Abstract
This study evaluated the safety, tolerability, and immunogenicity of an investigational quadrivalent meningococcal conjugate vaccine, MenACWY-CRM, when administered concomitantly with a combined tetanus, reduced diphtheria, and acellular pertussis (Tdap) vaccine, in subjects aged 11 to 25 years. Subjects received either MenACWY-CRM and Tdap, MenACWY-CRM and saline placebo, or Tdap and saline placebo. No significant increase in reactogenicity and no clinically significant vaccine-related adverse events (AEs) occurred when MenACWY-CRM and Tdap were administered concomitantly. Similar immunogenic responses to diphtheria, tetanus, and meningococcal (serogroups A, C, W-135, and Y) antigens were observed, regardless of concomitant vaccine administration. Antipertussis antibody responses were comparable between vaccine groups for filamentous hemagglutinin and were slightly lower, although not clinically significantly, for pertussis toxoid and pertactin when the two vaccines were administered concomitantly. These results indicate that the investigational MenACWY-CRM vaccine is well tolerated and immunogenic and that it can be coadministered with Tdap to adolescents and young adults.
Meningococcal disease has an impact that is belied by its overall incidence. Although the highest incidence of invasive meningococcal disease occurs in infants below the age of 12 months, most countries report a second incidence peak in individuals between the ages of 15 and 19 years (1, 8, 16, 19). In 2006, the case fatality rate was 7.8% in Europe and an average of 10 to 14% in the United States (2, 7). Survivors often suffer serious sequelae, including deafness, neurological problems and amputations. While the overall population-based mortality rate is highest in infants, adolescents have the highest case fatality rate (1).
Although serogroups B and C were the most frequently reported causes of meningococcal disease in Europe in 2006, out of a total of 4,910 cases of confirmed or probable meningococcal disease, 102 (2%) and 149 (3%) cases were caused by serogroups W-135 and Y, respectively (7). In the United States, the proportion of cases of meningococcal disease caused by serogroup Y rose from 2% in 1989 to 1991 to 28% in 1997 to 2003 (12). The dynamic nature of meningococcal disease epidemiology is also highlighted by the recent emergence of disease due to serogroup W-135 in some regions, including large outbreaks among Hajj pilgrims in 2000 and subsequent outbreaks in sub-Saharan Africa, where serogroup A has more typically been the cause (14).
New vaccines have been licensed for use in adolescents; these are increasingly recommended for routine use and include vaccines against tetanus, diphtheria, pertussis, meningococcal disease, human papillomavirus, and, under some circumstances, varicella (15). In the United States, the Advisory Committee on Immunization Practices (ACIP) recommends that the combined tetanus (T), reduced-antigen diphtheria (d), and reduced-antigen acellular pertussis (ap) vaccine (Tdap) should be administered together with the quadrivalent conjugate meningococcal vaccine to adolescents aged 11 to 18 years during the same office visit, if both vaccines are indicated (3). Furthermore, Tdap is included in the recommended adolescent schedules of several European countries, including Austria, Belgium, Finland, Germany, Iceland, Italy, Luxemburg, and Sweden (9). It is therefore important that studies on concomitant use be performed in adolescents to ascertain the compatibility of new vaccines alongside Tdap. To date, two published studies have investigated the interaction between meningococcal C conjugate (MenC) vaccines and tetanus and reduced-antigen diphtheria (Td) boosters in adolescents 13 to 18 years old; these found no clinically relevant impact on the reactogenicity or immunogenicity of the MenC vaccines (4, 21).
An investigational quadrivalent meningococcal CRM197 conjugate vaccine (MenACWY-CRM; Novartis Vaccines) has recently been developed, and its safety and immunogenicity in infants (20) and adolescents (13) have been evaluated. When the present study was performed, there was no licensed quadrivalent MenACWY vaccine in the European Union, though in the United States the use of the quadrivalent MenACWY vaccine was recommended. In this study, the safety, tolerability, and immunogenicity of MenACWY-CRM, when administered concomitantly with Tdap vaccine, were evaluated in adolescents and young adults.
MATERIALS AND METHODS
This phase III, observer-blind, multicenter, randomized, controlled study compared the safety and immunogenicity of MenACWY-CRM, when administered either alone or concomitantly with Tdap, with the safety and immunogenicity of a single dose of Tdap for healthy subjects 11 to 25 years old. Ethics Committee approval of the protocol was obtained before enrollment, and written informed consent was obtained from every subject ≥18 years of age, or from the parents or guardians of all subjects 11 to 17 years of age.
Subjects.
Healthy subjects aged 11 to 25 years who had undergone primary vaccination with a vaccine containing diphtheria and tetanus (DT) or Tdap antigens and a T, Td, or Tdap booster at least 5 years before enrollment were eligible for inclusion in the study. Subjects were excluded from the study if they had either received a meningococcal vaccination previously, been vaccinated with any licensed vaccines ≤1 month before enrollment, received any investigational agents or vaccines ≤90 days before enrollment, had any serious acute, chronic, or progressive disease, or had a known or suspected impairment/alteration of immune function.
