Abstract
In a previous immunoproteome analysis of Borrelia hermsii, candidate antigens that bound IgM antibodies from mice and patients infected with relapsing fever spirochetes were identified. One candidate that was identified is a hypothetical protein with a molecular mass of 57 kDa that we have designated Borrelia immunogenic protein A (BipA). This protein was further investigated as a potential diagnostic antigen for B. hermsii given that it is absent from the Borrelia burgdorferi genome. The bipA locus was amplified and sequenced from 39 isolates of B. hermsii that had been acquired from western North America. bipA was also expressed as a recombinant fusion protein. Serum samples from mice and patients infected with B. hermsii or B. burgdorferi were used to confirm the immunogenicity of the recombinant protein in patients infected with relapsing fever spirochetes. Lastly, in silico and experimental analysis indicated that BipA is a surface-exposed lipoprotein in B. hermsii. These findings enhance the capabilities of diagnosing infection with relapsing fever spirochetes.
The first outbreak of tick-borne relapsing fever in the United States was observed in Colorado in 1915 (39). Since then this disease continues to be under-reported and misdiagnosed, while regions where the disease is endemic continue to be identified in the Western and Southern United States (20, 51, 53). The three causative agents of relapsing fever in the United States are Borrelia hermsii, Borrelia turicatae, and Borrelia parkeri; the Borrelia spirochetes are maintained in enzoonotic cycles with their respective tick vectors Ornithodoros hermsi, Ornithodoros turicata, and Ornithodoros parkeri (17, 22). Most reported cases of this disease and all human isolates collected in the United States are due to B. hermsii. Although B. parkeri and B. turicatae have not been isolated from a human, B. turicatae has been implicated in several outbreaks in Texas because of the association of its tick vector with an area where an outbreak occurred (46). B. turicatae has also been isolated from sick dogs (52, 58), further supporting the likelihood of infection to humans.
The spirochetemia during mammalian infection is cyclic with spirochetes escaping the immune response by genetic variation of surface proteins known as variable major proteins (Vmps) (5, 8, 16, 25, 47, 56). During episodes of spirochetemia, the bacteria can reach upwards of 107 spirochetes per ml of blood (13, 15). However, spirochetes are cleared by IgM antibody primarily generated against these Vmps (1-5, 7, 10, 40, 56).
Given the ability to reach high spirochete densities in the blood (13, 15), infection can be diagnosed by microscopy. However, microscopic observation lacks sensitivity if spirochete densities are <104 spirochetes per ml of blood, for instance, when the examination is performed between high spirochetemic episodes (20). Further complicating diagnostic testing in the United States is the presence of B. burgdorferi in areas where relapsing fever spirochetes occur (19-21, 26, 32, 42, 49, 54, 59), with reports of serological cross-reactivity from patients infected with B. burgdorferi to other Borrelia species (35-38, 45).
Previously, a proteomic analysis identified 31 candidate antigens that reacted with IgM from mice and patients infected with B. hermsii (33). Searching GenBank using the basic local alignment search tool (BLAST) indicated that one candidate antigen with a molecular mass of 57 kDa (GenBank accession no. FJ446703) is unique to relapsing fever spirochetes; this has been designated Borrelia immunogenic protein A (BipA). The locus of this antigen was amplified and sequenced from 39 isolates to confirm the presence of bipA in B. hermsii isolates and to determine whether bipA segregated B. hermsii isolates into genomic group I (GGI) or genomic group II (GGII) isolates as previously reported for other loci (43, 52). Also, this gene was expressed in Escherichia coli as a recombinant fusion protein, and its antigenicity was determined using serum samples from mice and patients infected with B. burgdorferi or B. hermsii. Serum generated against this recombinant protein was tested with lysates from Borrelia species and GGI and GGII isolates of B. hermsii. Lastly, in silico and experimental analysis of the protein was performed to determine the surface localization of this protein. These findings identify a new surface protein of B. hermsii that can aid in serodiagnosing infection by these spirochetes.
MATERIALS AND METHODS
Borrelia spirochetes.
We used 23 GGI and 16 GGII isolates of B. hermsii in the present study; the origins of the isolates have previously been described (43, 53, 54). Also, B. turicatae 91E135, B. parkeri RML, Borrelia crocidurae CR2A, Borrelia recurrentis 132, and B. burgdorferi B31 were used in the present study. Relapsing fever spirochetes were maintained in mBSK medium containing 12% rabbit serum (6, 9) and passaged every 3 days when the spirochetes were ∼108 spirochetes per ml as previously described (34).
Immune sera.
Pooled immune sera from three mice infected with B. hermsii DAH were collected 7 days after infection and used in the present study as previously described (33). Also, serum samples were collected 4 weeks after infecting mice by tick bite or needle inoculation with 105 GGII spirochetes YOR or MTW-4. A total of 10 human serum samples were available from patients infected with B. hermsii, and 7 samples were available from patients infected with B. burgdorferi as previously reported (55).
Sequence and in silico analysis of bipA.
The entire locus was amplified in 23 GGI and 16 GGII isolates of B. hermsii using primers flanking the gene (Table 1) . DNA sequences from B. hermsii DAH (for GGI isolates) and MTW-2 (for GGII isolates) were used to design sequencing primers for conserved regions in both genomic group isolates (Table 1). PCR amplification was performed by using a DNA Engine Tetrad (Bio-Rad, Hercules, CA) consisting of 1 cycle at 96°C for 5 min and 35 cycles with a denaturing temperature of 94°C for 30 s, an annealing temperature of 58°C for 30 s, and an extension temperature of 72°C for 2.5 min using a GoTaq Flexi DNA polymerase kit (Promega, Madison, WI). Amplicons were visualized in an agarose gel containing GelRed (Phenix Research Products, Candler, NC) and processed with a PCR purification kit (Qiagen, Inc., Valencia, CA). Sequencing bipA and DNA alignments were performed as previously described (52). In silico translation of bipA and amino acid alignments were performed by using the MacVector 6.0 software package (Oxford Molecular, Oxford, United Kingdom). The prediction algorithms used to indicate if BipA contained a signal peptide, was a surface protein, or contained any conserved motifs were Motif Scan (28) on the MyHits web server, LipoP 1.0 (29), and ScanProsite (18). Also, the EMBOSS-Lite-Sequence Comparison Tool was used for amino acid alignments of BipA (48).
