Abstract
The pathogenesis of sickle vaso-occlusive crisis (VOC) in sickle cell disease (SCD) patients involves the accumulation of rigid sickle cells and the stimulation of an ongoing inflammatory response, as well as the stress of infections. The immune response, via cytokine imbalances and deregulated T-cell subsets, also has been proposed to contribute to the development of VOC. In this study, a panel of high-sensitivity cytokine kits was used to investigate cytokines in the sera of SCD patients in VOC. The results were compared primarily with those for stable SCD patients and secondarily with those for normal healthy people who served as controls. The cytokines studied included interleukin-2 (IL-2), IL-4, and IL-10. Lymphocyte subsets of patients with VOC were also studied and were compared with those of both control groups (20 stable patients without crisis [SCD group] and 20 normal healthy controls [NHC]). The VOC group was notable for remarkably elevated levels of IL-4, among the three cytokines tested, compared with those for the SCD and NHC groups. Patients with VOC also differed from stable SCD patients and NHC by having notably lower IL-10 levels, as well as the lowest ratio of CD4+ to CD8+ T cells (0.7). The patterns of the proinflammatory cytokine IL-2 did not differ between VOC and stable SCD patients, but NHC had significantly lower IL-2 levels than both the VOC and SCD groups. Our results demonstrate coexisting levels, both high and low, of TH1- and TH2-type cytokines, as well as diminished levels of T-cell subsets in VOC. These results are discussed in an effort to better understand the importance of the immune system profile in the pathogenesis of sickle cell VOC. Since the possibility that a cytokine imbalance is implicated in the pathogenesis of sickle cell crisis has been raised, our results should prompt further investigation of the host immune response in terms of TH1 and TH2 balance in sickle cell crisis.
Sickle cell disease (SCD) is a chronic, incurable condition presenting primarily as anemia (sickle cell anemia [SCA]) in people homozygous for hemoglobin S (HbS). This abnormal hemoglobin, resulting from the replacement of glutamic acid at position 6 of the β-globin chain by valine, is responsible for erythrocyte distortion and fragility in these patients, as well as for thrombosis, fever, splenomegaly, joint pain, lethargy, and weakness. Sickle cell crises refer to the sudden attacks of pain, at various levels of severity, that occur during the lifetime of the patient with sickle cell disease (1, 3). Of these, the painful vaso-occlusive crisis (VOC) is the most common and is characterized by fever, leukocytosis, joint effusions, and tenderness, which occur in about 50% of patients at initial presentation (2), as well as by susceptibility to infection. It is a medical emergency and an acute crisis state. Patients in a state of well-being between these episodes are referred to as “steady-state” SCD patients.
The sequence of pathophysiological events that lead to the sickle cell VOC is not well understood. Several authors (8, 13, 27, 28) have outlined a sequence of steps occurring in the microcirculation that culminate in this painful sickle cell crisis. Polymerization of HbS, decreased blood red cell flexibility, microvascular occlusion, hypoxia of tissue involved with the occluded microvascular network, and tissue damage triggering painful stimuli have been mentioned (26), although the precise dynamics of these events and their interrelationships are poorly understood. Tissue ischemia due to vascular occlusion causing infarctive tissue damage, which in turn initiates secondary inflammatory responses, has also been mentioned (3, 4). Ischemic events produced by the occlusion of both large and small blood vessels are stressful and involve intricate interactions between red blood cells, the endothelium, and leukocytes (7). These interactions are known to be regulated by cytokines secreted by T cells as well as by adhesion molecules, and consequently, the immune response is implicated in the initiation and development of the sickle cell crisis. Indeed, studies now show that immune subsets are operative in sickle cell disease (9, 14, 16, 25), and the susceptibility of sickle cell disease patients in crisis to infections that specifically require the help of T cells to be cleared, such as Salmonella enterica serovar Typhimurium osteomyelitis (14), is suggestive.
CD4+ T cells, subdivided based on their associated cytokines, play a crucial role in inflammatory responses and the elimination of infection. TH1 cells provide immunity against intracellular pathogens by secreting the cytokines interleukin-2 (IL-2), IL-12, and gamma interferon (IFN-γ), whereas commitment to the TH2 lineage programs the clearance of extracellular pathogens and the secretion of cytokines such as IL-10, IL-4, and IL-13. This balance of TH1/TH2 cytokine responses is believed to play an important role in coordinating an effective immune response, even under inflammatory conditions, although very limited data exist on their roles in sickle cell VOC.
This study thus hypothesizes that the balance between TH1- and TH2-type cytokines might explain the differences in clinical outcomes in sickle cell disease. It was undertaken with patients with SCD in VOC in Zaria, Nigeria, a town in the zone of sickle cell endemicity of West Africa (17). The study analyzed numerical values for CD3+, CD4+, and CD8+ T cells and levels of selected serum cytokines in patients in VOC, and it compared these values with those obtained for steady-state SCD patients and unaffected hemoglobin AA homozygotes who served as normal healthy controls (NHC). This was done in an effort to understand if any imbalance in the immune response is important in the pathogenesis of sickle cell disease.
MATERIALS AND METHODS
Study site.
The study site was the Ahmadu Bello University Teaching Hospital (ABUTH), Zaria, Kaduna State, Nigeria. Since it is a tertiary health facility, although it provides substantial primary care to its immediate environs, patients requiring specialized emergency attention, such as those in sickle cell crisis, are usually referred here.
Subjects.
