Abstract
Myeloma is associated with suppression of osteoblastogenesis, consequentially resulting in increased osteoclast activity and induction of typical osteolytic bone disease. The molecular mechanisms by which myeloma cells suppress osteoblastogenesis and the consequences of increased osteoblast activity on myeloma cell growth have been partially delineated only recently. Reduced osteoblastogenesis is a consequence of abnormal properties and impaired osteogenic potential of osteoprogenitor cells from myeloma patients and is also the result of production of multiple osteoblastogenesis inhibitors by myeloma cells and by microenvironmental cells within the myelomatous bone. Nevertheless, novel osteoblast-activating agents (e.g. proteasome inhibitor bortezomib) are capable of inducing bone formation in myeloma animal models and clinically. These agents induce increased osteoblast activity, often coupled with a concomitant reduction in osteoclastogenesis, that is strongly associated with reduced myeloma tumor burden. In vitro, osteoblasts, in contrast to osteoclasts, attenuate the growth of myeloma cells from a large subset of patients; potential molecular mechanisms are discussed. These studies suggest that myeloma cells suppress osteoblastogenesis to their advantage and that increased osteoblast activity is a promising approach to treat myeloma bone disease and simultaneously control myeloma development and progression.
Keywords: Myeloma, bone formation, osteoblasts, proteoglycans, bortezomib, Wnt
Introduction
The coupling of osteoclastic bone resorption to osteoblastic bone formation is tightly controlled by communication between osteogenic cells and osteoclasts via cell surface and systemic factors [1]. In multiple myeloma (MM) the coupling is disrupted, resulting in severe osteolytic bone disease in ~80% of patients [2–4]. It is now evident that myeloma cells suppress osteoblastogenesis and that osteolytic bone disease in MM is also a reflection of osteoblast deactivation. Emerging evidence also indicates that increasing the number of bone-building osteoblasts directly and indirectly negatively affects the progression of MM in bone. Understanding the mechanisms by which myeloma cells impair osteoblastogenesis will help in development of effective interventions for MM bone disease. Shifting myelomatous bone turnover to an anabolic state may negatively impact MM progression and increase the susceptibility of malignant myeloma cells to conventional and novel drugs.
Suppression of osteoblastogenesis in myeloma
Studies on the status of bone remodeling at the early stage of MM development may shed light on the relationship between osteoblastogenesis and myeloma tumor growth. Patients with monoclonal gammopathy of undetermined significance (MGUS), a benign disease with an annual conversion rate of 1% to overt myeloma, do not have typical osteolytic lesions seen in patients with active MM [5]. Bataille et al. [2] were the first to report that increased osteoblast activity coupled with increased bone resorption rate is an early event in conversion from MGUS to MM and that patients who maintained high osteoblast activity did not develop osteolytic disease. Recent intriguing clinical studies provided evidence of altered bone microstructure and impaired bone formation in patients with MGUS [6]. In light of a recent prospective study suggesting that an asymptomatic MGUS stage consistently preceded MM [7], these findings suggest that osteoblastogenesis and bone formation rates play a role in MM progression.
The notion that myeloma cells actively suppress osteoblastogenesis through production of soluble factors was suggested 20 years ago [8], and subsequent data have supported this. These early findings showed that myeloma cells downregulate osteoblast secretion of osteocalcin, a typical osteoblastic marker, and upregulate osteoblast production of interleukin (IL)- 6, a central myeloma growth factor [9]. It is now evident that osteoblast differentiation is inhibited by factors secreted by both myeloma cells (e.g. Wnt-signaling inhibitors DKK1 [10] and secreted frizzled-related protein-2 [11], IL-7 [12], and hepatocyte growth factor (HGF) [13]) and by microenvironmental cells within myelomatous bone (e.g. IL-3 [14,15]).
Osteolytic lesions in MM often occur in areas adjacent to the tumor area [16], suggesting that myeloma-induced suppression of osteoblastogenesis is mediated by, in addition to soluble factors, direct cell contact between myeloma cells and mesenchymal stem cells (MSCs), as well as dysregulation of cell-surface factors that mediate coupling of bone remodeling. Indeed, myeloma cells downregulate osteocalcin in osteogenic cells partially via cell contact [6]. Giuliani et al. [12] demonstrated that myeloma cells induce MSCs to underexpress the critical osteoblast transcription factor RUNX2 (Runt-related transcription factor 2), partially via interactions between cell-surface molecules very late antigen 4 (VLA-4) and vascular cell adhesion molecule 1 (VCAM-1).
Eph receptors and ephrin ligands are cell-surface molecules capable of bidirectional signaling that control cell–cell interactions, immune regulation, neuronal development, tumor growth, metastasis, and angiogenesis [17]. Recent studies indicate that bidirectional signaling between ligand ephrinB2 and its receptor EphB4 also mediates communication between osteoblasts and osteoclasts [18–20]. Whereas osteoblasts and their precursors, MSCs, express ephrinB2 and EphB4, osteoclasts mainly express ephrinB2. Forward signaling in MSCs promotes osteogenic differentiation, and reverse signaling in osteoclast precursors inhibits their differentiation into multinucleated bone-resorbing osteoclasts [19]. It has been proposed that signaling through this axis regulates osteoblastogenesis also via an autocrine or paracrine loop [20]. We have recently shown that ephrinB2 and EphB4 are underexpressed in MSCs from patients with MM. In vitro, myeloma cells downregulated expression of these factors in normal MSCs and had no effect on expression of ephrinB2 in osteoclast precursors. In severe combined immunodeficiency (SCID) mice implanted with fetal human bone (the SCID-hu mouse model), treatment with myeloma-cell conditioned medium downregulated the expression of EphB4, but immunohistochemical analysis of myelomatous bones in this model revealed the downregulation of both of these factors by osteoblasts and osteoclasts [21]. These studies suggest that myeloma cells and the microenvironmental conditioning they induce influence expression of cell-surface bone-coupling factors in myelomatous bone.
