Abstract
The bacterium Mycobacterium tuberculosis remains one of the world's most successful pathogens, a situation that is aggravated by the fact that the existing vaccine, Mycobacterium bovis BCG, is not effective in adults. As with any vaccine, the purpose of giving BCG vaccination is to establish a long-lived state of memory immunity, but whether this is successfully completely established is still unclear. It is generally accepted that memory T cells can be divided into central and effector memory populations by function and by phenotype; however, the majority of data supporting this division have been generated using transgenic mouse models or mice that have recovered from acute viral infections. Tuberculosis, on the other hand, represents a persistent, chronic state of immunity in which the presence of memory T cells is far less well defined. We show here that mice vaccinated with BCG or chronically infected with M. tuberculosis establish antigen-specific populations of cells within the lungs that predominantly express a cellular phenotype consistent with their being effector or effector memory cells. In contrast, cells with a central memory phenotype exist in much lower numbers in the lungs but can be found in significantly larger numbers in the spleen, where they may represent a potential reservoir. These data suggest that the effector-to-central-memory T-cell transition may well be minimal in these persisting mycobacterial infections, and they support a novel hypothesis that this may explain the fundamental basis of the failure of the BCG vaccine in humans.
Mycobacterium tuberculosis remains one of the world's most successful bacterial pathogens, causing in excess of 2 million deaths and 8 million new cases each year (9). A troublesome aspect of this epidemic is the increasing incidence of isolates that are resistant to multiple drugs, now estimated at approaching half a million new cases each year (4, 26, 30). The seriousness of this situation is amplified by the fact that the existing Mycobacterium bovis BCG vaccine is not effective in adults (7, 23, 26).
The purpose of vaccination is to establish a long-lived state of immunological memory. In the context of tuberculosis this is the central ambition of vaccination, and it has been assumed to date that such a state can be established and that it is mediated by a specific subset of T cells. This arose from various early studies in the field, particularly from our laboratory (24) and from that of Andersen (1), in which mice infected with M. tuberculosis were found to generate long-lived memory immunity which provided a heightened state of acquired resistance to a secondary infection. Mice immunized with the BCG vaccine were also able to rapidly generate an acquired cellular immune response to this infection, characterized primarily by the emergence of a T-cell population capable of adoptively transferring substantial levels of protection against a challenge infection with M. tuberculosis (29). In studies conducted with a mouse model of memory immunity in which mice were rendered immune by a primary infection followed by antibiotic treatment and rest, tuberculosis-specific memory cells were recruited from the recirculating pool, leading to rapidly increasing precursor frequencies in the liver and a simultaneous decrease in the blood (2).
That the reality is probably more complicated, however, is shown by current information that indicates that there are at least two subsets of memory T cells, both for CD4 and CD8 cells, based upon the anatomical locations of these subsets, their expression of various cell surface markers, and various functional responses based on cytokine secretion and the rapidity of their response (11, 21, 37). A primary distinction is based upon homing receptors that allow entry into lymphoid tissues. Hence, those that lack or have low expression of CD62L and CCR7 and are found in peripheral tissues, where they seem to provide a first line of defense, are designated “effector memory” cells or TEM. A second population, which is high in expression of CD62L and CCR7, is found in lymph nodes and seems to represent a second line of defense; these cells are designated “central memory” T cells or TCM (18, 33-35).
Much of what is known about these cells, however, is based upon responses to defined antigens, as often as not in T-cell receptor (TCR) transgenic mice or in mice capable of efficiently clearing acute viral infections. Even today, far more is known about the CD8 memory T-cell response than about its CD4 counterpart (13, 19, 36, 38-40). In contrast, what happens in mycobacterial infections, where there is extensive inflammation and immune stimulation and in which the infection is often chronic, ensuring continued production of antigens, is only just starting to be studied. In previous work we found that BCG vaccination of mice established a pool of CD62Llo memory T cells in the lungs that seemed to persist for a very long period of time (17) and that similar populations of cells persisted in the lungs of mice chronically infected with M. tuberculosis (15). In the present study we revisited this observation, and we show here that both chronically infected mice and BCG-vaccinated (but not challenged) mice generate CD4 and CD8 T-cell populations in the lungs that have a phenotype known to be associated with cells that have the potential function of TEM. Since these cells predominate, it is reasonable to assume they mediate the accelerated resistance of the animal to challenge infection with M. tuberculosis (1, 2, 17, 24, 25). In the case of the chronically infected mice this probably represents a mixture of memory cells and effector cells, because a percentage of these cells could be stained for gamma interferon (IFN-γ). In contrast, cells with a phenotype characteristic of TCM represented a very small population that were found primarily in the spleen but not in the lungs. In addition, other populations of cells were observed, some of them apparently previously unnoticed. Based on these observations, we propose the novel hypothesis that an apparent failure by BCG to induce significant numbers of TCM may be the fundamental reason for its now generally recognized weakness as a vaccine.
MATERIALS AND METHODS
Animals.
Specific-pathogen-free female, 6- to 8-week-old C57BL/6 mice were purchased from Jackson Laboratories, Bar Harbor, ME. They were kept under barrier conditions in an ABL-III laboratory and fed sterile water and chow. All experimental protocols were approved by the Animal Care and Usage Committee of Colorado State University.
Experimental infections.
M. tuberculosis strain H37Rv was grown in Proskauer-Beck liquid medium containing 0.05% Tween 80 to mid-log phase and then frozen in aliquots at −70°C until needed. For low-dose aerosol infections, bacterial stocks were diluted in 5 ml of sterile distilled water to 2 × 106 CFU/ml and placed in a nebulizer attached to an airborne infection system (Glass-Col, Terre Haute, IN). Mice were exposed to an aerosol infection in which approximately 100 bacteria were deposited in the lungs of each animal. This established a chronic disease in the range of 5.0 to 5.6 log10 bacilli in the lungs over the course of the study. In the vaccination studies, animals were immunized with 106 CFU BCG Pasteur subcutaneously (note that these animals were not challenged).
