Abstract
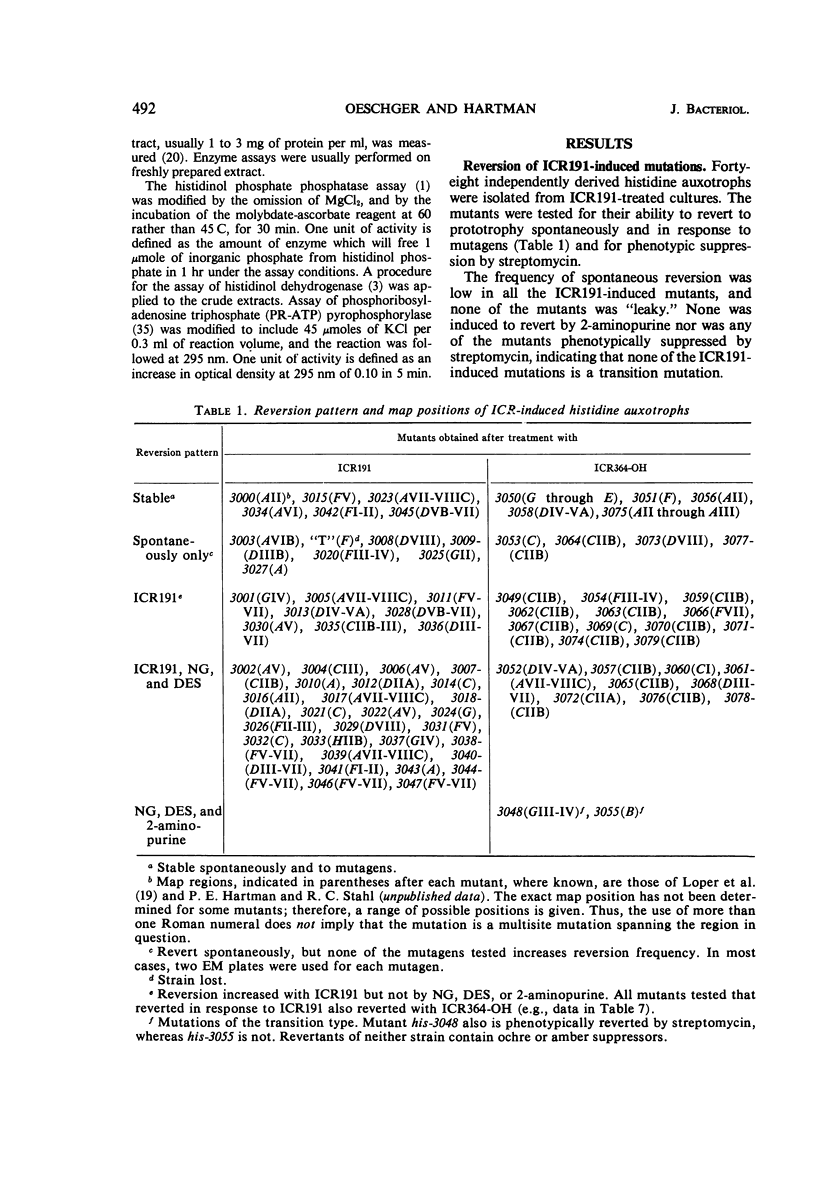
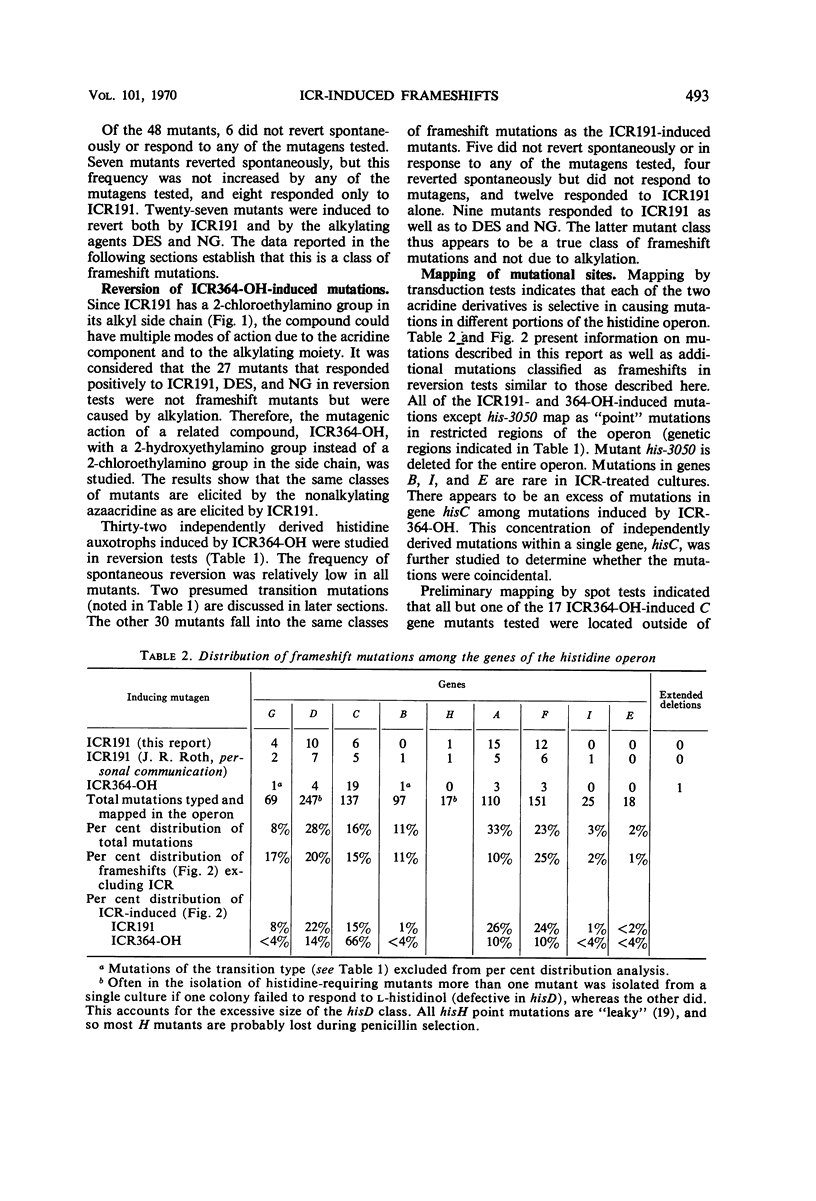
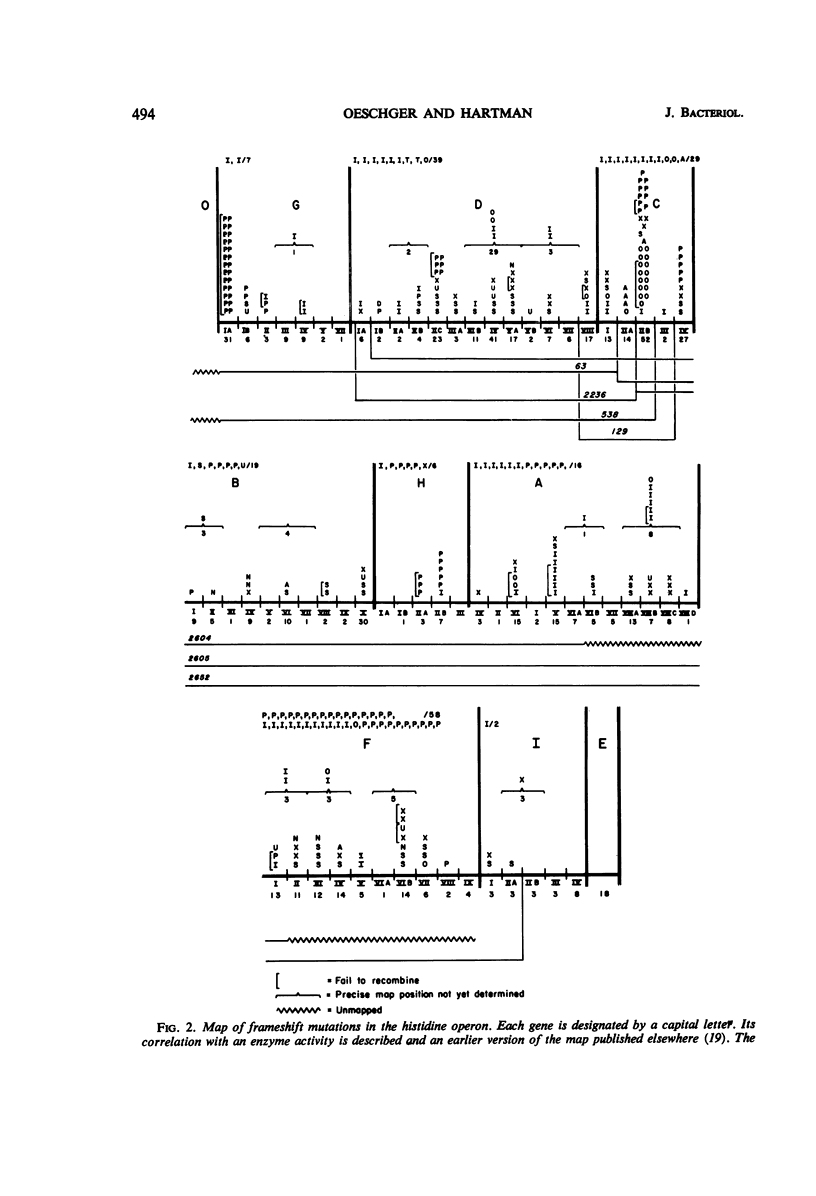
Both the acridine half-mustard, ICR191, and the nonalkylating azaacridine derivative, ICR364-OH, induce three classes of frameshift mutations in the histidine operon of Salmonella typhimurium. (i) One class is completely stable in reversion tests and is presumed to represent deletion of one or a few critical nucleotide pairs or two nearby frameshifts. One extended deletion was found out of 11 stable mutations. (ii) Of two spontaneously reverting classes which also are considered to predominantly involve base deletions, one is unaffected in reversion with ICR191, nitrosoguanidine, and diethylsulfate, and the other is induced to revert with ICR191. (iii) A third class, considered to predominantly involve base additions, responds in reversion tests with ICR191 as well as with nitrosoguanidine and diethylsulfate. Other investigators have shown that one mutant of this class is a “plus” frameshift and that nitrosoguanidine acts in reversion to delete a guanine plus cytosine base pair. Although such plus frameshifts are found with high frequency among mutations selected from acridine-treated bacteria or when strong selection pressure is applied for their detection in reversion tests, data from this laboratory indicate that this class of plus frameshifts is rare among mutations derived spontaneously or after treatment with a variety of other mutagens. Finally, we demonstrate that the alkylating ICR191 and the nonalkylating ICR364-OH preferentially cause mutations in different chromosome regions and that their spectra of activity only partially overlap that found for spontaneous frameshift mutations.
