Abstract
The symptoms of chronic fatigue syndrome (CFS) are consistent with cytokine dysregulation. This has led to the hypothesis of immune dysregulation as the cause of this illness. To further test this hypothesis, we did repeated blood sampling for cytokines while patients and matched healthy controls slept in the sleep lab. Because no one method for assaying cytokines is acknowledged to be better than another, we assayed for protein in serum, message in peripheral blood lymphocytes (PBLs), and function in resting and stimulated PBLs. We found no evidence of proinflammatory cytokine upregulation. Instead, in line with some of our earlier studies, we did find some evidence to support a role for an increase in interleukin-10, an anti-inflammatory cytokine. Although the changes were small, they may contribute to the common complaint in CFS patients of disrupted sleep.
Chronic fatigue syndrome (CFS) is a medically unexplained illness lasting at least 6 months and characterized by severe fatigue that produces a substantial reduction in activity accompanied by at least four of the following symptoms: sore throat, tender lymph nodes, headache, myalgia, arthralgia, unrefreshing sleep, difficulty concentrating, and the report of syndromic exacerbation following mild exertion (7). One major hypothesis for the cause of CFS is an immune dysregulation of unknown etiology with high levels of proinflammatory cytokines producing the CFS symptom complex. Two findings fostered this idea: first, approximately a third of CFS patients report a sudden, influenza-like onset of their illness (37), and second, administration of proinflammatory cytokines leads to many of the same symptoms seen in CFS (26). However, we recently reviewed the literature on this hypothesis and found relatively little empirical data to support it (20); a more recent small study did report higher levels of one such cytokine, serum transforming growth factor β, in patients than in controls (35); however, another group did not confirm this result (30). Other work extending the research to cellular production of proinflammatory cytokines was also negative (1).
However, a very different and alternative hypothesis focuses on the common complaint in CFS of unrefreshing sleep (4). Recent work suggests that sleep is under the control of a cytokine/sleep network where normal sleep follows a balanced secretion of pro- and anti-inflammatory cytokines (13). We hypothesized that CFS might result from an imbalance of this network in favor of the anti-inflammatory cytokines which are sleep disrupting (14). Supporting this possibility is the result of a recent study (28) in which we sampled blood for cytokines every 20 min across a 24-h day in patients with fibromyalgia (FM) (28), a medically unexplained, diffuse pain syndrome that has substantial overlap with CFS (5). Of several pro- and anti-inflammatory cytokines studied, the only one to show differences from controls, i.e., increases, was the anti-inflammatory cytokine interleukin-10 (IL-10), and that was true only for nocturnal data. Because no information was provided as to whether this patient group also fulfilled criteria for CFS, it is not appropriate to extend the results of that study to patients with CFS. Thus, one purpose of the current study was to determine if the results we obtained in patients with FM could be extended to those with CFS. Moreover, because we hypothesized disturbances in the cytokine/sleep network, we decided to study cytokines during sleep with the expectation of finding maximum differences at this time.
In a recent study, we assayed cytokines in plasma (28). However, assaying cytokines in plasma is only one of several methods that currently exist to determine cytokine production, and differences in plasma cytokines between patients and controls could reflect differences in distribution of cytokines over time. Unfortunately, there is no single “gold standard” as to which method best reflects cytokine production and levels in a person. Therefore, in this study, we extended our assay methods beyond assaying cytokines in plasma to include an analysis of cytokine gene expression in whole peripheral blood cells and cytokine release by in vitro-stimulated peripheral blood mononuclear cells (PBMCs) using enzyme-linked immunospot (ELISPOT) assays.
MATERIALS AND METHODS
Subjects.
The subjects were 62 women, 47 with CFS and 36 healthy controls, matched for age (range, 27 to 56 years old) and body mass index (BMI). Subjects older or younger than those selected were excluded because of possible age effects on sleep and on cytokines. Subjects were recruited from either our data set of prior research subjects or from the clinical practice of B. H. Natelson, who specializes in the care of these patients. Other patients were referred by their physician or were self-referred based on media reports about our research. All subjects initially completed an extensive health screening form (Pain and Fatigue Study Center, UMDNJ) which over the years has proven effective in identifying CFS patients with approximately a 5% margin of error. This screening vehicle was also used to exclude patients taking antidepressants, opiates, steroids, hypnotics, or other sedatives, including benzodiazepines. Patients screening positive for CFS and controls indicating their health to be excellent or good, not fair or poor, and not taking any medications besides birth control pills arrived at our center, where they signed the informed consent, approved by the medical school's Institutional Review Board, to participate in this research.
Subsequently, each research subject underwent a complete medical history and physical examination, including a tender point evaluation to allow for the diagnosis of FM (36) and a psychiatric diagnostic interview (Q-DIS), all administered by the study's advanced practice nurse under the supervision of B. H. Natelson. The psychiatric interview (24) was used to identify DSM-IV-based exclusionary disorders, including schizophrenia, eating disorders, substance abuse, or bipolar disorder (8) as well as major depressive disorder, a psychiatric disorder that can disrupt sleep (17). In addition, based on their history, patients were also categorized as having “severe” CFS or “all other” CFS (22). Finally, a set of blood tests was done to eliminate patients with medical causes of fatigue; these causes included anemia, increased sedimentation rate, and abnormal liver or thyroid function tests and the presence of Lyme antibody, antinuclear antibody, rheumatoid factor, or elevated C-reactive protein.
Following this evaluation, 21 patients and 12 healthy control subjects were dropped from further study participation for the following reasons: inadequate criteria for CFS (three patients); use of exclusionary drugs (six patients); previously unappreciated medical illness (one patient and two controls); current depression (five patients); obesity (one patient); abnormal lab results (one patient and five controls); moved or no longer interested (three patients and two controls); technical or other problem (one patient and three controls). The remaining patients all fulfilled the 1994 case definition for CFS (8); of these patients, 14 also fulfilled the American College of Rheumatology's 1990 criteria for FM (36) (Table 1).
