Abstract
Although hypertension is a major risk factor for cerebrovascular disease (CVD) and is highly prevalent in African Americans, little is known about how blood pressure (BP) affects brain behavior relationships in this population. In predominantly Caucasian populations, high BP is associated with alterations in frontal-subcortical white matter and in executive functioning aspects of cognition. We investigated associations among BP, brain structure, and neuropsychological functioning in 52 middle-older aged African Americans without diagnosed history of CVD. All participants underwent diffusion tensor imaging (DTI) for examination of white matter integrity, indexed by fractional anisotropy (FA). Three regions of interest (ROI's) were derived in the anterior corpus callosum (genu), posterior corpus callosum (splenium), and across the whole brain. A brief neuropsychological battery was administered from which composite scores of executive function and memory were derived. Blood pressure was characterized by mean arterial blood pressure (MABP), an indicator of end-organ perfusion pressure. When controlling for age, higher MABP was associated with lower FA in the genu, and there was a trend for this sample relationship with regard to whole brain FA. When the sample was broken into groups based on treatment for BP regulation (medicated / nonmedicated), MABP was related to genu and whole-brain FA only in the non-medicated group. There were no associations in those individuals who reported taking medication to control blood pressure. Neither MABP nor FA was significantly related to either neuropsychological composite score regardless of medication use. These data provide important evidence that variation in BP may contribute to significant alterations in specific neural regions of white matter in non-medicated individuals without symptoms of overt CVD.
Elevated blood pressure (BP) is a significant risk factor for cerebrovascular disease (CVD), and it is becoming more prevalent as the population grows proportionally older and more sedentary. Complications arising from high BP are more widespread in African American communities relative to other racial groups (Howard et al., 2006; Taylor et al., 2008), and there is evidence to suggest that as a group, African Americans are more susceptible to the serious consequences of CVD, such as stroke and vascular-related cognitive decline (Singh, Cohen, Krupp, & Abedi, 1998; Whitfield et al., 2008). It is possible that this increased susceptibility to vascular events may also result in heightened risk for more subtle brain changes and neuropsychological impairment.
Several studies have now provided substantial evidence that long-standing hypertension can cause significant alterations to brain structure (den Heijer et al., 2003; Firbank et al., 2007; Guo et al., 2009; Taki et al., 2004; Wiseman et al., 2004). Damage to white matter, in particular, is well documented, with many reports that high blood pressure is associated with a greater percentage of white matter lesions (WML; WM hyperintensities measured on T2 or fluid-attenuated inversion-recovery (FLAIR) imaging) (Murray et al., 2005; Verdelho et al., 2007). While these lesions are commonly found in periventricular brain areas (Henskens et al., 2009), they have also been observed in frontal lobe white matter and subcortical brain regions (Hoptman et al., 2009; Raz, Rodrigue, Kennedy, & Acker, 2007; van Es et al., 2008). In addition to causing white matter lesions, hypertension and/or general indices of CVD risk can result in damage to white matter fiber tracts. For example, a recent study found that “stroke risk” (a classification that includes blood pressure as part of its formula) was associated with reduced tissue integrity in the genu of the corpus callosum, a major white matter pathway providing interhemispheric connectivity (Delano-Wood et al., 2008). The vulnerability of white matter pathways, such as the corpus callosum, to vascular compromise has also been documented in more severe cerebrovascular disorders such as vascular dementia (Schmahmann, Smith, Eichler, & Filley, 2008; Zarei et al., 2009). Schmahmann and colleagues (Schmahmann et al., 2008) have suggested that these changes may arise from thickening of arterial walls, followed by consistently restricted blood flow, particularly to microvascular brain regions. White matter is thought to be particularly vulnerable because of the fact that many pathways are fed by these smaller blood vessels, making them more susceptible to ischemic injury (Schmahmann et al., 2008). Reduced flow results in lower oxygenation, which could ultimately contribute to tissue degeneration and neuronal death (Havlik et al., 2002; Knopman, Mosley, Catellier, & Sharrett, 2005; Skoog, 2005).
Regionally-specific hypertension-related alterations to white matter tissue have been reported, including frontal and prefrontal brain regions (Raz, Rodrigue, & Acker, 2003; Raz, Rodrigue, Kennedy et al., 2007), connections associated with subcortical nuclei (Jokinen et al., 2009; van Es et al., 2008), as well as more posterior brain areas (Raz, Rodrigue, & Haacke, 2007). However, despite evidence of seemingly widespread regional impact to brain structure, the predominant finding has been that high blood pressure has at least an initial direct and selective impact on anterior brain regions, particularly in the absence of overt dementia or severe cognitive impairment. This impact includes damage to white matter connecting frontal and subcortical brain regions (Debette et al., 2007; Gouw et al., 2008; Jouvent et al., 2008); (Gold et al., 2005; Wiseman et al., 2004) as well as anterior corpus callosum fibers (Chen et al., 2009; Delano-Wood et al., 2008). The vulnerability of anterior brain regions to high blood pressure is supported by studies in patients with more advanced cerebrovascular diseases, such as vascular dementia (Chen et al., 2009; Hallam et al., 2008; Zarei et al., 2009). For example, a recent report found that patients with subcortical ischemic vascular dementia, a disease for which hypertension is a risk factor, have evidence of damage to the anterior (genu) corpus callosum, in addition to white matter in bilateral frontal-subcortical brain regions (Chen et al., 2009). However, the majority of these studies examined high blood pressure as a group classification as opposed to direct association with blood pressure values.
Given the evidence of structural alterations to anterior brain regions, it is not unexpected that there are associated decrements in cognition, primarily in executive functions, a neuropsychological domain that in aging has been linked to the frontal cortex and its associated subcortical structures (Brady, Spiro, & Gaziano, 2005; Sorond, Schnyer, Serrador, Milberg, & Lipsitz, 2008). Notably, individuals with at least one CVD risk factor, such as elevated blood pressure, perform poorly on neuropsychological measures of attention, concentration, and higher-order thinking (Brady et al., 2005; Raz et al., 2003; Raz, Rodrigue, Kennedy et al., 2007). Supporting the structure-function connection, these impairments have also been reported in conjunction with white matter tissue changes. For example, Raz et al. (2007) found a greater percentage of frontal lobe white matter hyperintensities in individuals with higher blood pressure, and these markers were associated with executive functioning impairments (Raz, Rodrigue, Kennedy et al., 2007). A more recent study found decreased executive functioning in untreated hypertensives that correlated with indicators of white matter integrity (mean diffusivity as assessed through DTI) (Hannesdottir et al., 2009). Thus, in summary, the preponderance of evidence points to the anterior brain and executive functioning as primary targets of elevated blood pressure.
