By adjusting for iris color and central foveal retinal thickness, the authors show that macular pigment optical density is directly related to serum lutein in a large cohort of patients with retinitis pigmentosa. In addition, macular pigment optical density in this disease tends to be reduced in eyes with moderate to marked swelling due to cystoid macular edema.
Abstract
Purpose.
To determine whether macular pigment optical density (MPOD) is related to serum lutein or serum zeaxanthin in patients with retinitis pigmentosa.
Methods.
The authors measured MPOD with heterochromatic flicker photometry, serum lutein and serum zeaxanthin by high performance liquid chromatography, and central foveal retinal thickness by optical coherence tomography (OCT) in 176 patients (age range, 18–68 years) with typical forms of retinitis pigmentosa; 37 (21%) of these patients had cystoid macular edema (CME) by OCT. The authors performed multiple regression analysis with MPOD as the dependent variable and with loge serum lutein and loge serum zeaxanthin as independent variables adjusting for age, sex, iris color, central foveal retinal thickness, and, in some analyses, serum total cholesterol.
Results.
MPOD increased with increasing serum lutein (P = 0.0017) and decreased with increasing serum total cholesterol (P = 0.0025) but was unrelated to serum zeaxanthin. MPOD was higher in patients with brown irides than in patients with lighter irides (P = 0.014) and was nonmonotonically related to central foveal retinal thickness (P < 0.0001), being lower in eyes with more photoreceptor cell loss and in eyes with moderate to marked CME.
Conclusions.
MPOD is independently related to serum lutein, serum total cholesterol, iris color, and central foveal retinal thickness in patients with retinitis pigmentosa.
Lutein and zeaxanthin, carotenoids found in dark green, leafy vegetables, are transported in the plasma exclusively by lipoproteins.1–4 Both are concentrated in and around the foveal depression in cone axons as yellow macular pigment,5,6 which partially screens the photoreceptors from short-wavelength light and thereby minimizes the effect of chromatic aberration on visual acuity and perhaps protects these cells from oxidative light damage.7,8
Although macular pigment optical density (MPOD) has been found to be directly related to serum lutein in healthy volunteers,9–12 it was reported to be unrelated to serum lutein in patients with the typical forms of retinitis pigmentosa.12 We hypothesized that the absence of a significant association between MPOD and serum lutein in patients with retinitis pigmentosa was due, at least in part, to variable incorporation of lutein as macular pigment in cone axons as a result of photoreceptor degeneration. In the present study, we compared MPOD to serum lutein and to serum zeaxanthin in a large cohort of patients with typical retinitis pigmentosa, adjusting for the relationships of MPOD to central foveal retinal thickness and to other potentially confounding factors (age, sex, iris color, and serum total cholesterol). This afforded us an opportunity also to test the hypothesis that cystoid macular edema (CME), which occurs in more than 25% of patients with this disease,13–15 reduces MPOD; this hypothesis was raised but not answered by a previous study.12
Patients and Methods
Patients
The protocol was approved by the institutional review boards of the Massachusetts Eye and Ear Infirmary and Harvard Medical School and conformed to the tenets of the Declaration of Helsinki and to HIPAA regulations. Informed consent was obtained from all patients. This population included 176 unrelated adults with typical forms of retinitis pigmentosa (58% male; age range, 18–68 years), best-corrected visual acuities of 20/80 or better, and sufficiently large central visual fields for measurement of MPOD. These patients with typical retinitis pigmentosa had elevated dark-adapted thresholds, retinal arteriolar attenuation, and reduced and delayed full-field electroretinograms; most had intraretinal bone spicule pigmentation in the peripheral retina. Our cohort included only patients who denied smoking, which removed one potential source of variability with respect to MPOD.16 In addition, all denied taking a separate lutein supplement over the past year based on their answers to a food-frequency questionnaire.17 There were 41 dominant cases (23.3%), 23 recessive cases (13.1%), 8 X-linked cases (4.5%), 97 simplex cases (55.1%), and 7 cases with undetermined inheritance (4.0%). Thirty-seven (21%) of the patients had CME in the study eye by optical coherence tomography (OCT), as defined by one or more intraretinal cysts measuring at least 50 μm.15
MPOD Measurements
MPOD was measured by heterochromatic flicker photometry using a commercial tabletop instrument (Macular Metrics Corp., Rehoboth, MA)18 in the eye with better visual acuity (or the right eye, if the two eyes were equal) after pupillary dilation to maximize sensitivity. Before testing, the patients watched a training video provided by the instrument manufacturer (macularmetrics.com/demovideo.html) that showed how a subject should make the manual adjustments. After the patients were optically corrected and aligned with the instrument, the examiner had them look at the red fixation LED with the 2° stimulus flickering at a 5° eccentricity, the reference location. This eccentricity was chosen as a reference because the retina there should have had sufficiently low macular pigment absorbance in our patients with retinitis pigmentosa to serve as a reference location yet should have retained adequate light sensitivity for the patients to perform the task. Furthermore, this reference eccentricity was compatible with methods used previously to measure MPOD in patients with retinitis pigmentosa.12 All patients included in this report confirmed that they could visualize the entire flickering stimulus at the 5° eccentricity, indicating that their central fields were sufficiently large to measure MPOD.