TABLE 1.
Primers used for amplifying, sequencing, and generating the Southern blot probe for bipA
| Primer | Sequence (5′→3′) | Function |
|---|---|---|
| FJ446703-R | AGGACTTACCCTAAAGCTTG | Primer flanking bipA that was used to amplify the gene |
| FJ446703-F | GGAATTTGTATAGGAGGATGG | Primer flanking bipA that was used to amplify the gene |
| FJ446703-R1 | GTCTGAAGCTCTCTTGAGTTGG | Reverse internal sequencing primer |
| FJ446703-R2 | CATTAGCAATCCCATCAGCAAC | Reverse internal sequencing primer |
| FJ446703-R3 | CTCCACCAGCAGCTATAACTCC | Reverse internal sequencing primer |
| FJ446703-F1 | GAGTTTCAGTTGGTGGTTCTGG | Forward internal sequencing primer |
| FJ446703-F2 | ACTTTTGTTACAGCCGCTATGC | Forward internal sequencing primer |
| FJ446703-F3 | TGGAGATGATGATACTGTTGTTGAC | Forward internal sequencing primer |
| FJ446703-R4 | TCTCTATCAACGTCAACAACAGTATCATCA | Primer used for generating the Southern blot probe |
| FJ446703-F4 | TTCTAGGCAGAGTAAATTAGAGAATGGACG | Primer used for generating the Southern blot probe |
Southern blot analysis.
Total genomic DNA was separated by reverse-field agarose gel electrophoresis and transferred to a MagnaGraph nylon transfer membrane (Osmonics, Inc., Minnetonka, MN) as previously described (34, 43). Primers for the hybridization probe were designed from genomic DNA of B. hermsii DAH in a conserved region of the gene so the probe would hybridize with DNA from both genomic group isolates (Table 1). Hybridization probes were produced with the PCR DIG probe synthesis kit according to the manufacturer's instructions (Roche Applied Science, Indianapolis, IN). PCR amplification for probes, hybridization, and developing Southern blots were performed as previously described (34, 52).
Producing recombinant BipA (rBipA).
The forward and reverse primers used to amplify bipA from B. hermsii DAH genomic DNA were 5′-CACCATGGATATGAGGAATCTTGGTATTG-3′ and 5′-AGCTTGACTTTTATCTTCAATTATGGATTTA-3′, respectively. PCR amplification was performed by using a DNA Engine Tetrad (Bio-Rad) consisting of 1 cycle at 96°C for 5 min and 35 cycles with a denaturing temperature of 94°C for 30 s, an annealing temperature of 58°C for 30 s, and an extension temperature of 72°C for 2.5 min. Amplicons were cloned into the pET102/Directional TOPO vector (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions, and correct orientation within the vector and nucleotide sequence were confirmed by using the Lasergene 8 software package (DNASTAR, Madison, WI). Chemically competent E. coli BL21 Star (DE3) cells (Invitrogen) were transformed with 10 ng of purified vector containing bipA according to the manufacturer's instructions (Invitrogen). E. coli BL21 Star (DE3) cells were induced with a final concentration of 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) to produce rBipA. The recombinant-fusion protein was expected to be ∼72 kDa and was purified by using a His-Bind Quick Column (EMD, Gibbstown, NJ) following the manufacturer's instructions.
Producing antisera to rBipA.
Three Rocky Mountain Laboratory (RML) mice (a closed colony of Swiss-Webster mice used at the RML since 1937) were immunized twice intraperitoneally with 4 weeks between immunizations with 50 μg of rBipA that was excised from an acrylamide gel. Serum was collected prior to immunizations and 2 weeks after each immunization and tested for reactivity to the 57-kDa protein in B. hermsii lysates and rBipA as described below. All animal handling was in compliance with the Rocky Mountain Laboratories Animal Care and Use Committee.
SDS-PAGE and immunoblotting.
SDS-PAGE and immunoblotting were performed as previously described (50). Whole-cell lysates of uninduced or induced E. coli BL21 Star (DE3) cells, several Borrelia species, GGI and GGII isolates of B. hermsii, or 1 μg of purified rBipA were electrophoresed on 12% Tris-glycine gels (Invitrogen) and transferred onto nitrocellulose membranes by using the iBlot dry blotting system (Invitrogen). Membranes were probed with anti-poly-histidine peroxidase conjugate (Sigma-Aldrich, St. Louis, MO) at a dilution of 1:2,000, pooled murine preimmunization or preinfection sera at a dilution of 1:200, pooled sera from mice immunized with rBipA at a dilution of 1:200, or human serum samples at a dilution of 1:200. To determine titers to rBipA, serum samples from mice and patients infected with relapsing fever spirochetes were diluted 5-fold starting at a dilution of 1:500. The secondary molecule used in immunoblotting was HRP-rec-protein A (Invitrogen) at 1:4,000. Immunoblots were performed three times, and images were captured by using the GelDoc imager (Bio-Rad).
Proteinase K treatment of B. hermsii.
B. hermsii DAH was grown to 108 spirochetes per ml and treated with proteinase K as previously described (55). A final concentration of 5 mg of phenylmethylsulfonyl fluoride/ml in isopropanol was added to spirochetes immediately after incubating with proteinase K or after 30-, 60-, 90-, and 120-min incubations. Samples were electrophoresed and transferred to nitrocellulose membranes, and membranes were probed with pooled mouse immune sera generated to rBipA as described above. Assays were performed three times.
Nucleotide sequence accession numbers.
Nucleotide sequences of bipA from GGI and GGII isolates of B. hermsii have been deposited in the GenBank database under accession numbers GQ869786 to GQ869824 and GU270942 for B. turicatae.
RESULTS
Plasmid mapping and sequence analysis of bipA from GGI and GGII isolates of B. hermsii.
Originally BipA was identified by mass spectrometry in B. hermsii DAH as an 57-kDa protein with an unknown function (33). Searching the B. hermsii DAH chromosome (GenBank accession no. CP000048) and partially assembled plasmid sequences indicated that bipA was plasmid encoded. Also, Southern blotting of nine GGI and seven GGII isolates mapped bipA to the 200-kb linear plasmid (data not shown).
