Abstract
In the diurnal unstriped Nile grass rat (Arvicanthis niloticus) access to a running wheel can trigger a shift in active phase preference, with some individuals becoming night-active (NA), while others continue to be day-active (DA). To investigate the contributions of different neural systems to the support of this shift in locomotor activity, we investigated the association between chronotype and Fos expression during the day and night in three major nuclei in the basal forebrain (BF) cholinergic (ACh) arousal system – medial septum (MS), vertical and horizontal diagonal band of Broca (VDB and HDB respectively) –, and whether neural activation in these areas was related to neural activity in the orexinergic system. We also measured Fos expression in dopaminergic and non-dopaminergic cells of two components of the reward system that also participate in arousal – the ventral tegmental area (VTA) and supramammillary nucleus (SUM). NAs and DAs were compared to animals with no wheels. NAs had elevated Fos expression at night in ACh cells, but only in the HDB. In the non-cholinergic cells of the BF of NAs, enhanced nocturnal Fos expression was almost universally seen, but only associated with activation of the orexinergic system for the MS/ VDB region. For some of the areas and cell types of the BF, the patterns of Fos expression of DAs appeared similar to those of NAs, but were never associated with activation of the orexinergic system. Also common to DAs and NAs was a general increase in Fos expression in non-dopaminergic cells of the SUM and anterior VTA. Thus, in this diurnal species, voluntary exercise and a shift to a nocturnal chronotype changes neural activity in arousal and reward areas of the brain known to regulate a broad range of neural functions and behaviors, which may be also affected in human shift workers.
Keywords: acetylcholine, diurnal, dopamine, orexin, wheel running
Most of the research done in the field of circadian biology involves the use of nocturnal species as experimental subjects. In contrast, relatively little has been investigated in diurnal species, mainly due to the lack of suitable models of diurnality. The unstriped Nile grass rat, Arvicanthis niloticus (grass rat), shows robust diurnal rhythms in both the laboratory and in the field (Blanchong et al., 1999), and thus represents an ideal animal model for the study of mechanisms underlying circadian rhythmicity in diurnal species (Katona and Smale, 1997). Interestingly, when a running wheel becomes available, a subset of these diurnal animals shifts to be predominantly active during the night (night-active individuals, NA), whereas others remain active during the day (day-active individuals, DA; Blanchong et al., 1999). The shift in locomotor activity pattern seen in NA grass rats does not appear to reflect a change in the functioning of the master oscillator of the suprachiasmatic nucleus (SCN; Blanchong et al., 1999; Rose et al., 1999; Schwartz and Smale, 2005), but rather a change in the coupling between the SCN and the neural mechanisms that control activity or a complete dissociation of these mechanisms and the master pacemaker. Because voluntary activity during the resting phase is a phenomenon commonly seen in human shift workers, the grass rat also represents a useful model to study the physiological effects exerted by temporal shifts in activity. This model contrasts with other animal models of shift work in which temporal shifts in activity (e.g. Murphy et al., 2003; Salgado-Delgado et al., 2008) are not produced voluntarily by the animal but induced by the researcher.
In sedentary grass rats (i.e. singly-housed with no wheels) wakefulness is associated with enhanced neural activity in the histaminergic (Novak et al., 2000) and orexinergic (Martinez et al., 2002) brain arousal systems, such that there is an increase in Fos expression in those cellular groups during the active phase as compared to the resting phase of the 24-hour cycle. Likewise, the orexinergic system of grass rats housed with wheels shows an increase in Fos expression at times when animals are actively running, regardless of the phase of the light-dark cycle where the locomotor activity occurs (Nixon and Smale, 2004). It is unclear, however, how other arousal systems of the brain might respond to this temporal segregation of activity. Of particular interest is the cholinergic (ACh) system of the basal forebrain (BF), since it participates in cortical arousal (reviewed in Jones, 2008). This system is involved in the generation of the hippocampal theta wave (HPC-θ), a rhythm that is displayed during wakefulness and paradoxical sleep (reviewed in Buzsaki, 2002) and has been associated with the execution of voluntary activity, such as wheel-running (Oddie et al., 1996). Also, when laboratory rats are stimulated to stay awake during their resting phase, ACh neurons of the BF show increased Fos expression (Greco et al., 2000) supporting the role of these neurons in arousal. In addition, neurons of the BF that secrete gamma-amino-n- butyric acid (GABA) and glutamate appear to play a modulatory role in cortical activity, as well as in the HPC-θ (Leung and Shen, 2004; Yoder and Pang, 2005; Henny and Jones, 2008).
It is likely that neural activity in the ACh BF is influenced by arousal systems, such as the orexinergic system. This system, composed of neurons producing orexin A (OXA), orexin B (OXB) and their receptors, has been postulated to play an important role in the regulation of other arousal systems, including the ACh BF (Saper et al., 2001). In fact, orexinergic neurons project densely to the latter cellular group and these cells are responsive to orexin stimulation (Wu et al., 2004). Moreover, blockade of orexin receptors in ACh cells of the medial septum (MS), a component of the BF, attenuates the HPC-θ rhythm (Gerashchenko et al., 2001). Thus, the orexinergic system may play a role in the modulation of neural activity of the ACh BF in grass rats with access to wheels.
An additional system of the brain that might be stimulated by the temporal segregation of activity in DA and NA grass rats is the reward system. It has been suggested that wheel running is rewarding and possibly addictive (Eikelboom and Lattanzio, 2003), since animals not only work for wheel access, but also compromise vital activities such as food and water intake in order to engage in wheel running (reviewed in Sherwin, 1998). The reward system includes the ventral tegmental area (VTA) and the supramammillary nucleus of the hypothalamus (SUM). These areas show increased neural activation upon exposure to rewarding stimuli (e.g. Asmus and Newman, 1994; Balfour et al., 2004; Marcangione and Rompre, 2008), including acute access to a running wheel (Yanagita et al., 2007). The rewarding effects mediated by these systems depend on direct (VTA; reviewed in Ikemoto and Panksepp, 1999) and indirect (SUM; Ikemoto et al., 2004) efferent projections to the nucleus accumbens (NAc), and require the release of dopamine in this nucleus (Yoshida et al., 1992; Schilstrom et al., 1998; Ikemoto et al., 2004). In addition to their role in reward, the VTA and SUM are also involved in the modulation of vigilance states. For example, there is increased activation of dopamine cells of the VTA during paradoxical sleep and during the execution of appetitive behaviors as compared to slow wave sleep and quiet wakefulness (Dahan et al., 2007). The SUM in turn is involved in the control of the HPC-θ rhythm, and thus may participate in learning and memory (reviewed in Pan and McNaughton, 2004). Additionally, the SUM contributes to emotional responses (Pan and McNaughton, 2004). Thus, given the roles of the VTA and SUM in motivation and arousal, it appears likely that neural activation of these regions is associated with wheel access in ways that may differ in animals that run at different times of day.
In the present study we investigated the relationship between neural activation in the BF, VTA, and SUM, and the voluntary shifts in locomotor activity seen in DA and NA grass rats. First, we evaluated whether neural activity in three major components of the ACh system of the grass rat BF – the MS, vertical diagonal band of Broca (VDB), and horizontal diagonal band of Broca (HDB) – is associated with temporal patterns of wheel running. Second, we evaluated the hypothesis that orexinergic cells might modulate neural activity of the grass rat BF by looking at whether activation of cells in these two systems is correlated and at whether orexin cells send direct axonal projections to the BF. Finally, we investigated whether neural activity in the reward system is associated with wheel running at different phases of the light-dark cycle in DA and NA grass rats. To address this question, we focused on dopaminergic and non-dopaminergic cells of the VTA and SUM. Thus, the present study expands our current understanding of voluntary temporal shifts in activity and their associations with neural activation in arousal, as well as in brain reward areas in a diurnal mammal.
Experimental procedures
Animals
Thirty-six adult male grass rats were used in this study. Twenty-four had access to wheels (n = 12 DA, 12 NA), while the remaining 12 served as no-wheel controls (C). All animals were born in our laboratory and derived from a group brought from Kenya in 1993 (Katona and Smale, 1997). Animals were kept on a 12:12 light-dark cycle with a red light (< 5 lux) on at all times, and were provided with ad libitum access to water and food (Harlan Teklad 8640 rodent diet, Harlan Teklad Laboratory, Madison, WI, or PMI Nutrition Prolab RMH 2000, PMI Nutrition International, Brentwood, MO). The approach used to determine the chronotype of DA and NA individuals, as well as their housing conditions, were described previously, as these same animals were used in prior work from this lab (Nixon and Smale, 2004). Briefly, the animals were housed with a wheel (26 cm diameter, 8 cm width) in plexiglass cages (17 × 34 × 28 cm) and wheel-running rhythms were monitored using DSI Dataquest 3 system (MiniMitter, Sunriver, OR). After the rhythms were stable for at least one week, animals were classified as DA if activity ceased within 2 hours after lights-out, or as NA if activity continued for more than 4 hours after lights-out. The third group of animals used in this study, the C group, consisted of singly-housed (plexiglass cages 17 × 34 × 28 cm) grass rats with no wheels. Under those conditions grass rats show patterns of sleep, general activity, and body temperature that are characteristic of diurnal species (McElhinny et al., 1997; Novak et al., 1999). All animals were kept under the aforementioned conditions for at least one month before sacrifice. All experiments were performed in compliance with guidelines established by the Michigan State University Institutional Animal Care and Use Committee, and the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Tissue collection and preparation
Animals (n = 6 per group) were perfused at Zeitgeber times (ZT) 4 and 16. At the time of perfusion animals were deeply anesthetized with intraperitoneal injections of sodium pentobarbital (Ovation Pharmaceutical, Deerfield, IL). An aluminum hood was placed over the head of the animals euthanized during the dark phase to prevent exposure to light. Animals were perfused intracardially with 0.01 M phosphate-buffered saline (PBS; pH 7.4), followed by 4% paraformaldehyde with 75 mM lysine and 10 mM sodium periodate (PLP). Brains were post-fixed in PLP for 4 – 8 h, and then transferred to 20% sucrose solution in 0.1 M PBS overnight at 4°C. Then, coronal sections (30 µm) were cut on a freezing sliding microtome, and alternate sections were collected in three series in cryoprotectant solution at −20°C until further processing.