The task was for the patient to adjust the radiances of a 460-nm stimulus and an alternating 570-nm stimulus to achieve a brightness match by eliminating flicker. These stimuli were centered on a 6° background of 475 nm to desensitize rods and short-wavelength–sensitive cones so that they would not contribute to the patient's judgment. After a practice session during which the examiner set the frequency of flicker to elicit a small “no flicker zone,” the patients adjusted the radiances to eliminate flicker within the central 1°, where macular pigment absorbance is maximal, and at the reference location. During testing, the examiner continually reinforced the principles of converging on the “no flicker zone” and of periodic blinking to reduce stimulus fading, particularly when the stimulus was at the reference location. The adjusted log10 radiance of the 460-nm stimulus minus the adjusted log10 radiance of the 570 nm stimulus for the central fovea minus the same difference for the reference location provided a psychophysical estimate of MPOD.
We assessed the intervisit variability of MPOD measurements in the first 66 patients who were invited and able to return for follow-up within 2 months of their original visits. These patients appeared to be a representative sample because at their first visit they did not differ significantly from the remaining 110 patients with respect to mean age (P = 0.10), sex distribution (P = 0.31), mean Snellen acuity in the study eye (P = 0.79), likelihood of having CME in the study eye (P = 0.67), or mean MPOD in the study eye (P = 0.17).
OCT Evaluations
We used a high-resolution optical coherence tomographer (Stratus, model 3000; Zeiss Meditec, Dublin, CA) to assess retinal structure and to measure retinal thickness after pupillary dilation, as described previously.15,19 Central foveal retinal thickness was routinely measured by the automated OCT software as the distance between the high-reflectance vitreoretinal interface and the retinal pigment epithelium (RPE)/choriocapillaris complex at the intersection of six radial scans oriented at 30° intervals. Every tomogram in this study was inspected to verify that the algorithm correctly defined these two high-reflective boundaries at the foveal center. In only one instance—an eye with 20/80 visual acuity without CME—was an interface (the vitreoretinal border) incorrectly designated, and one of us (MAS) used the manual software calipers to redo the thickness measurement.
Serum Lutein and Zeaxanthin Measurements
Fasting blood was drawn, and serum was stored in the dark under nitrogen at −80°C. Serum was analyzed by high-performance liquid chromatography (HPLC) for lutein and zeaxanthin (Alliance 2695; Waters, Milford, MA), as described previously with echinenone as the internal standard.20 Using this method, cis lutein, all-trans lutein, cis zeaxanthin, all-trans zeaxanthin, cryptoxanthin, α-carotene, 13-cis β-carotene, all-trans β-carotene, 9-cis β-carotene, cis lycopene, and trans lycopene were separated. Lutein and zeaxanthin were quantified by determining peak areas in the HPLC chromatograms calibrated against known amounts of standards. Lutein and zeaxanthin standards, provided by DSM Nutritional Products (Basel, Switzerland), were dissolved in ethanol and used as references to quantify the peak areas for these carotenoids in the HPLC chromatograms. Data were collected and analyzed (Millenium32, version 3.05.01, Windows NT; Waters) with the lower limit of detection for carotenoids at 0.2 pmol.
Statistical Analysis
We performed multiple regression analysis with MPOD as the dependent variable and serum trans lutein, serum trans zeaxanthin, age, sex, iris color, and central foveal retinal thickness as independent variables. Because MPOD was not normally distributed in this population (see Results), the analysis was repeated after density values were converted to ranks and then to the standard normal distribution with the Van Der Waerden approximation to test the validity of the original model's findings. Given that both analyses led to substantially the same conclusions, only the model based on actual density values is considered below.
