Abstract
Viruses are among the most common causes of acute gastroenteritis. In recent years, new viruses causing outbreaks of acute gastroenteritis have been described. Among these, Aichi virus was identified in Japan in 1989. Aichi virus belongs to the Kobuvirus genus in the family Picornaviridae. This virus has been detected in outbreaks of gastroenteritis associated with oyster consumption and in pediatric stool samples, but little is known about its epidemiology or pathogenesis. In the present study, the prevalence of antibodies to Aichi virus in a Spanish population was determined between 2007 and 2008 by using an enzyme-linked immunosorbent assay (ELISA). As in previous studies, a high seroprevalence of antibodies to Aichi virus (70%) was observed, with levels differing according to age. We observed significant differences in titers of antibody to Aichi virus among different age groups, grouped by decades. We report high ELISA and neutralizing antibody titers, and both titers fitted a sigmoid curve significantly. However, this virus is seldom detected; therefore, further studies are needed to gain a better understanding of its importance as a pathogenic agent.
Viruses are a common cause of gastroenteritis and affect humans of all ages. Rotavirus (mainly group A), calicivirus (including norovirus and sapovirus), adenovirus, and astrovirus are considered the major causes of viral gastroenteritis. However, in many cases of gastroenteritis, no specific pathogen can be identified, and other viruses, such as Aichi virus, may be involved. This virus was proposed as a probable cause of nonbacterial gastroenteritis associated with oyster consumption in Aichi, Japan, in 1989 (16). Aichi virus belongs to the genus Kobuvirus, in the family Picornaviridae (9, 19). The major differences between the genus Kobuvirus and other genera of the same family are found in the coding region of the L protein, in the absence of a VP0 cleavage site, and in the distinct morphology of the 2A protein (14).
The Aichi virus genome is a single-stranded, positive-sense RNA molecule of 8,260 nucleotides and has a poly(A) tail. The single large open reading frame encodes a polyprotein of 2,432 amino acids. This polyprotein, like those of the other members of the family, is cleaved into the structural proteins VP0, VP3, and VP1 and the nonstructural proteins 2A, 2B, 2C, 3A, 3B, 3C, and 3D (11, 19). Aichi virus has been classified into two genotypes (A and B) by phylogenetic analysis of a 519-bp sequence at the 3C-3D (3CD) junction (20), and recently (in 2008), a new genotype, C, was proposed by Ambert-Balay et al. (1). These authors have also genotyped Aichi virus based on phylogenetic analysis of a 699-bp sequence of the gene encoding the VP1 protein. The results of this analysis correlate well with the 3CD sequence classification and also give rise to A, B, and C genotypes.
Little is known about the incidence of Aichi virus infection in humans. Aichi virus antigen or viral RNA was first detected in fecal samples collected in Japan (17). The virus was later isolated from patients with gastroenteritis, comprising Pakistani children and Japanese travelers from Southeast Asia (18), and among patients from Japan, Bangladesh, Thailand, and Vietnam (8). In 2006, the virus was isolated for the first time in the Americas (Brazil) and Europe (Germany) (7), and since then, Aichi virus has been detected in France (6), Tunisia (12, 13), Hungary (10), and Finland (5).
The first study of Aichi virus seroprevalence was performed in Japan and revealed a high rate of antibodies to Aichi virus (17). Other studies in Germany (7) and in France (3) have given similar results.
The purpose of the present study was to determine the seroprevalence of antibodies to Aichi virus in Valencia, Spain, during the years 2007 to 2008.
MATERIALS AND METHODS
Serum samples.
A total of 364 serum samples from healthy individuals were randomly collected at the Hospital Clinico Universitario, Valencia, Spain, from 2007 to 2008. Samples were divided into 10 groups according to the ages of the individuals as follows: under the age of 2 years (6 sera), between the ages of 2 and 4 years (63 sera), between 5 and 9 years (49 sera), between 10 and 14 years (38 sera), between 15 and 19 years (62 sera), between 20 and 24 years (42 sera), between 25 and 29 years (25 sera), between 30 and 39 years (42 sera), between 40 and 49 years (21 sera), and over the age of 50 years (16 sera). Serum samples were stored at −20°C.
Virus.