Following informed consent and approval by the Ahmadu Bello University Teaching Hospital Research and Ethics Board, peripheral blood was obtained from 27 subjects in sickle cell VOC, 20 steady-state SCD patients, and 20 normal healthy controls. There were no established country prevalence rates for patients in acute medical crisis states (sickle cell VOC), who were the focus of this study. They were taken into the study purposively and consecutively as soon as they presented to the hospital. Due to the expense involved in the scientific analysis of these patients' samples and the difficulty in sourcing these patients within a limited time frame, after due consultation with a statistician, the minimum sample size for patients was calculated using the statistical formula n = [Z2 × p × (1 − p)]/d2, where n is the sample size, p is the prevalence of the disease in the population (2% for sickle cell crisis), Z2 is equal to 1.96, and d is the tolerable sampling error (0.05).
This gave a minimum sample size of 28 sickle cell crisis patients at a 95% confidence level, a 5% sampling error, and a prevalence rate of 2.0% for sickle cell crisis.
Case definitions for patients in sickle cell crisis, as well as the appropriate controls, included the following clinical criteria. (i) Patients were considered to be in sickle cell VOC if they were known sickle cell anemic patients with bone or joint pain, or multiple sites of pain, necessitating hospital admission and analgesic administration (23). (ii) Steady-state sickle cell anemia patients taken as controls included those with steady hematocrit and hemoglobin values over 2 to 3 months and a state of well-being, without any symptoms or signs of HIV or other overt infection, pain, or any other acute episode suggestive of crisis, as established by a careful history and a complete physical examination (23). (iii) Normal healthy controls included 20 healthy age- and sex-matched individuals with no signs of sickle cell crisis or any other definition of ill health. All NHC were assessed medically by competent clinicians and had their HIV status, height, weight, blood pressure, and medical history properly documented. All individuals who met one of the criteria described above and were included in the study gave consent.
Exclusion criteria included (i) refusal of consent and (ii) concurrent HIV or overt infection.
Clinical assessment of patients.
For all candidates, clinical, infection, and pain information was assessed by the hematologists (A.I.M. and A.H.I.) using an established pro forma questionnaire for the study. Basically, for clinical infection diagnosis, this entailed taking a history for possible exposure to infectious disease and recording clinical signs and symptoms of fever, rashes, jaundice, or swelling, as well as organ-specific signs and symptoms, such as coughing, diarrhea, and dysuria. Assessment of pain included noting the site of pain (hip, chest, abdomen, lower or upper limbs), indicating its character (deep-seated, boring, or “toothache” like), estimating its duration (transient [5 to 10 min] or persistent [lasting 24 h to days]), and noting its severity (mild, moderate, or severe) based on the doses of analgesics needed or used.
Reagents.
The following materials were essential for the study: Ficoll-Hypaque, monoclonal antibodies against human T lymphocytes, enzyme linked immunosorbent assay (ELISA) kits for cytokine assays, and minimal essential medium (MEM). All were obtained commercially. Ficoll-Hypaque (Histopaque-1077; Sigma Diagnostics Co., St. Louis, MO) was a mixture of sodium metrizoate (9.6%, wt/vol) and Ficoll (5.6%, wt/vol) with a final density of 1.077 to 1.078 g/ml. It was obtained in 100-ml aliquots and was stored protected from light at 4 to 6°C. The monoclonal antibodies against human T lymphocytes used in this study included anti-human CD3, CD4, and CD8 antibodies (UCHT1, QS4120, and QS4122, respectively) conjugated with fluorescein isothiocyanate (FITC) at 0.5 mg/ml, with a fluorescein/IgG molar ratio of 10.0. They were obtained from Ancell Immunology Research Products and were stored in the dark at 2 to 5°C. Functionally, UCHT1 defines all peripheral T cells, QS4120 defines the CD4+ helper/inducer T-cell subset, and QS4122 defines the CD8+ suppressor/cytotoxic T-cell subset. Highly sensitive ELISA kits for the evaluation of serum IL-2, IL-4, IL-10, and tumor necrosis factor alpha (TNF-α) levels (Quantikine ELISA kits) were obtained from R&D Systems Inc., Minneapolis, MN. The kits were stored in the dark at 2 to 5°C. MEM was obtained from Sigma Diagnostics (St. Louis, MO) and was prepared in 100-ml aliquots, autoclaved, and preserved with streptomycin and penicillin.
Specimen collection.
Twenty milliliters of blood was drawn from each subject. Of this amount, 3 ml was transferred to an EDTA bottle for determination of the basic hematological indices. Seven milliliters was dispensed into a plain sterile bottle for separation of the serum into two aliquots. Both aliquots were stored in tubes containing drops of Trasylol (aprotinin) to inhibit the degradation of cytokines and were subsequently stored at −20°C for cytokine assays. Ten milliliters of blood was collected into preservative-free heparin tubes (Sigma) for the extraction of peripheral blood lymphocytes. Blood samples were taken from all research subjects upon receipt of consent, at confirmation of diagnosis, and before the administration of any drugs. Blood samples were also taken from normal healthy controls after consent was obtained and were treated similarly. In support of the clinical diagnosis, blood, stool, urine, and sputum samples were assessed for microbiological evidence of infection in patients where possible.
Complete blood count.
Complete blood counts were done for all patients and controls in the study according to the standard procedures of Dacie and Lewis (6), currently in use in the hematology laboratory of the teaching hospital. These standard procedures included hemoglobin and hematocrit determinations, differential and total white blood cell counts, determination of sickling, hemoglobin genotyping, and platelet and reticulocyte counts. Blood films for malaria parasites were also taken. Absolute lymphocyte counts were deduced from the total white blood cell counts and the differential lymphocyte percentages as follows: A/100 × B = C, where A is the differential lymphocyte percentage, B is the total white blood cell count (expressed as 109 cells/liter), and C is the absolute number of cells (109/liter).