While there is substantial evidence indicating brain structural and neuropsychological consequences of high blood pressure and vascular risk, there are few studies that have examined these associations across a range of risk indexed quantitatively, as opposed to grouping individuals dichotomously by the presence or absence of risk (i.e., hypertensive or nonhypertensive; low versus high vascular risk), and none of these studies, to our knowledge, have examined these associations in an exclusively African American cohort. In the current study, we examined relationships among continuous quantitative measures of white matter integrity, cognition, and blood pressure. We used advanced DTI acquisition and analysis procedures to investigate these quantitative relationships with three aims:
(1) Determine how blood pressure, a measure that is commonly used to gauge CVD risk, is associated with brain structure (white matter). Specific regions of interest (ROIs) included indices of integrity of anterior (anterior corpus callosum, or genu), posterior (posterior corpus callosum, or splenium), as well as a whole brain diffusion tensor imaging (DTI) measure. We predicted that higher levels of blood pressure and CVD risk would be associated with reduced fractional anisotropy (FA), an index of white matter integrity, in the genu and on a whole brain level, but not in the splenium because of its posterior location.
(2) Determine whether variation in blood pressure impacts cognition, specifically executive and memory function, as these are associated with anterior and posterior brain regions, respectively. We predicted that higher blood pressure would be associated with executive, but not memory functioning.
(3) Determine whether variation in each of the three diffusion ROIs relates to cognition. We predicted that higher FA in the genu of the corpus callosum, as well as whole-brain FA, would be associated with higher scores (and thus better performance) on the executive functioning composite score.
These collective predictions were based on the preponderance of evidence in prior literature documenting frontal brain changes and executive function deficits in individuals with relatively high risk for CVD, particularly in those without any evidence of overt disease or early dementia, as is the case in the current sample. Ultimately, we expect that findings from this study would contribute significantly to knowledge regarding risk in this specific population, as well as to the larger literature on the relationship of quantitative indicators of CVD risk such as blood pressure to brain structure and cognition.
Methods
Participants
The study sample was comprised of fifty-two participants (23M /29F) who were recruited by the Harvard Cooperative Program on Aging (HCPA) Claude Pepper Older American Independence Center (OAIC). Participants in this program were recruited from the community in response to an advertisement appearing in the HCPA newsletter asking for healthy community-dwelling older African Americans to participate in a study to examine physical health and cognition. Participants from this larger group who agreed to have a magnetic resonance imaging (MRI) scan were included in the current study. Inclusion criteria included age of 50–85. Participants were excluded for the following reasons: a history of head trauma of “mild” severity or greater according to the criteria of Fortuny et al. (Fortuny, Briggs, Newcombe, Ratcliff, & Thomas, 1980) (e.g., loss of consciousness for greater than 10 minutes), any history of more than one head injury (due to possible cumulative neuropathological effects), diagnosis of any form of dementia (i.e., Parkinson's, Alzheimer's), any severe psychiatric illness, or any history of brain surgery. All participants were literate with at least a 6th grade education. Fifty of the participants were right-handed. Mini-mental state examination (MMSE) scores ranged from 24 to 30. These scores are in a range outside of a dementia diagnosis, according to normative data in this particular racial group (Bohnstedt, Fox, & Kohatsu, 1994; Wong & Baden, 2001).
MRI Image Acquisition
Each participant received a whole-head high resolution DTI scan (Siemens; 1.5 Tesla Sonata System), collected using the following parameters: repetition time (TR)=7200 ms echo time (TE)=77 ms, 60 slices total, acquisition matrix = 128 × 128 (field of view; FOV=256 × 256 mm), slice thickness = 2 mm (for 2 mm3 isotropic voxels) with 0 mm gap, with a b value=700 s/mm2, 10 T2 and 60 diffusion weighted images, and one image, the T2-weighted “low b” image with a b-value=0 s/mm2 as an anatomical reference volume. Total acquisition time for the DTI scan was 8 minutes 31 seconds. Acquisitions used a twice-refocused balanced echo to reduce eddy current distortions (Reese, Heid, Weisskoff, & Wedeen, 2003).
Image Analysis and DTI Processing
Diffusion data were processed using a multistep procedure involving the FreeSurfer image analysis suite (http://surfer.nmr.mgh.harvard.edu) and FSL (http://www.fmrib.ox.ac.uk.fsl/) processing streams. A T2-weighted structural volume, collected using identical sequence parameters as the directional volumes with no diffusion-weighting and thus in register with the final diffusion maps, was used for all registration and motion correction using a 12-parameter affine mutual information procedure in FMRIB's Linear Image Registration Tool (FLIRT) (Jenkinson, 2003; Jenkinson, Bannister, Brady, & Smith, 2002; Jenkinson & Smith, 2001). The diffusion tensor was calculated for each voxel using a least-squares fit to the diffusion signal (Pierpaoli & Basser, 1996), and brain-extracted using BET (Smith, 2002). The FA metric was derived from the diffusion tensor as described previously (Pierpaoli & Basser, 1996). Each FA image was mapped to Talairach space. Then, FA data were prepared for statistical analyses using TBSS (Tract-Based Spatial Statistics, (Smith et al., 2006)), part of FSL. First, all participants' FA data were aligned into a common space using the nonlinear registration tool FNIRT (Andersson, Jenkinson, & Smith, 2007a, 2007b), which uses a b-spline representation of the registration warp field (Rueckert et al., 1999). Next, the mean FA image was created and thinned to create a mean FA skeleton that represents the centers of all fiber tracts common to the entire sample. Each subject's aligned FA data were then projected onto this skeleton and then transformed back to native space, from which whole brain and ROI voxel-based FA data were derived.
Regional Analysis of Diffusion Data
Regions for ROI analysis included the anterior corpus callosum (genu), posterior corpus callosum (splenium), and a global whole brain FA average. These regions were selected based on the premise that higher levels of CVD risk factors would have an impact on major white matter pathways (such as the corpus callosum) and on a whole brain level, at least in the early stages of cerebrovascular disease. The corpus callosum is a major white matter pathway in the brain that runs from anterior to posterior brain regions, and thus is an appropriate structure in which to examine the differential effects of CVD risk on these specific brain regions.
Whole-brain FA was measured by segmenting the entire FA skeleton for each individual to ensure that only white matter was included and to avoid potential partial voluming. Genu and splenium ROIs were derived from the Johns Hopkins University white-matter tractography atlas, a probabilistic atlas available as part of the FSL toolbox (Hua et al., 2008; Mori, Wakana, Nagae-Poetscher, & van Zijl, 2005; Wakana et al., 2007). Mean FA values were extracted for these atlas-defined regions on each volume. All ROI analyses were performed on native space data by inverting the transform to standard TBSS space (see above) and then applying the inverted (deprojected) transform to the regional labels (genu, splenium, whole brain).
