Abstract
Shiga-like toxin 2 (Stx2)-producing enterohemorrhagic Escherichia coli (referred to as EHEC or STEC) strains are the primary etiologic agents of hemolytic-uremic syndrome (HUS), which leads to renal failure and high mortality rates. Expression of Stx2 is the most relevant virulence-associated factor of EHEC strains, and toxin neutralization by antigen-specific serum antibodies represents the main target for both preventive and therapeutic anti-HUS approaches. In the present report, we describe two Salmonella enterica serovar Typhimurium aroA vaccine strains expressing a nontoxic plasmid-encoded derivative of Stx2 (Stx2ΔAB) containing the complete nontoxic A2 subunit and the receptor binding B subunit. The two S. Typhimurium strains differ in the expression of flagellin, the structural subunit of the flagellar shaft, which exerts strong adjuvant effects. The vaccine strains expressed Stx2ΔAB, either cell bound or secreted into the extracellular environment, and showed enhanced mouse gut colonization and high plasmid stability under both in vitro and in vivo conditions. Oral immunization of mice with three doses of the S. Typhimurium vaccine strains elicited serum anti-Stx2B (IgG) antibodies that neutralized the toxic effects of the native toxin under in vitro conditions (Vero cells) and conferred partial protection under in vivo conditions. No significant differences with respect to gut colonization or the induction of antigen-specific antibody responses were detected in mice vaccinated with flagellated versus nonflagellated bacterial strains. The present results indicate that expression of Stx2ΔAB by attenuated S. Typhimurium strains is an alternative vaccine approach for HUS control, but additional improvements in the immunogenicity of Stx2 toxoids are still required.
Shiga-like toxins (Stx) play a crucial role in the pathogenesis of enterohemorrhagic Escherichia coli (EHEC) strains, which may lead to hemorrhagic colitis, central nervous system disturbances, and hemolytic-uremic syndrome (HUS) (27, 33). HUS involves acute renal failure, thrombocytopenia, and microangiopathic hemolytic anemia, with mortality rates ranging from 1% to 4% (45, 50). EHEC strains may express different serotypes, including the widely distributed O157:H7 serotype, and infection correlates with the ingestion of contaminated ground beef and cow manure-contaminated water, vegetables, juices, and other products (13, 18). The incidence of EHEC-associated HUS cases is particularly high in developed countries, and high incidence rates have been recorded in Argentina, where cultural and diverse epidemiological factors contribute to the widespread dissemination of the disease among children and teenagers (38).
EHEC strains may express two different Stx types. Stx1 is virtually identical to Stx produced by Shigella dysenteriae, while Stx2 shows only 56% homology to Stx1 at the amino acid sequence level (14, 33, 51). Both toxin types are formed by one A subunit and five B subunits, which bind to glycosphingolipid receptors, such as globotriaosyl ceramide (Gb3), on host cell membranes and promote retrograde toxin transport through the Golgi complex and endoplasmic reticulum. In the cell cytoplasm, Stx2 subunit A is processed into two fragments; one of them (A1) is endowed with N-glycosidase activity, which depurinates a specific adenine residue of the eukaryotic 28S rRNA, inhibits protein synthesis, and induces apoptosis of the target cell (18, 51).
After ingestion and gut colonization, Stx molecules are released by the bacterial cells and translocate across the gut epithelium to reach, via the bloodstream, capillary endothelial cells at renal glomeruli, where the most relevant tissue damage occurs (33, 45, 50). Epidemiological data indicate that individuals infected with Stx2-producing bacterial strains, and some closely related variants, have a high probability of developing HUS (45, 50). In addition, Stx2 expression has been shown to increase gut colonization by bacterial cells due to induction of increased receptor expression by enterocytes (39).
So far, there is no effective prophylactic or therapeutic approach for the prevention of HUS development among EHEC-infected individuals. The treatments available involve platelet transfusion in cases of severe anemia, hemodialysis, and supportive care (7, 50). A more direct anti-Stx treatment under clinical or preclinical evaluation involves the use of synthetic Stx glycolipid receptor analogs and humanized anti-Stx monoclonal antibodies (44, 52).
Attempts to develop prophylactic anti-HUS vaccines are focused on the generation of Stx-neutralizing antibodies or the blockade of gut colonization. The vaccine strategies based on Stx2 that have been tested under experimental conditions have included DNA vaccines (5, 12), protein-conjugated polysaccharides (28), purified recombinant B subunits (24, 25, 29, 30, 47, 53, 55, 58), and B-subunit-derived synthetic peptides (19, 20). Anti-EHEC vaccine approaches based on the blockade of gut colonization have employed intimin and type III secreted proteins, such as EspA and EspB (3, 37, 54).
Live bivalent anti-Stx vaccines based on genetically modified, attenuated Vibrio cholerae or Salmonella enterica serovar Typhimurium strains have been reported to induce anti-StxB antibody responses following oral administration to mice or rabbits (1, 10, 49). Attenuated Salmonella strains, used as orally administered vaccine vectors for the expression of heterologous antigens, show several advantages over conventional parenterally delivered cellular or acellular vaccine formulations (15, 16). Attenuated Salmonella strains are safe, are easily administered by untrained personnel, and, more relevantly, may induce systemic and secreted antigen-specific antibody and cell-based immune responses against self and heterologous antigens. In addition, whole bacterial cells carry on their surfaces several molecular structures known to activate both innate and adaptive immune responses. These molecules, such as lipopolysaccharide and flagellin, act as strong adjuvants, both systemically and at mucosal surfaces.
Flagellins, the structural subunit of flagellar filaments, contribute both to the virulence of bacterial pathogens and to the activation of inflammatory responses in mammalian hosts. Bacterial flagellins have been shown to bind both extracellular and intracellular receptors of antigen-presenting cells, leading to inflammation and increased adaptive immune responses, including the generation of antigen-specific antibodies and T cells (2, 26). The strong adjuvant effects of Salmonella flagellins, either when admixed with purified antigens or when used as hybrid proteins genetically fused to the target antigens, have been demonstrated recently (4, 8, 22, 23, 36). However, there is no clear evidence that the expression of flagellin affects the immunogenicity of heterologous antigens expressed by attenuated Salmonella vaccine strains.
In the present study, we generated new experimental anti-HUS vaccine formulations based on two recombinant attenuated S. Typhimurium aroA vaccine strains differing in the expression of flagellin. The two strains were genetically modified in order to express a nontoxic Stx2 derivative consisting of the whole Stx2 B subunit and a partially deleted A subunit encompassing the first amino acid of the A1 subunit genetically fused to the whole A2 subunit (Stx2ΔAB). The Stx2ΔAB protein was previously tested in mice immunized with a DNA vaccine (5). The results of the present study show that the S. Typhimurium vaccine strains express and secrete the recombinant toxin and induce both systemic and mucosal anti-StxB antibodies with anti-Stx2 neutralization activity, conferring partial protection against intravenous (i.v.) challenge with Stx2.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The bacterial strains employed in the present study are listed in Table 1. S. Typhimurium SL3261 and LDV321 are isogenic strains differing in the expression of the fliC and fljB genes, which were specifically deleted by nonpolar site-directed mutagenesis (31). The S. Typhimurium aroA strains were routinely cultivated in Luria-Bertani (LB) broth or on LB agar plates containing 100 μg of dihydroxybenzoic acid (DHB) in the culture medium. Plasmid-transformed bacterial strains were cultivated in medium with ampicillin added (100 μg/ml). All cultures were grown at 37°C with shaking.
TABLE 1.