Preparation of cells.
Mice were euthanized by CO2 asphyxiation, and the thoracic cavity was opened. The lung was cleared of blood by perfusion through the pulmonary artery with 10 ml of ice-cold phosphate-buffered saline (PBS) containing 50 U/ml of heparin (Sigma, St. Louis, MO). Lungs were aseptically removed, teased apart and treated with a solution of DNase IV (DNase) (Sigma Chemical; 30 μg/ml) and collagenase XI (Sigma Chemical; 0.7 mg/ml) for 45 min at 37°C. Spleens and pulmonary lymph nodes (LN) were also harvested. Bone marrow (BM) cells were harvested from the femurs of the mice, any possible erythrocyte contamination was lysed with Gey's solution (0.15 M NH4Cl, 10 mM HCO3), and the cells were washed with Dulbecco's modified Eagle's minimal essential medium. Total cell numbers were determined by flow cytometry using BD liquid counting beads, as described by the manufacturer (BD Pharmingen, San Jose, CA).
Flow cytometry for surface markers and intracellular cytokines.
For flow cytometry analysis, single-cell suspensions of lung from each mice were resuspended in PBS (Sigma-Aldrich) containing 0.1% of sodium azide. Cells were incubated in the dark for 25 min at 37°C with predetermined optimal titrations of specific antibodies. Cell surface expression was analyzed for CD44, CCR7, CD4, CD8α, CD62L, CD127, and CD45RB. Measurement of intracellular IFN-γ was conducted using a commercial kit. All antibodies and reagents were purchased from BD Pharmingen or eBioscience (eBioscience, San Diego, CA). To detect antigen-specific cells, cells were stained with a soluble pMHCII tetramer recognizing a major epitope of ESAT-6, generated as described elsewhere (22), or with a class I tetramer recognizing a major CD8 epitope of the M. tuberculosis protein MTb32, as we previously demonstrated (14). All the samples were analyzed on a Becton Dickinson LSR-II instrument, and data were analyzed using FACSDiva v5.0.1 software. Cells were gated on lymphocytes based on characteristic forward and side scatter profiles. Individual cell populations were identified according to the presence of specific fluorescence-labeled antibodies. All the analyses were performed with acquisition of a minimum of 300,000 events.
Statistics.
Statistical significance was determined using two-way analysis of variance (ANOVA) with Bonferroni post tests using GraphPad Prism v4.00 for Windows, (GraphPad Software, San Diego, CA).
RESULTS
Gating and analysis of memory T-cell subsets.
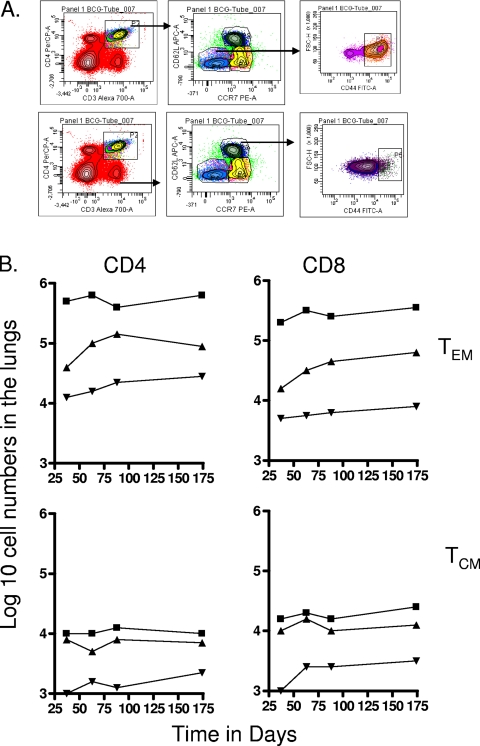
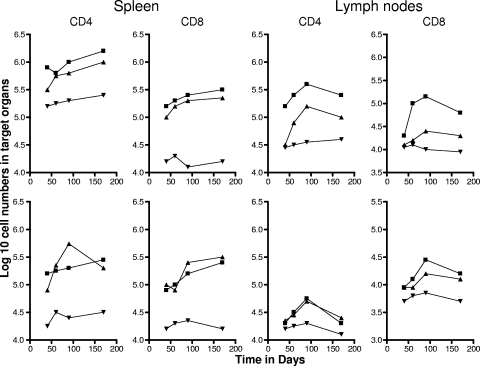
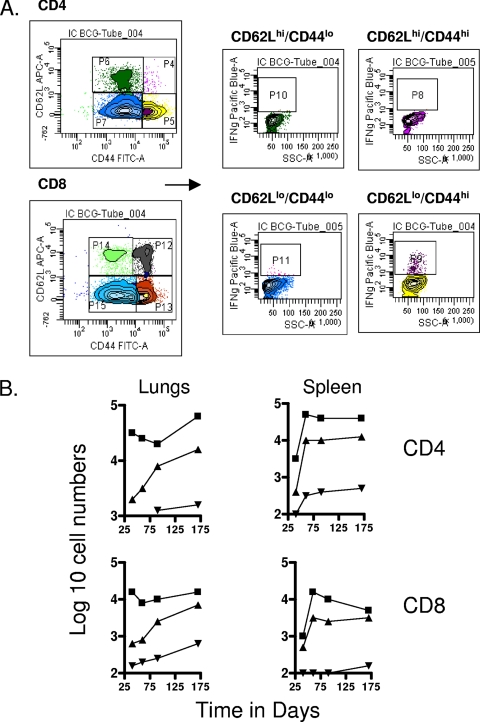
To differentiate between cells having the phenotype of TEM and TCM, we compared a variety of flow cytometry gating strategies based upon expression of the multiple markers CD62L, CCR7, CD44, CD45RB, and CD127, using previously published studies as a guide (31, 41, 42). The two last markers did not allow us to clearly distinguish between the different subsets (most cells were CD45RBmid/lo and CD127lo/mid), so we based our analysis on the distinctions between CD62L and CCR7 expression versus CD44hi expression (31, 33). As shown in Fig. 1, mice chronically infected with M. tuberculosis had approximately 50 to 70-fold-higher numbers of activated CD4 and CD8 cells expressing a CD62Llo CCR7lo CD44hi phenotype in the lungs compared to uninfected controls (P < 0.01 or lower). In this case this is almost certainly a mixture of activated effector T cells (AET) and TEM, whereas cells with this phenotype in the BCG-vaccinated mice, which were also significantly increased (P < 0.04 or lower), were probably mostly true TEM since the BCG vaccine itself never reaches the lung tissues after subcutaneous inoculation. Similar distributions were seen in the draining lymph nodes, whereas in the spleen there were essentially the same numbers seen in both infected groups (Fig. 2), suggesting that this organ was acting as a major reservoir of the two TEM subsets.
