile biology (temperatures in the 20°C to 37°C range) has given us a misimpression of the importance of cold on earth. However, 70% of the Earth's surface is covered by oceans with average temperatures between 1°C and 5°C (11), 20% of the Earth's terrestrial surface is permafrost (47), and a larger portion of the surface undergoes seasonal freezing, making our planet a predominantly cold environment. Hence, cold adaptation in the microbial world should be expected (55).
Permafrost is defined as soils or sediments that are continuously exposed to a temperature of 0°C or less for at least 2 years (44). Permafrost temperatures range from −10°C to −20°C in the Arctic and from −10°C to −65°C in the Antarctic, and permafrost has low water activity, often contains small amounts of carbon (0.85 to 1%), and is subjected to prolonged exposure to damaging gamma radiation from 40K in soil minerals (49). Liquid water occurs as a very thin, salty layer surrounding the soil particles in the frozen layer. Despite the challenges of the permafrost, a variety of microorganisms successfully colonize this environment, and many microorganisms have been isolated from it (54, 70). The bacterial taxa most frequently isolated from the Kolyma permafrost of northeast Siberia include Arthrobacter, Exiguobacterium, Flavobacterium, Sphingomonas, and Psychrobacter (71). Rhode and Price (56) proposed that microorganisms can survive in frozen ice for very long periods due to the very thin film of water surrounding each cell that serves as a reserve of substrates. Permafrost is a more favorable environment than ice as a result of its heterogeneous soil particles and larger reservoirs of nutrients.
The genus Psychrobacter comprises a group of Gram-negative, rod-shaped, heterotrophic bacteria, and many Psychrobacter species are capable of growth at low temperatures. Members of this genus can grow at temperatures between −10°C and 42°C, and they have frequently been isolated from various cold environments, including Antarctic sea ice, ornithogenic soil and sediments, the stomach contents of Antarctic krill (Euphausia), deep seawater, and permafrost (9, 36, 57, 70, 71, 76; http://www.bacterio.cict.fr/p/psychrobacter.html). Psychrobacter arcticus 273-4 is a recently described species (4) that was isolated from a 20,000- to 30,000-year-old continuously frozen permafrost horizon in the Kolyma region in Siberia that was not exposed to temperatures higher than 4°C during isolation (70). This strain, the type strain of the species, grows at temperatures ranging from −10°C to 28°C, has a generation time of 3.5 days at −2.5°C, exhibits excellent long-term survival under freezing conditions, and has temperature-dependent physiological modifications in membrane composition and carbon source utilization (50). The fact that Psychrobacter has been found to be an indicator genus for permafrost and other polar environments (66) suggests that many of its members are adapted to low temperatures and increased levels of osmotica and have evolved molecular-level changes that aid survival at low temperatures.
Early studies on cold adaptation in microorganisms revealed physiological strategies to deal with low temperatures, such as changes in membrane saturation, accumulation of compatible solutes, and the presence of cold shock proteins (CSPs) and many other proteins with general functions (62). However, many of the studies were conducted with mesophilic microorganisms, which limits the generality of the conclusions. We addressed the question of cold adaptation by studying microorganisms isolated from subzero environments using physiologic and genomic methods. We chose P. arcticus as our model because of its growth at subzero temperatures and widespread prevalence in permafrost. This paper focuses on the more novel potential adaptations.
MATERIALS AND METHODS
Cell preparation and genome sequencing.
The genome of P. arcticus 273-4 (= ATCC BAA1226) was sequenced by the Joint Genome Institute (Walnut Creek, CA) using its standard shotgun method and Sanger sequencing (13). Coding sequences (CDS) were identified by combining the results from Critica (3) and Glimmer (17) gene modelers using the Oakridge National Laboratory Genome Analysis Pipeline. CDS identification was confirmed and polished by comparison of amino acid translations with GenBank's nonredundant database using the basic local alignment search tool for proteins (BLASTP) (2) and manual identification of ribosomal binding sites using Artemis (60). Genes encoding tRNAs were identified with the tRNAScanSE tool (34), while 16S and 23S rRNAs were identified by comparing genome fragments with an rRNA database using the BLASTN tool. Structural RNAs (e.g., 5S rRNA, RnpB, transfer-messenger RNA, signal recognition particle RNA) were identified using the Infernal search tool (18).
A functional assignment of each CDS was made manually by comparing the CDS with the KEGG (74), InterPro (77), TIGRFams (24), PFams (65), and Clusters of Orthologous Groups of Proteins (COGs) (67) databases and using a hierarchy system that considered database ranking, sequence identity, and alignment quality (available upon request). Finally, the annotation was polished by the Integrated Microbial Genomes annotation group (http://img.jgi.doe.gov/cgi-bin/pub/main.cgi).
Amino acid usage analysis.
Cold adaptation at the amino acid level was examined by comparison with the Swiss-Prot database using several indicators of cold adaptation (23). Briefly, P. arcticus amino acid sequences were searched against the Swiss-Prot database (7) using BLAST, and the five most similar protein sequences with an expect value of <10−15 were extracted from the database. The Swiss-Prot database was used for this analysis because it is a manually curated database with high-quality annotations. Cold adaptation was measured for each P. arcticus amino acid sequence and the corresponding Swiss-Prot database proteins using the following parameters known to contribute to cold adaptation (55, 58): grand average of hydropathicity (GRAVY) and aliphacity, which were calculated using the ExPASy website (http://us.expasy.org/); proline content; acidic residue content; and the Arg/Lys ratio, which was calculated using in-house PERL scripts. A one-sample t test was used to evaluate if there were statistically significant differences between the P. arcticus amino acid sequence queried and the five most similar proteins of the Swiss-Prot database for a given parameter. Amino acid sequences for which there were significant differences were classified as cold or hot adapted depending on the direction of the change.
To determine if there was enrichment of cold-adapted genes in the P. arcticus genome, a ratio of the total number of cold-adapted genes to the total number of hot-adapted genes was calculated for each cold adaptation descriptor. A chi-square analysis was used to determine if there was a significant difference between the number of cold-adapted genes and the number of hot-adapted genes in the P. arcticus genome using R version 2.3.1 (25). Cluster analysis using Euclidian distance and average linkage was performed with the significance data using Cluster 3.0 (16), and the results were visualized using Java Treeview (61).
Repeat analysis.
RepeatFinder was used to identify and classify repeated sequences that were >25 bp long (33, 72). The MultiFASTA output from RepeatFinder.pl was BLASTed against the P. arcticus genome sequence using blastall 2.2.5 with word size 7 and low-complexity sequence filters turned off. Repeated sequences in the genome were identified by the presence of alignments with more than 94% nucleotide sequence identity for more than 85% of the length of the query sequence. Hits for multiple repeat sequences to the same base pair coordinates in a genome were resolved by deleting the shorter repeat. If a pair of repeat loci overlapped by more than 25% of the length of either member of the pair, the repeats were merged into a single sequence. The most abundant repeat class, class 1, was subclassified by multiple-sequence alignment analysis using ClustalW 1.81 (68). The secondary structure of the class 1 repeats was predicted using MFOLD 3.1.2 (79). Predictions were made by assuming that the temperature was 22°C, and the default settings were used for all other parameters. Code is available upon request.