Aichi virus strain A846/88, isolated by T. Yamashita (16), was kindly provided by Pierre Pothier (University Hospital of Dijon, Dijon, France). This strain was propagated in Vero cells, recovered from cell lysates, and clarified by centrifugation, and the supernatant was divided into aliquots, which were stored at −80°C. The stock virus was titrated by immunofluorescence on Vero cells.
Antigen purification.
Viral antigen was partially purified from Aichi virus-infected cells by ultracentrifugation. The Aichi virus was propagated on Vero cells. When the cytopathic effect was 80 to 90%, the cell cultures were frozen and thawed three times and were then clarified by low-speed centrifugation (15,450 × g for 25 min). The supernatants were concentrated by ultracentrifugation at 50,000 rpm for 2 h at 4°C, using a Beckman 70 Ti rotor. A 300-μl aliquot of TNC (0.05 M Tris-HCl, 0.15 M NaCl, 0.01 M CaCl2) was added to the resulting pellets, which were then resuspended. The protein concentration was determined by the Bradford method (Bio-Rad), and the viral antigen preparation was stored at −80°C.
Detection of Aichi virus-specific antibodies by ELISA.
The presence and levels of antibodies against Aichi virus were determined by enzyme-linked immunosorbent assays (ELISA). Ninety-six-well polystyrene microtiter plates (Costar) were coated with 100 μl/well of partially purified antigens of Aichi virus (prepared as described above) diluted in carbonate/bicarbonate buffer (pH 9.0) and were incubated for 2 h at 37°C. Wells were washed three times with 0.5% Tween 20 in phosphate-buffered saline (PBS-T), and 100 μl of serially diluted serum samples in PBS containing 1% bovine serum albumin (PBS-BSA) was added. The plates were incubated for 2 h at 37°C, washed three times, and incubated for 2 h at 37°C with 100 μl/well of horseradish peroxidase (HRP)-conjugated anti-human IgG antibody (Sigma) diluted 1/2,000 in PBS-BSA. After the plates were washed, color was developed by the addition of 50 μl of o-phenylenediamine (OPD), and the reaction was stopped with 3 M H2SO4. The absorbance was read at 492 nm (SpectraMax Plus384 spectrophotometer; Molecular Devices). Since the semipurified Aichi virus antigen preparation could contain cellular debris, serum samples were tested simultaneously against the semipurified Aichi virus antigen and against the antigens of uninfected Vero cells, extracted by the same procedure. The absorbance of each serum sample against the cellular antigen was subtracted from the absorbance obtained against the viral antigen.
Calculation of percentages of positivity.
The absorbance data of different plates were standardized by calculating the percentage of positivity (PP) for each sample, as described previously (2). For this purpose, all assays included the same positive control (a human serum sample with high anti-Aichi virus antibody levels) and the same negative controls (three human sera with absorbance values three times lower than that of the PBS-T well) obtained in a previous study. The PP of each sample corresponds to the ratio of the Aichi virus-specific antibodies in that sample to the Aichi virus-specific antibodies in the positive control. Thus, 0% positivity represents no binding of antibody to the antigen, and 100% positivity is equivalent to the binding of the antibody to the antigen in the positive control. The PP of each sample was calculated as 100 × (specific absorbance of the serum sample/specific absorbance of the positive control), where the specific absorbance of the serum sample is equal to (absorbance of serum sample − absorbance of negative controls) and the specific absorbance of the positive control is equal to (absorbance of positive control − absorbance of negative controls).
A negative cutoff value was determined by the following equation: cutoff value = (average PP of all negative controls) + [3 × (standard deviation of the PP of all negative controls)].
Any serum sample with a PP above this cutoff was considered to have a significant antibody response in this test and was therefore regarded as a positive sample.
Seroneutralization assay.
Twenty-three randomly selected serum samples were inactivated for 30 min at 56°C and were serially diluted 4-fold in cell culture medium (minimum essential medium [MEM]; Gibco). All dilutions were incubated in the presence of 50 to 100 immunoperoxidase-stained focus-forming units of Aichi virus for 1 h at 37°C. Then the mixtures were added in duplicate to Vero cell monolayers growing in 96-well plates (Costar), and the plates were incubated for 1 h at 37°C. Viral inocula were removed; the cells were incubated for another 8 h and were then fixed with methanol-acetone; and viral antigens were detected by an immunoperoxidase assay. Briefly, fixed cell monolayers were rinsed with PBS and were then incubated for 1 h at 37°C with a hyperimmune human serum (primary antibody) against Aichi virus diluted 1/500 in PBS-BSA. Following incubation, the cells were washed twice with PBS for 10 min each time and were then incubated for 1 h at 37°C with an HRP-conjugated antibody against human IgG (Sigma) diluted 1/2,000 in PBS-BSA. The cells were then washed three times with PBS, stained with diaminobenzidine (Fast DAB; Sigma), washed again with deionized water, and observed under an inverted light microscope. Neutralizing titers were considered the inverse of the sample dilution that showed a >60% reduction in the number of peroxidase-stained Aichi virus focus-forming units.