Consequences of increasing osteoblastogenesis on myeloma progression
The combination of inhibited osteogenic differentiation along with direct effects on expression of myeloma cell growth factors (e.g. IL-6, VEGF) [9,22,23] and osteoclastogenic factors (e.g. RANKL [receptor activator of NF-κB ligand]) [24,25] create an efficient niche for cultivating myeloma cells and inducing osteolysis [22,26,27]. Accumulating pre-clinical studies support the notion that increasing osteoblast activity in myelomatous bone helps not only to restore bone turnover but also to create an inhospitable environment for myeloma cells because osteoclasts uniformly support survival of myeloma cells [28] while osteoblasts have variable effects on myeloma cells, depending of the source of myeloma cells [29].
Bone-building osteoblasts differ from MSCs and immature osteoblasts by their expression of different levels of bone-building products, cytokines, osteoclastogenic factors, and so-called `coupling factors'. Bone-building osteoblasts produce high levels of osteoprotegerin (OPG) and reduced levels of RANKL, thereby altering the RANKL/OPG ratio in bone and resulting in reduced osteoclastogenesis [25,30,31]. Bone-building osteoblasts may also produce reduced levels of cytokines, such as IL-6, IGF-1, and VEGF, that are involved in bone remodeling [32] and are recognized as myeloma growth factors [33]. These data suggest that approaches to increase numbers of bone-building osteoblasts will reduce levels of critical myeloma growth factors and osteoclastogenic factors in the bone marrow milieu, indirectly affecting survival and growth of myeloma cells.
Do bone-building osteoblasts also directly affect growth and survival of myeloma cells? Primary myeloma cells from most patients have poor survival and growth in vitro unless they are cocultured with osteoclasts [29]. However, when cocultured with osteoblasts or with both osteoblasts and osteoclasts, myeloma cells from a large subset of patients had reduced survival and proliferation, but myeloma cells from certain patients had unaffected or even increased growth and survival [29]. Intriguingly, of patients whose myeloma plasma cells were responsive to osteoblasts, 69% had lytic bone disease; in contrast, of patients whose myeloma plasma cells were not suppressed by osteoblasts, only 33% had bone disease. This study also revealed that the anti-myeloma effect of osteoblasts is not specific to the source of osteoblasts but, rather, reflects inter-patient heterogeneity of myeloma cells [29]. These data suggest that osteoblasts produce factors that can directly restrain growth of myeloma cells.
As an initial step to understand the molecular mechanisms associated with interactions of myeloma cells with osteoblasts, we looked for potential anti-neoplastic factors highly produced by osteoblasts. Bone-building osteoblasts are known to secrete high levels of small leucine-rich proteoglycans (SLRPs) [34], which are implicated in regulation of organic matrix assembly, remodeling of osteoid, and mineral deposition [35–37]. SLRPs such as decorin [38–42] and lumican [43,44] suppress tumor cell growth, and their expression levels in certain malignancies are negatively associated with progression stage [45], suggesting that they could be part of the mechanisms by which osteoblasts attenuate myeloma cell growth. Indeed, blocking activity or expression of decorin reduced osteoblasts' inhibitory effects on myeloma cell growth and survival, and recombinant decorin directly induced myeloma cell apoptosis and attenuated osteoclasts' stimulatory effects on myeloma cells [34]. Overexpression of decorin in MSCs lessened the ability of these cells to support myeloma cell survival [34].
MM is a heterogeneous disease, and myeloma cells from different patients often respond differently to a given intervention, so it is not surprising that decorin had variable effects on primary myeloma cells and did not affect growth of stroma-independent myeloma cell lines. This suggests that the tumor cells adapt pathways to overcome decorin-induced growth suppression [46]. Potential mechanisms by which decorin inhibits tumor cell growth may be related to binding and degradation of growth-factor receptors such as EGFR, Erb2/Erb4, and Met and subsequent upregulation of p21WAF and activation of caspase-3 (for review, see Goldoni and Iozzo [47]). Interestingly, the main Met ligand, HGF, is an autocrine growth factor highly produced by myeloma cells [48] and is involved in myeloma-induced suppression of osteoblastogenesis [13]. These studies suggest that increased levels of decorin, and probably other bone-building factors, in myelomatous bone that result from increased osteoblastogenesis may alter progression of myeloma cells directly and indirectly by alterating activity of supportive bone marrow microenvironmental components such as neomicrovessels and osteoclasts [34].
Pharmacological and cytotherapy approaches to promote bone formation in myeloma
Further understanding of the association between osteoblast activity and myeloma cell growth has been further revealed by in vivo studies. Potentially relevant interventions to activate osteoblasts in MM include agents that promote Wnt signaling in bone indirectly (e.g. neutralizing activity of MM-produced Wnt antagonists) or directly (e.g. Wnt ligands [49], lithium chloride [50]), proteasome inhibitors [51–53], and infusion of MSCs [29].