Bacterial strains and plasmids used in the present study
| Strain or plasmid | Genotype or characteristics | Source or reference |
|---|---|---|
| Strains | ||
| S. Typhimurium | ||
| SL3261 | aroA his | 21 |
| LDV321 | aroA his ΔfliC ΔfljB | 31 |
| LDV326 | LDV321(pGEM-T) | This study |
| LDV327 | LDV321(pCVT-2) | This study |
| LDV328 | SL3261(pGEM-T) | This study |
| LDV329 | SL3261(pCVT-2) | This study |
| E. coli | ||
| JM109 | recA1 supE44 endA1 hsdR17 gyrA96 relA1 thiΔ(lac-proAB) F [traD36 proAB+ lacIqlacZΔM15] | Stratagene |
| LDV15 | JM109(pCVT-1) | This study |
| DH5α | recA1 endA1 gyrA96 glnV44 supE44 relA1 deoR Δ(lacZ-argF)U169 hsdR17 thi-1 λ− φ80dlac Δ(lacZ)M15 F− | Invitrogen |
| Plasmids | ||
| pGEMT | Ampr | Promega |
| pCVT-1 | pGEM-T with complete stx2 operon sequence | This study |
| pCVT-2 | pGEM-T with cloned sequence encoding StxΔAB2 | This study |
Generation of recombinant S. Typhimurium strains encoding StxΔAB2.
The complete nucleotide sequence of the gene encoding Stx2, including the native promoter region, was amplified by PCR from total DNA from E. coli O157:H7 with primers Ds (5′-GAATTCATTATGCGTTGTTAG-3′) and R1 (5′-GAATTCTCAGTC ATTATTAAACTG-3′), each containing an EcoRI restriction site, and the product was cloned into the EcoRI-cleaved pGEM-T Easy vector. The selected recombinant vector was named pCVT-1 and encompassed the complete stx2 operon, including the promoter sequence. After the transformation of E. coli strain DH5α and the selection of a recombinant clone, the plasmid was digested with AvaI and StuI, treated with Klenow DNA polymerase, and ligated with T4 DNA ligase to produce the vector named pCVT-2. This vector encodes the Stx2 B subunit (signal peptide and mature protein sequences) plus the A2 subunit fused to the signal sequence and the first N-terminal amino acid of the A1 subunit. The experimental steps related to the cloning and construction of the genes encoding Stx2 and Stx2ΔAB, as well as the final nucleotide sequences of these constructs, have been reported previously (5). After screening and selection of a clone with the desired genes, the purified pCVT-2 vector was electroporated into S. Typhimurium strain SL3261 and the nonflagellated strain LDV321, resulting in strains LDV329 and LDV327, respectively.
Detection of Stx (Stx2ΔAB) by immunoblot and dot blot assays.
Whole-cell protein extracts and concentrated culture supernatants of the recombinant S. Typhimurium strains were sorted in 15% polyacrylamide gels and were electrotransferred to nitrocellulose sheets (pore size, 0.45 μm) at 200 mA for 1 h. After being blocked overnight with 1% (wt/vol) bovine serum albumin (BSA) in phosphate-buffered saline (PBS) at 4°C, the nitrocellulose sheets were probed with mouse anti-Stx2 B subunit sera, followed by secondary antibodies (horseradish peroxidase-conjugated rabbit antibodies against mouse IgG [Sigma]), according to procedures described previously (43). The membranes were developed with a chemiluminescent kit (Pierce) and were exposed to Kodak X-Omat film. Dot blot assays were performed with culture supernatants or with whole-cell extracts of cultures at the exponential-growth phase. The quantity of Stx2ΔAB produced by each vaccine strain was determined by dot blot assays using purified recombinant Stx2B as a standard. Aliquots containing approximately 108 CFU (10 μg of total protein) were 2-fold serially diluted in PBS and were subsequently spotted (10 μl) onto nitrocellulose sheets using a vacuum manifold device (Millipore). Aliquots of bacterial culture supernatant were collected, precipitated with 10% trichloroacetic acid (TCA), washed with cold acetone, and suspended in PBS (pH 7.4). An aliquot (2 μl) of each sample was spotted onto nitrocellulose sheets and was subsequently incubated with mouse anti-Stx2 B subunit sera, followed by secondary antibodies (horseradish peroxidase-conjugated rabbit antibodies against mouse IgG [Sigma]). Mouse anti-Stx2B sera have been generated in our laboratory by producing the recombinant Stx2 B subunit in E. coli and purifying it by affinity chromatography with a nickel-containing resin. The blots were developed by the same procedure used for Western blots.
Motility of S. Typhimurium vaccine strains.
Motility assays with the recombinant S. Typhimurium strains were carried out by stabbing cells at the centers of motility agar plates, followed by inoculation at 37°C for 24 h, as described previously (31).
Determination of in vitro and in vivo stabilities of recombinant plasmids carried by the S. Typhimurium vaccine strains.
The in vitro segregational stabilities of the pGEM-T and pCVT-2 vectors in the transformed S. Typhimurium strains were measured during growth in LB medium supplemented with DHB but without ampicillin. The strains were cultivated for 18 h, and aliquots were diluted in PBS and plated onto LB agar plates without ampicillin. After overnight incubation, colonies were replica plated onto LB agar plates containing 100 μg/ml of ampicillin, and the number of resistant colonies was determined. The initial culture was repeated daily over a period of 5 days in LB medium without ampicillin. The numbers of antibiotic-resistant colonies were determined daily. The in vivo plasmid stabilities of S. Typhimurium strains were determined with bacterial colonies recovered from female BALB/c mice orally inoculated with a single 1010-CFU dose of one of the recombinant S. Typhimurium vaccine strains. The animals were euthanized 24, 48, and 72 h after oral administration of the bacterial strains, and their small intestines and spleens were removed under aseptic conditions. Five Peyer's patches (PP) and whole spleens from each mouse were homogenized and serially diluted in PBS. Aliquots were plated onto MacConkey agar or LB agar supplemented with novobiocin (5 μg/ml) and streptomycin (50 μg/ml) and were incubated at 37°C for 24 h to select for the S. Typhimurium colonies. Recovered colonies were replica plated onto LB agar plates containing 100 μg/ml of ampicillin, and the number of ampicillin-resistant colonies was determined after overnight incubation at 37°C.
Mouse immunization with S. Typhimurium vaccine strains.
BALB/c mice were supplied by the Isogenic Mouse Breeding Facility of the Department of Parasitology, Institute of Biomedical Sciences, São Paulo University (USP). All animal-handling procedures were in accordance with the principles of the Brazilian code for the use of laboratory animals and were approved by the ethics committee on the use of laboratory animals of the Institute of Biomedical Sciences at the University of São Paulo. Oral immunizations were carried out using viable bacterial cells harvested during the exponential-growth phase (optical density at 600 nm, 0.8). Bacteria were washed once with PBS and were resuspended in sodium bicarbonate at a concentration of 1010 CFU/ml. Mice were immunized with 0.5-ml aliquots of the bacterial suspensions with a stainless-steel round-tip gavage cannula on days 1, 22, and 36. Blood, collected from the retro-orbital plexus, and feces were collected on day zero (preimmune samples) and on days 21, 35, and 50 after immunization. Fecal homogenates were prepared as described previously (42).
Enzyme-linked immunosorbent assays (ELISA).