Analysis was performed using the trans isomers of serum lutein and serum zeaxanthin because only very small amounts of the cis isomers have been detected in macular pigment.21 Because their distributions showed moderate positive skew (see Results), we converted serum lutein and serum zeaxanthin values to natural logarithms to minimize the effect of high leverage values, as performed in earlier studies with large cohorts.11,22
We included age in the model because of its possible association with MPOD in healthy volunteers,23–25 and we included sex because MPOD has been found to be higher in men than in women.23,26 We included an indicator variable for iris color (brown versus blue/green/hazel) because higher MPOD has been associated with darker irises in healthy volunteers.27 Central foveal retinal thickness was included because previous studies found MPOD to be directly related to foveal retinal thickness in healthy volunteers28 and in patients with retinitis pigmentosa without CME.12 Central foveal retinal thickness was given the attribute of a spline with three knots so that we could fit MPOD to this variable by a nonmonotonic function, if needed, given that our cohort included patients with CME who might have had low MPOD values associated with marked retinal swelling.
We also performed an analysis including serum total cholesterol in the model, as in one previous study,11 because serum carotenoids are exclusively transported on lipoproteins and, therefore, variation in serum total cholesterol could confound the relationships of MPOD to serum lutein and serum zeaxanthin. Fasting serum cholesterol was measured by the clinical laboratory of the Massachusetts Eye and Ear Infirmary.
Lastly, we performed three subset analyses. In the first two analyses, we removed sex from the model and evaluated the relationship of MPOD to loge serum lutein separately in men and in women because a study of healthy volunteers found a stronger relationship in men than in women.11 In the third subset analysis, we excluded the 37 patients with CME and assumed a linear relationship between MPOD and central foveal retinal thickness to better relate our findings to an earlier study of patients with retinitis pigmentosa that excluded those with CME and failed to find a significant relationship between MPOD and serum lutein.12 We even performed a bivariate analysis correlating MPOD with serum lutein to match what had been done previously.12 Analyses were performed with JMP, version 6 (SAS Institute, Cary, NC).
Results
Distribution and Reproducibility of Macular Pigment Optical Density
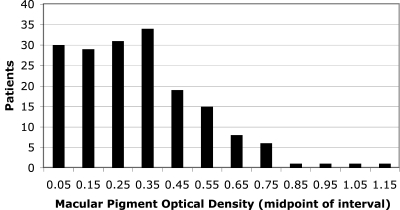
The frequency distribution of MPOD in the 176 patients with retinitis pigmentosa is shown in Figure 1. The distribution had a mean ± SEM of 0.32 ± 0.02 log10-unit, only slightly higher than the mean (0.29 log10-unit) found in a previous study of patients with retinitis pigmentosa.12 The distribution also had a small skewness coefficient (0.83) that did not affect the conclusions from the regression analyses reported here (see Patients and Methods).
Figure 1.
Frequency distribution of macular pigment optical density based on 176 patients with retinitis pigmentosa. In 10 patients, recorded density values between −0.2 and 0 were recoded as 0.
MPOD test-retest data based on the subset of 66 patients who returned within 2 months yielded a standard deviation for the absolute value of the between-visit differences (SD) of 0.06. Compared with other measurements done with heterochromatic flicker photometry, our SD was higher than that (0.04) reported by a previous study of patients with retinitis pigmentosa12 but within the range (0.02–0.10) based on five studies of healthy volunteers.29–33
Distributions of Serum Lutein and Serum Zeaxanthin
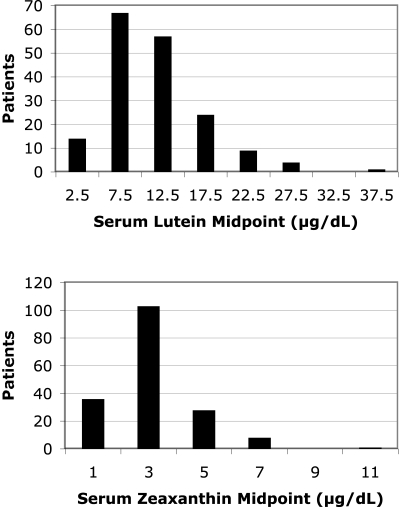
Figure 2 illustrates the frequency distributions for serum trans lutein and for serum trans zeaxanthin based on the 176 patients. The distribution of serum lutein had a mean ± SEM of 11.5 ± 0.4 μg/dL, the distribution of serum zeaxanthin had a mean ± SEM of 3.1 ± 0.1 μg/dL, and both distributions had moderate positive skew (1.3 and 1.6, respectively). When converted to a log scale to minimize the effect of high leverage values in the regression analyses, neither distribution differed significantly from normal (Shapiro-Wilk W test for goodness-of-fit, P = 0.82 for serum lutein and P = 0.62 for serum zeaxanthin).