Neuropsychological Testing
All participants completed a neuropsychological battery designed to assess both memory and executive functions. The following measures were included in the current study: the Trailmaking Test part B (Spreen & Strauss, 1998); California Verbal Learning Test-Second Edition (CVLT-II) short-delay free recall (SDFR) and long-delay free recall (LDFR) (Delis, Kramer, Kaplan, & Ober, 2000); and Controlled Oral Word Association (COWA) or Verbal Fluency (Spreen & Strauss, 1998). All raw scores were converted to z-scores based on the current sample. Z-scores from measures in each domain were added together to create a composite score. The executive function composite score comprised scores from COWA and Trailmaking part B. The memory function composite score was created by adding scores from the CVLT-II SDFR and LDFR. Two individuals did not complete COWA and two did not complete Trailmaking part B; thus, four individuals were not included in analyses with the executive function subscore.
Cerebrovascular Disease Risk Assessment
Blood pressure (BP) was recorded in a seated position after five minutes of rest, with the arm at rest at the level of the heart using a standard sphygmomanometer. A second measurement was obtained 5 minutes later, and an average systolic and average diastolic pressure were computed across the two measurements. Blood pressure was always measured by the study physician (JLR). Systolic and diastolic blood pressure were then considered together to create a mean arterial blood pressure (MABP) using the following formula: MABP: 1/3 (Systolic − diastolic) + diastolic. MABP is a metric commonly used in clinical settings to obtain an accurate metric of overall BP, due to the fact that it contains both systolic and diastolic measurements in its formula. MABP is believed to indicate perfusion pressure, particularly in body organs. Thus, it is an appropriate metric to use when examining associations between blood pressure and brain structure, or blood pressure and function. Prior studies have utilized MABP, particularly when examining blood pressure in the context of cognition or brain structure in older adults (Brown et al., 2008; Guo et al., 2009). In our sample, systolic BP ranged from 105 to 172 millimeters of pressure (mm HG) and diastolic BP ranged from 56 to 109. MABP ranged from 74 to 130. Current convention considers a MABP of approximately 106 to be indicative of mild hypertension, and a MABP of 126 to be indicative of moderate hypertension (American Heart Association). Nine percent (5 out of 53) of the sample would thus be considered to have mild hypertension, and .2 % (1 out of 53) would be classified as having moderate hypertension.
Medication Usage
An additional, potentially important variable relates to use of medication to control blood pressure. In the current sample, 25 individuals (48%) were taking blood pressure regulating medicine (such as beta blockers, ace inhibitors, or calcium channel blockers). Given the fact that approximately half of the sample was taking blood pressure medications, we divided the sample further and conducted analyses both across and in each group separately (medicated and non-medicated). These individuals are also represented separately in Figures (see Figures 1 & 2) of significant results, demonstrating how participants taking blood pressure medication fell across the distribution of each association.
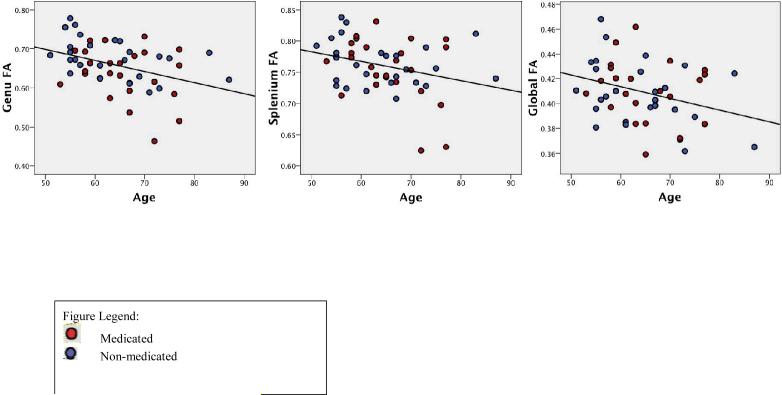
Figure 1.
Relationship of age to diffusion ROIs.
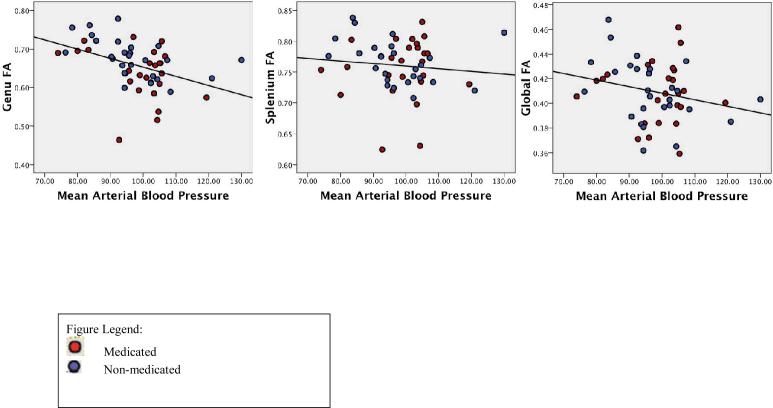
Figure 2.
Relationship of MABP to diffusion ROIs.
Statistical Analyses
Statistical analyses initially involved a series of bivariate correlations to determine the relationship of age to all primary variables (including MABP, genu FA, splenium FA, whole-brain FA, executive function composite score, and memory composite score). Next, one-way analyses of variance (ANOVAs) were conducted to determine any group differences (yes/no medication use) on all primary variables. Primary analyses then involved a series of partial correlation analyses examining: 1) the relationship of MABP to genu FA, splenium FA, and whole-brain FA, 2) the relationship of MABP to each neuropsychological composite score, and 3) the relationship of all three diffusion ROIs to each neuropsychological composite score. Age was included as a covariate in those partial correlation analyses for which there was a significant correlation with each dependent variable.
Results
Demographic, Physiological, Neuropsychological and Brain Structural Data
Demographic data, blood pressure data (MABP), and mean neuropsychological test scores (including individual tests as well as composite z-scores) for the entire sample are presented in Table 1. Table 2 presents mean FA values for diffusion ROIs.
Table 1.
Demographic, Physiological and Neuropsychological Data (n=52)
| Mean | Standard Deviation | |
|---|---|---|
| Age | 66.02 | 7.63 |
| Education (years) | 13.60 | 2.30 |
| MMSE score | 27.19 | 2.26 |
| Physiological | ||
| Systolic Blood Pressure (mm HG) | 135.68 | 16.37 |
| Diastolic Blood Pressure (mm HG) | 80.70 | 8.66 |
| MABP | 99.03 | 10.19 |
| Neuropsychological | ||
| Trailmaking part B (seconds) (n=50) | 113.50 | 57.62 |
| COWA (n=50) | 36.97 | 12.84 |
| Executive function composite z-score (n=48)^ | −.07 | 1.09 |
| CVLT SDFR | 8.41 | 2.48 |
| CVLT LDFR | 9.24 | 2.86 |
| Memory composite z-score | .05 | 1.81 |
MABP=mean arterial blood pressure; CVLT=California Verbal Learning Test; SDFR=short-delay free recall; LDFR=long delay free recall; COWA=controlled oral word association; MMSE=Mini-Mental State Examination (score is out of 30 possible points)
CVLT SDFR and LDFR: score is out of 16 total words; COWA: total words beginning with F, A and S given in 3 minutes
total N is reduced to 48 for the executive function composite score due to 4 individuals not completing all tests.