Although the tissue from all groups was processed at the same time, the time elapsed between perfusion and immunocytochemical procedures was not the same for all groups. Whereas the brains of DAs and NAs were stored in cryoprotectant for over seven years, the brains of our Cs were stored in this solution only for one month. This difference in exposure to cryoprotectant, however, was not likely to have an effect on our results since tissue can be stored for many years in this solution without losing antigenicity (Hoffman and Le, 2004). Furthermore, our results were area and sample-time specific, and did not indicate reduced antigenicity in the tissue kept the longest in cryoprotectant. Thus, the data presented here are likely to reflect only neuronal activation associated with the conditions of the study pertinent to each group and not the effects of differential time since perfusion.
Experiment 1: Basal forebrain activation and its relation to orexinergic activation
1.1. Basal forebrain activation
Tissue was rinsed 3 times (10 min/rinse) in 0.01 M PBS between all steps of the immunocytochemical procedures and all steps were carried out at room temperature unless indicated otherwise. In addition, all incubations included 0.3% Triton X-100. Free-floating sections containing the MS, VDB, and the HDB were rinsed (6 times, 10 min/rinse) in 0.01 M PBS, blocked for 1 h using 5% normal donkey serum (NDS; Jackson ImmunoResearch Laboratories, West Grove, PA) in PBS and incubated for 48 h in a rabbit anti-Fos antibody (Santa Cruz Biotechnology, Santa Cruz, CA; diluted 1:20,000 in PBS and 3% NDS). The sections were then incubated for 1 h 30 min in a donkey anti-rabbit biotinylated antibody (Jackson; diluted 1:200 in PBS and 3% NDS), and then for 1 h 30 min in avidin-biotin peroxidase complex (AB complex, Vector Laboratories, Burlingame, CA; in PBS). After 3 rinses (10 min/rinse) in Tris buffer (pH = 7.2), the sections were reacted with 0.025% diaminobenzidine (DAB; Sigma-Aldrich, St. Louis, MO; enhanced with 2.5% nickel sulfate in Tris buffer with 3% hydrogen peroxide) for 7 min. This reaction was followed by four 10 min rinses in PBS. Then, sections were blocked for 1 h in 5% normal horse serum (NHS; Vector; in PBS) and incubated for 48 h in a goat anti-choline acetyltransferase (ChAT) antibody (Chemicon, Temecula, CA; diluted 1:10,000 in PBS and 3% NHS). Following primary incubation, the sections were incubated for 1 h 30 min in a horse anti-goat biotinylated antibody (Vector; diluted 1:200 in PBS and 3% NHS). Then, the tissue was incubated for 1 h 30 min with AB complex (Vector; in PBS). After 3 rinses (10 min/rinse) in Tris buffer, the sections were reacted with 0.02% DAB in the same buffer (with 0.35 µl 30% hydrogen peroxide/ml buffer) for 5 minutes, and then rinsed in 0.01 M PBS (4 times, 5 min/rinse). All sections were mounted onto gelatin-coated slides, dehydrated, and coverslipped with DPX.
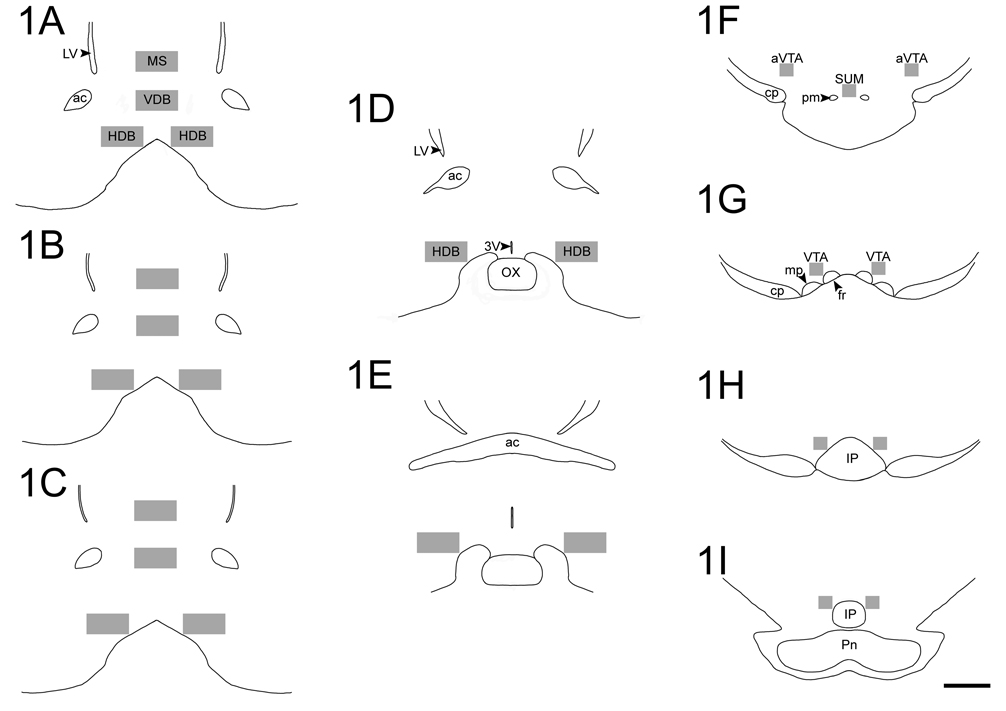
In order to quantify Fos and ChAT expression, we selected three sections throughout the MS and VDB and five sections for the HDB. Sections for the MS and VDB corresponded approximately to plates 15 through 17 of the rat brain atlas by Paxinos and Watson (1997), whereas sections for HDB corresponded to plates 15 through 20. For every section selected, cells expressing Fos, ChAT, and Fos+ChAT were counted within a region defined by a 600 µm (width) × 300 µm (height) box placed in the center of the studied areas (see Fig. 1A–1E). Stereo Investigator software (MBF Bioscience Inc, 2007) was used to place the box and perform counts. All counts were made by an investigator unaware of the source of the tissue. After the cell counts were completed, the total cell counts were divided by the area occupied by the box (0.18 mm2) and that represented the data that were analyzed. Adjustments were done when small portions of the counting box fell outside the studied areas. For the HDB, we averaged data from the two sides of the brain per section. The MS and VDB did not require this procedure since they are located close to the midline (see Fig. 1A–1C).
Figure 1.
Rostro-caudal illustrations depicting representative sampling areas used to quantify Fos expression in ACh and nACh cells of the MS, VDB, and HDB (A–E), as well as Fos expression in nTH and TH cells of the VTA and SUM (F–I). Boxes used for cellular counts had the following dimensions: (A–E) 600 µm × 300 µm and (F–I) 160 µm × 160 µm. Scale bar= 1 mm.
To analyze the data obtained in the BF, we used three-way analysis of variance (ANOVA). These analyses had ZT (4 and 16) and group (DA, NA, and C) as between-subjects factors and level of the section (3 levels for MS and VDB, and 5 levels for HDB) as a within-subjects factor. The dependent variables analyzed per area were: (1) number of ACh cells/mm2, (2) number of non-cholinergic (nACh) cells expressing Fos/mm2, and (3) proportion of ACh cells expressing Fos. Because this latter variable was expressed as proportions, it was squared root transformed, and then, arcsine transformed to normalize its distribution. For all comparisons differences were considered significant when p was less than 0.05. Significant interaction effects were followed by analyses of simple effects and post hoc comparisons using t- tests. The software used for the statistical analyses was SAS v 9.1 (SAS Institute, 2002).
1.2. BF activation and orexin activity in DA and NA grass rats
To determine whether activation of orexin cells was correlated with BF activation, we used correlational analyses between Fos expression in OXA and OXB cells and Fos expression in ACh and nACh cells of the BF. Data for Fos in OXA and OXB cells in the same animals came from Nixon and Smale (2004; see Animals section above). We did not test whether orexin activation was related to BF activation in Cs since for this group we did not have data on the former variable. The program used to analyze the correlations was SPSS v. 15.0 (SPSS; SPSS, Chicago, IL).
To complement the correlational analyses, we evaluated whether the grass rat BF might receive orexinergic inputs. Particularly, we wanted to determine if orexin-containing fibers formed putative appositions with ACh cells. We limited the anatomical analyses to OXA only, since the distribution of orexin-containing fibers in the BF of grass rats is very similar for the two orexins (Nixon and Smale, 2007). For this analysis, sections containing the BF from 2 animals per group (DA, NA, and C) were reacted for OXA, as described in Nixon and Smale (2004), and for ChAT. Briefly, tissue was blocked using NDS (Jackson), then reacted with goat anti-OXA (Santa Cruz; diluted 1:10,000), and donkey anti-goat secondary (Santa Cruz; diluted 1:500). Following PBS rinses after the OXA/nickel-DAB reaction (7 minutes), the tissue was blocked using NHS, and then incubated in goat anti-ChAT primary following the same procedure described above for BF activation. Following DAB reaction (3 minutes), tissue was mounted and coverslipped with DPX. Putative contacts between OXA fibers and ACh cells were verified with a 100x objective.
Experiment 2: Activation of reward systems
Free- floating sections throughout the VTA were processed for Fos and tyrosine hydroxylase (TH) following procedures similar to the ones described above with the exceptions noted below. For the Fos staining, the tissue was incubated in 5% NDS (in PBS) for 30 min and with a rabbit anti-Fos antibody (Santa Cruz; diluted 1:20,000 in PBS and 3% NDS) for 24 h. The incubations with a donkey anti-rabbit biotinylated antibody (Jackson; diluted 1:200 in PBS and 3% NDS), as well as with the AB complex (in PBS) were for 1 h. The DAB-nickel sulfate reaction lasted 6.5 min. For TH staining, the tissue was incubated in 5% NHS (Vector; in PBS) for 30 minutes. Tissue was then incubated with mouse anti-TH (Immunostar, Hudson, WI; diluted 1:20,000 in PBS and 3% NHS) for 24 h. The biotinylated secondary antibody incubation with horse anti-mouse (Vector; diluted 1:200 in PBS and 3% NHS), as well as the AB complex incubation (in PBS) were for 1 h. The reaction time with the DAB solution lasted 2 min.