FIG. 1.
Changes in CD4 and CD8 T-cell subsets expressing markers for TEM and TCM in target organs over the course of the study. (A) Representative gating strategy to identify and quantitate CD62Llo CCR7lo CD44hi TEM and CD62Lhi CCR7hi CD44hi TCM populations. (B) Numbers of CD4 TEM, CD8 TEM, CD4 TCM, and CD8 TCM in the lungs versus time of the infection. Test groups were mice chronically infected with M. tuberculosis (▪), mice that had been subcutaneously vaccinated with BCG (▴), or age-matched uninfected controls (▾). In each case, the data point represents the log10 mean value for cells recovered from the lungs or other organs of four or five mice; standard errors of the means (SEM) are omitted for clarity and did not exceed 10%. In all cases, numbers of cells were increased in the infected animals compared to uninfected age-matched controls (for M. tuberculosis infection at all time points, P < 0.01 or lower for TEM values and P < 0.02 for TCM values; for BCG, P < 0.04 [day 40 CD4 and CD8 TEM] and P < 0.03 or lower [for all other data points]).
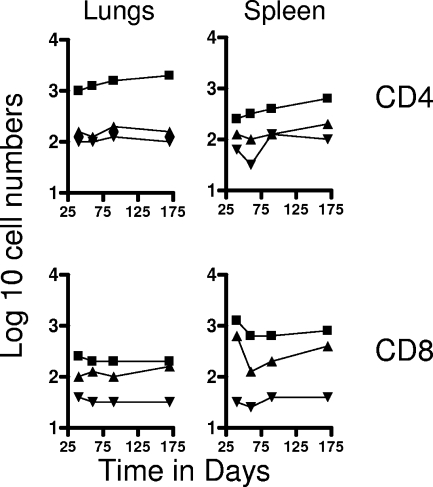
FIG. 2.
Numbers of CD4 TEM and CD8 TEM (top panels) and CD4 TCM and CD8 TCM (bottom panels) in the spleen and draining mediastinal lymph nodes versus time. Test groups were mice chronically infected with M. tuberculosis (▪), mice that had been subcutaneously vaccinated with BCG (▴), or age-matched uninfected controls (▾). In each case, the data point represents the log10 mean value for cells recovered from the lungs or other organs of four or five mice; SEM are omitted for clarity and did not exceed 10%. All data points for the spleen were significantly higher than for uninfected controls (P < 0.02 or lower). In the lymph nodes, TEM data points were significant (P < 0.04 or lower), but BCG data points did not reach significance (P < 0.05) until the day 90 time point. Similarly, values for TCM did not reach significance until day 90 and were borderline (P < 0.05).
In contrast, much lower numbers of cells expressing the TCM phenotype CD62Lhi CCR7hi CD44hi were observed in the lungs and lymph nodes (Fig. 1 and 2). However, much higher numbers (>105) were seen in the spleens of both test groups throughout the study (P < 0.02), again suggesting a significant reservoir.
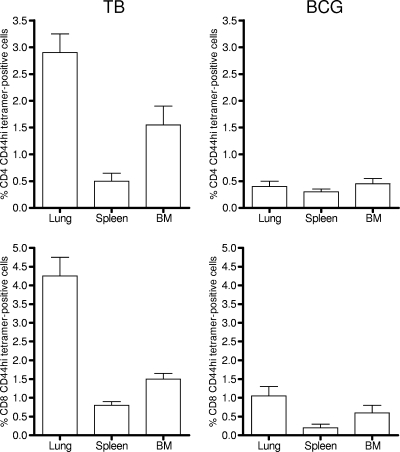
To be certain that the cells we were detecting were antigen-specific cells and not just some nonspecific polyclonal cellular expansion in response to the presence of the infections, lung cells were also stained with tetramers loaded with a dominant epitope of the major immunogenic protein ESAT-6, or with a major CD8 epitope from the Mtb32 protein (14, 22). As shown in Fig. 3, in chronically infected mice about 3% of CD4 CD44hi cells costained with the pMHCII tetramer, whereas staining of cells from the BCG-vaccinated mice was at background levels (BCG does not have the ESAT-6 gene). Similarly, positive staining for the CD8 epitope was in about 4% of total CD8 CD44hi cells harvested from the chronically infected animals. Based on these frequencies, we can reasonably conclude that most of the cell subsets we are measuring in this study are indeed antigen-specific T cells acquired in response to the M. tuberculosis infection.