MaxiSorp microtiter plates (Nalge Nunc) were coated with the purified recombinant Stx2 B subunit (1 μg/ml) in PBS (pH 7.4) and were incubated overnight at 4°C, as previously described (17). The plates were blocked with PBS with 1% BSA for 1 h at 37°C, and aliquots of serum and fecal extracts, previously diluted in PBS with 0.05% Tween 20 (PBST), were added to the plates in a series of 2-fold dilutions, followed by incubation for a further 2 h at 37°C. The plates were washed twice with PBST and were incubated for 1 h with a peroxidase-conjugated rabbit antibody specifically reacting with mouse IgG or IgA isotypes and IgG1 or IgG2a subclasses (Southern Biotechnology). After being washed with PBST, plates were developed with O-phenylenediamine dihydrochloride and with H2O2 as a substrate. Reactions were stopped with H2SO4, and absorbance values were measured at 492 nm in a microtiter plate spectrophotometer (Multiscan MS; LabSystems). Endpoint titers were automatically calculated with the Microcal Origin (version 6.0) Professional program as the reciprocal values of the last dilutions with an optical density of 0.1. Results are expressed as arithmetic means of at least duplicate determinations of the endpoint titers and standard errors of the means (SEM).
In vitro and ex vivo Stx2-neutralizing tests and challenge assay.
Both in vitro and ex vivo Stx2 neutralization activities of sera collected from mice immunized with the different S. Typhimurium vaccine strains were determined as described previously (5, 12, 17). The in vitro test was carried out with equal volumes of whole E. coli LDV15 extracts containing twice the dose of Stx2 that leads to 50% cytotoxicity (CD50) in Vero cells, as described previously (5, 12). Ex vivo Stx2 neutralization tests with immune sera were carried out after incubation of Stx2 aliquots with diluted serum samples for 1 h at 37°C and 1 h at 4°C before i.v. inoculation into naïve nonimmunized mice. The in vivo lethality studies were carried out with Stx2 aliquots inoculated i.v. at a dose corresponding to 53 ng/mouse, which resulted in 100% mortality in nonvaccinated mice 4 days after injection.
Determination of blood urea nitrogen and creatinine levels.
Concentrations of blood urea nitrogen and creatinine in serum samples from mice challenged with Stx2 were determined with a colorimetric-enzymatic kit (Labtest, Brazil). Results were measured in an Ultrospec 2100 Pro spectrophotometer (Amersham Biosciences).
Statistical analysis.
Immunized mouse groups were compared using unpaired t test analysis. To estimate P values, all statistical analyses were interpreted in a two-tailed manner. P values of <0.05 were considered statistically significant.
RESULTS
Construction of S. Typhimurium vaccine strains encoding a nontoxic form of Stx2 (Stx2ΔAB).
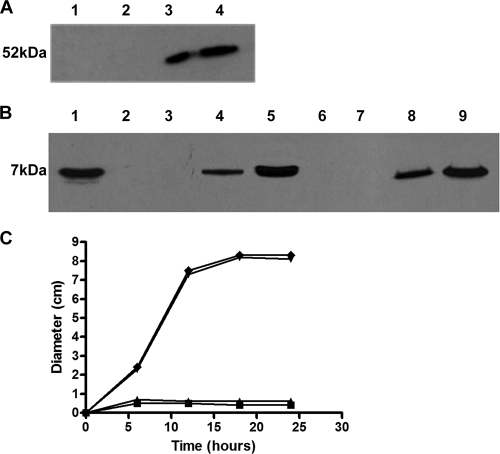
A nontoxic genetically engineered form of Stx2, named Stx2ΔAB, encompassing the complete Stx2 B subunit (signal peptide and mature protein sequences) plus the A2 subunit fused to the signal sequence of the A1 subunit, was generated as a nontoxic antigen with enhanced immunogenicity with regard to the purified B subunits (5). Initial attempts to clone the gene encoding Stx2ΔAB under the control of the nirB promoter, activated by low oxygen levels (35), resulted in extensive cell death and plasmid segregation in S. Typhimurium strains under both in vitro and in vivo conditions (data not shown). On the other hand, cloning of the DNA fragment containing the native stx2 promoter and the gene encoding Stx2ΔAB into the pGEM-T vector allowed expression of the heterologous antigen without affecting the cell viability and plasmid stability of the transformed S. Typhimurium aroA strains. The recombinant plasmid, named pCVT-2, was electroporated into the attenuated S. Typhimurium strains SL3261 and LDV321, differing in the expression of flagellin, resulting in the selection of the two vaccine strains, named LDV329 and LDV327, respectively. S. Typhimurium strains LDV326 and LDV328 were obtained after the introduction of the pGEM-T vector into strains LDV321 and SL3261, respectively. No significant growth delay was detected in S. Typhimurium strains harboring pCVT-2 relative to the growth of strains transformed with pGEM-T (data not shown). Moreover, S. Typhimurium strain LDV329 showed complete plasmid stability after continuous growth for 4 days under nonselective conditions, while 52% of the LDV327 colonies retained the plasmid after the same period (Table 2). As expected, flagellin was detected in whole-cell extracts of strains LDV328 and LDV329 but not in extracts of strains LDV326 and LDV327 (Fig. 1A). A 7.5-kDa band reacting with an anti-Stx2B serum was detected in whole-cell extracts and culture supernatants of both S. Typhimurium LDV327 and S. Typhimurium LDV329 (Fig. 1B). Seeding of the vaccine strains in soft agar plates showed that strains LDV328 and LDV329 were motile while strains LDV326 and LDV327 were nonmotile (Fig. 1C).
TABLE 2.
In vitro and in vivo stabilities of recombinant plasmids carried by S. Typhimurium vaccine strains
| Condition and time | Plasmid stabilitya |
|||
|---|---|---|---|---|
| LDV326 | LDV327 | LDV328 | LDV329 | |
| In vitrob | ||||
| 1 day | 50/50 (100) | 50/50 (100) | 50/50 (100) | 50/50 (100) |
| 2 days | 43/50 (86) | 35/50 (70) | 45/50 (91) | 50/50 (100) |
| 3 days | 40/50 (79) | 32/50 (64) | 45/50 (90) | 50/50 (100) |
| 4 days | 32/50 (63) | 26/50 (52) | 43/50 (86) | 50/50 (100) |
| In vivoc | ||||
| 24 h | 46/50 (92) | 50/50 (100) | 50/50 (100) | 50/50 (100) |
| 48 h | 34/50 (68) | 36/50 (72) | 36/50 (72) | 46/50 (92) |
| 72 h | 21/50 (42) | 31/50 (62) | 35/50 (70) | 39/50 (78) |
Expressed as number of Ampr colonies/total number of colonies tested (percentage of Ampr colonies).
S. Typhimurium strains transformed with pGEM-T (strains LDV326 and LDV328) or pCVT-2 (strains LDV327 and LDV329) were screened for ampicillin resistance on LB plates following daily cultures carried out under nonselective conditions.
S. Typhimurium colonies isolated from 5 Peyer's patches recovered from inoculated mice were screened for ampicillin resistance following oral administration of 1010 cells of the vaccine strains tested.
FIG. 1.
Expression of FliC flagellin and Stx2ΔAB by the S. Typhimurium vaccine strains. (A) Detection of FliC expression in the S. Typhimurium vaccine strains. FliC was detected in Western blots developed with polyclonal anti-FliC antibodies and whole-cell extracts of the bacterial strains. Lanes: 1, LDV326; 2, LDV327; 3, LDV328; 4, LDV329. (B) Detection of Stx2ΔAB, encoded by the S. Typhimurium vaccine strains, in culture supernatants (lanes 2, 4, 6, and 8) and whole-cell extracts (lanes 3, 5, 7, and 9) of the strains. Lanes: 1, purified recombinant StxB; 2 and 3, LDV326; 4 and 5, LDV327; 6 and 7, LDV328; 8 and 9, LDV329. (C) In vitro motilities of recombinant S. Typhimurium vaccine strains LDV326 (▴), LDV327 (▾), LDV328 (▪), and LDV329 (⧫) at 37°C measured by the diameter of the bacterial growth halos.