In order to quantify Fos and TH expression, we selected four sections throughout the VTA (rostral to caudal VTA). These sections corresponded approximately to plates 37 through 43 of the rat brain atlas by Paxinos and Watson (1997). For every section selected, cells expressing Fos, TH, and Fos+TH were counted within a region defined by a 160 µm × 160 µm box that was placed lateral to the medial border of the VTA (see Fig. 1F–1I), in an area rich in TH staining. Counts were done bilaterally in the VTA and cell numbers were averaged per section. In addition, the counting box was placed in one section of the caudal SUM (see Fig. 1F). All counts were done using a 25x objective and performed by an investigator unaware of the source of the tissue.
To analyze the data obtained in the VTA, we could not use repeated measures ANOVA, because some sections were missing for a few animals. Instead, we used two-way ANOVAs per VTA region. The independent variables were ZT and group whereas the dependent variables analyzed were (1) number of Fos cells in non-TH (nTH) cells and (2) number of TH cells. Data on TH cells expressing Fos were not analyzed because double-labeled cells were very rare across all groups. Significant main effects were followed by post hoc comparisons using t-tests. The software used for the statistical analyses was SAS v 9.1 (SAS Institute, 2002).
Results
Experiment 1: Basal forebrain activation and its relation to orexinergic activation
1.1. Basal forebrain activation
The total number of ACh cells in the MS, VDB, and HDB did not differ significantly for any comparison (all p values > 0.08). Also, the proportions of ACh cells that contained Fos in the MS and VDB, were not significantly affected by time of day or by chronotype or by an interaction between these factors (all p values > 0.14; data not shown). The patterns of results for which ANOVAs revealed significant effects of our independent variables on cells within the BF are summarized in Figure 2–Figure 5.
Figure 2.
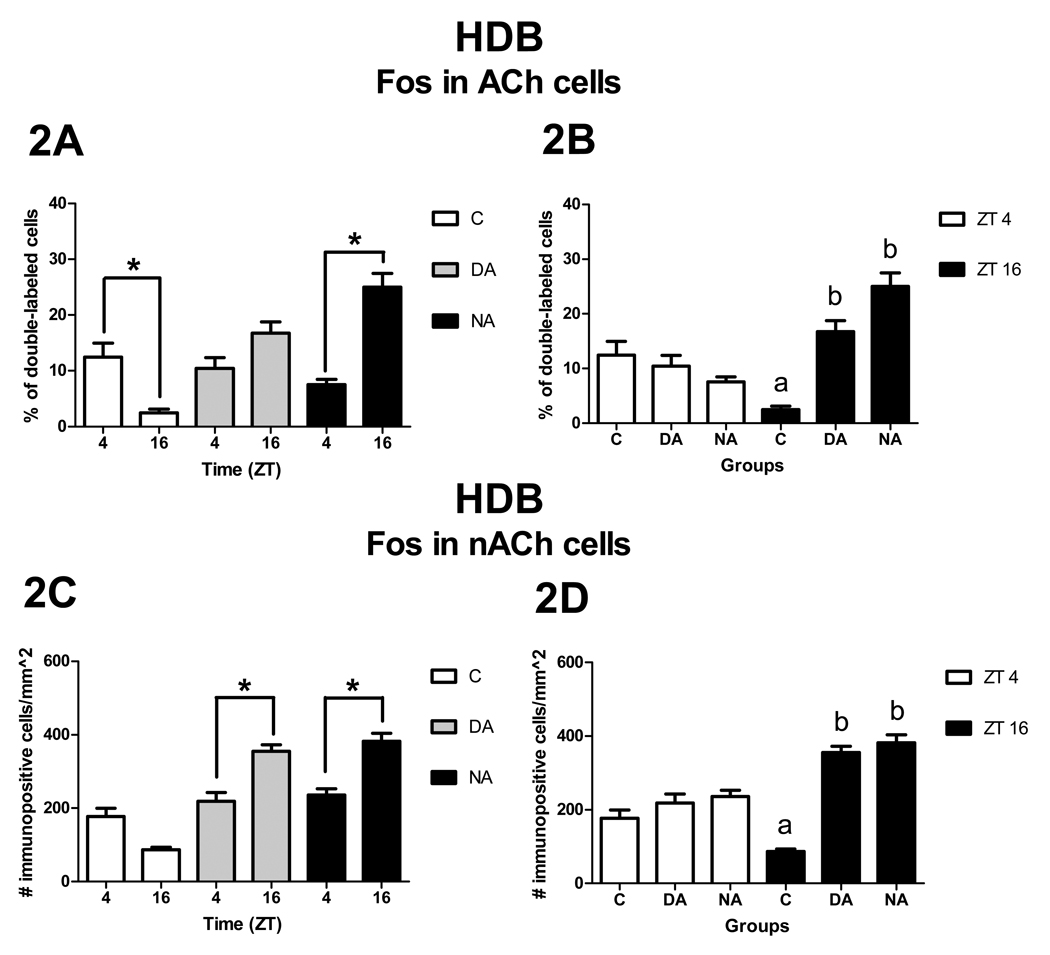
Patterns of Fos expression in ACh cells (A, B) and nACh cells (C, D) in the HDB. Panels A and C show significant ZT differences (asterisks) within groups, whereas panels B and D show significant group differences within ZT (p< 0.05). Note that group means with different letters are significantly different from each other, here as well as in Figure 3, Figure 4, and Figure 7.
Figure 5.
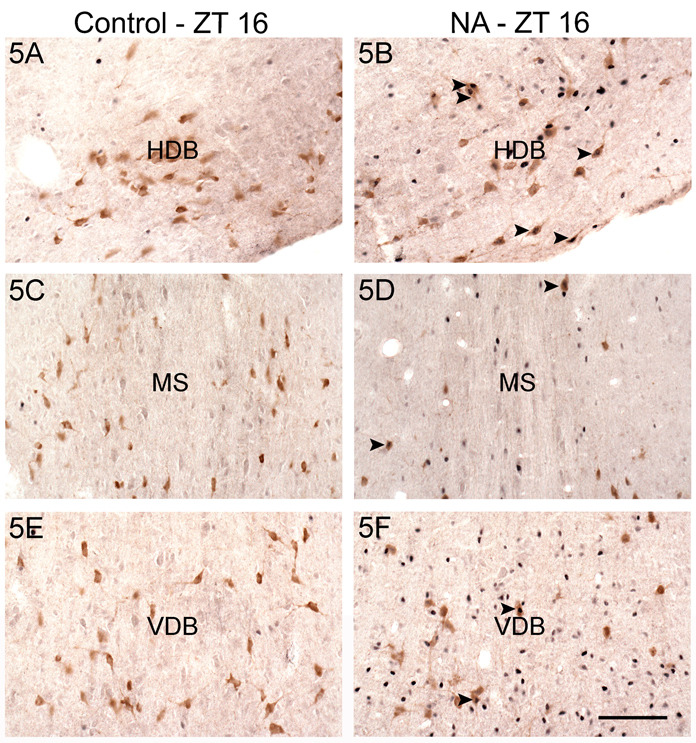
Photomicrographs of ACh cells (ChAT- positive) expressing Fos in the HDB (A, B), MS (C, D), and VDB (E, F) of a control (A, C, E) and a NA grass rat (B, D, F) euthanized at ZT 16. Arrowheads indicate some double-labeled cells. Fos: blue nuclear staining. ChAT: brown cell body staining. Scale bar= 100 µm.
HDB
Patterns of Fos expression in the HDB were not significantly affected by level of the HDB being analyzed nor by any of the interactions involving this factor (p > 0.27; data not shown). Thus, the data were averaged across sections and analyzed using two-way ANOVAs.
Patterns of Fos expression in ACh cells
The interaction between ZT and group was significant [F2, 27 = 6.76, p < 0.01]. Analysis of the simple effect of ZT within group revealed that NAs had more double-labeled cells at ZT 16 than at ZT 4 [F1, 27 = 8.80, p < 0.01, Fig. 2A], whereas Cs had more double-labeled cells at ZT 4 than at ZT 16 [F1, 27 = 4.63, p = 0.04, Fig. 2A]. In contrast, there was no significant effect of ZT for DAs [F1, 27 = 1.22, p = 0.28, Fig. 2A]. Analysis of the simple effect of group detected no significant differences across groups at ZT 4 [F2, 27 = 0.14, p = 0.87], whereas a significant difference was observed at ZT 16 [F2, 27 = 11.19, p < 0.01]. At ZT 16, NAs and DAs had more double-labeled cells than Cs [t27 = −4.62, p <0.01 and t27 = −2.98, p <0.01, respectively, Fig. 2B). No differences were observed between DAs and NAs [t27 = −1.16, p = 0.26, Fig. 2B].
Patterns of Fos expression in nACh cells
As the raw data were found to violate the normality assumption of the ANOVA, they were squared root transformed prior to analysis. The same transformation was done for all data sets involving nACh neurons. There was a significant interaction between ZT and group [F2, 27 = 5.53, p < 0.01]. Analysis of the simple effect of ZT revealed that DAs and NAs had more Fos expression at ZT 16 than at ZT 4 [F1, 27 = 4.40, p = 0.05 and F1, 27 = 5.11, p = 0.03, respectively, Fig. 2C], whereas no differences were observed for Cs [F1, 27 = 3.36, p = 0.08, Fig. 2C]. Analysis of the simple effect of group revealed that at ZT 4 there were no differences in Fos expression across groups [F2, 27 = 0.99, p = 0.39], whereas at ZT 16 differences were observed [F2, 27 = 17.96, p < 0.01]. At ZT 16, NAs and DAs had more Fos expression in nACh cells than Cs [t27 = −5.48, p < 0.01 and t27 = −4.62, p < 0.01, respectively, Fig. 2D]. No difference was observed between DAs and NAs [t27 = −0.28, p = 0.78, Fig. 2D].