TABLE 1.
Subject characteristics for controls, all CFS patients, and CFS patient subgroupsa
| Group | n | Age (yrs) | BMI (kg/m2) | % (no. in group) with severe CFS | SL (min) | SE (%) |
|---|---|---|---|---|---|---|
| Controls | 24 | 37.75 ± 8.51 | 24.10 ± 4.34 | 0 | 14.44 ± 17.19 | 85.77 ± 8.07 |
| All CFS | 26 | 41.62 ± 8.20 | 24.81 ± 5.15 | 38 (10) | 24.26 ± 31.43 | 79.68 ± 10.89* |
| CFS with FM | 15 | 44.80 ± 5.67 | 22.64 ± 3.80 | 40 (6) | 25.33 ± 40.49 | 79.08 ± 11.95 |
| CFS without FM | 11 | 37.27 ± 9.34 | 26.40 ± 5.53 | 36 (4) | 22.90 ± 15.34 | 80.43 ± 9.90 |
Values are means ± standard deviations. SL, sleep latency; SE, sleep efficiency. *, significant difference between control subjects and CFS groups (P < 0.05; t test). Results shown in boldface indicate significant cases.
All of these patients then underwent a diagnostic sleep study, for which data have been reported previously (29). Within a 6-month period of this diagnostic evaluation, those subjects willing to participate further in our studies returned to the sleep lab for a second night during which blood was periodically sampled via an indwelling catheter that allowed remote sampling without disturbing the subject. On this night, subjects were also set up with instruments to allow recording of electroencephalogram (EEG; C3/A2, O1/A2, and FZ/A2), electrooculogram (EOG), submental electromyogram (EMG), and a lead II electrocardiogram (ECG). Prior to going to sleep and upon awakening the next morning, subjects completed visual analog scales (0 to 15.5 cm) to estimate perceived sleepiness and fatigue. Sleep was scored by a single scorer according to the standard criteria of Rechtschaffen and Kales (23) every 30 s.
Blood sampling.
Within 1 h after installation of the venous catheter, subjects had approximately 2 ml of blood removed with replacement of volume using heparinized saline. Patients were then allowed to go to sleep between 10:30 and 11:00 p.m. while being monitored. Three additional blood samples were taken at approximately 1:00 a.m., 3:00 a.m., and 5:00 a.m. Subjects then awoke between 7:00 and 7:30 a.m., after which a final blood sample was taken. Whole heparinized blood samples were kept at room temperature, and PBMCs were generated, counted, and plated in ELISPOT plates within 6 h of collection. Samples in PAXgene tubes (Qiagen Inc., Valencia, CA) were collected at the time of the venipuncture and stored frozen (−20°C) until further processing (RNA isolation for quantitative reverse transcription-PCR [qRT-PCR]).
Analysis of plasma cytokines using Luminex.
Immediately after collection, an aliquot of blood was centrifuged, and then plasma was removed and snap-frozen on dry ice with subsequent storage at −80°C until time of assay. Samples were then thawed, diluted 1:1 with assay buffer, and assayed with Millipore's Beadlyte human multicytokine detection system 2 (Billerica, MA) according to the instructions in the kit, with some modifications. This kit simultaneously measured the proinflammatory cytokines IL-1β, IL-6, IL-8, and tumor necrosis factor alpha (TNF-α) and the anti-inflammatory cytokines IL-4 and IL-10. There were two modifications to the assay protocol. To stop the reaction and remove the unbound luminescence, the wells in the 96-well plates were rinsed twice with assay buffer instead of using the stop solution. In addition, the standard solution was serially diluted to obtain a standard curve with concentrations of 0, 0.49, 0.98, 1.95, 3.90, 7.80, 15.60, 31.25, and 62.5 pg/ml. Data reduction was performed using the MasterPlex-QT software (Miraibio, Alameda, CA); the standard curves were fit with a linear model using those standards that encompassed the values for each cytokine obtained from the samples in each assay. The r2 values for the linear fits were always above 0.95 and very often above 0.99. In each 96-well plate, the standards and a quality control pool were run in triplicate, and 33 samples were run in duplicate. Additional details about this assay have been published elsewhere (28).
Measurement of ELISPOT data.
Because the ELISPOT approach measures cytokine production within a maximum period of 48 h (9), it establishes frequencies of Th1 and Th2 cells as present in vivo, allowing induction of memory cells to produce cytokines but not their priming, proliferation, or in vitro differentiation. We (S. K. Schwander) have been involved in developing a sensitive IL-10 ELISPOT assay (9). Individual antigen-specific cytokine-producing cells can be detected with high sensitivity (18) directly ex vivo within a large pool (1/300,000 cells) of pharmacologically untreated cells. In the case of nonspecific stimuli that were used in this assay, proportions of responder cells are expected to be considerably higher and represent maximally inducible and functional cell numbers.
Preservation of nocturnal whole blood samples.
Extended pilot experiments were performed to assess optimal short-term storage conditions for whole blood samples for ELISPOT-based cytokine assessments. Nonspecific ELISPOT assay results were minimized by keeping whole blood samples in upright standing Vacutainer tubes (BD, Franklin Lakes, NJ) at room temperature until PBMCs were generated. To ensure comparability of the assays between samples drawn at around midnight and samples drawn in the early morning hours, all whole blood samples were kept for 4 to 6 h at room temperature until preparation of their respective PBMCs. Plating of PBMCs into the ELISPOT assay plates started at 9:00 a.m. with the sample from the earliest venipuncture time point and ended at 1:00 p.m with the sample from the latest (7:00 a.m.) venipuncture time point.
Preparation of PBMCs.