Anti-DKK1-based therapy to indirectly promote Wnt signaling
Canonical Wnt ligands promote osteoblast differentiation by triggering phosphorylation of the GSK3/Axin complex and subsequent prevention of β-catenin degradation by the proteasome [54]. Canonical Wnt signaling seems to be inhibited in the MM microenvironment mainly through production of DKK1 by myeloma cells, which inhibits Wnt signaling by binding to Wnt receptors LRP5/6 and Kermen [55]. Circulating DKK1 levels are highly correlated with degree of bone loss in patients with MM [10,56] and MGUS [6]. Patient with MM sera that contain high levels of DKK1 effectively suppress Wnt3a-induced β-catenin stabilization in osteoblast precursors and prevent their differentiation [57]. DKK1 inhibition of Wnt signaling indirectly promotes osteoclastogenesis by increasing the RANKL/OPG ratio in preosteoblasts [25]. These studies suggest that DKK1 is a potential target for interventions aimed at treating MM bone disease.
We tested the effects of DKK1-neutralizing antibody on MM bone disease and tumor growth in the SCID-rab model, constructed by implanting a SCID mouse with bone from a 4-week-old rabbit [58]. As in the well-established SCID-hu model [59–61], primary myeloma cells grow restrictively in the implanted rabbit bones and induce typical manifestations of MM, including suppression of osteoblastogenesis, stimulation of osteoclastogenesis and angiogenesis, and induction of osteolysis [62]. In this study, myeloma cells from 11 patients were engrafted in SCID-rab mice. Anti-DKK1 therapy stimulated osteoblastogenesis, inhibited osteoclastogenesis, and subsequently resulted in increased bone mass of the myelomatous bone in 8 of 11 experiments [58]. In 4 of the 11 experiments, increased bone formation was associated with reduced tumor burden from pre-treatment levels [58], reflecting the often heterogeneous clinical response of primary myeloma. These findings were subsequently confirmed in SCID-hu mice engrafted with the INA-6 human myeloma cell line, which is dependent on IL-6 [63]. Interestingly, in the 5T2MM murine model of MM, DKK1-neutralizing antibody abolished suppression of osteoblastogenesis but had no effect on osteoclastogenesis or tumor growth [64]. Taken together, these studies emphasize the central role of DKK1 in myeloma-induced bone disease and myeloma's heterogeneous responses to anti-microenvironmental interventions.
Activation of canonical Wnt signaling
Therapeutically increasing Wnt signaling to induce osteoblastogenesis in myelomatous bone should be evaluated with caution due to continuing controversy over direct effects of Wnt signaling on myeloma cell growth. Edwards et al. [50] investigated the consequences of increasing Wnt signaling in the 5TGM1 myeloma mouse model by using lithium chloride to inhibit GSK-3β. Increased Wnt signaling effectively prevented bone disease and reduced myeloma burden in the bone marrow microenvironment, but it also stimulated tumor growth when 5TGM1 cells were inoculated subcutaneously [50]. Qiang et al. [49] demonstrated that Wnt3a similarly reduced bone disease and growth of H929 myeloma cells and primary myeloma cells that were engrafted in the human bone environment in SCID-hu mice, but Wnt3a had no effect when H929 myeloma cells were grown subcutaneously in SCID mice. Although several laboratories suggested that Wnt3a stimulates myeloma cell growth [65,66], the in vivo studies of Edwards et al. [50] and Qiang et al. [49] indicate that, in the context of medullary myeloma cells growing within the bone marrow microenvironment, increasing Wnt signaling effectively prevents MM bone disease and reduces MM tumor burden in bone, partially through simultaneously increasing osteoblastogenesis and reducing osteoclastogenesis.
Proteasome inhibition
The ubiquitin–proteasome pathway plays a critical role in regulating growth and survival of myeloma cells, providing strong rationale for proteasome targeting and the successful introduction of bortezomib into clinical management of myeloma [67]. Inhibition of the ubiquitin–proteasome pathway reportedly modulates osteoblast differentiation through upregulating expression of bone morphogenetic protein 2 (BMP-2) [51] and preventing proteolytic degradation of RUNX2 [68,69] and β-catenin in a mechanism that is independent of canonical Wnt ligands [70]. Proteasome inhibitor bortezomib may also promote bone formation, through inhibition of DKK1 expression in osteogenic cells [71]. It is noteworthy that bortezomib also directly inhibits osteoclast differentiation, presumably via inhibition of NF-κB activity in osteoclast precursors [72].
Pennisi et al. [52] investigated the effects of bortezomib on myeloma-induced bone resorption and tumor growth in SCID-rab mice engrafted with myeloma cells from 16 patients. Bortezomib heterogeneously affected myeloma tumor growth and overall reduced myeloma tumor burden from pre-treatment levels in 9 of the 16 experiments. Importantly, the anti-myeloma effects of bortezomib were associated with marked increase in bone mass in responding hosts. Inhibition of myeloma growth by frontline clinical agent melphalan was not associated with bone anabolism, suggesting that bortezomib's effects on skeletal homeostasis are not a consequence of reduced tumor burden but rather of direct effects on bone cells [52]. When myeloma cells were located within the bone marrow, bortezomib seemed to induce greater reductions in tumor burden than in extra-osseous sites, further suggesting that this agent's effects on bone cells significantly contribute to its anti-myeloma properties [73].
The association between the anti-myeloma activity and increased osteoblast activity induced by bortezomib is strongly supported by clinical evidence. Zangari et al. [53,74] were the first to demonstrate that the anti-myeloma response of bortezomib is associated with increased bone mass and bone alkaline phosphatase [75] and that increased levels of this osteoblastic marker was a power predictor of treatment response [76]. Additional clinical observations supported the notion that bortezomib is a bone-anabolic agent in patients with MM [77,78]. These clinical studies also suggest that, although MSCs from patients with myeloma reportedly are genetically, phenotypically, and functionally abnormal [26,79,80], these cells are still capable of building bone in patients upon reduced myeloma burden, removal of deleterious factors produced by myeloma cells, and treatment with osteoblast-activating agents.