Figure 2.
Distributions of serum lutein and serum zeaxanthin based on 176 patients with retinitis pigmentosa.
Relationships of MPOD to Age, Sex, Iris Color, and Central Foveal Retinal Thickness
Mean MPOD fell by 0.0022 log10-unit (0.5%) for each increasing year of age and was 0.04 log10-unit (10%) higher in males than females, values that were not significantly different from zero (Table 1). On the other hand, MPOD was significantly related to iris color, averaging 0.08 log10-unit (22%) higher in eyes with brown irides than in eyes with lighter irides, and to central foveal retinal thickness (Table 1). Figure 3 illustrates the spline regression of MPOD on central foveal retinal thickness. Based on the fitted curve, MPOD first increased with increasing retinal thickness and then declined for greater retinal thicknesses. The data show that the increase primarily reflected eyes without CME and that the decrease primarily reflected eyes with moderate to marked CME.
Table 1.
Multiple Regression of Macular Pigment Optical Density on Age, Sex, Iris Color, Central Foveal Retinal Thickness, Serum Lutein, and Serum Zeaxanthin in Patients with Retinitis Pigmentosa
| Characteristic | Estimate | SE | P |
|---|---|---|---|
| Age, y | −0.0022 | 0.0014 | 0.13 |
| Sex (male–female) | 0.0398 | 0.0302 | 0.19 |
| Iris color (brown–not brown) | 0.0811 | 0.0326 | 0.0138 |
| Central foveal retinal thickness, μm* | — | — | <0.0001 |
| Serum lutein, loge μg/dL | 0.1510 | 0.0474 | 0.0017 |
| Serum zeaxanthin, loge μg/dL | −0.0716 | 0.0523 | 0.17 |
Spline function with three knots.
Figure 3.
Macular pigment optical density by central foveal retinal thickness for 176 patients with retinitis pigmentosa, 37 of whom had CME in the study eye. Solid curve: fitted spline function with three knots.
Relationship of MPOD to Serum Lutein and to Serum Zeaxanthin
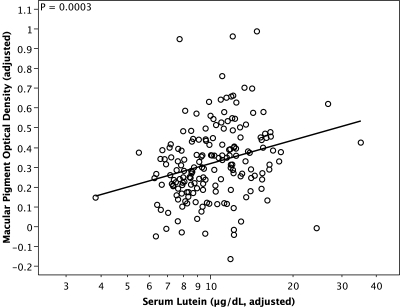
With the model adjusted for age, sex, iris color, central foveal retinal thickness, and loge serum zeaxanthin, MPOD increased significantly with increasing loge serum lutein (Table 1), and the partial correlation between the two variables was 0.24. When added to the model, serum total cholesterol was a negative predictor of MPOD (P = 0.0025). Because serum cholesterol was positively correlated with loge serum lutein in our patients (r = 0.21, P = 0.0046) and because MPOD was inversely related to serum cholesterol, adding serum cholesterol to the model strengthened the relationship of MPOD to serum lutein (rpartial = 0.27, P = 0.0003). Figure 4 illustrates the relationship of MPOD to serum lutein adjusted for the other terms in the model, including serum cholesterol. The fitted line indicates that a 10-fold increase in serum lutein was associated with an average MPOD increase of ∼0.4 log10-unit. In contrast to loge serum lutein, MPOD was not significantly related to loge serum zeaxanthin (Table 1). This was also true after serum total cholesterol was included in the model (data not shown).
Figure 4.
Partial regression plot of macular pigment optical density on serum lutein for 176 patients with retinitis pigmentosa. Serum lutein is displayed on a log scale because loge serum lutein was used in the regression analysis. Both the x-coordinates and the y-coordinates have been adjusted for the relationships of macular pigment optical density to age, sex, iris color, a spline function of central foveal retinal thickness, loge serum zeaxanthin, and serum total cholesterol. As a result of adjusting for these variables, for example, eight macular pigment optical density values ranging from 0 to 0.08 have shifted in this plot to negative values ranging from −0.01 to −0.17.