PBMCs were prepared using standard procedures (2). Briefly, heparinized venous whole blood diluted 1:1 with supplemented RPMI medium (BioWhittaker, Walkersville, MD) was centrifuged (1,200 rpm, 45 min, 21°C) over a Ficoll-Paque density gradient. PBMCs were removed from the interface, washed twice in RPMI, and resuspended in complete culture medium (RPMI with 2% pooled human AB serum). PBMCs were counted in a hemacytometer, and PBMCs numbers were adjusted to various concentrations. Viability of PBMCs was 100% based on trypan blue exclusion.
Constitutive and stimulant-induced IL-1β, IL-4, IL-6, IL-8, IL-10, and TNF-α production based on ELISPOT assays.
To assess constitutive or maximally stimulated cytokine production, PBMCs were cultured in complete culture medium only or in the presence of lipopolysaccharide (LPS; 1 μg/ml, final concentration) or phytohemagglutinin (PHA; 5 μg/ml, final concentration), respectively. Because no definitive etiopathogenic agent is known for chronic fatigue syndrome, it was not possible to assess antigen-specific cytokine induction. Aliquots of 100 μl of the stimuli in complete culture medium were added to the wells. Aliquots (100 μl) of the PBMC suspensions were then transferred into each well of the ELISPOT plates by using large-orifice pipette tips. ELISPOT plates (MultiscreenHTS high protein binding 96-well plates; Millipore, Bedford, MA) were coated with primary monoclonal antibodies against IL-1β, IL-4, IL-6, IL-8, IL-10, and TNF-α. IL-1β, IL-6, and IL-8 ELISPOT assays were done using commercial kits (Cell Sciences, Canton, MA) according to the manufacturer's instructions. IL-4- and IL-10-producing as well as TNF-α-producing cells were assessed using in-house ELISPOT assays and capture and biotinylated detection antibodies from BD Pharmingen, San Diego, CA (IL-4 and IL-10) and Endogen Pierce, Rockford, IL (TNF-α), respectively. Constitutive and stimulant-induced cytokine production levels by the PBMCs were assessed in triplicate wells. The optimal concentrations of PBMCs to be used per well differed for the various cytokine ELISPOT assays and had been identified in pilot experiments. Final PBMC concentrations per well were as follows: IL-1β, 100,000; IL-4, 200,000; IL-6, 2,000; IL-8, 2,000; IL-10, 200,000; TNF-α, 20,000 and 40,000 (dual setup).
Similarly, optimal short-term culture incubation periods varied by cytokine and had been identified in pilot experiments or were according to manufacturers' specifications: IL-1β, 20 h; IL-4, 48 h; IL-6, 24 h; IL-8, 24 h; IL-10, 48 h; TNF-α, 4 h.
Following the short-term cultures, PBMCs were washed off the plates with phosphate-buffered saline (PBS; three times) and PBS-0.05% Tween (three times), and detection antibodies were added overnight at 4°C. On the next day plates were washed and streptavidin/horseradish peroxidase (HRP) was added for 2 h. Plates were then washed again and spots visualized using color reagents (kits) or with peroxidase-conjugated streptavidin (Dako, Glostrup, Denmark) and the chromogen 1% 3-amino-9-ethylcarbazole (AEC; Pierce, Rockford, IL) in the in-house assays. Following an additional washing step, plates were dried overnight.
Measurement of cytokine production.
Plates were then scanned, and spot-forming units (cytokine spots) were enumerated using an automated ELISPOT reader (Immunospot series I analyzer, software version 3.0; Cellular Technology, Ltd., Cleveland, OH) that permits single-cell analysis of size, chromatic density, and shape of the cytokine spots by employing objective definition criteria for comparison of separate wells. Frequencies of cytokine-producing cells were calculated by averaging the number of spots from triplicate wells per condition.
Measurement of cytokine gene expression. (i) cDNA synthesis.
RNA was isolated from PAXgene tubes according to the manufacturer's guidelines. cDNA synthesis was performed using the following method. For each reaction mixture, 10 μl of RNA was added to the following reagents in 0.2-ml PCR tubes: 2.0 μl reaction buffer (10×), 0.8 μl of deoxynucleoside triphosphate mix, 2.0 μl random primers, and 1.0 μl reverse transcriptase. The volume was brought to 20 μl by adding 4.2 μl of PCR-grade water. The samples were incubated at 25°C for 10 min and the at 37°C for 120 min, followed by 85°C for 5 s. All samples were then held at 4°C and stored at −70°C until further use.
(ii) Real-time PCR assay.
The TaqMan universal PCR was performed in 96-well optical plates using an ABI 7500 sequence detection system (Applied Biosystems, Foster City, CA). Table 2 provides a list of genes that were evaluated: human glyceraldehyde 3-phosphate dehydrogenase (GAPDH), IL-10, IL-1B, TNF, and IL-4. Each run contained, in duplicate, one cDNA sample, a no-template control (NTC), and one untreated and one treated cell line control sample. Each 25-μl reaction mixture contained 12.5 μl 2× master mix (Applied Biosystems, Foster City, CA), 1 μl primer/probe mix, 1 μl cDNA, 10.5 μl PCR-grade water. The samples were incubated at 50°C for 2 min, followed by 95°C for 10 min, and then 40 cycles at 95°C for 15 s, 60°C for 1 min.
TABLE 2.
Genes targeted and the gene-specific primers and probes used
Data preprocessing.
Based on the fact that cytokines display burst-like changes in level over time (10) and our preliminary observation that every data set, for every cytokine and from every assay, had some extreme outlier values, producing non-Gaussian distributions, data preprocessing was necessary. We considered linear and second-order polynomial detrenders and selected the former for two reasons: first because our earlier work found no evidence of higher-order trends in 24-h data, and next because nonlinear detrending did not adequately identify outliers. Thus, we developed a filtering method based on LOWESS, a locally weighted regression, to eliminate extreme values from the data (6). The following are the details of our procedure: we repeatedly computed a linear least-squares regression of a given time series {xi}, i = 1,…, m (m = 5 for this study) using weights {δi}, i = 1,…, m calculated from the weight function B(x). The weight function B(x) is a bisquare function defined by B(x) = (1 − x2)2 for x < 1 and B(x) = 0 for x ≥ 1.