HIV screening assays.
HIV infection in the subjects was ruled out by rapid screening for HIV using the established national algorithm of double parallel rapid tests. Commercially procured kits, the Determine (Abbott Laboratories, Japan) and ImmunoComb II HIV 1 & 2 BiSpot (Orgenics, Yavne, Israel) rapid parallel tests, were used according to the manufacturers' instructions. Individuals whose samples were positive for HIV-1/2 by these tests were dropped from the study.
Enumeration of CD3+, CD4+, and CD8+ T cells. (i) Separation of lymphocytes.
The differential-gradient centrifugation method of Gupta and Good (12) was employed. Ten milliliters of heparinized blood was diluted 1:2 with Eagle's MEM adsorbed with fetal calf serum. This was layered in 7-ml aliquots on 3 ml of Ficoll-Histopaque (Sigma) density gradient medium in 10-ml tissue culture tubes (Sigma). The mixture was centrifuged (MSE, United Kingdom) at 1,800 rpm for 30 min at room temperature, and pure lymphocytes were harvested by gentle removal of the lymphocyte layer at the Ficoll-MEM interface with a Pasteur pipette and transfer to another 10-ml tissue culture tube. Lymphocyte viability was tested by a trypan blue exclusion test. The cells were then washed three times with MEM by suspension in the medium and were centrifuged at 1,800 rpm for 10 min at room temperature. After the final wash, 4 × 106 cells were suspended in 1 ml of MEM containing 100 μg/ml penicillin, 100 μg/ml streptomycin, and 5% heat-inactivated adsorbed fetal calf serum (Wellcome, United Kingdom) for lymphocyte enumeration by immunofluorescence microscopy.
(ii) Immunofluorescence microscopy technique.
Lymphocyte subsets (CD3, CD4, and CD8) were enumerated by the method described in the Becton Dickinson (BD Pharmingen) source book by using the corresponding monoclonal antibodies (UCHT1, QS4120, and QS4122; Ancell Corporation) conjugated to FITC. Cells were incubated for 30 min in crushed ice in 50-μl aliquots. Cells were then taken from the lymphocyte suspension, separated as described above, and placed in small plastic tubes, and 20 μl of the appropriate monoclonal antibody was added. The cells were then settled by centrifugation at 1,000 rpm for 5 min at 4°C (Chilspin; MSE, United Kingdom) and were washed twice. After the final wash, the cells were suspended in 50 μl of MEM and were placed on a sterile glass slide, to be kept in the dark at 4°C for 15 min. Direct fluorescence analysis was carried out using a fluorescent microscope (Orthoplan; Leitz, Germany). Random fields were screened in the dark by selecting a field, viewing it under normal light to record the total number of cells in the area, and then viewing the same field under a UV light filter and recording the number of cells that stained bright apple-green peripherally as positive. Several fields were scanned until a total of 200 lymphocytes were counted. The number of positive cells was then expressed as a percentage of the total count, and the absolute number of cells was calculated.
Serum cytokine assays.
Due to financial constraints, cytokine studies were not done on all samples. The aim was to include at least one inflammatory and one anti-inflammatory cytokine in the study group. Consequently, samples from 26 patients in sickle cell VOC, 20 stable SCD patients, and 18 normal healthy controls were analyzed for the cytokines IL-2, IL-4, and IL-10.
Batched samples stored at −20°C in Trasylol (Sigma) were assayed for cytokines in serum with high-sensitivity commercial enzyme linked immunosorbent assay kits (R&D Systems Inc., Minneapolis, MN) by following the immunoassay protocol outlined by Pathare et al. (25). Briefly, all serum samples that had been frozen at −20°C were thawed once at the time of assay. Samples were dispensed diluted (1:50) into 96-well microtiter ELISA plates precoated with monoclonal antibodies to human interleukins (e.g., IL-2, IL-4, IL-10), and the plates were incubated at room temperature for 2 h. The plates were then washed four times with wash buffer (phosphate-buffered saline [PBS]-0.05% Tween 20) and were incubated for 2 h with horseradish peroxidase (HRP)-conjugated anticytokine antibodies that corresponded to each of the cytokines tested. The bound enzyme was then detected by incubation in the dark with tetramethylbenzidine (TMB) and with hydrogen peroxide as a substrate. The plates were scanned using a microplate reader (Bio-Rad) set at the appropriate wavelength for the color-forming reaction (optical density at 450 mm). A standard curve was then generated, and concentrations of cytokines in the specimens were determined by comparing the optical density of the sample with the values on the standard curve.
Statistical analysis of immunological responses.
Data were analyzed by use of the SPSS (version 11.5) and GraphPad Prism (version 4.0) statistical programs, and statistical significance was calculated using Student's unpaired two-tailed t test (for hematology and T-cell subset values) or analysis of variance (ANOVA) with least square difference (LSD) posthoc tests (for cytokines; a normal distribution was observed from the cytokine study). Comparisons were made between crisis patients and steady-state patients, between crisis patients and normal healthy controls, and between steady-state patients and normal healthy controls.
Level of significance.
Statistical test results with a P value of <0.05 were considered to be significant.
RESULTS
Demographic and clinical features in the sickle cell crisis study.