Statistical analysis.
The 95% confidence intervals for the seroprevalence levels detected by age groups were estimated by the binomial exact method. A preliminary test for normality showed a skewed distribution of PP values. Thus, results were analyzed by a nonparametric Kruskal-Wallis test. Multiple comparisons were performed by using a nonparametric unpaired Mann-Whitney U test, and a Bonferroni adjustment of the significance level was applied for multiple comparisons (P, 0.00139). Comparison of multiple proportions was also performed by the Marascuilo procedure. The analyses were performed by using SPSS software (version 14.0; LEAD Technologies Inc.). Analysis of variance to assess the relationship between ELISA titers and seroneutralization titers was performed using SigmaPlot software (version 8.0; Systat Software Inc.) with a significance level (P) of 0.05.
RESULTS
Aichi virus seroprevalence.
The seroprevalence of antibodies against Aichi virus was analyzed in a total of 364 human serum samples obtained in Valencia, Spain, by using an indirect IgG enzyme-linked immunosorbent assay. To normalize the results of different experiments, the absorbance values were transformed into PP data. Finally, a cutoff PP of 11% was obtained. Any sample with a PP equal to or greater than this cutoff was considered positive.
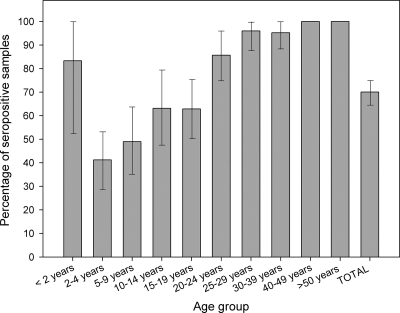
As a whole, 70% of serum samples were found positive for antibodies to Aichi virus in this study (Fig. 1). Among children aged 0 to 2 years, 5 out of 6 samples (83%) were found positive. Seroprevalence increased from 41% in the 2- to 4-year age group to 49% in the 5- to 9-year age group. In the 10- to 14-year and 15- to 19-year age groups, seroprevalence was about 63%; it rose to 86% in the 20- to 24-year age group. In 25- to 29-year-old and 30- to 39-year-old individuals, seroprevalence was around 95%, and it rose to 100% in individuals more than 40 years old.
FIG. 1.
Aichi virus seroprevalence. Percentages of sera positive for antibodies against Aichi virus by age group in Valencia, Spain, between 2007 and 2008 are shown. Error bars indicate 95% confidence intervals.
Age distribution of levels of antibody to Aichi virus.
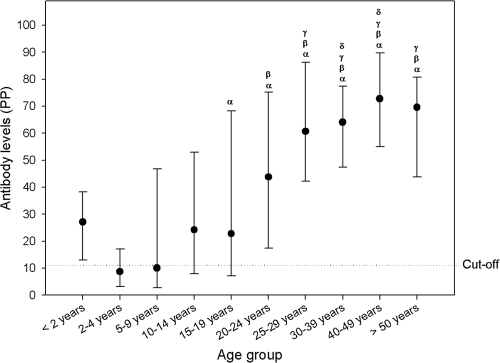
The PP was used to measure the levels of antibodies to Aichi virus by age. The age distribution of PPs was analyzed by nonparametric statistical methods. The antibody levels represented by the PP increased gradually and significantly with age (P, <0.001 by the Kruskal-Wallis test) (Fig. 2). This quantification correlated well with the distribution of seroprevalence by age. Comparisons between age groups were performed with the nonparametric Mann-Whitney U test with Bonferroni's adjustment for multiple comparisons (Fig. 2). The levels of antibody to Aichi virus were observed to increase every 10 years. The median PP of the 2- to 4-year-old group was significantly lower than those of older groups. The 5- to 9-year age group differed significantly from the 20- to 24-year age group and older groups. The 10- to 14-year age group also showed significant differences in the median PP from the 25- to 29-year age group and older groups. The 15- to 19-year age group differed from the 30- to 39-year and 40- to 49-year age groups, but not from the >50-year age group.