Subset Analyses
We also tested whether the relationship between MPOD and loge serum lutein was stronger in men than in women, as found in a previous study of healthy observers.11 When we removed sex from the multiple regression model (but included serum cholesterol11), we found that the relationship of MPOD to loge serum lutein was significant for the 102 men (P = 0.0048) and borderline for the 74 women (P = 0.0504) and that the slope for men (0.188 ± 0.065 MPOD/loge serum lutein in μg/dL) was steeper than the slope for women (0.137 ± 0.069 MPOD/loge serum lutein in μg/dL), albeit not significantly different.
A previous study of 48 patients with retinitis pigmentosa that excluded those with CME found a nonsignificant relationship between MPOD and serum lutein (r = 0.14, P = 0.44).12 In our third subset analysis we regressed MPOD on age, sex, iris color, central foveal retinal thickness, loge serum lutein, loge serum zeaxanthin, and serum total cholesterol, excluding the patients with CME in the study eye. For the remaining 139 patients, MPOD increased linearly with increasing central foveal retinal thickness (P < 0.0001), and we again found that MPOD increased with increasing loge serum lutein (P < 0.0001); the partial correlation between MPOD and loge serum lutein was 0.33 without serum cholesterol and 0.37 with serum cholesterol in the model. Even for a simple bivariate relationship, MPOD was significantly correlated with serum lutein in this patient subset (r = 0.23, P = 0.0068).
Discussion
MPOD has been reported by others to be related to serum lutein in healthy volunteers9–12 but not in a group of 48 patients with retinitis pigmentosa without CME, in whom the correlation was 0.14.12 After adjusting for age, sex, iris color, central foveal retinal thickness, loge serum zeaxanthin, and serum total cholesterol and excluding eyes with CME, we found a higher correlation (0.37) between MPOD and loge serum lutein in our patients with retinitis pigmentosa. This correlation was highly significant for the large sample size in this analysis (n = 139) and would also have been statistically significant for the smaller sample size of the previous study.12 Although a simple correlation between MPOD and serum lutein in our patients was also significant and was higher (0.23) than what was obtained before,12 it would not have been statistically significant for the smaller sample size of the previous study. The present study, therefore, illustrates the value of reducing the unexplained MPOD variance by removing the influence of confounding factors in detecting a significant relationship between MPOD and serum lutein in retinitis pigmentosa.
Including serum total cholesterol in the multiple regression model revealed an inverse relationship between MPOD and serum cholesterol and strengthened the positive relationship between MPOD and serum lutein. The inference from these two observations is that a higher serum cholesterol level impedes the transport of lutein into the retina. Because these findings relating MPOD to serum lutein and to serum cholesterol were unchanged when we excluded patients with CME from the analysis, this conclusion does not appear to hinge on a partial breakdown of the distal blood-retinal barrier.
We also found that the relationship between MPOD and serum lutein tended to be stronger in men than in women, compatible with previous results in healthy subjects based on measurements of serum lutein11 or of plasma lutein plus zeaxanthin.26 The latter study hypothesized that hormonally controlled variations in lipid transport used by carotenoids in women might weaken the relationship between MPOD and plasma lutein plus zeaxanthin.
On the other hand, we did not find that MPOD was positively related to loge serum zeaxanthin. The absence of a significant positive association between MPOD and serum zeaxanthin was reported previously for healthy volunteers.11 These negative results may be explained by the observations that lutein outweighs zeaxanthin in diet and serum,34–36 and, though zeaxanthin predominates in the center of the fovea,21,37 that approximately half of this zeaxanthin is derived from lutein.21
We did not find significant relationships between MPOD and age or sex in our patients with retinitis pigmentosa, although the trends were in the same direction as in some previous reports based on healthy volunteers.23–26 We did find that MPOD was significantly related to iris color. MPOD averaged 22% higher in patients with brown irides than in patients with lighter irides, slightly smaller than the 26% difference found in healthy volunteers27 and consistent with the observation that patients with retinitis pigmentosa and light irides were more likely to have a low MPOD than a high MPOD.12
We found that MPOD was nonmonotonically related to central foveal retinal thickness in our cohort, which included patients without CME and patients with CME in the study eye. MPOD initially increased with increasing retinal thickness in eyes without CME or with minimal swelling caused by CME and then decreased with further increasing retinal thickness in eyes primarily with moderate to marked CME. The initial increase is consistent with the idea that xanthophyll incorporation is proportional to the number of central foveal cone photoreceptors,12,28 and we confirmed a significant linear relationship between MPOD and central foveal retinal thickness when we excluded the data from patients with CME in the study eye. The subsequent decrease in MPOD based on our total cohort could mean that moderate to marked edema hinders the uptake of lutein into the macula or distorts the radial arrangement of the foveolar cone axons, whose macular pigment molecules are normally oriented perpendicularly to the fiber axes,6 reducing their effective absorbance of incident blue light. Part of the decline in MPOD also could reflect CME coexisting with central foveal photoreceptor cell loss, which would compound the deficiency of MPOD. Still, by comparing the two slopes in Figure 3, photoreceptor cell loss clearly has a greater impact than CME on MPOD in retinitis pigmentosa.