MS
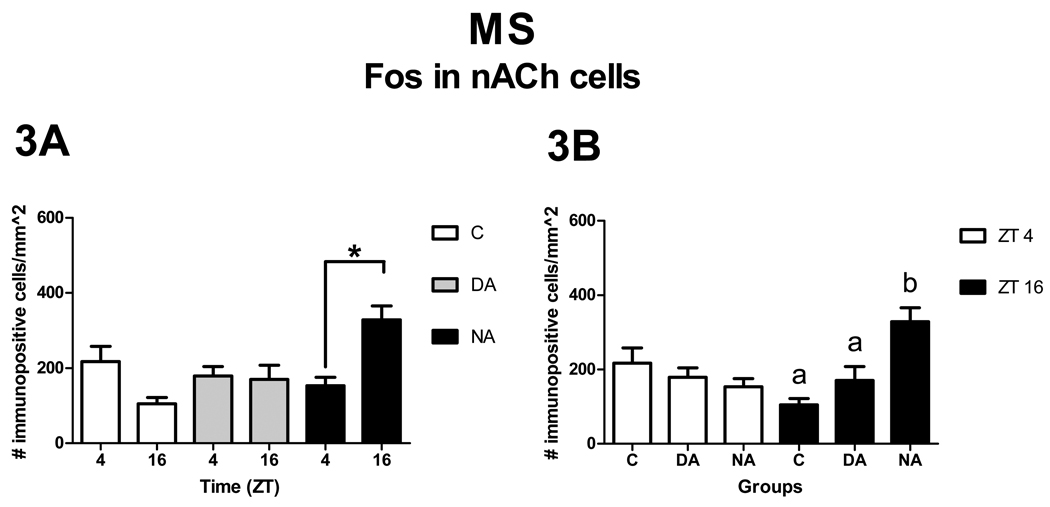
Patterns of Fos expression in nACh cells
The main effect of level was significant [F2, 54 = 9.67, p < 0.01], with the caudal level showing more Fos expression in nACh cells than the rostral level [t54 = −4.39, p < 0.01]. The middle level had more Fos expression than the rostral level [t54 = −2.08, p = 0.04], but lower expression than the caudal level [t54 = −2.32 p = 0.02]. These effects of level had no significant interactions with the effects of ZT or group. The interaction between ZT and group, however, was significant [F2, 27 = 5.38, p = 0.01]. Analysis of the simple effects of ZT revealed that NAs had more Fos expression in nACh cells at ZT 16 than at ZT 4 [F1, 27 = 7.14, p = 0.01 Fig, 3A]. No differences were observed for Cs [F1, 27 = 3.77, p = 0.06, Fig, 3A] or DAs [F1, 27 = 0.01, p = 0.94, Fig, 3A]. Analysis of the simple effects of group revealed that at ZT 4 there were no statistical differences across groups [F2, 27 = 0.37, p = 0.69], but at ZT 16 there was a significant effect of group [F2, 27 = 7.22, p < 0.01]. Paired comparisons revealed that at ZT 16, NAs expressed more Fos in nACh cells than DAs and Cs [t27 = −2.18, p = 0.04 and t27 = −3.76, p < 0.01, respectively, Fig. 3B]. No differences were observed between DAs and Cs [t27 = −1.18, p = 0.25, Fig. 3B].
Figure 3.
Patterns of Fos expression in nACh cells in the MS. Panel A shows significant ZT differences (asterisks) within groups, whereas panel B shows significant group differences (letters) within ZT (p< 0.05).
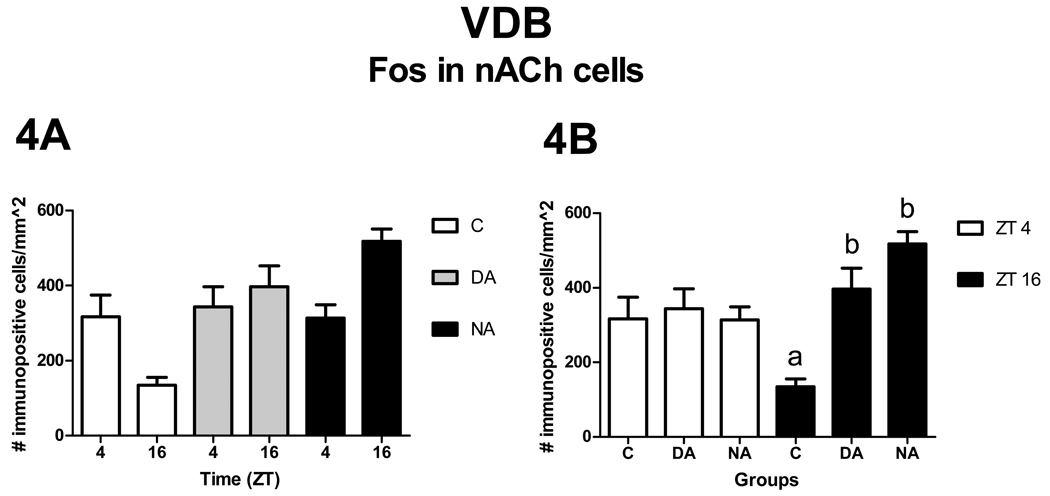
VDB
Patterns of Fos expression in nACh cells
There was a significant main effect of level [F2, 54 = 4.60, p = 0.01], and t-tests revealed that the rostral level contained fewer nACh cells expressing Fos than the caudal level [t54 = −3.03, p < 0.01]. In contrast, no differences were observed between the middle and rostral [t54 = −1.48, p = 0.15] or the middle and caudal [t54 = −1.56 p = 0.12] levels. These effects of level had no significant interactions with the effects of ZT or group. In contrast, a significant interaction was observed between ZT and group [F2, 27 = 3.82, p = 0.03]. Analysis of the simple effects of ZT revealed that none of the groups had differential expression of Fos at the two ZTs [C: F1, 27 = 3.77, p = 0.06; DA: F1, 27 = 0.26, p = 0.62; and NA F1, 27 = 3.68, p = 0.07, Fig. 4A]. Analysis of the simple effects of group, revealed that at ZT 4 there were no differences across groups [F2, 27 = 0.12, p = 0.89], whereas at ZT 16 significant differences were observed [F2, 27 = 9.29, p < 0.01]. At ZT 16, NAs and DAs had more Fos expression in nACh cells than Cs [t27 = −4.21, p < 0.01 and t27 = −2.69, p = 0.01, respectively, Fig. 4B], whereas no differences were observed between DAs and NAs [t27 = −1.08, p = 0.29, Fig. 4B].
Figure 4.
Patterns of Fos expression in nACh cells in the VDB. Panel A shows comparisons within groups (no significant effects of ZT). Panel B shows significant group differences (letters) within ZT (p< 0.05).
1.2. BF activation and orexin activity in DA and NA grass rats
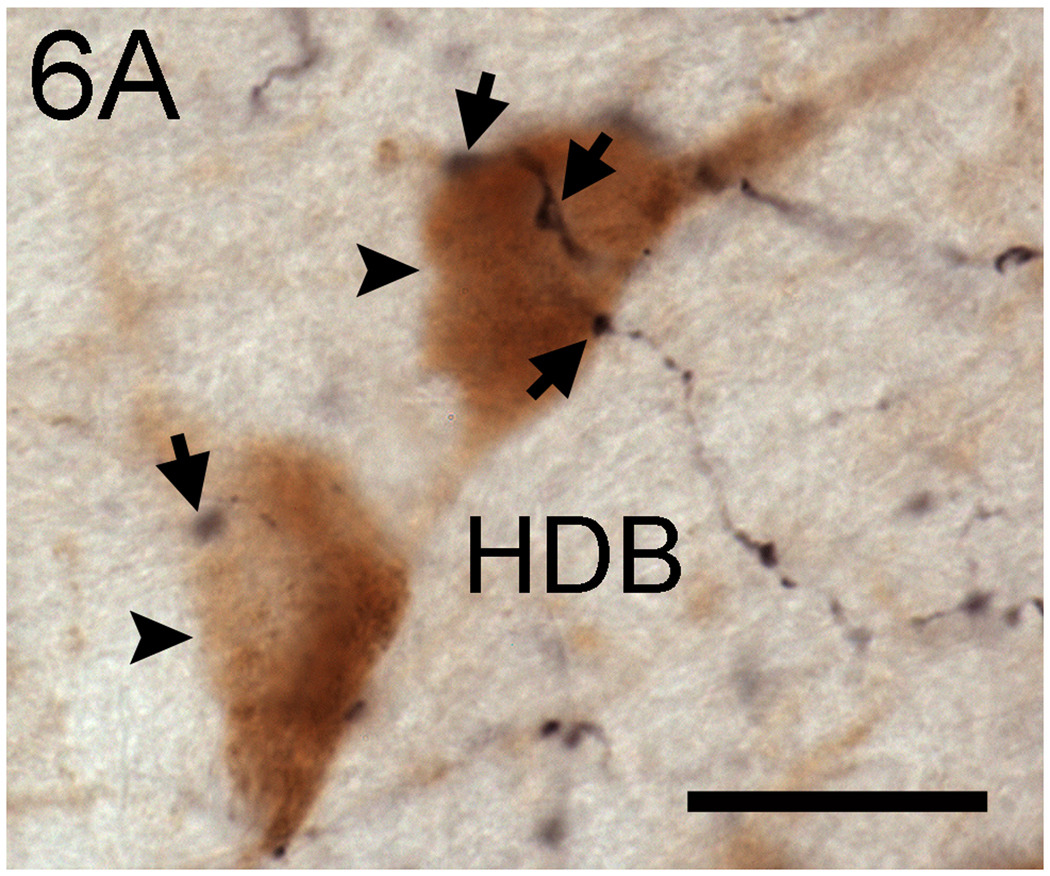
In the MS and VDB, Fos expression in ACh and nACh cells, was positively correlated with Fos expression in OXA cells, but only in NAs (Table 1). The results were identical for OXB (data not shown). In contrast, in the HDB no significant correlations between these measures were observed for either group. OXA fibers were visible in the MS, VDB, and HDB of all groups. Furthermore, the pattern and characteristics of OXA projections to those areas were similar across groups. In the three brain regions, OXA fibers were distributed sparsely and contained numerous varicosities. Those varicosities were observed in close proximity to ACh cells, as well as to nACh cells of the BF (Fig. 6).