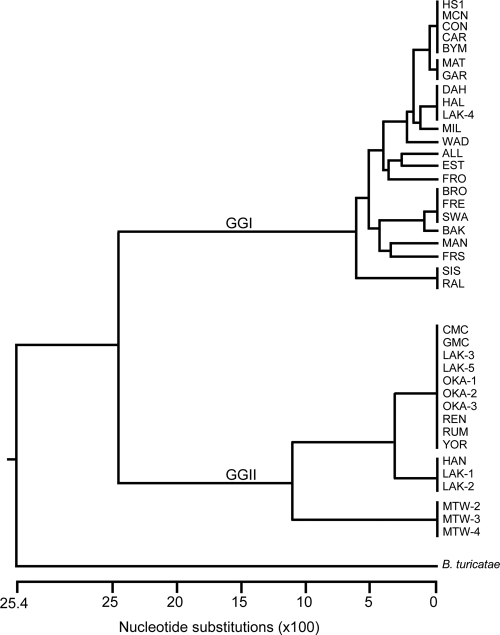
Amplifying the bipA locus from 23 GGI and 16 GGII isolates produced a product of the expected size (data not shown), indicating the probable presence of this gene in all isolates of B. hermsii tested. DNA sequencing of these amplicons confirmed the presence of the gene, and a phylogenetic tree differentiated bipA isolates into the two genomic groups (Fig. 1). These findings are consistent with most loci that have been sequenced in B. hermsii (34, 43, 53).
FIG. 1.
Phylogram of bipA sequences from GGI and GGII isolates of B. hermsii. The tree was constructed with CLUSTAL V, and the outgroup used was B. turicatae.
Phylogenetic analysis (Fig. 1) and determining the nucleotide identity of bipA between isolates (see Fig. S1 in the supplemental material) identified identical sequences from isolates with diverse geographical locations, an observation consistent with other genetic analyses of B. hermsii (43, 53). For example, CON, a GGI isolate originating from the Sierra Nevada Mountains in California, grouped more closely with HS1, MCN, CAR, and BYM isolates originating from Washington State and Idaho (Fig. 1 and Fig. S1 in the supplemental material). In addition, MAT and GAR had identical bipA sequences (Fig. 1 and Fig. S1 in the supplemental material); their origins were the Bitteroot Selway mountains, ID, and the Okanagan Valley, British Columbia, Canada, respectively.
The bipA locus demonstrated more genetic heterogeneity in GGI isolates with 13 alleles, compared to GGII isolates with only three alleles (Fig. 1). Sequence analysis also identified multiple indels (gaps resulting in insertions or deletions) between genomic groups that are reflected in the amino acid sequence alignments, with one isolate representing each allele (Fig. 2). The amino acid sequences shown from GGI isolates are HS1, MAT, DAH, MIL, WAD, ALL, EST, FRO, BRO, BAK, MAN, FRS, and SIS, and the sequences from GGII isolates are CMC, LAK-1, and MTW-2 (Fig. 2). Furthermore, BipA from GGII isolates was predicted to be larger with a molecular mass of ∼62 kDa.
FIG. 2.
Amino acid alignments from isolates of B. hermsii that represent the 13 and 3 alleles in GGI and GGII isolates, respectively. Identical amino acids are shaded with a gray background.
Expressing bipA in E. coli and reactivity of immune serum to rBipA.
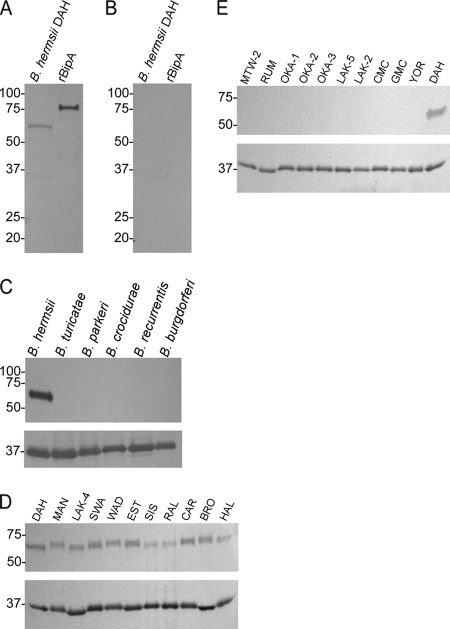
Coomassie stained gels of uninduced and induced E. coli lysates expressing bipA from the GGI isolate DAH produced a recombinant fusion protein of the expected molecular mass (see Fig. S2A in the supplemental material). Also, anti-poly-histidine peroxidase MAb bound to the recombinant protein of the expected molecular mass as rBipA with minimal protein degradation (Fig. S2B in the supplemental material).
Immunoblotting using pooled serum samples from three mice infected with B. hermsii DAH (Fig. 3A) or serum samples from human patients (Fig. 3B to D) infected with unknown isolates of B. hermsii confirmed the immunogenicity of rBipA. Furthermore, preinfection serum samples from mice were negative to B. hermsii DAH lysates and rBipA (data not shown). Serum samples from patients infected with B. burgdorferi did not react with rBipA (Fig. 3E and F), indicating that rBipA has the potential to discriminate between infection by B. burgdorferi and B. hermsii. In addition, immunoblots from seven additional human patients infected with B. hermsii and five patients infected with B. burgdorferi displayed similar results (data not shown). The serum titers to rBipA from mice and human patients infected with relapsing fever spirochetes ranged between 1:2,500 to 1:12,500.
FIG. 3.
