Abstract
Taeniasis/cysticercosis caused by Taenia solium is a frequent parasitic infection of the human brain in most of the world. Rapid and simple screening tools to identify taeniasis and cysticercosis cases are needed for control programs, mostly to identify tapeworm carriers which are the source of infection and need to be treated, or as tools for point-of-care case detection or confirmation. These screening assays should be affordable, reliable, rapid, and easy to perform. Immunochromatographic tests meet these criteria. To demonstrate proof of principle, we developed and evaluated two magnetic immunochromatographic tests (MICTs) for detection of human Taenia solium taeniasis antibodies (ES33-MICT) and neurocysticercosis antibodies (T24-MICT). These assays detected stage-specific antibodies by using two recombinant proteins, rES33 for detection of taeniasis antibodies and rT24H for detection of cysticercosis antibodies. The sensitivity and specificity of the ES33-MICT to detect taeniasis infections were 94.5% and 96%, respectively, and those of the T24-MICT to detect cases of human cysticercosis with two or more viable brain cysts were 93.9% and 98.9%, respectively. These data provide proof of principle that the ES33- and T24-MICTs provide rapid and suitable methods to identify individuals with taeniasis and cysticercosis.
Humans can be infected with both the adult worm and larval forms of the cestode Taenia solium, causing taeniasis and cysticercosis, respectively. Humans, who are the only definitive host for the adult tapeworm, develop taeniasis after eating raw or undercooked pork infested with the larval stages of the parasite. Both pigs and humans can develop cysticercosis by ingesting eggs passed in the feces of a tapeworm carrier. Consequently, cysticercosis can be acquired under any variety of cultural and socioeconomic conditions where there is close contact with a taeniasis carrier. Because tapeworm-infected humans are the only source of transmission of both human and pig cysticercosis, diagnosis and treatment of taeniasis are crucial for control and elimination of cysticercosis in both humans and pigs (18). Therefore, accurate and rapid diagnostic methods to identify tapeworm carriers are critical tools needed in cysticercosis/taeniasis control and elimination programs. Although diagnosis of porcine or human cysticercosis is not crucial in the context of control programs, the availability of rapid diagnosis for human cysticercosis would be useful for estimating the burden of disease and for determining seroprevalence rates in pigs.
Diagnosis of taeniasis usually is established by microscopic observation of T. solium eggs in stool specimens, but this method is insensitive and cannot differentiate between T. solium and Taenia saginata ova. Other methods to detect taeniasis include coproantigen detection methods, which are more sensitive than microscopy, and serodiagnosis of taeniasis by the use of excretory-secretory proteins in an enzyme-linked immunoelectrotransfer blot (EITB) (1, 14, 23). Two specific T. solium excretory-secretory (TSES) proteins, ES33 and ES38, were identified for specific taeniasis serodiagnosis. Recombinant forms of ES33 and ES38 (rES33 and rES38) have been evaluated, and the diagnostic performances of these two proteins were determined to be comparable (11).
The gold standard for serological identification of cysticercosis is the EITB, which relies on antibody reactivity with seven diagnostic lentil lectin purified glycoproteins (LLGP) (20). Recombinant or synthetic peptide forms of these proteins are now available (7-9), and after comparing the performances of these diagnostic proteins, rT24H (which corresponds to a 24,000-Da protein of the LLGP extract) (9) was identified as the recombinant protein with the best sensitivity and specificity for detecting neurocysticercosis cases (11).
The gold standard tests described above are not field friendly, not widely available, and do not exist in point-of-care formats. In contrast, lateral flow immunochromatographic tests, can be used at the point of care and in settings with minimal infrastructure (5, 21). Lateral flow methods have been used for several decades for clinical and veterinary diagnosis (16). In conventional lateral flow assays, reactions are detected visually, generating a qualitative result, or with a detection device that measures reflectance, contrast, color change, or fluorescence (16). Several studies have demonstrated that magnetic particle-labeled detection systems have the potential to improve lateral flow assay sensitivity and to provide a means of quantification (12, 17, 25). In this study, we describe the development and evaluation of two magnetic immunochromatographic tests (MICTs) for serologic detection of taeniasis (based on rES33 antigen) and cysticercosis (based on the rT24H antigen).
MATERIALS AND METHODS
Chemicals and reagents.
All reagents were reagent grade or better and unless otherwise noted were obtained from Mallinckrodt (St. Louis, MO). Guinea pig IgG and goat anti-guinea pig IgG(H+L) were purchased from Biomeda Corporation (Foster City, CA). EDC (1-ethyl-3-[3-dimethylaminopropyl] carbodiimide HCl) and sulfo-NHS (N-hydroxy-sulfosuccinimide) were purchased from Pierce (Thermo Scientific, Rockford, IL). Carboxyl superparamagnetic particles (200 nm) and particle storage buffer were purchased from Ademtech (Pessac, France).