Table 2.
Fractional anisotropy (FA) data for diffusion ROIs*
| Diffusion ROIs** | Mean | Standard Deviation |
|---|---|---|
| Genu FA | .77 | .07 |
| Splenium FA | .83 | .06 |
| Whole Brain FA | .35 | .03 |
FA values range from .1 (lowest anisotropy) to .9 (highest anisotropy)
Relationship of Age to all Variables
Bivariate correlation analyses were conducted to determine if age was significantly related to any of the dependent variables. All three diffusion ROIs were all significantly related to age (genu: r=−.42, p < .01; splenium: r=−.34, p < .05; whole-brain FA: r=−.39, p < .05). As expected, in all three regions, age was negatively associated with FA such that FA decreased as age increased across the sample. These results are presented in Figure 1; medication groups are represented separately. Age was not associated with MABP (r=−.007, p > .05) or with either neuropsychological variable (executive function: r=.23, p > .05; memory: r=−0.20, p > .05). Thus, age was included as a covariate only in analyses with diffusion ROIs.
Effects of Medication
As an initial means to determine the effect of medication usage on all variables, we conducted a series of ANOVAs to determine if there were significant differences between individuals taking or not taking BP medication. Seven one-way ANOVAs were conducted with BP medication as a between subjects variable and either MABP, genu FA, splenium FA, whole-brain FA, executive function score, or memory score as the within-subjects dependent variable. Group differences were found only for the genu (F (1, 50) = 5.49, p < .05). Means and standard deviations for all variables by group are presented in Table 3. Four participants did not complete one of the two measures used for the executive function composite score due to testing time constraints (two in each group). Thus, analyses with the executive function variable contained two fewer individuals per group (four total).
Table 3.
Demographic, MABP, diffusion, and neuropsychological data by BP medication group
| Medicated (N=25) Mean (SD) | Non-Medicated (N=27) Mean (SD) | |
|---|---|---|
| Age | 65.38 (6.92) | 63.48 (9.23) |
| MABP | 98.64 (9.82) | 97.00 (11.64) |
| Genu FA* | .77 (.08) | .81 (.05) |
| Splenium FA | .82 (.06) | .84 (.05) |
| Whole-brain FA | .35 (.03) | .36 (.03) |
| Executive Function Composite z-Score (n=23) | −.16 (.03) | .03 (1.02) |
| Memory Composite z-Score | .31 (1.50) | −.17 (2.04) |
p < .05 between groups (one-way ANOVA)
Correlation Analyses with MABP
Due to the fact that approximately half of the total sample reported taking blood pressure medications at the time of evaluation, correlation analyses were run in the entire sample as well as separately for those individuals taking (“medicated”) and not taking (“non-medicated”) BP medications.
1. Relationship of MABP to Diffusion ROIs
Partial correlations, controlling for age, were computed relating MABP to all three diffusion ROIs in the entire sample and in each group. Age was included as a covariate because of its significant negative association with all three brain regions. In the entire sample, the relationship between MABP and genu FA was significant (p < .01), and the relationship between MABP and whole-brain FA approached significance (p=.08). The correlation between MABP and splenium FA was non-significant (p > .05) (Table 4a). In the non-medicated group, analyses revealed that MABP was significantly related to the genu and to whole-brain FA, but not to FA in the splenium (Figure 2; Table 4b). In the medicated group, there were no significant relationships between MABP and spelnium or whole-brain FA; however, the relationship between MABP and FA in the genu approached significance (p=.07) (Table 4c), and trends in this group were similar to those in the non-medicated group such that higher FA was associated with lower MABP values. In order to more directly compare correlation coefficients across the two samples, Fisher r-to-z transformations were conducted for the significant correlations, including the genu/MABP and the whole-brain/MABP comparisons. These analyses revealed a z of .91 (p > .05) for the genu correlation, and a z of 1.34 (p < .10) for the whole-brain comparison, indicating that these correlations were not statistically significantly different across medicated and non-medicated samples.
Table 4a.
Partial and Bivariate Correlations between MABP, Diffusion ROIs, and Neuropsychological Variables in the entire sample (n=52)
| MABP | Genu FA | Splenium FA | Whole-brain FA | |
|---|---|---|---|---|
| MABP | --- | −.458** | −.115 | −.248^ |
| Executive function score (n=48) | −.253^ | .014 | .002 | .200 |
| Memory score | −.104 | −.060 | .039 | .107 |
Correlations with all diffusion ROIs (genu FA, splenium FA and whole-brain FA) were partial correlations controlling for age
MABP=Mean arterial blood pressure
p < .01
approached significance (p < .10)
Table 4b.
Partial and Bivariate Correlations between MABP, Diffusion ROIs, and Neuropsychological Variables in the medicated group (n=25)
| MABP | Genu FA | Splenium FA | Whole-brain FA | |
|---|---|---|---|---|
| MABP | --- | −.376^ | .048 | −.044 |
| Executive function score (n=23) | −.173 | −.161 | −.112 | .029 |
| Memory score | .036 | .231 | .268 | .284 |
Correlations with all diffusion ROIs (genu FA, splenium FA and whole-brain FA) were partial correlations controlling for age
MABP=Mean arterial blood pressure
approached significance (p < .10)
Table 4c.
Partial and Bivariate Correlations between MABP, Diffusion ROIs, and Neuropsychological Variables in the non-medicated group (n=27)
| MABP | Genu FA | Splenium FA | Whole-brain FA | |
|---|---|---|---|---|
| MABP | --- | −.581** | −.321 | −.413* |
| Executive function score (n=25) | −.318 | .141 | .237 | .342 |
| Memory score | −.206 | −.331 | −.251 | −.004 |
Correlations with all diffusion ROIs (genu FA, splenium FA and whole-brain FA) were partial correlations controlling for age
MABP=Mean arterial blood pressure
p < .05
p < .01
2. Relationship of MABP to Neuropsychological Composite Sores
Bivariate correlation analyses revealed no significant relationships between MABP and either cognitive score in the entire sample, or in either group, although the negative correlation between MABP and executive function score approached significance in the entire sample (p=.06), such that higher MABP was associated with poorer executive function. This was also true in the non-medicated group (p=.11) (Tables 4a, 4b & 4c).