Vaccines and vaccinations.
A total of 1,072 subjects were randomized 1:1:1 to receive concomitant administration of either MenACWY-CRM and Tdap (Boostrix; GlaxoSmithKline) (n = 361), Tdap and saline placebo (n = 354), or MenACWY-CRM and saline placebo (n = 357). This allowed assessment of the safety and immunogenicity of MenACWY-CRM, of Tdap, and of both vaccines administered concomitantly. Study vaccines (0.5-ml dose of each) were injected separately into the deltoid muscles of opposite arms in an observer-blinded fashion.
Safety monitoring.
Subjects were observed for 30 min postvaccination for local and systemic (particularly anaphylactic) reactions. Axillary temperatures were recorded for 7 days postvaccination. Subjects (or their parents/guardians, when applicable) recorded on diary cards any local reactions (pain, erythema, or induration) or systemic reactions (chills, nausea, malaise, myalgia, arthralgia, or headache) that occurred between day 1 and day 7 postvaccination. Any adverse events (AEs) requiring medical attention were recorded during the first month after vaccination, while medically significant and serious AEs (SAEs) were recorded for 6 months postvaccination (a serious adverse event was defined as any untoward medical occurrence that, at any dose, resulted in death, was life-threatening, required inpatient hospitalization, resulted in persistent or significant disability/incapacity, required intervention to prevent permanent impairment or damage, or was an important and significant medical event that may not be immediately life-threatening and may not result in death or hospitalization but, on the basis of appropriate medical judgment, may jeopardize the patient/subject or may require intervention to prevent one of the other outcomes listed).
Serology.
Blood samples (10 ml) were taken prevaccination and 1 month (28 to 43 days) postvaccination. The immunogenicity of MenACWY-CRM was inferred from the presence of bactericidal antibodies to Neisseria meningitidis serogroups A, C, W-135, and Y in the sera by using human complement (12, 20). The serological correlate of protection for meningococcal serogroups was a human serum bactericidal assay (hSBA) titer of ≥1:4 (11); the methods used have been described elsewhere (20). Antibodies against diphtheria and tetanus toxoids and against pertussis antigens were assessed by enzyme-linked immunosorbent assays (ELISA). A cutoff measurement of ≥1.0 IU/ml was used to demonstrate protection against diphtheria and tetanus toxoids, while a 4-fold increase in titers of antibodies against pertussis toxoid (PT), filamentous hemagglutinin (FHA), and pertactin (PRN) was used.
Statistical methods and analyses.
The immunogenicity of Tdap, when administered concomitantly with MenACWY-CRM, was considered noninferior to the immunogenicity of Tdap given separately if, for all five antigens (diphtheria, tetanus, PT, FHA, and PRN), the lower limit (LL) of the two-sided 95% confidence interval (95% CI) for the difference in the vaccine response rate was ≥−10%.
With regard to local and systemic reaction rates, postvaccination differences among the groups were analyzed by means of Pearson's chi-square test or Fisher's exact test. Statistical analyses were performed with SAS software, version 9.1 or higher (SAS Institute, Cary, NC).
RESULTS
Subject characteristics.
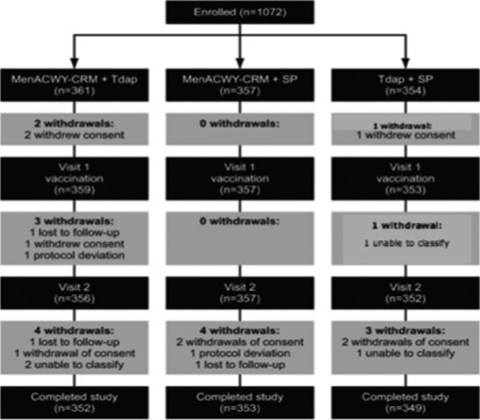
Subject demographics were similar in all groups (Table 1). Of the 1,072 subjects enrolled, 1,054 completed the study in line with the protocol. Of the 18 subjects who did not complete the study, 9 withdrew their consent, 3 were lost to follow-up, 2 had study protocol violations, and 4 were defined as “unable to classify” (Fig. 1).
TABLE 1.