CA and model-based clustering.
The FactoMineR R package was used to perform a correspondence analysis (CA) of amino acid frequencies to identify the factors that determine amino acid usage (53). After selection of protein sequences with more than 100 residues, the first 10 and last 5 residues were removed, and normalized amino acid frequencies were generated using a custom PERL script. Model-based clustering, as described by Riley et al. (53), was performed using the scores from the first six dimensions from the CA and the Mclust R package.
Development of a defined medium.
Using the genome sequence information, a basal defined medium that was also suitable for transcriptome experiments was developed for growth of P. arcticus. This medium contained 20 mM dl-lactic acid or 100 mM sodium pyruvate, 5 mM glutamate or 5 mM NH4Cl, 1 mM K2HPO4, 1× Wolin's vitamins (75), morpholinepropanesulfonic acid (MOPS), and 1× trace minerals (31). The buffers tested to determine whether they supported growth included HEPES, piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES), 3-(n-morpholino)-2-hydroxypropanesulfonic acid (MOPSO), MOPS, and 1 and 2 mM K2HPO4 buffer, all at pH 7.0. P. arcticus was cultured serially in marine broth containing 5% sea salts and then in the basal defined medium containing 20 mM lactate. A 1% inoculum was used to inoculate the basal defined medium containing the carbon source being tested, and growth was measured by determining the optical density at 600 nm (OD600). Growth rates were estimated from the data obtained for the OD600 range from 0.03 to 0.3. Cultures were considered negative for growth on a substrate if no growth was observed after 5 days of incubation at 22°C or after 10 days of incubation at 4°C.
Nucleotide sequence accession number.
The complete genome sequence of P. arcticus 273-4 has been deposited in the GenBank database under accession number CP000082.
RESULTS AND DISCUSSION
Genome summary.
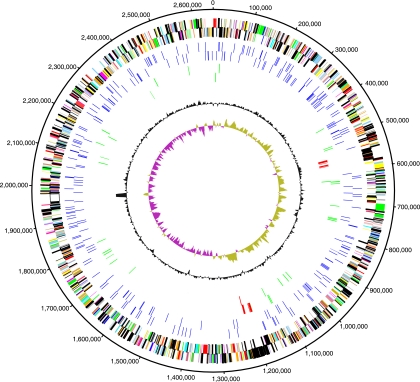
P. arcticus 273-4 contains a single replication unit consisting of 2,650,701 bp, which encodes 2,147 putative proteins (see Table S1 in the supplemental material). Genes were evenly distributed between the forward (53.5%) and reverse (46.5%) strands, and the average CDS length was 994 bp. Eighty-two percent of the coding bases were in sequences encoding putative proteins. rRNA operons were found in the positive (1 copy) and negative (3 copies) strands. The GC content was constant across the genome, and the average value was 42.8% (Fig. 1). An unusually high GC content was detected in a 20,147-bp region between bp 1959325 and 1979472 in which the average value was 56.9%. This region encodes a putative protein consisting of 6,715 amino acids with a possible membrane function. The genome is divided into two symmetric replichores (Fig. 1), as indicated by GC skew analysis. The GC skew was weakly correlated with spikes in the GC content. Mobile elements (e.g., phages, transposons, and insertion sequences) could be responsible for the changes in GC content and GC skew since some of these elements were in the areas where GC spikes occurred.
FIG. 1.
Diagram of the P. arcticus 273-4 genome. The outermost two circles (circles 1 and 2) show the genes in the forward and reverse strands, respectively; different colors indicate different function categories. The next two circles (circles 3 and 4) show the class 1 repeat elements in the forward and reverse strands, respectively. Circles 5 and 6 show transposons and IS elements (green) and prophage (red) in the forward and reverse strands, respectively; circle 7 shows the GC content; and circle 8 shows the GC skew.
The genome contains 48 transposons or insertion sequence (IS) elements and 25 phage-related genes. The majority of the IS elements were classified in one of four distinct major families, the IS3 (27 elements), IS4 (10 elements), IS30 (4 elements), and IS mutator (3 elements) families. Although many of these sequences seem to be truncated, based on small open reading frames (ORFs) (35), they could still be active (45). IS elements seem to be randomly distributed in the genome (Fig. 1), except for the IS3 family elements that were found in pairs that were from 50 bp to 12 kb apart. The close proximity of the IS3 elements could indicate that were acquired via a transposition event or that they still actively contribute to genome rearrangements.
There are two almost complete phage genomes in the P. arcticus genome. The first phage genome is the genome of a 33.3-kb lambda-like phage located between bp 551715 and 585095, and the second is most similar to the genome of a 45.7-kb MU phage and is located between bp 1177957 and 1223710. Both phage regions encode many, but not all, phage proteins needed for replication. A total of 57 hypothetical proteins were encoded by genes in the phage regions, accounting for 17% of all hypothetical proteins. In the phage MU region a gene encoding a possible neutral zinc metallopeptidase (gi 71065546), which is most similar to a hypothetical protein from Haemophilus influenzae, was identified, indicating that phages have likely helped shape the current genome structure by addition of genes that are unique to P. arcticus as far as we know. Although there were indications that gene transfer occurred due to IS elements and phage genes, the GC skew data indicate that no major genome rearrangements or horizontal movement of genes has occurred (22). This finding is surprising since the genus Psychrobacter was characterized as a taxon that is naturally transformable (27). The lack of horizontal transfer and gene rearrangements could be an indication of the strong selective pressure exerted by the cold niche early in the evolution of the genome, resulting in the current structure.
Correspondence analysis (CA) revealed differences in the amino acid frequencies in the P. arcticus genome. P. arcticus proteins grouped in two clusters along the first dimension (see Fig. S1 in the supplemental material). An inertia analysis of the amino acids (i.e., an analysis of their relative contributions in the CA) indicated that hydrophobic (F, L, and W) and charged (E, D, and K) residues were important for generation of clusters A and B, respectively (see Table S2 in the supplemental material). Pascal and coworkers observed the same amino acid distributions that resulted in two similar clusters when model genomes were analyzed using CA (46). They concluded that hydrophobicity is a discriminant factor for protein location, since all proteins in the cluster equivalent to cluster A were integral inner membrane proteins. The separation is likely due to the greater abundance of hydrophobic residues. Interestingly, for Psychromonas ingrahamii 37 the proteins determined from the genome sequence did not form two clusters, possibly because of lower hydrophobicity (53). No well-defined clusters were formed along the second and third dimensions in the P. arcticus CA. The inertia of these two axes indicates that aromatic residues and glutamine, respectively, influence the protein distribution along these two dimensions. Compared with the findings for P. ingrahamii, asparagine did not occur as frequently in the third dimension, suggesting that differences in the habitats of the two microorganisms (terrestrial versus marine) could affect amino acid preferences.