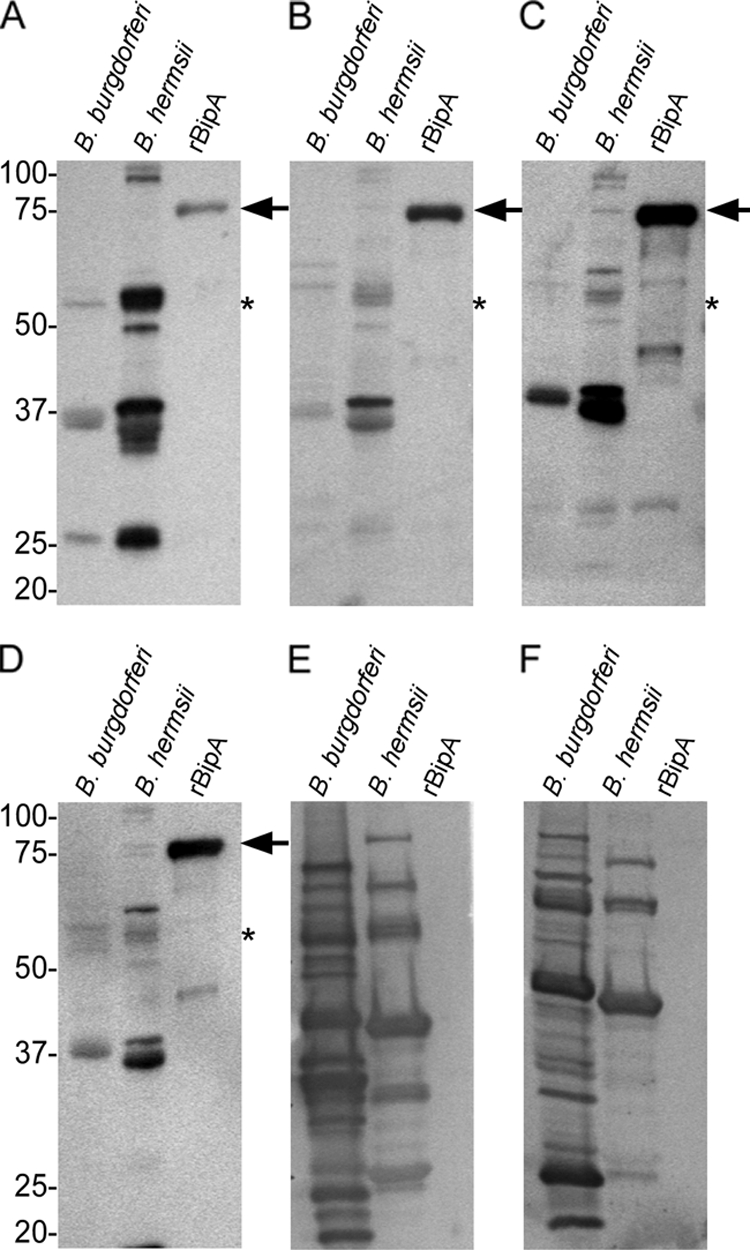
Immunoblotting to determine antibody reactivity to rBipA and borrelia lysates. The serum samples used included pooled immune sera from three mice (A) and three human patients infected with B. hermsii (B to D) or two human patients infected with B. burgdorferi (E and F). Arrows indicate protein bands of the expected size for rBipA, and the asterisk indicates the expected size of native BipA in B. hermsii DAH lysates. Molecular mass standards are indicated in kilodaltons.
Detecting BipA in Borrelia species and GGI and GGII isolates of B. hermsii.
Pooled immune sera from three mice immunized with rBipA reacted with the 57-kDa protein in spirochete lysates of B. hermsii DAH (Fig. 4A), while preimmunization sera did not (Fig. 4B). Also, the B. recurrentis (31) and B. turicatae genomes contain an orthologue to the B. hermsii DAH bipA, with amino acid identities of 28.1 and 39.7%, respectively. However, anti-rBipA immune sera did not bind to a protein in these spirochete lysates (Fig. 4C) or with lysates from B. parkeri, B. crocidurae, and B. burgdorferi (Fig. 4C, top panel). Interestingly, whereas anti-rBipA immune sera bound to the protein in lysates of GGI isolates, there was no reactivity with lysates from GGII isolates (Fig. 4D and E, top panels), even though the amino acid identity of BipA between the DAH and GGII isolates is ca. 68%. Probing immunoblots with an anti-flagellin MAb indicated that equal concentrations of protein were used from each species of Borrelia and GGI and GGII isolates (Fig. 4C to E, lower panels).
FIG. 4.
Immunoblotting with pooled sera from mice immunized with rBipA. Immune sera against rBipA (A) and preimmunization sera (B) were used to determine seroconversion to BipA in B. hermsii lysates. Five species of Borrelia (C), GGI (D), and GGII (E) isolates of B. hermsii were also probed with anti-rBipA sera (C to E, top panels) and a monoclonal antibody generated against B. hermsii flagellin (C to E, lower panels). Molecular mass standards are indicated on the left of each immunoblot in kilodaltons.
Serum reactivity to rBipA from mice infected with GGII isolates of B. hermsii.
Immune sera generated against a GGI rBipA did not bind to a protein in GGII isolates; however, when mice were infected with YOR or MTW-4 they seroconverted to rBipA (Fig. 5A, B, D, and E). Also, preinfection serum samples from these mice were negative (Fig. 5C and F). These results indicate that BipA is produced in GGII isolates and is immunogenic during infection.
FIG. 5.
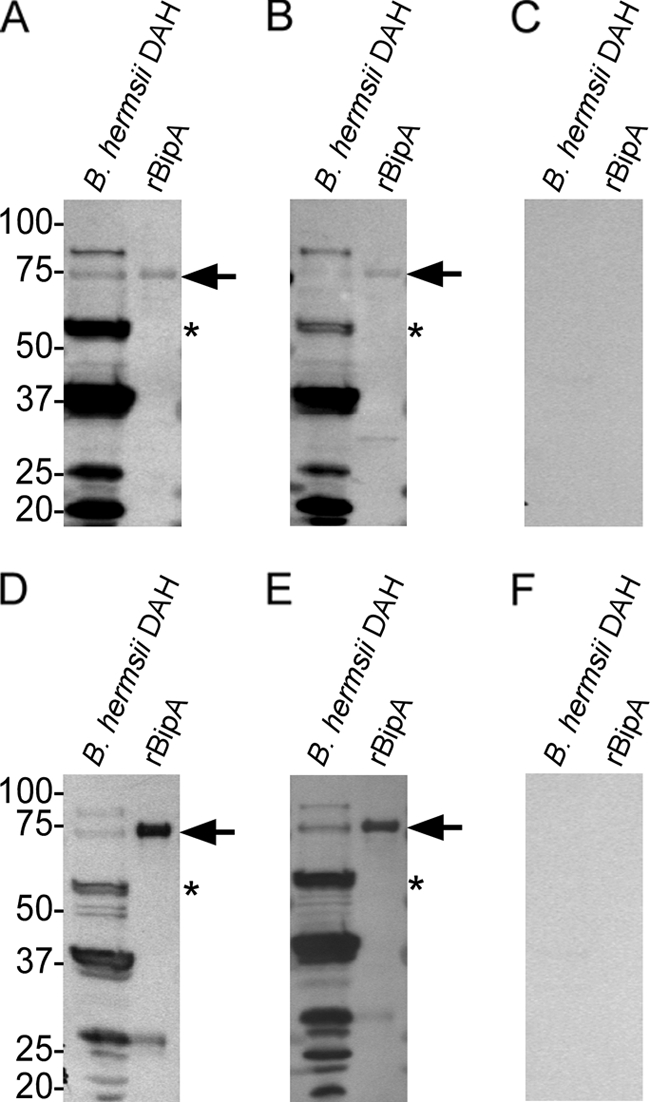
Immunoblotting against B. hermsii DAH lysates and DAH rBipA using serum samples from two mice infected with the GGII isolate YOR (A and B) and two mice infected with MTW-4 (D and E). Pooled preinfection serum samples from mice infected with YOR (C) and MTW-4 (F) were also used. Arrows indicate the protein bands of the expected size for rBipA, and the asterisks indicate the expected sizes of native BipA in B. hermsii DAH lysates. Molecular mass standards are indicated in kilodaltons.