The amounts of antigen expressed by the S. Typhimurium vaccine strains were determined in exponentially growing cells cultivated in LB medium at 37°C using both whole-cell extracts and culture supernatants. Under such conditions, 1010 CFU of S. Typhimurium strain LDV327 or LDV329 accumulated approximately 3.5 μg of Stx2ΔAB intracellularly. In addition, for both strains, the amount of secreted antigen detected in culture supernatants of spent medium following incubation for 12 h was approximately 150 ng/ml.
In vivo plasmid stability of, and gut colonization by, S. Typhimurium strains expressing Stx2ΔAB.
Mice were orally administered a single dose containing 1010 CFU of each S. Typhimurium vaccine strain. S. Typhimurium colonies recovered from Peyer's patches were replica plated onto a medium with ampicillin for determination of the number of plasmid-containing ampicillin-resistant clones. The numbers of S. Typhimurium colonies recovered from mice orally inoculated with a strain expressing Stx2ΔAB (LDV327 or LDV329) were significantly higher than those detected in the Peyer's patches of mice inoculated with LDV326 or LDV328, strains that do not express Stx2ΔAB (Fig. 2). No statistically significant difference was observed between the numbers of bacterial colonies recovered from the guts of mice inoculated with flagellated versus nonflagellated bacterial strains (Fig. 2). No statistically significant differences were observed in the numbers (<10 CFU) of bacterial colonies detected in the spleens of mice 24 h after oral administration of the bacterial strains tested (data not shown). The in vivo stabilities of plasmids in the S. Typhimurium LDV327 and LDV329 cells recovered from Peyer's patches of inoculated mice, measured by the antibiotic resistance phenotype, ranged from 100%, after 24 h postinoculation, to approximately 65%, 72 h after oral administration. Similar results were also obtained with strains LDV326 and LDV328, which had been transformed with the pGEM-T vector (Table 2).
FIG. 2.
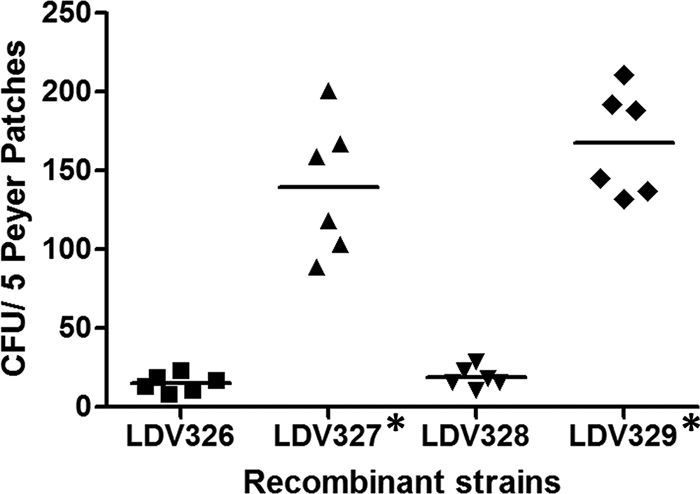
Colonization of mouse guts by the S. Typhimurium vaccine strains. Five Peyer's patches (PP) obtained from each mouse orally dosed with 1010 bacterial cells were homogenized and serially diluted in PBS before being plated onto ampicillin-LB plates. The graph represents the number of CFU obtained 72 h after the initial inoculation of the S. Typhimurium strain. Asterisks indicate statistically significant differences from results for mice immunized with S. Typhimurium strain LDV326 or LDV328 (P < 0.05).
Induction of anti-Stx2 antibodies in mice orally immunized with the S. Typhimurium vaccine strains.
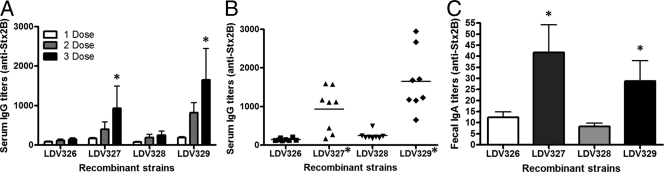
The immunogenicity of Stx expressed by the S. Typhimurium vaccine strains was determined in mice after three oral administrations given on days 1, 22, and 36. Following immunizations, serum and fecal samples from vaccinated mice were analyzed for the presence of anti-Stx2B antibodies (IgG in serum and IgA in fecal extracts) by using ELISA plates treated with purified Stx2 B subunits (Fig. 3). As shown in Fig. 3A, anti-StxB serum IgG responses were detected in mice orally immunized with three doses of S. Typhimurium strain LDV327 or LDV329, expressing the recombinant Stx2ΔAB antigen. The average serum anti-Stx2B IgG titer in mice immunized with three doses of S. Typhimurium strain LDV327 or LDV329 reached 931.15 ± 274 or 1,654.7 ± 252, respectively (Fig. 3B; Table 3). Low fecal anti-Stx2B IgA responses were detected only in mice orally immunized with S. Typhimurium strain LDV327 or LDV329 (Fig. 3C).
FIG. 3.
Serum IgG and fecal IgA responses to Stx2B elicited in mice orally immunized with the recombinant S. Typhimurium vaccine strains. The anti-Stx2B antibody titers were determined by ELISA using the purified recombinant Stx2 B subunit. (A) Kinetics of anti-Stx2B IgG responses detected in pools of sera from mice immunized with one of the three S. Typhimurium strains. Mice immunized with one (open bars), two (shaded bars), or three (filled bars) doses of the vaccine strain are distinguished. (B) Individual serum anti-Stx2B IgG titers elicited in mice immunized with three doses of one of the tested S. Typhimurium vaccine strains. (C) Fecal anti-Stx2B IgA titers detected in mice immunized with three doses of one of the tested S. Typhimurium vaccine strains. Fecal IgA levels were measured in sample pools collected from animals of the same immunization group. Asterisks indicate statistically significant differences from results for mice immunized with S. Typhimurium strain LDV326 or LDV328 (P < 0.05).
TABLE 3.
Anti-Stx2 neutralization activities of recombinant S. Typhimurium vaccine strains measured under in vitro, ex vivo, and in vivo conditions
| Vaccine strain | In vitro anti-Stx2 neutralization titera | Neutralization effect (no. of mice surviving challenge/total no. of mice [%]) |
|
|---|---|---|---|
| Ex vivob | In vivoc | ||
| LDV326 | <5d | 0/12 | 0/8 (0) |
| LDV327 | 30 (931.1 ± 274.2) | 0/12 | 1/8 (12.5) |
| LDV328 | <5 | 0/12 | 0/8 (0) |
| LDV329 | 60 (1,654.7 ± 252.4) | 3/12 (25) | 2/8 (25) |
Anti-Stx2 neutralizing titers of pooled sera harvested from mice immunized with one of the different S. Typhimurium vaccine strains and measured by the Vero assay. The Stx2 neutralization titer was defined as the maximal serum dilution that abolished the cytotoxic effects of Stx2-containing bacterial extracts on in vitro-cultured Vero cells. Values in parentheses are mean titers of IgG antibodies in the sera of mice (n = 8) immunized with the indicated recombinant strain ± standard deviations.
Mice were challenged with Stx2 that had previously been incubated with pooled sera harvested from mice immunized with one of the S. Typhimurium vaccine strains. Toxin samples were incubated with serum samples at a final dilution of 1:10, as described in Materials and Methods. Survivors were monitored up to 96 h after challenge.
