Summary
Metabolic processes that regulate muscle energy use are major determinants of bodily energy balance. Here we find that sarcolemmal ATP-sensitive K+ (KATP) channels, which couple membrane excitability with cellular metabolic pathways, set muscle energy expenditure under physiological stimuli. Disruption of KATP channel function provoked, in conditions of unaltered locomotor activity and blood substrate availability, an extra energy cost of cardiac and skeletal muscle performance. Inefficient fuel metabolism in KATP channel-deficient striated muscles reduced glycogen and fat body depots promoting a lean phenotype. The propensity to lesser body weight imposed by KATP channel deficit persisted under a high-fat diet, yet obesity restriction was achieved at the cost of compromised physical endurance. Thus, sarcolemmal KATP channels govern muscle energy economy, and their down-regulation in a tissue-specific manner could present an anti-obesity strategy by rendering muscle increasingly thermogenic at rest and less fuel efficient during exercise.
Body weight reflects the balance between energy intake and consumption. Biological systems have evolved in an environment with ample demand for physical activity and restricted food supply presenting a selection bias for mechanisms that conserve energy (Celi, 2009). While naturally protective, however, these energy conserving systems under conditions of hyperalimentation and sedentary lifestyle promote obesity (Schwartz et al., 2003; Ogden et al., 2006). Hence, comprehension of energy conserving mechanisms and interference with their efficiency could advance obesity treatment and prevention.
The ATP-sensitive K+ (KATP) channel, due to a unique ability to integrate energy cues with membrane excitability-dependent processes, may represent such an energy controlling mechanism (Miki and Seino, 2005; Ashcroft, 2005; Alekseev et al., 2005; Nichols, 2006). Widely expressed in excitable tissues, KATP channels are formed by tissue-specific multimerization of pore-forming Kir6.x with regulatory SURx subunits (Inagaki et al., 1995; Yamada et al., 1997; Babenko et al., 1998), yet their adenine nucleotide sensing function remains consistent throughout the body (Nichols et al., 1996; Aguilar-Bryan et al., 2001; Ashcroft, 2005; Zingman et al., 2003).
Sarcolemmal KATP channels (Kir6.2/SUR2A) are increasingly recognized as safety valves protecting muscle function under stress (Matar et al., 2000; Zingman et al., 2002; Renaud, 2002; Kane et al., 2004; Miki and Seino, 2005; Nichols, 2006). In response to stress-induced modulation of intracellular nucleotide levels (Nichols and Lederer, 1991; Miki and Seino, 2005), KATP channel opening limits the duration or amplitude of cardiac and skeletal muscle action potentials, reducing Na+/K+-, Ca2+- and myosin-ATPase operation to prevent energy depletion (Zingman et al., 2002; Thabet et al., 2005; Cifelli et al., 2007, 2008). While skeletal and cardiac muscles account for 10–20% of sedentary daily energy use, during physical activity their energy consumption increases 20–100 times over basal levels (McArdle et al., 1996). Therefore, by integrating with the intracellular energy network (Weiss and Lamp, 1989; Nichols and Lederer, 1990; Carrasco et al., 2001; Abraham et al., 2002; Selivanov et al., 2004), KATP channels may set the performance of cellular energy-sparing systems and control muscle energy expenditure not only under stress conditions but at any level of activity.
Here, genetic disruption of KATP channel function was found to raise energy expenditure in cardiac and skeletal muscles, generating a lean phenotype resistant to diet-induced obesity albeit with compromised physical endurance. The KATP channel is thus identified as a safeguard of bodily energy economy, mapping a molecular regulator of obesity risk.
RESULTS
KATP channel-dependent control of body weight
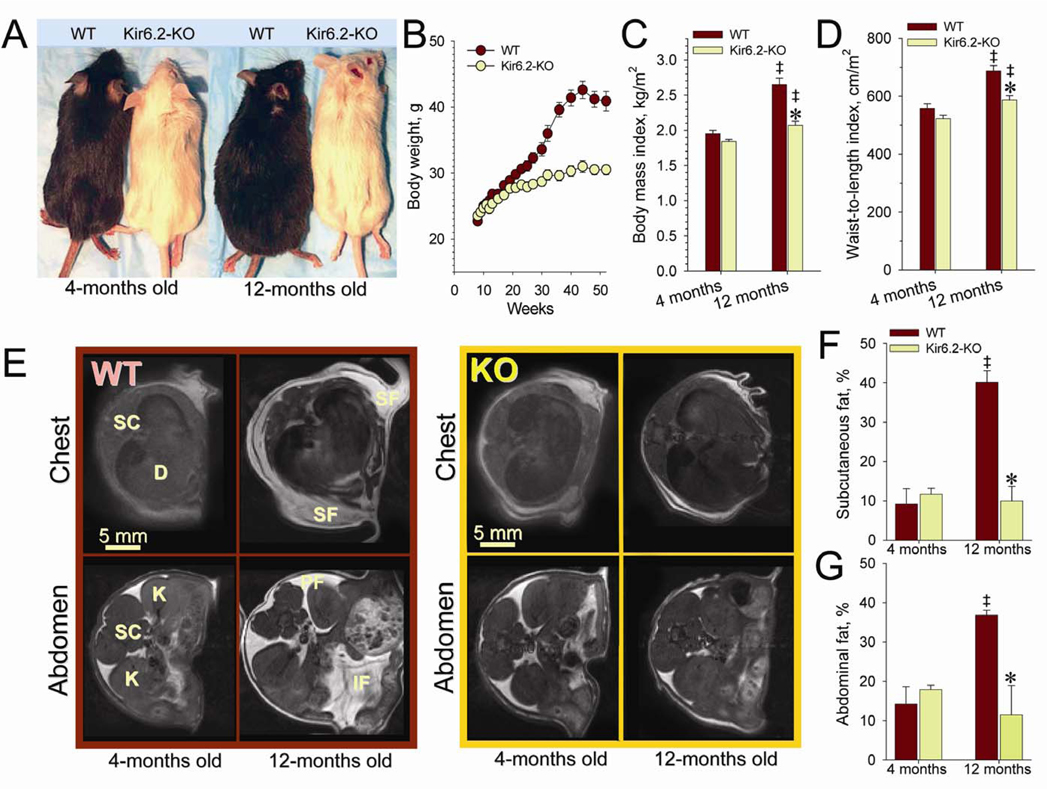
Mice lacking KATP channels (Kir6.2-KO), individually housed and fed ad libitum with regular chow diet, demonstrated lower body weights than age- and gender-matched wild-type (WT; see Experimental Procedures). Compared to WT, until 4 months of age, Kir6.2-KO had similar body weight and fat distribution (Figure 1B and 1E–G), as well as adipose tissue-related endocrine status (Figure S1). Divergence in body weight was manifested at 20 weeks of age, and was maintained throughout the 50-week follow-up (Figure 1A and 1B). The reduced body weight in Kir6.2-KO was not a consequence of altered growth, as both cohorts displayed similar heights and reached 12.2±0.2 cm (n=10) for Kir6.2-KO and 12.4±0.1 cm (n=10) for WT by 12 months of age. As a result, the body mass index and waist-to-height ratio of Kir6.2-KO were significantly reduced compared to WT (Figure 1C and 1D). Whole body magnetic resonance imaging (MRI) indicated that the lower body weight of 1-year old Kir6.2-KO was associated with depletion of subcutaneous and abdominal fat depots (Figure 1E–1G). Thus, the mature Kir6.2-KO demonstrated a phenotype characterized by low body weight and reduced fat storage, suggesting an altered balance between energy availability and consumption induced by KATP channel gene knockout.
Figure 1. Kir6.2-KO mice inherit low body weight.
(A) WT display larger trunk size compared to Kir6.2-KO at 1-year follow up.
(B) Divergence in body weight in individually caged WT (n=10) and Kir6.2-KO (n=10) by 5 months of age.
(C and D) Body mass index (BMI) calculated as the ratio of body weight to the square of body nasal-to-anal length, and waist-to-length index (WLI) calculated as the ratio of abdomen diameter to the square of body nasal-to-anal length, increased during maturation of WT and Kir6.2-KO (‡, p<0.05 at 12 versus 4-months within each cohort; n=10 in each group), with BMI and WLI significantly lower at 12 months in Kir6.2-KO compared to WT (*, p<0.05; n=10 in each group).
(E) Transverse MRI scans through thorax and abdomen of WT (left) and Kir6.2-KO (right) at 4 and 12 months. Abbreviations: D, diaphragm; IF, interstitial fat; K, kidney; PF, retroperitoneal fat; SC, spinal cord; SF, subcutaneous fat. Note prominent fat deposition in WT compared to Kir6.2-KO at 1-year of age.
(F and G) Mean areas of subcutaneous and abdominal fat, expressed relative to total cross-sectional area, were significantly increased in 12-months old WT compared to either 4-month-old WT (‡, p<0.05; n=4) or 12-month-old Kir6.2-KO (*, p<0.05; n=4). In contrast to WT, fat stores in Kir6.2-KO were statistically indistinguishable at both ages.
Data are mean±SEM.