Then, the weight δi of each data point was decided by δi = B(ek/6s), where ei = ŷi − yi is residual between the current fitted value ŷi and given data point yi at each iteration and s is the median of ei . The weights {δi} were updated at each iteration step. We repeated the above weighted regression until the chi-square statistic marked the minimum value. After estimating the proper trend, the 95% confidence areas of the trend were also determined. Then we defined outliers as the data which exceeded these areas and excluded them from the data for the later analyses. Secondly, in order to improve the normality of the data, the data were log transformed after adding a value of 1 to each datum. To improve understanding of the results, however, we have prepared the tables using the actual numerical data (see Tables 3 to 5, below).
TABLE 3.
Group comparisons of mean cytokine levels based on Luminex assay
| Cytokine | Mean cytokine level (pg/ml)a |
|||
|---|---|---|---|---|
| Control (n = 20) | CFS (n = 19) | CFS with FM (n = 11) | CFS without FM (n = 8) | |
| IL-1β | 0.19 ± 0.08 | 0.07 ± 0.03 | 0.04 ± 0.02 | 0.12 ± 0.06 |
| IL-4 | 2.08 ± 0.56 | 1.78 ± 0.59 | 1.14 ± 0.54 | 2.66 ± 1.17 |
| IL-6 | 0.55 ± 0.20 | 0.23 ± 0.07 | 0.29 ± 0.11 | 0.14 ± 0.08 |
| IL-8 | 2.60 ± 0.47 | 2.06 ± 0.31 | 1.90 ± 0.38 | 2.29 ± 0.54 |
| IL-10 | 0.20 ± 0.07 | 0.35 ± 0.10 | 0.19 ± 0.07 | 0.57 ± 0.18*† |
| TNF-α | 0.75 ± 0.22 | 0.56 ± 0.19 | 0.42 ± 0.19 | 0.76 ± 0.37 |
Values are means ± standard errors of the means of raw data. The statistical tests were conducted with logarithmic-transformed data. *, significant difference from control subjects (P < 0.05; ANOVA); †, significant difference between subjects with CFS plus FM versus those with CFS without FM (P < 0.05; ANOVA). Values in boldface indicate significant cases.
TABLE 5.
Group comparisons of mean cytokine levels based on qRT-PCR
| Cytokine | Mean cytokine level (relative quantity)a |
|||
|---|---|---|---|---|
| Control (n = 20) | CFS (n = 24) | CFS with FM (n = 13) | CFS without FM (n = 11) | |
| IL-1β | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| IL-4 | 1.00 ± 0.27 | 0.73 ± 0.21 | 0.53 ± 0.16 | 0.96 ± 0.41 |
| IL-10 | 0.65 ± 0.18 | 0.39 ± 0.05 | 0.43 ± 0.07 | 0.34 ± 0.08 |
| TNF-α | 0.82 ± 0.19 | 0.64 ± 0.07 | 0.58 ± 0.09 | 0.71 ± 0.13 |
Values are means ± standard errors of the means of raw data. The statistical tests were conducted with logarithmic-transformed data.
For almost all data, one or two iterations of weighted regression were enough to obtain the minimum chi-square value. Of the 1,380 samples evaluated on Luminex and ELISPOT, 2 and 24, respectively, were excluded as outliers; of the 920 samples evaluated by qRT-PCR, 9 were excluded. We detected no temporal pattern for outliers in any of the data sets. If more than two of five data points were missed or eliminated, we excluded the data for the analysis. Thus, the number of subjects whose samples were analyzed (i.e., those listed in Tables 3 to 5) is not the same as the total number of subjects who entered the study. From the preprocessed data, we calculated a mean cytokine value for each cytokine of each subject. This provided us final data sets comprised of the mean cytokine values for six different cytokines × n subjects for the following analyses.
Correlation and statistical analysis.
We adopted Spearman's correlation coefficients to examine the correlation properties between two variables as a conservative measure, in order to avoid the effects of extreme values. For similar reasons we used both the nonparametric Mann-Whitney test and the conventional t test to determine the significance of differences in cytokine expression. One-way analysis of variance (ANOVA) followed by the Tukey post hoc test was used for group mean comparisons. Furthermore, in order to examine the group differences in the expression levels of each cytokine over time, we used a linear mixed model, which can deal with missing cytokine data (<6% of total data points which include outliers). In the model, we treated group and sampling times as fixed effects and subjects as random effects. Bonferroni post hoc analyses were performed when the main effects (group or times) or the interaction effect (group × time) were significant. P values of <0.05 were considered statistically significant.
RESULTS
Table 1 provides demographic data for the subjects studied. There were no significant differences in age, BMI, or sleep latency (i.e., time to fall asleep) among the groups. CFS patients as a group had significantly lower mean sleep efficiency than control subjects, reflecting more-disturbed sleep. There was no difference in rates of severe CFS between patients with or without comorbid FM.
Correlations across different assays.
We first examined correlations of expression levels among the three cytokine detection assay types for each cytokine by using Spearman's correlation coefficients. The only significant correlation was an inverse one for IL-10 between the results from the Luminex and unstimulated ELISPOT assays. However, this effect was principally due to the group difference in IL-10 between patients and controls, with patients having higher levels than controls in the Luminex assay and lower levels than controls in the ELISPOT assay. We also evaluated the correlations by using Pearson's correlation coefficients, an independent component analysis (ICA), and the alternating conditional expectations (ACE) method (3); however, these analyses revealed no significant correlations. Thus, expression levels of cytokines in the various assays were independent of each other.
Luminex assay: group differences in cytokine production.