3. Relationship of Diffusion ROIs to Neuropsychological Composite Scores
Partial correlation analyses, controlling for age, also revealed no significant relationships between neuropsychological composite scores and any diffusion ROI for the whole sample or for either group, although the relationship between the memory score and genu FA approached significance in the non-medicated group (p=.11) (Tables 4a, 4b & 4c).
Discussion
The present data demonstrate associations between MABP and imaging measures of neural integrity. Increased mean arterial blood pressure (MABP) was associated with decreased FA in the genu of the corpus callosum; this relationship was the most significant in individuals who did not report taking any blood pressure medications. MABP was also associated with FA on a whole brain level in the non-medicated group, and there was a trend for this same relationship in the entire sample. Splenium FA and MABP were not significantly correlated in the whole sample, or in either group. Neither neuropsychological composite score was related to MABP or to any of the diffusion ROIs, regardless of medication status. These data are in part consistent with predictions and suggest that CVD risk (i.e., blood pressure) may have a regional impact to anterior brain white matter, as well as a global impact. Our findings are particularly compelling because of the increased prevalence of various CVD risk factors in the African American community, and they raise the additional possibility of structural and cognitive changes in this population, whose clinical significance may be underreported. To our knowledge, this is the first study of its kind to investigate this set of issues in a predominantly African American sample, and the nuances of the findings must be further explored and replicated in a larger sample with appropriate controls. Nonetheless, our study represents a novel investigation of the neural and cognitive consequences across a range of blood pressure values, and it provides the first steps in understanding the relationships among these complex systems.
The relationship of white matter integrity (FA) to MABP is consistent with prior reports of a negative impact on the integrity of white matter microstructure (Holtmannspotter et al., 2005; Taki et al., 2004; van Dijk et al., 2004), and furthermore, it appears that blood pressure is selectively related to anterior brain regions, evidenced by a significant relationship between MABP and the anterior (genu), but not posterior (splenium), corpus callosum. Degeneration of anterior callosal fibers has previously been described in association with hypertension and vascular risk, and is also supported by prior studies demonstrating hypertension-related white matter signal abnormalities primarily in frontal and subcortical brain regions (van Dijk et al., 2004; Wu et al., 2006). Our findings are also consistent with reports of a CVD-related impact on the anterior callosum in more severe dementing disorders with vascular etiology (Hallam et al., 2008), supporting the idea that the genu may be vulnerable to CVD risk before a disease process is evident. White matter disease is indeed thought to be an indicator of worsening disease and cognitive function, particularly in individuals with a history of CVD and CVD risk (Dufouil et al., 2009).
The fact that there were no significant findings between MABP and FA in the group taking medications suggests that treatment potentially has a modifying effect on the associations between blood pressure and brain structure. Prior studies have reported similar neuroanatomical and functional consequences of high blood pressure, regardless of medication use. For example, Raz et al. (2003) found reduced prefrontal brain volumes and lower executive functioning in both treated and untreated high blood pressure, suggesting that hypertension may be detrimental even when controlled by medication (Raz et al., 2003). However, past work has also described circumstances under which hypertensive medication may harbor a protective effect on brain structure and function, and may serve to prevent or slow cognitive decline (Dufouil et al., 2001). Alzheimer's disease patients with a history of blood pressure medication use have even demonstrated less neuropathology on autopsy when compared to those with no such history (Hoffman et al., 2009). Supporting the idea that treatment may at the very least result in differences compared to non-treatment, a recent study found that when compared with normotensive individuals, medically-treated hypertensives exhibited deficits on tests of executive functions, while those with untreated high blood pressure demonstrated worse memory performance (Hannesdottir et al., 2009). The only association with brain structure was found in the untreated group, in which poorer scores on tests of executive function correlated with lower DTI mean diffusivity values (Hannesdottir et al., 2009). Thus, there is certainly a sufficient amount of evidence to suggest that medication use may influence brain-behavior relationships. Our data are consistent with this idea, as we found that blood pressure only significantly related to brain structure in the absence of controlling medication; in addition, there were also significant between-group differences only in the genu such that those taking medication had a higher mean FA than those who were not. The fact that medication groups did not differ significantly with regard to any other variable provides further support for the idea that blood pressure primarily has an effect on anterior white matter. Despite these relatively consistent findings, our sample does differ from prior work in that it did not focus exclusively on hypertension, and instead, contained a fairly broad range of blood pressure readings, from “normotensive” to mild and moderately “hypertensive.” In fact, the non-medicated group represented a larger range of BP values, while the medicated group was, not surprisingly, more restricted in range. Taken together with the fact that the association with FA was similar, and with the fact that the actual correlations were not significantly different, this suggests that group differences may be in part due to differences in blood pressure variability. It may be that with larger sample sizes, correlation magnitudes would become more statistically different in medicated versus non-medicated groups. While medication may influence the BP-brain structure association, there might still be a relationship whereby higher BP, even in a range that would not warrant treatment by clinical standards, may have a negative impact on white matter. However, based on these results alone, we are not able to make statements regarding the potentially protective role of BP medication, other than to speculate that it may prevent against white matter disease (such as degeneration), as longitudinal studies of the relationship between BP and white matter have demonstrated (Guo et al., 2009). Our findings do shed additional light on the impact that blood pressure has on brain structure even in the mild to moderate range of risk, and at the very least suggest that medication to control blood pressure may mediate this physiology-structure relationship.
We did not find any significant relationships between brain structure (diffusion) and neuropsychological variables, which was inconsistent with predictions and prior studies that have found associations between FA and cognition even in samples with no CVD or hypertensive medication usage (Madden et al., 2004; Schiavone, Charlton, Barrick, Morris, & Markus, 2009). One possibility for the lack of findings is that an observable structure-function relationship with diffusion markers of white matter integrity is not apparent until more advanced stages of cerebrovascular disease. Indeed, there is substantial evidence to indicate that white matter abnormalities such as “silent” lacunar infarcts often do not manifest themselves clinically (Kramer, Kenenoff, & Chui, 2001; Kurata, Okura, Watanabe, & Higaki, 2005; Takahashi et al., 2006). As such, our findings of higher blood pressure in relation to both global and regionally reduced FA are potentially early indicators of silent subclincal CVD. However, it is important to note that despite these non-significant correlations, there were two instances in which the direction of the observed correlation was in the opposite direction of what was expected, such that higher splenium FA was associated with lower performance; this was the finding for executive function in the medicated group and for memory function in the non-medicated group, while the correlation direction in the whole sample was in the expected direction. Thus, it may be that medication for blood pressure impacts associations specifically between the splenium and cognition, and it is possible that with a larger sample size, such relationships would become more apparent and significant. However, in the present study it is merely speculative to comment on these potential findings.