FIG. 2.
Distribution of levels of antibodies to Aichi virus by age. Levels of IgG antibody against Aichi virus were quantified by calculating the PP for each sample. In the graph, the dots represent median values, and the error bars represent the 25th-to-75th-percentile intervals. The dotted line marks the cutoff level. Greek letters represent significant differences (by the Mann-Whitney U test with Bonferroni's correction for multiple comparisons) from the 2- to 4-year-old group (α), from the 5- to 9-year-old group (β), from the 10- to 14-year-old group (γ), and from the 15- to 19-year-old group (δ). The 0- to 2-year age group was not included in the statistical analysis due to the low number of samples.
Relationship between ELISA and neutralization assay results.
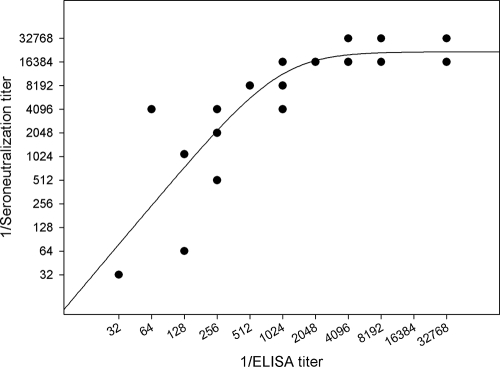
The relationship between the antibody titers determined by the neutralization assay and those determined by ELISA was evaluated. ELISA titers as high as 1/32,768 were achieved (Fig. 3). We performed a seroneutralization assay to ensure the specificity of the recognition of Aichi virus by the antibody. This assay was applied to 23 randomly selected sera. Several serum samples tested also showed high neutralizing antibody titers (up to 1/32,768) (Fig. 3).
FIG. 3.
Relationship between ELISA titers and seroneutralization titers. The ELISA titers and seroneutralization titers of 23 randomly selected serum samples were compared. The graphic representation of ELISA and neutralizing titers fits the sigmoid curve well. This relationship was assessed by analysis of variance (P < 0.0001; R2 = 0.7046). Only 18 dots are shown, because several samples yielded the same values for ELISA and neutralizing titers.
There is a relationship between ELISA and seroneutralization titers. Although the linear correlation was statistically significant according to the analysis of variance (P, 0.0151), both titers were better fitted to the sigmoid curve (R2, 0.25 and 0.70, respectively) (Fig. 3). The fit of the data to the sigmoid curve was assessed by analysis of variance (P, <0.0001). ELISA and neutralizing titers increased linearly until the ELISA titer reached 1/1,024 and until the neutralization titer reached 1/16,384. From these values, the neutralizing titers grew only to 1/32,768, and ELISA titers increased steadily to 1/32,768.
DISCUSSION
To our knowledge, this is the first study performed in Spain, and the third conducted in Europe, to analyze the seroprevalence of antibodies to Aichi virus (3, 7). A high prevalence of antibodies against Aichi virus was observed in the Spanish population under study (70%), results similar to those obtained by previous studies (76% in Germany and 55% in Japan) (7, 17). The seroprevalence in children between the ages of 2 and 4 years is 41%, and seroprevalence increases to reach values close to 100% by the age of 25. The group of samples from children under the age of 2 years was excluded, because their sera can retain antibodies to Aichi virus derived from their mothers, as previously reported (7). Despite the low number of samples analyzed, we found a high percentage of positive sera at this age (83% [5 of 6 samples tested]). Our data are the first to show a seroprevalence of 100% in individuals over the age of 40 years. This high percentage may reflect differences in the prevalence of antibodies to Aichi virus between countries. However, we cannot exclude the possibility that methodological discrepancies explain this variation. In the studies by Oh et al. (7) and Yamashita et al. (17), the seroprevalence of antibodies to Aichi virus was determined by a seroneutralization assay, while in the study by Goyer et al. (3) and in our study, seroprevalence was calculated by ELISA.