Table 1.
Correlations between Fos expression in MS, VDB, and HDB cells and Fos expression in OXA cells.
| OXA | ||
|---|---|---|
| DA | NA | |
| HDB | ||
| Fos in Ach cells | r= 0.032 | r= 0.558 |
| p= 0.935 | p= 0.059 | |
| Fos in nAch cells | r= −0.132 | r= 0.533 |
| p= 0.736 | p= 0.074 | |
| MS | ||
| Fos in Ach cells | r= 0.307 | r= 0.625* |
| p= 0.421 | p= 0.030 | |
| Fos in nAch cells | r= 0.037 | r= 0.758* |
| p= 0.925 | p= 0.004 | |
| VDB | ||
| Fos in Ach cells | r= 0.302 | r= 0.782* |
| p= 0.429 | p= 0.003 | |
| Fos in nAch cells | r= 0.295 | r= 0.640* |
| p= 0.441 | p= 0.025 | |
Significant correlation (p <0.05).
Figure 6.
Photomicrographs of putative OXA fiber appositions (arrows) with ACh cells (arrowheads) in the HDB of a representative animal. Note that putative appositions are also observed in the vicinity of ACh cells. Scale bar= 30 µm.
Experiment 2: Activation of reward systems
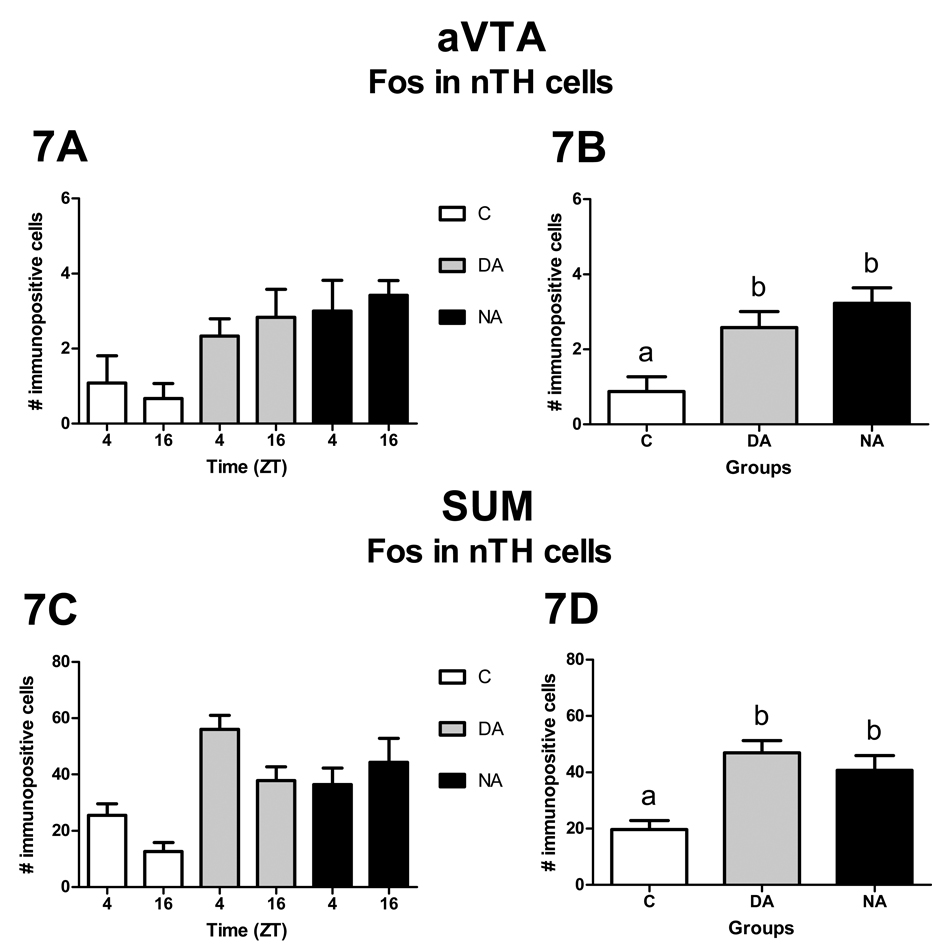
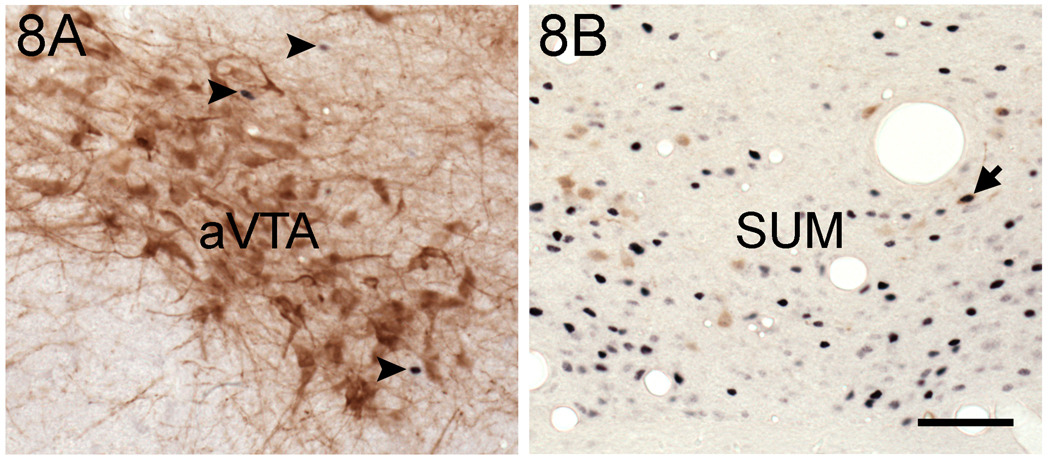
The number of TH cells in the VTA (p > 0.23) and SUM (p > 0.06) was not significantly affected by any of the factors analyzed (data not shown). The pertinent results for the VTA and SUM are summarized in Figure 7 and Figure 8.
Figure 7.
Patterns of Fos expression in nTH cells of the aVTA (A, B) and SUM (C, D). Panels A and C show the distribution of Fos expression in nTH cells for each group at each sampled time. Neither the effect of ZT nor the interaction between ZT and group were statistically significant. Panels B and D show the overall distribution of Fos expression in nTH cells for each group. The effect of group was statistically significant (p< 0.05).
Figure 8.
Photomicrographs of Fos and TH cells in the aVTA (A) and SUM (B). Double-labeled cells were scarce (arrow in B) or absent in both areas. In addition, note that TH cells were abundant in the aVTA. However, these cells were not positive for Fos, even though this protein was observed in the vicinity of those neurons (arrowheads). Fos: blue nuclear staining. TH: brown cell body staining. Scale bar= 100 µm.
VTA
In the most rostral level of the VTA (anterior VTA, aVTA) neither the effect of ZT [F1, 29 = 0.11, p = 0.74] nor the interaction between ZT and group [F2, 29 = 0.36, p = 0.70] were significant, whereas the main effect of group [F2, 29 = 7.94, p < 0.01] was significant, such that DAs and NAs expressed more Fos than Cs [t29 = −2.86, p < 0.01 and t29 = −3.82, p < 0.01, respectively, Fig. 7A and 7B]. No significant difference was observed between DAs and NAs [t29 = −1.02, p = 0.32, Fig. 7A and 7B]. For the rest of the VTA none of the factors analyzed or their interactions had a significant effect on Fos expression (data not shown).
SUM
Neither the main effect of ZT [F1, 28 = 2.80, p = 0.11] nor the interaction between ZT and group [F2, 28= 2.98, p = 0.07] were significant. The main effect of group [F2, 28= 13.30, p < 0.01], however, was significant, such that DAs and NAs expressed more Fos than Cs [t28 = −4.97, p < 0.01 and t28= −3.72, p < 0.01, respectively, Fig. 7C and 7D]. No significant difference was observed between DAs and NAs [t28= −1.17, p = 0.25, Fig. 7C and 7D].
Discussion
Experiment 1: Basal forebrain activation and its relation to orexinergic activation
HDB
In the HDB, we found that regardless of wheel access and chronotype Fos expression in ACh and nACh cells was similar across groups at ZT 4 (Fig. 2), even though NAs sleep more at this time (Schwartz and Smale, 2005). A likely explanation for these results is that a common experience (i.e., sleep) produces the return of neural activation to baseline levels in the BF. In fact, both DA and NA grass rats housed with wheels (Schwartz and Smale, 2005), as well as sedentary grass rats (Novak et al., 1999), increase the display of behavioral sleep late in the dark phase (between ZT 20 and 22). This universal display of sleep late at night could be the common experience that induces the return of neural activity to baseline levels in the grass rat BF, even if the sleep debt of NAs is not completely dissipated at ZT 4. Evidence for this claim comes from experiments using laboratory rats (Greco et al., 2000) and mice (Basheer et al., 1997). In both species, forced wakefulness produces an increase in Fos expression in the BF, but this pattern declines rapidly following 1–2 hours of recovery sleep. Furthermore, in laboratory rats, recovery sleep particularly decreases activation of ACh cells of the VDB and HDB (Greco et al., 2000). Thus, it is possible that at ZT 4 the similar pattern of Fos expression across groups in nACh and ACh cells of the HDB is a consequence of the sleep that occurs towards the end of the dark phase.
In sharp contrast to the results obtained during the day, during the night Fos expression in ACh and nACh cells in the HDB was higher in both groups of animals with wheels than in animals without them (Fig. 2 and 5). This was likely due to a decrease in Fos expression from day to night in the Cs, combined with either an increase in Fos expression at night (i.e., in ACh and nACh cells of NAs and in the nACh cells of DA animals) or the maintenance of day time levels of expression when the animals were sampled at ZT 16 (i.e., in ACh cells of DAs). Thus, in general enhanced Fos expression in the HDB corresponded to the pattern of wakefulness or activity for the NAs and the Cs, but that relationship was absent in the DAs. This suggests that after many days of access to a running wheel the functioning of these cells becomes decoupled from wheel running.
