Abstract
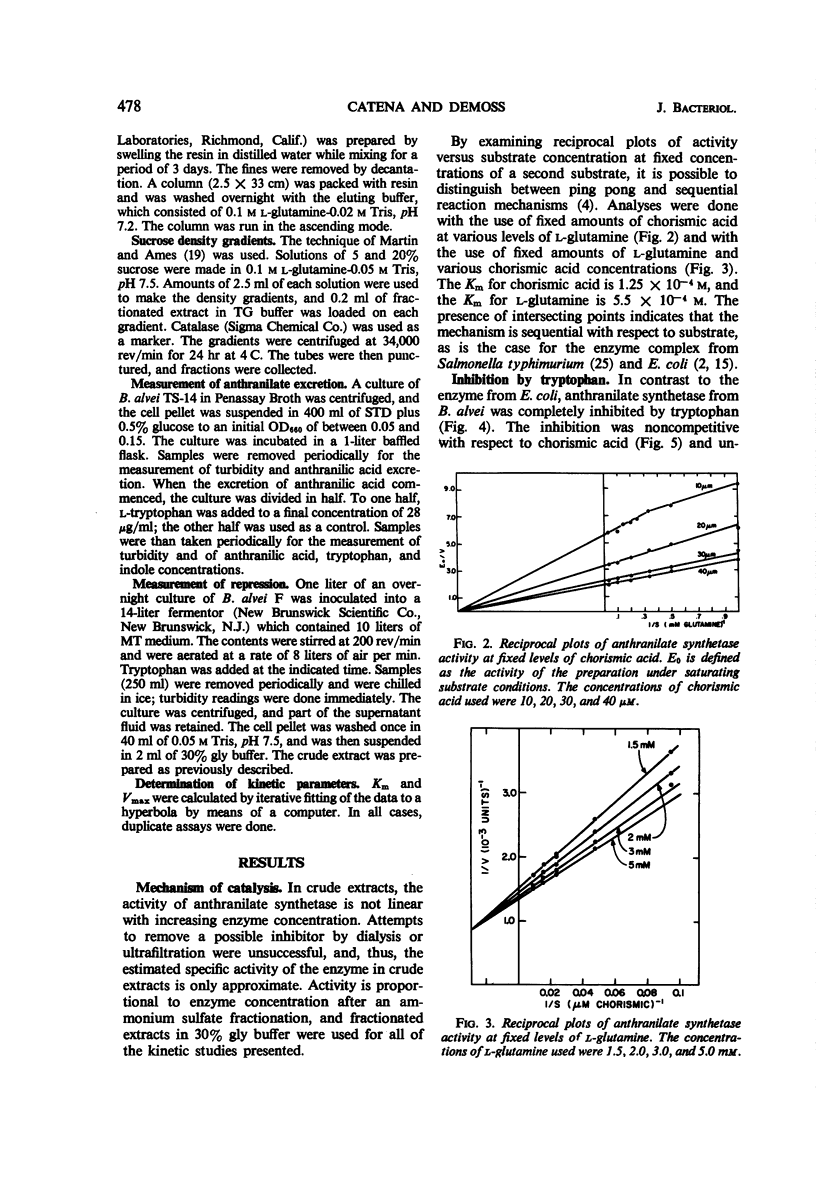
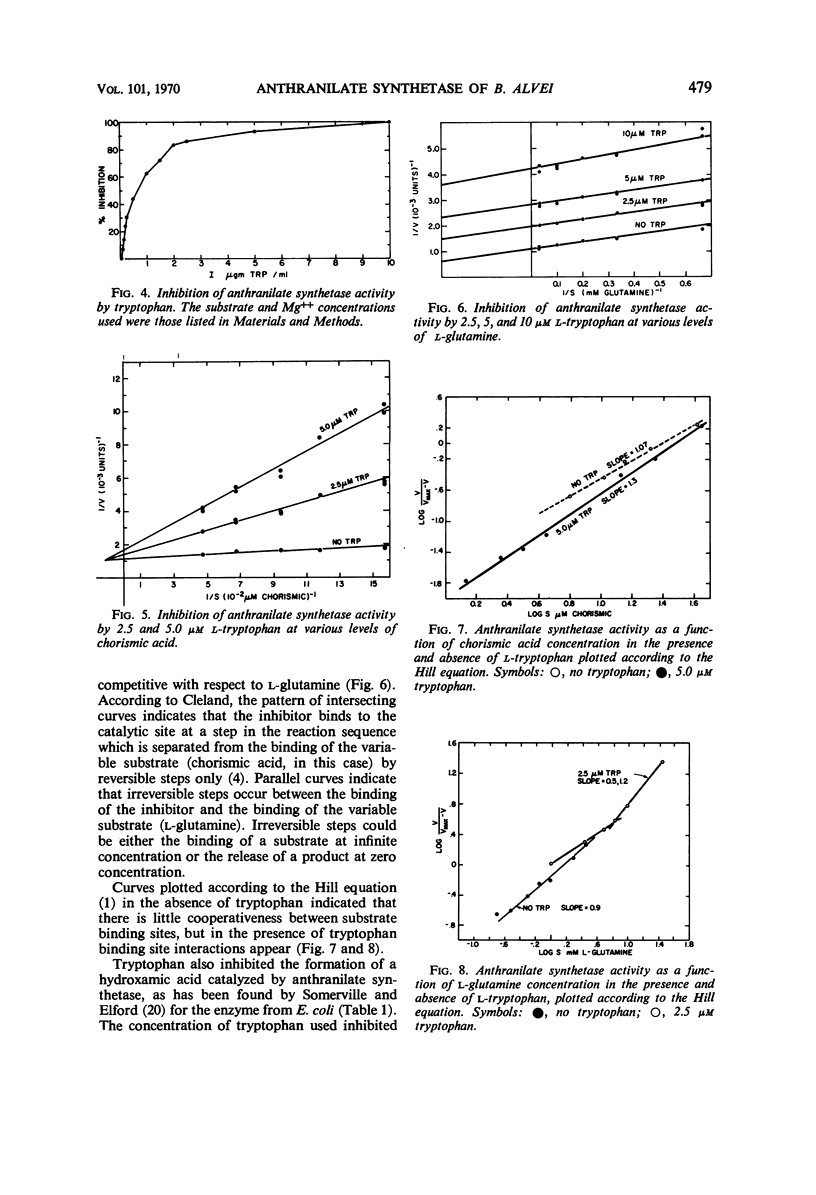
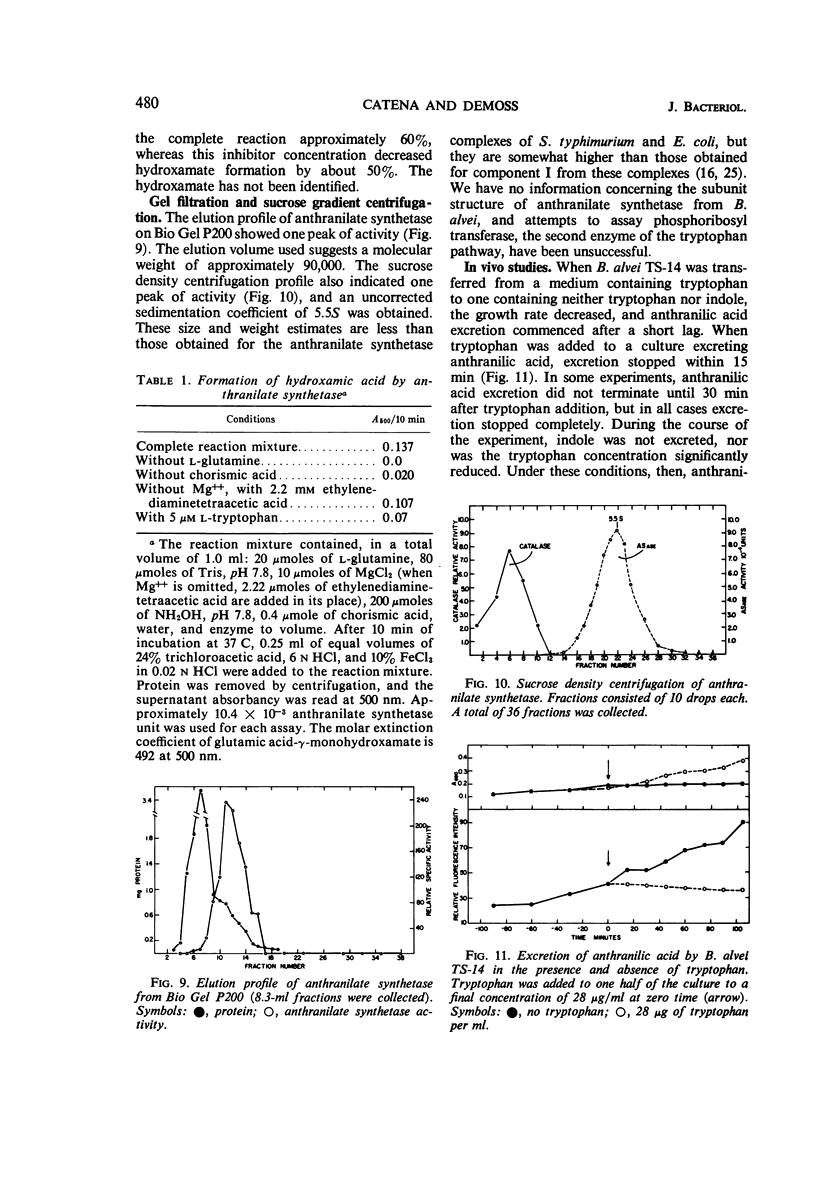
Anthranilate synthetase from Bacillus alvei was partially purified by ammonium sulfate fractionation and was stabilized by glycerol. The reaction mechanism of the enzyme was found to be sequential with respect to substrate, and the enzyme formed a hydroxamic acid in the absence of Mg++. The Km for chorismic acid was 1.25 × 10−4m, and the Km for l-glutamine was 5.5 × 10−4m. Enzyme activity was inhibited by tryptophan noncompetitively with respect to chorismic acid and uncompetitively with respect to l-glutamine. An analysis of the inhibition patterns indicated that tryptophan may act as a dead end inhibitor and bind at the catalytic site. Enzyme activity could be completely inhibited in vitro and in vivo under the appropriate conditions, and enzyme synthesis was sensitive to repression by tryptophan. A sedimentation coefficient of 5.5S and an estimated molecular weight of 90,000 were obtained for the enzyme.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATKINSON D. E., HATHAWAY J. A., SMITH E. C. KINETICS OF REGULATORY ENZYMES. KINETIC ORDER OF THE YEAST DIPHOSPHOPYRIDINE NUCLEOTIDE ISOCITRATE DEHYDROGENASE REACTION AND A MODEL FOR THE REACTION. J Biol Chem. 1965 Jun;240:2682–2690. [PubMed] [Google Scholar]