Serum samples. (i) Sera for assay optimization.
A serum pool constructed by pooling five serum samples from human cases with confirmed cysticercosis was used for optimizing both the MICT for detection of human Taenia solium taeniasis antibodies (ES33-MICT) and the MICT for detection of neurocysticercosis antibodies (T24-MICT). This pool contained antibodies that reacted to the native excretory-secretory (ES) proteins in the original TSES EITB and the diagnostic proteins in the LLGP-EITB (13, 14, 20). Three polyclonal anti-rES33 hyperimmune goat serum samples with low, medium, and high antibody titers (based on enzyme-linked immunosorbent assay [ELISA] findings) were also used. A serum pool constructed from five serum samples collected from pigs in Peru with confirmed cysticercosis was also used in the T24-MICT analyses. A serum sample from a human case of alveolar echinococcosis (Echinococcus multilocularis) was used as a heterologous infection serum control. A negative serum pool was constructed from five serum samples from U.S. residents with no history of international travel.
(ii) Serum panels for determinations of diagnostic sensitivity and diagnostic specificity.
A total of 161 serum samples from persons with taeniasis were collected at the Instituto de Ciencias Neurologicas, Lima, Peru, and in epidemiological studies. The definitive diagnosis of taeniasis was made by identifying a T. solium adult worm in feces after a purgative treatment using PCR and/or morphological characteristics of the proglottids, scolex, and number of uterine branches (15). Defined neurocysticercosis serum samples were also collected at the Instituto de Ciencias Neurologicas, Lima, Peru, from patients presenting with clinical symptoms of neurocysticercosis. The definitive diagnosis of neurocysticercosis was confirmed by computed tomography (CT) or magnetic resonance imaging (MRI) of the brain (4). Serum samples from the neurocysticercosis cases were sorted into two categories based on the imaging data from each patient: (i) serum samples from patients with two or more viable cysts (n = 100) (these included serum samples from patients with multiple viable cysts or a racemose cyst) and (ii) those from patients with a single, viable cyst (n = 15). All serum samples were collected in compliance with protocols approved by the ethical review boards of all participating institutions with specific permission for future use of stored samples.
A total of 281 serum samples were used to assess specificity (Table 1). A panel of 164 serum samples was assembled from healthy residents of the United States or Egypt (one serum sample was not available for T24-MICT evaluation). The donors from Egypt were tested for the presence of intestinal parasites by stool examination, and all were negative. In addition, a panel of serum samples collected from patients with heterologous infections (117 samples) was used (four serum samples were not available for the ES33-MICT evaluation). All of these serum samples were collected from regions where transmission of cysticercosis/taeniasis does not occur.
TABLE 1.
Diagnostic specificity of the ES33-MICT and T24-MICT
| Serum panel | Category | Total no. | % Reactivity |
|
|---|---|---|---|---|
| ES33-MICT | T24-MICT | |||
| Normal human serum | U.S. resident | 145a | 0.7 | 0.7 |
| Egypt | 19b | 6.2 | 0 | |
| Heterologous infection serum | Taenia saginata | 7 | 0 | 0 |
| Ascaris lumbricoides | 1 | 100 | 100 | |
| Echinococcus granulosus | 14c | 6.3 | 0 | |
| Echinococcus multilocularis | 1 | 0 | 0 | |
| Plasmodium falciparum | 8 | 12.5 | 0 | |
| Schistosoma mansoni | 80 | 5 | 1.3 | |
| Schistosoma haematobium | 5 | 0 | 0 | |
| Trichinella sp. | 1 | 100 | 0 | |
| Total | 281 | |||
ES33-MICT had one more serum sample.
ES33-MICT had three less serum samples.
ES33-MICT had one less serum sample.
Recombinant Taenia solium proteins and controls.
rES33 was expressed in Sf21/Sf9 cells, using a baculovirus system. Similarly, the hydrophilic extracellular domain of T24, rT24H, was expressed in the Tni cell line, as described previously (9, 13). Both proteins were solubilized in striping buffer (10 mM KH2PO4/K2HPO4 [pH 7.4]) and protein coupling buffer (50 mM borate buffer [pH 8.5] plus 0.05% Tween 20 plus 0.15 M NaCl). Goat anti-guinea pig IgG (used as the assay positive control) was solubilized into striping buffer, and guinea pig IgG was solubilized into coupling buffer.
Coupling of rES33 and rT24H to superparamagnetic particles.