The seroprevalence of antibodies to Aichi virus differs with age, but this study also supports the hypothesis that the seroprevalence of antibodies to Aichi virus changes geographically, as previously proposed (3). In Japan (17) and in France (3), a plateau effect was clearly observed, at the age of 30 years in France (84% of positive sera) and at 35 years in Japan (83.3% of positive sera). This plateau effect is also apparent in the present study. However, in Germany, 86% positivity was achieved at the age of 15 years (7), and in Spain, 85% of sera were positive at the age of 20 years. In addition, seroprevalences in German and Spanish children are higher than those in Japanese and French children. These differences may reflect differences in the epidemiology of Aichi virus in these countries, with two possible patterns of antibody distribution, one in Japan and France and another in Spain and Germany.
The distribution of positive serum samples by age was not homogeneous. Previous studies (3, 7, 17) and our results suggest that younger individuals have a lower prevalence of antibodies and that this prevalence rises gradually with age. This increase may reflect reinfections, most of which may be asymptomatic (3). Aichi virus transmission seems to be associated with oyster consumption (6, 20). However, the virus has been isolated in pediatric stool samples (1, 7-8, 10, 12-13) and in an outbreak unconnected with oyster consumption (20), suggesting that Aichi virus may be transmitted by other means.
In this study, the conversion of absorbance values into PPs was used to quantify antibody levels by age. The results indicate that there is a good correlation between the distribution of positive sera and antibody levels by age, and this good correlation has enabled us to analyze the differences between age groups statistically. The levels of specific anti-Aichi virus antibodies increase gradually and significantly with age. According to our data, every 10 years, the levels of antibodies increase significantly up to the age of 30 years, with the absence of a significant increase after that age. These variations in antibody levels support the hypothesis that reinfections occur, thus increasing antibody levels with age.
To confirm the specificity of the antibodies against Aichi virus determined by ELISA, a neutralization assay was also performed. The observation that these antibodies do neutralize the infectivity of Aichi virus in vitro excludes the possibility that these antibodies were induced by other picornaviruses, such as polioviruses or enteroviruses. A similar conclusion was reached by Yamashita et al. (17).
We obtained high ELISA titers in several selected serum samples (up to 1/32,768). Our results also show the presence of neutralizing antibodies against Aichi virus in serum samples by neutralization assays in cell culture. In fact, the seroneutralization titers were much higher than those in previous studies (up to 1/32,768) (7, 17). This variation may be due to differences in the method applied, because we used an immunoperoxidase focus reduction assay, while other studies used the 50% tissue culture infective dose (TCID50) reduction assay. Yamashita et al. (17) detected seroconversion to Aichi virus in patients affected by outbreaks of gastroenteritis, which may reflect a primary infection, whereas we have titrated sera from patients who may have suffered repeated reinfections. There is a good correlation between ELISA and neutralization titers in the serum samples, and this correlation fits a sigmoid curve. This could mean that the ability of the sera to neutralize Aichi virus is limited, at least in cell culture. Besides, we cannot exclude the production of nonneutralizing antibodies during infection. We observed that ELISA titers higher than 1/1,024 did not increase the neutralization titers of the sera. This could mean that high titers of antibody do not necessarily indicate in vivo protection against Aichi virus.
The ELISA analysis specifically focuses on the IgG class of anti-Aichi virus antibodies, and other classes of antibodies can also be produced. Goyer et al. (3) also investigated IgM antibodies in sera positive for IgG, randomly chosen from samples collected at ages where the prevalence progressed quickly, but none of the samples gave a positive result, even at the 1/10 dilution. The high seroprevalence found contrasts with the low frequency of Aichi virus detection in sporadic cases and in outbreaks of gastroenteritis, since a low incidence of Aichi virus outbreaks has been reported. In Europe, Aichi virus has been detected only in 6 of 110 outbreaks in France (1) and in 1 of 65 children investigated in Hungary (10). Aichi virus was not found in 188 outbreaks of gastroenteritis in which norovirus was previously excluded in the Netherlands (15), and in Finland, 68 samples from patients with gastroenteritis proved negative for Aichi virus by reverse transcription-PCR (RT-PCR) (4). Even in Southeast Asia, where the virus was first identified, Aichi virus has seldom been diagnosed (8, 16, 18-19). According to these results, the pathogenesis of Aichi virus has been questioned (15, 19-20). Nevertheless, recent studies have identified Aichi virus as the only pathogen isolated in 3 outbreaks in Germany (7), in 6 of 13 patients in France (1), in 16 of 234 (12) and in 25 of 788 (13) Tunisian children, in 1 outbreak of gastroenteritis associated with oyster consumption (6), and in 1 case in a study of 65 stool samples in Hungary (10). Furthermore, Aichi virus has often been associated with other gastrointestinal viruses in diarrhea outbreaks. These results suggest that Aichi virus infection may be asymptomatic or may cause mild clinical symptoms (3). In Finland, a recent study investigating a total of 1,063 stool specimens reported the finding of Aichi virus infection in 5 children (0.5%) (5). Although the children suffered acute gastroenteritis, in 4 cases coinfection with other enteric viruses (rotavirus or norovirus) was found. However, most recent studies suggest that the virulence of this virus should be reconsidered. Reuter et al. (10) describe the case of a child who was shedding Aichi virus and suffered, in addition to diarrhea, respiratory symptoms and purulent conjunctivitis. Sdiri-Loulizi et al. (13) observed a significantly higher frequency of Aichi virus monoinfections among hospitalized children than among outpatients, and they suggest that this virus could be virulent enough to require hospitalization.