Table 3 summarizes mean cytokine levels for each study group. There were no significant differences between CFS patients either with or without FM as a single group versus control subjects for any of the cytokines. To further evaluate the data, we stratified the CFS data into two subgroups (CFS with FM [n = 15] and CFS without FM [n = 11]) and compared the group differences among groups (Table 3). The expression level of IL-10 for the CFS without FM subgroup was significantly higher than that of healthy control subjects or the group with CFS and FM. In addition, CFS patients with FM tended to have decreased IL-1β (P = 0.068) compared with control subjects.
Exploration for correlations between cytokine data and clinical variables was negative except for a tendency toward lower IL-8 values with higher CFS severity score (r = −0.44; P = 0.058). Scores for patients with CFS alone did not differ in severity from those with CFS plus FM (Table 1). There was a significant correlation between total sleep period and IL-10 (r = −0.33; P = 0.042) in patients.
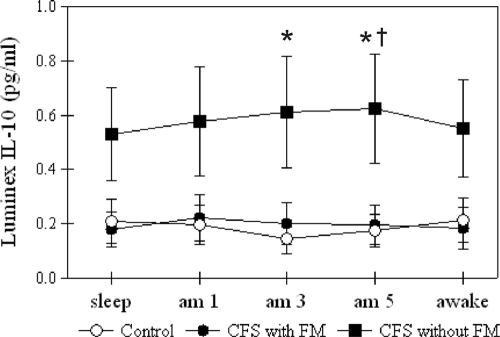
The mixed model analysis reiterated our group analysis by showing a significant difference in only IL-10 levels. Figure 1 shows the distribution of IL-10 data over time. CFS patients without FM showed higher IL-10 values than controls at 3:00 and 5:00 a.m. and higher values than for subjects with CFS and FM at 5:00 a.m.
FIG. 1.
Group-averaged IL-10 levels (in pg/ml) from the Luminex assay for control, CFS with FM, and CFS without FM groups. The error bars indicate standard errors of means. *, significant difference from control; †, significant difference between CFS with FM and CFS without FM. Note that the statistical tests were conducted with logarithmic-transformed data.
ELISPOT assay: group differences in cytokines under basal conditions.
Table 4 summarizes mean cytokine levels for all groups from the ELISPOT assay. There were no significant group differences among any of the cytokines for patients compared to controls under either stimulated or unstimulated conditions. As had been the case with plasma protein, IL-1β tended to be lower in CFS subjects than controls (P = 0.099). We found no significant correlation between mean cytokine levels in the ELISPOT assay and clinical variables for the patients. For the mixed model analysis, there were no significant differences in any cytokines under either stimulated or unstimulated conditions.
TABLE 4.
Group comparisons of cytokine-producing cells based on ELISPOT assaya
| Cytokine | Mean no. of cytokine-producing cells |
|||
|---|---|---|---|---|
| Control (n = 23) | CFS (n = 23) | CFS with FM (n = 13) | CFS without FM (n = 10) | |
| IL-1β | 105.15 ± 57.45 | 20.68 ± 8.25 | 27.36 ± 13.87 | 12.00 ± 5.91 |
| IL-4 | 7.40 ± 1.71 | 10.87 ± 2.01 | 10.92 ± 2.33 | 10.80 ± 3.65 |
| IL-6 | 71.77 ± 19.57 | 82.17 ± 18.28 | 66.02 ± 22.37 | 103.16 ± 30.42 |
| IL-8 | 35.60 ± 6.96 | 56.53 ± 9.17 | 54.37 ± 12.19 | 59.33 ± 14.60 |
| IL-10 | 58.17 ± 19.87 | 30.56 ± 12.17 | 44.91 ± 20.58 | 11.92 ± 5.53 |
| TNF-α | 156.71 ± 27.33 | 118.25 ± 27.26 | 129.84 ± 35.71 | 103.18 ± 43.84 |
| ST IL-1β | 539.7 ± 88.1 | 463.8 ± 79.5 | 407.2 ± 91.5 | 537.4 ± 141.3 |
| ST IL-4 | 262.2 ± 28.5 | 263.4 ± 26.3 | 269.6 ± 31.0 | 255.3 ± 47.0 |
| ST IL-6 | 106.7 ± 18.4 | 112.7 ± 16.1 | 104.5 ± 19.4 | 123.4 ± 28.0 |
| ST IL-8 | 40.9 ± 7.2 | 66.6 ± 8.9 | 55.0 ± 12.2 | 81.6 ± 11.9 |
| ST IL-10 | 571.7 ± 47.5 | 521.6 ± 45.9 | 526.5 ± 61.7 | 515.1 ± 72.5 |
| ST TNF-α | 471.4 ± 35.9 | 460.1 ± 41.2 | 452.5 ± 44.7 | 470.1 ± 77.7 |
Values are means ± standard errors of the means of raw data. ST, stimulant-induced data. The statistical tests were conducted with logarithmic-transformed data.
qRT-PCR assay.
Table 5 summarizes mean cytokine levels for all groups based on results from the qRT-PCR assay. There were no significant group differences among any of the cytokines for patients compared to control subjects. We also examined relationships between cytokine levels of CFS patients and clinical variables. IL-1β showed a tendency toward increased levels in patients with higher severity scores (r = 0.34; P = 0.094); however, we found no significant correlation between IL-1β and CFS severity in the PCR assay. For the mixed model analysis, there were no significant differences in any cytokines.
In summary, correlations among the methods were not significant. The Luminex assay revealed increases in IL-10 for patients with CFS alone and a tendency for decreases in IL-1β in the CFS plus FM group. No differences in cytokines were found between patients and controls in either the ELISPOT or the qRT-PCR assays.