Mice were challenged i.v. with Stx2. Each group of mice was vaccinated with three doses of the indicated S. Typhimurium vaccine strain.
There was no visible Stx2 neutralization activity at the lowest serum dilution tested (1:5).
Stx2 neutralization effects of antibodies raised in mice immunized with S. Typhimurium vaccine strains.
Stx2 aliquots promoting cytotoxic effects on in vitro-cultured Vero cells or causing 100% lethality in BALB/c mice were incubated with different dilutions of serum samples collected from vaccinated mice in order to evaluate the anti-Stx2 neutralization titers. As indicated in Table 3, pooled serum samples collected from mice immunized with S. Typhimurium strain LDV327 or LDV329 neutralize the toxic effects of Stx2, as evaluated with Vero cells, up to a final dilution of 30 or 60, respectively. In addition, incubation of Stx2 aliquots with diluted serum pools collected from mice immunized with S. Typhimurium strain LDV329 protected 3 out of 12 (25%) mice inoculated with a lethal Stx2 load. On the other hand, no in vivo protection was observed in mice inoculated with Stx2 incubated with serum samples from mice immunized with S. Typhimurium strain LDV327. Similarly, no Stx2 neutralization activity was detected in serum samples collected from mice immunized with S. Typhimurium strain LDV326 or LDV328 (Table 3).
The protective anti-Stx2 immunity raised in mice immunized with the S. Typhimurium vaccine strains was determined after challenge of vaccinated mice with Stx2. Two out of 8 mice (25% protection level) vaccinated with three doses of strain LDV329 were protected from a lethal Stx2 challenge for more than 15 days, while 1 out of 8 (12.5%) mice immunized with three doses of strain LDV327 survived the same lethal challenge (Table 3). No protection was recorded for animals immunized with S. Typhimurium strain LDV326 or LDV328.
Blood urea nitrogen and creatinine levels in mice challenged with native Stx2.
Blood urea nitrogen and creatinine levels were determined in mice immunized with the S. Typhimurium vaccine strains and challenged with native Stx2. The increased levels of urea and creatinine in serum are indicative of defective renal function caused by Stx2 (9, 41) Significant increases in serum urea and creatinine concentrations were observed after administration of Stx2 to mice immunized with strain LDV326 or LDV328. On the other hand, mice surviving the ex vivo Stx2 challenge after immunization with S. Typhimurium strain LDV327 (one animal) or LDV329 (two animals) had normal serum urea and creatinine levels (Table 4).
TABLE 4.
Blood urea nitrogen and creatinine levels after challenge with Stx2 in mice immunized with the different S. Typhimurium vaccine strainsa
| Substance and time | Concn in serum (mg/dl) for mice immunized with: |
|||
|---|---|---|---|---|
| LDV326 | LDV327 | LDV328 | LDV329 | |
| Blood urea nitrogen | ||||
| 0 h | 33.3 ± 2.4 | 29.54 ± 3.45 | 35.19 ± 3.58 | 34.3 ± 4.62 |
| 24 h | 43.9 ± 7.4 | 34.49 ± 6.49 | 37.28 ± 6.2 | 45.06 ± 6.72 |
| 72 h | 204.71 ± 21.8 | 197.09 ± 67.80b | 199.09 ± 27.20 | 148.52 ± 58.96b |
| Creatinine | ||||
| 0 h | 0.25 ± 0.09 | 0.27 ± 0.01 | 0.32 ± 0.09 | 0.29 ± 0.01 |
| 24 h | 0.24 ± 0.01 | 0.35 ± 0.01 | 0.43 ± 0.01 | 0.34 ± 0.01 |
| 72 h | 1.67 ± 0.13 | 1.34 ± 0.36b | 1.54 ± 0.04 | 1.18 ± 0.50b |
Blood urea nitrogen and creatinine levels in serum were analyzed in groups of mice (n = 8) before the Stx2 challenge (0 h) and 24 h and 72 h after the toxin challenge. Values are averages ± standard deviations for individually tested sera in each immunization group.
The blood urea nitrogen concentration in the sera of mice immunized with strain LDV329 that survived the Stx2 challenge (n = 2) was 36.05 ± 1.07 mg/dl, while that for the surviving mouse immunized with strain LDV327 (n = 1) was 39.13. The creatinine concentrations in the same serum samples were 0.37 ± 0.015 mg/dl (for animals immunized with strain LDV329) and 0.47 mg/dl (for the animal immunized with strain LDV327).
DISCUSSION
Attenuated S. Typhimurium aroA vaccine strains have been intensively investigated as safe and effective mucosally delivered bivalent immunization vectors leading to activation of both humoral (mucosal and systemic) and cellular immune responses against self and passenger antigens following oral administration and transient colonization of the intestinal mucosa (15, 48). In this study, we generated two S. Typhimurium aroA strains, genetically modified to express a nontoxic Stx2 derivative (Stx2ΔAB), as orally delivered vectors for the control of HUS associated with Stx2-producing EHEC strains. Our experiments are the first to successfully report that a genetically modified Stx2 toxoid can be expressed and actively secreted by S. Typhimurium strains under conditions leading to specific immunologic responses among orally vaccinated mice. More relevantly, the S. Typhimurium vaccine strains induced systemic and mucosal Stx2-specific antibody responses with toxin neutralization activity following oral administration to mice. The present evidence demonstrated that the bivalent Salmonella-EHEC vaccine strains are potential tools for the generation of preventive or therapeutic strategies against Stx-associated sequelae, but further improvements in the generation of toxin-neutralizing antibody titers are required.
Bacterial flagellins show strong adjuvant effects when delivered to mammalian hosts, via mucosal or parenteral routes, either in the form of purified protein admixed with target antigens or as hybrid proteins genetically fused to the antigen (4, 8, 22, 23, 36). Our results demonstrate that derivatives of S. Typhimurium strain SL3261, proficient in the expression of both FliCI and FljB, and strain LDV321, in which the expression of both flagellar phases is specifically blocked (31), did not differ significantly in their immunogenicity as vaccine vectors for passenger antigens regarding activation of either Stx2-specific systemic or mucosal antibody responses in orally vaccinated mice. Since the adjuvant effects of bacterial flagellins require the depolymerization of flagella into monomers (46), we can assume that during transit through the gastrointestinal tract, the number of released flagellin monomers is not sufficiently high to promote a strong inflammatory response and, consequently, to exert adjuvant effects for a target heterologous antigen carried by the attenuated Salmonella strain. Indeed, our own observations and results reported by other groups indicate that oral administration of flagellated Salmonella vaccine strains, in contrast to parenteral delivery of bacteria, did not activate antibody responses to flagellin and genetically fused heterologous antigen (31, 40, 43). Such immunological behavior is coherent with the evolution of an immunologically suppressive environment in the mouse gastrointestinal tract that, under healthy conditions, did not allow the activation of strong inflammatory responses by flagellin, a highly conserved protein found among several enterobacterial species (32, 40, 56, 57).