Mesenchymal stem cells cytotherapy
The utility of MSC cytotherapy for building and generating bone has been extensively studied and clinically implicated [81,82]. We have shown that injecting MSCs into primary myelomatous bones in SCID-hu mice had heterogeneous effects on MM bone disease and that myeloma cell growth was attenuated only in cases associated with increased bone formation [29]. Interestingly, the effects of exogenous MSCs on MM bone disease seemed to be mainly mediated through `trophic' mechanisms, inhibiting osteoclastogenesis and promoting endogenous osteogenesis, rather than through direct participation in bone building. MSCs may also be used as a vehicle to deliver therapeutic transgenes. For instance, Rabin et al. [83] demonstrated in β2m NOD/SCID mice engrafted with KMS-12-BM myeloma cell line that myeloma bone disease is inhibited by systemically infusing MSCs that overexpress OPG.
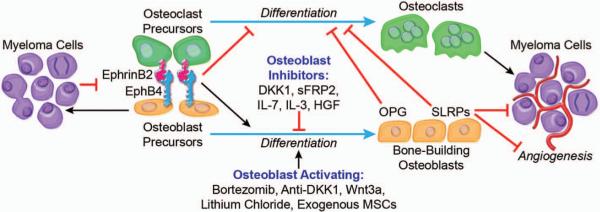
Collectively, these studies suggest that increasing bone formation in myelomatous bone is feasible. Results from studies of agents with unknown direct inhibitory effects on myeloma cell growth (e.g. anti-DKK1, Wnt3a, lithium chloride) also indicate that increasing osteoblast activity and subsequent reduction in osteoclastogenesis may also negatively impact medullary growth of myeloma in a subset of patients (Figure 1).
Figure 1.
Interactions of myeloma cells with osteoblasts and their precursors. Soluble factors (e.g. DKK1) and dysregulated cell-surface molecules (e.g. ephrinB2/EphB4) are involved in MM-induced suppression of osteoblastogenesis and subsequent stimulation of osteoclastogenesis. Osteoblast precursors and osteoclasts provide sanctuary to and stimulate growth of myeloma cells through cell–cell contact interactions and production of growth factors. Osteoblast-activating agents (e.g. bortezomib, anti-DKK1) increase the number of bone-building osteoblasts, resulting in normalization of osteoclastogenesis through increased production of anti-osteoclastogenic factors (e.g. OPG). Bone-building osteoblasts attenuate myeloma cell growth by increasing levels of potential anti-myeloma and anti-angiogenic factors (e.g. certain SLRPs) in the MM bone marrow microenvironment.
Concluding remarks
Multiple factors – both related and unrelated to MM – result in impairment of osteoblastogenesis in this disease. Patients with MM typically are elderly people who are susceptible to osteoporosis. These patients are often treated with the glucocorticoid dexamethasone, a clinical frontline drug known to reduce the life span of osteoblasts and osteocytes and to enhance survival of osteoclasts [84,85]. Patients with MM with bone disease also routinely receive bisphosphonates that block osteoclast activity, but the concomitant effects on osteoblastogenesis in these patients are unclear. MSCs from patients with MM possess abnormal properties and impaired abilities for osteogenic differentiation, at least in vitro [26,79,80]. Finally, myeloma cells produce or induce secretion of various factors known to inhibit osteoblastogenesis. Nevertheless, experimental studies demonstrate that bone formation and increased bone mass can be induced in myelomatous bones [21,49,52,58] and in patients with MM who responded to bortezomib [75]. Although large lytic lesions may not be repaired by conventional treatment, even in patients with long-term remission, osteoblast-activating agents such as bortezomib may recover small and microlytic lesions in these patients.
The consequences of increasing bone formation in MM involve not only reducing skeletal complications and improving patients' quality of life but also, importantly, restraining myeloma cell growth and increasing duration of complete remission. Whether altered bone remodeling also influences the progression from benign to overt MM remains an open question. As discussed here, patients with MGUS have altered bone turnover and microstructure. In contrast to osteoclasts or MSCs, mature osteoblasts produce high levels of bone-building factors, some of which exhibit anti-myeloma effects and may increase susceptibility of myeloma cells to conventional and novel clinical agents.
Acknowledgments
This work was supported by grants from the National Cancer Institute (CA-093897) (S. Y.) and from the Multiple Myeloma Research Foundation (Senior and Translational Research Award) (S. Y.). The author would like to recognize the efforts of the members of Dr. Yaccoby's laboratory: Xin Li, Angela Pennisi, Wen Ling, Sharmin Khan, Jianmei Chen, Yuping Yang. The author thanks the faculty, staff, and patients of the Myeloma Institute for Research and Therapy for their support, as well as the Office of Grants and Scientific Publications at the University of Arkansas for Medical Sciences for editorial assistance during the preparation of this manuscript.