Augmented energy expenditure underlies KATP channel-deficient lean phenotype
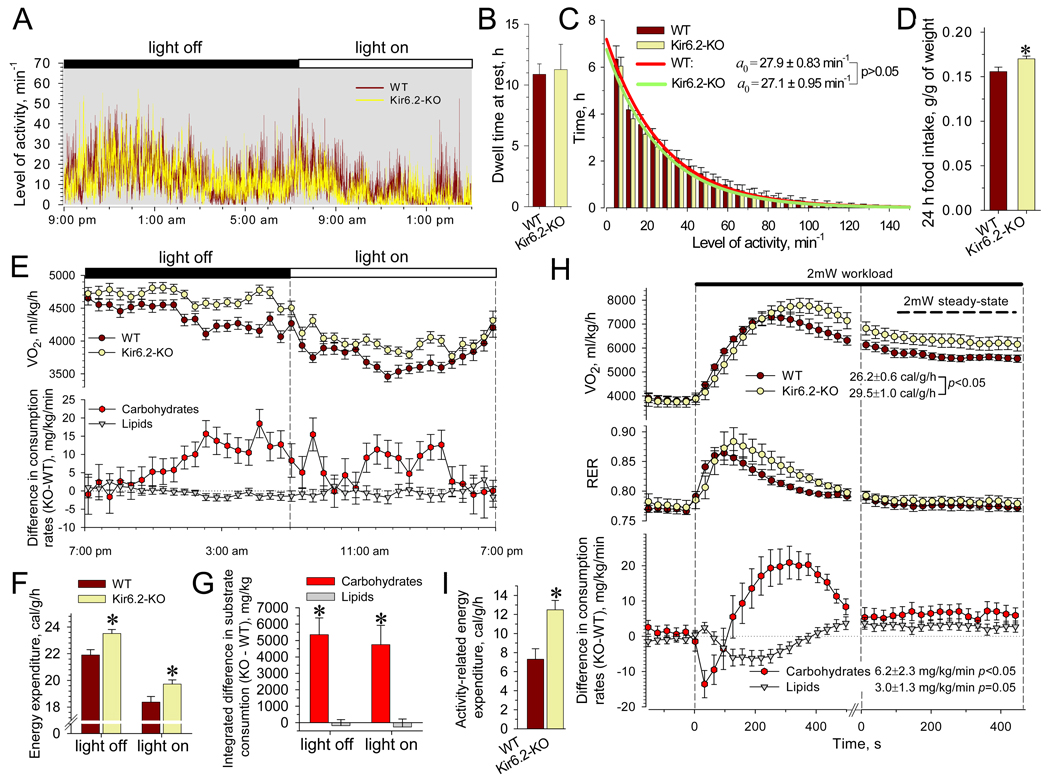
To assess KATP channel-dependent regulation of energy balance, Kir6.2-KO and WT mice were studied at the age of 3–4 months when effects of senescence or differences in adiposity could be excluded. Locomotor activity, compared by telemetry, was indistinguishable between cohorts (Figure 2A). Analysis of motion patterns, accumulated during day cycles, revealed equivalent dwell times at rest (Figure 2B) and comparable time periods at all levels of detected activity (Figure 2C) for WT and Kir6.2-KO. Specifically, the in-action pattern was analyzed as a memory-less Poisson process, with the time spent at activity level k proportional to e−αk, where the mean characteristic level of activity (1/α) was 27.9±0.8 movements/min for WT and 27.1±0.9 movements/min for Kir6.2-KO (n=8 in each group; Figure 2C). Kir6.2-KO had an elevated calory intake from food, cumulatively measured during 24 h and corrected for body weight (Figure 2D). Blood samples revealed similar glucose, triglyceride and free fatty acid concentrations in WT and Kir6.2-KO (Figure S1) indicating equal energy substrate availability for tissue uptake, excluding disproportional energy supply as a basis for distinct trends in body weight.
Figure 2. Activity, food intake and EE in WT and Kir6.2-KO.
(A) Records of continuous locomotive activity monitored using implanted transmitting devices.
(B and C) Dwell time spent without movement was indistinguishable between WT and Kir6.2-KO (n=8 for each group). Fitting of the activity distributions, solid curves, revealed mean characteristic activities indistinguishable between WT and Kir6.2-KO.
(D) Food intake with regular chow diet, accumulated over 24 h and corrected for body weight, was higher in Kir6.2-KO (n=14) versus WT (n=16).
(E) Average time-course of oxygen consumption rates (V̇O2), and difference of substrate consumption rate calculated for Kir6.2-KO (n=19) relative to WT (n=17). Data points represent the average of 3 measurements from each animal.
(F) EE calculated based on V̇O2 and V̇CO2 values and averaged separately for light-on and light-off periods was higher in Kir6.2-KO versus WT.
(G) Cumulative substrate utilization obtained by integration over light-on and light-off periods revealed elevated carbohydrate consumption in Kir6.-KO compared to WT. Lipid utilization remained equivalent.
(H) V̇O2, respiratory exchange ratio (RER) and substrate consumption rates in WT (n=8) and Kir6.2-KO (n=8) were obtained within 30 s intervals during 2 mW treadmill test. At steady-state, Kir6.2-KO displayed an elevated EE compared to WT, accompanied with raised rates of substrate utilization.
(I) Activity-related EE was higher in Kir6.2-KO versus WT (n=8 in each group).
Data are mean±SEM; *, p<0.05.
Equal body weight, under comparable levels of activity and elevated caloric input, suggests raised energy expenditure (EE). During continuous 24-h monitoring under sedentary conditions indirect calorimetry revealed a higher rate of O2 consumption in Kir6.2-KO compared to WT (Figure 2E). Concomitantly, Kir6.2-KO demonstrated a higher rate of caloric output normalized to whole body weight, during day periods, reflecting increased EE compared to WT (Figure 2F). The higher caloric output in Kir6.2-KO was associated with an elevated rate of carbohydrate consumption relative to WT, and equal rates of lipid use (Figure 2E), estimated using Ferrannini’s equations (Ferrannini, 1988). Cumulative carbohydrate utilization in Kir6.2-KO during each 12-h day period, was 4.5–5 g per kg of weight above that of WT (Figure 2G). Assuming a 4 kcal energy yield from 1 g of carbohydrates, this would account for an additional 1.5–1.6 kcal/kg/h EE measured in Kir6.2-KO (Figure 2F).
Under sedentary conditions, EE comprises basal metabolic rate and thermogenesis aimed at non-exercise activity such as upholding posture, fidgeting and involuntary muscle contraction (Levine et al., 1999). To evaluate the contribution of KATP channels to the energy cost of physical activity, indirect calorimetry was further performed under a low exercise regimen that imposed walking on a treadmill runway. Upon acquisition of baseline EE during sleep, treadmill speed was adjusted to body weight to achieve a workload rate of 2 × 10−3 Joules/s (2 mW) at 5° inclination. In response to initiation of exercise both WT and Kir6.2-KO displayed a transient, ~10 min-long, stress-induced elevation of O2 consumption rates until matching the imposed workload at steady-state (Figure 2H). With exception of the first 2 min, when activation of aerobic metabolism in Kir6.2-KO was delayed relative to WT, a deficit in KATP channel function resulted in increased steady-state EE along with elevated rates of carbohydrate and lipid utilization (Figure 2H). Subtraction of baseline from steady-state EE defined the 2 mW activity-specific component, which was significantly higher in Kir6.2-KO than WT (n=8 in each group; p<0.05, Figure 2I). KATP channel deficit thus raises EE through an extra energy cost of physical activity.
KATP channels determine EE in muscle tissues governing body energy stores
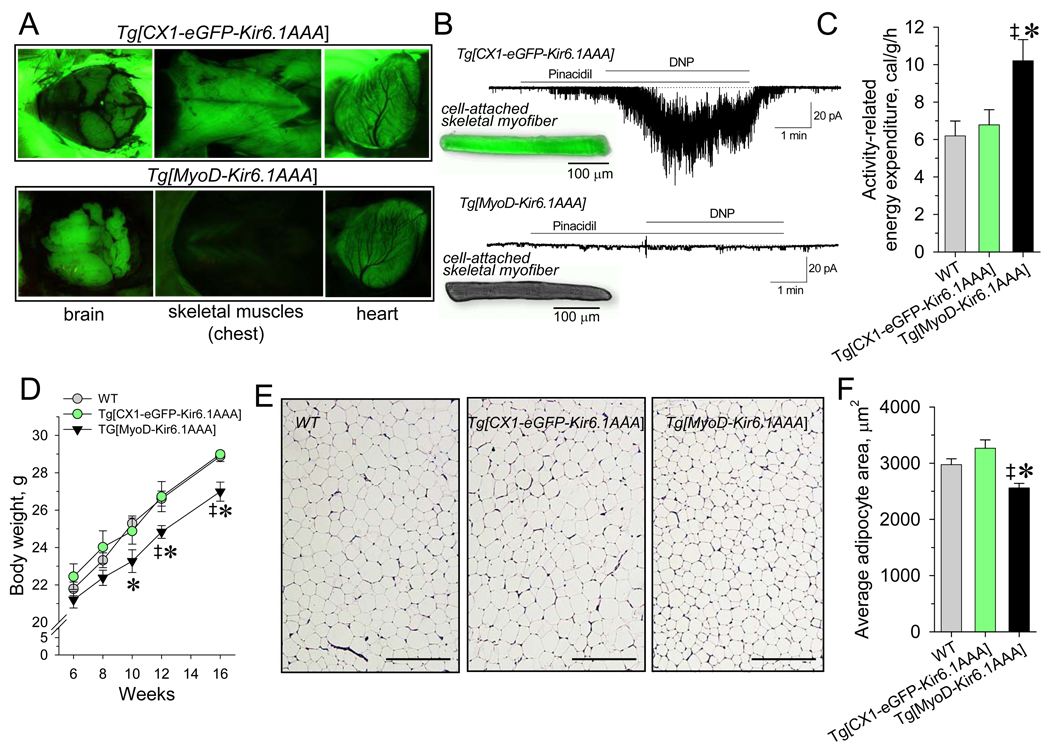
The main determinant of activity-related energy cost is the performance of skeletal and cardiac muscles (Kunz, 2001; Ghanassia et al., 2007). To dissect KATP channel-dependent regulation of skeletal muscle EE, the transgenic Tg[MyoD-Kir6.1AAA] model lacking channel function in a tissue-specific manner was developed (Figure 3A and 3B). Crossing Tg[CX1-eGFP-Kir6.1AAA] mice, which express eGFP in all somatic cells (Figure 3A, upper panel) but are otherwise phenotypically normal (Malester et al, 2007), with MyoD-cre transgenic animals (Chen et al., 2005) generated Tg[MyoD-Kir6.1AAA] progeny with skeletal muscle-specific Kir6.1AAA transgene expression tracked by selective elimination of eGFP fluorescence (Figure 3A, lower panel). Accordingly, patch-clamp recordings demonstrated lack of KATP channel activity in skeletal myofibers isolated from Tg[MyoD-Kir6.1AAA] in response to the potassium channel opener pinacidil or to metabolic challenge initiated by the mitochondria uncoupler 2,4-dinitrophenol (Figure 3B). Skeletal muscle-specific ablation of KATP channel function resulted in an elevated cost of physical activity, estimated by subtraction of baseline EE from the 2 mW steady-state EE, in Tg[MyoD-Kir6.1AAA] compared to littermate FVB/N WT and Tg[CX1-eGFP-Kir6.1AAA] controls (Figure 3C). This aggravated energy use was accompanied by relatively lower body weight and adipocyte area in Tg[MyoD-Kir6.1AAA] compared to WT or Tg[CX1-eGFP-Kir6.1AAA] littermates (Figure 3D–3F).
