Abstract
Deleting individual genes for outer surface c-type cytochromes in Geobacter sulfurreducens partially inhibited the reduction of humic substances and anthraquinone-2,6,-disulfonate. Complete inhibition was obtained only when five of these genes were simultaneously deleted, suggesting that diverse outer surface cytochromes can contribute to the reduction of humic substances and other extracellular quinones.
Humic substances can play an important role in the reduction of Fe(III), and possibly other metals, in sedimentary environments (6, 34). Diverse dissimilatory Fe(III)-reducing microorganisms (3, 5, 7, 9, 11, 19-22, 25) can transfer electrons onto the quinone moieties of humic substances (38) or the model compound anthraquinone-2,6-disulfonate (AQDS). Reduced humic substances or AQDS abiotically reduces Fe(III) to Fe(II), regenerating the quinone. Electron shuttling in this manner can greatly increase the rate of electron transfer to insoluble Fe(III) oxides, presumably because soluble quinone-containing molecules are more accessible for microbial reduction than insoluble Fe(III) oxides (19, 22). Thus, catalytic amounts of humic substances have the potential to dramatically influence rates of Fe(III) reduction in soils and sediments and can promote more rapid degradation of organic contaminants coupled to Fe(III) reduction (1, 2, 4, 10, 24).
To our knowledge, the mechanisms by which Fe(III)-reducing microorganisms transfer electrons to humic substances have not been investigated previously for any microorganism. However, reduction of AQDS has been studied using Shewanella oneidensis (17, 40). Disruption of the gene for MtrB, an outer membrane protein required for proper localization of outer membrane cytochromes (31), inhibited reduction of AQDS, as did disruption of the gene for the outer membrane c-type cytochrome, MtrC (17). However, in each case inhibition was incomplete, and it was suggested that there was a possibility of some periplasmic reduction (17), which would be consistent with the ability of AQDS to enter the cell (40).
The mechanisms for electron transfer to humic substances in Geobacter species are of interest because molecular studies have frequently demonstrated that Geobacter species are the predominant Fe(III)-reducing microorganisms in sedimentary environments in which Fe(III) reduction is an important process (references 20, 32, and 42 and references therein). Geobacter sulfurreducens has routinely been used for investigations of the physiology of Geobacter species because of the availability of its genome sequence (29), a genetic system (8), and a genome-scale metabolic model (26) has made it possible to take a systems biology approach to understanding the growth of this organism in sedimentary environments (23).
AQDS and humic substance reduction in various G. sulfurreducens mutants.
In order to obtain insight into the mechanisms for electron transfer to humic substances in G. sulfurreducens, the impact of various deletions in outer membrane proteins on the reduction of AQDS and soil humic acids was investigated. The focus in this study was on the redox-active proteins that have previously been shown to be most abundant on the outer surface of cells. Cells were cultured as previously described (8) with acetate as the electron donor and fumarate as the electron acceptor. Cell suspensions were prepared from 200-ml cultures under anoxic conditions as previously described (39) and suspended in an osmotically balanced solution (OBS) (2.5 g/liter NaHCO3, 0.25 g/liter NH4Cl, 0.006 g/liter NaH2PO4·H2O, 0.1 g/liter KCl, 1.75 g/liter NaCl) (39).
Washed cell suspensions were added to 10 ml of 5 mM AQDS in OBS with or without 10 mM acetate as the electron donor, and the preparations were incubated at 37°C. Production of anthrahydroquinone-2,6-disulfonate was monitored at 450 nm as previously described (19), and the amount was normalized to the amount of protein determined by the bicinchoninic acid method (41). Studies with soil humic acids were conducted in a similar manner with the AQDS omitted and 2 mg/ml Elliott soil humic acid standard (International Humic Substances Society) added. The electrons transferred to humic substances after 2 h were determined as previously described (19).