References
- 1.Datta HK, Ng WF, Walker JA, Tuck SP, Varanasi SS. The cell biology of bone metabolism. J Clin Pathol. 2008;61:577–587. doi: 10.1136/jcp.2007.048868. [DOI] [PubMed] [Google Scholar]
- 2.Bataille R, Chappard D, Marcelli C, et al. Recruitment of new osteoblasts and osteoclasts is the earliest critical event in the pathogenesis of human multiple myeloma. J Clin Invest. 1991;88:62–66. doi: 10.1172/JCI115305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taube T, Beneton MN, McCloskey EV, et al. Abnormal bone remodelling in patients with myelomatosis and normal biochemical indices of bone resorption. Eur J Haematol. 1992;49:192–198. doi: 10.1111/j.1600-0609.1992.tb00046.x. [DOI] [PubMed] [Google Scholar]
- 4.Roodman GD. Pathogenesis of myeloma bone disease. Leukemia. 2009;23:435–441. doi: 10.1038/leu.2008.336. [DOI] [PubMed] [Google Scholar]
- 5.Kyle RA, Therneau TM, Rajkumar SV, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346:564–569. doi: 10.1056/NEJMoa01133202. [DOI] [PubMed] [Google Scholar]
- 6.Drake M, Ng A, Kumar S, et al. Increases in serum levels of dickkopf 1 are associated with alterations in skeletal microstructure in monoclonal gammopathy of undetermined significance [abstract]. The 31st Annual Meeting of the American Society for Bone and Mineral Research; Denver USA. September 2009. [Google Scholar]
- 7.Landgren O, Kyle RA, Pfeiffer RM, et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: a prospective study. Blood. 2009;113:5412–5417. doi: 10.1182/blood-2008-12-194241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evans CE, Galasko CS, Ward C. Does myeloma secrete an osteoblast inhibiting factor? J Bone Joint Surg Br. 1989;71:288–290. doi: 10.1302/0301-620X.71B2.2925748. [DOI] [PubMed] [Google Scholar]
- 9.Barille S, Collette M, Bataille R, Amiot M. Myeloma cells upregulate interleukin-6 secretion in osteoblastic cells through cell-to-cell contact but downregulate osteocalcin. Blood. 1995;86:3151–3159. [PubMed] [Google Scholar]
- 10.Tian E, Zhan F, Walker R, et al. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med. 2003;349:2483–2494. doi: 10.1056/NEJMoa030847. [DOI] [PubMed] [Google Scholar]
- 11.Oshima T, Abe M, Asano J, et al. Myeloma cells suppress bone formation by secreting a soluble Wnt inhibitor, sFRP-2. Blood. 2005;106:3160–3165. doi: 10.1182/blood-2004-12-4940. [DOI] [PubMed] [Google Scholar]
- 12.Giuliani N, Colla S, Morandi F, et al. Myeloma cells block RUNX2/CBFA1 activity in human bone marrow osteoblast progenitors and inhibit osteoblast formation and differentiation. Blood. 2005;106:2472–2483. doi: 10.1182/blood-2004-12-4986. [DOI] [PubMed] [Google Scholar]
- 13.Standal T, Abildgaard N, Fagerli UM, et al. HGF inhibits BMP-induced osteoblastogenesis: possible implications for the bone disease of multiple myeloma. Blood. 2007;109:3024–3030. doi: 10.1182/blood-2006-07-034884. [DOI] [PubMed] [Google Scholar]
- 14.Lee JW, Chung HY, Ehrlich LA, et al. IL-3 expression by myeloma cells increases both osteoclast formation and growth of myeloma cells. Blood. 2004;103:2308–2315. doi: 10.1182/blood-2003-06-1992. [DOI] [PubMed] [Google Scholar]
- 15.Ehrlich LA, Chung HY, Ghobrial I, et al. IL-3 is a potential inhibitor of osteoblast differentiation in multiple myeloma. Blood. 2005;106:1407–1414. doi: 10.1182/blood-2005-03-1080. [DOI] [PubMed] [Google Scholar]
- 16.Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350:1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 17.Pasquale EB. Eph-ephrin bidirectional signaling in physiology and disease. Cell. 2008;133:38–52. doi: 10.1016/j.cell.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Edwards CM, Mundy GR. Eph receptors and ephrin signaling pathways: a role in bone homeostasis. Int J Med Sci. 2008;5:263–272. doi: 10.7150/ijms.5.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao C, Irie N, Takada Y, et al. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab. 2006;4:111–121. doi: 10.1016/j.cmet.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 20.Allan EH, Hausler KD, Wei T, et al. EphrinB2 regulation by parathyroid hormone (PTH) and PTHrP revealed by molecular profiling in differentiating osteoblasts. J Bone Miner Res. 2008;23:1170–1181. doi: 10.1359/jbmr.080324. [DOI] [PubMed] [Google Scholar]
- 21.Pennisi A, Ling W, Li X, et al. The ephrinB2/EphB4 axis is dysregulated in osteoprogenitors from myeloma patients and its activation affects myeloma bone disease and tumor growth. Blood. 2009;114:1803–1812. doi: 10.1182/blood-2009-01-201954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gunn WG, Conley A, Deininger L, et al. A crosstalk between myeloma cells and marrow stromal cells stimulates production of DKK1 and IL-6: a potential role in the development of lytic bone disease and tumor progression in multiple myeloma. Stem Cells. 2006;24:986–991. doi: 10.1634/stemcells.2005-0220. [DOI] [PubMed] [Google Scholar]
- 23.Podar K, Anderson KC. The pathophysiologic role of VEGF in hematologic malignancies: therapeutic implications. Blood. 2005;105:1383–1395. doi: 10.1182/blood-2004-07-2909. [DOI] [PubMed] [Google Scholar]