Forty-seven patients with a diagnosis of sickle cell disease (SCD) who met the inclusion criteria, comprising 27 males (57.4%) and 20 females (42.6%), were recruited. Twenty volunteers, hemoglobin AA homozygotes who were relatives of the patients or hospital workers, served as a group of normal healthy controls. Of the SCD patients, 20 (42.6%) were in steady state and represented our stable controls (group 2), while 27 (57.4%) were in vaso-occlusive crisis (VOC) and constituted the study group (group 1). The mean age for all SCD patients was 22.43 (±6.28) years, with a range of 12 to 39 years. In group 1 (SCD patients in VOC), there were 17 (63%) males and 10 (37%) females, with a mean age of 23.3 ± 6.15 years.
The average number of crisis episodes for SCD patients and patients in VOC before the study was 2. The predominant tribe among these patients in group 1 was Hausa (63%), followed by Yoruba (18.5%) and Ibo (7.4%). A total of 25 (92.6%) patients in group 1 were homozygous for HbS (HbSS), while only 2 (7.4%) presented with HbSS plus HbF. In group 2 there were 10 males and 10 females, with a mean age of 21.3 ± 6.4 years. There was no significant difference in the age range between group 1 and group 2 (P, 0.05). In group 2 also, Hausa was the major tribe (80%), followed by Yoruba (10%) and Ibo (5%). The majority of these patients were also SS homozygotes (90%). The mean blood pressure for both groups was 110/70, and there was no significant difference in the mean body mass index (BMI) between the groups (19. 2 ± 3.14 versus 18.7 ± 3.97 [P, >0.05]). The numbers of recorded episodes of VOC in groups 1 and 2 were similar, about 1 to 2 per year, with increases upon exposure to stressful conditions, such as exams or extremely cold weather. While pain occurred in bones and joints for most patients in VOC in this study, the major sites of pain encountered were the upper and lower limbs (50%), the back (25%), and the chest (25%). A few (25%) of the patients in VOC had generalized body pains, while another 25% presented with severe abdominal pains.
Infection status of VOC patients.
Eleven patients in VOC recorded fever (body temperatures above 39.0°C). All 11 of these patients had malaria parasites. Two other patients had coughs, and 2 had pain on urination, making for a total of 15 patients with suspected infections. However, none of the samples submitted for microbiological culture from these 15 patients yielded any growth. All patients and controls in the study were also seronegative for HIV by double rapid testing with the Determine (Trinity Biotech, Japan) and ImmunoComb II HIV 1 & 2 BiSpot (Orgenics) parallel tests.
Hematological indices in sickle cell crisis.
The hematological values obtained for patients in steady state and VOC are shown in Table 1. There were statistically significant differences in total white blood cell (WBC) counts and neutrophil percentages, which were higher in patients in VOC (63.3% ± 10.41%) and steady-state SCD patients (57.3% ± 9.73%) than in normal healthy controls (50.45% ± 9.58%) (P, <0.05 for each analysis). There was a similar significant difference in differential lymphocyte counts between SCD patients in steady state (40.30% ± 11.44%) or VOC (34.66% ± 10.1%) and healthy controls (46.10% ± 9.11%) (P, <0.05). While there were notable differences in platelet counts between groups, these differences were not statistically significant (P, >0.05). The blood films of patients in VOC also frequently showed poikilocytes, target cells, polychromasia, and hypochromia, as well as circulating sickling cells. Malaria parasites were detected in 11 (41%) VOC patients' blood films but not in those of SCD patients in steady state or of NHC. Some degree of reticulocytosis (6 to 13%) was seen in patients in VOC compared to patients in steady state.
TABLE 1.
Hematological values for sickle cell disease patients in steady state or VOC and for NHC
| Variable | Value (mean ± SD) for: |
Pa | ||
|---|---|---|---|---|
| HbSS patients |
NHC (n = 20) | |||
| VOC (n = 27) | Steady state (n = 20) | |||
| Packed cell vol | 24.7 ± 5.2 | 25.1 ± 5.1 | 40.0 ± 5.2 | <0.05* |
| White blood cell count (109/liter) | 11.2 ± 6.4 | 9.9 ± 4.6 | 6.7 ± 2.1 | <0.05*§ |
| Differential count (% of white blood cells) | ||||
| Neutrophils | 63.3 ± 10 | 57.3 ± 9.7 | 50.45 ± 9.6 | <0.05*§ |
| Lymphocytes | 34.7 ± 10 | 40.3 ± 11 | 46.1 ± 9 | <0.05*§ |
| Eosinophils | 3.00 ± 2 | 3.86 ± 3 | 3.01 ± 2 | >0.05 |
| Basophils | 3.4 ± 2 | 2.75 ± 1 | 2.9 ± 0.9 | >0.05 |
| Platelet count (109/liter) | 242 ± 51 | 233 ± 42 | 267 ± 56 | >0.05 |
| Reticulocytes (%) | 4.4 ± 4 | 3.6 ± 1.6 | ||
Determined by two-tailed Student t tests. *, significant (P < 0.05) statistical difference between values for patients in VOC and those for normal healthy controls; §, significant (P < 0.05) statistical difference between values for SCD patients in steady state or VOC and those for NHC.
T-lymphocyte subsets in sickle cell crisis.
As shown in Table 2, the percentages of total T cells (CD3) and the absolute CD3 lymphocyte counts were not significantly different between patients in VOC and steady-state SCD patients (P, >0.05). However, while there were significant differences in the percentages of CD4+ and CD8+ T cells between patients in VOC and steady-state patients (P, <0.05), these differences were corroborated only for CD4+ T cells (P, <0.05) when cell numbers were expressed as absolute values. The absolute counts of CD8 cells did not differ significantly between the two groups. The ratio of mean CD4+ T cells to mean CD8+ T cells (Table 2) in patients in VOC (0.7) was lower than that in steady-state SCD patients (1.1), but not significantly so. However, the difference between patients in VOC and normal healthy controls (1.4) was significant.