Riley and coworkers (53) analyzed the CA results for P. ingrahamii using model-based clustering (MBC) and found six classes of proteins. MBC of P. arcticus CA results classified proteins in four clusters, similar to the findings for many microorganisms (see Fig. S2 in the supplemental material). In contrast to the findings for P. ingrahamii, which showed that the bulk of the proteins formed three clusters, for P. arcticus 60% of the proteins were in cluster 2 and 19.6% of the proteins were in cluster 4 (see Table S3 in the supplemental material). Representatives of all COG categories were present in both cluster 2 and cluster 4 and were very abundant in cluster 2, except for transport proteins, which were more abundant in cluster 3. Cluster A proteins in the CA corresponded to cluster 3 proteins in the MBC analysis, supporting the finding that a higher number of transport proteins were in cluster 3. In contrast to the findings for P. ingrahamii, the good separation of proteins in cluster 3 is indicative of the distinct frequencies of hydrophobic residues in this cluster compared with the frequencies in the entire proteome of P. arcticus. Finally, cluster 1 contains most transposons and a moderate number of hypothetical and conserved hypothetical proteins. The number of clusters and their compositions show that there are differences in amino acid frequencies between P. arcticus and P. ingrahamii, although both bacteria are cold-adapted microorganisms.
Known adaptations to cold.
Based on previous work on cold adaptation, we expected membrane modifications, compatible solute accumulation, and cold shock proteins to be features encoded in the P. arcticus genome. Lipid analyses of P. arcticus grown at warm and cold temperatures revealed that in P. arcticus the acyl chain length and saturation of the membrane fatty acids decrease at low temperatures (50). Analysis of the P. arcticus genome sequence revealed genes for synthesis of saturated and unsaturated fatty acids and genes for control of the acyl chain length. Unsaturated fatty acids can be synthesized either de novo or via a fatty acid desaturase, suggesting that fatty acid unsaturation is an important characteristic for growth of P. arcticus at low temperatures. Colwellia psychrerythraea 34H, a psychrophile from a marine environment, uses a similar strategy by employing multiple polyunsaturated fatty acid synthases (40). Redundancy of pathways ensures that an essential phenotypic trait is expressed when it is needed, as expected in low-nutrient and/or harsh environments like soil (12). Low temperatures also increase accumulation of compatible solutes, such as proline, glutamate, and glycine betaine, in P. arcticus (48). Transcriptomic analysis of the P. arcticus genome revealed that genes related to transport and synthesis of compatible solutes were upregulated at low temperatures in the presence of salt (48). Since P. arcticus lives in a low-temperature and low-water-activity soil environment, compatible solute accumulation likely represents an important adaptation to life in the permafrost niche. Finally, the cold shock genes cspE, cspA, and capB and a gene encoding an unnamed cold shock binding domain protein were identified in the P. arcticus genome. Transcriptome analysis of cspA revealed that it is constitutively expressed regardless of the temperature (48). Hence, cspA appears to be required for protein synthesis in P. arcticus.
Distinctive features.
We reconstructed the central carbon metabolism to better understand the potential metabolism of P. arcticus. Although P. arcticus grows on complex media, Psychrobacter spp. in general do not grow on carbohydrates (8). Reconstruction of the metabolism revealed that P. arcticus lacks the genes necessary for any of the known versions of glycolysis (see the Discussion in the supplemental material) and phosphotransferase system (PTS)-type sugar transporters, suggesting that P. arcticus is not able to utilize sugars. However, both of the “committed” gluconeogenic enzymes, fructose-1,6-bisphosphatase and phosphoenolpyruvate synthase, are present in P. arcticus (see Fig. S3 in the supplemental material), which indicates that oxidized substrates, such as acetate, malate, oxaloacetate, and other mono- and dicarboxylic acids, could be the preferred carbon sources.
A defined medium was developed to test hypothesis that P. arcticus prefers oxidized substrates instead of sugars. Acetate supported more than 2-fold-faster growth than lactate (Table 1 ). No growth was observed on glucose, isovalerate, isobutyrate, glycerol, malate, succinate, citrate, glycolate, glyoxylate, serine, glycine, or propionate at 22°C or 4°C after repeated attempts using inocula grown on acetate or lactate or in marine broth (Table 1). Transporters are present for the dicarboxylates (TRAP-T dicarboxylate uptake transporter [gi 71066517 to gi 71066519]), glycolate (glycolate:H+ symporter [gi 71066208]), and glycine (BCCT uptake transporter [gi 71065858]). However, it is possible that culture conditions could not induce use of these substrates for growth or that unpredicted aspects of the metabolism prevented growth on these substrates despite the presence of the complete gene complement. Growth on palmitic acid was observed only at 22°C for unknown reasons, although this result could have been due to a combination of reduced water solubility of the compound at low temperatures and reduced uptake of the compound due to decreased membrane fluidity at 4°C.
TABLE 1.
P. articus 273-4 growth responses to diverse growth substrates
| Substrate(s)a | Growth rate (h−1) at: |
|
|---|---|---|
| 22°C | 4°C | |
| Acetate | 0.159 ± 0.007 | 0.037 ± 0.002 |
| Acetate + 5 mM glutamate | 0.168 ± 0.002 | NDd |
| Malonate | 0.154 ± 0.007 | ND |
| Pyruvate | 0.007 ± 0.002 | 0.005 ± 0.0006 |
| Lactate | 0.062 ± 0.005 | 0.023 ± 0.005 |
| Lactate + 5 mM glutamate | 0.064 ± 0.009 | ND |
| Butanoic acid | 0.094 ± 0.002 | 0.025 ± 0.002 |
| Decanoic acidc | 0.032 ± 0.009 | 0.014 ± 0.005 |
| Palmitic acidc | 0.014 | NGb |
| Glutamate | 0.092 ± 0.009 | ND |
| Marine broth with 5% sea salts | 0.244 ± 0.014 | 0.060 ± 0.002 |
| 0.5× tryptic soy broth + 5% NaCl | 0.044 ± 0.0009 | 0.012 ± 0.002 |
Unless otherwise noted, all carbon sources were used at a concentration of 20 mM.
NG, no growth after 10 days of incubation at 4°C.
No significant growth was observed on 0.5% ethanol added to cultures as a solvent for decanoic and palmitic acids.
ND, no data collected.