In silico analysis and cellular localization of BipA.
Prosite Pattern and LipoP 1.0 predicted a signal sequence for the first 22 amino acids of BipA, and Motif Scan predicted a lipid attachment site from amino acids 1 to 23 (data not shown). Also, scanning the amino acid sequence of BipA in the Motif Scan database detected the presence of the Mlp lipoprotein family motif (statistically significant E-value of 0.00064), pfam03304, for a section of 48 amino acids toward the C terminus of the protein (amino acids 392 to 440 of DAH BipA). These amino acids had 40.8% amino acid identity to a 43-amino-acid portion of a 23-kDa protein annotated as the Mlp lipoprotein family protein in B. burgdorferi (YP_002776332). Based on these in silico analyses suggesting the surface localization of BipA, the prediction that BipA was a surface protein of B. hermsii DAH was experimentally tested.
Intact spirochetes were incubated with proteinase K and immunoblotted with anti-rBipA immune sera, which demonstrated that the protein was digested within 30 min of enzymatic treatment (Fig. 6, top panel). Also, BipA did not degrade in untreated spirochetes incubated at 37°C for the duration of the assay (Fig. 6, top panel). Immunoblots were also probed for the periplasmic protein flagellin (Fig. 6, lower panel), indicating that the spirochetes were not permeabilized during the assay. These results suggest that spirochetes were intact during this assay and that BipA is exposed on the outer surface of B. hermsii DAH.
FIG. 6.
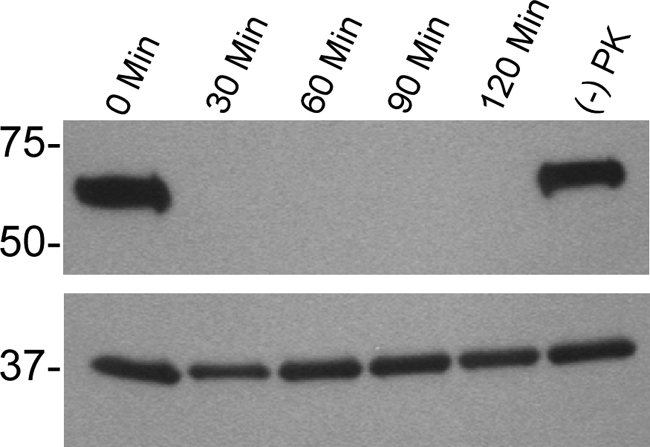
Analysis of B. hermsii DAH BipA after 0-, 30-, 60-, 90-, and 120-min incubations with proteinase K. The top panel was probed with anti-rBipA immune sera, while the bottom panel was probed with an anti-flagellin monoclonal antibody. Also, (−) PK indicates untreated spirochetes that were incubated at 37°C for the duration of the assay. Molecular mass standards are indicated on the left of the immunoblot in kilodaltons.
DISCUSSION
For the ecological niches where relapsing fever and Lyme disease causing spirochetes overlap (11, 12, 30, 32, 42, 60) and the serological cross-reactivity from infected patients occurs (37), identifying antigens that are species specific is important. We demonstrate here that a protein, which we have designated Borrelia immunogenic protein A (BipA), is surface localized and immunogenic in patients and mice infected with B. hermsii. Also, with no orthologue of bipA in B. burgdorferi (14, 24), lack of reactivity to rBipA from serum samples of patients infected with Lyme disease causing spirochetes was expected.
Interestingly, pooled sera from mice immunized with a GGI rBipA bound to the protein only in GGI isolates. Due to the regions of amino acid deletions in GGI isolates, the quaternary structure of the protein may differ between genomic groups. Thus, the mice used to produce antisera to rBipA may have generated antibodies against an epitope of the protein that is unique in GGI isolates. In addition, the amino acid alignments of BipA from GGI and GGII isolates indicated conservation at the C terminus of the protein. Therefore, mice infected with the GGII isolates YOR and MTW-4 may have generated antibodies to the C terminus of BipA, suggesting differential antigen processing of BipA in mice immunized with the recombinant protein and during natural infection.
Although GGI isolates displayed more nucleotide diversity in the bipA locus, there was no evidence for the horizontal gene transfer of bipA between GGI and GGII isolates, as shown for another plasmid-encoded gene, the variable tick protein (43). The nucleotide diversity of the bipA locus in GGI isolates implies a selective pressure on this gene. The surface localization and immunogenicity of BipA supports the hypothesis that immune pressure may be responsible for the observed genetic heterogeneity in GGI isolates. Furthermore, GGII isolates may represent a more recent derivative from GGI isolates, possibly explaining the lesser amount of nucleotide diversity of bipA in GGII isolates.