It should be pointed out that the derivation of MPOD by heterochromatic flicker photometry assumes that the brightness match between the alternating blue and green test stimuli is mediated by the same proportion and relative sensitivities of long-wavelength–sensitive and middle-wavelength–sensitive cones at the test and reference locations within a given patient. The method also assumes that cone photopigment optical density is the same, or nearly the same, at the two locations.38 Although there is no evidence that long-wavelength–sensitive cones become more or less affected than middle-wavelength–sensitive cones as retinitis pigmentosa progresses, it is likely that cone photopigment optical density was reduced more in the parafovea than at the central fovea in most of our patients given that cone sensitivity39 and cone directionality40 are typically lost in the parafovea before the fovea in this disease. This difference in cone photopigment optical density would be expected to reduce the measured MPOD.38 On the other hand, the significant relationships we found between MPOD and serum lutein concentration, iris color, and central foveal retinal thickness are concordant with previous findings in healthy observers, suggesting, at least for these comparisons, that the validity of the MPOD measurements was not appreciably compromised in our patient cohort.
Although it was based on cross-sectional data, our finding that MPOD increased significantly with increasing serum lutein and not with increasing serum zeaxanthin raises the possibility that an increase in macular pigment in the axons of cone photoreceptors in patients with retinitis pigmentosa could be more easily achieved in the diet by increasing serum lutein than by increasing serum zeaxanthin. Of course, this hypothesis can only be verified by a longitudinal study involving supplementation. Because we also noted that the between-session reproducibility of our MPOD data was similar to that observed in healthy observers,29–33 we propose that an increase in MPOD could be used as a biomarker for lutein uptake by the retina in this disease. In this regard, an open-label pilot study in which 23 patients with retinitis pigmentosa were asked to take a 20-mg lutein supplement each day for 6 months found that mean MPOD increased significantly in the fovea over follow-up.12 Although that pilot study did not detect any significant improvement in visual acuity or in foveal sensitivity with lutein supplementation,12 it remains to be determined whether an increase in MPOD with lutein supplementation in retinitis pigmentosa might further shield cone photoreceptors from ambient light and reduce oxidative damage over the long term, possibly slowing the rate of cone photoreceptor degeneration.41,42
Footnotes
Supported by National Eye Institute Grant EY00169, the United States Department of Agriculture under agreement number 58–1950-7–707, and The Foundation Fighting Blindness.
Disclosure: M.A. Sandberg, None; E.J. Johnson, None; E.L. Berson, None
References
- 1.Parker R. Absorption, metabolism and transport of carotenoids. FASEB J 1996; 10: 542–551 [PubMed] [Google Scholar]
- 2.Holden JM, Eldridge AL, Beecher GR, et al. Carotenoid content of US foods: an update of the database. J Food Comp Anal 1999; 12: 169–196 [Google Scholar]
- 3.Clevidence BA, Bieri JG. Association of carotenoids with human plasma lipoproteins. Methods Enzymol 1993; 214: 3–17 [DOI] [PubMed] [Google Scholar]
- 4.Forman MR, Johnson EJ, Lanza E, et al. Effect of menstrual cycle phase on the concentration of individual carotenoids in lipoproteins of premenopausal: a controlled dietary study. Am J Clin Nutr 1998; 67: 81–87 [DOI] [PubMed] [Google Scholar]
- 5.Snodderly DM, Brown PK, Delori FC, Auran JD. The macular pigment, I: absorbance spectra, localization, and discrimination from other yellow pigments in primate retinas. Invest Ophthalmol Vis Sci 1984; 25: 660–673 [PubMed] [Google Scholar]