P. arcticus may be optimized to conserve energy by utilizing acetate, a substrate expected to be present under the waterlogged conditions during the tundra summer, as the basis for its biosynthesis and energy metabolism. Addition of acetate resulted in the highest growth rate and highest yield of all the carbon sources tested, and the growth rate was 65% of the growth rate observed in the complex, rich marine broth with 5% sea salts. Supplementation of the medium with 5 mM glutamate resulted in only a slight increase in the growth rate compared with that in medium containing acetate or lactate alone. Acetate metabolism has several features that may be desirable at low temperatures. Uptake of acetate occurs through the cytoplasmic membrane with no transporter required, although transporters may assist uptake (5). Utilization of the glyoxylate shunt can allow P. arcticus to conserve all of the carbon from this substrate. Metabolism of acetate requires only one or two enzymes to generate acetyl coenzyme A (acetyl-CoA) from acetate and HS-CoA.
A less known but likely important factor in cold adaptation is the synthesis of wax esters. Wax esters are commonly found in plants and animals (30). Acinetobacter sp. accumulates large amounts of wax esters that are used later as a carbon source for growth (26, 28, 52). The P. arcticus genome has a wax ester synthase/acyl-CoA:diacylglycerol acyltransferase (gi 71064803). In Psychrobacter urativorans (formerly Micrococcus cryophilus) wax esters account for 14% of the total lipid content and were found to be associated with the cell membrane (59). Furthermore, a decrease in the growth temperature from 20 to 1°C resulted in a significant increase in unsaturation and a decrease in the average chain length of the wax-associated fatty acids, suggesting that these compounds are important for low-temperature growth (59). The wax ester synthase of P. arcticus shares 52% amino acid identity with the wax synthase of Acinetobacter sp. strain ADP1, and our transcriptomic studies showed that the gene encoding this protein is expressed constitutively regardless of the growth temperature (48). The wax ester synthase may play a role in membrane fluidity in P. arcticus.
Production of extracellular polymeric substances (EPS) has been observed for many prokaryotic species. Typically, EPS is either closely associated with the cell wall, forming a capsule, or loosely attached, forming slime (73). Although there are many possible functions for EPS, one general trend is that it increases cell survival by forming biofilms, retaining water (especially important in a frozen soil), and serving as a cryoprotectant (29). Similar to the psychrophiles C. psychrerythraea 34H (40) and P. ingrahamii 37 (53), P. arcticus possesses genes for production of capsular type EPS (gi 71065213, 71065215, 71065219, and 71065223). P. arcticus formed a capsule in the presence of salt (48), suggesting that this could be an adaptation to growth in the permafrost environment.
P. arcticus has a highly repetitive genome that contains the following four superfamilies of dispersed repeat loci that are >25 bp long: the long tandem repeat constituent sequences (total length, ∼16.7 kb), transposon and insertion sequence (IS) repetitive elements (1.86% of repeated sequences), repeats internal to nonmobile genes (3 genes), and AT-rich intergenic repeated loci. The Shannon-Weaver index and evenness estimator, which were calculated to test for dominance of particular repeat sequences, were 0.79 and 0.11, respectively, indicating that the repeat sequence group was dominated by one or a few sequences.
A single group of AT-rich intergenic repeat sequences that were 69 to 149 bp long (the class 1 repeats) accounted for 305 of 336 AT-rich repeat loci or 1.92% of the genome sequence. Class 1 sequences were characterized by inverted terminal repeats that were at least 34 bp long and ended in TA dinucleotide repeats at both ends. Class 1 repeats were predicted to form secondary structures at 22°C (see Fig. S4 in the supplemental material) by MFOLD 3.1.2 (37, 79). The 6-bp terminal sequence, TATAGT, and the predicted hairpin loop structures of the class 1 repeats are similar to Neisseria miniature insertion sequences (nemis elements) of pathogenic Neisseria spp. (10, 14, 15, 38). Nemis elements of Neisseria spp. are thought to participate in recombination and are known to bind to the integration host factor (IHF) and to be cleaved by RNase III where they are transcribed (10, 15). The predicted secondary structures of the nemis elements and the class 1 repeat elements are similar, suggesting that class 1 P. arcticus repeats may exhibit the activities described above. It has long been known that salt stress, growth temperature, and temperature shock strongly affect DNA topology (21, 32, 42, 43). Hence, the dominant repeats in P. arcticus may associate with DNA binding proteins to alter the temperature and salt effect on the topology of the chromosome in as-yet-unknown ways.
Traits with evidence of cold adaptation.
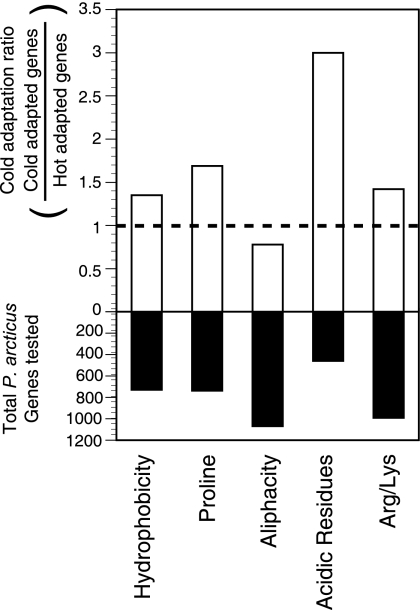
Comparisons of P. arcticus proteome substitutions with the Swiss-Prot database revealed strategies used to cope with the limitations due to low temperatures. Between 476 (38%) and 1,074 (84%) of the P. arcticus amino acid sequences were statistically significantly different from sequences in the Swiss-Prot database for all cold adaptation indicators; i.e., they were less hydrophobic, had fewer proline residues, were less aliphatic, had fewer acidic residues, or had low Arg and increased Lys contents (Fig. 2). The ratios of the total number of cold-adapted genes to the total number of hot-adapted genes calculated for hydrophobicity, the number of proline residues, the number of acidic residues, and the Arg and Lys contents were 1.35, 1.69, 3.0, and 1.42, respectively, indicating that there were more cold-adapted genes in the P. arcticus genome. Aliphacity was the only indicator with a ratio less than 1 (0.78). A total of 1,212 genes (56% of the genome) had at least one adaptation, and the average number was three cold-adaptive qualities per gene. In contrast to other studies (1, 39), which used proportional changes, we evaluated cold adaptation by measuring the substitutions that are more likely to be present in cold-adapted microbes than in mesophilic microorganisms. Measurement of amino acid substitutions proved to be very sensitive compared to examination of proportional changes in the amino acid composition, since four of the five indicators used suggested that the P. arcticus proteome is cold adapted. Metpally and Reddy (41) compared amino acid substitution patterns for psychrophilic and mesophilic organisms, and the psychrophilic organisms used included Psychrobacter cryohalolentis K5, C. psychrerythraea 34H, and P. ingrahamii 37. They concluded that psychrophile proteins contained fewer hydrophilic, acidic, and proline residues, consistent with our findings (41, 69).
FIG. 2.