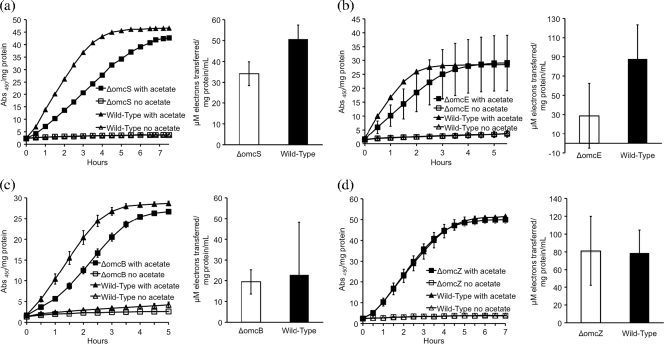
Single-knockout deletions of genes for the outer membrane c-type cytochromes OmcS and OmcE (28) slightly impaired the ability of G. sulfurreducens to reduce AQDS and humic substances. Deletion of omcS had the greatest impact on AQDS reduction (Fig. 1a), whereas deletion of omcE had the most significant impact on humic substance reduction (Fig. 1b). OmcS and OmcE are only loosely bound to the outer surface of G. sulfurreducens (28), and previous studies indicated that both of these cytochromes are involved in electron transfer to Fe(III) and Mn(IV) oxides (28) and electrodes (12). Neither protein is required for reduction of soluble, chelated Fe(III) (28). The finding that deleting omcS or omcE did not significantly inhibit the reduction of AQDS is consistent with the finding that addition of AQDS to omcS or omcE deletion mutants restored the capacity to reduce Fe(III) oxide (28).
FIG. 1.
Impact of deletion of single c-type cytochrome genes on reduction of AQDS (left panels) and the Elliott soil humic acid standard (right panels) for strains with the (a) omcS, (b) omcE, (c) omcB, or (d) omcZ gene deleted. The rates of reduction for the mutant strains were compared with the rate of reduction for a batch of wild-type cells grown and analyzed simultaneously. Incubation was carried out with acetate as the electron donor and (in controls) without added acetate. The data are the means ± standard errors for triplicate incubations.
Deletion of the gene for OmcB (16) only had a slight impact on the potential for AQDS or humic substance reduction (Fig. 1c). OmcB, which is embedded in the outer membrane (35), is essential for reduction of Fe(III) oxide, although the capacity to reduce some soluble Fe(III) is retained (15, 16). These results suggest that there are additional routes for electron transfer to AQDS and humic substances that are not available for reduction of Fe(III) oxides.
OmcZ is a loosely bound outer surface c-type cytochrome that is essential for transfer of electrons to electrodes but not for Fe(III) reduction (33). Deleting OmcZ (33) had no impact on AQDS or humic substance reduction (Fig. 1d). In a similar manner, deleting the gene for PilA, the structural pilin protein, did not inhibit the reduction of AQDS or humic substances (data not shown), consistent with the finding that although deletion of pilA prevents the production of electrically conductive pili that are required for Fe(III) oxide reduction, these pili are not required for reduction of soluble Fe(III) (36).
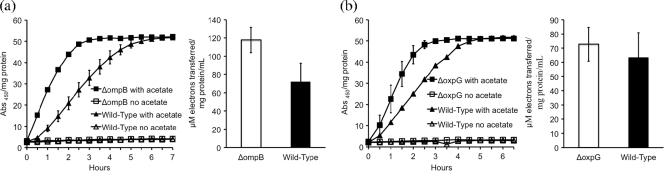
OmpB is representative of a class of putative multicopper, outer surface proteins that are required for reduction of Fe(III) oxide but not for reduction of soluble, chelated Fe(III) (13, 27). Surprisingly, deletion of the gene for OmpB (27) enhanced the capacity for AQDS and humic substance reduction (Fig. 2a). Proper localization of OmpB to the outer surface requires a type II secretion system. Deletion of the gene which encodes the pseudopilin protein OxpG results in accumulation of OmpB in the periplasm (27). A strain of G. sulfurreducens in which oxpG was deleted (27) also reduced AQDS and humic substances faster than wild-type cells (Fig. 2b). These results clearly indicate that, unlike Fe(III) oxide, OmpB is not required for AQDS and humic substance reduction. Why a failure to localize OmpB on the outer surface of the cell enhances AQDS and humic substance reduction requires further study.
FIG. 2.
Impact of deletion of the gene for OmpB (a) or a gene that affects its secretion to the outer surface, oxpG (b). Results for AQDS (left panels) and the Elliott soil humic acid standard (right panels) are shown. The data are the means ± standard errors for triplicate incubations.
The finding that deletion of individual genes for outer surface c-type cytochromes only partially inhibited AQDS or humic substance reduction suggested that there are multiple routes for transfer of electrons to these acceptors. Furthermore, the degree of inhibition of AQDS and humic substance reduction associated with deletion of any one outer surface c-type cytochrome gene could not necessarily be directly related to the relative flow of electrons to AQDS and humic substances in wild-type cells because of the potential for increased electron flow through alternative routes once a single cytochrome gene was deleted. Therefore, in order to investigate this further, the impact of multiple gene deletions was investigated.