The coupling of rES33, rT24H, and guinea pig IgG to superparamagnetic particles used standard cross-linking chemistries. Briefly, magnetic particles were washed in 0.1 M 2-(N-morpholino)ethanesulfonic acid (MES; pH 6) plus 0.5 M NaCl plus 0.05% Tween 20 buffer, then activated by EDC and sulfo-NHS solution to form an amine reactive sulfo-NHS ester. The solution was then mixed in a rotator (<60 rpm) at room temperature (RT) for 30 min. After washing, the protein to be coupled was added to the particle suspension, forming a stable amide bond with the activated beads. Cross-linking was allowed to proceed for 3 h at RT. One-tenth volume of 10% bovine serum albumin (BSA) in 50 mM borate buffer (pH 8.5) was added for 30 min at RT to block residual activated carboxyl groups. After washing, the beads were reconstituted in particle storage buffer to a concentration of 10 mg/ml and stored at 4°C.
Preparation of lateral flow cassettes. (i) Protein striping.
Goat anti-guinea pig IgG (positive control) and rES33 or rT24H were striped onto HF135 nitrocellulose membrane (Hi-Flow Plus HF13504; Millipore, Billerica, MA) by using an IsoFlow reagent dispenser (Imagene Technology, Hanover, NH). After striping, the membranes were dried at 37°C for 2 h and then stored in a sealed plastic bag with a desiccant at RT until use.
(ii) Device assembly.
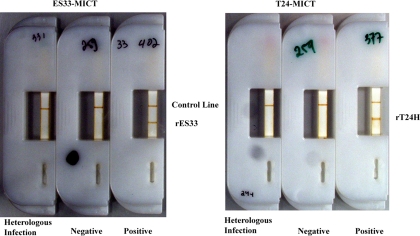
The device consisted of the HF135 membrane attached onto a MICT base card (MagnaBioSciences, San Diego, CA). A wide glass fiber conjugate pad (Millipore) was placed so that it overlapped with the membrane by ∼2 mm, and at the other end, a cellulose fiber sample pad (Millipore) was attached and also overlapped with the membrane to act as the “wick” or reservoir for transported fluids. The exposed part of the membrane was then covered with laminating plastic (MagnaBioSciences). The card was cut into 5-mm individual strips by using a Matrix 2360 programmable shearer (Kinematic Automation, Twain Harte, CA). The strip was then placed into a cassette (MagnaBioSciences) that facilitated the detection of the magnetic field at the assay reaction lines (Fig. 1). The device was stored at RT inside a sealed plastic bag with a desiccant until used.
FIG. 1.
Fully assembled ES33- and T24-MICT devices. MICT devices were fully assembled and used to test heterologous infection (cross-reactor), negative, and positive serum samples.
Optimization and evaluation of the ES33- and T24-MICT methods. (i) Optimization.
The MICT methods were optimized by varying the concentration of protein antigens on the test line (evaluating both the concentration and volume of antigen used), assessing blocking agents (Tween 20 in combination with concentrations of BSA in chase buffers), and defining the reaction time. The optimum conditions were determined to be those that generated the highest signal-to-noise ratio between positive and negative serum, without increasing the signal of a serum sample from a heterologous infection, i.e., alveolar echinococcosis.
(ii) Assay evaluation.
For the ES33-MICT, 5 μl of serum (1:10 dilution) was added to a 96-well plate with 43.4 μl of chase buffer (phosphate-buffered saline [PBS] plus 0.5% Tween 20 plus 0.25% BSA plus 0.1% NaN3) plus 0.6 μl of rES33 conjugate (rES33 coupled to superparamagnetic particles) plus 1 μl of guinea pig IgG conjugate. For the T24-MICT, 5 μl of serum was added to 43 μl of chase buffer plus 1 μl of rT24H conjugate (rT24H coupled superparamagnetic particles) plus 1 μl guinea pig IgG conjugate. The mixture was then loaded onto the sample well of the MICT device. Directly after sample addition, 120 μl of chase buffer was added. Reactions were read using a magnetic assay reader (MAR) (catalog no. 8094-600-1; MAR assay development system; MagnaBioSciences) (12). Results were also read visually by two independent readers 7 days after the run; discrepant results were resolved by a third reader.
(iii) Inter- and intra-assay reproducibility.
Because MICT requires a new set of reagents for each test, batchwise quality control traditionally employed in clinical laboratories is generally not applicable to MICT (22). Seven batches of seven individual cassettes were tested using independently prepared test solutions for each batch. These 49 tests results were used to determine intertest variation, and the values obtained from the seven batches were used to determine batch-to-batch variation. These values were generated using a serum sample with moderate reactivity from a pig with porcine cysticercosis for the T24-MICT evaluation and a polyclonal anti-rES33 hyperimmune goat serum with medium antibody titer for the ES33-MICT assessment.
Data analysis.
The MAR assay development system software yielded results expressed as relative magnetic units (RMU), based on the strength of the magnetic signal read by the system (12). For controlling variation and normalization of the data, a guinea pig IgG conjugate and goat anti-guinea pig IgG pair was used as the MICT internal control, and the ratio of the RMU of the test line against the RMU of the control line (called MAR value) was used for all the calculations.
