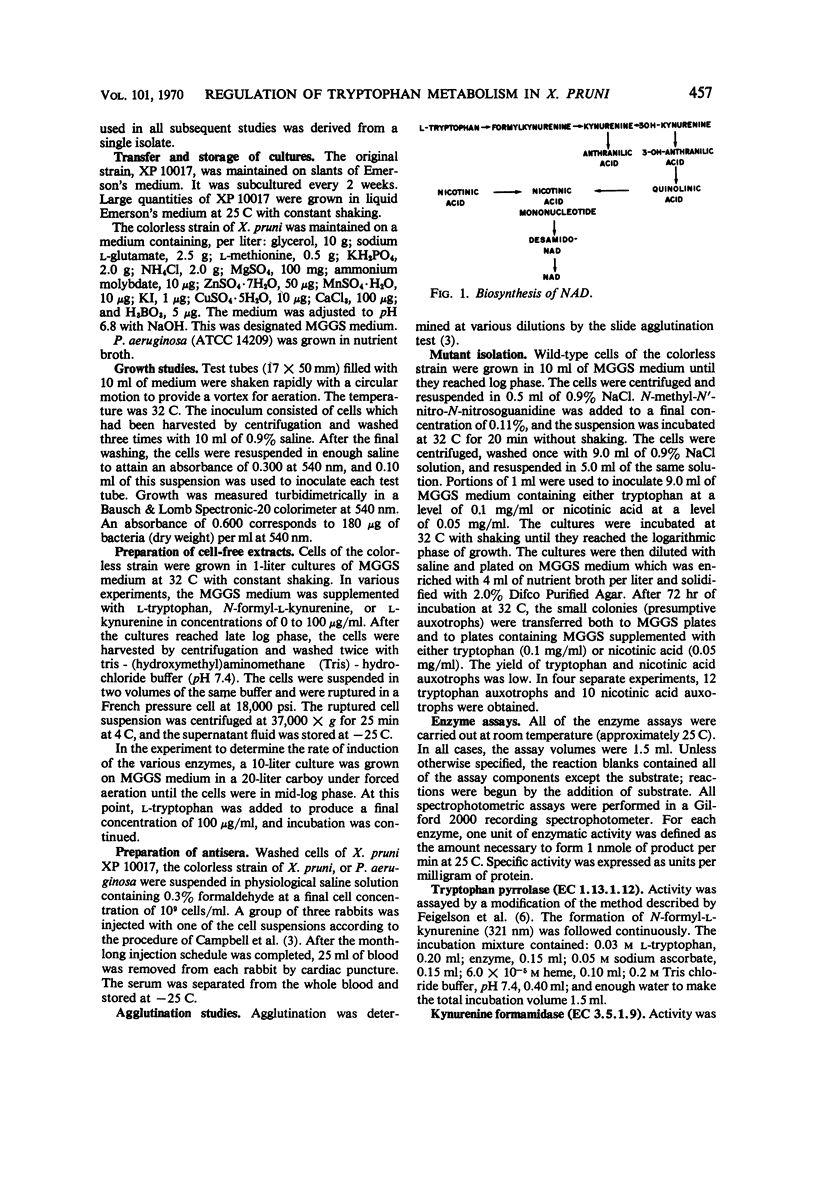
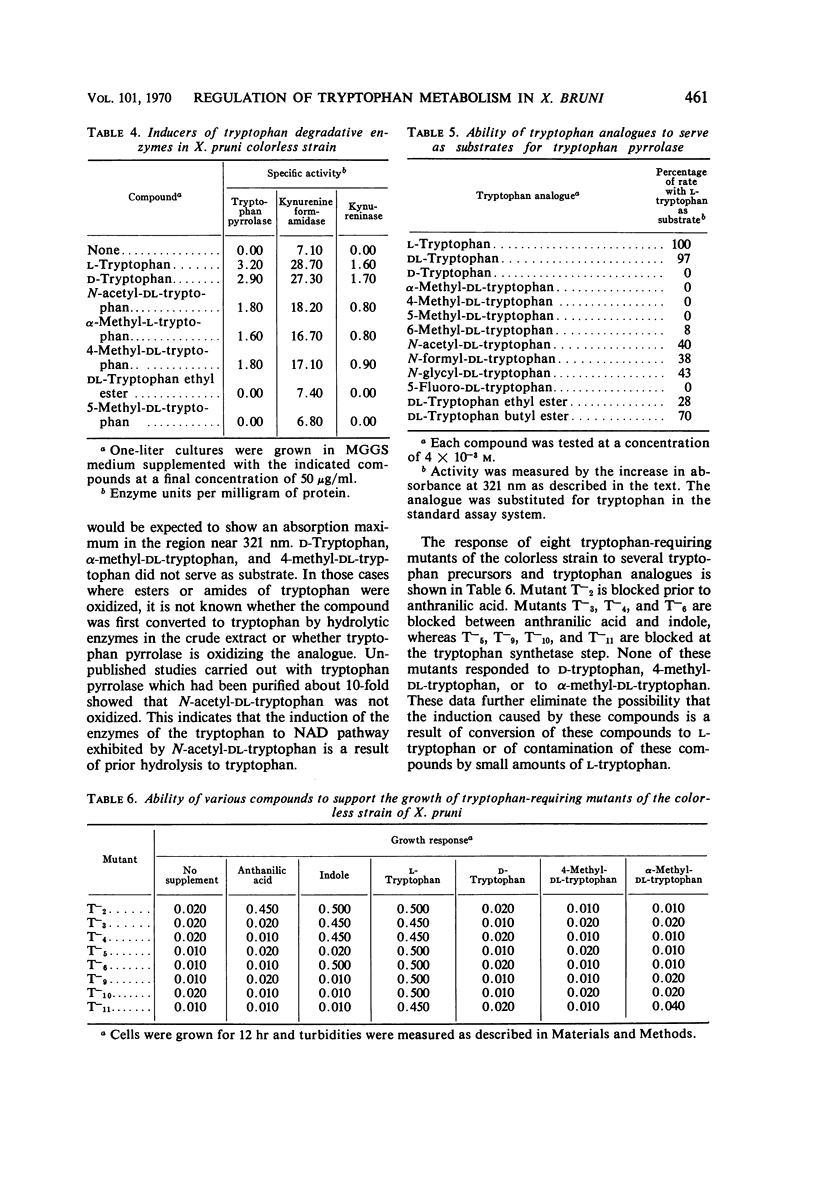
Abstract
A colorless strain of Xanthomonas pruni was isolated which is capable of converting tryptophan to nicotinamide adenine dinucleotide (NAD). The enzymes responsible for the conversion of tryptophan to quinolinic acid were shown to be present. Nicotinic acid-requiring mutants were isolated, and it was found that the growth of these mutants can be supported by various intermediates on the pathway from tryptophan to NAD. The first three enzymes on this pathway are induced coordinately by l-tryptophan. Gratuitous inducers of these enzymes include d-tryptophan, α-methyl-dl-tryptophan, and 4-methyl-dl-tryptophan; formyl-l-kynurenine and l-kynurenine were not effective as inducers. These data suggest that at least the first three enzymes in the pathway from tryptophan to NAD are under common regulatory control.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad F., Moat A. G. Nicotinic acid biosynthesis in prototrophs and tryptophan auxotrophs of Saccharomyces cerevisiae. J Biol Chem. 1966 Feb 25;241(4):775–780. [PubMed] [Google Scholar]
- Beadle G. W., Mitchell H. K., Nyc J. F. Kynurenine as an Intermediate in the Formation of Nicotinic Acid from Tryptophane by Neurospora. Proc Natl Acad Sci U S A. 1947 Jun;33(6):155–158. doi: 10.1073/pnas.33.6.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS D., HENDERSON L. M., POWELL D. The niacin-tryptophan relationship in the metabolism of Xanthomonas pruni. J Biol Chem. 1951 Apr;189(2):543–549. [PubMed] [Google Scholar]