Full text
PDF
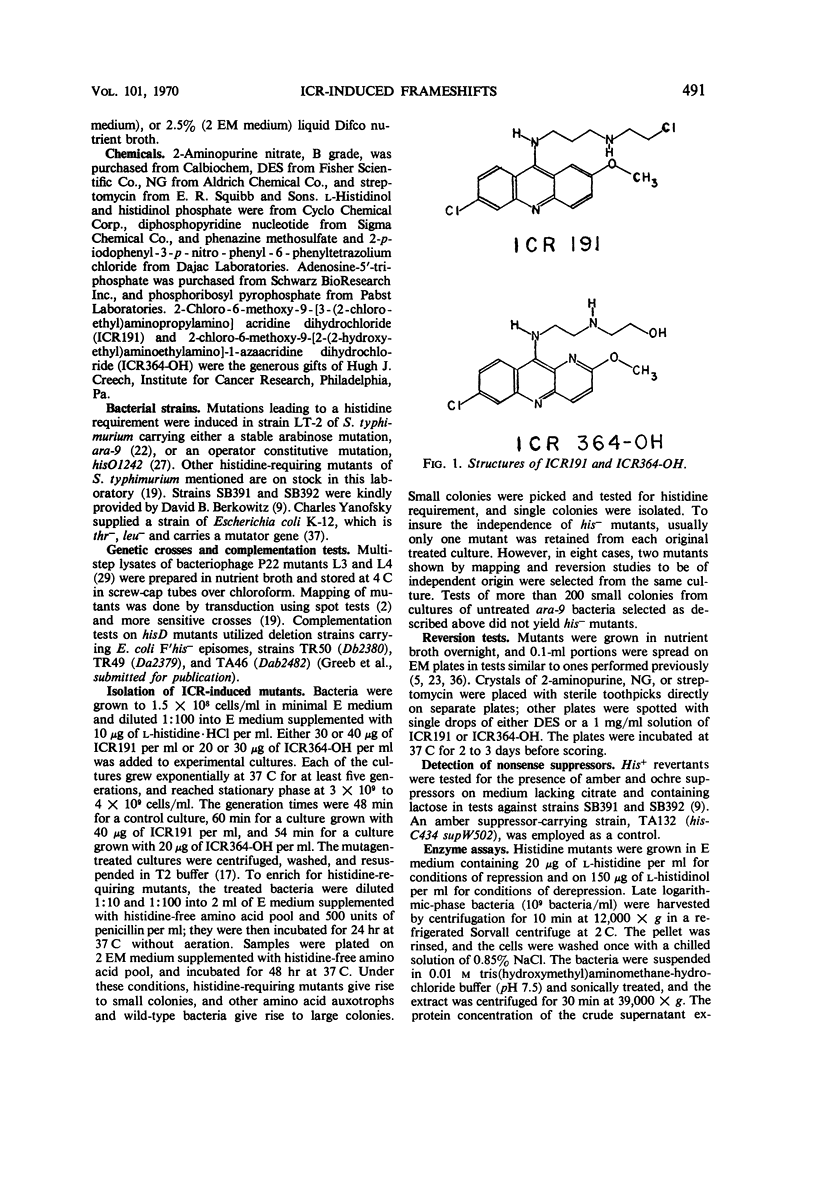





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., GARRY B., HERZENBERG L. A. The genetic control of the enzymes of histidine biosynthesis in Salmonella typhimurium. J Gen Microbiol. 1960 Apr;22:369–378. doi: 10.1099/00221287-22-2-369. [DOI] [PubMed] [Google Scholar]
- AMES B. N., HARTMAN P. E., JACOB F. Chromosomal alterations affecting the regulation of histidine biosynthetic enzymes in Salmonella. J Mol Biol. 1963 Jul;7:23–42. doi: 10.1016/s0022-2836(63)80016-9. [DOI] [PubMed] [Google Scholar]
- AMES B. N., MARTIN R. G., GARRY B. J. The first step of histidine biosynthesis. J Biol Chem. 1961 Jul;236:2019–2026. [PubMed] [Google Scholar]
- Ames B. N., Whitfield H. J., Jr Frameshift mutagenesis in Salmonella. Cold Spring Harb Symp Quant Biol. 1966;31:221–225. doi: 10.1101/sqb.1966.031.01.030. [DOI] [PubMed] [Google Scholar]
- Bautz E., Freese E. ON THE MUTAGENIC EFFECT OF ALKYLATING AGENTS. Proc Natl Acad Sci U S A. 1960 Dec;46(12):1585–1594. doi: 10.1073/pnas.46.12.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger H., Brammar W. J., Yanofsky C. Analysis of amino acid replacements resulting from frameshift and missense mutations in the tryptophan synthetase A gene of Escherichia coli. J Mol Biol. 1968 Jul 14;34(2):219–238. doi: 10.1016/0022-2836(68)90248-9. [DOI] [PubMed] [Google Scholar]
- Berger H., Brammar W. J., Yanofsky C. Spontaneous and ICR191-A-induced frameshift mutations in the A gene of Escherichia coli tryptophan synthetase. J Bacteriol. 1968 Nov;96(5):1672–1679. doi: 10.1128/jb.96.5.1672-1679.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz D., Hushon J. M., Whitfield H. J., Jr, Roth J., Ames B. N. Procedure for identifying nonsense mutations. J Bacteriol. 1968 Jul;96(1):215–220. doi: 10.1128/jb.96.1.215-220.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brammar W. J., Berger H., Yanofsky C. Altered amino acid sequences produced by reversion of frameshift mutants of tryptophan synthetase A gene of E. coli. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1499–1506. doi: 10.1073/pnas.58.4.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRICK F. H., BARNETT L., BRENNER S., WATTS-TOBIN R. J. General nature of the genetic code for proteins. Nature. 1961 Dec 30;192:1227–1232. doi: 10.1038/1921227a0. [DOI] [PubMed] [Google Scholar]
- Carlson E. A., Sederoff R., Cogan M. Evidence favoring a frame shift mechanism for ICR-170 induced mutations in Drosophila melanogaster. Genetics. 1967 Feb;55(2):295–313. doi: 10.1093/genetics/55.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Serres F. J., Brockman H. E. Homology tests on presumed multilocus deletions in the ad-3 region of Neurospora crassa induced by the acridine mustard ICR-170. Genetics. 1968 Jan;58(1):79–83. doi: 10.1093/genetics/58.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink G. R., Klopotowski T., Ames B. N. Histidine regulatory mutants in Salmonella typhimurium. IV. A positive selection for polar histidine-requiring mutants from histidine operator constitutive mutants. J Mol Biol. 1967 Nov 28;30(1):81–95. doi: 10.1016/0022-2836(67)90245-8. [DOI] [PubMed] [Google Scholar]