Assay performance, diagnostic sensitivity, and diagnostic specificity for the ES33- and T24-MICT methods were calculated using the Youden J index as previously described (10). All analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC). Agreement between two readers was determined by calculation of the κ value (2).
RESULTS
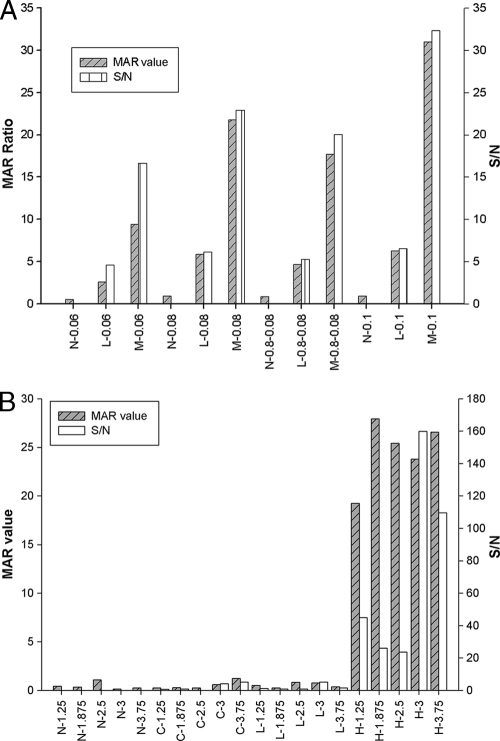
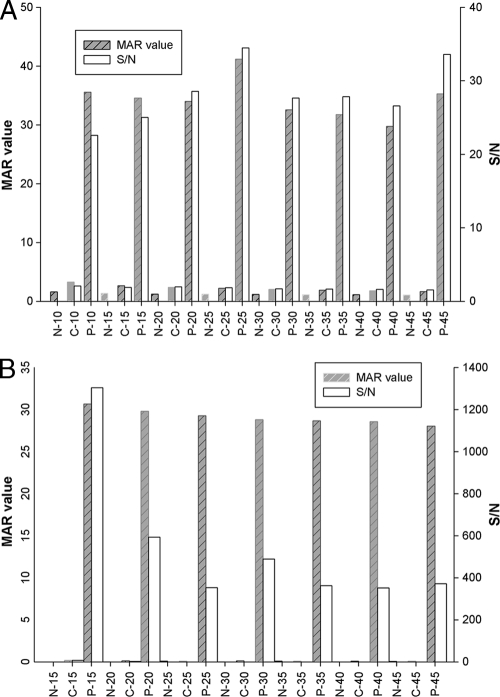
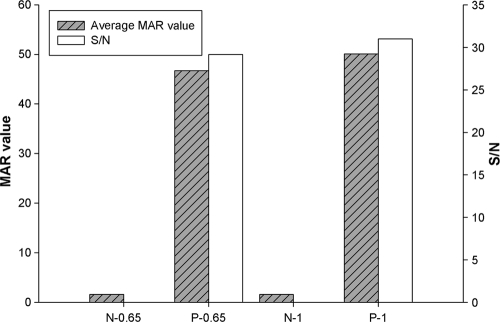
Fully assembled ES33- and T24-MICT devices are shown in Fig. 1. Several parameters were optimized before evaluation of assay performance, as follows: the concentration of protein antigens on the test line, the selection of a blocking agent, the conjugate volume, and reaction time. The concentration of the striped proteins tested in the ES33-MICT ranged from 60 to 100 ng/mm. For the T24-MICT, the protein concentrations evaluated ranged from 125 to 375 ng/mm. The optimum concentration of rES33 was 100 ng/mm, and that of rT24H was 300 ng/mm (Fig. 2). The reaction time was evaluated in 5-min increments, beginning at 10 min for the ES33-MICT assay and at 15 min for the T24 assay, through 45 min. The optimal reaction times for the ES33- and T24-MICTs were 25 min and 15 min, respectively (Fig. 3).
FIG. 2.