Figure 3. Elevated EE decreases body weight in Tg[MyoD-Kir6.1AAA].
(A) Brain and muscle tissues of Tg[CX1-eGFP-Kir6.1AAA] and Tg[MyoD-Kir6.1AAA] mice exposed to 525 nm wavelength light. Tg[MyoD-Kir6.1AAA], due to Cre-recombination of eGFP-coding region, lacked eGFP fluorescence exclusively in skeletal muscles.
(B) Expression of Kir6. 1AAA suppressed KATP channel function in skeletal muscle cells from Tg[MyoD-Kir6. 1AAA] as assessed by electrophysiology with no KATP channel response to pinacidil (50 µM) and 2,4-dinitrophenol (DNP, 200 µM), which induced vigorous KATP channel activation in Tg[CX1-eGFP-Kir6.1AAA] myofibers.
(C) Activity-related EE defined in 8 weeks old Tg[MyoD-Kir6.1AAA] (n=5) was significantly higher compared to FVB/N WT (‡, p<0.05; n=6) or Tg[CX1-eGFP-Kir6.1AAA] littermates (*, p<0.05; n=4).
(D) Body weight in Tg[MyoD-Kir6.1AAA] (n=6) was reduced compare to Tg[CX1-eGFP-Kir6.1AAA] (n=5;*, p<0.05) and FVB/N WT (n=8; ‡, p<0.05) littermates.
(E and F) Average cross-sectional area of individual adipocytes was reduced in Tg[MyoD-Kir6.1AAA] (n=3) compared to Tg[CX1-eGFP-Kir6.1AAA] (n=3;*, p<0.05) and FVB/N WT (n=3; ‡, p<0.05); scale bars, 200 µm.
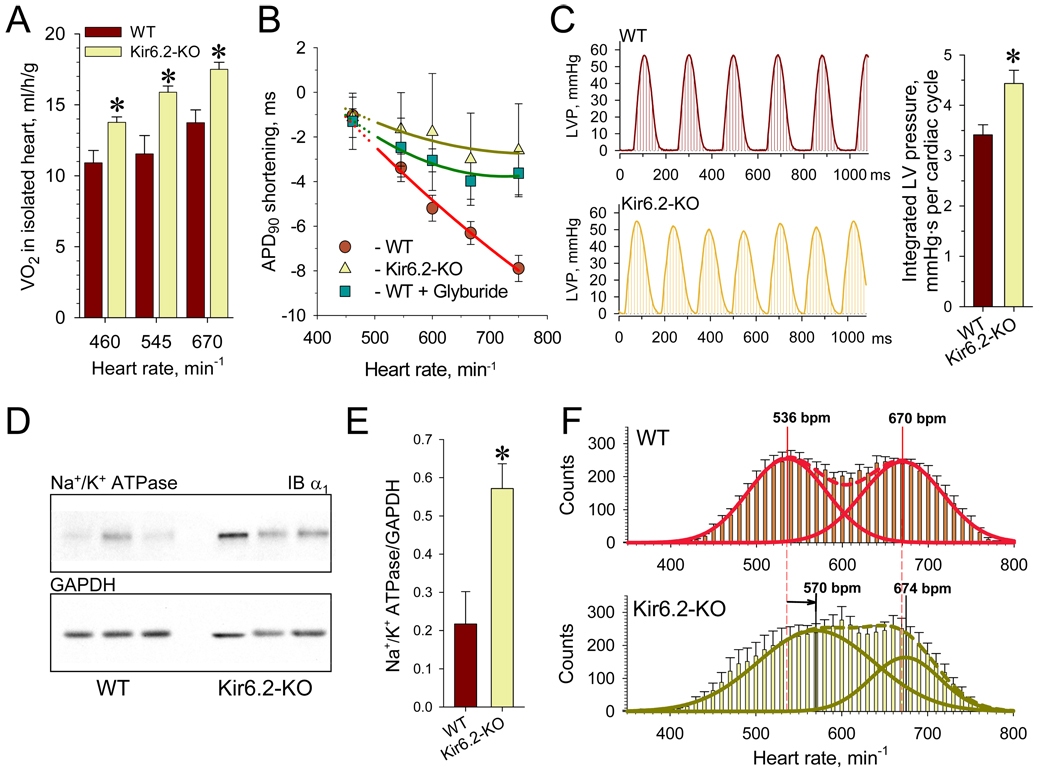
The contribution of KATP channels to myocardial energy consumption was assessed in hearts isolated from Kir6.2-KO under workloads set by pacing within the physiological range of 460–670 beats/min. Hearts lacking KATP channels exhibited augmented oxygen consumption, indicating an elevated energy cost of cardiac performance compared to WT (Figure 4A). At imposed workloads, progressive reduction of myocardial action potential duration (APD90), characteristic for WT, was absent in Kir6.2-KO hearts as assessed from monophasic action potential recordings (Figure 4B). In WT hearts, workload-induced shortening of action potentials was reversed by the KATP channel blocker glyburide (10µM) underscoring the contribution of KATP channels in modulating myocardial excitability across the range of physiological cardiac rates. Absence of KATP channel function augmented cardiac mechanical work, measured as an integrated left ventricular pressure per cardiac cycle (Figure 4C), and induced overexpression of Na+/K+ pumps (Figure 4D and 4E), identifying these and myosin ATPase as molecular contributors to increased energy use.
Figure 4. Increased EE and mechanical function of Kir6.2-KO isolated hearts.
(A) Rate of oxygen consumption, measured in isolated perfused hearts revealed a significantly elevated energy demand in Kir6.2-KO versus WT (n=5 for each group).
(B) Pacing rates above 130 ms (460 beats/min) induced significant shortening of APD90 in WT (n=8) that was sensitive to the sulfonylurea glyburide (10 µM, n=4) but not in hearts from Kir6.2-KO (n=5). Solid lines were constructed as results of quadratic polynomial fitting of experimental data relative to APD90 at 150 ms pacing interval.
(C)Integrated left ventricular pressure was defined for 3–4 s long periods and divided by number of cardiac cycles in unpaced Kir6.2-KO and WT hearts (n=7 for each group).
(D and E) Overexpression of sarcolemmal Na+/K+ pumps in Kir6.2-KO compared to WT (n=3 for each group) revealed by Western blots.
(F) Histograms summarizing heart rates during 24-h long monitoring using telemetry in WT (n=11, top) and Kir6.2-KO (n=10, bottom). Dotted lines represent the fit of distributions with the sum of two Gaussian functions; solid lines indicate individual functions. Arrow indicates right shift of the lower mean heart rate in Kir6.2-KO relative to the corresponding value in WT.
Data are mean±SEM;*, p<0.05.