The relationship of age to all diffusion ROIs is not surprising, given prior evidence of a negative relationship between age and FA in global and regional brain regions (Salat et al., 2005), including anterior white matter (Yoon, Shim, Lee, Shon, & Yang, 2008). Although not a primary focus of the paper, there were also no significant relationships between blood pressure and neuropsychological functioning, with the exception of a trend for higher MABP and lower executive function composite scores. This is consistent with hypotheses and with what has been reported previously, and perhaps it is the case that these relationships also become more evident as CVD progresses. Additionally, it is certainly possible that with greater power (i.e., larger sample size), the associations between neuropsychological functioning and both brain structure and blood pressure will become more significant.
Our results also provide important implications for the current conceptualization of the phrase “normal cognitive aging.” Much of the literature examining normal aging employs chronological age as a primary independent variable for exploring age-related brain and cognitive changes; historically these studies have used self-report of diseases such as hypertension and diabetes to define groups of assumed healthy participants. The current study provides evidence that these variables may be uncontrolled covariates that should be considered as standard biological factors contributing to variation in neural and cognitive aging. We present critical data implying that a wide range of levels, including those in the subclinical range, can have measurable effects on cognition and brain structure in middle-older aged adults who are self-reported “healthy,” the standard population for studies of normal aging.
It is important to note that the present study was cross-sectional, and as such, does not allow for causal inferences to be made regarding the directionality of the relationship between blood pressure and brain structure. For example, it is certainly possible that changes in white matter precede any blood pressure variation, and alternatively, that the two are not as intrinsically connected as is believed (Jennings & Zanstra, 2009). As suggested by Jennings and Zanstra (2009), the mechanisms underlying vasculature and the brain are still not completely understood, and future studies, particularly those with a longitudinal component, will be necessary to make more precise statements (Jennings & Zanstra, 2009). Nonetheless, we present preliminary but novel findings of a relationship between a physiological risk factor and neural structure in a population that is especially vulnerable to cerebrovascular disease. The fact that our data indicate that manifestations of CVD risk factors such as blood pressure may be operating before a disease process is evident further underscores the importance of early detection, treatment, and monitoring. It may be that over time, the brain becomes even more vulnerable to long-standing elevated blood pressure, and thus, longitudinal investigations are critical. Furthermore, future studies focusing on how aging affects the brain should consider blood pressure, with and without medication usage, as a potential mediator of change.
Acknowledgements
This research was supported by grants from the National Institute of Neurologic Disorders and Stroke (F32NS051942 and K23NS062148), a grant from the National Institute of Nursing Research (R01NR010827), grants from the National Institute on Aging (P60AG08812 and K01AG24898), and by Medical Research Service VA Merit Review Awards to William Milberg and Regina McGlinchey. The authors would like to thank Marge Ahlquist for her assistance with blood pressure collection on all participants.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/neu
References
- American Heart Association from www.americanheart.org.
- Andersson JLR, Jenkinson M, Smith SM. Non-linear optimisation. Oxford: 2007a. [Google Scholar]
- Andersson JLR, Jenkinson M, Smith SM. Non-linear registration, aka Spatial normalisation. Oxford: 2007b. www.fmrib.ox.ac.uk/analysis/techrep. [Google Scholar]
- Bohnstedt M, Fox PJ, Kohatsu ND. Correlates of Mini-Mental Status Examination scores among elderly demented patients: the influence of race-ethnicity. J Clin Epidemiol. 1994;47(12):1381–1387. doi: 10.1016/0895-4356(94)90082-5. [DOI] [PubMed] [Google Scholar]
- Brady CB, Spiro A, Gaziano JM. Effects of age and hypertension on cognition: the Veterans Affairs Normative Aging Study. Neuropsychology. 2005;19(6):770–777. doi: 10.1037/0894-4105.19.6.770. [DOI] [PubMed] [Google Scholar]
- Brown AD, McMorris CA, Longman RS, Leigh R, Hill MD, Friedenreich CM, et al. Effects of cardiorespiratory fitness and cerebral blood flow on cognitive outcomes in older women. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Chen TF, Lin CC, Chen YF, Liu HM, Hua MS, Huang YC, et al. Diffusion tensor changes in patients with amnesic mild cognitive impairment and various dementias. Psychiatry Res. 2009;173(1):15–21. doi: 10.1016/j.pscychresns.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Debette S, Bombois S, Bruandet A, Delbeuck X, Lepoittevin S, Delmaire C, et al. Subcortical hyperintensities are associated with cognitive decline in patients with mild cognitive impairment. Stroke. 2007;38(11):2924–2930. doi: 10.1161/STROKEAHA.107.488403. [DOI] [PubMed] [Google Scholar]
- Delano-Wood L, Bondi MW, Jak AJ, Horne NR, Schweinsburg BC, Frank LR, et al. Stroke risk modifies regional white matter differences in mild cognitive impairment. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kramer J, Kaplan E, Ober B. California Verbal Learning Test-Second Edition. The Psychological Corporation; San Antonio, TX: 2000. [Google Scholar]
- den Heijer T, Skoog I, Oudkerk M, de Leeuw F-E, Cees de Groot J, Hofman A, et al. Association between blood pressure levels over time and brain atrophy in the elderly. Neurobiology of Aging. 2003;24:307–313. doi: 10.1016/s0197-4580(02)00088-x. [DOI] [PubMed] [Google Scholar]
- Dufouil C, de Kersaint-Gilly A, Besancon V, Levy C, Auffray E, Brunnereau L, et al. Longitudinal study of blood pressure and white matter hyperintensities: the EVA MRI Cohort. Neurology. 2001;56(7):921–926. doi: 10.1212/wnl.56.7.921. [DOI] [PubMed] [Google Scholar]