- 24.Pearse RN, Sordillo EM, Yaccoby S, et al. Multiple myeloma disrupts the TRANCE/ osteoprotegerin cytokine axis to trigger bone destruction and promote tumor progression. Proc Natl Acad Sci USA. 2001;98:11581–11586. doi: 10.1073/pnas.201394498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qiang YW, Chen Y, Stephens O, et al. Myeloma-derived Dickkopf-1 disrupts Wnt-regulated osteoprotegerin and RANKL production by osteoblasts: a potential mechanism underlying osteolytic bone lesions in multiple myeloma. Blood. 2008;112:196–207. doi: 10.1182/blood-2008-01-132134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corre J, Mahtouk K, Attal M, et al. Bone marrow mesenchymal stem cells are abnormal in multiple myeloma. Leukemia. 2007;21:1079–1088. doi: 10.1038/sj.leu.2404621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stewart JP, Shaughnessy JD., Jr. Role of osteoblast suppression in multiple myeloma. J Cell Biochem. 2006;98:1–13. doi: 10.1002/jcb.20774. [DOI] [PubMed] [Google Scholar]
- 28.Yaccoby S, Wezeman MJ, Henderson A, et al. Cancer and the microenvironment: myeloma-osteoclast interactions as a model. Cancer Res. 2004;64:2016–2023. doi: 10.1158/0008-5472.can-03-1131. [DOI] [PubMed] [Google Scholar]
- 29.Yaccoby S, Wezeman MJ, Zangari M, et al. Inhibitory effects of osteoblasts and increased bone formation on myeloma in novel culture systems and a myelomatous mouse model. Haematologica. 2006;91:192–199. [PMC free article] [PubMed] [Google Scholar]
- 30.Glass DA, Bialek P, Ahn JD, et al. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell. 2005;8:751–764. doi: 10.1016/j.devcel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 31.Spencer GJ, Utting JC, Etheridge SL, Arnett TR, Genever PG. Wnt signalling in osteoblasts regulates expression of the receptor activator of NFkB ligand and inhibits osteoclastogenesis in vitro. J Cell Sci. 2006;119:1283–1296. doi: 10.1242/jcs.02883. [DOI] [PubMed] [Google Scholar]
- 32.Canalis E. Growth factor control of bone mass. J Cell Biochem. 2009;108:769–777. doi: 10.1002/jcb.22322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Podar K, Chauhan D, Anderson KC. Bone marrow microenvironment and the identification of new targets for myeloma therapy. Leukemia. 2009;23:10–24. doi: 10.1038/leu.2008.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li X, Pennisi A, Yaccoby S. Role of decorin in the antimyeloma effects of osteoblasts. Blood. 2008;112:159–168. doi: 10.1182/blood-2007-11-124164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waddington RJ, Roberts HC, Sugars RV, Schonherr E. Differential roles for small leucine-rich proteoglycans in bone formation. Eur Cell Mater. 2003;6:12–21. doi: 10.22203/ecm.v006a02. [DOI] [PubMed] [Google Scholar]
- 36.Balint E, Lapointe D, Drissi H, et al. Phenotype discovery by gene expression profiling: mapping of biological processes linked to BMP-2-mediated osteoblast differentiation. J Cell Biochem. 2003;89:401–426. doi: 10.1002/jcb.10515. [DOI] [PubMed] [Google Scholar]
- 37.Ameye L, Young MF. Mice deficient in small leucine-rich proteoglycans: novel in vivo models for osteoporosis, osteoarthritis, Ehlers-Danlos syndrome, muscular dystrophy, and corneal diseases. Glycobiology. 2002;12:107R–116R. doi: 10.1093/glycob/cwf065. [DOI] [PubMed] [Google Scholar]
- 38.De LA, Santra M, Baldi A, Giordano A, Iozzo RV. Decorin-induced growth suppression is associated with up-regulation of p21, an inhibitor of cyclin-dependent kinases. J Biol Chem. 1996;271:18961–18965. doi: 10.1074/jbc.271.31.18961. [DOI] [PubMed] [Google Scholar]
- 39.Moscatello DK, Santra M, Mann DM, et al. Decorin suppresses tumor cell growth by activating the epidermal growth factor receptor. J Clin Invest. 1998;101:406–412. doi: 10.1172/JCI846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reed CC, Gauldie J, Iozzo RV. Suppression of tumorigenicity by adenovirus-mediated gene transfer of decorin. Oncogene. 2002;21:3688–3695. doi: 10.1038/sj.onc.1205470. [DOI] [PubMed] [Google Scholar]
- 41.Reed CC, Waterhouse A, Kirby S, et al. Decorin prevents metastatic spreading of breast cancer. Oncogene. 2005;24:1104–1110. doi: 10.1038/sj.onc.1208329. [DOI] [PubMed] [Google Scholar]
- 42.Zhu JX, Goldoni S, Bix G, et al. Decorin evokes protracted internalization and degradation of the epidermal growth factor receptor via caveolar endocytosis. J Biol Chem. 2005;280:32468–32479. doi: 10.1074/jbc.M503833200. [DOI] [PubMed] [Google Scholar]
- 43.Naito Z. Role of the small leucine-rich proteoglycan (SLRP) family in pathological lesions and cancer cell growth. J Nippon Med.Sch. 2005;72:137–145. doi: 10.1272/jnms.72.137. [DOI] [PubMed] [Google Scholar]
- 44.Li Y, Aoki T, Mori Y, et al. Cleavage of lumican by membrane-type matrix metalloproteinase-1 abrogates this proteoglycan-mediated suppression of tumor cell colony formation in soft agar. Cancer Res. 2004;64:7058–7064. doi: 10.1158/0008-5472.CAN-04-1038. [DOI] [PubMed] [Google Scholar]