The results of neuronal activation in the HDB of animals with wheel access contrast with those reported for the orexinergic system of these animals. In the orexigenic arousal system, expression of Fos is predominantly determined by the temporal display of activity: it is high at night for NAs and high during the day for DAs (Nixon and Smale, 2004). Thus, these results, together with the lack of significant correlations between Fos expression in the HDB and Fos expression in the orexin cells (Table 1), serve to argue against a prominent role for the orexinergic system in inducing Fos expression in the HDB of grass rats with access to wheels. This is in spite of anatomical evidence that orexinergic fibers appear to make contacts with ACh and nACh cell in the HDB of grass rats (Fig. 6). Considering the role of the orexins in the activation of serotonergic, histaminergic, and norepinephrinergic arousal systems (reviewed in Saper et al., 2001), our results raise the possibility that other arousal systems independent of orexinergic influences play a role in the activation of HDB cells seen here.
An outcome of enhanced neural activity at night in the HDB of both DAs and NAs could be neural changes in areas that receive input from the HDB. The olfactory bulbs (OBs) and piriform cortex (Pir), which are components of the olfactory system, receive ACh and GABAergic projections from the HDB (Luskin and Price, 1982; Mesulam et al., 1983; Zaborszky et al., 1986). In laboratory rats, Fos expression in ACh cells of the BF is associated with release of acetylcholine at their terminals (Greco et al., 1999; Greco et al., 2000). If this is also true for our animals with wheel access, it suggests that the OBs and Pir are being stimulated by the release of acetylcholine during the normal resting phase. Likewise, the GABAergic cellular group of DAs and NAs could be stimulated to release GABA at night in animals with wheel access, independently of the preferred phase of the day-night cycle for the display of activity. The release of these neurotransmitters in the OBs and Pir could alter functions modulated by these areas, such as olfactory processing in the case of the Pir and OBs (reviewed in Wilson et al., 2004), and thermoregulation, sexual behavior, and circadian rhythmicity in the case of the OBs (Brunjes, 1992). In laboratory rats, ACh cells of the BF also send projections to the main circadian pacemaker of the suprachiasmatic nucleus (SCN; Bina et al., 1993), and nocturnal release of ACh in the SCN can shift its functioning in a time dependent manner (i.e. phase advances throughout the night; Buchanan and Gillette, 2005). In grass rats, the shift to a nocturnal pattern of activity does not affect the phase of SCN rhythms (Blanchong et al., 1999; Rose et al., 1999; Schwartz and Smale, 2005), although it appears to activate at least some ACh cells of the BF (i.e., those of the HDB, as shown here). A lack of a phase shift in SCN functioning may be due to the fact that in sharp contrast to laboratory rats, the SCN of grass rats receives only a few ACh projections (Castillo-Ruiz and Nunez, 2007), and these are not likely to originate in the BF (A. Castillo-Ruiz, unpublished observations).
MS and VDB
In the MS and VDB of all groups there was a rostro-caudal gradient of Fos expression in nACh cells, with more labeled cells present in the caudal sections. The BF, including the MS and VDB, is organized according to rostro-caudal bands that coil along the axis, and each band is proposed to contain sub-bands of neurons that produce specific neurotransmitters and peptides (Zaborszky and Duque, 2003). Therefore, our results may reflect that different cell groups across the rostro-caudal axis of the BF contribute differentially to neural activation of the BF, which may have in turn differential effects in the functioning of brain areas that receive inputs from these cellular groups.
Since in laboratory rats, ACh cells of the VDB, but not those of the MS, show increased Fos expression after prolonged waking (Greco et al., 2000), we expected to find that our NAs would show increased Fos expression in ACh cells of the VDB during their active time: the dark phase. However, we did not find effects of group or ZT on Fos expression in ACh cells in either the VDB or the MS. The discrepancy between Greco’s results and ours in the VDB could be because the physiological effects produced in the VDB by voluntary activity maybe fundamentally different from those produced by forced wakefulness. That is, forced wakefulness is likely to be more stressful and could induce more Fos expression in the BF than voluntary wakefulness during the normal resting phase. The MS and VDB of mice and laboratory rats contain the corticotropin-releasing hormone (CRH) receptor 1 (Radulovic et al., 1998), and in mice there is a high degree of colocalization of this receptor and ACh in neurons in both areas (Sauvage and Steckler, 2001). Furthermore, in laboratory rats CRH stimulates neuronal activity in the MS and VDB (Osada, 1997). Thus, experimental wakefulness paradigms may induce widespread Fos expression in ACh cells of the VDB due to the actions of CRH on these neurons, but only when stress responses are involved. Another possibility is that an acute episode of wakefulness during the resting phase activates more ACh neuronal groups than chronic exposure does. In Greco’s study the animals were exposed to two hours of sleep deprivation, whereas in this study our NA animals were running in wheels and showing wheel running during the night for a month. In addition, our animals were not likely to be sleep deprived, due to their redistribution of sleep, which involves a compensatory increase in daytime sleep bouts (Schwartz and Smale, 2005).
In sharp contrast to our results for the ACh cells of the MS and VDB, we found statistically significant group differences in Fos expression in nACh cells in these areas, but only at night (Fig. 3–5). As discussed previously for the HDB, a potential explanation for the lack of group differences at ZT 4 is the resetting effect of the sleep that occurs late at night in all grass rats, and that may result in a return to baseline neural activity in the BF, regardless of group differences in nocturnal locomotor activity. During the night, Fos expression in nACh cells of the VDB was higher in both groups of animals with wheels than in those without wheels, but in the MS nocturnal Fos expression was high only in NAs. As with the HDB, group differences in the VDB at ZT 16 appear to be due to a relative reduction in Fos expression between ZT 4 and ZT 16 for the Cs, combined with the opposite trend occurring during that time in NAs, and with the maintenance of daytime levels in DAs.
The MS and VDB project densely to the hippocampus, and ACh, GABAergic, as well as glutamatergic neurons of the MS and VDB modulate the HPC-θ (Leung and Shen, 2004; Yoder and Pang, 2005). The HPC-θ is seen when animals engage in voluntary behaviors, including wheel running , and in laboratory rats integrity of the MS connection to the hippocampus is needed not only to maintain the HPC-θ, but also to support wheel running (Oddie et al., 1996). These data suggest that the septal-hippocampal pathway is important for the modulation of wheel-running intensity. Interestingly, NA grass rats show substantially higher levels of wheel running when compared to DAs (Blanchong et al., 1999); and in the present study, that group had the highest levels of Fos expression in the MS. Thus, our results suggest that the high levels of running seen in NAs might be supported by this septal-hippocampal pathway.
In contrast to the results for the HDB, there appears to be a relationship between the expression of Fos in orexin producing cells and Fos expression in the ACh and nACh of the MS and VDB, but only for NAs (Table 1). Thus for these regions of the BF, the somewhat similar profiles of Fos expression seen in NAs and DAs may in fact reflect the activation of different neural systems or that those neural systems are modulating BF neural activation in different ways. A role for the orexins in inducing Fos expression in the BF of NAs at ZT 16 is not surprising since at that time these animals are awake and very active (Blanchong et al., 1999). The DAs, on the other hand, show frequent bouts of sleep at night (Schwartz and Smale, 2005) and little Fos expression in orexin neurons at ZT 16 (Nixon and Smale, 2004). This is consistent with the view that neural systems independent of orexinergic activation drive Fos expression in the BF of the DAs. One possibility related to this idea is that at least for DAs Fos patterns may be induced by the activation of cells of the BF that play a role in paradoxical sleep. This is because cells of the BF are not only active during wakefulness, but also during this sleep stage (Lee et al., 2005).
Our anatomical data revealed that OXA containing fibers form putative appositions with ACh and nACh neurons in all of the areas studied, regardless of wheel access or chronotype, suggesting the potential for functional synaptic relationships between OXA-containing fibers and ACh or nACh neurons in these regions. It is possible, however, that differences between groups exist at the quantitative level, in that the number of synaptic contacts between OXA fibers and cell groups of the BF might differ. That possibility could explain differences in BF activation between DAs and NAs. However, in the present study we did not attempt to quantify the number of apparent contacts between OXA-containing fibers and ACh or nACh neurons.
Experiment 2: Activation of reward systems
We examined whether temporal patterns of wheel running were associated with patterns of Fos expression in TH cells of the VTA and SUM. However, none of the comparisons revealed significant differences. Furthermore, double-labeled cells were very rare (Fig. 8). While there are several potential explanations for the lack of double-labeled cells in the VTA and SUM of all groups, it is unlikely that the lack of double-labeling was due to a failure of the immunocytochemical procedure, since double-labeled cells were easily identified in the periaqueductal gray of the same sections (data not shown). One possible explanation for these results is that neural activation in dopaminergic cells of the reward system is only seen during the initial stage of running wheel access, and not after continuous access for several days or weeks. In laboratory rats, Fos expression in TH cells of the VTA is seen after acute exposure to voluntary wheel-running (Yanagita et al., 2007), as well as after acute exposure to other rewarding stimuli, especially in the rostral VTA (Asmus and Newman, 1994; Balfour et al., 2004). However, when animals are exposed repeatedly for several days to rewarding stimulus such as cocaine, there is a reduction in c-fos mRNA in the caudate putamen, an area which receives dopaminergic inputs (Ennulat et al., 1994). These data suggest that even though Fos expression in dopaminergic neurons is seen after acute reward presentations, repeated exposure to a rewarding stimulus can produce attenuation of the induction of Fos expression in dopamine cells of areas related to the reward system, which might be related to habituation to the stimulus. Thus, our results might reflect attenuation in Fos expression in the dopaminergic reward system induced by chronic exposure to the wheel, after an initial increase when the wheels were first introduced, resulting in uniform low Fos expression in TH cells across groups. Finally, another potential explanation is that in dopaminergic cells, early genes other than Fos are expressed as a result of voluntary wheel running.