- Dufouil C, Godin O, Chalmers J, Coskun O, MacMahon S, Tzourio-Mazoyer N, et al. Severe cerebral white matter hyperintensities predict severe cognitive decline in patients with cerebrovascular disease history. Stroke. 2009;40(6):2219–2221. doi: 10.1161/STROKEAHA.108.540633. [DOI] [PubMed] [Google Scholar]
- Firbank MJ, Wiseman RM, Burton EJ, Saxby BK, O'Brien JT, Ford GA. Brain atrophy and white matter hyperintensity change in older adults and relationship to blood pressure. Journal of Neurology. 2007;254(6):713–721. doi: 10.1007/s00415-006-0238-4. [DOI] [PubMed] [Google Scholar]
- Fortuny LA, Briggs M, Newcombe F, Ratcliff G, Thomas C. Measuring the duration of post traumatic amnesia. Journal of Neurology, Neurosurgery and Psychiatry. 1980;43(5):377–379. doi: 10.1136/jnnp.43.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold SM, Dziobek I, Rogers K, Bayoumy A, McHugh PF, Convit A. Hypertension and hypothalamo-pituitary-adrenal axis hyperactivity affect frontal lobe integrity. Journal of Clinical Endocrinology and Metabolism. 2005;90(6):3262–3267. doi: 10.1210/jc.2004-2181. [DOI] [PubMed] [Google Scholar]
- Gouw AA, van der Flier WM, Fazekas F, van Straaten EC, Pantoni L, Poggesi A, et al. Progression of white matter hyperintensities and incidence of new lacunes over a 3-year period: the Leukoaraiosis and Disability study. Stroke. 2008;39(5):1414–1420. doi: 10.1161/STROKEAHA.107.498535. [DOI] [PubMed] [Google Scholar]
- Guo X, Pantoni L, Simoni M, Bengtsson C, Bjorkelund C, Lissner L, et al. Blood pressure components and changes in relation to white matter lesions: a 32-year prospective population study. Hypertension. 2009;54(1):57–62. doi: 10.1161/HYPERTENSIONAHA.109.129700. [DOI] [PubMed] [Google Scholar]
- Hallam BJ, Brown WS, Ross C, Buckwalter JG, Bigler ED, Tschanz JT, et al. Regional atrophy of the corpus callosum in dementia. J Int Neuropsychol Soc. 2008;14(3):414–423. doi: 10.1017/S1355617708080533. [DOI] [PubMed] [Google Scholar]
- Hannesdottir K, Nitkunan A, Charlton RA, Barrick TR, MacGregor GA, Markus HS. Cognitive impairment and white matter damage in hypertension: a pilot study. Acta Neurol Scand. 2009;119(4):261–268. doi: 10.1111/j.1600-0404.2008.01098.x. [DOI] [PubMed] [Google Scholar]
- Havlik RJ, Foley DJ, Sayer B, Masaki K, White L, Launer LJ. Variability in Midlife Systolic Blood Pressure is Related to Late-Life Brain White Matter Lesions. Stroke. 2002;33:26–30. doi: 10.1161/hs0102.101890. [DOI] [PubMed] [Google Scholar]
- Henskens LH, Kroon AA, van Oostenbrugge RJ, Gronenschild EH, Hofman PA, Lodder J, et al. Associations of ambulatory blood pressure levels with white matter hyperintensity volumes in hypertensive patients. J Hypertens. 2009;27(7):1446–1452. doi: 10.1097/HJH.0b013e32832b5204. [DOI] [PubMed] [Google Scholar]
- Hoffman LB, Schmeidler J, Lesser GT, Beeri MS, Purohit DP, Grossman HT, et al. Less Alzheimer disease neuropathology in medicated hypertensive than nonhypertensive persons. Neurology. 2009;72(20):1720–1726. doi: 10.1212/01.wnl.0000345881.82856.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmannspotter M, Peters N, Opherk C, Martin D, Herzog J, Bruckmann H, et al. Diffusion magnetic resonance histograms as a surrogate marker and predictor of disease progression in CADASIL: a two-year follow-up study. Stroke. 2005;36(12):2559–2565. doi: 10.1161/01.STR.0000189696.70989.a4. [DOI] [PubMed] [Google Scholar]
- Hoptman MJ, Gunning-Dixon FM, Murphy CF, Ardekani BA, Hrabe J, Lim KO, et al. Blood pressure and white matter integrity in geriatric depression. J Affect Disord. 2009;115(1–2):171–176. doi: 10.1016/j.jad.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard VJ, McClure LA, Meschia JF, Pulley L, Orr SC, Friday GH. High prevalence of stroke symptoms among persons without a diagnosis of stroke or transient ischemic attack in a general population: the REasons for Geographic And Racial Differences in Stroke (REGARDS) study. Arch Intern Med. 2006;166(18):1952–1958. doi: 10.1001/archinte.166.18.1952. [DOI] [PubMed] [Google Scholar]
- Hua K, Zhang J, Wakana S, Jiang H, Li X, Reich DS, et al. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage. 2008;39(1):336–347. doi: 10.1016/j.neuroimage.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M. Fast, automated, N-dimensional phase-unwrapping algorithm. Magn Reson Med. 2003;49(1):193–197. doi: 10.1002/mrm.10354. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimization method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jennings JR, Zanstra Y. Is the brain the essential in hypertension? Neuroimage. 2009;47(3):914–921. doi: 10.1016/j.neuroimage.2009.04.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokinen H, Kalska H, Ylikoski R, Madureira S, Verdelho A, Gouw A, et al. MRI-Defined Subcortical Ischemic Vascular Disease: Baseline Clinical and Neuropsychological Findings. The LADIS Study. Cerebrovasc Dis. 2009;27(4):336–344. doi: 10.1159/000202010. [DOI] [PubMed] [Google Scholar]
- Jouvent E, Mangin JF, Porcher R, Viswanathan A, O'Sullivan M, Guichard JP, et al. Cortical changes in cerebral small vessel diseases: a 3D MRI study of cortical morphology in CADASIL. Brain. 2008;131(Pt 8):2201–2208. doi: 10.1093/brain/awn129. [DOI] [PubMed] [Google Scholar]
- Knopman DS, Mosley TH, Catellier DJ, Sharrett AR. Cardiovascular risk factors and cerebral atrophy in a middle-aged cohort. Neurology. 2005;65:876–881. doi: 10.1212/01.wnl.0000176074.09733.a8. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Kenenoff LA, Chui HC. The Neuropsychology of Subcortical Ischemic Vascular Dementia. In: Waldstein SR, Elias MF, editors. Neuropsychology of Cardiovascular Disease. Lawrence Erlbaum Associates; Mahwah, New Jersey: 2001. pp. 279–300. [Google Scholar]
- Kurata M, Okura T, Watanabe S, Higaki J. Association between carotid hemodynamics and asymptomatic white and gray matter lesions in patients with essential hypertension. Hypertension Research. 2005;28(10):797–803. doi: 10.1291/hypres.28.797. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Whiting WL, Huettel SA, White LE, MacFall JR, Provenzale JM. Diffusion tensor imaging of adult age differences in cerebral white matter: relation to response time. Neuroimage. 2004;21(3):1174–1181. doi: 10.1016/j.neuroimage.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Mori S, Wakana S, Nagae-Poetscher LM, van Zijl PCM. MRI Atlas of Human White Matter. Elsevier; Amsterdam: 2005. [Google Scholar]