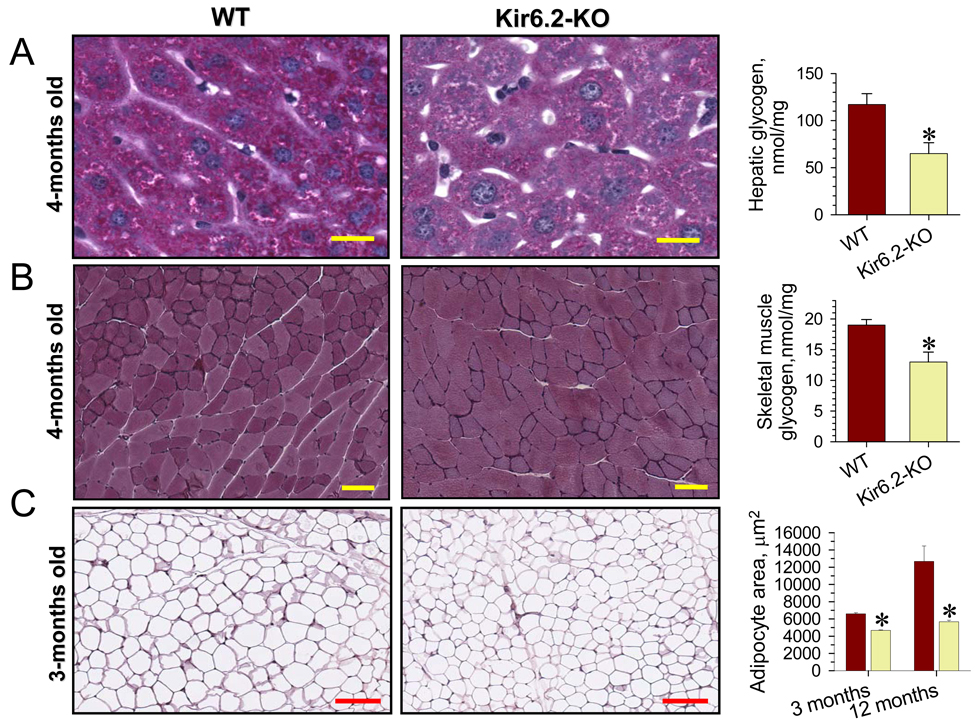
Heightened muscle performance requires mobilization of bodily energy stores (Ghanassia et al., 2007). Analysis of telemetry-recorded heart rates revealed a two-peak distribution in the 400 to 800 beats/min range for WT and Kir6.2-KO during continuous daily activity (Figure 4F). This profile reflected normally-distributed heart rate variability that characterizes low versus high activity periods in each cohort. Fitting these distributions with the sum of two Gaussian functions indicated that while the highest mean heart rate was equivalent for Kir6.2-KO and WT (674 versus 670 beats/min, respectively), the lower characteristic mean value in Kir6.2-KO (570 beats/min) was right-shifted relative to WT (536 beats/min). The absence of KATP channels thereby provoked a partial redistribution of heart rate events from the region of 500 beats/min to that of 600 beats/min (Figure 4F). Thus, even in periods of low activity, Kir6.2-KO hearts are prompted to perform at rates higher than the WT, a feature characteristic of a hypermetabolic state (Bachman et al., 2004) characterized by augmented demand for oxygen and substrate delivery. In fact, the increased EE in Kir6.2-KO striated muscles intensified the mobilization of glycogen and fat energy depots, reducing muscular and hepatic glycogen stores within 16 h of fasting compared to WT (Figure 5A and 5B). This glycogen change in Kir6.2-KO is in line with increased muscle glucose uptake (Miki et al., 2002), augmented insulin sensitivity and a prominent drop of blood glucose levels under fasting and after exhaustion (Figure S1 and S2). Further, increased lipolysis in Kir6.2-KO revealed by fasting or in post-exercise recovery (Figure S1) caused a reduction of adipocyte size compared to WT (Figure 5C). Thus, lack of KATP channel function that precipitated inefficient EE in skeletal and cardiac muscles compromised bodily energy stores.
Figure 5. Reduced energy depots in Kir6.2-KO.
(A) Left: PAS histology of hepatic tissue indicates depletion of glycogen stores in liver from Kir6.2-KO compared to WT. Scale bar; 100 µm (40x image magnification). Right: biochemical evaluation confirmed liver glycogen deficit in Kir6.2-KO compared to WT n=9 in each group).
(B) Left: PAS staining of skeletal muscle revealed significant depletion of glycogen in certain fibers from Kir6.2-KO gastrocnemius compared to WT. Scale bar: 200 µm (20x image magnification). Right: biochemical evaluation confirmed glycogen deficit in gastrocnemius from Kir6.2-KO (n=4) compared to WT (n=5).
(C) Left: Adipose tissue histology in WT and Kir6.2-KO (scale bars, 200 µm). Right: Average cross-sectional area of individual adipocytes was increased in WT compared to Kir6.2-KO by 3 months of age, and the difference progressed by 12 months (n=3 for each group).
Data are mean±SEM;*, p<0.05.
Inefficient energetics in KATP channel knockout fails to support intense workload
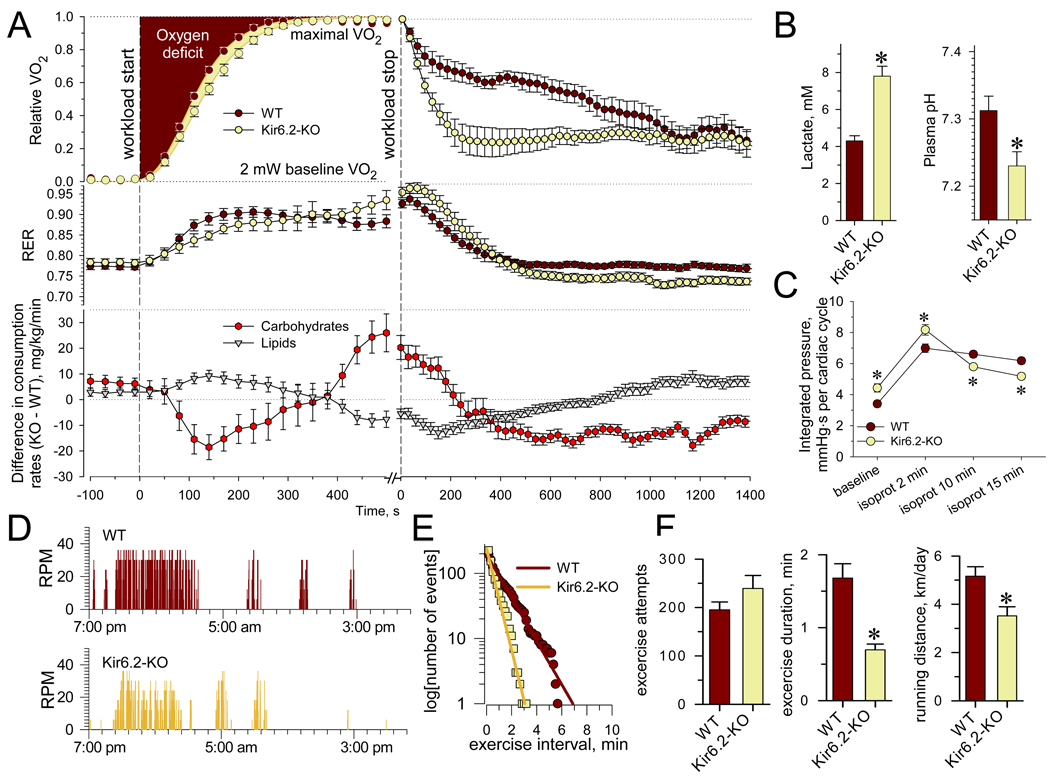
Endurance, or the ability to withstand activity-related workload, relies on the efficiency of body energetics. Maximal oxygen consumption (V̇O2max) reflects the maximal rate of energy production and the ability of the cardiovascular system to support physical performance at highest workloads by ensuring oxygen delivery to skeletal muscle (Joyner, 1991). Here, under treadmill testing, WT and Kir6.2-KO achieved similar V̇O2max , 7817±364 ml/kg/h (n=7) versus 8311±253 ml/kg/h (n=9) respectively, indicating equivalent oxidative phosphorylation potentials. Although V̇O2max sets the upper limit for energy production, it does not determine ultimate performance (Zoll et al., 2002). Indeed, despite similar energy consumption rates at V̇O2max , i.e., 38.6±0.9 cal/g/h and 37.4±0.9 cal/g/h, KATP channel knockouts attained a total work of 17.9±3.2 Joules (20 m/min at 15° inclination; n=8) below the 27.0±2.8 Joules (22.5 m/min at 20° inclination; n=9; p<0.05) achieved by more energy-efficient WT counterparts.
The efficiencies of the cardiovascular system and body energy resource recruitment were assessed in response to maximal workload (Barbato et al., 1998). Calorimetry-derived data normalized as R(t)=(V̇O2 - V̇O2min) / (V̇O2max - V̇O2min), where the oxygen consumption rate (V̇O2) was accelerated from V̇O2min (at 2 mW) to V̇O2max (at 30 mW workload), were fitted by the Gompertz function, R(t)=exp[-exp(-(t-tmax)/tr)] where 1/tr is the maximum growth rate at time tmax (Figure 6A). Both parameters were significantly slower for Kir6.2-KO (tr=68±3.4 s and tmax=204±5.6 s, n=11) compared to WT (tr=56±3.0 s and tmax=187±3.5 s, n=10; p<0.05). Oxygen deficit at the onset of exercise, calculated as the area above corresponding Gompertz curves, was higher for Kir6.2-KO (242.2±7.2 s, n=11) compared to WT (220.1±5.2 s, n=10; ANOVA p<0.05; Figure 6A). The increased oxygen deficit in Kir6.2-KO was accompanied by reversed substrate utilization dynamics compared to WT. On exercise initiation, mice lacking KATP channels demonstrated deficit of carbohydrate and raised lipid oxidation (Figure 2H and Figure 6A). These trends were reversed upon approaching V̇O2max and exhaustion (Figure 6A), when Kir6.2-KO exhibited an energetically less efficient combination of increased carbohydrate and depressed lipid oxidation. Impaired mobilization of aerobic systems in Kir6.2-KO was linked to stimulation of anaerobic metabolic pathways manifested by development of lactic acidosis (Figure 6B). At exercise termination due to exhaustion, precipitous drop of oxygen consumption, fast decline of carbohydrate and lipid oxidation (Figure 6A), bradycardia and reduced mobility (Figure S3) collectively indicated that the circulatory system of Kir6.2-KO was unable to sustain substrate and oxygen supply. In fact, in response to stimulation with the sympathomimetic isoproterenol (10 µM), Kir6.2-KO hearts were unable to maintain performance for as long as WT hearts (Figure 6C).
Figure 6. Reduced endurance and voluntary performance in Kir6.2-KO.