In contrast to our observations in TH cells, animals with wheels showed an increase in Fos expression in nTH cells of the aVTA, and did so regardless of chronotype (Fig. 7). This result is in agreement with those of other studies, which found that after acute exposure to a rewarding stimulus there is a region-specific expression of Fos in nTH cells of the VTA, with more Fos seen in rostral areas than in caudal areas (Hunt and McGregor, 1998; Balfour et al., 2004). Similarly in the SUM, we observed that DAs and NAs expressed more Fos in nTH cells than did the Cs (Fig. 7). These results are interesting, given the role that the SUM appears to play in the reward pathway. For example, administration of the glutamate receptor agonist alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) into the SUM, but not the VTA, produces rewarding effects (Ikemoto et al., 2004). Furthermore, they suggest that reward experienced over a relatively long interval may not involve activation of dopaminergic cell bodies. However, since some of the rewarding effects mediated by the SUM involve enhance release of dopamine from axonal terminals in the NAc (Ikemoto et al., 2004), we can not rule out a role for dopamine in the rewarding properties of long-term access to a running wheel.
Expected consequences of the neural activation of components of the reward system induced by wheel access are neural changes in regions that receive inputs from these areas. Components of the reward system, including the SUM and the VTA, send efferent projections to the BF (Gaykema and Zaborszky, 1996; Pan and McNaughton, 2004), and modulate the neural activity of that region. For example, stimulation of the VTA induces Fos expression in the MS and VDB (Sandner et al., 1992). Moreover, stimulation of the SUM is likely to have effects on the BF since it sends projections to cells of the MS and VDB (Pan and McNaughton, 2004). Thus, the patterns of Fos expression seen in some regions of the BF of grass rats with access to wheels may be in part due to a tonic increase in the activity of the SUM and aVTA. This contribution may be of particular significance with respect to Fos expression at night in the BF of DAs, which appears to be independent of the orexinergic system. In addition, changes in modulation of vigilance states are expected, given the participation of the VTA and SUM in this function.
Summary and conclusions
One of the main findings of this study is that when diurnal grass rats shift to a night-active pattern of activity, the effects of that change are not uniform across arousal systems. Thus, although ACh cells of the HDB show a nocturnal increase in Fos expression in animals that are voluntarily active at night, that effect is not seen in other ACh cell groups of the BF known to contribute to the support of wakefulness (i.e., the MS and the VDB). An increase in nocturnal Fos expression in NA animals, similar to that seen in the orexinergic system (Nixon and Smale, 2004) was almost universally seen in the nACh cells of all regions of the BF, but apparently only associated with activation of the orexinergic system for the MS and the VDB, thus providing further evidence for functional differences in how brain arousal systems respond to a shift in phase preference.
One unexpected observation of this study was that for some of the areas and cell types, but not all (see the data for the MS) the temporal patterns, and/or overall levels, of Fos expression of DA animals with wheel access appear similar to those of NAs. This may be due to an effect of voluntary exercise that is seen regardless of a shift in phase preference for the display of activity. These similarities in patterns of Fos expression between DA and NA animals may nevertheless stem from the activation of different neural systems, since Fos expression in the MS and VDB was only correlated with Fos expression in the orexinergic system in the case of NAs, and activation of the BF has been associated with the display of paradoxical sleep as well as wakefulness (Lee et al., 2005). Also common to both groups with wheels was an increase in Fos expression in two specific areas of the brain reward system that also participate in arousal, the SUM and aVTA. This effect appears to reflect a tonic upregulation of these brain regions, and was not affected by either ZT or by the display of day-or night-active patterns of activity.
Because the BF and the brain reward system project widely throughout the brain, the effects of altered neural activation in those regions are likely to be seen in a broad range of neural functions and behaviors. For example, since the MS, VDB and SUM project to the hippocampus, effects on learning and memory processes may be expected. In addition, changes in sensory processes and motivation are likely to occur given the projections of the HDB and those of the reward system, respectively. The present study suggests these effects may also occur in other diurnal species, including humans, since voluntary exercise and voluntary display of activity during the night (e.g., shift work) are widespread practices around the world (Rajaratnam and Arendt, 2001). Therefore, the diurnal grass rat represents an interesting model to investigate how daily amount and temporal distribution of voluntary activity affects the functioning of brain systems that modulate a variety of important functions including vigilance states and motivation.
Acknowledgements
We would like to thank Dr. Cynthia Jordan, Dr. Sharleen Sakai, Dr. Chidambaram Ramanathan, Molly Skaer, Anna Baumgras, and Jessica Schrader for their valuable technical assistance and comments on earlier versions of this paper. This study was supported by the National Institute of Mental Health grant MH 53433 and Minnesota Craniofacial Research Training Program Grant T32DE007288 from the National Institute of Dental & Craniofacial Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Dental & Craniofacial Research or the National Institutes of Mental Health.
Abbreviations
- 3V
third ventricle
- AB complex
avidin-biotin peroxidase complex
- ac
anterior commissure
- ACh
cholinergic
- AMPA
alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid
- ANOVA
analysis of variance
- aVTA
anterior ventral tegmental area
- BF
basal forebrain
- C
control
- ChAT
choline acetyltransferase
- cp
cerebral peduncle
- CRH
corticotropin-releasing hormone
- DA
day-active
- DAB
diaminobenzidine
- fr
fasciculus retroflexus
- GABA
gamma-amino-n-butyric acid
- HDB
horizontal diagonal band of Broca
- HPC-θ
hippocampal theta wave
- IP
interpeduncular nucleus
- LV
lateral ventricle
- mp
mammillary peduncle
- MS
medial septum
- NA
night-active
- NAc
nucleus accumbens
- nACh
non-cholinergic
- NDS
normal donkey serum
- NHS
normal horse serum
- nTH
non-tyrosine hydroxylase
- OB
olfactory bulb
- OX
optic chiasm
- OXA
orexin A
- OXB
orexin B
- PBS
phosphate-buffered saline
- Pir
piriform cortex
- PLP
paraformaldehyde-lysine-sodium periodate
- pm
principal mammillary tract
- Pn
pontine nuclei
- SCN
suprachiasmatic nucleus
- SUM
supramammillary nucleus
- TH
tyrosine hydroxylase
- VDB
vertical diagonal band of Broca
- VTA
ventral tegmental area
- ZT
Zeitgeber time
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asmus SE, Newman SW. Colocalization of tyrosine hydroxylase and Fos in the male Syrian hamster brain following different states of arousal. J Neurobiol. 1994;25:156–168. doi: 10.1002/neu.480250207. [DOI] [PubMed] [Google Scholar]
- Balfour ME, Yu L, Coolen LM. Sexual behavior and sex-associated environmental cues activate the mesolimbic system in male rats. Neuropsychopharmacology. 2004;29:718–730. doi: 10.1038/sj.npp.1300350. [DOI] [PubMed] [Google Scholar]
- Basheer R, Sherin JE, Saper CB, Morgan JI, McCarley RW, Shiromani PJ. Effects of sleep on wake-induced c-fos expression. J Neurosci. 1997;17:9746–9750. doi: 10.1523/JNEUROSCI.17-24-09746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bina KG, Rusak B, Semba K. Localization of cholinergic neurons in the forebrain and brainstem that project to the suprachiasmatic nucleus of the hypothalamus in rat. J Comp Neurol. 1993;335:295–307. doi: 10.1002/cne.903350212. [DOI] [PubMed] [Google Scholar]
- Blanchong JA, McElhinny TL, Mahoney MM, Smale L. Nocturnal and diurnal rhythms in the unstriped Nile rat, Arvicanthis niloticus. J Biol Rhythms. 1999;14:364–377. doi: 10.1177/074873099129000777. [DOI] [PubMed] [Google Scholar]
- Brunjes PC. Lessons from lesions: the effects of olfactory bulbectomy. Chemical senses. 1992;17:729–763. [Google Scholar]
- Buchanan GF, Gillette MU. New light on an old paradox: site-dependent effects of carbachol on circadian rhythms. Exp Neurol. 2005;193:489–496. doi: 10.1016/j.expneurol.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Castillo-Ruiz A, Nunez AA. Cholinergic projections to the suprachiasmatic nucleus and lower subparaventricular zone of diurnal and nocturnal rodents. Brain Res. 2007;1151:91–101. doi: 10.1016/j.brainres.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Dahan L, Astier B, Vautrelle N, Urbain N, Kocsis B, Chouvet G. Prominent burst firing of dopaminergic neurons in the ventral tegmental area during paradoxical sleep. Neuropsychopharmacology. 2007;32:1232–1241. doi: 10.1038/sj.npp.1301251. [DOI] [PubMed] [Google Scholar]
- Eikelboom R, Lattanzio SB. Wheel access duration in rats: II. Day-night and within-session changes. Behav Neurosci. 2003;117:825–832. doi: 10.1037/0735-7044.117.4.825. [DOI] [PubMed] [Google Scholar]
- Ennulat DJ, Babb S, Cohen BM. Persistent reduction of immediate early gene mRNA in rat forebrain following single or multiple doses of cocaine. Brain Res Mol Brain Res. 1994;26:106–112. doi: 10.1016/0169-328x(94)90080-9. [DOI] [PubMed] [Google Scholar]