- Baker T. I., Crawford I. P. Anthranilate synthetase. Partial purification and some kinetic studies on the enzyme from Escherichia coli. J Biol Chem. 1966 Dec 10;241(23):5577–5584. [PubMed] [Google Scholar]
- Bauerle R. H., Margolin P. A multifunctional enzyme complex in the tryptophan pathway of Salmonella typhimurium: comparison of polarity and pseudopolarity mutations. Cold Spring Harb Symp Quant Biol. 1966;31:203–214. doi: 10.1101/sqb.1966.031.01.028. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. III. Prediction of initial velocity and inhibition patterns by inspection. Biochim Biophys Acta. 1963 Feb 12;67:188–196. doi: 10.1016/0006-3002(63)91816-x. [DOI] [PubMed] [Google Scholar]
- DEMOSS J. A. THE CONVERSION OF SHIKIMIC ACID TO ANTHRANILIC ACID BY EXTRACTS OF NEUROSPORA CRASSA. J Biol Chem. 1965 Mar;240:1231–1235. [PubMed] [Google Scholar]
- FRANK L. H., DEMOSS R. D. Specific enzymic method for the estimation of L-tryptophan. Arch Biochem Biophys. 1957 Apr;67(2):387–397. doi: 10.1016/0003-9861(57)90293-x. [DOI] [PubMed] [Google Scholar]
- Gibson F. Chorismic acid: purification and some chemical and physical studies. Biochem J. 1964 Feb;90(2):256–261. doi: 10.1042/bj0900256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson F., Pittard J. Pathways of biosynthesis of aromatic amino acids and vitamins and their control in microorganisms. Bacteriol Rev. 1968 Dec;32(4 Pt 2):465–492. [PMC free article] [PubMed] [Google Scholar]