To construct strains deficient in multiple cytochromes, linear DNA fragments for single-step gene replacement were constructed with the primers listed in Table 1 as previously described (14, 18, 30). An omcB-omcS double mutant was constructed by replacing the omcS gene of strain DL6 (16) with a kanamycin resistance cassette. The genes for OmcS and OmcT are adjacent on the G. sulfurreducens chromosome (28), and both of these genes were replaced with a kanamycin resistance cassette in strain DL6 (16) in order to generate an omcB-omcS-omcT triple mutant. An omcB-omcS-omcT-omcE quadruple mutant was constructed by replacing the omcE gene of the omcB-omcS-omcT triple mutant (this study) with a gentamicin resistance cassette. An omcB-omcS-omcT-omcE-omcZ quintuple mutant was constructed by replacing the omcZ gene of the omcB-omcS-omcT-omcE quadruple mutant (this study) with a spectinomycin resistance cassette. The orientation of the antibiotic resistance cassettes was the same as that of the disrupted genes. Electroporation, isolation of mutants, and genotype confirmation were performed as previously described (8, 18).
TABLE 1.
Primers used in this study
| Primer | Use(s) | Sequence | Reference |
|---|---|---|---|
| omcS-1 | Recombinant PCR for omcS deletion and omcS-omcT deletion | CTCCGACAAGCTCAGATGCG | This study |
| omcS-2 | Recombinant PCR for omcS deletion and omcS-omcT deletion | GCTGCTGCCACGGAAAGACTT | This study |
| omcS-3 | Recombinant PCR for omcS deletion and omcS-omcT deletion | AAGTCTTTCCGTGGCAGCAGCAGTGCCACCTGGGATGAATG | This study |
| omcS-4 | Recombinant PCR for omcS deletion | GTTGCAGAGAGCGCGCTGGTATGGCAGGTTGGGCGTCGC | This study |
| omcS-5 | Recombinant PCR for omcS deletion | ACCAGCGCGCTCTCTGCAAC | This study |
| omcS-6 | Recombinant PCR for omcS deletion | CTTGAGCCAGCCGAAATCGC | This study |
| omcT-4 | Recombinant PCR for omcS-omcT deletion | GTTGCACAGGACCCGTTGATATGGCAGGTTGGGCGTCGC | This study |
| omcT-5 | Recombinant PCR for omcS-omcT deletion | ATCAACGGGTCCTGTGCAAC | This study |
| omcT-6 | Recombinant PCR for omcS-omcT deletion | CGGGCATCAGGGAATAGAGG | This study |
| omcE-1 | Recombinant PCR for omcE deletion | TTGTAGCGAATTGCGGTTGG | This study |
| omcE-2 | Recombinant PCR for omcE deletion | GCTTGACCGGCACGTTATTC | This study |
| omcE-3 | Recombinant PCR for omcE deletion | GAATAACGTGCCGGTCAAGCGGCCCGGTACCGAGGAC | This study |
| omcE-4 | Recombinant PCR for omcE deletion | CATTGCTCAGATCGGTGCCCGCGGTGGAGCTCGAATTG | This study |
| omcE-5 | Recombinant PCR for omcE deletion | GGGCACCGATCTGAGCAATG | This study |
| omcE-6 | Recombinant PCR for omcE deletion | GCCAAGACCGACACTGACG | This study |
| 2076-1 | Recombinant PCR for omcZ deletion | ATGTGATGCGATATCCCGGC | 45 |
| 2076-2 | Recombinant PCR for omcZ deletion | CGCTGACGTGACACTCGAGAC | 45 |
| 2076spec-3 | Recombinant PCR for omcZ deletion | GTCTCGAGTGTCACGTCAGCGGGAGCACAGGATGACGCCTAAC | This study |
| 2076spec-4 | Recombinant PCR for omcZ deletion | GGTGATGCGGAGCTCGTAGCGCATAGTCTCCCCAGCTCTC | This study |
| 2076-5 | Recombinant PCR for omcZ deletion | CTACGAGCTCCGCATCACC | 45 |
| 2076-6 | Recombinant PCR for omcZ deletion | CACCCAGAGGAGGCAGCAGG | 45 |
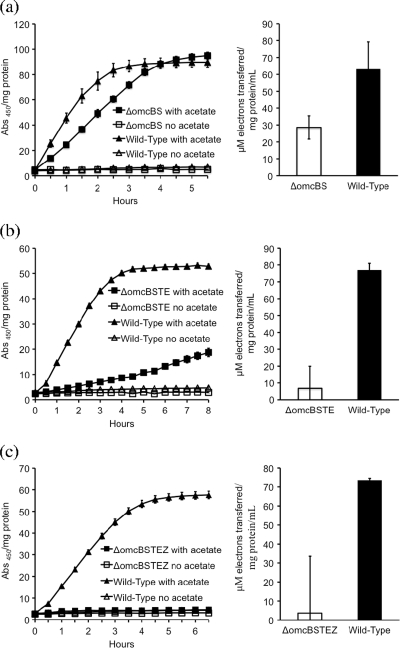