Subject demographics
| Parameter | Value for the group receiving the following vaccinationa: |
||
|---|---|---|---|
| MenACWY-CRM + Tdap (n = 361) | MenACWY-CRM + saline placebo (n = 357) | Tdap + saline placebo (n = 354) | |
| Mean age (yr) (SD) | 14.4 (3.4) | 14.3 (3.2) | 14.1 (3.2) |
| No. (%) male | 172 (47.6) | 192 (53.7) | 190 (53.6) |
| Race (no. [%]) | |||
| Asian | 1 (0.3) | 1 (0.3) | 1 (0.3) |
| Black | 1 (0.3) | 0 | 1 (0.3) |
| Caucasian | 350 (96.5) | 350 (98.0) | 346 (97.7) |
| Hispanic | 4 (1.1) | 1 (0.3) | 4 (1.1) |
| Other | 5 (1.3) | 5 (1.4) | 2 (0.3) |
MenACWY-CRM, quadrivalent meningococcal conjugate vaccine; Tdap, combined tetanus, reduced diphtheria, and acellular pertussis vaccine.
FIG. 1.
Flowchart of subjects enrolled.
The subjects who did not complete the study consented, both when informed consent was given and when it was withdrawn, to the use of their partial data for the study.
Withdrawal of consent, follow-up drop-out, and protocol violations were due mainly to the difficulty in respecting examination times, especially when they coincided with summer holidays. In one case of protocol violation, the subject no longer fulfilled the recruitment requirements, because his situation had changed during the study. Finally, because of incomplete source data, four subjects could not be assigned with certainty to one or another of the three groups. The volunteers and their parents were aware that they could withdraw their consent at any time without having to provide any explanation; however, none of the parents and subjects who did offer an explanation, in response to a specific question, stated that withdrawal was due to the reactogenicity of the vaccination.
Safety and tolerability.
No clinically significant vaccine-related AEs were reported. Local reactions were reported by 50 to 80% of subjects across all three vaccine groups (Table 2). The number of local reactions reported was highest at the injection site where Tdap was administered; while local reactogenicity rates at the MenACWY-CRM injection site were not affected by concomitant Tdap administration. The most frequently reported injection site reaction was pain; it was reported more frequently in the Tdap group (63 to 70%) than in the MenACWY-CRM group (23 to 32%) or the saline placebo group (12 to 16%) (Table 2). Severe reactions were uncommon, whether the study vaccines were given concomitantly or with the saline placebo (<1 to 3% for MenACWY-CRM; 1 to 8% for Tdap) (Table 2).
TABLE 2.
Solicited local and systemic reactions following vaccination with MenACWY-CRM administered concomitantly with Tdapa or with saline placebo
| Reactionb | No. (%) of subjects with reactions after receiving: |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| MenACWY-CRM + Tdap (n = 359) |
MenACWY-CRM + saline placebo (n = 357) |
Tdap + saline placebo (n = 353) |
|||||||
| Total or nonlocal reaction | Injection site reaction |
Total or nonlocal reaction | Injection site reaction |
Total or nonlocal reaction | Injection site reaction |
||||
| MenACWY-CRM | Tdap | MenACWY-CRM | Saline placebo | Tdap | Saline placebo | ||||
| Local | |||||||||
| Total | |||||||||
| Any | 278 (77.4) | 179 (50.1) | 282 (79.9) | ||||||
| Severe | 39 (10.8) | 16 (4.5) | 27 (7.6) | ||||||
| Pain | |||||||||
| Any | 82 (23.8) | 227 (63.2) | 116 (32.5) | 57 (15.9) | 246 (69.7) | 41 (11.6) | |||
| Severe | 6 (1.6) | 30 (8.4) | 10 (2.8) | 3 (<1) | 17 (4.8) | 5 (1.4) | |||
| Erythema | |||||||||
| Any | 48 (13.4) | 103 (28.7) | 66 (18.5) | 37 (10.4) | 103 (29.2) | 34 (9.6) | |||
| Severe | 1 (<1) | 3 (<1) | 1 (<1) | 0 (0) | 3 (<1) | 1 (<1) | |||
| Induration | |||||||||
| Any | 41 (11) | 110 (30.6) | 59 (16.5) | 23 (6.4) | 118 (33.4) | 27 (7.6) | |||
| Severe | 1 (<1) | 6 (1.7) | 4 (1.1) | 0 (0) | 6 (1.7) | 2 (<1) | |||
| Systemic | |||||||||
| Total | |||||||||
| Any | 198 (55.2) | 171 (47.9) | 202 (57.2) | ||||||
| Severe | 41 (11.4) | 28 (7.8) | 23 (6.5) | ||||||
| Chills | |||||||||
| Any | 39 (10.9) | 47 (13.2) | 41 (11.6) | ||||||
| Severe | 3 (<1) | 6 (1.7) | 3 (<1) | ||||||
| Nausea | |||||||||
| Any | 43 (11.9) | 28 (7.8) | 35 (9.9) | ||||||
| Severe | 5 (1.4) | 4 (1.1) | 2 (<1) | ||||||
| Malaise | |||||||||
| Any | 65 (18.1) | 43 (12.0) | 57 (16.1) | ||||||
| Severe | 9 (2.5) | 5 (1.4) | 5 (1.4) | ||||||
| Myalgia | |||||||||
| Any | 118 (32.9) | 79 (22.1) | 127 (35.9) | ||||||
| Severe | 16 (4.5) | 9 (2.5) | 9 (2.5) | ||||||
| Arthralgia | |||||||||
| Any | 57 (15.9) | 40 (11.2) | 60 (16.9) | ||||||
| Severe | 10 (2.8) | 9 (2.5) | 4 (1.1) | ||||||
| Headache | |||||||||
| Any | 129 (35.9) | 128 (35.8) | 110 (31.2) | ||||||
| Severe | 25 (6.9) | 19 (5.3) | 11 (3.1) | ||||||
| Fever | |||||||||
| ≥38°C | 11 (3.1) | 14 (3.9) | 7 (1.9) | ||||||
| ≥40°C | 0 (0) | 0 (0) | 0 (0) | ||||||
| Other | |||||||||
| Analgesic/antipyretic used | 38 (10.6) | 31 (8.7) | 38 (10.8) | ||||||
| Stayed at home | 12 (3.4)c | 12 (3.4) | 15 (4.0) | ||||||
MenACWY-CRM, quadrivalent meningococcal conjugate vaccine; Tdap, combined tetanus, reduced diphtheria, and acellular pertussis vaccine.