FIG. 3.
Percentages of tetramer-positive CD4 and CD8 cells (means ± SEM; n = 4 or 5) in target organs in the two infection groups, analyzed on day 100 of each infection. BM, bone marrow aspirates. CD4 cells against a major epitope of ESAT-6 and CD8 cells against a major epitope of Mtb32 were detected.
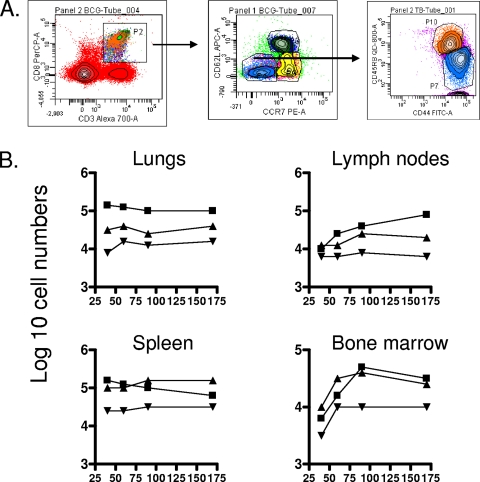
Evidence for a CD8 CD45RBhi subset.
In our earliest studies on this topic, which represented the very first application of the flow cytometry technique to tuberculosis infection in mice, we observed a rapid expansion of CD45RBhi cells, which we interpreted at the time as blast cells given their large size (12). In the current study, in which we looked much later on in the infection (Fig. 4), a subset of CD8 CD62Llo cells expressing CD45RBhi was distinctly observed and appeared to clearly be a separate subset from CD45RBmid/lo cells. At this time we can only speculate that these cells, which were found in substantial numbers in the lungs, lymph nodes, and bone marrow (P < 0.03), could potentially represent a subset of TEM in a state of activation and possible division and could potentially be in a state of transition to secondary effector cells.
FIG. 4.
Changes in numbers in a CD8 subset expressing the CD62Llo CCR7lo CD44hi CD45RBhi phenotype. (A) Gating strategy. (B) Numbers of cells recovered from organs of mice chronically infected with M. tuberculosis (▪), mice that had been subcutaneously vaccinated with BCG (▴), or age-matched uninfected controls (▾). In each case, the data point represents the log10 mean value for cells recovered from the organs of four or five mice; SEM are omitted for clarity and did not exceed 10%. All data points for the lungs and spleen were raised over uninfected control values (P < 0.03 or lower). Similar ranges of P values were seen in the lymph nodes and bone after day 90 of the study.
IFN-γ-producing T-cell subsets.
Since it is highly likely that the CD62Llo subsets we were detecting were in fact a potential mixture of effector and memory cells, we further stained cells for the effector cytokine IFN-γ. To analyze CD4 or CD8 T cells producing IFN-γ, we started by gating on CD44 and CD62L (Fig. 5). Populations representing CD44hi CD62Llo cells were the biggest producers of IFN-γ, as might be anticipated. Comparable numbers of cytokine-positive CD4 cells were seen in the lungs and spleens of chronically infected mice, whereas in the BCG group there was evidence of gradual expansion of the numbers of these cells in the lungs and spleen (P < 0.02). A small number of such cells were also detected in the lymph nodes and bone marrow (data not shown). Similar patterns were seen in the CD8 subset, albeit at approximately 10-fold-lower levels.
FIG. 5.
Changes in numbers of CD4 and CD8 TEM cells that stained positive for IFN-γ. (A) Gating strategy. (B) Numbers of CD4 cells and CD8 cells recovered from the indicated organs from mice chronically infected with M. tuberculosis (▪), mice that had been subcutaneously vaccinated with BCG (▴), or age-matched uninfected controls (▾). In each case, the data point represents the log10 mean value for cells recovered from the organs of four or five mice; SEM are omitted for clarity and did not exceed 10%. All data points were higher than uninfected control values (P < 0.02 or lower), except for the lungs of BCG-vaccinated mice at the first two time points.
In contrast, very few cytokine-positive CD4 or CD8 cells expressing the TCM phenotype CD44hi CD62Lhi could be detected in these tissues (Fig. 6), and indeed the data shown are at the very lowest limit of detection and should thus be treated with due caution. In addition, the numbers of cells remained flat with time, with no evidence indicating any degree of expansion. Similarly, levels of such cells in the lymph nodes and bone marrow were at the very limit of detection (data not shown).
FIG. 6.
Changes in numbers of CD4 and CD8 TCM cells that stained positive for IFN-γ. Data shown are from mice chronically infected with M. tuberculosis (▪), mice that had been subcutaneously vaccinated with BCG (▴), or age-matched uninfected controls (▾). In each case, the data point represents the log10 mean value for cells recovered from the organs of four or five mice; SEM are omitted for clarity and did not exceed 10%. In the case of CD4 cells, only values for the M. tuberculosis-infected group were raised above control values (P < 0.03 or lower), whereas for CD8 cells, these were raised in both test groups after day 60 (P < 0.04). As noted, however, these values were at the limit of assay detection and should be viewed with caution.
Expansion of CD62Llo CCR7hi T cells in the organs of infected animals.
In current models of memory immunity, high- or low expression of CD62L and CCR7 are usually parallel, with CD62Llo CCR7lo representing TEM and CD62Lhi CCR7hi representing TCM. It has been reported, however, that following antigen stimulation TEM can transiently upregulate CCR7 (32), and there is evidence that CD62L is rapidly shed after TCR triggering or following lymph node migration (6), possibly as a result of signaling via CD127.