Cold adaptation. The upper graph shows cold adaptation ratios that were calculated by using all of the genes whose data were statistically significantly different from data in the Swiss-Prot database in favor (cold adapted) or against (hot adapted). A ratio of 1 indicates that the proportions of genes in the two categories are equal. The lower graph shows the total numbers of genes used to generate the ratios.
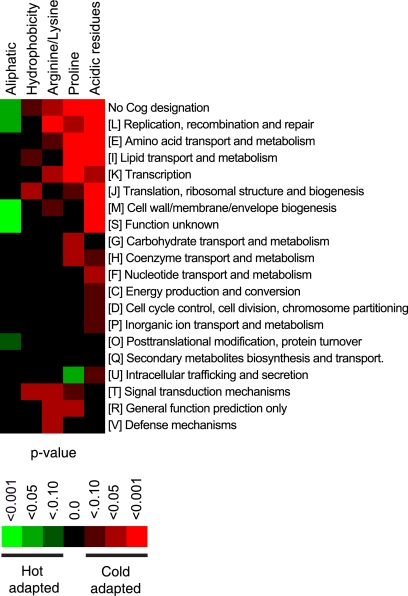
To examine the specific groups of genes that show signs of cold adaptation, all the genes from the previous analysis were separated according to COG categories. A chi-square analysis of the numbers of cold- and hot-adapted genes (see Table S4 in the supplemental material) and a cluster analysis of the results revealed that acidic residues (14 categories) and proline (10 categories) are the two most frequent cold adaptation indicators across all COG categories (Fig. 3) (see Table S5 in the supplemental material). The categories with higher numbers of adaptations include the following COG categories: no COG designation; replication, recombination, and repair; amino acid transport and metabolism; lipid transport and metabolism; transcription; translation, ribosomal structure, and biogenesis; and signal transduction mechanisms (Fig. 3).
FIG. 3.
Distribution of cold adaptation qualities across COG categories. The statistical significance of differences between the number of genes with cold qualities and the number of genes with hot qualities in each COG category was determined using the chi-square test. The results were ranked using a scale from 0 to 3, where a P value of <0.001 was 3, a P value of <0.05 was 2, a P value of <0.10 was 1, and a P value of >0.10 was 0. Enrichment of cold-adapted qualities was indicated by a plus sign, and enrichment of hot-adapted qualities was indicated by a minus sign. Rankings were used for cluster analysis of the COG categories.
One of the biggest challenges for proteins at low temperatures is having sufficient flexibility so that they can increase their interactions with substrates, reducing the required activation energy. An abundance of proline residues has been related to increased protein stability due to the rigidity of the N-Cα bond (19, 51). Hence, the decrease in the amount of proline observed suggests that there is cold adaptation, which supports a trend seen in smaller-scale studies (69). Arginine is also considered an amino acid that stabilizes the structurally since it forms salt bridges and hydrogen bonds with side chains (1). Substituting lysine for arginine has been proposed to be a substitution that results in more flexibility. The negatively charged (acidic) amino acids glutamic acid and aspartic acid favor salt bridge formation on protein surfaces, thus favoring a stable protein structure (23). Removal of acidic residues increases protein flexibility (20). Finally, an increase in the hydrophobicity of core amino acids increases protein stability at higher temperatures (64), while an overall reduction in stability has been observed in cold-active enzymes (63). Our results suggest that the adaptation of P. arcticus to low temperatures involved multiple amino acid substitutions that decreased protein stability, presumably yielding enzymes that are more active at low temperatures. The only parameter not consistent with this conclusion was aliphacity since it showed the opposite response to cold. This inconsistent result, which has been observed previously, could be due to the strategy used for analysis, which did not separate exposed and buried residues (23, 41, 69). In support of this, Metpally and Reddy (41) recently showed that there is a high frequency of aliphatic residues in coil-loop regions of proteins encoded by psychrophile genomes.
To further validate our cold adaptation indicators, we analyzed genes that were upregulated at 4°C as measured by two-dimensional liquid (proteome) mapping (78). A total of 11 of 14 proteins with at least 2-fold-greater expression when cells were grown at 4°C than when cells were grown at 22°C show at least two cold adaptations per gene and an average of 3.6 cold adaptations per gene. Proteins in COG categories related to essential processes, such as transcription, translation, repair, amino acid metabolism, and lipid metabolism, had a higher number of adaptations, which is consistent with the importance of these processes for life in cold environments. Considering that microbial metabolism in permafrost is controlled by substrate diffusion from a thin layer of water around the cell (56), it seems logical that genes essential for survival have several cold adaptation strategies. Overall, the results suggest that increased protein flexibility, due to amino acids that promote structural instability, is a major adaptation employed by P. arcticus to maintain activity at low temperatures.
Conclusions.
P. arcticus 273-4 is a Siberian permafrost psychroactive bacterium that is capable of growth at −10°C. Members of the genus Psychrobacter have been isolated frequently from cold environments, and recent work on this genus using quantitative PCR indicated that its distribution and density are strongly skewed toward cold environments and that its densities are highest in Antarctic sediments (55). To survive the continuous gamma radiation emitted by soil particles, it is necessary to invest ATP in repair processes. P. arcticus prefers acetate, a simple ubiquitous substrate, as the basis for its biosynthesis and energy metabolism. Acetate easily diffuses into the cell without costly transport systems. Low temperatures in the permafrost make mRNAs more stable and less efficient for translation. P. arcticus possesses three CSPs, which are RNA chaperones that enhance translation processes by eliminating the formation of secondary structures in the mRNA. Multiple pathways to increase the unsaturation of membrane lipids and to increase acyl chain length maintain the membrane fluidity. P. arcticus compensates for the effects of low temperatures on enzyme activity by structural modifications that increase the flexibility of at least 50% of its proteome, which reduces the energetic requirements for activity. Transcriptome analysis following growth at subzero temperatures detected increased expression for fatty acid unsaturation, growth rate control mechanisms, and isozyme exchange, along with a more structurally flexible DEAD box RNA helicase and a d-alanyl-d-alanine carboxypeptidase that was upregulated at cold temperatures (6). The low levels of nutrients, low water activities, and low temperatures characteristic of the permafrost environment should favor microorganisms with multiple, low-cost coping strategies. The combination of the strategies described above and probably more strategies, especially strategies involving many cold-induced genes with unknown functions, apparently allows P. arcticus to live for a long time at subzero temperatures.
Supplementary Material
Acknowledgments
This work was supported by NASA Astrobiology Institute cooperative agreement NCC2-1274 and by DOE's Joint Genome Institute.
We thank Iván Dávila Marcano for statistical advice and Ting Zhang Wang and Antoine Danching for help with the correspondence analysis and model-based clustering.