Severe reactions are defined as symptoms preventing normal daily activities; severe erythema or induration is defined as >50 mm in diameter.
For this reaction in this group, a total of 358 subjects were considered.
Systemic reactions were reported by 48% of the MenACWY-CRM-plus-saline placebo group, 55% of the MenACWY-CRM-plus-Tdap group, and 57% of the Tdap-plus-saline placebo group (Table 2). Between 7% and 11% of subjects across all vaccine groups reported a systemic reaction that was rated as severe. The most commonly reported systemic reactions were headache (31 to 36%) and myalgia (22 to 36%). Severe headache was reported by 3 to 7% of all subjects and severe myalgia by 3 to 4% of all subjects (Table 2).
Statistical analysis showed no significant differences between the frequency of adverse events scored as severe among subjects who received the combined vaccines and the frequency of the same AEs among the total of the subjects of the two groups receiving MenACWY-CRM or Tdap with saline placebo (for instance, chi-square values were 2.94 for pain [P, >0.05], 0.10 for chills [P, >0.05], 0.26 for nausea [P, >0.05], 1.07 for malaise [P, >0.04], 2.26 for myalgia [P, >0.05], 0.62 for arthralgia [P, >0.05], and 3.12 for headache [P, >0.05]).
While myalgia and arthralgia were monitored, fainting or changes in motor function were not specifically investigated. However, no subjects spontaneously reported any severe impairment of motor functions, whether vaccination was concomitant or separate.
In general, the proportions of subjects reporting any local or systemic reaction were higher among those who received Tdap plus saline placebo and among those who received MenACWY- CRM plus Tdap than among recipients of MenACWY-CRM plus saline placebo.
Immunogenicity.
Immune responses to diphtheria, tetanus, and meningococcal serogroups A, C, W-135, and Y were similar, whether vaccine administration was separate or concomitant.
(i) Meningococcal serogroups A, C, W-135, and Y.
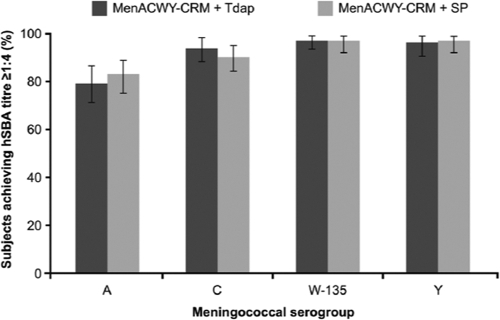
The proportions of subjects with an hSBA titer of ≥1:4 against all meningococcal serogroups were similar in the two MenACWY-CRM vaccine groups. For meningococcal serogroups C, W-135, and Y, values were in the 90 to 97% range; for serogroup A, they were in the 79 to 83% range (Fig. 2). Geometric mean titers (GMTs) were also similar in the two MenACWY-CRM vaccine groups (Table 3).
FIG. 2.
Proportions of subjects achieving human serum bactericidal assay (hSBA) titers of ≥1:4 against Neisseria meningitidis serogroups A, C, W-135, and Y 1 month after vaccination with a quadrivalent meningococcal conjugate vaccine (MenACWY-CRM) plus a combined tetanus, reduced diphtheria, and acellular pertussis vaccine (Tdap) or with MenACWY-CRM plus saline placebo (SP). Error bars represent 95% confidence intervals.
TABLE 3.