- Gaykema RP, Zaborszky L. Direct catecholaminergic-cholinergic interactions in the basal forebrain .II. Substantia nigra-ventral tegmental area projections to cholinergic neurons. Journal of Comparative Neurology. 1996;374:555–577. doi: 10.1002/(SICI)1096-9861(19961028)374:4<555::AID-CNE6>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Gerashchenko D, Salin-Pascual R, Shiromani PJ. Effects of hypocretin-saporin injections into the medial septum on sleep and hippocampal theta. Brain Res. 2001;913:106–115. doi: 10.1016/s0006-8993(01)02792-5. [DOI] [PubMed] [Google Scholar]
- Greco MA, McCarley RW, Shiromani PJ. Choline acetyltransferase expression during periods of behavioral activity and across natural sleep-wake states in the basal forebrain. Neuroscience. 1999;93:1369–1374. doi: 10.1016/s0306-4522(99)00201-8. [DOI] [PubMed] [Google Scholar]
- Greco MA, Lu J, Wagner D, Shiromani PJ. c-Fos expression in the cholinergic basal forebrain after enforced wakefulness and recovery sleep. Neuroreport. 2000;11:437–440. doi: 10.1097/00001756-200002280-00002. [DOI] [PubMed] [Google Scholar]
- Henny P, Jones BE. Projections from basal forebrain to prefrontal cortex comprise cholinergic, GABAergic and glutamatergic inputs to pyramidal cells or interneurons. Eur J Neurosci. 2008;27:654–670. doi: 10.1111/j.1460-9568.2008.06029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman GE, Le WW. Just cool it! Cryoprotectant anti-freeze in immunocytochemistry and in situ hybridization. Peptides. 2004;25:425–431. doi: 10.1016/j.peptides.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Hunt GE, McGregor IS. Rewarding brain stimulation induces only sparse Fos-like immunoreactivity in dopaminergic neurons. Neuroscience. 1998;83:501–515. doi: 10.1016/s0306-4522(97)00409-0. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res Brain Res Rev. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Witkin BM, Zangen A, Wise RA. Rewarding effects of AMPA administration into the supramammillary or posterior hypothalamic nuclei but not the ventral tegmental area. J Neurosci. 2004;24:5758–5765. doi: 10.1523/JNEUROSCI.5367-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BE. Modulation of cortical activation and behavioral arousal by cholinergic and orexinergic systems. Ann N Y Acad Sci. 2008;1129:26–34. doi: 10.1196/annals.1417.026. [DOI] [PubMed] [Google Scholar]
- Katona C, Smale L. Wheel-running rhythms in Arvicanthis niloticus. Physiol Behav. 1997;61:365–372. doi: 10.1016/s0031-9384(96)00407-6. [DOI] [PubMed] [Google Scholar]
- Lee MG, Hassani OK, Alonso A, Jones BE. Cholinergic basal forebrain neurons burst with theta during waking and paradoxical sleep. J Neurosci. 2005;25:4365–4369. doi: 10.1523/JNEUROSCI.0178-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung LS, Shen B. Glutamatergic synaptic transmission participates in generating the hippocampal EEG. Hippocampus. 2004;14:510–525. doi: 10.1002/hipo.10199. [DOI] [PubMed] [Google Scholar]
- Luskin MB, Price JL. The distribution of axon collaterals from the olfactory bulb and the nucleus of the horizontal limb of the diagonal band to the olfactory cortex, demonstrated by double retrograde labeling techniques. J Comp Neurol. 1982;209:249–263. doi: 10.1002/cne.902090304. [DOI] [PubMed] [Google Scholar]
- Marcangione C, Rompre PP. Topographical Fos induction within the ventral midbrain and projection sites following self-stimulation of time posterior mesencephalon. Neuroscience. 2008;154:1227–1241. doi: 10.1016/j.neuroscience.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Martinez GS, Smale L, Nunez AA. Diurnal and nocturnal rodents show rhythms in orexinergic neurons. Brain Res. 2002;955:1–7. doi: 10.1016/s0006-8993(02)03264-x. [DOI] [PubMed] [Google Scholar]
- McElhinny TL, Smale L, Holekamp KE. Patterns of body temperature, activity, and reproductive behavior in a tropical murid rodent, Arvicanthis niloticus. Physiol Behav. 1997;62:91–96. doi: 10.1016/s0031-9384(97)00146-7. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Wainer BH, Levey AI. Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1-Ch6) Neuroscience. 1983;10:1185–1201. doi: 10.1016/0306-4522(83)90108-2. [DOI] [PubMed] [Google Scholar]
- Murphy HM, Wideman CH, Nadzam GR. A laboratory animal model of human shift work. Integr Physiol Behav Sci. 2003;38:316–328. doi: 10.1007/BF02688860. [DOI] [PubMed] [Google Scholar]
- Nixon JP, Smale L. Individual differences in wheel-running rhythms are related to temporal and spatial patterns of activation of orexin A and B cells in a diurnal rodent (Arvicanthis niloticus) Neuroscience. 2004;127:25–34. doi: 10.1016/j.neuroscience.2004.04.052. [DOI] [PubMed] [Google Scholar]
- Nixon JP, Smale L. A comparative analysis of the distribution of immunoreactive orexin A and B in the brains of nocturnal and diurnal rodents. Behav Brain Funct. 2007;3:28. doi: 10.1186/1744-9081-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak CM, Smale L, Nunez AA. Fos expression in the sleep-active cell group of the ventrolateral preoptic area in the diurnal murid rodent, Arvicanthis niloticus. Brain Res. 1999;818:375–382. doi: 10.1016/s0006-8993(98)01319-5. [DOI] [PubMed] [Google Scholar]
- Novak CM, Smale L, Nunez AA. Rhythms in Fos expression in brain areas related to the sleep-wake cycle in the diurnal Arvicanthis niloticus. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1267–R1274. doi: 10.1152/ajpregu.2000.278.5.R1267. [DOI] [PubMed] [Google Scholar]
- Oddie SD, Stefanek W, Kirk IJ, Bland BH. Intraseptal procaine abolishes hypothalamic stimulation-induced wheel-running and hippocampal theta field activity in rats. J Neurosci. 1996;16:1948–1956. doi: 10.1523/JNEUROSCI.16-05-01948.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osada T. Effects of CRH and LHRH on rat septo-hippocampal neurons. Endocr J. 1997;44:519–525. doi: 10.1507/endocrj.44.519. [DOI] [PubMed] [Google Scholar]
- Pan WX, McNaughton N. The supramammillary area: its organization, functions and relationship to the hippocampus. Prog Neurobiol. 2004;74:127–166. doi: 10.1016/j.pneurobio.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 3rd Edition. San Diego: Academic Press; 1997. [Google Scholar]
- Radulovic J, Sydow S, Spiess J. Characterization of native corticotropin-releasing factor receptor type 1 (CRFR1) in the rat and mouse central nervous system. J Neurosci Res. 1998;54:507–521. doi: 10.1002/(SICI)1097-4547(19981115)54:4<507::AID-JNR8>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Rajaratnam SM, Arendt J. Health in a 24-h society. Lancet. 2001;358:999–1005. doi: 10.1016/S0140-6736(01)06108-6. [DOI] [PubMed] [Google Scholar]
- Rose S, Novak CM, Mahoney MM, Nunez AA, Smale L. Fos expression within vasopressin-containing neurons in the suprachiasmatic nucleus of diurnal rodents compared to nocturnal rodents. J Biol Rhythms. 1999;14:37–46. doi: 10.1177/074873099129000425. [DOI] [PubMed] [Google Scholar]
- Salgado-Delgado R, Angeles-Castellanos M, Buijs MR, Escobar C. Internal desynchronization in a model of night-work by forced activity in rats. Neuroscience. 2008;154:922–931. doi: 10.1016/j.neuroscience.2008.03.066. [DOI] [PubMed] [Google Scholar]
- Sandner G, Di Scala G, Rocha B, Angst MJ. C-fos immunoreactivity in the brain following unilateral electrical stimulation of the dorsal periaqueductal gray in freely moving rats. Brain Res. 1992;573:276–283. doi: 10.1016/0006-8993(92)90773-3. [DOI] [PubMed] [Google Scholar]
- Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24:726–731. doi: 10.1016/s0166-2236(00)02002-6. [DOI] [PubMed] [Google Scholar]
- Sauvage M, Steckler T. Detection of corticotropin-releasing hormone receptor 1 immunoreactivity in cholinergic, dopaminergic and noradrenergic neurons of the murine basal forebrain and brainstem nuclei--potential implication for arousal and attention. Neuroscience. 2001;104:643–652. doi: 10.1016/s0306-4522(01)00137-3. [DOI] [PubMed] [Google Scholar]
- Schilstrom B, Svensson HM, Svensson TH, Nomikos GG. Nicotine and food induced dopamine release in the nucleus accumbens of the rat: putative role of alpha7 nicotinic receptors in the ventral tegmental area. Neuroscience. 1998;85:1005–1009. doi: 10.1016/s0306-4522(98)00114-6. [DOI] [PubMed] [Google Scholar]
- Schwartz MD, Smale L. Individual differences in rhythms of behavioral sleep and its neural substrates in Nile grass rats. J Biol Rhythms. 2005;20:526–537. doi: 10.1177/0748730405280924. [DOI] [PubMed] [Google Scholar]
- Sherwin CM. Voluntary wheel running: a review and novel interpretation. Anim Behav. 1998;56:11–27. doi: 10.1006/anbe.1998.0836. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Fletcher ML, Sullivan RM. Acetylcholine and olfactory perceptual learning. Learn Mem. 2004;11:28–34. doi: 10.1101/lm.66404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Zaborszky L, Hajszan T, van den Pol AN, Alreja M. Hypocretin/orexin innervation and excitation of identified septohippocampal cholinergic neurons. J Neurosci. 2004;24:3527–3536. doi: 10.1523/JNEUROSCI.5364-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagita S, Amemiya S, Suzuki S, Kato Y, Kubota N, Kita I. The comparison of acute spontaneous and forced exercise on activation of several brain areas related to psychological responses. Neuroscience Research. 2007;58:S165–S165. [Google Scholar]
- Yoder RM, Pang KC. Involvement of GABAergic and cholinergic medial septal neurons in hippocampal theta rhythm. Hippocampus. 2005;15:381–392. doi: 10.1002/hipo.20062. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Yokoo H, Mizoguchi K, Kawahara H, Tsuda A, Nishikawa T, Tanaka M. Eating and drinking cause increased dopamine release in the nucleus accumbens and ventral tegmental area in the rat: measurement by in vivo microdialysis. Neurosci Lett. 1992;139:73–76. doi: 10.1016/0304-3940(92)90861-z. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Duque A. Sleep-wake mechanisms and basal forebrain circuitry. Front Biosci. 2003;8:d1146–d1169. doi: 10.2741/1112. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Carlsen J, Brashear HR, Heimer L. Cholinergic and GABAergic afferents to the olfactory bulb in the rat with special emphasis on the projection neurons in the nucleus of the horizontal limb of the diagonal band. J Comp Neurol. 1986;243:488–509. doi: 10.1002/cne.902430405. [DOI] [PubMed] [Google Scholar]