Further analysis of the bipA locus identified identical sequences from B. hermsii isolates spread over a large geographical area, a finding similar to that when the 16S-23S ribosomal DNA noncoding intergenic spacer region (IGS) was sequenced in these isolates (53). In addition, sequence analysis of B. hermsii detected in a squirrel in Montana was identical to that of a spirochete found in a Northern spotted owl in Kittias County, WA (23, 57). These data further support a role of birds in spreading relapsing fever spirochetes in nature as previously suggested (23, 53), possibly explaining the distant locations of B. hermsii with identical bipA sequences.
The current antigen used for serodiagnosis of infection by relapsing fever spirochetes in North American and Africa is recombinant glycerophosphodiester phosphodiesterase (rGlpQ) (41, 44, 55). In addition, the factor H binding protein may be a promising antigen for diagnosing infection caused by B. hermsii (27). Since BipA is unique to relapsing fever spirochetes with no identified homologues in other bacteria, and human patients produce high titers of antibody against rBipA, this protein also has potential as a diagnostic antigen. Also, with orthologues of glpQ in Yersinia, Clostridium, Salmonella, and Klebsiella species having ca. 45 to 50% amino acid identity to B. hermsii GlpQ (data not shown), using rBipA with rGlpQ or the factor H binding protein in an enzyme-linked immunosorbent assay could improve serodiagnosis. Furthermore, given the amino acid heterogeneity of BipA between species of relapsing fever spirochetes, expressing bipA orthologues as recombinant proteins may aid in determining the species causing infection.
Supplementary Material
Acknowledgments
We thank Patricia A. Rosa, B. Joseph Hinnebusch, and Dave Safronetz for reviewing the manuscript and Paul Policastro for immune serum samples.
This research was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Footnotes
Published ahead of print on 10 February 2010.
Supplemental material for this article may be found at http://cvi.asm.org/.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1.Alugupalli, K. R. 2008. A distinct role for B1b lymphocytes in T cell-independent immunity. Curr. Top. Microbiol. Immunol. 319:105-130. [DOI] [PubMed] [Google Scholar]
- 2.Alugupalli, K. R., R. M. Gerstein, J. Chen, E. Szomolanyi-Tsuda, R. T. Woodland, and J. M. Leong. 2003. The resolution of relapsing fever borreliosis requires IgM and is concurrent with expansion of B1b lymphocytes. J. Immunol. 170:3819-3827. [DOI] [PubMed] [Google Scholar]
- 3.Alugupalli, K. R., J. M. Leong, R. T. Woodland, M. Muramatsu, T. Honjo, and R. M. Gerstein. 2004. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity 21:379-390. [DOI] [PubMed] [Google Scholar]
- 4.Alugupalli, K. R., A. D. Michelson, I. Joris, T. G. Schwan, K. Hodivala-Dilke, R. O. Hynes, and J. M. Leong. 2003. Spirochete-platelet attachment and thrombocytopenia in murine relapsing fever borreliosis. Blood 102:2843-2850. [DOI] [PubMed] [Google Scholar]
- 5.Barbour, A. G. 1990. Antigenic variation of a relapsing fever Borrelia species. Annu. Rev. Microbiol. 44:155-171. [DOI] [PubMed] [Google Scholar]
- 6.Barbour, A. G. 1984. Isolation and cultivation of Lyme disease spirochetes. Yale J. Biol. Med. 57:521-525. [PMC free article] [PubMed] [Google Scholar]
- 7.Barbour, A. G., and V. Bundoc. 2001. In vitro and in vivo neutralization of the relapsing fever agent Borrelia hermsii with serotype-specific immunoglobulin M antibodies. Infect. Immun. 69:1009-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barbour, A. G., Q. Dai, B. I. Restrepo, H. G. Stoenner, and S. A. Frank. 2006. Pathogen escape from host immunity by a genome program for antigenic variation. Proc. Natl. Acad. Sci. U. S. A. 103:18290-18295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Battisti, J. M., S. J. Raffel, and T. G. Schwan. 2008. A system for site-specific genetic manipulation of the relapsing fever spirochete Borrelia hermsii. Methods Mol. Biol. 431:69-84. [DOI] [PubMed] [Google Scholar]
- 10.Belperron, A. A., C. M. Dailey, and L. K. Bockenstedt. 2005. Infection-induced marginal zone B-cell production of Borrelia hermsii-specific antibody is impaired in the absence of CD1d. J. Immunol. 174:5681-5686. [DOI] [PubMed] [Google Scholar]
- 11.Brown, R. N., and R. S. Lane. 1994. Natural and experimental Borrelia burgdorferi infections in woodrats and deer mice from California. J. Wildl. Dis. 30:389-398. [DOI] [PubMed] [Google Scholar]
- 12.Brown, R. N., M. A. Peot, and R. S. Lane. 2006. Sylvatic maintenance of Borrelia burgdorferi (Spirochaetales) in Northern California: untangling the web of transmission. J. Med. Entomol. 43:743-751. [DOI] [PubMed] [Google Scholar]
- 13.Bryceson, A. D., E. H. Parry, P. L. Perine, D. A. Warrell, D. Vukotich, and C. S. Leithead. 1970. Louse-borne relapsing fever. Q. J. Med. 39:129-170. [PubMed] [Google Scholar]
- 14.Casjens, S., N. Palmer, R. van Vugt, W. M. Huang, B. Stevenson, P. Rosa, R. Lathigra, G. Sutton, J. Peterson, R. J. Dodson, D. Haft, E. Hickey, M. Gwinn, O. White, and C. M. Fraser. 2000. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 35:490-516. [DOI] [PubMed] [Google Scholar]
- 15.Coffey, E. M., and W. C. Eveland. 1967. Experimental relapsing fever initiated by Borrelia hermsi. II. Sequential appearance of major serotypes in the rat. J. Infect. Dis. 117:29-34. [DOI] [PubMed] [Google Scholar]
- 16.Dai, Q., B. I. Restrepo, S. F. Porcella, S. J. Raffel, T. G. Schwan, and A. G. Barbour. 2006. Antigenic variation by Borrelia hermsii occurs through recombination between extragenic repetitive elements on linear plasmids. Mol. Microbiol. 60:1329-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis, G. E. 1942. Species unity or plurality of the relapsing fever spirochetes, p. 41-47. In F. R. Moulton (ed.), A symposium of relapsing fever in the Americas. American Association for the Advancement of Science, Washington, DC.