(A) Relative VO2 (upper trace) and RER (middle trace) in WT (n=10) and Kir6.2-KO (n=11) were constructed during 30 mW treadmill workload and post-exercise recovery, respectively. The differences in KO versus WT in carbohydrate and lipid consumption rates are presented for corresponding time points (lower trace). Data points were acquired at 30 s intervals.
(B) Plasma lactate and pH measured in blood samples taken immediately upon exhaustion from Kir6.2-KO (n=4) and WT (n=5).
(C) In response to isoproterenol (10 µM), Kir6.2-KO hearts were not able to sustain the integrated LV pressure per cardiac cycle compared to WT hearts (n=7 in each group).
(D) Representative recordings of voluntary performance during 24 h in WT and Kir6.2-KO housed in cages with attached running wheels.
(E) Distributions of activity intervals were fitted by single exponential functions (lines on the semi-logarithmic scale). The higher exponential slope indicates a shortened mean exercise duration for Kir6.2-KO (0.56±0.02 min) versus WT (1.31±0.02 min).
(F) Analysis of distributions for WT (n=10) and Kir6.2-KO (n=11) revealed similar number of voluntary exercise attempts (first bin), yet the mean exercise duration was double in WT compared to Kir6.2-KO. Accordingly, the running distance, defined based on wheel circumference and RPM, was compromised in Kir6.2-KO.
Data are mean±SEM;*, p<0.05.
Under voluntary activity, Kir6.2-KO also exhibited a low overall performance capacity compared to WT (Figure 6D). In contrast to coerced workload imposed by a treadmill regimen, free-access running wheels allow measurement of stress-free exercise capacity (Momken et al., 2005). Patterns of voluntary wheel runs were collected throughout 24-h intervals (Figure 6D), and bursts of activity, i.e., continuous periods of wheel rotations, accumulated in time-event distributions reflecting individual voluntary activity patterns (Figure 6E). Analysis of distributions revealed a similar total number of exercise attempts for both cohorts, yet a halved mean time of activity intervals for Kir6.2-KO compared to WT (Figure 6F). The superior ability of WT to sustain voluntary performance was further highlighted by a running distance that exceeded that of Kir6.2-KO counterparts (Figure 6F). Thus, lack of KATP channels impaired voluntary exercise, suggesting a critical role for these biosensors in optimizing activity-related energy use.
Kir6.2-KO are resistant to high-fat diet induced obesity
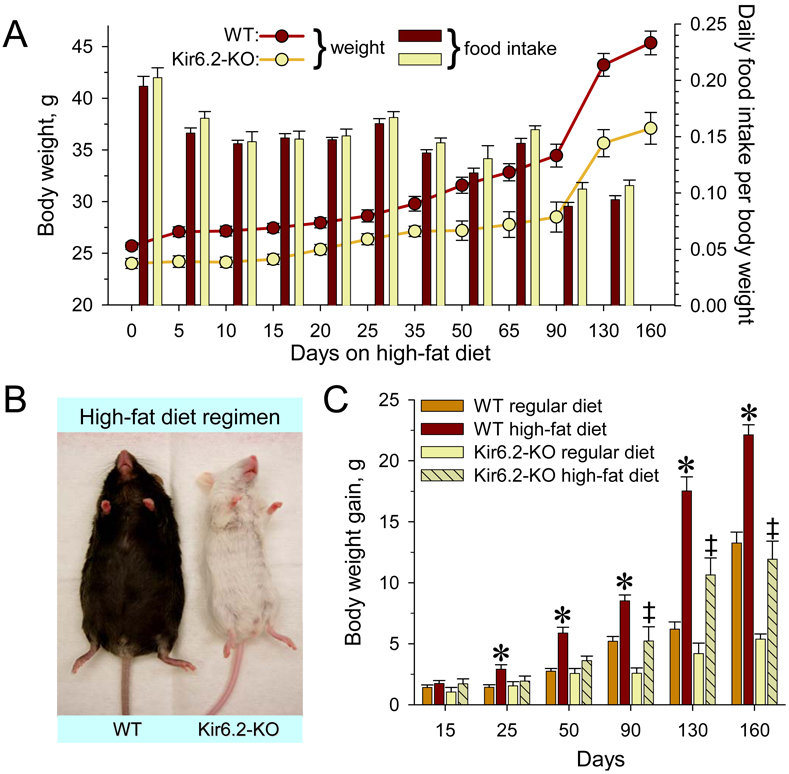
As disruption of KATP channel function raised the energy cost of exercise and non-exercise activity, resistance to diet-induced obesity was assessed. WT and Kir6.2-KO were challenged with a Western-type high-fat diet, starting at 11 weeks of age for 160 days of prospective monitoring (Figure 7A). Under continuous high-fat diet regimen, WT progressively acquired an obese phenotype in contrast to Kir6.2-KO that demonstrated obesity-resistance despite comparable food intake (Figure 7A and 7B). Throughout follow-up, Kir6.2-KO displayed lower body weight and rate of body weight gain than age-matched WT. High-fat diet compared to regular chow diet doubled the rate of weight gain in WT, i.e., 0.138 g/day (r2=0.96, n=10) versus 0.071 g/day (r2=0.87, n=10), respectively (Figure 7C). In Kir6.2-KO the rate of body weight gain was increased from 0.027 g/day under regular diet (r2=0.95, n=10) to 0.074 g/day under high-fat diet (r2=0.96, n=10) resulting in a body weight of Kir6.2-KO under high-fat diet that did not exceed that of WT under regular diet (Figure 7C). Thus, the anti-obesity effect evoked by KATP channel dysfunction was preserved under high-fat diet challenge.
Figure 7. Resistance to high-fat diet induced obesity in Kir6.2-KO.
(A) Under a high-fat diet regimen, Kir6.2-KO (n=10) gained less weight (left y-axis) than age- and gender-matched WT (n=10), despite comparable food consumption (right y-axis).
(B) Representative WT and Kir6.2-KO after 90 days of high-fat diet regimen.
(C) High-fat diet accelerated the rate of weight gain compared to regular chow diet in both WT and Kir6.2-KO, as determined by linear regression. However, the weight gain in WT was significantly higher compared to regular diet already at 25 days of the high fat regimen (*, p<0.05, n=10 for both groups), in contrast to 90 days for Kir6.2-KO (‡, p<0.05, n=10 in each group). Furthermore, the weight gained by Kir6.2-KO throughout the high-fat diet regimen was considerably lower compared to WT.
Data are mean±SEM.
DISCUSSION
Sarcolemmal KATP channels are recognized molecular metabolic sensors that modulate membrane excitability according to cellular energy availability, yet their actual homeostatic role remains partially understood. Metabolic sensing by KATP channels has been typically assigned to a protective response under severe energy insult, such as acute ischemia, sympathetic distress or muscle fatigue (Matar et al., 2000; Zingman et al., 2002; Renaud 2002; Gumina et al., 2003; Miki and Seino, 2005; Yamada et al, 2006; Cifelli et al., 2007; Reyes et al., 2007). This study establishes a previously unrecognized role for sarcolemmal KATP channels in the continuous regulation of cellular energy use under non-stressed, physiological states. In this way, KATP channels emerge as important components of a cellular energy-sparing system that optimizes muscle energy expenditure. Lack of this KATP channel-dependent energy-controlling mechanism exaggerated energy use by heart and skeletal muscle, dissipating bodily glycogen/fat depots and promoting lower weight. The observed resistance to body weight gain under a high-fat diet, induced by KATP channel-deficiency, provides a basis for potential anti-obesity treatment (Watt et al., 2006). The sarcolemmal KATP channel, suitable for manipulation to render skeletal muscle increasingly thermogenic at rest and less fuel efficient in exercise (Himms-Hagen, 2004), thus represents a molecular target to magnify the benefit of activity for obesity treatment or prevention.
The energy-controlling function of KATP channels is based on the unique ability to feedback bioenergetic cues into constraints on cellular energy expenses for myofibrillar contraction and re-sequestration of Ca2+ and Na+ ions by opposing myocyte depolarization (Nichols and Lederer, 1991; Gong et al., 2000; Zingman et al., 2002; Thabet et al., 2005; Cifelli et al., 2007, 2008). By virtue of sensing even minor changes in cellular nucleotide content (Abraham et al., 2002; Selivanov et al., 2004), KATP channels could decode cellular metabolic dynamics during regular daily activity. Indeed, KATP channel-driven shortening of action potentials demonstrated here, within the physiological range of cardiac rates, ensured reduced oxygen consumption. An overload of ATPases supporting ion homeostasis was exposed by Na+/K+ pump upregulation precipitated in the absence of sarcolemmal KATP channel function. Escalation of fuel metabolism in striated muscles translated to whole-body energy overuse in both Kir6.2-KO and Tg[MyoD-Kir6.1AAA] KATP channel-deficient models, manifested by reduction in fat depots and limited weight gain.
Characteristic of hypermetabolic states (Bachman et al., 2004), the increased energy demand in the absence of KATP channels entailed acceleration of cardiac rate, initiating a “vicious” cycle in which magnified cardiac mechanical work imposed further energy use and mobilization of glycogen and fat stores. Yet, while unchallenged, Kir6.2-KO did not exhibit a deficit in caloric intake or blood energy substrates despite the role of KATP channels in glucose homeostasis (Miki et al., 1998; Aguilar-Bryan et al., 2001; Ashcroft, 2005) and possibly in appetite control (Spanswick et al., 1997). The accelerated metabolism profile of Kir6.2-KO resembles transgenic models overexpressing mitochondria uncoupling proteins in skeletal muscle (UCP-TG; Li et al., 2000). Although sharing with UCP-TG properties of a lean phenotype, enhanced insulin sensitivity and glucose uptake (Li et al., 2000; Miki et al., 2002; Minami et al., 2003), Kir6.2-KO, by reaching V̇O2max equivalent to WT, exhibited an unaltered oxidative phosphorylation capacity (Mootha et al., 1997) and sarcoplasmic nucleotide levels (Zingman et al., 2002). Thus, enhanced mobilization of body energy depots in Kir6.2-KO, while maintaining phosphocreatine and nucleotide pools, limits body weight gain.