- 45.Troup S, Njue C, Kliewer EV, et al. Reduced expression of the small leucine-rich proteoglycans, lumican, and decorin is associated with poor outcome in node-negative invasive breast cancer. Clin Cancer Res. 2003;9:207–214. [PubMed] [Google Scholar]
- 46.Zafiropoulos A, Nikitovic D, Katonis P, et al. Decorin-induced growth inhibition is overcome through protracted expression and activation of epidermal growth factor receptors in osteosarcoma cells. Mol Cancer Res. 2008;6:785–794. doi: 10.1158/1541-7786.MCR-07-0165. [DOI] [PubMed] [Google Scholar]
- 47.Goldoni S, Iozzo RV. Tumor microenvironment: modulation by decorin and related molecules harboring leucine-rich tandem motifs. Int J Cancer. 2008;123:2473–2479. doi: 10.1002/ijc.23930. [DOI] [PubMed] [Google Scholar]
- 48.Borset M, Seidel C, Hjorth-Hansen H, Waage A, Sundan A. The role of hepatocyte growth factor and its receptor c-Met in multiple myeloma and other blood malignancies. Leuk Lymphoma. 1999;32:249–256. doi: 10.3109/10428199909167385. [DOI] [PubMed] [Google Scholar]
- 49.Qiang YW, Shaughnessy JD, Jr., Yaccoby S. Wnt3a signaling within bone inhibits multiple myeloma bone disease and tumor growth. Blood. 2008;112:374–382. doi: 10.1182/blood-2007-10-120253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Edwards CM, Edwards JR, Lwin ST, et al. Increasing Wnt signaling in the bone marrow microenvironment inhibits the development of myeloma bone disease and reduces tumor burden in bone in vivo. Blood. 2008;111:2833–2842. doi: 10.1182/blood-2007-03-077685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garrett IR, Chen D, Gutierrez G, et al. Selective inhibitors of the osteoblast proteasome stimulate bone formation in vivo and in vitro. J Clin Invest. 2003;111:1771–1782. doi: 10.1172/JCI16198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pennisi A, Li X, Ling W, et al. The proteasome inhibitor, bortezomib suppresses primary myeloma and stimulates bone formation in myelomatous and nonmyelomatous bones in vivo. Am J Hematol. 2009;84:6–14. doi: 10.1002/ajh.21310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zangari M, Esseltine D, Lee CK, et al. Response to bortezomib is associated to osteoblastic activation in patients with multiple myeloma. Br J Haematol. 2005;131:71–73. doi: 10.1111/j.1365-2141.2005.05733.x. [DOI] [PubMed] [Google Scholar]
- 54.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 55.Pinzone JJ, Hall BM, Thudi NK, et al. The role of Dickkopf-1 in bone development, homeostasis, and disease. Blood. 2009;113:517–525. doi: 10.1182/blood-2008-03-145169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaiser M, Mieth M, Liebisch P, et al. Serum concentrations of DKK-1 correlate with the extent of bone disease in patients with multiple myeloma. Eur J Haematol. 2008;80:490–494. doi: 10.1111/j.1600-0609.2008.01065.x. [DOI] [PubMed] [Google Scholar]
- 57.Qiang YW, Barlogie B, Rudikoff S, Shaughnessy JD., Jr. Dkk1-induced inhibition of Wnt signaling in osteoblast differentiation is an underlying mechanism of bone loss in multiple myeloma. Bone. 2008;42:669–680. doi: 10.1016/j.bone.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 58.Yaccoby S, Ling W, Zhan F, et al. Antibody-based inhibition of DKK1 suppresses tumor-induced bone resorption and multiple myeloma growth in vivo. Blood. 2007;109:2106–2111. doi: 10.1182/blood-2006-09-047712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yaccoby S, Pearse RN, Johnson CL, et al. Myeloma interacts with the bone marrow microenvironment to induce osteoclastogenesis and is dependent on osteoclast activity. Br J Haematol. 2002;116:278–290. doi: 10.1046/j.1365-2141.2002.03257.x. [DOI] [PubMed] [Google Scholar]
- 60.Yaccoby S, Epstein J. The proliferative potential of myeloma plasma cells manifest in the SCID-hu host. Blood. 1999;94:3576–3582. [PubMed] [Google Scholar]
- 61.Yaccoby S, Barlogie B, Epstein J. Primary myeloma cells growing in SCID-hu mice: a model for studying the biology and treatment of myeloma and its manifestations. Blood. 1998;92:2908–2913. [PubMed] [Google Scholar]
- 62.Yata K, Yaccoby S. The SCID-rab model: a novel in vivo system for primary human myeloma demonstrating growth of CD138-expressing malignant cells. Leukemia. 2004;18:1891–1897. doi: 10.1038/sj.leu.2403513. [DOI] [PubMed] [Google Scholar]
- 63.Fulciniti M, Tassone P, Hideshima T, et al. Anti-DKK1 mAb (BHQ880) as a potential therapeutic agent for multiple myeloma. Blood. 2009;114:371–379. doi: 10.1182/blood-2008-11-191577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Heath DJ, Chantry AD, Buckle CH, et al. Inhibiting Dickkopf-1 (Dkk1) removes suppression of bone formation and prevents the development of osteolytic bone disease in multiple myeloma. J Bone Miner Res. 2009;24:425–436. doi: 10.1359/jbmr.081104. [DOI] [PubMed] [Google Scholar]
- 65.Derksen PW, Tjin E, Meijer HP, et al. Illegitimate WNT signaling promotes proliferation of multiple myeloma cells. Proc Natl Acad Sci USA. 2004;101:6122–6127. doi: 10.1073/pnas.0305855101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dutta-Simmons J, Zhang Y, Gorgun G, et al. Aurora kinase A is a target of Wnt/{beta}-catenin involved in multiple myeloma disease progression. Blood. 2009;114:2699–2708. doi: 10.1182/blood-2008-12-194290. [DOI] [PubMed] [Google Scholar]