- 6.Snodderly DM, Auran JD, Delori FC. The macular pigment, II: spatial distribution in primate retinas. Invest Ophthalmol Vis Sci 1984; 25: 674–684 [PubMed] [Google Scholar]
- 7.Ham WT., Jr Ocular hazards of light sources: review of current knowledge. J Occup Med 1983; 25: 101–103 [PubMed] [Google Scholar]
- 8.Ham WT, Jr, Mueller A, Ruffolo JJ, Jr, et al. Basic mechanisms underlying the production of photochemical lesions in the mammalian retina. Curr Eye Res 1984; 3: 165–174 [DOI] [PubMed] [Google Scholar]
- 9.Ciulla TA, Curran-Celantano J, Cooper DA, et al. Macular pigment optical density in a midwestern sample. Ophthalmology 2001; 108: 730–737 [DOI] [PubMed] [Google Scholar]
- 10.Bone RA, Landrum JT, Dixon Z, et al. Lutein and zeaxanthin in the eyes, serum and diet of human subjects. Exp Eye Res 2000; 71: 239–245 [DOI] [PubMed] [Google Scholar]
- 11.Broekmans WM, Berendschot TT, Klopping-Ketelaars IA, et al. Macular pigment density in relation to serum and adipose tissue concentrations of lutein and serum concentrations of zeaxanthin. Am J Clin Nutr 2002; 76: 595–603 [DOI] [PubMed] [Google Scholar]
- 12.Aleman TS, Duncan JL, Bieber ML, et al. Macular pigment and lutein supplementation in retinitis pigmentosa and Usher syndrome. Invest Ophthalmol Vis Sci 2001; 42: 1873–1881 [PubMed] [Google Scholar]
- 13.Adackapara CA, Sunness J, Dibernardo CW, et al. Prevalence of cystoid macular edema and stability in OCT retinal thickness in eyes with retinitis pigmentosa during a 48-week lutein trial. Retina 2008; 28: 103–110 [DOI] [PubMed] [Google Scholar]
- 14.Hajali M, Fishman GA, Anderson RJ. The prevalence of cystoid macular oedema in retinitis pigmentosa patients determined by optical coherence tomography. Br J Ophthalmol 2008; 92: 1065–1068 [DOI] [PubMed] [Google Scholar]
- 15.Sandberg MA, Brockhurst RJ, Gaudio AR, Berson E. Visual acuity is related to parafoveal retinal thickness in patients with retinitis pigmentosa and macular cysts. Invest Ophthalmol Vis Sci 2008; 49: 4568–4572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hammond BR, Jr, Wooten BR, Snodderly DM. Cigarette smoking and retinal carotenoids: implications for age-related macular degeneration. Vision Res 1996; 36: 3003–3009 [DOI] [PubMed] [Google Scholar]
- 17.Willett WC, Sampson L, Browne ML, et al. The use of a self-administered questionnaire to assess diet four years in the past. Am J Epidemiol 1988; 127: 188–199 [DOI] [PubMed] [Google Scholar]
- 18.Wooten BR, Hammond BR, Land RI, Snodderly DM. A practical method for measuring macular pigment optical density. Invest Ophthalmol Vis Sci 1999; 40: 2481–2489 [PubMed] [Google Scholar]
- 19.Sandberg MA, Brockhurst RJ, Gaudio AR, Berson EL. The association between visual acuity and central retinal thickness in retinitis pigmentosa. Invest Ophthalmol Vis Sci 2005; 46: 3349–3354 [DOI] [PubMed] [Google Scholar]
- 20.Johnson EJ, Hammond BR, Yeum K-J, et al. Relation among serum and tissue concentrations of lutein and zeaxanthin and macular pigment density. Am J Clin Nutr 2000; 71: 1555–1562 [DOI] [PubMed] [Google Scholar]
- 21.Johnson EJ, Neuringer M, Russell RM, et al. Nutritional manipulation of primate retinas, III: effects of lutein or zeaxanthin supplementation on adipose and retina of xanthophylls-free monkeys. Invest Ophthalmol Vis Sci 2005; 46: 696–702 [DOI] [PubMed] [Google Scholar]
- 22.Mares-Perlman JA, Fisher AI, Klein R, et al. Lutein and zeaxanthin in the diet and serum and their relation to age-related maculopathy in the Third National Health and Nutrition Examination Survey. Am J Epidemiol 2001; 153: 424–432 [DOI] [PubMed] [Google Scholar]
- 23.Hammond BR, Jr, Caruso-Avery M. Macular pigment optical density in a southwestern sample. Invest Ophthalmol Vis Sci 2000; 41: 1492–1497 [PubMed] [Google Scholar]