Effect of antigen concentration on reactivity in the ES33- and T24-MICTs. (A) Different concentrations of rES33 were tested to determine the optimum signal-to-noise ratio of the MAR value for the ES33-MICT. MAR value, the ratio of the RMU of test line divided by the RMU of control line; S/N, signal-to-noise ratio (the MAR value of the positive serum divided by the MAR value of the negative serum); N, negative serum; L, low-positive serum; M, medium-positive serum; 0.06, 1 mg/ml of rES33 applied at 0.06 μl/mm; 0.08, 1 mg/ml of rES33 applied at 0.08 μl/mm; 0.1, 1 mg/ml of rES33 applied at 0.1 μl/mm; 0.8-0.08, 0.8 mg/ml of rES33 applied at 0.08 μl/mm. (B) Different concentrations of rT24H were used to determine the optimum signal-to-noise ratio of the MAR value for the T24-MICT. 1.25, 2.5 mg/ml of rT24H applied at 0.05 μl/mm; 1.875, 2.5 mg/ml of rT24H applied at 0.075 μl/mm; 2.5, 2.5 mg/ml of rT24H applied at 0.1 μl/mm; 3, 2.5 mg/ml of rT24H applied at 1.25 μl/mm; 3.75, 2.5 mg/ml of rT24H applied at 1.5 μl/mm.
FIG. 3.
Reactivity versus reading time for ES33-MICT (A) and T24-MICT (B). Three serum samples, N (normal), C (cross-reactor), and P (positive), were added to five devices each for the ES33- and T24-MICTs. Reactivity was measured for each device in 5-min intervals. MAR value, the ratio of the RMU of test line divided by the RMU of control line; S/N, signal-to-noise ratio (the MAR value of the positive serum divided by the MAR value of the negative serum).
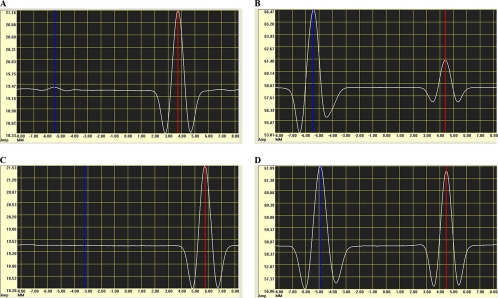
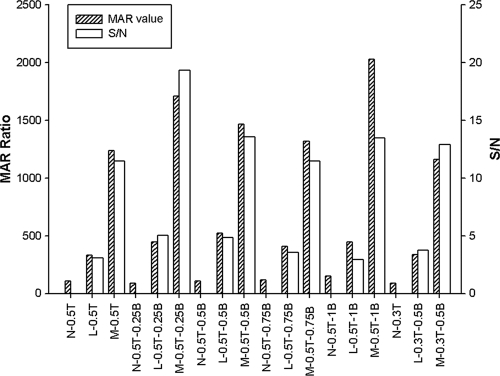
Although the striped concentration of rES33 was lower than that of rT24H, a detectable signal was found in negative samples in the ES33-MICT; this was not the case in the T24-MICT (Fig. 4). To decrease those nonspecific signals in the ES33-MICT, different chase buffers and lower conjugate concentrations/volumes were evaluated. Chase solutions consisting of 0.5% and 0.3% Tween 20 were tested, as well as concentrations of BSA between 0.25% and 1%; 0.1% NaN3 was added to prevent microbial growth in all chase buffers. The chase solution consisting of PBS plus 0.5% Tween 20 plus 0.25% BSA plus 0.1% NaN3 resulted in the best signal-to-noise ratio (Fig. 5). A reduced volume of conjugate was examined, 0.65 μl versus 1 μl, but this did not decrease the background signal of the normal sera (Fig. 6).
FIG. 4.
Graphic output generated by the magnetic assay reader. The blue line shows the reading of a test line, and the red line represents a control line. (A) Output from a negative taeniasis sample. (B) Output from a positive taeniasis sample. (C) Output from a cysticercosis negative sample. (D) Output from a cysticercosis positive sample.
FIG. 5.
Optimization of the chase buffers to reduce background signal in the ES33-MICT. MAR value, the ratio of the RMU of test line divided by the RMU of control line; S/N, signal-to-noise ratio (the MAR value of the positive serum divided by the MAR value of the negative serum); N, negative serum; L, low-positive serum; M, medium-positive serum; T, Tween 20; 0.5T, 0.5% Tween 20; 0.3T, 0.3% Tween 20; B, BSA; 0.25B, 0.25% BSA; 0.5B, 0.5% BSA; 0.75B, 0.75% BSA; 1B, 1% BSA.
FIG. 6.
Effect of conjugate concentration on reactivity in the ES33-MICT. N, negative; P, positive; MAR value, the ratio of the RMU of test line divided by the RMU of control line; S/N, signal-to-noise ratio (the MAR value of the positive serum divided by the MAR value of the negative serum); 0.65, 0.65 μl of rES33-magnetic particle conjugate at a concentration of 10 mg/ml; 1, 1 μl of rES33-magnetic particle conjugate at a concentration of 10 mg/ml.
Intra- and interassay variabilities were determined. For the ES33-MICT, the intra-assay variability was 8.7%, and the interassay variability was 15.8%. For the T24-MICT, the intra-assay and interassay variabilities were 13.1% and 12.3%, respectively.