Geometric mean titers and geometric mean concentrations of antibodies against vaccine antigens 1 month after vaccination
| Parametera | Value (95% CI) for the groupb vaccinated with: |
||
|---|---|---|---|
| MenACWY-CRM + Tdap (n = 356) | MenACWY-CRM + saline placebo (n = 357) | Tdap + saline placebo (n = 352) | |
| Geometric mean titers | |||
| MenA | 34 (23-50) | 50 (34-74) | NAc |
| MenC | 89 (58-136) | 92 (60-140) | NA |
| MenW-135 | 73 (54-98) | 77 (57-103) | NA |
| MenY | 73 (54-98) | 70 (52-94) | NA |
| Geometric mean concns | |||
| Anti-diphtheria (IU/ml) | 8 (6-10) | NA | 3 (2-4) |
| Anti-tetanus (IU/ml) | 12 (10-14) | NA | 15 (13-18) |
| Anti-PT (EU/ml) | 62 (52-74) | NA | 79 (66-93) |
| Anti-FHA (EU/ml) | 186 (165-209) | NA | 219 (194-246) |
| Anti-PRN (EU/ml) | 157 (131-189) | NA | 254 (211-304) |
GMTs and GMCs were rounded down when the first decimal was ≤0.5 and were rounded up when the first decimal was >0.5. GMTs are all SBA inverse titers. PT, pertussis toxoid; FHA, filamentous hemagglutinin; PRN, pertactin.
MenACWY-CRM, quadrivalent meningococcal conjugate vaccine; Tdap, combined tetanus, reduced diphtheria, and acellular pertussis vaccine.
NA, not applicable.
(ii) Diphtheria.
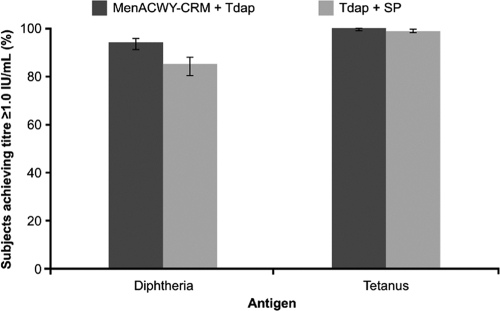
The proportion of subjects with seroprotective anti-diphtheria antibody concentrations (≥1.0 IU/ml) 1 month postvaccination was significantly higher when Tdap was administered concomitantly with MenACWY-CRM than when Tdap was administered with saline placebo (94% versus 85%; LL of 95% CI, >0%) (Fig. 3) and was associated with higher geometric mean concentrations (GMCs) of anti-diphtheria antibodies (Table 3).
FIG. 3.
Proportions of subjects achieving antibody concentrations of ≥1.0 IU/ml against diphtheria and tetanus antigens 1 month after vaccination with a quadrivalent meningococcal conjugate vaccine (MenACWY-CRM) plus a combined tetanus, reduced diphtheria, and acellular pertussis vaccine (Tdap) or vaccination with Tdap plus saline placebo (SP). Error bars represent 95% confidence intervals.
(iii) Tetanus.
One month postvaccination, 100% of subjects who had received concomitant MenACWY-CRM plus Tdap and 99% of those who had received Tdap plus saline placebo achieved a seroprotective anti-tetanus antibody concentration (≥1.0 IU/ml) (Fig. 3). The anti-tetanus immune response to MenACWY-CRM plus Tdap was noninferior to the response to Tdap plus saline placebo (LL of 95% CI, −1%). Similarly, the GMCs of anti-tetanus antibodies in subjects receiving MenACWY plus Tdap were noninferior to those in subjects receiving Tdap plus saline placebo (12 versus 15 IU/ml, respectively; LL of 95% CI, 0.69) (Table 3).
(iv) Pertussis.
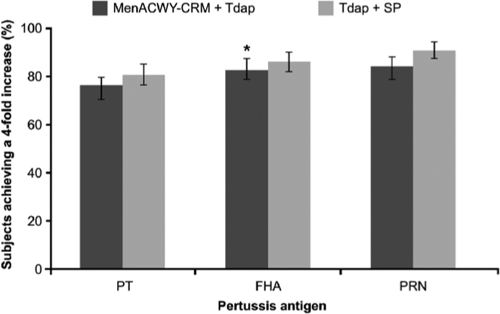
The proportion of subjects achieving a 4-fold increase in antibody concentrations against the pertussis antigens was lower for all three antigens when Tdap was administered concomitantly with MenACWY-CRM than when it was administered with saline placebo (Fig. 4). Noninferiority was achieved for FHA, as demonstrated by the LL of the two-sided 95% CI (−9%) of the vaccine group difference, but not for the PRN or PT antigen (Table 3). However, for PT the difference was slight (−14%) and not statistically significant, while for PRN the difference was moderate.
FIG. 4.