DISCUSSION
A major hypothesis for the cause of CFS is an immune dysfunction characterized by a general upregulation of proinflammatory cytokines. This hypothesis is based, in part, on clinical reports that administration of proinflammatory cytokines in the treatment of disease in humans produces a flu-like syndrome resembling that of CFS (26). However, there have been few empirical data supporting this hypothesized link between proinflammatory cytokines and CFS, and our own prior studies in CFS patients did not find any cytokine abnormalities (15, 38). The results of this study using nocturnal samples also do not support the hypothesis of an upregulated inflammatory immune system mediated by cytokines in the genesis of CFS. A similar conclusion has been reached in a study of a sample of patients with postinfectious fatigue (32).
Experimental evidence exists to support the notion of a cytokine/sleep network in which proinflammatory cytokines induce sleep and anti-inflammatory cytokines disturb sleep (13, 14). The existence of this immunological homeostatic system controlling sleep led us to generate an alternative hypothesis to the proinflammatory one as a cause of CFS, namely, that disturbances in this cytokine/sleep network could lead to disturbed sleep with resulting fatigue. Our own earlier study looking at cytokine secretory patterns in plasma across a 24-h day supported that hypothesis. We found elevated levels of IL-10 in the blood of FM patients occurring during the nighttime (28). The data collected here support that result for the CFS-only group and not for the CFS plus FM group, as might have been expected. But the magnitude of this difference was small, less than 1 pg/ml, and thus may be of only limited biological significance. Moreover, our analyses covered multiple cytokines and multiple methods, and so this one significant finding could reflect a type 1 error.
However, plasma levels of IL-10 in our patients did correlate inversely with their total sleep period, and our own earlier work using neural nets (12) as well as that of other groups studying cytokines in CFS also reported a shift of the cytokine network away from a proinflammatory and toward an anti-inflammatory balance (25, 27). Thus, the modest change in IL-10 reported here is consistent with the suggestion that patients with CFS show a shift toward Th2-type cytokines.
The argument that CFS is caused by immune dysregulation has also been extended to patients with FM (33). In FM, the best evidence is for elevations in IL-8, a chemokine involved in the early stages of the genesis of an inflammatory response (10, 33, 34). Those results were based on single daytime samples of either serum or supernatants of stimulated or unstimulated PBMCs. We did not find this result in our sample of CFS patients with FM, despite our sampling repeatedly during the nighttime. We cannot explain this discrepancy except to suggest that the differences found previously may reflect an altered circadian pattern of IL-8, with increases just during the waking period. Another group reported no decrease in either serum level or gene expression of mRNA for IL-8 in patients with chronic widespread pain who were taking various medicines for their pain (31). However, those researchers did find that the chronic pain patients had both reduced gene expression and reduced serum levels of the anti-inflammatory cytokines IL-4 and IL-10 compared to healthy controls, a different result from that found in the current study. It is also possible that differences in medication usage among patients in these different studies might have been responsible for this discrepant result.
The fact that both CFS and FM patients have some alterations in the balance of Th1 and Th2 cytokines toward a Th2 or sleep-disrupting response suggests a possible role for cytokine-induced, disturbed sleep in the pathogenesis of both syndromes. We have recently studied the sleep of subgroups of patients with CFS alone or with CFS plus FM after excluding patients with diagnosable sleep pathology (29). We found that both subgroups had evidence of disrupted sleep as manifested by shorter bouts of sleep than healthy controls (29). In a subsequent study, we sleep deprived some of these patients for an entire night and found that one-third of them showed delays in falling asleep the next morning compared to healthy control subjects (unpublished data). Sleep deprivation is an obvious cause of severe fatigue, and several studies have shown that disrupted sleep can also lead to reduced pain thresholds in healthy volunteers (11, 16, 19). Combined, these data suggest that sleep disturbances, perhaps related to an altered sleep/cytokine network favoring a Th2 response, may lead to the symptoms consistent with the diagnoses of CFS and FM.
Because the literature did not indicate which of the several methodologies available to assay cytokines would be the best in identifying abnormalities in CFS and FM, we used a comprehensive approach and measured cytokine protein concentrations in plasma, constitutive and stimulated protein release from PBMCs, and cytokine mRNA expression in whole blood cells. Importantly, our findings indicate that cytokine data using each of the assay methods did not correlate well with cytokine data generated by the other methods, suggesting that each method captures different aspects of cytokine properties or function. The relatively poor correlation between assay methods has been noted previously (21).
The only method that revealed a significant result between patients and controls was measurement of cytokines in plasma using the Luminex technology. The fact that more basic measures of cytokine secretion and release did not reveal significant differences between patients and controls may mean that the increases in IL-10 observed here and in our previous work may reflect systemic factors influencing cytokine levels in blood from CFS patients rather than frank increases in anti-inflammatory cytokine manufacture.
In summary, we designed this study to examine two alternative hypotheses: one, that CFS and FM are caused by increases in proinflammatory cytokines, and two, that CFS and FM are caused by disrupted sleep produced by increases in anti-inflammatory cytokines. Since disparate results have come from studies sampling the blood once during the daytime, we decided to sample blood several times during the nighttime while subjects slept. We reduced variability by studying women with CFS alone or with CFS plus FM, at the same menstrual phase, and after excluding any subjects with current major depression. Despite using these “pure” patient groups, we found evidence for a rather small elevation of one anti-inflammatory cytokine, IL-10, only in those patients with CFS alone, and in only one of three assays, the one quantifying cytokines in plasma. Obvious limitations of this study are the relatively small sample size and our focus on female patients only. These data provide additional experimental evidence against the hypothesis that CFS is a manifestation of an upregulated proinflammatory state and leave open the role for anti-inflammatory cytokines in the genesis of CFS.
Acknowledgments
This work was supported by NIH AI-54478 and NIH HL-077462. Additional support to extend the work to IL-6 and IL-8 was provided by the American Fibromyalgia Syndrome Association.
Footnotes
Published ahead of print on 24 February 2010.