The J index, which reflects the optimum diagnostic sensitivity and diagnostic specificity, was used to determine the cutoff value for each assay (Table 2). Alternative cutoff values were determined for each test that reflected greater sensitivity without dramatically reducing the specificity.
TABLE 2.
Assay performance of the ES33-MICT and T24-MICT
| MICT | Reading method and time | Conditiona | Optimum J index | Cutoff point | % Sensitivity (no.) | % Specificity (no.) |
|---|---|---|---|---|---|---|
| ES33 | Reader, time zero | Taeniasis | 0.91 | 1.10 | 95 (161) | 96 (278) |
| Reader, day 7 | Taeniasis | 0.87 | 0.23 | 93 (161) | 93 (278) | |
| Visual, day 7 | Taeniasis | 0.72 | NA | 73 (161) | 99 (278) | |
| T24 | Reader, time zero | NCC, ≥2 viable cysts | 0.92 | 0.40 | 93 (100) | 99 (281) |
| NCC, single viable cyst | 0.40 | 33 (15) | ||||
| Reader, day 7 | NCC, ≥2 viable cysts | 0.92 | 0.04 | 94 (100) | 99 (281) | |
| NCC, single viable cyst | 0.04 | 47 (15) | ||||
| Visual, day 7 | NCC, ≥2 viable cysts | 0.72 | NA | 72 (100) | 100 (281) | |
| NCC, single viable cyst | NA | 7 (15) |
NCC, neurocysticercosis.
To determine if immediate reading of the results was necessary, visual reading was conducted 7 days after running the assays. There was good agreement between the two readers, with a κ value of 0.96 for the ES33-MICT and 0.98 for the T24-MICT. The diagnostic sensitivity and diagnostic specificity of the visual reading were also evaluated (Table 2).
DISCUSSION
A rapid test for detecting taeniasis cases will provide a key tool needed for cysticercosis control programs. The MICT developed here represents the first report of a rapid test for taeniasis and establishes proof of principle that a rapid MICT using recombinant proteins for serodetection of taeniasis and cysticercosis is possible. The ES33-MICT performed with characteristics similar to those of the ES33 EITB (14) but required less time to perform (45 min versus 3.5 h) and little technical expertise. This would make the MICT suitable as a point-of-care test.
The ES33- and T24-MICTs, as developed, are quantitative and reproducible. Generating quantitative results allows the use of a cutoff value, thus improving sensitivity compared to that of visual reading. The best performance of the ES33-MICT was achieved with a cutoff point of the MAR ratio value at 1.1, which resulted in a sensitivity of 94.5% and a specificity of 96%. However, even better sensitivity is desirable for taeniasis screening. When the cutoff point was adjusted in this evaluation to increase sensitivity (to ∼97%), the specificity decreased (to 94%), as expected. However, this concomitant decrease in specificity may not be a problem, since treatment of taeniasis is both safe and inexpensive. Unfortunately, the sensitivity of the ES33-MICT could not be improved further without a drastic decrease in specificity.
One clear advantage of this method is the use of magnetic particles as the detection system in conjunction with the MAR system. The magnetic particle conjugate does not bleach across the time, as is the case with other reporters, such as gold and fluorescence (12), and the assay performances conducted seven days later were similar to those calculated from the data generated at the time of testing (Table 2).
Two disadvantages of the current version of ES33- or T24-MICTs were recognized. First is the requirement for a liquid conjugate. Drying the conjugate onto the pad will improve the usefulness of the test because it will reduce the number of steps needed to carry out the assay and possibly eliminate the need to maintain a cold chain. This is only a matter of process development and can be achieved by industrial manufacturers in proper manufacturing environments. Second, optimal sensitivity and specificity are dependent on the benchtop magnetic assay reader. This hinders the portability of the tests. Although it was possible to identify taeniasis cases based on visual observation, the sensitivity of the test decreased (Table 2). A possible solution is use of a hand-held reader—a prototype, which is already constructed by the proprietary company MagnaBioSciences but is not commercially available yet. If quantification is needed, as was the case in this study, after reading visually in the field, the devices can be brought back to a centralized station, where a benchtop reader is available. As demonstrated in this study, the time interval from testing to reading should not exceed 7 days.
We also developed a MICT to detect human cysticercosis antibodies; the performance of the T24-MICT was comparable to the performance of other methods using rT24H. Like other serological methods for detection of neurocysticercosis, such as the LLGP-EITB (the current standard assay of reference) (24), a cysticercosis antigen specific EITB (9, 19), and ELISAs (3), the T24-MICT has reduced sensitivity for detection of cases with a single viable cyst. Although this T24-MICT assay was evaluated using human sera, in principle, the test could be used for screening pig sera, as this method is species independent. Rapid assessment of human cysticercosis may not be needed in control programs, but pen-side testing for porcine cysticercosis may be useful to monitor control (6).