Inefficient energy control in the absence of KATP channels endowed resistance to diet-induced obesity, although at the cost of decreased workload endurance. In fact, the oxygen deficit identified in Kir6.2-KO at workload initiation indicated delayed activation of enzymatic reactions and substrate mobilization required for skeletal muscle oxidative metabolism (McArdle, 1996). This was counterbalanced by anaerobic processes that, although compensating for energy deficit, provoked excessive lactate accumulation. The developed lactic acidosis suppressed lipolysis at peak exercise (Liu et al., 2009), and further compromised cardiac function critical in maintaining physical activity (Nielsen et al., 2002; Zingman et al., 2002; Kane et al., 2004). Accordingly, the Kir6.2-KO was unable to sustain stress-free voluntary performance despite an equal-with-WT number of exercise attempts.
In summary, this study demonstrates that sarcolemmal KATP channels not only respond to metabolic stress but continuously control energy use by cardiac and skeletal muscles, thereby promoting body energy conservation. Positive energy balance favoring obesity would be therefore moderated by tissue-specific interruption of KATP channel-driven optimization of energy use, offering a paradigm in weight control.
Experimental Procedures
Animal models
KATP channel-deficient mice were generated by targeted disruption of the KCNJ11 gene (Miki et al., 1998). Due to the proximity of the mutated gene with the gene encoding albino hair color in SV129 embryonic stem cells used to create the knockout, Kir6.2-knockout (Kir6.2-KO) mice remained white upon backbreeding into the black C57BL/6 line (WT) for 10 generations. Experiments were performed with male mice, 3–4 months of age preceding divergence in body weight and fat distribution, unless otherwise indicated. Transgenic Tg[CX1-eGFP-Kir6.1-AAA] mice were obtained from the FVB/N mouse strain using a non-conducting Kir6.1AAA pore mutant where a Gly-Phe-Gly motif critical for K+ permeation was replaced by Ala-Ala-Ala (Malester et al., 2007). Kir6.1AAA was subcloned downstream of the loxP sites flanking eGFP coding region and a stop codon, in such a way that dominant negative suppression of Kir6.2/SUR2A could be initiated by expression of Cre recombinase. Accordingly, skeletal muscle-specific ablation of KATP channel activity was achieved by crossing Tg[CX1-eGFP-Kir6.1-AAA] and MyoD-cre transgenic mice that express Cre recombinase under control of the MyoD promoter (Chen et al., 2005). Protocols were approved by Mayo Clinic and University of Iowa Institutional Animal Care and Use Committees.
Animal maintenance and diets
Mice were maintained in a temperature-controlled (25°C) facility on a 12-h light/dark cycle and fed with regular chow diet (PicoLab® Rodent Diet 20 5053; PMI Nutrition International, St Louis, MO) containing 5% fat accounting for ~13% of caloric content. A subset of animals was fed a high-fat diet containing 9% fat (~22% of the diet’s caloric content; PicoLab® Mouse Diet 20 5058). Food intake was measured using a food consumption monitoring system (Columbus Instruments; Columbus, OH).
Magnetic resonance imaging
Transverse sections at the T3–T7 and L1–L5 levels were obtained under isoflurane anesthesia using a 7 Tesla scanner (Bruker; Billerica, MA) by a spin-echo method (Yamada et al., 2006). Analyze (Mayo Foundation; Rochester, MN) and MetaMorph (Molecular Devices; Sunnyvale, CA) softwares were used for image analysis.
Histochemistry
Liver and skeletal muscle samples were harvested from 16-h fasting animals, flash-frozen in isopentane/liquid nitrogen, and Periodic Acid Schiff (PAS) used for staining of glycogen content. Gluteal subcutaneous white fat was fixed in 10% buffered formalin, and stained with hematoxylin-eosin (Kane et al., 2004). Micrographs were taken by a KS-4000 Axiocam digital camera with an Axioplan 2 light microscope (Carl Zeiss MicroImaging, Thornwood, NY) and analyzed using MetaMorph.
Glycogen biochemical assay
Hepatic tissue and gastrocnemius muscles were harvested from fasting animals, homogenized in chilled 0.03 N HCl, sonicated for 45 s using a Branson Sonifier 450 (Branson, Danbury, CT), and boiled for 5 min. Samples were separated for measurement of free glucose or boiled in 2 N HCl for 3 h to hydrolyze glycogen content. Samples were then centrifuged for 1 min at 13,000 rpm and hydrolysis stopped by alkalization. Glucose levels were measured using a Glucose Assay Kit (Biovision Inc, Mountain View, CA), and free glucose concentration subtracted from glycogen-produced glucose. Results were normalized to wet tissue weights.
Analytical procedures
Blood lactate and pH were determined using a 5700 Gem-Primier3000 Gas/Electrolyte analyzer (Instrumentation Laboratory; Lexington, MA).
Locomotor activity
Activity was monitored using implanted telemetric devices (Data Science International; St. Paul, MN). Variations in signal strength due to change in animal orientation or distance from the receiver were averaged for each 10 s periods, and counted as movements per min (min−1) revealing the level of activity. All activity levels were collected into histograms with 6 min−1 bin width implying that a certain activity level ai was achieved with attaining all preceding activity levels, i.e., all bins from a1 to ai were incremented by 10 s. Mean activity level, a0, was obtained by fitting each histogram with a single exponential function, T=T0 · exp(-k/a0), where T0 denotes total time of activity (the first histogram bin); and k, the activity level variable.
Indirect calorimetry and treadmill exercise
Indirect calorimetry was performed using metabolic chambers (Oxymax, Columbus Instruments). Experiments were synchronized with a start-time of 19:00 h, for continuous monitoring under ad libitum chow diet. EE was determined based on rate of oxygen consumption (V̇O2) and carbon dioxide production (V̇CO2):
The calorimeter was calibrated before each measurement with a standard span gas (0.501% CO2, 20.53% O2 balanced with N2), and cross-calibrated against room air at every fifth measurement. Maximal rates of oxygen consumption (V̇O2max) and EE during physical activity were measured using a modular treadmill connected to the calorimetry system. Ten days before exercise tests, mice were acclimated daily for a nonmoving treadmill followed by 15 min at a velocity of 3.5 m/min. The treadmill velocity, set for a constant workload rate (W), was adjusted to body weight for each animal as W/(m g)/sin(α), where m is body weight; g, acceleration due to gravity (9.8 m/s2);α, treadmill inclination. The exercise-endurance protocol was performed as described (Zingman et al, 2002). Differences in the rate of carbohydrate and lipid consumption between cohorts were calculated based on V̇O2 and 2 V̇CO2 values using Ferrannini’s equations (Ferrannini, 1988):
where N is the rate of urinary nitrogen excretion used to estimate protein oxidation, with the assumption that urinary nitrogen is equivalent in experimental groups. Errors for the difference in substrate utilization between Kir6.2-KO and WT mean values were calculated using the quadrature rule according to the propagation of uncertainties for normally distributed independent measures (Tayler, 1997).
Patch-clamp recordings
Skeletal muscle cells from musculus flexor digiti brevis were enzymatically isolated (Lueck et al., 2007), and single channel currents were recorded in the cell-attached mode of the patch-clamp technique at a holding command potential of +60 mV set in a pipette electrode using an Axopatch 200B amplifier (Molecular Devices; Sunnyvale, CA). Patch pipettes, filled with a solution containing (in mM): KCl 140, CaCl2 1, MgCl2 1, HEPES-KOH 5 (pH 7.3), had a resistance of 5–7 MΩ. Cells were bathed in (in mM): KCl 140, MgCl2 1, EGTA 5, HEPES-KOH 5 (pH 7.3). Measurements were performed at 30°C.
Western blotting
Protein extracts were prepared by homogenizing ventricular tissue in NaCl 150 mM, Tris-HCl 50 mM, pH 7.8 supplemented with 1% Triton X-100 and protease inhibitors. Electrophoresis was performed on 3–8% gradient Nu-Page Tris-Acetate gel and transferred to polyvinylidene difluoride membranes (InVitrogen). Mouse monoclonal antibodies against Na+/K+ ATPase (Afinity Bioreagents, Rockford, IL) and anti-mouse HP-conjugated secondary antibody (Santa Cruz Biotechnology, Inc. Sant-Cruz, CA) were used.
Monophasic action potential (MAP), left ventricular (LV) pressure and oxygen consumption
Isolated hearts were retrogradely perfused at 90 mmHg with Krebs–Henseleit buffer bubbled with 95% O2/5% CO2 (37°C, pH 7.4). Hearts were paced (A310 Accupulser; World Precision Instruments; Sarasota, FL) using a catheter positioned in the right ventricular apex (NuMed; Hopkinton, NY). A MAP probe (EP Technologies; Sunnyvale, CA) was maintained at the LV epicardium, and amplified signals (IsoDam; World Precision Instruments; Sarasota, FL) were acquired at 11.8 kHz (Zingman et al., 2002). MAPs were analyzed at 90% of repolarization (APD90). LV pressure was measured using a fluid-filled balloon attached to a pressure transducer (Harvard Apparatus; Holliston, MA), and diastolic pressure was set to 10 mmHg. Oxygen consumption was calculated based on Henry’s Law as the difference between the oxygen partial tension measured, using the 5700 GemPrimier3000 Gas/Electrolyte analyzer, in the perfusate prior (PO2cntr) and after (PO2) heart passage. The rate of oxygen consumption (V̇O2)was calculated as:
where ν is rate of coronary flow measured by a T106 flow-meter (Transonic System Inc.; Ithaca, NY); α = (0.024 ml O2 per ml H2O)/760 mmHg denotes the Bunsen’s solubility coefficient (Sinaasappel et al., 1999); and mh is heart wet weight.