TABLE 2.
Values for total T cells and T-cell subsets in sickle cell patients in steady state, sickle cell patients in VOC, and NHC
| Variablea | Value (mean ± SD) for: |
Pb | ||
|---|---|---|---|---|
| HbSS patients |
Normal healthy controls (n = 20) | |||
| VOC (n = 27) | Steady state (n = 20) | |||
| CD3 cells | ||||
| % | 43 ± 4.7 | 43 ± 4.6 | 52 ± 6.5 | >0.05* |
| Abs count (109/liter) | 1.6 ± 1.0 | 1.7 ± 1.0 | 1.6 ± 0.6 | >0.05# |
| CD4 cells | ||||
| % | 17 ± 3.4 | 22 ± 2.9 | 30 ± 4.1 | <0.05*§# |
| Abs count (109/liter) | 0.6 ± 0.4 | 0.9 ± 0.5 | 0.9 ± 0.4 | <0.05*§ |
| CD8 cells | ||||
| % | 26 ± 3.7 | 20 ± 4.1 | 22 ± 3.5 | <0.05*§ |
| Abs count (109/liter) | 1.0 ± 0.5 | 0.8 ± 0.5 | 0.7 ± 0.2 | >0.05 |
| CD4/CD8 ratio | 0.7 | 1.1 | 1.4 | <0.05* |
CD3 cells represent total T cells; subsets are CD4 and CD8 cells. Cell numbers are given as percentages of total cells counted and as absolute (Abs) counts.
Determined by two-tailed Student t tests. Symbols indicate significant differences (P < 0.05) between HbSS patients in VOC and normal healthy controls (*), between HbSS patients in VOC and steady-state HbSS patients (§), and between steady-state HbSS patients and normal healthy controls (#).
Serum cytokine levels in SCD.
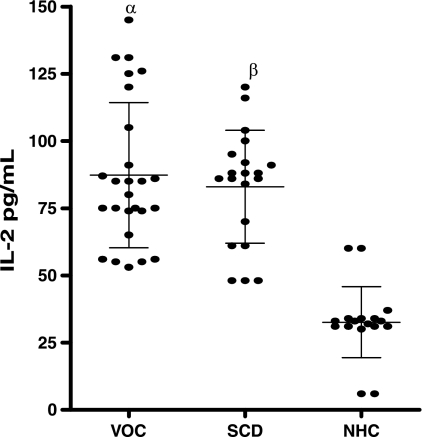
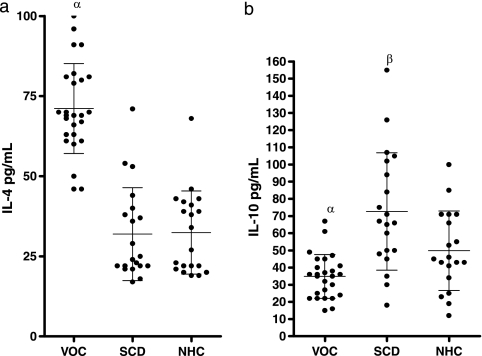
The data in Fig. 1 and 2 show the levels of IL-2, IL-4, and IL-10 in the sera of patients with VOC compared to those for steady-state SCD patients and normal healthy controls. The mean level of IL-2 in the sera of normal healthy control subjects was 31.00 ± 11.29 pg/ml (range, 6.0 to 60.00 pg/ml), while levels in SCD patients in steady state and VOC were 83.00 ± 21.02 pg/ml (range, 48 to 120 pg/ml) and 87.23 ± 26.99 pg/ml (range, 53 to 145 pg/ml), respectively, showing a significant difference between SCD patients and normal healthy controls (P, <0.05). However, there was no significant difference in mean serum IL-2 levels between stable SCD patients and those in VOC (P, >0.05) (Fig. 1). The mean serum IL-4 level was significantly higher in SCD patients in VOC (71.15 ± 14.05 pg/ml; range, 46.0 to 100.0 pg/ml) than in steady-state, stable SCD patients (31.95 ± 14.28 pg/ml; range, 17.0 to 71.0 pg/ml) and normal healthy controls (33.4 ± 12.39 pg/ml; range, 19.0 to 68.0 pg/ml) (P, <0.05). There was, however, no significant difference between the levels in stable SCD patients and those in normal healthy controls (P, >0.05) (Fig. 2).
FIG. 1.
Serum IL-2 levels in patients in vaso-occlusive crisis (VOC) (n = 26), stable SCD patients (n = 20), and normal healthy controls (NHC) (n = 18) were assessed by ANOVA and posthoc tests. Bars represent means and standard deviations. Greek letters indicate statistically significant (P < 0.05) differences between either VOC patients (α) or stable SCD patients (β) and NHC.
FIG. 2.
Distribution of serum IL-4 and IL-10 levels in patients in VOC, steady-state SCD patients, and normal healthy controls. (a) Serum IL-4 concentrations in patients in VOC (n = 26), steady-state SCD patients (n = 20), and NHC (n = 18) were assessed by ANOVA and posthoc tests. α, significant differences (P < 0.05) for VOC patients versus steady-state SCD patients and normal healthy controls. (b) Serum IL-10 concentrations in VOC patients, SCD patients, and NHC. Greek letters indicate significant differences (P < 0.05) between either VOC patients (α) or steady-state SCD patients (β) and NHC. Bars represent means and standard deviations.