- Murray AD, Staff RT, Shenkin SD, Deary IJ, Starr JM, Whalley LJ. Brain white matter hyperintensities: relative importance of vascular risk factors in nondemented elderly people. Radiology. 2005;237(1):251–257. doi: 10.1148/radiol.2371041496. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magnetic Resonance Medicine. 1996;36:893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Acker JD. Hypertension and the brain: vulnerability of the prefrontal regions and executive functions. Behavioral Neuroscience. 2003;117(1169–1180) doi: 10.1037/0735-7044.117.6.1169. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Haacke EM. Brain aging and its modifiers: insights from in vivo neuromorphometry and susceptibility weighted imaging. Ann N Y Acad Sci. 2007;1097:84–93. doi: 10.1196/annals.1379.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Kennedy KM, Acker JD. Vascular health and longitudinal changes in brain and cognition in middle-aged and older adults. Neuropsychology. 2007;21(2):149–157. doi: 10.1037/0894-4105.21.2.149. [DOI] [PubMed] [Google Scholar]
- Reese TG, Heid O, Weisskoff RM, Wedeen VJ. Reduction of eddy-current-induced distortion in diffusion MRI using a twice-refocused spin echo. Magnetic Resonance Medicine. 2003;49:177–182. doi: 10.1002/mrm.10308. [DOI] [PubMed] [Google Scholar]
- Rueckert D, Sonoda LI, Hayes C, Hill DL, Leach MO, Hawkes DJ. Nonrigid registration using free-form deformations: application to breast MR images. IEEE Trans Med Imaging. 1999;18(8):712–721. doi: 10.1109/42.796284. [DOI] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Greve DN, van der Kouwe A, Hevelone ND, Zaleta AK, et al. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiology of Aging. 2005;26:1215–1227. doi: 10.1016/j.neurobiolaging.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Schiavone F, Charlton RA, Barrick TR, Morris RG, Markus HS. Imaging age-related cognitive decline: A comparison of diffusion tensor and magnetization transfer MRI. J Magn Reson Imaging. 2009;29(1):23–30. doi: 10.1002/jmri.21572. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Smith EE, Eichler FS, Filley CM. Cerebral white matter: neuroanatomy, clinical neurology, and neurobehavioral correlates. Ann N Y Acad Sci. 2008;1142:266–309. doi: 10.1196/annals.1444.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Cohen SN, Krupp R, Abedi AG. Racial differences in ischemic cerebrovascular disease. J Stroke Cerebrovasc Dis. 1998;7(5):352–357. doi: 10.1016/s1052-3057(98)80054-2. [DOI] [PubMed] [Google Scholar]
- Skoog I. The Role of Blood Pressure in Dementia. In: Copeland JRM, Abou-Saleh MT, Blazer DG, editors. Principles and Practice of Geriatric Psychiatry. John Wiley & Sons, Ltd.; 2005. pp. 256–257. [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Sorond FA, Schnyer DM, Serrador JM, Milberg WP, Lipsitz LA. Cerebral blood flow regulation during cognitive tasks: effects of healthy aging. Cortex. 2008;44(2):179–184. doi: 10.1016/j.cortex.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreen O, Strauss E. A Compendium of Neuropsychological Tests. Oxford University Press; New York: 1998. [Google Scholar]
- Takahashi T, Murata T, Narita K, Hamada T, Kosaka H, Omori M, et al. Multifractal analysis of deep white matter microstructural changes on MRI in relation to early-stage atherosclerosis. Neuroimage. 2006;32(3):1158–1166. doi: 10.1016/j.neuroimage.2006.04.218. [DOI] [PubMed] [Google Scholar]
- Taki Y, Goto R, Evans A, Zijdenbos A, Neelin P, Lerch J, et al. Voxel-based morphometry of human brain with age and cerebrovascular risk factors. Neurobiology of Aging. 2004;25(4):455–463. doi: 10.1016/j.neurobiolaging.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Taylor H, Liu J, Wilson G, Golden SH, Crook E, Brunson CD, et al. Distinct component profiles and high risk among African Americans with metabolic syndrome: the Jackson Heart Study. Diabetes Care. 2008;31(6):1248–1253. doi: 10.2337/dc07-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk EJ, Breteler MMB, Schmidt R, Berger K, Nilsson L, Oudkerk M, et al. The Association Between Blood Pressure, Hypertension, and Cerebral White Matter Lesions. Hypertension. 2004;44:625. doi: 10.1161/01.HYP.0000145857.98904.20. [DOI] [PubMed] [Google Scholar]
- van Es AC, van der Grond J, de Craen AJ, Admiraal-Behloul F, Blauw GJ, van Buchem MA. Risk factors for cerebral microbleeds in the elderly. Cerebrovasc Dis. 2008;26(4):397–403. doi: 10.1159/000151680. [DOI] [PubMed] [Google Scholar]
- Verdelho A, Madureira S, Ferro JM, Basile AM, Chabriat H, Erkinjuntti T, et al. Differential impact of cerebral white matter changes, diabetes, hypertension and stroke on cognitive performance among non-disabled elderly. The LADIS study. J Neurol Neurosurg Psychiatry. 2007;78(12):1325–1330. doi: 10.1136/jnnp.2006.110361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36(3):630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield KE, Allaire JC, Gamaldo A, Aiken-Morgan AT, Sims R, Edwards C. Blood pressure and memory in older African Americans. Ethn Dis. 2008;18(2):181–186. [PubMed] [Google Scholar]
- Wiseman RM, Saxby BK, Burton EJ, Barber R, Ford GA, O'Brien JT. Hippocampal atrophy, whole brain volume, and white matter lesions in older hypertensive subjects. Neurology. 2004;63:1892–1897. doi: 10.1212/01.wnl.0000144280.59178.78. [DOI] [PubMed] [Google Scholar]
- Wong G, Baden AL. Multiculturally Sensitive Assessment with Older Adults. In: Suzuki LA, Ponterotto JG, Meller PJ, editors. Handbook of Multicultural Assessment. John Wiley & Sons; San Francisco: 2001. pp. 497–522. [Google Scholar]
- Wu Y, Storey P, Cohen BA, Epstein LG, Edelman RR, Ragin AB. Diffusion alterations in corpus callosum of patients with HIV. American Journal of Neuroradiology. 2006;27(3):656–660. [PMC free article] [PubMed] [Google Scholar]
- Yoon B, Shim YS, Lee KS, Shon YM, Yang DW. Region-specific changes of cerebral white matter during normal aging: a diffusion-tensor analysis. Arch Gerontol Geriatr. 2008;47(1):129–138. doi: 10.1016/j.archger.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Zarei M, Damoiseaux JS, Morgese C, Beckmann CF, Smith SM, Matthews PM, et al. Regional white matter integrity differentiates between vascular dementia and Alzheimer disease. Stroke. 2009;40(3):773–779. doi: 10.1161/STROKEAHA.108.530832. [DOI] [PubMed] [Google Scholar]