The double mutant in which omcS and omcB were deleted was impaired in AQDS and humic substance reduction compared to the wild type, but only marginally more than the strain with the single mutation in omcS (Fig. 3a). A triple mutant in which the gene for the OmcS homolog OmcT was also deleted had a phenotype similar to that of the omcB-omcS double mutant (data not shown). However, the mutations in a quadruple mutant in which the gene for OmcE was also deleted resulted in substantially greater inhibition of AQDS and humic substance reduction (Fig. 3b). Deletion of the genes for all five outer surface c-type cytochromes, including omcZ, completely inhibited the reduction of AQDS, and there was little, if any, reduction of humic substances (Fig. 3c). This result suggests that although deletion of just omcZ did not inhibit AQDS and humic substance reduction, OmcZ can play a minor role in transfer of electrons to these electron acceptors, at least when alternative routes for electron transfer are eliminated.
FIG. 3.
Impact of deletion of multiple c-type cytochromes on reduction of AQDS (left panels) and the Elliott soil humic acid standard (right panels). The strains evaluated were deficient in the genes for (a) OmcB and OmcS; (b) OmcB, OmcE, OmcS, and OmcT; or (c) OmcB, OmcE, OmcS, OmcT, and OmcZ. The data are the means ± standard errors for triplicate incubations.
Implications.
The results described above suggest that AQDS and humic substances are reduced at the outer surface of G. sulfurreducens and that a number of outer surface c-type cytochromes previously identified as proteins that are important in transfer of electrons to Fe(III) and/or electrodes contribute to this process. The fact that AQDS reduction was completely inhibited in the quintuple outer surface cytochrome mutant suggests that there is no significant reduction of AQDS in the cells of this mutant. The fact that a number of outer surface cytochromes appear to be capable of transferring electrons to AQDS and humic substances suggests that the reduction of quinone moieties in these molecules is a rather nonspecific redox reaction. This lack of specificity for AQDS and humic substance reduction contrasts with the specific requirement for the outer surface cytochrome OmcZ for high levels of current production in microbial fuel cells (33, 37) and the requirement for OmcS for Fe(III) oxide reduction (28). However, G. sulfurreducens can adapt to the loss of most outer surface cytochromes in order to reduce soluble, chelated Fe(III) (15, 28). It is likely that the similarities between AQDS reduction, humic substance reduction, and soluble Fe(III) reduction and their differences with insoluble electron acceptors, such as Fe(III) oxides and electrodes, are related to access. Electrons can be transferred readily from low-potential hemes to quinones or Fe(III), and mere contact between the two types of molecules is probably sufficient for reduction of soluble quinones or Fe(III). However, accessing insoluble electron acceptors is likely to be more difficult and may require specific arrangements of the outer surface molecules in order to bring hemes and electrodes or Fe(III) oxides into sufficient proximity for electron transfer.
Long-range extracellular electron transfer via quinone-containing electron shuttles can allow microorganisms to reduce not only Fe(III) oxides but also a variety of contaminant metals and organic compounds, as well as electrodes. The finding that diverse outer surface c-type cytochromes are capable of extracellular quinone reduction suggests that it may be rather simple to engineer microorganisms which have other desirable properties for this form of extracellular electron transfer.
Acknowledgments
This research was supported by the Office of Science (BER), U.S. Department of Energy (cooperative agreement DE-FC02-02ER63446), and was partially supported by a grant from the KRIBB Research Initiative Program and by grant M10437010001 from the Ministry of Education, Science and Technology (MEST) of the Republic of Korea.