Stx2ΔAB expression resulted in increased colonization of the mouse gastrointestinal tract by S. Typhimurium strains LDV327 and LDV329, as determined by the number of bacteria recovered from Peyer's patches of mice orally inoculated with one of the tested vaccine strains. Stx2 expression has been shown to enhance gut colonization by EHEC O157:H7 strains due to the increased expression by mouse enterocytes of nucleolin, which, together with the bacterially derived Tir protein, acts as a surface-exposed receptor for intimin-mediated adhesion of EHEC as well as of other pathogenic intimin-expressing enteric bacteria (34, 39). The observation that Stx2ΔAB expression suffices to enhance gut colonization and/or invasion by S. Typhimurium strains suggests that the presence of an active A1 subunit is not required for the upregulation of nucleolin or of other, undetermined host cell proteins. Since S. Typhimurium strains, in contrast to EHEC and other enteropathogenic E. coli strains, do not express intimin, the enhanced gut colonization by Stx2ΔAB-expressing S. Typhimurium vaccine strains was an unexpected finding and raises interesting questions regarding the role of Stx2 in bacterial adhesion to mouse epithelial cells. Certainly, further studies addressing this interesting observation are warranted.
Oral administration of the recombinant S. Typhimurium strains LDV327 and LDV329 resulted in systemic and secreted anti-Stx2 antibody responses in all vaccinated mice. Mice immunized with S. Typhimurium strain LDV327 showed lower, but not statistically different, serum anti-Stx2 antibody responses than mice immunized with the S. Typhimurium vaccine strain LDV329. In both groups, the anti-Stx2B titers detected did not rise above 2,000, which may reflect the low intrinsic immunogenicity of Stx2, particularly the B subunit, in different mammalian hosts (6, 11, 29, 30). Our previous attempts to use the same target antigen encoded by a DNA vaccine also resulted in rather low specific serum anti-Stx2 responses in vaccinated mice (5). Based on the available evidence, the concomitant expression of the A2 and B subunits (as represented in Stx2ΔAB), although A1 subunit-dependent toxicity is lacking, does not enhance the immunogenicity of Stx2 in mammalian hosts. Stx2-derived toxoids with reduced toxicity but preserved immunogenicity in mice have been generated by in vitro mutagenesis (47). The expression of Stx2 toxoids containing a nontoxic A1 subunit derivative by Salmonella vaccine strains may thus represent an alternative way to enhance both the immunogenicity and the toxin-neutralizing activities of anti-Stx2 antibodies induced in vaccinated animals.
Anti-Stx2 antibodies induced in mice immunized with S. Typhimurium strains LDV327 and LDV329 inhibited Stx2 toxicity toward Vero cells. Nonetheless, sera harvested from mice immunized with S. Typhimurium strain LDV329 conferred only partial (25%) protection on mice challenged with Stx2 previously treated with the lowest tested dilution of the immune sera. In addition, no protection was recorded for mice inoculated with native Stx2 incubated with sera from mice immunized with S. Typhimurium strain LDV327. The differences observed under in vitro and ex vivo conditions may be explained by the higher sensitivity of Vero cells to the cytotoxic effects of Stx2 and, therefore, higher sensitivity for the detection of toxin-neutralizing antibodies. In contrast, the lethal challenge assay requires higher toxin loads and consequently needs Stx2-neutralizing antibody concentrations not achieved in the sera of mice immunized with strain LDV327 or LDV329. The same interpretation applies to the partial protection observed in i.v. challenged mice following immunization with the S. Typhimurium vaccine strains. Further studies should also evaluate putative differences in the quality of the induced Stx2-specific antibody responses regarding antigen avidity and capacity to form immune complexes. It should also be emphasized that the i.v. challenge with Stx2 that was used does not reproduce the actual conditions observed during bacterial infection, in which secreted anti-Stx2 IgA antibodies may reduce the amount of toxin reaching the circulation.
Comparison of anti-Stx2B IgG titers and Stx2 neutralization titers shows that only a small fraction of the antibodies elicited in mice vaccinated with the S. Typhimurium vaccine strains effectively bind and inactivate the native toxin. Such a functional feature may reflect not only the low immunogenicity of Stx2-derived toxoids but also the absence of epitopes of the A1 subunit that play a relevant role in the generation of antibodies required for inactivation of the native toxin. Collectively, these results indicate that further attempts to improve the performance of attenuated S. Typhimurium strains as bivalent vaccine vectors for the control of Stx2-associated HUS should include the expression of the detoxified A1 subunit as a target antigen. In conclusion, the present results demonstrated that the bivalent Salmonella-EHEC vaccine formulations are potential tools for the generation of preventive or therapeutic strategies for EHEC infection-associated sequelae but that further improvements in the generation of toxin-neutralizing antibodies among vaccinated mice are still required.
Acknowledgments
This work was supported by Capes/SECyT and FAPESP grants.
We gratefully acknowledge the invaluable technical assistance of L. C. da Silva and J. P. Afonso.
Footnotes
Published ahead of print on 10 February 2010.
REFERENCES
- 1.Acheson, D. W., M. M. Levine, J. B. Kaper, and G. T. Keusch. 1996. Protective immunity to Shiga-like toxin I following oral immunization with Shiga-like toxin I B subunit-producing Vibrio cholerae CVD 103-HgR. Infect. Immun. 64:355-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akira, S., S. Uematsu, and O. Takeuchi. 2006. Pathogen recognition and innate immunity. Cell 124:783-801. [DOI] [PubMed] [Google Scholar]
- 3.Babiuk, S., D. J. Asper, D. Rogan, G. K. Mutwiri, and A. A. Potter. 2008. Subcutaneous and intranasal immunization with type III secreted proteins can prevent colonization and shedding of Escherichia coli in mice. Microb. Pathog. 45:7-11. [DOI] [PubMed] [Google Scholar]
- 4.Bargieri, D. Y., D. S. Rosa, C. J. M. Braga, B. O. Carvalho, F. T. Costa, N. M. Spíndola, A. J. Vaz, I. S. Soares, L. C. S. Ferreira, and M. M. Rodrigues. 2008. New malaria vaccine candidates based on the Plasmodium vivax Merozoite Surface Protein-1 and the TLR-5 agonist Salmonella Typhimurium FliC flagellin. Vaccine 26:6132-6142. [DOI] [PubMed] [Google Scholar]