In this study, we have confirmed the high seroprevalence of Aichi virus in a Spanish population, similar to the seroprevalences in Japan and in other European countries, such as France and Germany. Seroprevalence increases with age from 2 to 25 years, an age at which the majority of the population has specific antibodies to Aichi virus. Moreover, neutralizing antibodies are produced. These results indicate that reinfections occur; however, Aichi virus is rarely found in outbreaks of gastroenteritis. Thus, further studies are needed to examine the epidemiology, pathology, and clinical significance of Aichi virus.
Acknowledgments
This work was supported by the “Enteric Viruses Emergence, New Tools” (EVENT) (SP22-CT-2004-502571) and DG SANCO (DIVINE-NET, 2003213) projects of the European Union.
We thank Pierre Pothier (University Hospital of Dijon, Dijon, France) for providing the A846/88 prototype strain of Aichi virus, originally isolated by T. Yamashita, and we thank Francisco Montes (Department of Statistics, Universitat de València) for supervising the statistical analyses. We thank Fabiola Barraclough for reviewing the English.
We declare no conflict of interest with regard to the conduct of the study and the writing of the manuscript.
Footnotes
Published ahead of print on 17 February 2010.
REFERENCES
- 1.Ambert-Balay, K., M. Lorrot, F. Bon, H. Giraudon, J. Kaplon, M. Wolfer, P. Lebon, D. Gendrel, and P. Pothier. 2008. Prevalence and genetic diversity of Aichi virus strains in stool samples from community and hospitalized patients. J. Clin. Microbiol. 46:1252-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colby, L. A., G. G. Schurig, and P. H. Elzer. 2002. An indirect ELISA to detect the serologic response of elk (Cervus elaphus nelsoni) inoculated with Brucella abortus strain RB51. J. Wildl. Dis. 38:752-759. [DOI] [PubMed] [Google Scholar]
- 3.Goyer, M., L. S. Aho, J. B. Bour, K. Ambert-Balay, and P. Pothier. 2008. Seroprevalence distribution of Aichi virus among a French population in 2006-2007. Arch. Virol. 153:1171-1174. [DOI] [PubMed] [Google Scholar]
- 4.Jokela, P., P. Joki-Korpela, M. Maaronen, V. Glumoff, and T. Hyypia. 2005. Detection of human picornaviruses by multiplex reverse transcription-PCR and liquid hybridization. J. Clin. Microbiol. 43:1239-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaikkonen, S., S. Räsänen, M. Rämet, and T. Vesikari. 2009. Aichi virus infection in children with acute gastroenteritis in Finland. Epidemiol. Infect. doi: 10.1017/S0950268809991300. [DOI] [PubMed]
- 6.Le Guyader, F. S., J. C. Le Saux, K. Ambert-Balay, J. Krol, O. Serais, S. Parnaudeau, H. Giraudon, G. Delmas, M. Pommepuy, P. Pothier, and R. L. Atmar. 2008. Aichi virus, norovirus, astrovirus, enterovirus, and rotavirus involved in clinical cases from a French oyster-related gastroenteritis outbreak. J. Clin. Microbiol. 46:4011-4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oh, D. Y., P. A. Silva, B. Hauroeder, S. Diedrich, D. D. Cardoso, and E. Schreier. 2006. Molecular characterization of the first Aichi viruses isolated in Europe and in South America. Arch. Virol. 151:1199-1206. [DOI] [PubMed] [Google Scholar]
- 8.Pham, N. T., P. Khamrin, T. A. Nguyen, D. S. Kanti, T. G. Phan, S. Okitsu, and H. Ushijima. 2007. Isolation and molecular characterization of Aichi viruses from fecal specimens collected in Japan, Bangladesh, Thailand, and Vietnam. J. Clin. Microbiol. 45:2287-2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pringle, C. R. 1999. Virus taxonomy at the XIth International Congress of Virology, Sydney, Australia, 1999. Arch. Virol. 144:2065-2070. [DOI] [PubMed] [Google Scholar]