Footnotes
Published ahead of print on 12 February 2010.
REFERENCES
- 1.Anderson, R. T., J. N. Rooney-Varga, C. V. Gaw, and D. R. Lovley. 1998. Anaerobic benzene oxidation in the Fe(III) reduction zone of petroleum-contaminated aquifers. Environ. Sci. Technol. 32:1222-1229. [Google Scholar]
- 2.Bhushan, B., A. Halasz, and J. Hawari. 2006. Effect of iron(III), humic acids and anthraquinone-2,6-disulfonate on biodegradation of cyclic nitramines by Clostridium sp. EDB2. J. Appl. Microbiol. 100:555-563. [DOI] [PubMed] [Google Scholar]
- 3.Bond, D. R., and D. R. Lovley. 2002. Reduction of Fe(III) oxide by methanogens in the presence and absence of extracellular quinones. Environ. Microbiol. 4:115-124. [DOI] [PubMed] [Google Scholar]
- 4.Bradley, P. M., and F. H. Chapelle. 1996. Anaerobic mineralization of vinyl chloride in Fe(III)-reducing, aquifer sediments. Environ. Sci. Technol. 30:2084-2086. [Google Scholar]
- 5.Cervantes, F. J., F. A. de Bok, T. Duong-Dac, A. J. Stams, G. Lettinga, and J. A. Field. 2002. Reduction of humic substances by halorespiring, sulphate-reducing and methanogenic microorganisms. Environ. Microbiol. 4:51-57. [DOI] [PubMed] [Google Scholar]
- 6.Chen, J., B. Gu, R. A. Royer, and W. D. Burgos. 2003. The roles of natural organic matter in chemical and microbial reduction of ferric iron. Sci. Total Environ. 307:167-178. [DOI] [PubMed] [Google Scholar]
- 7.Coates, J., D. Ellis, E. Blunt-Harris, C. Gaw, E. Roden, and D. R. Lovley. 1998. Recovery of humic-reducing bacteria from a diversity of environments. Appl. Environ. Microbiol. 64:1504-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coppi, M. V., C. Leang, S. J. Sandler, and D. R. Lovley. 2001. Development of a genetic system for Geobacter sulfurreducens. Appl. Environ. Microbiol. 67:3180-3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finneran, K. T., H. M. Forbush, C. V. VanPraagh, and D. R. Lovley. 2002. Desulfitobacterium metallireducens sp. nov., an anaerobic bacterium that couples growth to the reduction of metals and humic acids as well as chlorinated compounds. Int. J. Syst. Evol. Microbiol. 52:1929-1935. [DOI] [PubMed] [Google Scholar]
- 10.Finneran, K. T., and D. R. Lovley. 2001. Anaerobic degradation of methyl tert-butyl ether (MTBE) and tert-butyl alcohol (TBA). Environ. Sci. Technol. 35:1785-1790. [DOI] [PubMed] [Google Scholar]
- 11.He, Q., and R. A. Sanford. 2003. Characterization of Fe(III) reduction by chlororespiring Anaeromxyobacter dehalogenans. Appl. Environ. Microbiol. 69:2712-2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holmes, D. E., S. K. Chaudhuri, K. P. Nevin, T. Mehta, B. A. Methé, A. Liu, J. E. Ward, T. L. Woodard, J. Webster, and D. R. Lovley. 2006. Microarray and genetic analysis of electron transfer to electrodes in Geobacter sulfurreducens. Environ. Microbiol. 8:1805-1815. [DOI] [PubMed] [Google Scholar]
- 13.Holmes, D. E., T. Mester, R. A. O'Neil, L. A. Perpetua, M. J. Larrahondo, R. Glaven, M. L. Sharma, J. E. Ward, K. P. Nevin, and D. R. Lovley. 2008. Genes for two multicopper proteins required for Fe(III) oxide reduction in Geobacter sulfurreducens have different expression patterns both in the subsurface and on energy-harvesting electrodes. Microbiology 154:1422-1435. [DOI] [PubMed] [Google Scholar]