- Gibson M. I., Gibson F. Preliminary studies on the isolation and metabolism of an intermediate in aromatic biosynthesis: chorismic acid. Biochem J. 1964 Feb;90(2):248–256. doi: 10.1042/bj0900248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch J. A., DeMoss R. D. Evidence for a physiological maximal typtophan pool level in Bacillus alvei. J Bacteriol. 1966 Apr;91(4):1655–1656. doi: 10.1128/jb.91.4.1655-1656.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch J. A., DeMoss R. D. Physiological role of tryptophanase in control of tryptophan biosynthesis in Bacillus alvei. J Bacteriol. 1966 Feb;91(2):667–672. doi: 10.1128/jb.91.2.667-672.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch J. A., Demoss R. D. Physiological Effects of a Constitutive Tryptophanase in Bacillus alvei. J Bacteriol. 1965 Sep;90(3):604–610. doi: 10.1128/jb.90.3.604-610.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito J., Cox E. C., Yanofsky C. Anthranilate synthetase, an enzyme specified by the tryptophan operon of Escherichia coli: purification and characterization of component I. J Bacteriol. 1969 Feb;97(2):725–733. doi: 10.1128/jb.97.2.725-733.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito J., Yanofsky C. The nature of the anthranilic acid synthetase complex of Escherichia coli. J Biol Chem. 1966 Sep 10;241(17):4112–4114. [PubMed] [Google Scholar]