Normal healthy controls had higher mean serum IL-10 levels (48.1 ± 22.64 pg/ml; range, 12 to 100 pg/ml) than SCD patients in VOC (34.8 ± 12.76 pg/ml; range, 15 to 67 pg/ml), but this difference was not significant (P, >0.05). However, serum IL-10 levels in steady-state, stable SCD patients (72.65 ± 34.2 pg/ml; range, 18 to 155 pg/ml) (Fig. 2) were statistically significantly higher (P, <0.05) than those in both normal controls and SCD patients in VOC crisis. The ratio of mean IL-2 to IL-10 levels was 2.4 in patients in VOC, 1.13 in steady-state patients, and 0.78 in normal healthy controls. The ratio of mean IL-2 to mean IL-4 levels was 1.18 in patients in VOC, 2.56 in steady-state patients, and 1.00 in normal healthy controls.
DISCUSSION
The white blood cell counts and neutrophil percentages of patients with SCD in VOC and steady-state SCD patients were significantly higher than those of normal healthy controls. This was at the expense of markedly lower differential lymphocyte percentages and in contrast to the low circulating neutrophil levels normally seen in SCD patients. Beddall (5) states that people of African and Caribbean descent normally have lower neutrophil counts than people of other races due to a higher ratio of marginating to circulating neutrophils. The elevated neutrophil percentages in patients in VOC and steady-state SCD patients found in this study could be a response to chemotactic stimuli as a result of infection or inflammation and are buttressed by the finding of Duits et al. (7) that SCD patients in crisis had high levels of IL-8 (a potent neutrophil chemokine).
SCD patients in VOC and those in steady state showed similarly elevated IL-2 levels compared to those in normal healthy controls (P, <0.05), indicating the presence of cell-mediated inflammatory responses in SCD. Although IL-2 is produced mainly by CD4+ T cells, a small amount is produced by CD8+ T cells, levels of which were somewhat elevated (though not significantly) in this study. Reticulocytes, levels of which were marginally higher in VOC than in steady-state SCD in this study, also have a propensity for adherence, due to surface adhesion molecules such as CD36, and may contribute to elevated IL-2 levels in patients in VOC due to sequestration of T cells to sites of inflammation. IL-2 mainly affects lymphocytes, facilitating the production of other cytokines from T cells, promoting NK cell and B-cell growth, and enhancing the responsiveness of immature bone marrow cells to other cytokines (29). The fact that IL-2 levels were higher in SCD patients in crisis and in steady state suggests some degree of activation-induced cytokinemia, in keeping with the ischemic inflammatory profile in SCD. This may be due to subclinical microinfarctions induced by enhanced adhesiveness of sickle reticulocytes and reversibly sickled erythrocytes to the vascular endothelium. It may also be due to the numerous infections that are endemic in the environment in Zaria (24). Such infections include malaria, intestinal parasitic helminth infections, and mycobacterial infections. Infections affect the adhesion of sickled erythrocytes and contribute to the persistent inflammatory presence in SCD. While it was not possible to diagnose or substantiate any bacterial infection in most of the sickle cell patients, 41% of patients in VOC had malaria infections. No malaria or other infection was detected in steady-state SCD patients or in NHC. Malaria infection has been linked with the upregulation of cytokine production and inflammation in SCD (20). This indicates a possible link between infection-inflammation and endothelial dysfunction in SCD. In this study, malaria infection was linked with elevated serum IL-2 and IL-4 levels in VOC. However, there was no significant difference in cytokine results between subgroups of patients with or without malaria in VOC versus steady-state SCD patients and normal controls, despite the difference in malaria parasitemia between the groups (data not shown). Also, the absence of any other infectious cause in >60% of patients in VOC implies the presence of noninfectious mechanisms in the pathogenesis of VOC. This is reiterated by Kooy et al. (18), who indicate that in many cases of VOC, fever may be absent; the white blood cell differential rarely demonstrates the presence of immature nucleated red blood cells or leukocytes (left shift); and even cultures from the affected sites are often negative for bacterial pathogens such as Staphylococcus aureus or Salmonella enterica serovar Typhi.
Interestingly, despite the high IL-2 levels observed in patients with SCD in this study, whether in VOC or in steady state, high levels of CD3+ and CD4+ T cells were not observed in the same patients. Helper (CD4+) T cells, in the presence of antigen-presenting cells (APCs) and repeated stimulation by antigen, produce IL-2, which binds to its receptor, IL-2R, to result in total (CD3+) and helper (CD4+) T-cell proliferation (21). It is generally accepted that antigen-dependent proliferation of antigen-activated T lymphocytes expressing IL-2R plays a pivotal role in the regulation of cellular immune responses. Thus, the possibility exists that a defect in the expression of IL-2R on T-cell surfaces or in the binding of IL-2 to its receptor might be responsible for the low total and helper T-cell responses seen in SCD in this study. Besides, IL-2 is said to have both growth-promoting and growth-inhibitory effects (30), which are operative at different phases of the immune response. Early in a T-cell response, when IL-2 concentrations are low, T-cell proliferative effects may be dominant, but when IL-2 levels are high, the immune response may be terminated (19). The high IL-2 levels in steady-state SCD patients and patients in VOC may represent a bid to terminate a low-grade immune response to chronic stimuli or tissue ischemia, respectively, in these patients. Additionally, sequestration of CD4+ T lymphocytes to sites of inflammation during VOC may explain the reduction of peripheral CD4+ cell percentages in the face of increased IL-2 levels in patients in this study. Koffi et al. (16) indicated a decrease in CD4+ and even CD8+ T-cell subsets in patients with sickle cell anemia (SCA) with splenic defects but not in those with normal spleens. Patients in VOC in this environment are noted to have atrophied spleens (asplenia) due to ischemia and infection as a result of chronic sickling, and this could explain the marked reduction in the level of CD4+ T cells observed in this study.