- 14.Kim, B.-C., C. Leang, Y. H. Ding, R. H. Glaven, M. V. Coppi, and D. R. Lovley. 2005. OmcF, a putative c-type monoheme outer membrane cytochrome required for the expression of other outer membrane cytochromes in Geobacter sulfurreducens. J. Bacteriol. 187:4505-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leang, C., L. A. Adams, K. J. Chin, K. P. Nevin, B. A. Methé, J. Webster, M. L. Sharma, and D. R. Lovley. 2005. Adaptation to disruption of the electron transfer pathway for Fe(III) reduction in Geobacter sulfurreducens. J. Bacteriol. 187:5918-5926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leang, C., M. V. Coppi, and D. R. Lovley. 2003. OmcB, a c-type polyheme cytochrome, involved in Fe(III) reduction in Geobacter sulfurreducens. J. Bacteriol. 185:2096-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lies, D. P., M. E. Hernandez, A. Kappler, R. E. Mielke, J. A. Gralnick, and D. K. Newman. 2005. Shewanella oneidensis MR-1 uses overlapping pathways for iron reduction at a distance and by direct contact under conditions relevant for biofilms. Appl. Environ. Microbiol. 71:4414-4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lloyd, J. R., C. Leang, A. L. Hodges-Myerson, M. V. Coppi, S. Ciufo, B. A. Methé, S. J. Sandler, and D. R. Lovley. 2003. Biochemical and genetic characterization of PpcA, a periplasmic c-type cytochrome in Geobacter sulfurreducens. Biochem. J. 369:153-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lovley, D. R., J. Coates, E. L. Blunt-Harris, E. J. P. Phillips, and J. C. Woodward. 1996. Humic substances as electron acceptors for microbial respiration. Nature 382:445-448. [Google Scholar]
- 20.Lovley, D. R., D. E. Holmes, and K. P. Nevin. 2004. Dissimilatory Fe(III) and Mn(IV) reduction. Adv. Microb. Physiol. 49:219-286. [DOI] [PubMed] [Google Scholar]
- 21.Lovley, D. R., K. Kashefi, M. Vargas, J. M. Tor, and E. L. Blunt-Harris. 2000. Reduction of humic substances and Fe(III) by hyperthermophilic microorganisms. Chem. Geol. 169:289-298. [Google Scholar]
- 22.Lovley, D. R., J. L. Fraga, E. L. Blunt-Harris, L. A. Hayes, E. J. P. Phillips, and J. D. Coates. 1998. Humic substances as a mediator for microbially catalyzed metal reduction. Acta Hydrochim. Hydrobiol. 26:152-157. [Google Scholar]
- 23.Lovley, D. R., R. Mahadevan, and K. P. Nevin. 2008. Systems biology approach to bioremediation with extracellular electron transfer, p. 71-96. In E. Diaz (ed.), Microbial biodegradation, genomics and molecular biology. Caister Academic Press, Norfolk, United Kingdom.
- 24.Lovley, D. R., J. C. Woodward, and F. H. Chapelle. 1996. Rapid anaerobic benzene oxidation with a variety of chelated Fe(III) forms. Appl. Environ. Microbiol. 62:288-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luijten, M., S. Weelink, B. Godschalk, A. Langenhoff, M. van Eekert, G. Schraa, and A. Stams. 2004. Anaerobic reduction and oxidation of quinone moieties and the reduction of oxidized metals by halorespiring and related organisms. FEMS Microbiol. Ecol. 49:145-149. [DOI] [PubMed] [Google Scholar]
- 26.Mahadevan, R., D. R. Bond, J. E. Butler, A. Esteve-Nunez, M. V. Coppi, B. O. Palsson, C. H. Schilling, and D. R. Lovley. 2006. Characterization of metabolism in the Fe(III)-reducing organism Geobacter sulfurreducens by constraint-based modeling. Appl. Environ. Microbiol. 72:1558-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehta, T., S. E. Childers, R. Glaven, D. R. Lovley, and T. Mester. 2006. A putative multicopper protein secreted by an atypical type II secretion system involved in the reduction of insoluble electron acceptors in Geobacter sulfurreducens. Microbiology 152:2257-2264. [DOI] [PubMed] [Google Scholar]