Voluntary performance
Mice were housed in individual cages with free access to attached running wheels of 242 mm diameter (Mini Mitter Co.; Bend, OR). Wheel turns were counted with a Mini Mitter Magnetic Switch, averaged for each 10 s, converted to RPM (rotations per minute) by a DSI acquisition system (Data Sciences International; St. Paul, MN). Exercise intervals (bursts of activity) were accumulated in Poisson time-event distributions with 10 s bin width implying that a running burst duration corresponding to ni was associated with attainment of all preceding activity duration intervals, i.e. all bins from n1 to ni were incremented by a unit. Thus, the first bin consists of a total number of bursts (i.e. number of exercise attempts), and the probability of a running interval with duration t is proportional to e−αt, where 1/α is the mean time of activity burst duration. Running distance was calculated as 1/6πD·ΣRMPi, where D is wheel diameter and ΣRMPi is sum of averaged RPM values.
Statistical analysis
Results are expressed as mean±SEM. Student’s t-test was used to define p values unless otherwise indicated. A p value of <0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
The authors are particularly grateful to Dr. T. Miki (Chiba University) and Dr. S. Seino (Kobe University) for providing the Kir6.2-KO model. We further thank Drs. P. Mishra and S. Macura, Mayo Clinic Analytical NMR Facility, for expertise with NMR imaging, Drs. K. Campbell and J. Lueck (University of Iowa) for guidance with myofiber isolation, L. Rowe (Mayo Clinic) for assistance with histological specimens, E. Stepniak (University of Iowa) for technical assistance, and Dr. S.I. Loginov (Surgut University) for valuable discussion. This work was supported by Gerstner Family Career Development Award in Individualized Medicine (A.E.A.), Mayo Graduate School Fellow-ship (S.R.); American Society for Clinical Pharmacology and Therapeutics Young Investigator Award (S.Y.); NIH T32GM008685 (S.S.); NIH RO1HL64822 (A.T.); NIH AR052777 (D.J.G.), Marriott Heart Diseases Research Program, Marriott Foundation (A.T.); and in part by Medical Research Initiative Roy J. Carver Charitable Trust pilot grant and Fraternal Order of Eagles, Iowa Aerie grant (L.V.Z. and D.M.H-Z.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham MR, Selivanov VA, Hodgson D, Pucar D, Zingman LV, Wieringa B, Dzeja PP, Alekseev AE, Terzic A. Coupling of cell energetics with membrane metabolic sensing: Integrative signaling through creatine kinase phosphor-transfer disrupted by M-CK gene knockout. J. Biol. Chem. 2002;277:24427–24434. doi: 10.1074/jbc.M201777200. [DOI] [PubMed] [Google Scholar]
- Aguilar-Bryan L, Bryan J, Nakazaki M. Of mice and men: KATP channels and insulin secretion. Recent Prog. Horm. Res. 2001;56:47–68. doi: 10.1210/rp.56.1.47. [DOI] [PubMed] [Google Scholar]
- Alekseev AE, Hodgson DM, Karger AB, Park S, Zingman LV, Terzic A. ATP-sensitive K+ channel channel/enzyme multimer: metabolic gating in the heart. J. Mol. Cell. Cardiol. 2005;38:895–905. doi: 10.1016/j.yjmcc.2005.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft FM. ATP-sensitive potassium channelopathies: focus on insulin secretion. J. Clin. Invest. 2005;115:2047–2058. doi: 10.1172/JCI25495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babenko AP, Aguilar-Bryan L, Bryan J. A view of SUR/KIR6.X, KATP channels. Annu. Rev. Physiol. 1998;60:667–687. doi: 10.1146/annurev.physiol.60.1.667. [DOI] [PubMed] [Google Scholar]
- Barbato JC, Koch LG, Darvish A, Cicila GT, Metting PJ, Britton SL. Spectrum of aerobic endurance running performance in eleven inbred strains of rats. J. Appl. Physiol. 1998;85:530–536. doi: 10.1152/jappl.1998.85.2.530. [DOI] [PubMed] [Google Scholar]
- Bachman ES, Hampton TG, Dhillon H, Amende I, Wang J, Morgan JP, Hollenberg AN. Effect of hyperthyroidism on spontaneous physical activity and energy expenditure in rats. Endocrinology. 2004;145:2767–2774. doi: 10.1210/en.2003-1670. [DOI] [PubMed] [Google Scholar]
- Carrasco A, Dzeja P, Alekseev A, Pucar D, Zingman L, Abraham M, Hodgson D, Bienengraeber M, Puceat M, Janssen E, et al. Adenylate kinase phosphotransfer communicates cellular energetic signals to ATP-sensitive potassium channels. Proc. Natl. Acad. Sci. USA. 2001;98:7623–7628. doi: 10.1073/pnas.121038198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celi FS. Brown adipose tissue – when it pays to be inefficient. N. Engl. J. Med. 2009;360:1553–1556. doi: 10.1056/NEJMe0900466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JC, Mortimer J, Marley J, Goldhamer DJ. MyoD-cre transgenic mice: a model for conditional mutagenesis and lineage tracing of skeletal muscle. Genesis. 2005;41:116–121. doi: 10.1002/gene.20104. [DOI] [PubMed] [Google Scholar]
- Cifelli C, Bourassa F, Gariépy L, Banas K, Maria Benkhalti M, Renaud J-M. KATP channel deficiency in mouse flexor digitorum brevis causes fibre damage and impairs Ca2+ release and force development during fatigue in vitro. J. Physiol. 2007;582:843–857. doi: 10.1113/jphysiol.2007.130955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cifelli C, Boudreault L, Gong B, Bercier JP, Renaud J-M. Contractile dysfunctions in ATP-dependent K+ channel-deficient mouse muscle during fatigue involve excessive depolarization and Ca2+ influx through L-type Ca2+ channels. Exp. Physiol. 2008;93:1126–1138. doi: 10.1113/expphysiol.2008.042572. [DOI] [PubMed] [Google Scholar]
- Ferrannini E. The theoretical bases of indirect calorimetry: a review. Metabolism. 1988;37:287–301. doi: 10.1016/0026-0495(88)90110-2. [DOI] [PubMed] [Google Scholar]
- Ghanassia E, Brun J-F, Mercier J, Raynaud E. Oxidative mechanisms at rest and during exercise. Clinica Chimica Acta. 2007;383:1–20. doi: 10.1016/j.cca.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Gong B, Miki T, Seino S, Renaud J-M. A KATP channel deficiency affects resting tension, not contractile force, during fatigue in skeletal muscle. Am. J. Physiol. 2000;279:C1351–C1358. doi: 10.1152/ajpcell.2000.279.5.C1351. [DOI] [PubMed] [Google Scholar]
- Gumina RJ, Pucar D, Bast P, Hodgson DM, Kurtz CE, Dzeja PP, Miki T, Seino S, Terzic A. Knockout of Kir6.2 negates ischemic reconditioning-induced protection of myocardial energetics. Am. J. Physiol. 2003;284:H2106–H2139. doi: 10.1152/ajpheart.00057.2003. [DOI] [PubMed] [Google Scholar]
- Himms-Hagen J. Exercise in a pill: feasibility of energy expenditure targets. Curr. Drug Targets CNS Neurol. Disord. 2004;3:389–409. doi: 10.2174/1568007043337076. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Gonoi T, Clement JP, 4th, Namba N, Inazawa J, Gonzalez G, Aguilar-Bryan L, Seino S, Bryan J. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor. Science. 1995;270:1166–1170. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- Joyner MJ. Modeling: optimal marathon performance on the basis of physiological factors. J. Appl. Physiol. 1991;70:683–687. doi: 10.1152/jappl.1991.70.2.683. [DOI] [PubMed] [Google Scholar]
- Kane GC, Behfar A, Yamada S, Perez-Terzic C, O'Cochlain F, Reyes S, Dzeja PP, Miki T, Seino S, Terzic A. ATP-sensitive K+ channel knockout compromises the metabolic benefit of exercise training, resulting in cardiac deficits. Diabetes. 2004;53 Suppl 3:S169–S175. doi: 10.2337/diabetes.53.suppl_3.s169. [DOI] [PubMed] [Google Scholar]
- Kunz WS. Control of oxidative phosphorylation in skeletal muscle. Biochim. Biophys. Acta. 2001;1504:12–19. doi: 10.1016/s0005-2728(00)00235-8. [DOI] [PubMed] [Google Scholar]
- Levine JA, Eberhardt NL, Jensen MD. Role of nonexercise activity thermogenesis in resistance to fat gain in humans. Science. 1999;283:212–214. doi: 10.1126/science.283.5399.212. [DOI] [PubMed] [Google Scholar]
- Li B, Nolte LA, Ju J-S, Han DH, Coleman T, Holloszy JO, Semenkovich CF. Skeletal muscle respiratory uncoupling prevents diet-induced obesity and insulin resistance in mice. Nat. Med. 2000;6:1115–1120. doi: 10.1038/80450. [DOI] [PubMed] [Google Scholar]