- Feigelson P., Ishimura Y., Hayaishi O. On the activation and catalytic mechanism of microbial tryptophan pyrrolase. Biochem Biophys Res Commun. 1964;14:96–101. doi: 10.1016/0006-291x(63)90218-3. [DOI] [PubMed] [Google Scholar]
- JAKOBY W. B., BONNER D. M. Kynureninase from Neurospora: purification and properties. J Biol Chem. 1953 Dec;205(2):699–707. [PubMed] [Google Scholar]
- Lingens F., Vollprecht P. Zur Biosynthese der Nicotinsäure in Streptomyceten, Algen, Phycomyceten und Hefe. Hoppe Seylers Z Physiol Chem. 1964;339(1):64–74. [PubMed] [Google Scholar]
- MIYAKE A., BOKMAN A. H., SCHWEIGERT B. S. 3-Hydroxyanthranilic acid metabolism. VI. Chemical studies on intermediate. J Biol Chem. 1954 Nov;211(1):391–404. [PubMed] [Google Scholar]
- Malmström B. G., Reinhammar B., Vänngård T. Two forms of copper (II) in fungal laccase. Biochim Biophys Acta. 1968 Feb 1;156(1):67–76. doi: 10.1016/0304-4165(68)90105-0. [DOI] [PubMed] [Google Scholar]
- Ogasawara N., Gander J. E., Henderson L. M. Purification and properties of 3-hydroxyanthranilate oxygenase from beef kidney. J Biol Chem. 1966 Feb 10;241(3):613–619. [PubMed] [Google Scholar]
- PALLERONI N. J., STANIER R. Y. REGULATORY MECHANISMS GOVERNING SYNTHESIS OF THE ENZYMES FOR TRYPTOPHAN OXIDATION BY PSEUDOMONAS FLUORESCENS. J Gen Microbiol. 1964 May;35:319–334. doi: 10.1099/00221287-35-2-319. [DOI] [PubMed] [Google Scholar]