- 18.de Castro, E., C. J. Sigrist, A. Gattiker, V. Bulliard, P. S. Langendijk-Genevaux, E. Gasteiger, A. Bairoch, and N. Hulo. 2006. ScanProsite: detection of PROSITE signature matches and ProRule-associated functional and structural residues in proteins. Nucleic Acids Res. 34:W362-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dworkin, M. S., D. E. Anderson, Jr., T. G. Schwan, P. C. Shoemaker, S. N. Banerjee, B. O. Kassen, and W. Burgdorfer. 1998. Tick-borne relapsing fever in the Northwestern United States and Southwestern Canada. Clin. Infect. Dis. 26:122-131. [DOI] [PubMed] [Google Scholar]
- 20.Dworkin, M. S., T. G. Schwan, and D. E. Anderson. 2002. Tick-borne relapsing fever in North America. Med. Clin. North Am. 86:417-433. [DOI] [PubMed] [Google Scholar]
- 21.Dworkin, M. S., P. C. Shoemaker, C. L. Fritz, M. E. Dowell, and D. E. Anderson, Jr. 2002. The epidemiology of tick-borne relapsing fever in the United States. Am. J. Trop. Med. Hyg. 66:753-758. [DOI] [PubMed] [Google Scholar]
- 22.Felsenfeld, O. 1971. Borrelia: strains, vectors, human and animal borreliosis. Warren H. Green, Inc., St. Louis, MO.
- 23.Fischer, R., Johnson, T. L., S. J. Raffel, and T. G. Schwan. 2009. The identification of the relapsing fever spirochete Borrelia hermsii in a mammal and bird. Emerg. Infect. Dis. 15:2064-2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fraser, C. M., S. Casjens, W. M. Huang, G. G. Sutton, R. Clayton, R. Lathigra, O. White, K. A. Ketchum, R. Dodson, E. K. Hickey, M. Gwinn, B. Dougherty, J.-F. Tomb, R. D. Fleischmann, D. Richardson, J. Peterson, A. R. Kerlavage, J. Quackenbush, S. Salzberg, M. Hanson, R. V. Vugt, N. Palmer, M. D. Adams, J. Gocayne, J. Weidman, T. Utterback, L. Watthey, L. McDonald, P. Artiach, C. Bowman, S. Garland, C. Fujii, M. D. Cotton, K. Horst, K. Roberts, B. Hatch, H. O. Smith, and J. C. Venter. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390:580-586. [DOI] [PubMed] [Google Scholar]
- 25.Hayes, L. J., D. J. M. Wright, and L. C. Archard. 1988. Segmented arrangement of Borrelia duttonii DNA and location of variant surface antigen genes. J. Gen. Microbiol. 134:1785-1793. [DOI] [PubMed] [Google Scholar]
- 26.Holden, K., J. T. Boothby, S. Anand, and R. F. Massung. 2003. Detection of Borrelia burgdorferi, Ehrlichia chaffeensis, and Anaplasma phagocytophilum in ticks (Acari: Ixodidae) from a coastal region of California. J. Med. Entomol. 40:534-539. [DOI] [PubMed] [Google Scholar]
- 27.Hovis, K. M., M. E. Schriefer, S. Bahlani, and R. T. Marconi. 2006. Immunological and molecular analyses of the Borrelia hermsii factor H and factor-H like protein 1 binding protein, FhbA: demonstration of its utility as a diagnostic marker and epidemiological tool for tick-borne relapsing fever. Infect. Immun. 74:4519-4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hulo, N., A. Bairoch, V. Bulliard, L. Cerutti, B. A. Cuche, E. de Castro, C. Lachaize, P. S. Langendijk-Genevaux, and C. J. Sigrist. 2008. The 20 years of PROSITE. Nucleic Acids Res. 36:D245-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Juncker, A. S., H. Willenbrock, G. Von Heijne, S. Brunak, H. Nielsen, and A. Krogh. 2003. Prediction of lipoprotein signal peptides in Gram-negative bacteria. Protein Sci. 12:1652-1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lane, R. S., and R. N. Brown. 1991. Wood rats and kangaroo rats: potential reservoirs of the Lyme disease spirochete in California. J. Med. Entomol. 28:299-302. [DOI] [PubMed] [Google Scholar]
- 31.Lescot, M., S. Audic, C. Robert, T. T. Nguyen, G. Blanc, S. J. Cutler, P. Wincker, A. Couloux, J. M. Claverie, D. Raoult, and M. Drancourt. 2008. The genome of Borrelia recurrentis, the agent of deadly louse-borne relapsing fever, is a degraded subset of tick-borne Borrelia duttonii. PLoS Genet. 4:e1000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin, T., J. H. Oliver, Jr., L. Gao, T. M. Kollars, Jr., and K. L. Clark. 2001. Genetic heterogeneity of Borrelia burgdorferi sensu lato in the southern United States based on restriction fragment length polymorphism and sequence analysis. J. Clin. Microbiol. 39:2500-2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lopez, J. E., S. F. Porcella, M. E. Schrumpf, S. J. Raffel, C. H. Hammer, M. Zhao, M. A. Robinson, and T. G. Schwan. 2009. Identification of conserved antigens for early serodiagnosis of relapsing fever Borrelia. Microbiology 155:2641-2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lopez, J. E., M. E. Schrumpf, S. J. Raffel, P. F. Policastro, S. F. Porcella, and T. G. Schwan. 2008. Relapsing fever spirochetes retain infectivity after prolonged in vitro cultivation. Vector Borne Zoonotic Dis. 8:813-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Magnarelli, L. A., and J. A. Anderson. 1988. Enzyme-linked immunosorbent assays for the detection of class-specific immunoglobulins to Borrelia burgdorferi. Am. J. Epidemiol. 127:818-825. [DOI] [PubMed] [Google Scholar]
- 36.Magnarelli, L. A., J. F. Anderson, and A. G. Barbour. 1989. Enzyme-linked immunosorbent assays for Lyme disease: reactivity of subunits of Borrelia burgdorferi. J. Infect. Dis. 159:43-49. [DOI] [PubMed] [Google Scholar]
- 37.Magnarelli, L. A., J. F. Anderson, and R. C. Johnson. 1987. Cross-reactivity in serological tests for Lyme disease and other spirochetal infections. J. Infect. Dis. 156:183-187. [DOI] [PubMed] [Google Scholar]