Proportion of subjects achieving a 4-fold increase in concentrations of antibodies against pertussis antigens (pertussis toxoid [PT], filamentous hemagglutinin [FHA], and pertactin [PRN]) 1 month after vaccination with a quadrivalent meningococcal conjugate vaccine (MenACWY-CRM) plus a combined tetanus, reduced diphtheria, and acellular pertussis vaccine (Tdap) or with Tdap plus saline placebo. The asterisk indicates noninferiority (the lower limit of the 95% confidence interval was ≥−10%). Error bars represent 95% confidence intervals.
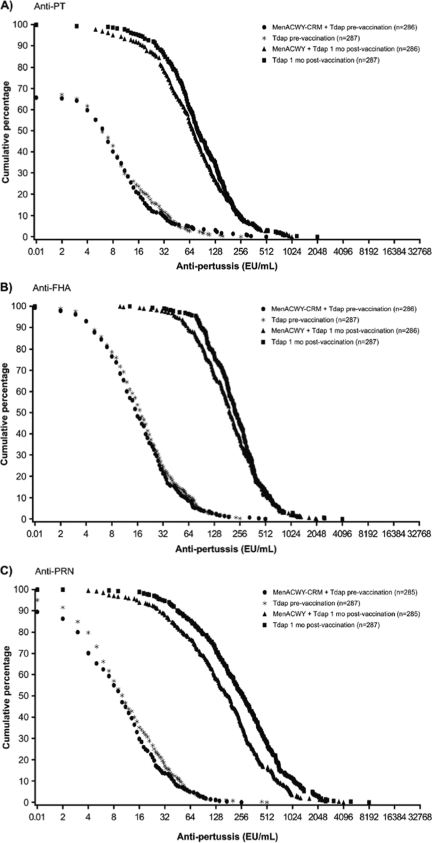
The reverse cumulative distribution curves, which show the distribution of the immune responses across the population of study subjects, are shown in Fig. 5.
FIG. 5.
Reverse cumulative distribution of anti-pertussis toxoid (anti-PT) (A), anti-filamentous hemagglutinin (anti-FHA) (B), and anti-pertactin (anti-PRN) (C) titers prevaccination and 1 month postvaccination. MenACWY-CRM, quadrivalent meningococcal conjugate vaccine; Tdap, combined tetanus, reduced diphtheria, and acellular pertussis vaccine.
DISCUSSION
Concomitant administration of MenACWY-CRM and Tdap did not result in an increase in reactogenicity over that found when the same vaccines were administered with a saline placebo. In addition, the immunogenicity of MenACWY-CRM was not impaired by concomitant administration of Tdap. Similarly, the immune responses to diphtheria and tetanus antigens were noninferior when Tdap was administered concomitantly with MenACWY-CRM. As expected, the anti-diphtheria immune response was higher when the two vaccines were coadministered, owing to the presence of the CRM197 carrier (a nontoxic variant of diphtheria toxin) in the investigational vaccine. In contrast, concomitant administration with MenACWY-CRM appeared to moderately attenuate the immune responses to two out of three of the pertussis antigens. Specifically, when a 4-fold increase in the antibody concentration was used as an endpoint, the administration of Tdap plus MenACWY-CRM elicited a response to FHA that proved to be noninferior to that elicited by Tdap plus saline placebo; with regard to PT, the response was blunted, though not statistically significantly different. These results are similar to those of another study on concomitant vaccine use in adolescents (11 to 18 years of age) (10), in which point estimates for all pertussis antigens were lower when Tdap was administered concomitantly with a quadrivalent meningococcal diphtheria-toxoid protein conjugated vaccine (Menactra; Sanofi Pasteur, Inc., Swiftwater, PA).
While the limitations of cross-study comparison must be acknowledged, the pertussis antigen GMCs following concomitant administration of Tdap and MenACWY-CRM in the current study compared favorably with historical data observed in the infant studies demonstrating pertussis vaccine (DTaP) efficacy (17, 18, 20, 21). The clinical relevance of this finding is not clear, since the correlates of protection for pertussis are not firmly established. In one attempt to define the correlates of immune protection for pertussis antigens, subjects with anti-PT antibody levels exceeding the authors' analytic level of 5 U/ml, but with anti-fimbria and anti-PRN antibodies below that threshold, had a computed vaccine efficacy of 85% (23). Although the absolute GMCs for pertussis antigens in our study were lower when MenACWY-CRM and Tdap vaccines were administered concomitantly, the levels of both anti-PT and anti-PRN were still appreciable (anti-PRN titer, >7 ELISA units [EU]/ml; anti-PT titer, >66 EU/ml [5, 22, 23]). Indeed, 95% of subjects who received concomitant vaccination showed anti-PRN titers of >7 EU/ml, and 50% showed anti-PT titers of >66 EU/ml, versus 98% and 60% of subjects who received Tdap alone (Fig. 5).