- Fink G. R., Martin R. G. Translation and polarity in the histidine operon. II. Polarity in the histidine operon. J Mol Biol. 1967 Nov 28;30(1):97–107. doi: 10.1016/0022-2836(67)90246-x. [DOI] [PubMed] [Google Scholar]
- HERSHEY A. D., CHASE M. Independent functions of viral protein and nucleic acid in growth of bacteriophage. J Gen Physiol. 1952 May;36(1):39–56. doi: 10.1085/jgp.36.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye M., Akaboshi E., Tsugita A., Streisinger G., Okada Y. A frame-shift mutation resulting in the deletion of two base pairs in the lysozyme gene of bacteriophage T4. J Mol Biol. 1967 Nov 28;30(1):39–47. doi: 10.1016/0022-2836(67)90241-0. [DOI] [PubMed] [Google Scholar]
- LOPER J. C., GRABNAR M., STAHL R. C., HARTMAN Z., HARTMAN P. E. GENES AND PROTEINS INVOLVED IN HISTIDINE BIOSYNTHESIS IN SALMONELLA. Brookhaven Symp Biol. 1964 Dec;17:15–52. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARGOLIN P. Genetic fine structure of the leucine operon in Salmonella. Genetics. 1963 Mar;48:441–457. doi: 10.1093/genetics/48.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malling H. V. The mutagenicity of the acridine mustard (ICR-170) and the structurally related compounds in Neurospora. Mutat Res. 1967 May-Jun;4(3):265–274. doi: 10.1016/0027-5107(67)90021-8. [DOI] [PubMed] [Google Scholar]
- Margolies M. N., Goldberger R. F. Correlation between mutation type and the production of cross-reacting material in mutants of the A gene of the histidine operon in Salmonella typhimurium. J Bacteriol. 1968 Feb;95(2):507–519. doi: 10.1128/jb.95.2.507-519.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. G. Frameshift mutants in the histidine operon of Salmonella typhimurium. J Mol Biol. 1967 Jun 14;26(2):311–328. doi: 10.1016/0022-2836(67)90300-2. [DOI] [PubMed] [Google Scholar]
- Martin R. G., Talal N. Translation and polarity in the histidine operon. IV. Relation of polarity to map position in hisC. J Mol Biol. 1968 Sep 14;36(2):219–229. doi: 10.1016/0022-2836(68)90377-x. [DOI] [PubMed] [Google Scholar]
- Okada Y., Streisinger G., Emrich J., Newton J., Tsugita A., Inouye M. Frame shift mutations near the beginning of the lysozyme gene of bacteriophage T4. Science. 1968 Nov 15;162(3855):807–808. doi: 10.1126/science.162.3855.807. [DOI] [PubMed] [Google Scholar]
- Roth J. R., Antón D. N., Hartman P. E. Histidine regulatory mutants in Salmonella typhimurium. I. Isolation and general properties. J Mol Biol. 1966 Dec 28;22(2):305–323. doi: 10.1016/0022-2836(66)90134-3. [DOI] [PubMed] [Google Scholar]