Footnotes
Published ahead of print on 12 February 2010.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Adekoya, O. A., R. Helland, N. P. Willassen, and I. Sylte. 2006. Comparative sequence and structure analysis reveal features of cold adaptation of an enzyme in the thermolysin family. Proteins Struct. Funct. Bioinformatics 62:435-449. [DOI] [PubMed] [Google Scholar]
- 2.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 3.Badger, J. H., and G. J. Olsen. 1999. CRITICA: coding region identification tool invoking comparative analysis. Mol. Biol. Evol. 16:512-524. [DOI] [PubMed] [Google Scholar]
- 4.Bakermans, C., H. L. Ayala-del-Rio, M. A. Ponder, T. Vishnivetskaya, D. Gilichinsky, M. F. Thomashow, and J. M. Tiedje. 2006. Psychrobacter cryohalolentis sp. nov. and Psychrobacter arcticus sp. nov., isolated from Siberian permafrost. Int. J. Syst. Evol. Microbiol. 56:1285-1291. [DOI] [PubMed] [Google Scholar]
- 5.Bakermans, C., C. Sloup, D. Zarka, J. M. Tiedje, and M. F. Thomashow. 2009. Development and use of genetic system to identify genes required for efficient low-temperature growth of Psychrobacter arcticus 273-4. Extremophiles 13:21-30. [DOI] [PubMed] [Google Scholar]
- 6.Bergholz, P. W., C. Bakermans, and J. M. Tiedje. 2009. Psychrobacter arcticus 273-4 uses resource efficiency and molecular motion adaptations for subzero temperature growth. J. Bacteriol. 191:2340-2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boeckmann, B., A. Bairoch, R. Apweiler, M. C. Blatter, A. Estreicher, E. Gasteiger, M. J. Martin, K. Michoud, C. O'Donovan, I. Phan, S. Pilbout, and M. Schneider. 2003. The SWISS-PROT protein knowledgebase and its supplement TrEMBL in 2003. Nucleic Acids Res. 31:365-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bowman, J. (ed.). 2006. The genus Psychrobacter, p. 920-930. In S. Falkow, E. Rosenberg, K.-H. Schleifer, E. Stackebrandt, and M. Dworkin (ed.), The prokaryotes. Springer-Verlag, New York, NY.
- 9.Bowman, J., D. Nichols, and T. McMeekin. 1997. Psychrobacter glacincola sp. nov, a halotolerant, psychrophilic bacterium isolated from Antarctic sea ice. Syst. Appl. Microbiol. 20:209-215. [Google Scholar]
- 10.Buisine, N., C. M. Tang, and R. Chalmers. 2002. Transposon-like Correia elements: structure, distribution and genetic exchange between pathogenic Neisseria sp. FEBS Lett. 522:52-58. [DOI] [PubMed] [Google Scholar]
- 11.Cavicchioli, R. 2006. Cold-adapted archaea. Nat. Rev. Microbiol. 4:331-343. [DOI] [PubMed] [Google Scholar]
- 12.Chain, P. S., V. J. Denef, K. T. Konstantinidis, L. M. Vergez, L. Agullo, V. L. Reyes, L. Hauser, M. Cordova, L. Gomez, M. Gonzalez, M. Land, V. Lao, F. Larimer, J. J. LiPuma, E. Mahenthiralingam, S. A. Malfatti, C. J. Marx, J. J. Parnell, A. Ramette, P. Richardson, M. Seeger, D. Smith, T. Spilker, W. J. Sul, T. V. Tsoi, L. E. Ulrich, I. B. Zhulin, and J. M. Tiedje. 2006. Burkholderia xenovorans LB400 harbors a multi-replicon, 9.73-Mbp genome shaped for versatility. Proc. Natl. Acad. Sci. U. S. A. 103:15280-15287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chain, P. S. G., P. Hu, S. A. Malfatti, L. Radnedge, F. Larimer, L. M. Vergez, P. Worsham, M. C. Chu, and G. L. Andersen. 2006. Complete genome sequence of Yersinia pestis strains Antiqua and Nepal516: evidence of gene reduction in an emerging pathogen. J. Bacteriol. 188:4453-4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Correia, F. F., S. Inouye, and M. Inouye. 1988. A family of small repeated elements with some transposon-like properties in the genome of Neisseria gonorrhoeae. J. Biol. Chem. 263:12194-12198. [PubMed] [Google Scholar]
- 15.De Gregorio, E., C. Abrescia, M. S. Carlomagno, and P. P. Di Nocera. 2003. Ribonuclease III-mediated processing of specific Neisseria meningitidis mRNAs. Biochem. J. 374:799-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Hoon, M. J., S. Imoto, J. Nolan, and S. Miyano. 2004. Open source clustering software. Bioinformatics 20:1453-1454. [DOI] [PubMed] [Google Scholar]
- 17.Delcher, A. L., D. Harmon, S. Kasif, O. White, and S. L. Salzberg. 1999. Improved microbial gene identification with GLIMMER. Nucleic Acids Res. 27:4636-4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eddy, S. R. 2002. A memory-efficient dynamic programming algorithm for optimal alignment of a sequence to an RNA secondary structure. BMC Bioinformatics 3:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fields, P. A. 2001. Review: protein function at thermal extremes: balancing stability and flexibility. Comp. Biochem. and Physiol. Part A Mol. Integr. Physiol. 129:417-431. [DOI] [PubMed] [Google Scholar]
- 20.Gianese, G., F. Bossa, and S. Pascarella. 2002. Comparative structural analysis of psychrophilic and meso- and thermophilic enzymes. Proteins 47:236-249. [DOI] [PubMed] [Google Scholar]
- 21.Goldstein, E., and K. Drlica. 1984. Regulation of bacterial DNA supercoiling: plasmid linking numbers vary with growth temperature. Proc. Natl. Acad. Sci. U. S. A. 81:4046-4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grigoriev, A. 1998. Analyzing genomes with cumulative skew diagrams. Nucleic Acids Res. 26:2286-2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grzymski, J. J., B. J. Carter, E. F. DeLong, R. A. Feldman, A. Ghadiri, and A. E. Murray. 2006. Comparative genomics of DNA fragments from six Antarctic marine planktonic bacteria. Appl. Environ. Microbiol. 72:1532-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haft, D. H., B. J. Loftus, D. L. Richardson, F. Yang, J. A. Eisen, I. T. Paulsen, and O. White. 2001. TIGRFAMs: a protein family resource for the functional identification of proteins. Nucleic Acids Res. 29:41-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ihaka, R., and R. Gentleman. 1996. R: a language for data analysis and graphics. J. Comput. Graph. Stat. 5:299-314. [Google Scholar]
- 26.Ishige, T., A. Tani, Y. Sakai, and N. Kato. 2000. Long-chain aldehyde dehydrogenase that participates in n-alkane utilization and wax ester synthesis in Acinetobacter sp. strain M-1. Appl. Environ. Microbiol. 66:3481-3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Juni, E., and G. A. Heym. 1980. Transformation assay for identification of psychrotrophic achromobacters. Appl. Environ. Microbiol. 40:1106-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalscheuer, R., and A. Steinbuchel. 2003. A novel bifunctional wax ester synthase/acyl-CoA:diacylglycerol acyltransferase mediates wax ester and triacylglycerol biosynthesis in Acinetobacter calcoaceticus ADP1. J. Biol. Chem. 278:8075-8082. [DOI] [PubMed] [Google Scholar]
- 29.Kim, S. J., and J. H. Yim. 2007. Cryoprotective properties of exopolysaccharide (P-21653) produced by the Antarctic bacterium, Pseudoalteromonas arctica KOPRI 21653. J. Microbiol. 45:510-514. [PubMed] [Google Scholar]
- 30.Kolattukudy, P. E. (ed.). 1976. Chemistry and biochemistry of natural waxes. Elsevier, Amsterdam, Netherlands.