Furthermore, with increasing numbers of vaccines recommended for all age-groups, concomitant administration (and data to support its utility) is an important means of simplifying vaccination campaigns. Concomitant administration of MenACWY-CRM and Tdap can provide the adolescent with the required protection in one vaccination visit. Indeed, coadministration has only one small potential disadvantage, namely, an additional, and as yet little known, increased risk of susceptibility to pertussis infection. However, taking into account the findings of Stehr et al. (22) and Cherry et al. (5), the small differences we observed suggest that the slightly higher risk of Bordetella pertussis infection is amply balanced by the reduced risk of missed opportunities.
In conclusion, considering the immune response against the pertussis antigens, the noninferiority of the response for FHA, the moderate responses for PT and PRN, the better immune response to diphtheria antigen, the results obtained by other authors in efficacy studies and clinical trials, and the greater compliance through concomitant administration, we suggest that MenACWY-CRM should be coadministered with the Tdap combined vaccine.
Acknowledgments
This study was funded by Novartis Vaccines, Siena, Italy.
We thank Sarah Angus (Alpharmaxim Healthcare Communications) and Donatella Panatto (Department of Health Sciences, University of Genoa, Genoa, Italy) for assistance in the preparation of the manuscript.
R. Gasparini, M. Conversano, G. Bona, and G. Gabutti declare no conflicts of interest. A. Anemona, P. M. Dull, and F. Ceddia were all Novartis Vaccines employees at time of the study. F. Ceddia has since joined GlaxoSmithKline Biologicals.
Footnotes
Published ahead of print on 17 February 2010.
Trial registration no. NCT00329901.
REFERENCES
- 1.American Academy of Pediatrics Committee on Infectious Diseases. 2005. Prevention and control of meningococcal disease: recommendations for use of meningococcal vaccines in pediatric patients. Pediatrics 116:496-505. [DOI] [PubMed] [Google Scholar]
- 2.Bilukha, O. O., N. Rosenstein, et al. 2005. Prevention and control of meningococcal disease. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recommend. Rep. 54(RR-7):1-21. [PubMed] [Google Scholar]
- 3.Broder, K. R., M. M. Cortese, J. K. Iskander, K. Kretsinger, B. A. Slade, K. H. Brown, C. M. Mijalski, T. Tiwari, E. J. Weston, A. C. Chon, P. U. Srivastava, J. S. Moran, B. Schwartz, and T. V. Murphy. 2006. Preventing tetanus, diphtheria, and pertussis among adolescents: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccines. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recommend. Rep. 55(RR-3):1-34. [PubMed] [Google Scholar]
- 4.Burrage, M., A. Robinson, R. Borrow, N. Andrews, J. Southern, J. Findlow, S. Martin, C. Thorton, D. Goldblatt, M. Corbel, D. Sesardic, K. Cartwight, P. Richmond, and E. Miller. 2002. Effect of vaccination with carrier protein on response to meningococcal C conjugate vaccines and value of different immunoassays as predictors of protection. Infect. Immun. 70:4946-4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cherry, J. D., J. Gornbein, U. Heininger, and K. Stehr. 1998. A search for serologic correlates of immunity to Bordetella pertussis cough illnesses. Vaccine 16:1901-1906. [DOI] [PubMed] [Google Scholar]
- 6.Edwards, K. M., and M. D. Decker. 2008. Pertussis vaccines, p. 467-517. In S. Plotkin, W. Orenstein, and P. Offit (ed.), Vaccines, 5th ed. Saunders/Elsevier, Philadelphia, PA.
- 7.European Union Invasive Bacterial Infections Surveillance Network (EU-IBIS). 2006. Invasive Neisseria meningitidis in Europe 2006. Health Protection Agency, London, United Kingdom. http://www.euibis.org/documents/2006_meningo.pdf.
- 8.European Union Invasive Bacterial Infections Surveillance Network (EU-IBIS). 9 January 2009, accession date. Age distribution of culture-confirmed Neisseria meningitidis cases in all reporting countries, by year, 1999-2006. http://www.euibis.org/php/meningo_age_chart.php?item=culture&country=All+countries.
- 9.EUVAC.NET. 9 January 2009, accession date. National childhood vaccination schedules. http://www.euvac.net/graphics/euvac/vaccination/vaccination.html.
- 10.Friedland, L., W. Weston, and B. Howe. 2007. Immunogenicity of coadministered Tdap and MCV4 vaccines compared to separately administered vaccines, poster G-1699, p. 282. 47th Annu. Intersci. Conf. Antimicrob. Agents Chemother., Chicago, IL, 17 to 20 September 2007.