- Sesnowitz-Horn S., Adelberg E. A. Proflavin treatment of Escherichia coli: generation of frameshift mutations. Cold Spring Harb Symp Quant Biol. 1968;33:393–402. doi: 10.1101/sqb.1968.033.01.045. [DOI] [PubMed] [Google Scholar]
- Smith H. O., Levine M. A phage P22 gene controlling integration of prophage. Virology. 1967 Feb;31(2):207–216. doi: 10.1016/0042-6822(67)90164-x. [DOI] [PubMed] [Google Scholar]
- Stewart C. R. Mutagenesis by acridine yellow in Bacillus subtilis. Genetics. 1968 May;59(1):23–31. doi: 10.1093/genetics/59.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss B., Coyle M., Robbins M. Alkylation damage and its repair. Cold Spring Harb Symp Quant Biol. 1968;33:277–287. doi: 10.1101/sqb.1968.033.01.032. [DOI] [PubMed] [Google Scholar]
- Streisinger G., Okada Y., Emrich J., Newton J., Tsugita A., Terzaghi E., Inouye M. Frameshift mutations and the genetic code. This paper is dedicated to Professor Theodosius Dobzhansky on the occasion of his 66th birthday. Cold Spring Harb Symp Quant Biol. 1966;31:77–84. doi: 10.1101/sqb.1966.031.01.014. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Voll M. J., Appella E., Martin R. G. Purification and composition studies of phosphoribosyladenosine triphosphate:pyrophosphate phosphoribosyltransferase, the first enzyme of histidine biosynthesis. J Biol Chem. 1967 Apr 25;242(8):1760–1767. [PubMed] [Google Scholar]
- Voll M. J. Translation and polarity in the histidine operon. 3. The isolation of prototrophic polar mutations. J Mol Biol. 1967 Nov 28;30(1):109–124. doi: 10.1016/0022-2836(67)90247-1. [DOI] [PubMed] [Google Scholar]
- Whitfield H. J., Jr, Martin R. G., Ames B. N. Classification of aminotransferase (C gene) mutants in the histidine operon. J Mol Biol. 1966 Nov 14;21(2):335–355. doi: 10.1016/0022-2836(66)90103-3. [DOI] [PubMed] [Google Scholar]
- Yanofsky C., Cox E. C., Horn V. The unusual mutagenic specificity of an E. Coli mutator gene. Proc Natl Acad Sci U S A. 1966 Feb;55(2):274–281. doi: 10.1073/pnas.55.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky C., Drapeau G. R., Guest J. R., Carlton B. C. THE COMPLETE AMINO ACID SEQUENCE OF THE TRYPTOPHAN SYNTHETASE A PROTEIN (alpha SUBUNIT) AND ITS COLINEAR RELATIONSHIP WITH THE GENETIC MAP OF THE A GENE. Proc Natl Acad Sci U S A. 1967 Feb;57(2):296–298. doi: 10.1073/pnas.57.2.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yourno J., Barr D., Tanemura S. Externally suppressible frameshift mutant of Salmonella typhimurium. J Bacteriol. 1969 Oct;100(1):453–459. doi: 10.1128/jb.100.1.453-459.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yourno J., Heath S. Nature of the hisD3018 frameshift mutation in Salmonella typhimurium. J Bacteriol. 1969 Oct;100(1):460–468. doi: 10.1128/jb.100.1.460-468.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]