Patients with SCD in VOC had higher levels of IL-4 than normal healthy controls and steady-state SCD patients in this study, while patients in steady state had higher IL-10 levels than either patients in VOC or normal healthy controls. This was in contrast to the study by Pathare et al. (25), where higher levels of IL-4 were found in patients in steady state than in crisis patients, and the study of Graido-Gonzalez et al. (9), where a trend toward higher IL-10 levels in patients in VOC than in normal healthy controls was seen. Since both IL-4 and IL-10 are anti-inflammatory cytokines, it appears that their differing elevated levels in VOC and SCD represent a compensatory counterregulatory mechanism to an ongoing inflammatory state. Munford and Pugin (22), maintain that the body's normal response to stress is the activation of anti-inflammatory mechanisms and that immune cells and cytokines have both pathogenic and protective roles. While the secretion of the proinflammatory cytokine IL-2 may represent an acute-phase response to endothelial dysfunction or infection-inflammation in sickle cell crisis, the activation of IL-4 and IL-10 in this study would represent the triggering of a compensatory anti-inflammatory mechanism to effectively downregulate the inflammatory response. However, the finding of more of one type of anti-inflammatory cytokine in one SCD condition than in another is interesting and may represent the relatively complex process of T-cell differentiation and polarization by APCs (26). Further studies are necessary to show whether the preponderance of IL-4 in patients in VOC is an indication of a more-potent regulator of inflammatory responses than IL-10 or whether effective levels of IL-10 (which were poor in patients in VOC in this study, as seen in the IL-2/IL-10 ratios) are obligatory to counter the inflammatory mechanisms that mediate sickle cell VOC.
While increased levels of IL-4 in patients in VOC in this study indicate a compensatory shift in T-cell type toward an anti-inflammatory profile, IL-4 is also associated with antibody production as a contributing factor to microvascular occlusion and intravascular hemolysis (14, 15). However, while hypergammaglobulinemia has generally been recorded in HbSS anemia patients and is thought to be an expression of compensatory β-cell overreaction to a wide range of antigenic stimuli (10, 11), it was not possible to analyze immunoglobulin levels in our patients.
In conclusion, although CD4+ T-cell levels were low in SCD patients in VOC and steady state, there was no significant difference between patients in VOC and those in steady state in the distribution pattern of T lymphocytes in peripheral blood, reflecting an abnormal immunoregulatory mechanism in the acute phase continuing into the chronic phase of the disease. It would also seem that immunological reactivity in sickle cell crisis represents a balance between immune cells and the inflammatory and anti-inflammatory cytokines they secrete. Many cells of the body, including erythrocytes, respond to stimuli from immune cells and interact with immune cells; thus, immune cells become prime mediators of tissue physiology under such conditions. Excessive increases in levels of inflammatory cytokines, such as IL-2, were seen in both steady-state SCD patients and SCD patients in VOC in this study, and they imply an inflammatory response to both chronic low-grade tissue ischemia in SCD and enhanced challenge in VOC. Elevated IL-2 levels increase cell adhesion to the endothelium, vascular occlusion, and pain crisis, and they contribute to an unsteady inflammatory state in SCD. Upregulation of anti-inflammatory or counterinflammatory cytokines, such as IL-4 and IL-10 (which were prominent in normal healthy controls in the study), might be necessary to inhibit the development of further mechanisms that mediate crisis development. These anti-inflammatory cytokines might also have utility in the treatment of sickle cell vaso-occlusive crisis. Further studies will, however, be necessary before this is possible. In the present study, we could not compare more serum cytokines of TH1/TH2 types. We are only able to present profiles of the cytokines IL-2, IL-4, and IL-10 as a preliminary report. Whether these cytokine characteristics are specific for sickle cell crisis remains to be determined in larger studies. These issues would have important implications for our understanding of the pathological and immunological status of patients with sickle cell vaso-occlusive crisis.
Acknowledgments
We appreciate the excellent technical assistance of T. S. Kene in the statistical analysis of the data.
Footnotes
Published ahead of print on 3 February 2010.
REFERENCES
- 1.Akinola, N. O., S. M. E. Stevens, I. M. Franklin, G. B. Mash, and J. Stuart. 1992. Subclinical ischaemic episodes during the steady state of sickle cell disease. J. Clin. Pathol. 45:902-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballas, S. K., J. Larner, E. D. Smith, et al. 1988. Rheologic predictors of the severity of the painful sickle cell crisis. Blood 72:1216-1223. [PubMed] [Google Scholar]
- 3.Ballas, S. K. 1995. The sickle cell painful crisis in adults: phases and objective signs. Hemoglobin 19:323-333. [DOI] [PubMed] [Google Scholar]
- 4.Ballas, S. K., and N. Mohandas. 1996. Pathophysiology of vaso-occlusion. Hematol. Oncol. Clin. North Am. 10:1221-1239. [DOI] [PubMed] [Google Scholar]
- 5.Beddall, A. 1990. Anaemias. Practitioner 234:713-726. [PubMed] [Google Scholar]
- 6.Dacie, J. V., and S. M. Lewis. 1991. Practical haematology, 7th ed., p. 54-79. Churchill Livingstone, Edinburgh, United Kingdom.