- 10.Reuter, G., A. Boldizsar, G. Papp, and P. Pankovics. 2009. Detection of Aichi virus shedding in a child with enteric and extraintestinal symptoms in Hungary. Arch. Virol. 154:1529-1532. [DOI] [PubMed] [Google Scholar]
- 11.Sasaki, J., Y. Kusuhara, Y. Maeno, N. Kobayashi, T. Yamashita, K. Sakae, N. Takeda, and K. Taniguchi. 2001. Construction of an infectious cDNA clone of Aichi virus (a new member of the family Picornaviridae) and mutational analysis of a stem-loop structure at the 5′ end of the genome. J. Virol. 75:8021-8030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sdiri-Loulizi, K., H. Gharbi-Khelifi, A. de Rougemont, S. Chouchane, N. Sakly, K. Ambert-Balay, M. Hassine, M. N. Guediche, M. Aouni, and P. Pothier. 2008. Acute infantile gastroenteritis associated with human enteric viruses in Tunisia. J. Clin. Microbiol. 46:1349-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sdiri-Loulizi, K., M. Hassine, H. Gharbi-Khelifi, N. Sakly, S. Chouchane, M. N. Guediche, P. Pothier, M. Aouni, and K. Ambert-Balay. 2009. Detection and genomic characterization of Aichi viruses in stool samples from children in Monastir, Tunisia. J. Clin. Microbiol. 47:2275-2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stanway, G., T. Hovi, N. Knowles, and T. Hyypia. 2002. Molecular and biological basis of picornavirus taxonomy, p. 17-24. In B. L. Semler and E. Wimmer (ed.), Molecular biology of picornaviruses. ASM Press, Washington, DC.
- 15.Svraka, S., E. Duizer, H. Vennema, E. de Bruin, B. van der Veer, B. Dorresteijn, and M. Koopmans. 2007. Etiological role of viruses in outbreaks of acute gastroenteritis in The Netherlands from 1994 through 2005. J. Clin. Microbiol. 45:1389-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamashita, T., S. Kobayashi, K. Sakae, S. Nakata, S. Chiba, Y. Ishihara, and S. Isomura. 1991. Isolation of cytopathic small round viruses with BS-C-1 cells from patients with gastroenteritis. J. Infect. Dis. 164:954-957. [DOI] [PubMed] [Google Scholar]
- 17.Yamashita, T., K. Sakae, Y. Ishihara, S. Isomura, and E. Utagawa. 1993. Prevalence of newly isolated, cytopathic small round virus (Aichi strain) in Japan. J. Clin. Microbiol. 31:2938-2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamashita, T., K. Sakae, S. Kobayashi, Y. Ishihara, T. Miyake, A. Mubina, and S. Isomura. 1995. Isolation of cytopathic small round virus (Aichi virus) from Pakistani children and Japanese travelers from Southeast Asia. Microbiol. Immunol. 39:433-435. [DOI] [PubMed] [Google Scholar]
- 19.Yamashita, T., K. Sakae, H. Tsuzuki, Y. Suzuki, N. Ishikawa, N. Takeda, T. Miyamura, and S. Yamazaki. 1998. Complete nucleotide sequence and genetic organization of Aichi virus, a distinct member of the Picornaviridae associated with acute gastroenteritis in humans. J. Virol. 72:8408-8412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamashita, T., M. Sugiyama, H. Tsuzuki, K. Sakae, Y. Suzuki, and Y. Miyazaki. 2000. Application of a reverse transcription-PCR for identification and differentiation of Aichi virus, a new member of the Picornavirus family associated with gastroenteritis in humans. J. Clin. Microbiol. 38:2955-2961. [DOI] [PMC free article] [PubMed] [Google Scholar]