REFERENCES
- 1.Amel Kashipaz, M. R., D. Swinden, I. Todd, and R. J. Powell. 2003. Normal production of inflammatory cytokines in chronic fatigue and fibromyalgia syndromes determined by intracellular cytokine staining in short-term cultured blood mononuclear cells. Clin. Exp. Immunol. 132:360-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyum, A. 1976. Isolation of lymphocytes, granulocytes and macrophages. Scand. J. Immunol. Suppl. 5:9-15. [PubMed] [Google Scholar]
- 3.Breiman, L., and J. H. Friedman. 1985. Estimating optimal transformations for multiple regression and correlation. J. Am. Stat. Assoc. 80:580-586. [Google Scholar]
- 4.Brimacombe, M., D. Helmer, and B. H. Natelson. 2002. Clinical differences exist between patients fulfilling the 1988 and 1994 case definitions of chronic fatigue syndrome. J. Clin. Psychol. Med. Settings 9:309-314. [Google Scholar]
- 5.Ciccone, D. S., and B. H. Natelson. 2003. Comorbid illness in the chronic fatigue syndrome: a test of the single syndrome hypothesis. Psychosom. Med. 62:268-275. [DOI] [PubMed] [Google Scholar]
- 6.Cleveland, W. S. 1979. Robust locally weighted regression and smoothing scatterplots. J. Am. Stat. Assoc. 74:829-836. [Google Scholar]
- 7.Fukuda, K., R. Nisenbaum, G. Stewart, W. W. Thompson, L. Robin, R. M. Washko, D. L. Noah, D. H. Barrett, B. Randall, B. L. Herwaldt, A. C. Mawle, and W. C. Reeves. 1998. Chronic multisymptom illness affecting Air Force veterans of the Gulf War. JAMA 280:981-988. [DOI] [PubMed] [Google Scholar]
- 8.Fukuda, K., S. E. Straus, I. Hickie, M. C. Sharpe, A. Komaroff, A. Schluederberg, J. F. Jones, A. R. Lloyd, S. Wessely, N. G. Gantz, G. P. Holmes, L. Steele, M. Reyes, S. Abbey, J. Rest, H. Jolson, D. L. Peterson, J. H. M. M. Vercoulen, U. Tirelli, B. Evengard, B. H. Natelson, and W. C. Reeves. 1994. The chronic fatigue syndrome: a comprehensive approach to its definition and study. Ann. Intern. Med. 121:953-959. [DOI] [PubMed] [Google Scholar]
- 9.Guerkov, R. E., O. S. Targoni, C. R. Kreher, B. O. Boehm, M. T. Herrera, M. Tary-Lehmann, P. V. Lehmann, and S. K. Schwander. 2003. Detection of low-frequency antigen-specific IL-10-producing CD4(+) T cells via ELISPOT in PBMC: cognate vs. nonspecific production of the cytokine. J. Immunol. Methods 279:111-121. [DOI] [PubMed] [Google Scholar]
- 10.Gür, A., M. Karakoç, K. Nas, R. Çevik, A. Denli, and J. Saraç. 2002. Cytokines and depression in cases with fibromyalgia. J. Rheumatol. 29:358-361. [PubMed] [Google Scholar]
- 11.Hakkionen, S., A. Alloui, A. Gross, A. Eschallier, and C. Dubray. 2001. The effects of total sleep deprivation, selective sleep interruption and sleep recovery on pain tolerance in healthy subjects. J. Sleep Res. 10:35-42. [DOI] [PubMed] [Google Scholar]
- 12.Hanson, S. J., W. C. Gause, and B. H. Natelson. 2001. Detection of immunologically significant factors for chronic fatigue syndrome using neural network classifiers. Clin. Diagn. Lab. Immunol. 8:658-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krueger, J. M., F. Obál, J. Fang, T. Kubota, and P. Taishi. 2000. The role of cytokines in physiological sleep regulation. Ann. N. Y. Acad. Sci. 917:211-221. [DOI] [PubMed] [Google Scholar]
- 14.Kushikata, T., J. Fang, and J. M. Krueger. 1999. Interleukin-10 inhibits spontaneous sleep in rabbits. J. Interferon Cytokine Res. 19:1025-1030. [DOI] [PubMed] [Google Scholar]
- 15.LaManca, J. J., S. Sisto, J. E. Ottenweller, S. Cook, A. Peckerman, Q. Zhang, T. N. Denny, W. C. Gause, and B. H. Natelson. 1999. Immunological response in chronic fatigue syndrome following a graded exercise test to exhaustion. J. Clin. Immunol. 19:135-142. [DOI] [PubMed] [Google Scholar]
- 16.Lentz, M. J., C. A. Landis, J. Rothermel, and J. L. Shaver. 1999. Effects of selective slow wave sleep disruption on musculoskeletal pain and fatigue in middle aged women. J. Rheumatol. 26:1586-1592. [PubMed] [Google Scholar]
- 17.Maes, M., J. Lambrechts, E. Bosmans, J. Jacobs, E. Suy, C. Vandervorst, C. De Jonckheere, B. Minner, and J. Raus. 1992. Evidence for a systemic immune activation during depression: results of leukocyte enumeration by flow cytometry in conjunction with monoclonal antibody staining. Psychol. Med. 22:45-53. [DOI] [PubMed] [Google Scholar]
- 18.Matesic, D., P. V. Lehmann, and P. S. Heeger. 1998. High-resolution characterization of cytokine-producing alloreactivity in naive and allograft-primed mice. Transplantation 65:906-914. [DOI] [PubMed] [Google Scholar]
- 19.Moldofsky, H., and P. Scarisbrick. 1976. Induction of neurasthenic musculoskeletal pain syndrome by selective sleep stage deprivation. Psychosom. Med. 38:35-44. [DOI] [PubMed] [Google Scholar]
- 20.Natelson, B. H., M. H. Haghighi, and N. M. Ponzio. 2002. Evidence for the presence of immune dysfunction in chronic fatigue syndrome. Clin. Diagn. Lab. Immunol. 9:747-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pascal, L. E., L. D. True, D. S. Campbell, E. W. Deutsch, M. Risk, I. M. Coleman, L. J. Eichner, P. S. Nelson, and A. Y. Liu. 2008. Correlation of mRNA and protein levels: cell type-specific gene expression of cluster designation antigens in the prostate. BMC Genomics 9:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peckerman, A., J. J. LaManca, K. Dahl, B. Qureishi, and B. H. Natelson. 2003. Abnormal impedance cardiography predicts symptom severity in chronic fatigue syndrome. Am. J. Med. Sci. 326:55-60. [DOI] [PubMed] [Google Scholar]
- 23.Rechtshaffen, A., and A. Kales. 1968. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Brain Information/ Brain Research Institute, Los Angeles, CA.