- 67.Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348:2609–2617. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- 68.Bellido T, Ali AA, Plotkin LI, et al. Proteasomal degradation of Runx2 shortens parathyroid hormone-induced anti-apoptotic signaling in osteoblasts. A putative explanation for why intermittent administration is needed for bone anabolism. J Biol Chem. 2003;278:50259–50272. doi: 10.1074/jbc.M307444200. [DOI] [PubMed] [Google Scholar]
- 69.Giuliani N, Morandi F, Tagliaferri S, et al. The proteasome inhibitor bortezomib affects osteoblast differentiation in vitro and in vivo in multiple myeloma patients. Blood. 2007;110:334–338. doi: 10.1182/blood-2006-11-059188. [DOI] [PubMed] [Google Scholar]
- 70.Qiang YW, Hu B, Chen Y, et al. Bortezomib induces osteoblast differentiation via Wnt-independent activation of beta-catenin/TCF signaling. Blood. 2009;113:4319–4330. doi: 10.1182/blood-2008-08-174300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Oyajobi BO, Garrett IR, Gupta A, et al. Stimulation of new bone formation by the proteasome inhibitor, bortezomib: implications for myeloma bone disease. Br J Haematol. 2007;139:434–438. doi: 10.1111/j.1365-2141.2007.06829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zavrski I, Krebbel H, Wildemann B, et al. Proteasome inhibitors abrogate osteoclast differentiation and osteoclast function. Biochem Biophys Res Commun. 2005;333:200–205. doi: 10.1016/j.bbrc.2005.05.098. [DOI] [PubMed] [Google Scholar]
- 73.Edwards CM, Lwin ST, Fowler JA, et al. Myeloma cells exhibit an increase in proteasome activity and an enhanced response to proteasome inhibition in the bone marrow microenvironment in vivo. Am J Hematol. 2009;84:268–272. doi: 10.1002/ajh.21374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zangari M, Yaccoby S, Cavallo F, Esseltine D, Tricot G. Response to bortezomib and activation of osteoblasts in multiple myeloma. Clin Lymphoma Myeloma. 2006;7:109–114. doi: 10.3816/CLM.2006.n.047. [DOI] [PubMed] [Google Scholar]
- 75.Zangari M, Pappas L, Zhan F, et al. Parathyroid hormones (PTH) serum variations are associated with bortezomib response in multiple myeloma patients. The 50th Annual Meeting of the American Society of Hematology; San Francisco, USA. December 2008; (Abstract 2783) [Google Scholar]
- 76.Zangari M, Esseltine D, Cavallo F, et al. Predictive value of alkaline phosphatase for response and time to progression in bortezomib-treated multiple myeloma patients. Am J Hematol. 2007;82:831–833. doi: 10.1002/ajh.20961. [DOI] [PubMed] [Google Scholar]
- 77.Terpos E, Heath DJ, Rahemtulla A, et al. Bortezomib reduces serum dickkopf-1 and receptor activator of nuclear factor-κB ligand concentrations and normalises indices of bone remodelling in patients with relapsed multiple myeloma. Br J Haematol. 2006;135:688–692. doi: 10.1111/j.1365-2141.2006.06356.x. [DOI] [PubMed] [Google Scholar]
- 78.Heider U, Kaiser M, Muller C, et al. Bortezomib increases osteoblast activity in myeloma patients irrespective of response to treatment. Eur J Haematol. 2006;77:233–238. doi: 10.1111/j.1600-0609.2006.00692.x. [DOI] [PubMed] [Google Scholar]
- 79.Garayoa M, Garcia JL, Santamaria C, et al. Mesenchymal stem cells from multiple myeloma patients display distinct genomic profile as compared with those from normal donors. Leukemia. 2009;23:1515–1527. doi: 10.1038/leu.2009.65. [DOI] [PubMed] [Google Scholar]
- 80.Wallace SR, Oken MM, Lunetta KL, Panoskaltsis-Mortari A, Masellis AM. Abnormalities of bone marrow mesenchymal cells in multiple myeloma patients. Cancer. 2001;91:1219–1230. doi: 10.1002/1097-0142(20010401)91:7<1219::aid-cncr1122>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 81.Prockop DJ, Gregory CA, Spees JL. One strategy for cell and gene therapy: harnessing the power of adult stem cells to repair tissues. Proc Natl Acad Sci USA. 2003;100(Suppl 1):11917–11923. doi: 10.1073/pnas.1834138100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Prockop DJ, Olson SD. Clinical trials with adult stem/progenitor cells for tissue repair: let's not overlook some essential precautions. Blood. 2007;109:3147–3151. doi: 10.1182/blood-2006-03-013433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rabin N, Kyriakou C, Coulton L, et al. A new xenograft model of myeloma bone disease demonstrating the efficacy of human mesenchymal stem cells expressing osteoprotegerin by lentiviral gene transfer. Leukemia. 2007;21:2181–2191. doi: 10.1038/sj.leu.2404814. [DOI] [PubMed] [Google Scholar]
- 84.Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J Clin Invest. 1998;102:274–282. doi: 10.1172/JCI2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Weinstein RS, Chen JR, Powers CC, et al. Promotion of osteoclast survival and antagonism of bisphosphonate-induced osteoclast apoptosis by glucocorticoids. J Clin Invest. 2002;109:1041–1048. doi: 10.1172/JCI14538. [DOI] [PMC free article] [PubMed] [Google Scholar]