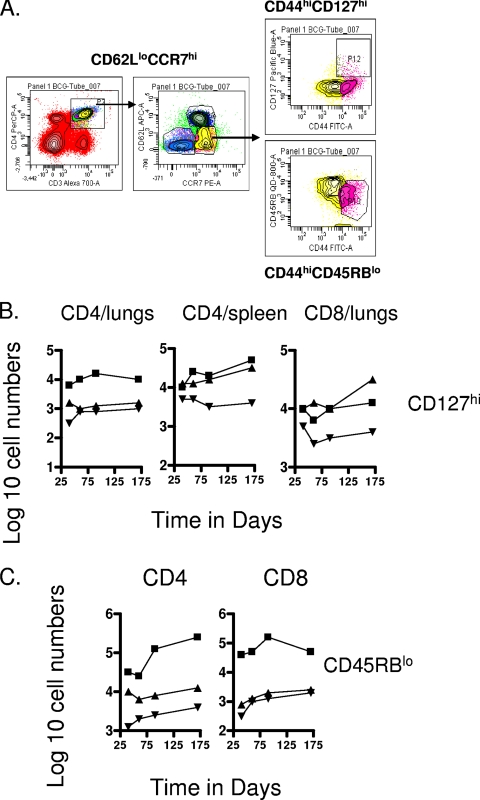
In our mycobacterial infection model a CD62Llo CCR7hi population was indeed clearly apparent, including a CD127hi subset, a further marker used to detect memory T cells. Gating on these cells revealed significant numbers (about 10-fold above those seen in uninfected controls) of CD4 cells in the lungs and spleen, as well as CD8 cells in the lungs (Fig. 7). A discrete second population of CD62Llo CCR7hi cells expressing the CD45RBlo phenotype was also detected within both the CD4 and CD8 subsets (Fig. 7C), although only for the M. tuberculosis-infected test group. Significant numbers of these were seen in the chronically infected lungs but not in any of the other tissues examined.
FIG. 7.
Analysis of CD127 and CD45RB subsets. (A) Gating strategies. (B) Changes in numbers of CD4 and CD8 T-cell subsets expressing the CD62Llo CCR7hi CD44hi CD127hi phenotype. Data shown are from mice chronically infected with M. tuberculosis (▪), mice that had been subcutaneously vaccinated with BCG (▴), or age-matched uninfected controls (▾). In each case, the data point represents the log10 mean value for cells recovered from the organs of four or five mice; SEM are omitted for clarity and did not exceed 10%. Numbers of CD4 cells in the lungs were not significant; all other data points were raised above control values (P < 0.04 or lower). (C) Changes in numbers of CD4 and CD8 T-cell subsets expressing the CD62Llo CCR7hi CD44hi CD45RBlo phenotype. Data shown are from mice chronically infected with M. tuberculosis (▪), mice that had been subcutaneously vaccinated with BCG (▴), or age-matched uninfected controls (▾). In each case the data point represents the log10 mean value for cells recovered from the organs of four or five mice; SEM are omitted for clarity and did not exceed 10%. Values for CD4 cells were raised above uninfected control values (P < 0.04 or lower), whereas only those in the M. tuberculosis-infected test group were raised (P < 0.01) in the case of CD8 cells.
DISCUSSION
The results of this study show that the immune response mediated by CD4 and CD8 T cells both in chronically infected and in BCG-vaccinated mice can be divided into further subsets, some of which clearly have phenotypes thought to be associated with TEM and TCM populations. Moreover, these subsets are widely distributed both in lung tissues and in lymphoid tissues, in which they are not static but show evidence of changes in numbers with time. In fact, several CD4 and CD8 subsets can be detected in these mice, particularly in the chronically infected animals, which we speculate potentially represent cells transitioning along the AET, TEM, and TCM pathways. We should also stress that we are using the term AET here in an operational sense, since these could represent effector cells arising from primed naïve T cells (which might then have the capacity to become memory T cells) or could instead be secondary effector cells arising from antigen-stimulated memory T-cell subsets.
The primary conclusions that can be drawn from these simple descriptive studies are 2-fold. The first is that the T-cell response to chronic mycobacterial infection or to BCG vaccination is dominated by cells expressing an AET/TEM response which is approximately 50- to 70-fold larger than a population of CD44hi CD62Lhi CCR7hi cells potentially representing the TCM response. Second, there appear to be additional T-cell subsets involved that have not previously been recognized as being of any importance and which do not exist in antimicrobial models in which antibody production is the key outcome.
Since BCG does not reach the lungs after subcutaneous injection (25, 28), it is reasonable that the increase in CD62Llo CCR7lo CD44hi CD4 and CD8 T cells in this organ up to 170 days later almost certainly can be regarded as representing a true TEM population. In contrast, cells with this phenotype in the M. tuberculosis-infected mice undoubtedly represent a mixture of AET as well as TEM. At this point we cannot distinguish between these further, but in preliminary studies in which we have treated these mice with chemotherapy to remove the chronic infection, we have seen a contraction of this cell population from about 6 × 105 to 7 × 105 cells to about 1 × 105 to 2 × 105, which may represent the pool of longer-lived cells; if so, then this indicates that the majority of the cells in the chronically infected mice are effector cells rather than memory cells. In both cases these not only represented the major IFN-γ+ population, but there was also a major reservoir of such cells in both the spleen and lymph nodes. Moreover, in the preliminary studies noted above we have observed that chemotherapy of infected mice does not reduce the numbers of TEM in the spleen further, thus suggesting that this organ could be a major reservoir of these cells. In contrast, there were far fewer CD44hi CD62Lhi CCR7hi cells that could be found, representing TCM; this may be a legacy of dissemination of the infection from the lungs to this organ, which is a primary facet of this infection model. A great proportion were of the CD8 phenotype, an observation similar to that of Kamath et al. (16), who found a preferential expansion of cells with this phenotype in drug-treated mice that were subsequently reinfected; hence, both of our studies support the existence of a pool of CD8 TCM generated under these conditions. In our hands, CD8 TCM accumulated primarily in the spleen and lymph nodes but could also be found in the lungs and in the bone marrow, the latter a known reservoir of TCM (5, 8, 20). Based on recent preliminary studies, we should also note that clearance of the infection by chemotherapy appears to substantially increase the numbers of TCM in the lungs, again very consistent with the observations of Kamath et al. (16).