In conclusion, we have shown that rapid test methods using recombinant proteins are feasible for immunodetection of taeniasis and human cysticercosis. Extensions of this work should focus on improved sensitivity for taeniasis detection that will allow determination of all true-positive cases in the field. Additionally, adaptation of this method for the immunodetection of porcine cysticercosis is needed.
Acknowledgments
We appreciate the technical assistance and suggestions from Shon Workman and Timothy C. Granade from National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, CDC. We also appreciate the help of Paige Gupton and Nathalie Jones in preparing samples for optimization and testing.
This work was supported in part by the Bill & Melinda Gates Foundation grant 23981, the CDC Epilepsy Program, and the Fogarty International Center training grant D43 TW001140 (to H.H.G.).
Footnotes
Published ahead of print on 24 February 2010.
REFERENCES
- 1.Allan, J. C., P. P. Wilkins, V. C. Tsang, and P. S. Craig. 2003. Immunodiagnostic tools for taeniasis. Acta Trop. 87:87-93. [DOI] [PubMed] [Google Scholar]
- 2.Altman, D. G. 1993. Practical statistics for medical research. Chapman & Hall, London, United Kingdom.
- 3.Bueno, E. C., C. M. Scheel, A. J. Vaz, L. R. Machado, J. A. Livramento, O. M. Takayanagui, V. C. Tsang, and K. Hancock. 2005. Application of synthetic 8-kD and recombinant GP50 antigens in the diagnosis of neurocysticercosis by enzyme-linked immunosorbent assay. Am. J. Trop. Med. Hyg. 72:278-283. [PubMed] [Google Scholar]
- 4.Del Brutto, O. H., V. Rajshekhar, A. C. White, Jr., V. C. Tsang, T. E. Nash, O. M. Takayanagui, P. M. Schantz, C. A. Evans, A. Flisser, D. Correa, D. Botero, J. C. Allan, E. Sarti, A. E. Gonzalez, R. H. Gilman, and H. H. Garcia. 2001. Proposed diagnostic criteria for neurocysticercosis. Neurology 57:177-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Girosi, F., S. S. Olmsted, E. Keeler, D. C. Hay Burgess, Y. W. Lim, J. E. Aledort, M. E. Rafael, K. A. Ricci, R. Boer, L. Hilborne, K. P. Derose, M. V. Shea, C. M. Beighley, C. A. Dahl, and J. Wasserman. 2006. Developing and interpreting models to improve diagnostics in developing countries. Nature 444(Suppl. 1):3-8. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez, A. E., R. Gilman, H. H. Garcia, J. McDonald, K. Kacena, V. C. Tsang, J. B. Pilcher, F. Suarez, C. Gavidia, and E. Miranda. 1994. Use of sentinel pigs to monitor environmental Taenia solium contamination. The Cysticercosis Working Group in Peru (CWG). Am. J. Trop. Med. Hyg. 51:847-850. [DOI] [PubMed] [Google Scholar]
- 7.Hancock, K., A. Khan, F. B. Williams, M. L. Yushak, S. Pattabhi, J. Noh, and V. C. Tsang. 2003. Characterization of the 8-kilodalton antigens of Taenia solium metacestodes and evaluation of their use in an enzyme-linked immunosorbent assay for serodiagnosis. J. Clin. Microbiol. 41:2577-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hancock, K., S. Pattabhi, R. M. Greene, M. L. Yushak, F. Williams, A. Khan, J. W. Priest, M. Z. Levine, and V. C. Tsang. 2004. Characterization and cloning of GP50, a Taenia solium antigen diagnostic for cysticercosis. Mol. Biochem. Parasitol. 133:115-124. [DOI] [PubMed] [Google Scholar]
- 9.Hancock, K., S. Pattabhi, F. W. Whitfield, M. L. Yushak, W. S. Lane, H. H. Garcia, A. E. Gonzalez, R. H. Gilman, and V. C. Tsang. 2006. Characterization and cloning of T24, a Taenia solium antigen diagnostic for cysticercosis. Mol. Biochem. Parasitol. 147:109-117. [DOI] [PubMed] [Google Scholar]
- 10.Handali, S., A. E. Gonzalez, K. Hancock, H. H. Garcia, J. M. Roberts, R. H. Gilman, and V. C. Tsang. 2004. Porcine antibody responses to taenia solium antigens rGp50 and sTs18var1. Am. J. Trop. Med. Hyg. 71:322-326. [PubMed] [Google Scholar]