- 24.Robbins, L. N., L. B. Cottler, K. K. Bucholz, W. M. Compton, C. S. North, and K. M. Rourke. 2000. Diagnostic interview schedule for the DSM-IV (DIS-IV). University of Washington School of Medicine, St. Louis, MO.
- 25.Skowera, A., A. Cleare, D. Blair, L. Bevis, S. C. Wessely, and M. Peakman. 2004. High levels of type 2 cytokine-producing cells in chronic fatigue syndrome. Clin. Exp. Immunol. 135:294-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smedley, H., M. Katrak, K. Sikora, and T. Wheeler. 1983. Neurological effects of recombinant human interferon. BMJ 286:262-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.ter Wolbeek, M., L. J. van Doornen, A. Kavelaars, E. M. Van de Putte, M. Schedlowski, and C. J. Heijnen. 2007. Longitudinal analysis of pro- and anti-inflammatory cytokine production in severely fatigued adolescents. Brain Behav. Immun. 21:1063-1074. [DOI] [PubMed] [Google Scholar]
- 28.Togo, F., B. H. Natelson, G. K. Adler, J. E. Ottenweller, D. L. Goldenberg, Z. R. Struzik, and Y. Yamamoto. 2009. Plasma cytokine fluctuations over time in healthy controls and patients with fibromyalgia. Exp. Biol. Med. (Maywood) 234:232-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Togo, F., B. H. Natelson, N. S. Cherniack, J. Fitzgibbons, C. Garcon, and D. M. Rapoport. 2008. Sleep structure and sleepiness in chronic fatigue syndrome with or without coexisting fibromyalgia. Arthritis Res. Ther. 10:R56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tomoda, A., T. Joudoi, E. Rabab, T. Matsumoto, T. H. Park, and T. Miike. 2005. Cytokine production and modulation: comparison of patients with chronic fatigue syndrome and normal controls. Psychiatry Res. 134:101-104. [DOI] [PubMed] [Google Scholar]
- 31.Üçeyler, N., R. Valenza, M. Stock, R. Schedel, G. Sprotte, and C. Sommer. 2006. Reduced levels of antiinflammatory cytokines in patients with widespread pain. Arthritis Rheum. 54:2656-2664. [DOI] [PubMed] [Google Scholar]
- 32.Vollmer-Conna, U., B. Cameron, D. Hadzi-Pavlovic, K. Singletary, T. Davenport, S. Vernon, W. C. Reeves, I. Hickie, D. Wakefield, and A. R. Lloyd. 2007. Postinfective fatigue syndrome is not associated with altered cytokine production. Clin. Infect. Dis. 45:732-735. [DOI] [PubMed] [Google Scholar]
- 33.Wallace, D. J., M. Linker-Israeli, D. Hallegue, S. Silverman, D. Silver, and M. H. Weisman. 2001. Cytokines play an aetiopathogenetic role in fibromyalgia: a hypothesis and pilot study. Rheumatology 40:743-749. [DOI] [PubMed] [Google Scholar]
- 34.Wang, H., M. Buchner, M. T. Moser, V. Daniel, and M. Schiltenwolf. 2009. The role of IL-8 in patients with fibromyalgia: a prospective longitudinal study of 6 months. Clin. J. Pain 25:1-4. [DOI] [PubMed] [Google Scholar]
- 35.White, P. D., K. E. Nye, A. J. Pinching, T. M. Yap, N. Power, V. Vleck, D. J. Bentley, J. M. Thomas, M. Buckland, and J. M. Parkin. 2004. Immunological changes after both exercise and activity in chronic fatigue syndrome: a pilot study. J. Chronic Fatigue Syndr. 12:51-66. [Google Scholar]
- 36.Wolfe, F., H. A. Smythe, M. B. Yunus, R. M. Bennett, C. Bombardier, D. L. Goldenberg, P. Tugwell, S. M. Campbell, M. Abeles, P. Clark, A. G. Fam, S. J. Farber, J. J. Fiechtner, C. M. Franklin, R. A. Gatter, D. Hamaty, J. Lessard, A. S. Lichtbroun, A. T. Masi, G. A. McCain, W. J. Reynolds, T. J. Romano, I. J. Russell, and R. P. Sheon. 1990. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia: report of the Multicenter Criteria Committee. Arthritis Rheum. 33:160-172. [DOI] [PubMed] [Google Scholar]
- 37.Zhang, Q., B. H. Natelson, J. E. Ottenweller, R. J. Servatius, J. J. Nelson, J. DeLuca, L. Tiersky, and G. Lange. 2000. Chronic fatigue syndrome beginning suddenly occurs seasonally over the year. Chronobiol. Int. 17:95-99. [DOI] [PubMed] [Google Scholar]
- 38.Zhang, Q., X. Zhou, T. Denny, J. Ottenweller, G. Lange, J. J. LaManca, M. H. Lavietes, C. Pollet, W. C. Gause, and B. H. Natelson. 1999. Changes in immune parameters in Gulf War veterans but not in civilians with chronic fatigue syndrome. Clin. Diagn. Lab. Immunol. 6:6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]