- Jensen R. A., Nasser D. S., Nester E. W. Comparative control of a branch-point enzyme in microorganisms. J Bacteriol. 1967 Nov;94(5):1582–1593. doi: 10.1128/jb.94.5.1582-1593.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Somerville R. L., Elford R. Hydroxamate formation by anthranilate synthetase of Escherichia coli K12. Biochem Biophys Res Commun. 1967 Aug 7;28(3):437–444. doi: 10.1016/0006-291x(67)90331-2. [DOI] [PubMed] [Google Scholar]
- Wegman J., Crawford I. P. Tryptophan synthetic pathway and its regulation in Chromobacterium violaceum. J Bacteriol. 1968 Jun;95(6):2325–2335. doi: 10.1128/jb.95.6.2325-2335.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolfolk C. A., Shapiro B., Stadtman E. R. Regulation of glutamine synthetase. I. Purification and properties of glutamine synthetase from Escherichia coli. Arch Biochem Biophys. 1966 Sep 26;116(1):177–192. doi: 10.1016/0003-9861(66)90026-9. [DOI] [PubMed] [Google Scholar]
- Zalkin H., Kling D. Anthranilate synthetase. Purification and properties of component I from Salmonella typhimurium. Biochemistry. 1968 Oct;7(10):3566–3573. doi: 10.1021/bi00850a034. [DOI] [PubMed] [Google Scholar]