Our data also suggested that additional cell subsets may exist. We found a population of CD8 cells that had the phenotype of AET/TEM but also had high expression of CD45RB. We had earlier noted such events in CD4 cells and showed that they were large (12), and hence we speculate that the cells we observed here, although low in number, might represent blasting CD8 TEM or CD8 cells transitioning from TEM to AET (secondary effectors) in response to the chronic infection and chronic antigen stimulation.
A second subset we noted had a CD4 CD44lo CD62Lhi phenotype. It seemed to exist only in the spleen and lymph nodes in any appreciable numbers, but it was capable of secreting IFN-γ. In a previous study (17) we hypothesized that CD4+ CD44hi CD62Llo cells contained a population of memory T cells, and so we used a high-speed cell sorter to select these and transferred them into aerosol-challenged Rag−/− mice. We used CD4+ CD44lo CD62Lhi cells as described above as the negative controls for the study. Surprisingly, we got exactly the reverse result from the one we expected, with the latter population showing evidence of substantial and rapid expansion and giving excellent protection. In fact, a similar observation had already been reported by Andersen's group (2), who selected cells using a magnetic column for high expression of CD45RB. Based upon these collective observations, we can now hypothesize that these cells are part of a discrete subset, and we speculate that they may represent a form of TCM which has lowered (or possibly shed) CD44 expression; certainly, the rapid expansion of these cells and significant expression of protection that we observed (17) is fully consistent with the rapid division and cytokine production exhibited by TCM cells (31). Since they can be found in the spleen, this indicates that they (or at least some of them) are part of the recirculating T-cell pool and hence would be expected to be able to reach the lungs as seen in our earlier transfer experiments. This of course still fails to explain why the CD44hi CD62Llo cells did not protect in that study, but they were initially harvested from the lungs and transferred intravenously and may have had difficulty in returning to that organ in sufficient numbers to express adequate resistance (we are currently looking at CXCR6 expression on these cells, the loss of which would prevent them from returning from the blood). We would note also that activation and IFN-γ production are not always a reliable measure of memory immune capacity, as shown by others (42).
As noted above, when injected subcutaneously BCG drains into the regional lymph nodes, where it grows for a brief time. Some bacilli reach the spleen in low numbers but there is no evidence they ever get any further. Despite this, we found significant numbers of T cells in the lungs compared to that in age-matched uninfected controls, consistent with our earlier observations (17), with additional evidence here that in some cases certain subsets of memory cells were obviously further expanding with time. This thus indicates that subcutaneous BCG inoculation establishes a persistent, apparently very long-lived population of T cells that is widely distributed in tissues, including the lungs and the bone marrow, as well as lymphoid tissues such as the spleen and the mediastinal lymph nodes. Some of these cells are presumably within the recirculating lymphocyte pool, but it also appears that they may represent a reservoir of potentially reactive cells ready to respond to active infection.
It has always been assumed that the BCG vaccine establishes some sort of state of memory immunity, and this is consistent with the accelerated response and quicker containment of M. tuberculosis infection previously demonstrated (26, 27). However, this earlier, more simple view of events is complicated by the newer concept that in mouse, memory itself may consist of the gradual differentiation of activated effector T cells to TEM and then possibly to TCM. In fact, the actual lineage relationship between these populations is still very unclear. Moreover, the current ground rules by which these memory T-cell populations are defined do not necessarily exist in the context of live mycobacterial infections. Indeed, it has been suggested (3) that true memory immunity to mycobacteria cannot be achieved because the organism cannot be fully eradicated by host immunity. Our data seem to suggest that BCG is adept at generating TEM cells but far less so in its ability to establish TCM. A similar situation seems to exist in terms of active tuberculosis infection, although here it is possible that both memory T-cell populations may be transitioning to an AET phenotype due to the continuing chronic infection and availability of antigen, a situation that provides a major challenge for analysis.
This inability to establish a significant CD4 or CD8 TCM population allows us to propose the novel hypothesis that this may be a root cause of the inability of the BCG vaccine to protect adult humans from tuberculosis. In this hypothesis, we would propose that TEM are generated as the primary memory pool after neonatal BCG vaccination and these cells function perfectly adequately over the first decade or so of life and protect the child from tuberculosis. However, we propose that over this period of time these cells are gradually lost by attrition or exhausted by exposure to the pathogen (or by exposure to environmental mycobacteria) in areas of endemicity or that they simply experience an age-related loss in activity over the 10- to 15-year span between neonatal BCG vaccination and the time at which the adult onset of active tuberculosis is observed in these people despite their vaccination status (in fact, in this regard whether such cells are lost over a long period of time has never been addressed even in the acute viral or transgenic mouse models of memory immunity). Once the TEM population had been lost or at least significantly reduced, then a lack of an adequate TCM population as a second line of defense (as it is currently regarded in viral infection models) would render the host now fully susceptible to tuberculosis infection. If this is the case, then it could thus provide a marker with which to guide rational vaccine design and could be applied to looking at which strategies, such as BCG prime boosting, can strengthen the longevity of the specific TEM response while more effectively driving a potential TEM-to-TCM transition pathway in order to establish a better and longer-lived recall response. Other vaccine candidates that are far better at inducing TCM responses may exist, and this could be looked for.