- 28.Mehta, T., M. V. Coppi, S. E. Childers, and D. R. Lovley. 2005. Outer membrane c-type cytochromes required for Fe(III) and Mn(IV) oxide reduction in Geobacter sulfurreducens. Appl. Environ. Microbiol. 71:8634-8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Methé, B. A., K. E. Nelson, J. A. Eisen, I. T. Paulsen, W. Nelson, J. F. Heidelberg, D. Wu, M. Wu, N. Ward, M. J. Beanan, R. J. Dodson, R. Madupu, L. M. Brinkac, S. C. Daugherty, R. T. DeBoy, A. S. Durkin, M. Gwinn, J. F. Kolonay, S. A. Sullivan, D. H. Haft, J. Selengut, T. M. Davidsen, N. Zafar, O. White, B. Tran, C. Romero, H. A. Forberger, J. Weidman, H. Khouri, T. V. Feldblyum, T. R. Utterback, S. E. Van Aken, D. R. Lovley, and C. M. Fraser. 2003. Genome of Geobacter sulfurreducens: metal reduction in subsurface environments. Science 302:1967-1969. [DOI] [PubMed] [Google Scholar]
- 30.Murphy, K. C., K. G. Campellone, and A. R. Poteete. 2000. PCR-mediated gene replacement in Escherichia coli. Gene 246:321-330. [DOI] [PubMed] [Google Scholar]
- 31.Myers, C. R., and J. M. Myers. 2002. MtrB is required for proper incorporation of the cytochromes OmcA and OmcB into the outer membrane of Shewanella putrefaciens MR-1. Appl. Environ. Microbiol. 68:5585-5594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nealson, K. H., and D. Saffarini. 1994. Iron and manganese in anaerobic respiration: environmental significance, physiology, and regulation. Annu. Rev. Microbiol. 48:311-343. [DOI] [PubMed] [Google Scholar]
- 33.Nevin, K. P., B.-C. Kim, R. H. Glaven, J. P. Johnson, T. L. Woodard, B. A. Methé, J. DiDonato, R. J. S. Covalla, A. Franks, A. Liu, and D. R. Lovley. 2009. Anode biofilm transcriptomics reveals outer surface components essential for high density current production in Geobacter sulfurreducens fuel cells. PLoS One 4:e5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nevin, K. P., and D. R. Lovley. 2002. Mechanisms for Fe(III) oxide reduction in sedimentary environments. Geomicrobiol. J. 19:141-159. [Google Scholar]
- 35.Qian, X., G. Reguera, T. Mester, and D. R. Lovley. 2007. Evidence that OmcB and OmpB of Geobacter sulfurreducens are outer membrane surface proteins. FEMS Microbiol. Lett. 277:21-27. [DOI] [PubMed] [Google Scholar]
- 36.Reguera, G., K. D. McCarthy, T. Mehta, J. S. Nicoll, M. T. Tuominen, and D. R. Lovley. 2005. Extracellular electron transfer via microbial nanowires. Nature 435:1098-1101. [DOI] [PubMed] [Google Scholar]
- 37.Richter, H., K. P. Nevin, H. Jia, D. A. Lowy, D. R. Lovley, and L. M. Tender. 2009. Cyclic voltammetry of biofilms of wild type and mutant Geobacter sulfurreducens on fuel cell anodes indicates possible roles of OmcB, OmcZ, type IV pili, and protons in extracellular electron transfer. Energy Environ. Sci. 2:506-516. [Google Scholar]
- 38.Scott, D. T., D. M. McKnight, E. L. Blunt-Harris, S. E. Kolesar, and D. R. Lovley. 1998. Quinone moieties act as electron acceptors in the reduction of humic substances by humics-reducing microorganisms. Environ. Sci. Technol. 32:2984-2989. [Google Scholar]
- 39.Shelobolina, E. S., M. V. Coppi, A. A. Korenevsky, L. N. Didonato, S. A. Sullivan, H. Konishi, H. Xu, C. Leang, J. E. Butler, B.-C. Kim, and D. R. Lovley. 2007. Importance of c-type cytochromes for U(VI) reduction by Geobacter sulfurreducens. BMC Microbiol. 7:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shyu, J. B., D. P. Lies, and D. K. Newman. 2002. Protective role of tolC in efflux of the electron shuttle anthraquinone-2,6-disulfonate. J. Bacteriol. 184:1806-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith, P. K., R. I. Krohn, G. T. Hermanson, A. K. Mallia, F. H. Gartner, E. K. Provenzano, N. M. Fujimoto, B. J. Goeke, and D. C. Klenk. 1985. Measurement of protein using bicinchonic acid. Anal. Biochem. 150:76-85. [DOI] [PubMed] [Google Scholar]
- 42.Weber, K. A., L. A. Achenbach, and J. D. Coates. 2006. Microorganisms pumping iron: anaerobic microbial iron oxidation and reduction. Nat. Rev. Microbiol. 4:752-764. [DOI] [PubMed] [Google Scholar]