- 7.Duits, A. J., J. B. Schnog, L. R. Lard, A. W. Saleh, and R. A. Rojer. 1998. Elevated IL-8 levels during sickle cell crisis. Eur. J. Haematol. 61(5):302-305. [DOI] [PubMed] [Google Scholar]
- 8.Francis, R. B., and C. S. Johnson. 1991. Vascular occlusion in sickle cell disease: current concepts and unanswered questions. Blood 77:1405-1414. [PubMed] [Google Scholar]
- 9.Graido-Gonzalez, E., J. C. Doherty, E. W. Bergreen, G. Organ, M. Telfer, and M. A. McMillen. 1998. Plasma endothelin-1, cytokine, and prostaglandin E2 levels in sickle cell disease and acute vaso-occlusive sickle crisis. Blood 92:2551-2555. [PubMed] [Google Scholar]
- 10.Green, G. A., M. M. Rehm, and V. K. Kalra. 1985. Cell bound autologous immunoglobulin in erythrocyte populations from patients with sickle cell disease. Blood 65:1127-1133. [PubMed] [Google Scholar]
- 11.Green, G. A. 1993. Autologous IgM, IgA, and complement binding to sickle erythrocytes in vivo. Evidence for the existence of dense sickle cell subsets. Blood 82:985-992. [PubMed] [Google Scholar]
- 12.Gupta, S., and R. A. Good. 1977. Subpopulations of human T lymphocytes. I. Studies in immunodeficient patients. Clin. Exp. Immunol. 30:222-228. [PMC free article] [PubMed] [Google Scholar]
- 13.Hebbel, R. P. 1991. Beyond hemoglobin polymerization: the red blood cell membrane and sickle cell disease pathophysiology. Blood 77:214-237. [PubMed] [Google Scholar]
- 14.Hernández, P., C. Cruz, M. N. Santos, and J. M. Ballester. 1980. Immunologic dysfunction in sickle cell anaemia. Acta Haematol. 63:156-161. [DOI] [PubMed] [Google Scholar]
- 15.Kirkpatrick, M. B., J. Haynes, and J. B. Bass. 1991. Results of bronchoscopically obtained lower airway cultures from adult sickle cell disease patients with acute chest syndrome. Am. J. Med. 90:206. [PubMed] [Google Scholar]
- 16.Koffi, K. G., D. Sawadogo, M. Meite, D. C. Nanho, E. S. Tanoh, A. K. Altia, I. Sanogo, and A. Sangare. 2003. Reduced levels of T-cell subsets CD4+ and CD8+ in homozygous sickle cell anaemia patients with splenic defects. Hematol. J. 4:363-365. [DOI] [PubMed] [Google Scholar]
- 17.Konotey-Ahulu, F. I. D. 1974. The sickle cell diseases. Arch. Intern. Med. 133:611-619. [PubMed] [Google Scholar]
- 18.Kooy, A., L. J. de Heide, A. ten Tije, et al. 1996. Vertebral bone destruction in sickle cell disease: infection, infarction or both. Neth. J. Med. 48:227-231. [DOI] [PubMed] [Google Scholar]
- 19.Lanzavecchia, A., and F. Sallusto. 2000. Dynamics of T cell responses: intermediates, effectors and memory cells. Science 290:92-97. [DOI] [PubMed] [Google Scholar]
- 20.Moore, C. M., M. D. Ehlayel, L. E. Leiva, and R. U. Sorensen. 1996. New concepts in the immunology of sickle cell disease. Ann. Allergy Asthma Immunol. 76:386-403. [DOI] [PubMed] [Google Scholar]
- 21.Mosmann, T. R. 1991. Cytokine secretion patterns and the cross regulation of T cell subsets. Immunol. Res. 10:183-188. [DOI] [PubMed] [Google Scholar]
- 22.Munford, R. S., and J. Pugin. 2001. Normal responses to injury prevent systemic inflammation and can be immunosuppressive. Am. J. Respir. Crit. Care Med. 163:316-321. [DOI] [PubMed] [Google Scholar]
- 23.Omoti, C. E. 2005. Haematological values in sickle cell anaemia in steady state and during vaso-occlusive crisis in Benin City, Nigeria. Ann. Afr. Med. 4(2):62-67. [Google Scholar]
- 24.Onyemelukwe, G. C., and B. O. P. Musa. 2001. T-lymphocyte subsets in patients with hookworm infection in Zaria, Nigeria. Afr. J. Med. Med. Sci. 30:255-259. [PubMed] [Google Scholar]
- 25.Pathare, A., S. Al Kindi, A. Alnaqdy, S. Daar, H. Knox-Macauly, and D. Dennison. 2004. Cytokine profile of sickle cell disease in Oman. Am. J. Hematol. 77:323-328. [DOI] [PubMed] [Google Scholar]
- 26.Platt, O. S., B. D. Thornington, D. J. Brambilla, et al. 1991. Pain in sickle cell disease. Rates and risk factors. N. Engl. J. Med. 325:11-16. [DOI] [PubMed] [Google Scholar]
- 27.Platt, O. S. 1994. Easing the suffering caused by sickle cell disease. N. Engl. J. Med. 330:783-784. [DOI] [PubMed] [Google Scholar]
- 28.Powars, D. R. 1990. Sickle cell anaemia and major organ failure. Hemoglobin 14:573-598. [DOI] [PubMed] [Google Scholar]
- 29.Roitt, I., J. Brostoff, and D. Male. 2001. Immunology, 6th ed., p. 126. Harcourt Publishers Ltd., Edinburgh, United Kingdom.
- 30.Van Parijs, L., and A. K. Abbas. 1998. Homeostasis and self tolerance in the immune system: turning lymphocytes off. Science 280:243-248. [DOI] [PubMed] [Google Scholar]