- 31.Kostka, J., and K. H. Nealson. 1998. Isolation, cultivation and characterization of iron- and manganese-reducing bacteria, p. 58-78. In R. S. Burlage, R. Atlas, D. Stahl, G. Geesey, and G. Sayler (ed.), Techniques in microbial ecology. Oxford University Press, New York, NY.
- 32.Krispin, O., and R. Allmansberger. 1995. Changes in DNA supertwist as a response of Bacillus subtilis towards different kinds of stress. FEMS Microbiol. Lett. 134:129-135. [DOI] [PubMed] [Google Scholar]
- 33.Kurtz, S., J. V. Choudhuri, E. Ohlebusch, C. Schleiermacher, J. Stoye, and R. Giegerich. 2001. REPuter: the manifold applications of repeat analysis on a genomic scale. Nucleic Acids Res. 29:4633-4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lowe, T. M., and S. R. Eddy. 1997. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 25:955-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mahillon, J., and M. Chandler. 1998. Insertion sequences. Microbiol. Mol. Biol. Rev. 62:725-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maruyama, A., D. Honda, H. Yamamoto, K. Kitamura, and T. Higashihara. 2000. Phylogenetic analysis of psychrophilic bacteria isolated from the Japan Trench, including a description of the deep-sea species Psychrobacter pacificensis sp. nov. Int. J. Syst. Evol. Microbiol. 50:835-846. [DOI] [PubMed] [Google Scholar]
- 37.Mathews, D. H., J. Sabina, M. Zuker, and D. H. Turner. 1999. Expanded sequence dependence of thermodynamic parameters improves prediction of RNA secondary structure. J. Mol. Biol. 288:911-940. [DOI] [PubMed] [Google Scholar]
- 38.Mazzone, M., E. De Gregorio, A. Lavitola, C. Pagliarulo, P. Alifano, and P. P. Di Nocera. 2001. Whole-genome organization and functional properties of miniature DNA insertion sequences conserved in pathogenic Neisseriae. Gene 278:211-222. [DOI] [PubMed] [Google Scholar]
- 39.Medigue, C., E. Krin, G. Pascal, V. Barbe, A. Bernsel, P. N. Bertin, F. Cheung, S. Cruveiller, S. D'Amico, A. Duilio, G. Fang, G. Feller, C. Ho, S. Mangenot, G. Marino, J. Nilsson, E. Parrilli, E. P. Rocha, Z. Rouy, A. Sekowska, M. L. Tutino, D. Vallenet, G. von Heijne, and A. Danchin. 2005. Coping with cold: the genome of the versatile marine Antarctica bacterium Pseudoalteromonas haloplanktis TAC125. Genome Res. 15:1325-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Methe, B. A., K. E. Nelson, J. W. Deming, B. Momen, E. Melamud, X. Zhang, J. Moult, R. Madupu, W. C. Nelson, R. J. Dodson, L. M. Brinkac, S. C. Daugherty, A. S. Durkin, R. T. DeBoy, J. F. Kolonay, S. A. Sullivan, L. Zhou, T. M. Davidsen, M. Wu, A. L. Huston, M. Lewis, B. Weaver, J. F. Weidman, H. Khouri, T. R. Utterback, T. V. Feldblyum, and C. M. Fraser. 2005. The psychrophilic lifestyle as revealed by the genome sequence of Colwellia psychrerythraea 34H through genomic and proteomic analyses. Proc. Natl. Acad. Sci. U. S. A. 102:10913-10918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Metpally, R. P., and B. V. Reddy. 2009. Comparative proteome analysis of psychrophilic versus mesophilic bacterial species: insights into the molecular basis of cold adaptation of proteins. BMC Genomics 10:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mizushima, T., K. Kataoka, Y. Ogata, R. Inoue, and K. Sekimizu. 1997. Increase in negative supercoiling of plasmid DNA in Escherichia coli exposed to cold shock. Mol. Microbiol. 23:381-386. [DOI] [PubMed] [Google Scholar]
- 43.Mizushima, T., S. Natori, and K. Sekimizu. 1993. Relaxation of supercoiled DNA associated with induction of heat shock proteins in Escherichia coli. Mol. Gen. Genet. 238:1-5. [DOI] [PubMed] [Google Scholar]
- 44.Mueller, S. 1973. Permafrost or permanently frozen ground and related engineering problems. United States Army, Washington, DC.
- 45.Normand, C., G. Duval-Valentin, L. Haren, and M. Chandler. 2001. The terminal inverted repeats of IS911: requirements for synaptic complex assembly and activity. J. Mol. Biol. 308:853-871. [DOI] [PubMed] [Google Scholar]
- 46.Pascal, G., C. Medigue, and A. Danchin. 2005. Universal biases in protein composition of model prokaryotes. Proteins 60:27-35. [DOI] [PubMed] [Google Scholar]
- 47.Pewe, T. 1995. Permafrost, p. 752-759. In Encyclopedia Britannica. Encyclopedia Britannica Inc., New York, NY.
- 48.Ponder, M. 2005. Characterization of physiological and transcriptome changes in the ancient Siberian permafrost bacterium Psychrobacter arcticum 273-4 with low temperature and increased osmotica. Ph.D. dissertation. Michigan State University, East Lansing, MI.
- 49.Ponder, M., T. Vishnivetskaya, J. McGrath, and J. M. Tiedje. 2004. Microbial life in permafrost: extended times in extreme conditions, p. 672. In B. Fuller, N. Lane, and E. E. Benson (ed.), Life in the frozen state. CRC Press, Boca Raton, FL.