We may also have to rethink ways in which vaccines are measured. To date, vaccine efficacy is usually selected on the basis of whether it can generate an IFN-γ response and then is tested in short-term assays in which the challenge is given 4 to 6 weeks after immunization. Given the obvious complexity of the memory response, this may be misleading. First, such short-term assays almost certainly do not give any pertinent information as to whether any TEM or TCM population can be generated by a given vaccine, and this may be critical to whether a new vaccine or prime boost strategy could establish memory immunity capable of lasting decades or more, which is what is obviously needed. Moreover, IFN-γ is considered another key readout, and this obviously differs between AET, TEM, and TCM populations, in both quantity and kinetics. Indeed, both our recent studies (17) and those of others (42) seem to indicate that in the context of memory immunity, at least, this is not always a reliable marker, and a recent review on this topic reaches a similar conclusion (10).
Acknowledgments
This work was supported by NIH grants AI40488, AI44072, and AI070456.
We are very grateful to Marc Jenkins and Andrea Cooper for provision of tetramer reagents.
Footnotes
Published ahead of print on 27 January 2010.
REFERENCES
- 1.Andersen, P., and I. Heron. 1993. Specificity of a protective memory immune response against Mycobacterium tuberculosis. Infect. Immun. 61:844-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen, P., and B. Smedegaard. 2000. CD4(+) T-cell subsets that mediate immunological memory to Mycobacterium tuberculosis infection in mice. Infect. Immun. 68:621-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bevan, M. J. 2002. Immunology: remembrance of things past. Nature 420:748-749. [DOI] [PubMed] [Google Scholar]
- 4.Bifani, P. J., B. Mathema, N. E. Kurepina, and B. N. Kreiswirth. 2002. Global dissemination of the Mycobacterium tuberculosis W-Beijing family strains. Trends Microbiol. 10:45-52. [DOI] [PubMed] [Google Scholar]
- 5.Cavanagh, L. L., R. Bonasio, I. B. Mazo, C. Halin, G. Cheng, A. W. van der Velden, A. Cariappa, C. Chase, P. Russell, M. N. Starnbach, P. A. Koni, S. Pillai, W. Weninger, and U. H. von Andrian. 2005. Activation of bone marrow-resident memory T cells by circulating, antigen-bearing dendritic cells. Nat. Immunol. 6:1029-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chao, C. C., R. Jensen, and M. O. Dailey. 1997. Mechanisms of L-selectin regulation by activated T cells. J. Immunol. 159:1686-1694. [PubMed] [Google Scholar]
- 7.Colditz, G. A., T. F. Brewer, C. S. Berkey, M. E. Wilson, E. Burdick, H. V. Fineberg, and F. Mosteller. 1994. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA 271:698-702. [PubMed] [Google Scholar]
- 8.Di Rosa, F., and R. Pabst. 2005. The bone marrow: a nest for migratory memory T cells. Trends Immunol. 26:360-366. [DOI] [PubMed] [Google Scholar]
- 9.Dye, C., S. Scheele, P. Dolin, V. Pathania, and M. C. Raviglione. 1999. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA 282:677-686. [DOI] [PubMed] [Google Scholar]
- 10.Goldsack, L., and J. R. Kirman. 2007. Half-truths and selective memory: interferon gamma, CD4(+) T cells and protective memory against tuberculosis. Tuberculosis (Edinb.) 87:465-473. [DOI] [PubMed] [Google Scholar]
- 11.Gourley, T. S., E. J. Wherry, D. Masopust, and R. Ahmed. 2004. Generation and maintenance of immunological memory. Semin. Immunol. 16:323-333. [DOI] [PubMed] [Google Scholar]
- 12.Griffin, J. P., and I. M. Orme. 1994. Evolution of CD4 T-cell subsets following infection of naive and memory immune mice with Mycobacterium tuberculosis. Infect. Immun. 62:1683-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hand, T. W., and S. M. Kaech. 2009. Intrinsic and extrinsic control of effector T cell survival and memory T cell development. Immunol. Res. 45:46-61. [DOI] [PubMed] [Google Scholar]
- 14.Irwin, S. M., A. A. Izzo, S. W. Dow, Y. A. Skeiky, S. G. Reed, M. R. Alderson, and I. M. Orme. 2005. Tracking antigen-specific CD8 T lymphocytes in the lungs of mice vaccinated with the Mtb72F polyprotein. Infect. Immun. 73:5809-5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Junqueira-Kipnis, A. P., J. Turner, M. Gonzalez-Juarrero, O. C. Turner, and I. M. Orme. 2004. Stable T-cell population expressing an effector cell surface phenotype in the lungs of mice chronically infected with Mycobacterium tuberculosis. Infect. Immun. 72:570-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamath, A., J. S. Woodworth, and S. M. Behar. 2006. Antigen-specific CD8+ T cells and the development of central memory during Mycobacterium tuberculosis infection. J. Immunol. 177:6361-6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kipnis, A., S. Irwin, A. A. Izzo, R. J. Basaraba, and I. M. Orme. 2005. Memory T lymphocytes generated by Mycobacterium bovis BCG vaccination reside within a CD4 CD44lo CD62 Ligandhi population. Infect. Immun. 73:7759-7764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lanzavecchia, A., and F. Sallusto. 2005. Understanding the generation and function of memory T cell subsets. Curr. Opin. Immunol. 17:326-332. [DOI] [PubMed] [Google Scholar]