- 5.Bentancor, L. V., M. Bilen, R. J. Fernández Brando, M. V. Ramos, L. C. S. Ferreira, P. D. Ghiringhelli, and M. S. Palermo. 2009. DNA vaccine encoding the enterohemorrhagic Escherichia coli (EHEC) Shiga-like toxin 2 (Stx2) A2 and B subunits confers protective immunity to Stx challenge in the murine model. Clin. Vaccine Immunol. 16:712-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bielaszewska, M., I. Clarke, M. A. Karmali, and M. Petric. 1997. Localization of intravenously administered verocytotoxins (Shiga-like toxins) 1 and 2 in rabbits immunized with homologous and heterologous toxoids and toxin subunits. Infect. Immun. 65:2509-2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bitzan, M. 2009. Treatment options for HUS secondary to Escherichia coli O157:H7. Kidney Int. Suppl. 112:S62-S66. [DOI] [PubMed] [Google Scholar]
- 8.Braga, C. J. M., G. M. G. Rittner, J. E. M. Henao, A. F. Teixeira, L. M. Massis, M. E. Sbrogio-Almeida, C. P. Taborda, L. R. Travassos, and L. C. S. Ferreira. 2009. Paracoccidioides brasiliensis vaccine formulations based on the gp43-derived P10 sequence and the Salmonella enterica FliC flagellin. Infect. Immun. 77:1700-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brando, R. J. F., E. Miliwebsky, L. V. Bentancor, N. Deza, A. Baschkier, M. V. Ramos, G. C. Fernández, R. Meiss, M. Rivas, and M. S. Palermo. 2008. Renal damage and death in weaned mice after oral infection with Shiga toxin 2-producing Escherichia coli strains. Clin. Exp. Immunol. 153:297-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butterton, J. R., E. T. Ryan, D. W. Acheson, and S. B. Calderwood. 1997. Coexpression of the B subunit of Shiga toxin 1 and EaeA from enterohemorrhagic Escherichia coli in Vibrio cholerae. Infect. Immun. 65:2127-2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Byun, Y., M. Omura, K. Fujihashi, S. Yamamoto, J. R. McGhee, S. Udaka, H. Kiyono, Y. Takeda, T. Kosaka, and Y. Yuki. 2001. Nasal immunization with E. coli verotoxin 1 (VT1)-B subunit and a nontoxic mutant of cholera toxin elicits serum neutralizing antibodies. Vaccine 19:2061-2070. [DOI] [PubMed] [Google Scholar]
- 12.Capozzo, A. V. E., V. P. Creydt, G. Dran, G. Fernández, S. Gómez, L. V. Bentancor, C. Rubel, C. Ibarra, M. Isturiz, and M. S. Palermo. 2003. Development of DNA vaccines against hemolytic-uremic syndrome in a murine model. Infect. Immun. 71:3971-3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caprioli, A., S. Morabito, H. Brugère, and E. Oswald. 2005. Enterohaemorrhagic Escherichia coli: emerging issues on virulence and modes of transmission. Vet. Res. 36:289-311. [DOI] [PubMed] [Google Scholar]
- 14.Friedrich, A. W., M. Bielaszewska, W. L. Zhang, M. Pulz, T. Kuczius, A. Ammon, and H. Karch. 2002. Escherichia coli harboring Shiga toxin 2 gene variants: frequency and association with clinical symptoms. J. Infect. Dis. 185:74-84. [DOI] [PubMed] [Google Scholar]
- 15.Galen, J. E., M. F. Pasetti, S. Tennant, P. Ruiz-Olvera, M. B. Sztein, and M. M. Levine. 2009. Salmonella enterica serovar Typhi live vector vaccines finally come of age. Immunol. Cell Biol. 87:400-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garmory, H. S., S. E. Leary, K. F. Griffin, E. D. Williamson, K. A. Brown, and R. W. Titball. 2003. The use of live attenuated bacteria as a delivery system for heterologous antigens. J. Drug Target. 11:471-479. [DOI] [PubMed] [Google Scholar]
- 17.Gomes, P. A. D. P., L. V. Bentancor, J. D. Paccez, M. E. Sbrogio-Almeida, M. S. Palermo, R. C. C. Ferreira, and L. C. S. Ferreira. 2009. Antibody responses elicited in mice immunized with Bacillus subtilis vaccine strains expressing Stx2B subunit of enterohaemorrhagic Escherichia coli O157:H7. Braz. J. Microbiol. 40:333-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Griffin, P. M., and R. V. Tauxe. 1991. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol. Rev. 13:60-98. [DOI] [PubMed] [Google Scholar]
- 19.Harari, I., and R. Arnon. 1990. Carboxy-terminal peptides from the B subunit of Shiga toxin induce a local and parenteral protective effect. Mol. Immunol. 27:613-621. [DOI] [PubMed] [Google Scholar]
- 20.Harari, I., A. Donohue-Rolfe, G. Keusch, and R. Arnon. 1988. Synthetic peptides of Shiga toxin B subunit induce antibodies which neutralize its biological activity. Infect. Immun. 56:1618-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoiseth, S. K., and B. A. Stocker. 1981. Aromatic-dependent Salmonella Typhimurium are non-virulent and effective as live vaccines. Nature 291:238-239. [DOI] [PubMed] [Google Scholar]
- 22.Honko, A. N., N. Sriranganathan, C. J. Lees, and S. B. Mizel. 2006. Flagellin is an effective adjuvant for immunization against lethal respiratory challenge with Yersinia pestis. Infect. Immun. 74:1113-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huleatt, J. W., A. R. Jacobs, J. Tang, P. Desai, E. B. Kopp, Y. Huang, L. Song, V. Nakaar, and T. J. Powell. 2007. Vaccination with recombinant fusion proteins incorporating Toll-like receptor ligands induces rapid cellular and humoral immunity. Vaccine 25:763-765. [DOI] [PubMed] [Google Scholar]
- 24.Imai, Y., R. Nagai, Y. Ono, T. Ishikawa, H. Nakagami, T. Tanikawa, and K. Kurohane. 2004. Production of secretory immunoglobulin A against Shiga toxin-binding subunits in mice by mucosal immunization. Infect. Immun. 72:889-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishikawa, S., K. Kawahara, Y. Kagami, Y. Isshiki, A. Kaneko, H. Matsui, N. Okada, and H. Danbara. 2003. Protection against Shiga toxin 1 challenge by immunization of mice with purified mutant Shiga toxin 1. Infect. Immun. 71:3235-3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Janeway, C. A., Jr., and R. Medzhitov. 2002. Innate immune recognition. Annu. Rev. Immunol. 20:197-216. [DOI] [PubMed] [Google Scholar]
- 27.Karmali, M. A. 2004. Infection by Shiga toxin-producing Escherichia coli: an overview. Mol. Biotechnol. 26:117-122. [DOI] [PubMed] [Google Scholar]
- 28.Konadu, E., A. Donohue-Rolfe, S. B. Calderwood, V. Pozsgay, J. Shiloach, J. B. Robbins, and S. C. Szu. 1999. Syntheses and immunologic properties of Escherichia coli O157 O-specific polysaccharide and Shiga toxin 1 B subunit conjugates in mice. Infect. Immun. 67:6191-6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marcato, P., G. Mulvey, R. J. Read, K. Vander Helm, P. N. Nation, and G. D. Armstrong. 2001. Immunoprophylactic potential of cloned Shiga toxin 2 B subunit. J. Infect. Dis. 183:435-443. [DOI] [PubMed] [Google Scholar]
- 30.Marcato, P., T. P. Griener, G. L. Mulvey, and G. D. Armstrong. 2005. Recombinant Shiga toxin B-subunit-keyhole limpet hemocyanin conjugate vaccine protects mice from shigatoxemia. Infect. Immun. 73:6523-6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Massis, L. M., C. J. Braga, M. E. Sbrogio-Almeida, C. Lauand, S. M. Newton, P. E. Klebba, and L. C. S. Ferreira. 2008. Anti-flagellin antibody responses elicited in mice orally immunized with attenuated Salmonella enterica serovar Typhimurium vaccine strains. Mem. Inst. Oswaldo Cruz 103:606-610. [DOI] [PubMed] [Google Scholar]
- 32.Mizel, S. B., and J. A. Snipes. 2002. Gram-negative flagellin-induced self-tolerance is associated with a block in interleukin-1 receptor-associated kinase release from toll-like receptor 5. J. Biol. Chem. 277:22414-22420. [DOI] [PubMed] [Google Scholar]