- 50.Ponder, M. A., S. J. Gilmour, P. W. Bergholz, C. A. Mindock, R. Hollingsworth, M. F. Thomashow, and J. M. Tiedje. 2005. Characterization of potential stress responses in ancient Siberian permafrost psychroactive bacteria. FEMS Microbiol. Ecol. 53:103-115. [DOI] [PubMed] [Google Scholar]
- 51.Reiersen, H., and A. R. Rees. 2001. The hunchback and its neighbours: proline as an environmental modulator. Trends Biochem. Sci. 26:679-684. [DOI] [PubMed] [Google Scholar]
- 52.Reiser, S., and C. Somerville. 1997. Isolation of mutants of Acinetobacter calcoaceticus deficient in wax ester synthesis and complementation of one mutation with a gene encoding a fatty acyl coenzyme A reductase. J. Bacteriol. 179:2969-2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Riley, M., J. T. Staley, A. Danchin, T. Z. Wang, T. S. Brettin, L. J. Hauser, M. L. Land, and L. S. Thompson. 2008. Genomics of an extreme psychrophile, Psychromonas ingrahamii. BMC Genomics 9:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rivkina, E. M., E. I. Friedmann, C. P. McKay, and D. A. Gilichinsky. 2000. Metabolic activity of permafrost bacteria below the freezing point. Appl. Environ. Microbiol. 66:3230-3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rodrigues, D. F., E. Conceição Jesus, Y. Baez, H. L. Ayala-del-Rio, V. H. Pellizari, D. Gilichinsky, L. Sepúlveda-Torres, and J. M. Tiedje. 2009. Biogeography of two cold-adapted genera: Psychrobacter and Exiguobacterium. ISME J. 3:658-665. [DOI] [PubMed] [Google Scholar]
- 56.Rohde, R., and P. B. Price. 2007. Diffusion-controlled metabolism for long-term survival of single isolated microorganisms trapped within ice crystals. Proc. Natl. Acad. Sci. U. S. A. 104:16592-16597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Romanenko, L. A., P. Schumann, M. Rohde, A. M. Lysenko, V. V. Mikhailov, and E. Stackebrandt. 2002. Psychrobacter submarinus sp. nov. and Psychrobacter marincola sp. nov., psychrophilic halophiles from marine environments. Int. J. Syst. Evol. Microbiol. 52:1291-1297. [DOI] [PubMed] [Google Scholar]
- 58.Russell, N. 2000. Toward a molecular understanding of cold activity of enzymes from psychrophiles. Extremophiles 4:83-90. [DOI] [PubMed] [Google Scholar]
- 59.Russell, N., and J. Volkman. 1980. The effect of growth temperature on wax ester composition in the psychrophilic bacterium Micrococcus cryophilus ATCC 15174. J. Gen. Microbiol. 118:131-141. [Google Scholar]
- 60.Rutherford, K., J. Parkhill, J. Crook, T. Horsnell, P. Rice, M.-A. Rajandream, and B. Barrell. 2000. Artemis: sequence visualization and annotation. Bioinformatics 16:944-945. [DOI] [PubMed] [Google Scholar]
- 61.Saldanha, A. J. 2004. Java Treeview—extensible visualization of microarray data. Bioinformatics 20:3246-3248. [DOI] [PubMed] [Google Scholar]
- 62.Scherer, S., and K. Neuhaus. 2006. Life at low temperatures, p. 210-262. In M. Dworkin, S. Falkow, E. Rosenberg, K.-H. Schleifer, and E. Stackebrandt (ed.), The prokaryotes, vol. 2. Springer, New York, NY. [Google Scholar]
- 63.Siddiqui, K. S., and R. Cavicchioli. 2006. Cold-adapted enzymes. Annu. Rev. Biochem. 75:403-433. [DOI] [PubMed] [Google Scholar]
- 64.Smalas, A. O., H. K. Leiros, V. Os, and N. P. Willassen. 2000. Cold adapted enzymes. Biotechnol. Annu. Rev. 6:1-57. [DOI] [PubMed] [Google Scholar]
- 65.Sonnhammer, E. L., S. R. Eddy, and R. Durbin. 1997. Pfam: a comprehensive database of protein domain families based on seed alignments. Proteins 28:405-420. [DOI] [PubMed] [Google Scholar]
- 66.Sul, W. 2009. Microbial community analyses by rRNA pyrosequencing: microbial community profiling of PCB-contaminated sites and bacterial communities responses to agricultural practices in tropical Africa. Ph.D. dissertation. Michigan State University, East Lansing, MI.
- 67.Tatusov, R. L., D. A. Natale, I. V. Garkavtsev, T. A. Tatusova, U. T. Shankavaram, B. S. Rao, B. Kiryutin, M. Y. Galperin, N. D. Fedorova, and E. V. Koonin. 2001. The COG database: new developments in phylogenetic classification of proteins from complete genomes. Nucleic Acids Res. 29:22-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thorvaldsen, S., E. Hjerde, C. Fenton, and N. P. Willassen. 2007. Molecular characterization of cold adaptation based on ortholog protein sequences from Vibrionaceae species. Extremophiles 11:719-732. [DOI] [PubMed] [Google Scholar]
- 70.Vishnivetskaya, T., S. Kathariou, J. McGrath, D. Gilichinsky, and J. M. Tiedje. 2000. Low-temperature recovery strategies for the isolation of bacteria from ancient permafrost sediments. Extremophiles 4:165-173. [DOI] [PubMed] [Google Scholar]
- 71.Vishnivetskaya, T. A., M. A. Petrova, J. Urbance, M. Ponder, C. L. Moyer, D. A. Gilichinsky, and J. M. Tiedje. 2006. Bacterial community in ancient Siberian permafrost as characterized by culture and culture-independent methods. Astrobiology 6:400-414. [DOI] [PubMed] [Google Scholar]
- 72.Volfovsky, N., B. J. Haas, and S. L. Salzberg. 2001. A clustering method for repeat analysis in DNA sequences. Genome Biol. 2:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.White, D. 2000. The physiology and biochemistry of prokaryotes. Oxford University Press, New York, NY.
- 74.Wixon, J., and D. Kell. 2000. The Kyoto encyclopedia of genes and genomes—KEGG. Yeast 17:48-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wolin, F. A., M. J. Wolin, and R. S. Wolfe. 1963. Formation of methane by bacterial extracts. J. Biol. Chem. 238:2882-2886. [PubMed] [Google Scholar]
- 76.Yumoto, I., K. Hirota, Y. Sogabe, Y. Nodasaka, Y. Yokota, and T. Hoshino. 2003. Psychrobacter okhotskensis sp. nov., a lipase-producing facultative psychrophile isolated from the coast of the Okhotsk Sea. Int. J. Syst. Evol. Microbiol. 53:1985-1989. [DOI] [PubMed] [Google Scholar]
- 77.Zdobnov, E. M., and R. Apweiler. 2001. InterProScan—an integration platform for the signature-recognition methods in InterPro. Bioinformatics 17:847-848. [DOI] [PubMed] [Google Scholar]
- 78.Zheng, S., M. A. Ponder, J. Y. Shih, J. M. Tiedje, M. F. Thomashow, and D. M. Lubman. 2007. A proteomic analysis of Psychrobacter articus 273-4 adaptation to low temperature and salinity using a 2-D liquid mapping approach. Electrophoresis 28:467-488. [DOI] [PubMed] [Google Scholar]
- 79.Zuker, M. 2003. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 31:3406-3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.