- 19.Lefrancois, L., and D. Masopust. 2009. The road not taken: memory T cell fate ‘decisions’. Nat. Immunol. 10:369-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Letsch, A., M. Knoedler, I. K. Na, F. Kern, A. M. Asemissen, U. Keilholz, M. Loesch, E. Thiel, H. D. Volk, and C. Scheibenbogen. 2007. CMV-specific central memory T cells reside in bone marrow. Eur. J. Immunol. 37:3063-3068. [DOI] [PubMed] [Google Scholar]
- 21.McKinstry, K. K., T. M. Strutt, and S. L. Swain. 2008. The effector to memory transition of CD4 T cells. Immunol. Res. 40:114-127. [DOI] [PubMed] [Google Scholar]
- 22.Moon, J. J., H. H. Chu, M. Pepper, S. J. McSorley, S. C. Jameson, R. M. Kedl, and M. K. Jenkins. 2007. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity 27:203-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orme, I. M. 1999. Beyond BCG: the potential for a more effective TB vaccine. Mol. Med. Today 5:487-492. [DOI] [PubMed] [Google Scholar]
- 24.Orme, I. M. 1988. Characteristics and specificity of acquired immunologic memory to Mycobacterium tuberculosis infection. J. Immunol. 140:3589-3593. [PubMed] [Google Scholar]
- 25.Orme, I. M. 1988. Evidence for a biphasic memory T-cell response to high dose BCG vaccination in mice. Tubercle 69:125-131. [DOI] [PubMed] [Google Scholar]
- 26.Orme, I. M. 2006. Preclinical testing of new vaccines for tuberculosis: a comprehensive review. Vaccine 24:2-19. [DOI] [PubMed] [Google Scholar]
- 27.Orme, I. M., P. Andersen, and W. H. Boom. 1993. T cell response to Mycobacterium tuberculosis. J. Infect. Dis. 167:1481-1497. [DOI] [PubMed] [Google Scholar]
- 28.Orme, I. M., and F. M. Collins. 1986. Aerogenic vaccination of mice with Mycobacterium bovis BCG. Tubercle 67:133-140. [DOI] [PubMed] [Google Scholar]
- 29.Orme, I. M., and F. M. Collins. 1983. Protection against Mycobacterium tuberculosis infection by adoptive immunotherapy. Requirement for T cell-deficient recipients. J. Exp. Med. 158:74-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raviglione, M. C., and I. M. Smith. 2007. XDR tuberculosis—implications for global public health. N Engl. J. Med. 356:656-659. [DOI] [PubMed] [Google Scholar]
- 31.Sallusto, F., J. Geginat, and A. Lanzavecchia. 2004. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu. Rev. Immunol. 22:745-763. [DOI] [PubMed] [Google Scholar]
- 32.Sallusto, F., E. Kremmer, B. Palermo, A. Hoy, P. Ponath, S. Qin, R. Forster, M. Lipp, and A. Lanzavecchia. 1999. Switch in chemokine receptor expression upon TCR stimulation reveals novel homing potential for recently activated T cells. Eur. J. Immunol. 29:2037-2045. [DOI] [PubMed] [Google Scholar]
- 33.Sallusto, F., A. Langenkamp, J. Geginat, and A. Lanzavecchia. 2000. Functional subsets of memory T cells identified by CCR7 expression. Curr. Top. Microbiol. Immunol. 251:167-171. [DOI] [PubMed] [Google Scholar]
- 34.Sallusto, F., D. Lenig, R. Forster, M. Lipp, and A. Lanzavecchia. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401:708-712. [DOI] [PubMed] [Google Scholar]
- 35.Sallusto, F., C. R. Mackay, and A. Lanzavecchia. 2000. The role of chemokine receptors in primary, effector, and memory immune responses. Annu. Rev. Immunol. 18:593-620. [DOI] [PubMed] [Google Scholar]
- 36.Schoenberger, S. P. 2009. T cell memory. Semin. Immunol. 21:51-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Surh, C. D., O. Boyman, J. F. Purton, and J. Sprent. 2006. Homeostasis of memory T cells. Immunol. Rev. 211:154-163. [DOI] [PubMed] [Google Scholar]
- 38.Tanel, A., S. G. Fonseca, B. Yassine-Diab, R. Bordi, J. Zeidan, Y. Shi, C. Benne, and R. P. Sekaly. 2009. Cellular and molecular mechanisms of memory T-cell survival. Expert Rev. Vaccines 8:299-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teixeiro, E., M. A. Daniels, S. E. Hamilton, A. G. Schrum, R. Bragado, S. C. Jameson, and E. Palmer. 2009. Different T cell receptor signals determine CD8+ memory versus effector development. Science 323:502-505. [DOI] [PubMed] [Google Scholar]
- 40.Vezys, V., A. Yates, K. A. Casey, G. Lanier, R. Ahmed, R. Antia, and D. Masopust. 2009. Memory CD8 T-cell compartment grows in size with immunological experience. Nature 457:196-199. [DOI] [PubMed] [Google Scholar]
- 41.Wherry, E. J., V. Teichgraber, T. C. Becker, D. Masopust, S. M. Kaech, R. Antia, U. H. von Andrian, and R. Ahmed. 2003. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 4:225-234. [DOI] [PubMed] [Google Scholar]
- 42.Wu, C. Y., J. R. Kirman, M. J. Rotte, D. F. Davey, S. P. Perfetto, E. G. Rhee, B. L. Freidag, B. J. Hill, D. C. Douek, and R. A. Seder. 2002. Distinct lineages of T(H)1 cells have differential capacities for memory cell generation in vivo. Nat. Immunol. 3:852-858. [DOI] [PubMed] [Google Scholar]