- Rosenfeld H., Feigelson P. Synergistic and product induction of the enzymes of tryptophan metabolism in Pseudomonas acidovorans. J Bacteriol. 1969 Feb;97(2):697–704. doi: 10.1128/jb.97.2.697-704.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STARR M. P., STEPHENS W. L. PIGMENTATION AND TAXONOMY OF THE GENUS XANTHOMONAS. J Bacteriol. 1964 Feb;87:293–302. doi: 10.1128/jb.87.2.293-302.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEPHENS W. L., STARR M. P. LOCALIZATION OF CAROTENOID PIGMENT IN THE CYTOPLASMIC MEMBRANE OF XANTHOMONAS JUGLANDIS. J Bacteriol. 1963 Nov;86:1070–1074. doi: 10.1128/jb.86.5.1070-1074.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr M. P. Nutrition of Phytopathogenic Bacteria: I. Minimal Nutritive Requirements of Genus Xanthomonas. J Bacteriol. 1946 Feb;51(2):131–143. [PMC free article] [PubMed] [Google Scholar]
- Tokuyama K. Further studies on bacterial and liver tryptophan pyrrolases. Biochim Biophys Acta. 1968 Jan 8;151(1):76–87. doi: 10.1016/0005-2744(68)90163-0. [DOI] [PubMed] [Google Scholar]
- Tremblay G. C., Gottlieb J. A., Knox W. E. Induction by L-tryptophan and an analogue, alpha-methyl-DL-tryptophan, of the enzymes catabolizing L-tryptophan in Pseudomonas. J Bacteriol. 1967 Jan;93(1):168–176. doi: 10.1128/jb.93.1.168-176.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON R. G., HENDERSON L. M. Tryptophan-niacin relationship in Xanthomonas pruni. J Bacteriol. 1963 Jan;85:221–229. doi: 10.1128/jb.85.1.221-229.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]