- 33.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nisole, S., B. Krust, and A. G. Hovanessian. 2002. Anchorage of HIV on permissive cells leads to coaggregation of viral particles with surface nucleolin at membrane raft microdomains. Exp. Cell Res. 276:155-173. [DOI] [PubMed] [Google Scholar]
- 35.Oxer, M. D., C. M. Bentley, J. G. Doyle, T. C. Peakman, I. G. Charles, and A. J. Makoff. 1991. High levels of heterologous expression in E. coli using the anaerobically-activated nirB promoter. Nucleic Acids Res. 19:2889-2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pino, O., M. Martin, and S. M. Michalek. 2005. Cellular mechanisms of the adjuvant activity of the flagellin component FljB of Salmonella enterica serovar Typhimurium to potentiate mucosal and systemic responses. Infect. Immun. 73:6763-6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Potter, A. A., S. Klashinsky, Y. Li, E. Frey, H. Townsend, D. Rogan, G. Erickson, S. Hinkley, T. Klopfenstein, R. A. Moxley, D. R. Smith, and B. B. Finlay. 2004. Decreased shedding of Escherichia coli O157:H7 by cattle following vaccination with type III secreted proteins. Vaccine 22:362-369. [DOI] [PubMed] [Google Scholar]
- 38.Rivas, M., E. Miliwebsky, I. Chinen, C. D. Roldán, L. Balbi, B. Garcia, G. Fiorilli, S. Sosa-Estani, J. Kincaid, J. Rangel, and P. M. Griffin. 2006. Characterization and epidemiologic subtyping of Shiga-toxin-producing Escherichia coli strains isolated from hemolytic uremic syndrome and diarrhea cases in Argentina. Foodborne Pathog. Dis. 3:88-96. [DOI] [PubMed] [Google Scholar]
- 39.Robinson, C. M., J. F. Sinclair, M. J. Smith, and A. D. O'Brien. 2006. Shiga toxin of enterohemorrhagic Escherichia coli type O157:H7 promotes intestinal colonization. Proc. Natl. Acad. Sci. U. S. A. 103:9667-9672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanders, C. J., Y. Yu, D. A. Moore III, I. R. Williams, and A. T. Gewirtz. 2006. Humoral immune response to flagellin requires T cells and activation of innate immunity. J. Immunol. 177:2810-2818. [DOI] [PubMed] [Google Scholar]
- 41.Sauter, K. A. D., A. R. Melton-Celsa, K. Larkin, M. L. Troxell, A. D. O'Brien, and B. E. Magun. 2008. Mouse model of hemolytic-uremic syndrome caused by endotoxin-free Shiga toxin 2 (Stx2) and protection from lethal outcome by anti-Stx2 antibody. Infect. Immun. 76:4469-4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sbrogio-Almeida, M. E., and L. C. S. Ferreira. 2001. Flagellin expressed by live Salmonella vaccine strains induces distinct antibody responses following delivery via systemic or mucosal immunization routes. FEMS Immunol. Med. Microbiol. 30:203-208. [DOI] [PubMed] [Google Scholar]
- 43.Sbrogio-Almeida, M. E., T. Mosca, L. M. Massis, I. A. Abrahamsohn, and L. C. S. Ferreira. 2004. Host and bacterial factors affecting induction of immune responses to flagellin expressed by attenuated Salmonella vaccine strains. Infect. Immun. 72:2546-2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sheoran, A. S., S. Chapman-Bonofiglio, B. R. Harvey, J. Mukherjee, G. Georgiou, A. Donohue-Rolfe, and S. Tzipori. 2005. Human antibody against Shiga toxin 2 administered to piglets after the onset of diarrhea due to Escherichia coli O157:H7 prevents fatal systemic complications. Infect. Immun. 73:4607-4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Siegler, R., and R. Oakes. 2005. Hemolytic uremic syndrome: pathogenesis, treatment, and outcome. Curr. Opin. Pediatr. 17:200-204. [DOI] [PubMed] [Google Scholar]
- 46.Smith, K. D., E. Andersen-Nissen, F. Hayashi, K. Strobe, M. A. Bergman, S. L. R. Barrett, B. T. Cookson, and A. Aderem. 2003. Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nat. Immunol. 4:1247-1253. [DOI] [PubMed] [Google Scholar]
- 47.Smith, J. J., L. D. Teel, H. M. Carvalho, A. R. Melton-Celsa, and A. D. O'Brien. 2006. Development of a hybrid Shiga holotoxoid vaccine to elicit heterologous protection against Shiga toxins types 1 and 2. Vaccine 24:4122-4129. [DOI] [PubMed] [Google Scholar]
- 48.Stocker, B. A. 2000. Aromatic-dependent Salmonella as anti-bacterial vaccines and as presenters of heterologous antigens or of DNA encoding them. J. Biotechnol. 83:45-50. [DOI] [PubMed] [Google Scholar]
- 49.Su, G. F., H. N. Brahmbhatt, J. Wehland, M. Rohde, and K. N. Timmis. 1992. Construction of stable LamB-Shiga toxin B subunit hybrids: analysis of expression in Salmonella typhimurium aroA strains and stimulation of B-subunit-specific mucosal and serum antibody responses. Infect. Immun. 60:3345-3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tarr, P. I., C. A. Gordon, and W. L. Chandler. 2005. Shiga toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet 365:1073-1086. [DOI] [PubMed] [Google Scholar]
- 51.Tesh, V. L., and A. D. O'Brien. 1991. The pathogenic mechanisms of Shiga toxin and the Shiga-like toxins. Mol. Microbiol. 5:1817-1822. [DOI] [PubMed] [Google Scholar]
- 52.Trachtman, H., A. Cnaan, E. Christen, K. Gibbs, S. Zhao, D. W. Acheson, R. Weiss, F. J. Kaskel, A. Spitzer, and G. H. Hirschman. 2003. Effect of an oral Shiga toxin-binding agent on diarrhea-associated hemolytic uremic syndrome in children: a randomized controlled trial. JAMA 290:1337-1344. [DOI] [PubMed] [Google Scholar]
- 53.Tsuji, T., T. Shimizu, K. Sasaki, H. Shimizu, K. Tsukamoto, H. Arimitsu, S. Ochi, S. Sugiyama, K. Tanaguchi, P. Neri, and H. Mori. 2008. Protection of mice from Shiga toxin-2 toxemia by mucosal vaccine of Shiga toxin 2B-His with Escherichia coli enterotoxin. Vaccine 26:469-476. [DOI] [PubMed] [Google Scholar]
- 54.Vilte, D. A., M. Larzábal, A. A. Cataldi, and E. C. Mercado. 2008. Bovine colostrum contains immunoglobulin G antibodies against intimin, EspA, and EspB and inhibits hemolytic activity mediated by the type three secretion system of attaching and effacing Escherichia coli. Clin. Vaccine Immunol. 15:1208-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wen, S. X., L. D. Teel, N. A. Judge, and A. D. O'Brien. 2006. Genetic toxoids of Shiga toxin types 1 and 2 protect mice against homologous but not heterologous toxin challenge. Vaccine 24:1142-1148. [DOI] [PubMed] [Google Scholar]
- 56.Winter, S. E., P. Thiennimitr, S. P. Nuccio, T. Haneda, M. G. Winter, R. P. Wilson, J. M. Russell, T. Henry, Q. T. Tran, S. D. Lawhon, G. Gomez, C. L. Bevins, H. Rüssmann, D. M. Monack, L. G. Adams, and A. J. Bäumler. 2009. Contribution of flagellin pattern recognition to intestinal inflammation during Salmonella enterica serotype Typhimurium infection. Infect. Immun. 77:1904-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yoshioka, A., R. Okamoto, S. Oshima, J. Akiyama, K. Tsuchiya, T. Nakamura, T. Kanai, and M. Watanabe. 2008. Flagellin stimulation suppresses IL-7 secretion of intestinal epithelial cells. Cytokine 44:57-64. [DOI] [PubMed] [Google Scholar]
- 58.Zhu, C., J. Yu, Z. Yang, K. Davis, H. Rios, I. B. Wang, G. Glenn, and E. C. Boedeker. 2008. Protection against Shiga toxin-producing Escherichia coli infection by transcutaneous immunization with Shiga toxin subunit B. Clin. Vaccine Immunol. 15:359-366. [DOI] [PMC free article] [PubMed] [Google Scholar]