- 11.Handali, S., M. Klarman, A. N. Gaspard, J. Noh, Y. M. Lee, S. Rodriguez, A. E. Gonzalez, H. H. Garcia, R. H. Gilman, V. C. Tsang, and P. P. Wilkins. 2010. Multiantigen print immunoassay for comparison of diagnostic antigens for Taenia solium cysticercosis and taeniasis. Clin. Vaccine Immunol. 17:68-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LaBorde, R. T., and B. O'Farrell. 2002. Paramagnetic-particle detection in lateral-flow assays. IVD Technol. 8:36-41. [Google Scholar]
- 13.Levine, M. Z., J. C. Calderon, P. P. Wilkins, W. S. Lane, J. M. Asara, K. Hancock, A. E. Gonzalez, H. H. Garcia, R. H. Gilman, and V. C. Tsang. 2004. Characterization, cloning, and expression of two diagnostic antigens for Taenia solium tapeworm infection. J. Parasitol. 90:631-638. [DOI] [PubMed] [Google Scholar]
- 14.Levine, M. Z., M. M. Lewis, S. Rodriquez, J. A. Jimenez, A. Khan, S. Lin, H. H. Garcia, A. E. Gonzales, R. H. Gilman, and V. C. Tsang. 2007. Development of an enzyme-linked immunoelectrotransfer blot (EITB) assay using two baculovirus expressed recombinant antigens for diagnosis of Taenia solium taeniasis. J. Parasitol. 93:409-417. [DOI] [PubMed] [Google Scholar]
- 15.Mayta, H., A. Talley, R. H. Gilman, J. Jimenez, M. Verastegui, M. Ruiz, H. H. Garcia, and A. E. Gonzalez. 2000. Differentiating Taenia solium and Taenia saginata infections by simple hematoxylin-eosin staining and PCR-restriction enzyme analysis. J. Clin. Microbiol. 38:133-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Farrell, B., and J. Bauer. 2006. Developing highly sensitive, more-reproducible lateral-flow assays. IVD Technol. 12:41-49. [Google Scholar]
- 17.Peck, R. B., J. Schweizer, B. H. Weigl, C. Somoza, J. Silver, J. W. Sellors, and P. S. Lu. 2006. A magnetic immunochromatographic strip test for detection of human papillomavirus 16 E6. Clin. Chem. 52:2170-2172. [DOI] [PubMed] [Google Scholar]
- 18.Schantz, P. M., M. Cruz, E. Sarti, and Z. Pawlowski. 1993. Potential eradicability of taeniasis and cysticercosis. Bull. Pan Am. Health Organ. 27:397-403. [PubMed] [Google Scholar]
- 19.Scheel, C. M., A. Khan, K. Hancock, H. H. Garcia, A. E. Gonzalez, R. H. Gilman, and V. C. Tsang. 2005. Serodiagnosis of neurocysticercosis using synthetic 8-kD proteins: comparison of assay formats. Am. J. Trop. Med. Hyg. 73:771-776. [PubMed] [Google Scholar]
- 20.Tsang, V. C., J. A. Brand, and A. E. Boyer. 1989. An enzyme-linked immunoelectrotransfer blot assay and glycoprotein antigens for diagnosing human cysticercosis (Taenia solium). J. Infect. Dis. 159:50-59. [DOI] [PubMed] [Google Scholar]
- 21.Urdea, M., L. A. Penny, S. S. Olmsted, M. Y. Giovanni, P. Kaspar, A. Shepherd, P. Wilson, C. A. Dahl, S. Buchsbaum, G. Moeller, and D. C. Hay Burgess. 2006. Requirements for high impact diagnostics in the developing world. Nature 444(Suppl. 1):73-79. [DOI] [PubMed] [Google Scholar]
- 22.von Lode, P. 2005. Point-of-care immunotesting: approaching the analytical performance of central laboratory methods. Clin. Biochem. 38:591-606. [DOI] [PubMed] [Google Scholar]
- 23.Wilkins, P. P., J. C. Allan, M. Verastegui, M. Acosta, A. G. Eason, H. H. Garcia, A. E. Gonzalez, R. H. Gilman, and V. C. Tsang. 1999. Development of a serologic assay to detect Taenia solium taeniasis. Am. J. Trop. Med. Hyg. 60:199-204. [DOI] [PubMed] [Google Scholar]
- 24.Wilson, M., R. T. Bryan, J. A. Fried, D. A. Ware, P. M. Schantz, J. B. Pilcher, and V. C. Tsang. 1991. Clinical evaluation of the cysticercosis enzyme-linked immunoelectrotransfer blot in patients with neurocysticercosis. J. Infect. Dis. 164:1007-1009. [DOI] [PubMed] [Google Scholar]
- 25.Workman, S., S. K. Wells, C. P. Pau, S. M. Owen, X. F. Dong, R. LaBorde, and T. C. Granade. 2009. Rapid detection of HIV-1 p24 antigen using magnetic immuno-chromatography (MICT). J. Virol. Methods 160:14-21. [DOI] [PubMed] [Google Scholar]