- 38.Magnarelli, L. A., J. M. Meegan, J. F. Anderson, and W. A. Chappell. 1984. Comparison of an indirect fluorescent-antibody test with an enzyme-linked immunosorbent assay for serological studies of Lyme disease. J. Clin. Microbiol. 20:181-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meader, C. N. 1915. Five cases of relapsing fever originating in Colorado, with positive blood findings in two. Colorado Med. 12:365-369. [Google Scholar]
- 40.Newman, K., Jr., and R. C. Johnson. 1984. T-cell-independent elimination of Borrelia turicatae. Infect. Immun. 45:572-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nordstrand, A., I. Bunikis, C. Larsson, K. Tsogbe, T. G. Schwan, M. Nilsson, and S. Bergström. 2007. Tickborne relapsing fever diagnosis obscured by malaria, Togo. Emerg. Infect. Dis. 13:117-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oliver, J. H., Jr., T. Lin, L. Gao, K. L. Clark, C. W. Banks, L. A. Durden, A. M. James, and F. W. Chandler, Jr. 2003. An enzootic transmission cycle of Lyme borreliosis spirochetes in the southeastern United States. Proc. Natl. Acad. Sci. U. S. A. 100:11642-11645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Porcella, S. F., S. J. Raffel, D. E. Anderson, Jr., S. D. Gilk, J. L. Bono, M. E. Schrumpf, and T. G. Schwan. 2005. Variable tick protein in two genomic groups of the relapsing fever spirochete Borrelia hermsii in western North America. Infect. Immun. 73:6647-6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Porcella, S. F., S. J. Raffel, M. E. Schrumpf, M. E. Schriefer, D. T. Dennis, and T. G. Schwan. 2000. Serodiagnosis of louse-borne relapsing fever with glycerophosphodiester phosphodiesterase (GlpQ) from Borrelia recurrentis. J. Clin. Microbiol. 38:3561-3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rath, P.-M., G. Rogler, A. Schonberg, H. D. Pohle, and F.-J. Fehrenbach. 1992. Relapsing fever and its serological discrimination from Lyme borreliosis. Infection 20:283-286. [DOI] [PubMed] [Google Scholar]
- 46.Rawlings, J. A. 1995. An overview of tick-borne relapsing fever with emphasis on outbreaks in Texas. Tex. Med. 91:56-59. [PubMed] [Google Scholar]
- 47.Restrepo, B. I., T. Kitten, C. J. Carter, D. Infante, and A. G. Barbour. 1992. Subtelomeric expression regions of Borrelia hermsii linear plasmids are highly polymorphic. Mol. Microbiol. 6:3299-3311. [DOI] [PubMed] [Google Scholar]
- 48.Rice, P., I. Longden, and A. Bleasby. 2000. EMBOSS: the European Molecular Biology Open Software Suite. Trends Genet. 16:276-277. [DOI] [PubMed] [Google Scholar]
- 49.Salkeld, D. J., S. Leonhard, Y. A. Girard, N. Hahn, J. Mun, K. A. Padgett, and R. S. Lane. 2008. Identifying the reservoir hosts of the Lyme disease spirochete Borrelia burgdorferi in California: the role of the western gray squirrel (Sciurus griseus). Am. J. Trop. Med. Hyg. 79:535-540. [PMC free article] [PubMed] [Google Scholar]
- 50.Schwan, T. G., K. K. Kime, M. E. Schrumpf, J. E. Coe, and W. J. Simpson. 1989. Antibody response in white-footed mice (Peromyscus leucopus) experimentally infected with the Lyme disease spirochete (Borrelia burgdorferi). Infect. Immun. 57:3445-3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwan, T. G., P. F. Policastro, Z. Miller, R. L. Thompson, T. Damrow, and J. E. Keirans. 2003. Tick-borne relapsing fever caused by Borrelia hermsii, Montana. Emerg. Infect. Dis. 9:1151-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schwan, T. G., S. J. Raffel, M. E. Schrumpf, P. F. Policastro, J. A. Rawlings, R. S. Lane, E. B. Breitschwerdt, and S. F. Porcella. 2005. Phylogenetic analysis of the spirochetes Borrelia parkeri and Borrelia turicatae and the potential for tick-borne relapsing fever in Florida. J. Clin. Microbiol. 43:3851-3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schwan, T. G., S. J. Raffel, M. E. Schrumpf, and S. F. Porcella. 2007. Diversity and distribution of Borrelia hermsii. Emerg. Infect. Dis. 13:436-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schwan, T. G., S. J. Raffel, M. E. Schrumpf, L. S. Webster, A. R. Marques, R. Spano, M. Rood, J. Burns, and R. Hu. 2009. Tick-borne relapsing fever and Borrelia hermsii, Los Angeles County, California, U. S. A. Emerg. Infect. Dis. 15:1026-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schwan, T. G., M. E. Schrumpf, B. J. Hinnebusch, D. E. Anderson, and M. E. Konkel. 1996. GlpQ: an antigen for serological discrimination between relapsing fever and Lyme borreliosis. J. Clin. Microbiol. 34:2483-2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stoenner, H. G., T. Dodd, and C. Larsen. 1982. Antigenic variation of Borrelia hermsii. J. Exp. Med. 156:1297-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thomas, N. J., J. Bunikis, A. G. Barbour, and M. J. Wolcott. 2002. Fatal spirochetosis due to a relapsing fever-like Borrelia sp. in a northern spotted owl. J. Wildl. Dis. 38:187-193. [DOI] [PubMed] [Google Scholar]
- 58.Whitney, M. S., T. G. Schwan, K. B. Sultemeier, P. S. McDonald, and M. N. Brillhart. 2007. Spirochetemia caused by Borrelia turicatae infection in 3 dogs in Texas. Vet. Clin. Pathol. 36:212-216. [DOI] [PubMed] [Google Scholar]
- 59.Wynns, H. L., and M. D. Beck. 1935. Epidemiological studies on relapsing fever in California. Am. J. Public Health 7:270-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zingg, B. C., R. N. Brown, R. S. Lane, and R. B. LeFebvre. 1993. Genetic diversity among Borrelia burgdorferi isolates from wood rats and kangaroo rats in California. J. Clin. Microbiol. 31:3109-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.