- Liu C, Wu J, Zhu J, Kuei C, Yu J, Shelton J, Sutton SW, Li X, Yun SJ, Mirzadegan T, et al. Lactate inhibits lipolysis in fat cells through activation of an orphan G-protein-coupled receptor, GPR81. J. Biol. Chem. 2009;284:2811–2822. doi: 10.1074/jbc.M806409200. [DOI] [PubMed] [Google Scholar]
- Lueck JD, Lungu C, Mankodi A, Osborne RJ, Welle SL, Dirksen RT, Thornton CA. Chloride channelopathy in myotonic dystrophy resulting from loss of posttranscriptional regulation for CLCN1. Am. J. Physiol. 2007;292:C1291–C1297. doi: 10.1152/ajpcell.00336.2006. [DOI] [PubMed] [Google Scholar]
- Malester B, Tong X, Ghiu I, Kontogeorgis A, Gutstein DE, Xu J, Hendricks-Munoz KD, Coetzee WA. Transgenic expression of a dominant negative KATP channel subunit in the mouse endothelium: effects on coronary flow and endothelin-1 secretion. FASEB J. 2007;21:2162–2172. doi: 10.1096/fj.06-7821com. [DOI] [PubMed] [Google Scholar]
- Matar W, Nosek TM, Wong D, Renaud J-M. Pinacidil suppresses contractility and preserves energy but glibenclamide has no effect during muscle fatigue. Am. J. Physiol. 2000;278:C404–C416. doi: 10.1152/ajpcell.2000.278.2.C404. [DOI] [PubMed] [Google Scholar]
- McArdle WD, Katch FI, Katch VL. Human energy expenditure during rest and physical activity. In: Balado D, editor. In Exercise Physiology. 4th edition. Baltimore, MD: Williams & Wilkins; 1996. pp. 151–164. [Google Scholar]
- Miki T, Seino S. Roles of KATP channels as metabolic sensors in acute metabolic changes. J. Mol. Cell. Cardiol. 2005;38:917–925. doi: 10.1016/j.yjmcc.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Miki T, Minami K, Zhang L, Morita M, Gonoi T, Shiuchi T, Minokoshi Y, Renaud J-M, Seino S. ATP-sensitive potassium channels participate in glucose uptake in skeletal muscle and adipose tissue. Am. J. Physiol. 2002;283:E1178–E1184. doi: 10.1152/ajpendo.00313.2002. [DOI] [PubMed] [Google Scholar]
- Miki T, Nagashima K, Tashiro F, Kotake K, Yoshitomi H, Tamamoto A, Gonoi T, Iwanaga T, Miyazaki J, Seino S. Defective insulin secretion and enhanced insulin action in KATP channel-deficient mice. Proc. Natl. Acad. Sci. USA. 1998;95:10402–10406. doi: 10.1073/pnas.95.18.10402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami K, Morita M, Saraya A, Yano H, Terauchi Y, Miki T, Kuriyama T, Kadowaki T, Seino S. ATP-sensitive K+ channel-mediated glucose uptake is independent of IRS-1/phosphatidylinositol 3-kinase signaling. Am. J. Physiol. 2003;285:E1289–E1296. doi: 10.1152/ajpendo.00278.2003. [DOI] [PubMed] [Google Scholar]
- Momken I, Lechêne P, Koulmann N, Fortin D, Mateo P, Doan BT, Hoerter J, Bigard X, Veksler V, Ventura-Clapier R. Impaired voluntary running capacity of creatine kinase-deficient mice. J. Physiol. 2005;565:951–964. doi: 10.1113/jphysiol.2005.086397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mootha V, Arai AE, Balaban RS. Maximum oxidative phosphorylation capacity of the mammalian heart. Am. J. Physiol. 1997;272:H769–H775. doi: 10.1152/ajpheart.1997.272.2.H769. [DOI] [PubMed] [Google Scholar]
- Nichols CG. KATP channels as molecular sensors of cellular metabolism. Nature. 2006;440:470–476. doi: 10.1038/nature04711. [DOI] [PubMed] [Google Scholar]
- Nichols CG, Lederer WJ. The regulation of ATP-sensitive K+ channel activity in intact and permeabilized rat ventricular myocytes. J. Physiol. 1990;423:91–110. doi: 10.1113/jphysiol.1990.sp018013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols CG, Lederer WJ. Adenosine triphosphate-sensitive potassium channels in the cardiovascular system. Am. J. Physiol. 1991;261:H1675–H1686. doi: 10.1152/ajpheart.1991.261.6.H1675. [DOI] [PubMed] [Google Scholar]
- Nichols CG, Shyng SL, Nestorowicz A, Glaser B, Clement JP, Gonzalez G, Aguilar-Bryan L, Permutt MA, Bryan J. Adenosine diphosphate as an intracellular regulator of insulin secretion. Science. 1996;272:1785–1787. doi: 10.1126/science.272.5269.1785. [DOI] [PubMed] [Google Scholar]
- Nielsen HB, Bredmose PP, Str000F8;mstad M, Volianitis S, Quistorff B, Secher NH. Bicarbonate attenuates arterial desaturation during maximal exercise in humans. J. Appl. Physiol. 2002;93:724–731. doi: 10.1152/japplphysiol.00398.2000. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- Renaud JM. Modulation of force development by Na+, K+, Na+/K+ pump and KATP channel during muscular activity. Can. J. Appl. Physiol. 2002;27:296–315. doi: 10.1139/h02-017. [DOI] [PubMed] [Google Scholar]
- Reyes S, Kane GC, Miki T, Seino S, Terzic A. KATP channels confer survival advantage in cocaine overdose. Mol Psychiatry. 2007;12:1060–1061. doi: 10.1038/sj.mp.4002083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Seeley RJ, Barash GS, Baskin DG, Leibel RL. Is the energy homeostasis system inherently biased toward weight gain? Diabetes. 2003;52:232–238. doi: 10.2337/diabetes.52.2.232. [DOI] [PubMed] [Google Scholar]
- Selivanov VA, Alekseev AE, Hodgson DM, Dzeja PP, Terzic A. Nucleotide-gated KATP channels integrated with creatine and adenylate kinases: amplification, tuning and sensing of energetic signals in the compartmentalized cellular environment. Mol. Cell. Biochem. 2004:256–257. 243–256. doi: 10.1023/b:mcbi.0000009872.35940.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinaasappel M, Donkersloot C, Van Bommel J, Ince C. PO2 measurements in the rat intestinal microcirculation. Am. J. Physiol. 1999;39:G1515–G1520. doi: 10.1152/ajpgi.1999.276.6.G1515. [DOI] [PubMed] [Google Scholar]
- Spanswick D, Smith MA, Groppi VE, Logan SD, Ashford MLJ. Leptin inhibits hypothalamic neurons by activation of ATP-sensitive potassium channels. Nature. 1997;390:521–525. doi: 10.1038/37379. [DOI] [PubMed] [Google Scholar]
- Tayler JR. An Introduction to Error Analysis: the Study of Uncertainties in Physical Measurements. 2nd ed. Sausalito, California: University Science Book; 1997. [Google Scholar]
- Thabet M, Miki T, Seino S, Renaud J-M. Treadmill running causes significant fiber damage in skeletal muscle of KATP channel-deficient mice. Physiol. Genomics. 2005;22:204–212. doi: 10.1152/physiolgenomics.00064.2005. [DOI] [PubMed] [Google Scholar]
- Watt MJ, Dzamko N, Thomas WG, Rose-John S, Ernst M, Carling D, Kemp BE, Febbraio MA, Steinberg GR. CNTF reverses obesity-induced insulin resistance by activating skeletal muscle AMPK. Nat. Med. 2006;12:541–548. doi: 10.1038/nm1383. [DOI] [PubMed] [Google Scholar]
- Weiss JN, Lamp ST. Cardiac ATP-sensitive K+ channels. Evidence for preferential regulation by glycolysis. J. Gen. Physiol. 1989;94:911–935. doi: 10.1085/jgp.94.5.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Isomoto S, Matsumoto S, Kondo C, Shindo T, Horio Y, Kurachi Y. Sulphonylurea receptor 2B and Kir6.1 form a sulphonylurea-sensitive but ATP-insensitive K+ channel. J. Physiol. 1997;499:715–720. doi: 10.1113/jphysiol.1997.sp021963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S, Kane GC, Behfar A, Liu XK, Dyer RB, Faustino RS, Miki T, Seino S, Terzic A. Protection conferred by myocardial ATP-sensitive K+ channels in pressure overload-induced congestive heart failure revealed in KCNJ11 Kir6.2-null mutant. J. Physiol. 2006;577:1053–1065. doi: 10.1113/jphysiol.2006.119511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingman L, Hodgson D, Bast P, Kane G, Perez-Terzic C, Gumina R, Pucar D, Bienengraeber M, Miki T, Seino S, et al. A. Kir6.2 required for adaptation to stress. Proc. Natl. Acad. Sci. USA. 2002;99:13278–13283. doi: 10.1073/pnas.212315199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingman LV, Hodgson DM, Alekseev AE, Terzic A. Stress without distress: homeostatic role for KATP channels. Mol. Psychiatry. 2003;8:253–254. doi: 10.1038/sj.mp.4001323. [DOI] [PubMed] [Google Scholar]
- Zoll J, Sanchez H, N'Guessan B, Ribera F, Lampert E, Bigard X, Serrurier B, Fortin D, Geny B, Veksler V, et al. Physical activity changes the regulation of mitochondrial respiration in human skeletal muscle. J. Physiol. 2002;543:191–200. doi: 10.1113/jphysiol.2002.019661. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.