- 11.Goldschneider, I., E. C. Gotschlich, and M. S. Artenstein. 1969. Human immunity to the meningococcus. I. The role of humoral antibodies. J. Exp. Med. 129:1307-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Granoff, D. M., L. H. Harrison, and R. Borrow. 2008. Meningococcal vaccines, p. 399-434. In S. Plotkin, W. Orenstein, and P. Offit (ed)., Vaccines, 5th ed. Saunders/Elsevier, Philadelphia, PA.
- 13.Jackson, L. A., R. M. Jacobson, K. S. Reisinger, A. Anemona, L. E. Danzig, and P. M. Dull. 2009. A randomized trial to determine the tolerability and immunogenicity of a quadrivalent meningococcal glycoconjugate vaccine in healthy adolescents. Pediatr. Infect. Dis. J. 28:86-91. [DOI] [PubMed] [Google Scholar]
- 14.Mayer, L. W., M. W. Reeves, N. Al-Hamdan, C. T. Sacchi, M. K. Taha, G. W. Ajello, S. E. Schmink, C. A. Noble, M. L. Tondella, A. M. Whitney, Y. Al-Mazrou, M. Al-Jefri, A. Mishkhis, S. Sabban, D. A. Caugant, J. Lingappa, N. E. Rosenstein, and T. Popovic. 2002. Outbreak of W135 meningococcal disease in 2000: not emergence of a new W135 strain but clonal expansion within the electrophoretic type-37 complex. J. Infect. Dis. 185:1596-1605. [DOI] [PubMed] [Google Scholar]
- 15.Middleman, A. B. 2007. New adolescent vaccination recommendations and how to make them “stick.” Curr. Opin. Pediatr. 19:411-416. [DOI] [PubMed] [Google Scholar]
- 16.Rosenstein, N. E., B. A. Perkins, D. S. Stephens, L. Lefkowitz, M. L. Cartter, R. Danila, P. Cieslak, K. A. Shutt, T. Popovic, A. Schuchat, L. H. Harrison, and A. L. Reingold. 1999. The changing epidemiology of meningococcal disease in the United States, 1992-1996. J. Infect. Dis. 180:1894-1901. [DOI] [PubMed] [Google Scholar]
- 17.Schmitt, H. J., A. Schuind, M. Knuf, K. Beutel, H. Schulte-Wissermann, M. Gahr, R. Schult, J. Folkens, W. Rauh, H. Bogaerts, H. L. Bork, and R. Clemens. 1996. Clinical experience of a tricomponent acellular pertussis vaccine combined with diphtheria and tetanus toxoids for primary vaccination in 22,505 infants. J. Pediatr. 129:695-701. [DOI] [PubMed] [Google Scholar]
- 18.Schmitt, H. J., C. H. von König, A. Neiss, H. Bogaerts, H. L. Bock, H. Schulte-Wissermann, M. Gahr, R. Schult, J. U. Folkens, W. Rauh, and R. Clemens. 1996. Efficacy of acellular pertussis vaccine in early childhood after household exposure. JAMA 275:37-41. [PubMed] [Google Scholar]
- 19.Shepard, C. W., I. R. Ortega-Sanchez, R. D. Scott II, N. E. Rosenstein, and the ABCs Team. 2005. Cost-effectiveness of conjugate meningococcal vaccination strategies in the United States. Pediatrics 115:1220-1232. [DOI] [PubMed] [Google Scholar]
- 20.Snape, M. D., K. P. Perrett, K. J. Ford, T. M. John, D. Pace, L. M. Yu, J. M. Langley, S. McNeil, P. M. Dull, F. Ceddia, A. Anemona, S. A. Halperin, S. Dobson, and A. J. Pollard. 2008. Immunogenicity of a tetravalent meningococcal glycoconjugate vaccine in infants. JAMA 299:173-184. [DOI] [PubMed] [Google Scholar]
- 21.Southern, J., D. Gelb, N. Andrews, P. A. Waight, R. Morris, K. Cartwright, and E. Miller. 2006. Reactogenicity of meningococcal C conjugate vaccines when administered at the same time as, or a month prior to or after, tetanus and diphtheria booster vaccinations. Hum. Vaccin. 2:237-242. [DOI] [PubMed] [Google Scholar]
- 22.Stehr, K., J. D. Cherry, U. Heininger, S. Schmitt-Grohé, M. Uberall, S. Laussucq, T. Eckhardt, M. Meyer, R. Engelhardt, and P. Christenson. 1998. A comparative efficacy trial in Germany in infants who received either the Lederle/Takeda acellular pertussis component DTP (DTaP) vaccine, the Lederle whole-cell component DTP vaccine, or DT vaccine. Pediatrics 101(1 Pt 1):1-11. [DOI] [PubMed] [Google Scholar]
- 23.Storsaeter, J., H. O. Hallander, L. Gustafsson, and P. Olin. 1998. Levels of anti-pertussis antibodies related to protection after household exposure to Bordetella pertussis. Vaccine 16:1907-1916. [DOI] [PubMed] [Google Scholar]