- 24.Beatty S, Murray IJ, Henson DB, et al. Macular pigment and risk for age-related macular degeneration in subjects from a northern European population. Invest Ophthalmol Vis Sci 2001; 42: 439–446 [PubMed] [Google Scholar]
- 25.Bernstein PS, Zhao DY, Wintch SW, et al. Resonance Raman measurement of macular carotenoids in normal subjects and in age-related macular degeneration patients. Ophthalmology 2002; 109: 1780–1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hammond BR, Curran-Celanto J, Judd S. Sex differences in macular pigment optical density: relation to plasma carotenoid concentrations and dietary patterns. Vision Res 1996; 36: 2001–2012 [DOI] [PubMed] [Google Scholar]
- 27.Hammond BR, Jr, Fuld K, Snodderly DM. Iris color and macular pigment optical density. Exp Eye Res 1996; 62: 293–297 [DOI] [PubMed] [Google Scholar]
- 28.Liew SHM, Gilbert CE, Spector TD, et al. Central retinal thickness is positively correlated with macular pigment optical density. Exp Eye Res 2006; 82: 915–920 [DOI] [PubMed] [Google Scholar]
- 29.Hammond BR, Jr, Fuld K, Curran-Celentano J. Macular pigment density in monozygotic twins. Invest Ophthalmol Vis Sci 1995; 36: 2531–2541 [PubMed] [Google Scholar]
- 30.Hammond BR, Jr, Fuld K. Interocular differences in macular pigment density. Invest Ophthalmol Vis Sci 1992; 33: 350–355 [PubMed] [Google Scholar]
- 31.Hammond BR, Jr, Johnson EJ, Russell RM, et al. Dietary modification of human macular pigment density. Invest Ophthalmol Vis Sci 1997; 38: 1795–1801 [PubMed] [Google Scholar]
- 32.Hammond BR, Jr, Wooten BR, Snodderly DM. Preservation of visual sensitivity of older subjects: association with macular pigment density. Invest Ophthalmol Vis Sci 1998; 39: 397–406 [PubMed] [Google Scholar]
- 33.Hammond BR, Jr, Wooten BR, Snodderly DM. Individual variations in the spatial profile of human macular pigment. J Opt Soc Am A 1997; 14: 1187–1196 [DOI] [PubMed] [Google Scholar]
- 34.Mares JA, LaRowe T, Snodderly DM, et al. Predictors of optical density of lutein and zeaxanthin in retinas of older women in the Carotenoids in Age-Related Eye Disease Study (CAREDS), an ancillary study of the Women's Health Initiative. Am J Clin Nutr 2006; 84: 1107–1122 [DOI] [PubMed] [Google Scholar]
- 35.Perry A, Rasmussen H, Johnson E. Xanthophyll (lutein, zeaxanthin) content in fruits, vegetables, and corn and egg products. J Food Composition Analysis 2009; 22: 9–15 [Google Scholar]
- 36.Talegawkar S, Johnson EJ, Carithers T, et al. Serum carotenoid and tocopherol concentrations vary by dietary pattern among African Americans. J Am Dietetic Assoc 2008; 108: 2013–2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bone RA, Landrum JT. Distribution of macular pigment components, zeaxanthin and lutein, in human retina. Methods Enzymol 1992; 213: 360–366 [DOI] [PubMed] [Google Scholar]
- 38.Werner JS, Bieber ML, Schefrin BE. Senescence of foveal and parafoveal cone sensitivities and their relations to macular pigment density. J Opt Soc Am A 2000; 17: 1918–1932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sandberg MA, Berson EL. Blue and green cone mechanisms in retinitis pigmentosa. Invest Ophthalmol Vis Sci 1977; 16: 149–157 [PubMed] [Google Scholar]
- 40.Birch DG, Sandberg MA, Berson EL. The Stiles-Crawford effect in retinitis pigmentosa. Invest Ophthalmol Vis Sci 1982; 22: 157–164 [PubMed] [Google Scholar]
- 41.Berson EL. Light deprivation for early retinitis pigmentosa: a hypothesis. Arch Ophthalmol 1971; 85: 521–529 [DOI] [PubMed] [Google Scholar]
- 42.Newsome DA, Berson EL, Bonner RF, et al. Possible role of optical radiation in retinal degenerations. In: Waxler M, Hitchins VM. eds. Optical Radiation and Visual Health Boca Raton, FL: CRC Press, Inc.; 1986: 89–101 [Google Scholar]