Abstract
The aim of this study was to analyze and compare the humoral immune responses in serum and cerebrospinal fluid (CSF) for 34 adult patients with clinically evident Lyme neuroborreliosis, 27 patients with clinically suspected Lyme neuroborreliosis, and 32 patients with tick-borne encephalitis. Additionally, we wanted to compare the findings of two methods for the detection of intrathecally synthesized borrelial antibodies: the IDEIA Lyme neuroborreliosis test using flagellar antigen and an approach based on the Liaison indirect chemiluminescence immunoassay using the OspC and VlsE antigens. Borrelial IgM and IgG antibodies were detected by at least one of these methods in the sera of 22/34 (64.7%) and 28/34 (82.4%) patients with clinically evident Lyme neuroborreliosis, respectively, and in the cerebrospinal fluid of 22/34 (64.7%) and 20/34 (58.8%) of these patients, respectively. Intrathecal synthesis of borrelial IgM and/or IgG was found in 19/34 (55.9%) patients: IgM in 17/34 (50%) patients and IgG in 15/34 (44.1%) patients. The relatively low proportion of intrathecal synthesis of borrelial antibodies and the high ratio of IgM positivity could be explained by the short duration of neurological disease as evidenced by reported symptoms (median, 10 days). Assessment of the humoral immune response in the sera and CSF of patients with early Lyme neuroborreliosis confirmed previous findings on the relationship between the duration of illness and the proportion of patients with detectable responses.
Lyme borreliosis is a multisystemic disease caused by the tick-transmitted spirochete Borrelia burgdorferi sensu lato. In the course of the disease, many different organs and organ systems may be affected, including the nervous system (Lyme neuroborreliosis) (20). In Europe, Borrelia garinii is the main cause of Lyme neuroborreliosis, whereas Borrelia afzelii is mostly associated with skin manifestations (17, 20).
Diagnosis of Lyme neuroborreliosis is usually based on isolation of B. burgdorferi sensu lato from cerebrospinal fluid (CSF), demonstration of borrelial DNA in CSF samples, and/or detection of specific borrelial antibodies (seroconversion and/or intrathecal production). Isolation of the etiological agent from CSF still represents the gold standard, although the method is demanding, time-consuming, and of low sensitivity (1, 4, 20). Detection of intrathecal synthesis of specific antibodies, a conventional diagnostic marker of Lyme neuroborreliosis (3), is convenient for routine laboratory work but has limitations in that the antibodies may be absent during the first few weeks (10), and a positive test result does not distinguish between acute infection and past infection (8).
The aim of this study was to assess the humoral immune responses in the sera and CSF of patients with Lyme neuroborreliosis and to compare the findings of two methods for the detection of intrathecally synthesized borrelial antibodies. We expected (i) that the proportions of patients with borrelial antibodies in serum would be similar in cases of clinically evident and clinically suspected Lyme neuroborreliosis and would be higher than those for patients with tick-borne encephalitis (TBE); (ii) that patients with clinically evident Lyme neuroborreliosis would have borrelial antibodies in CSF more often than those with suspected Lyme neuroborreliosis, and that these antibodies would be found only exceptionally in the CSF of patients with TBE; and (iii) that intrathecal borrelial antibody production would be limited to patients with clinically evident Lyme neuroborreliosis, with potential rare exceptions for patients with suspected Lyme neuroborreliosis.
MATERIALS AND METHODS
Patient groups.
Patients with a clinical diagnosis of Lyme neuroborreliosis comprised 34 adults (19 men and 15 women; ages, 18 to 77 years [median, 56 years]) with a working clinical diagnosis of evident Lyme neuroborreliosis (erythema migrans within 4 months before the appearance of neurological symptoms and/or signs, including radiculoneuritic pain and/or peripheral facial palsy, and pleocytosis) and 27 patients (10 men and 17 women; ages, 28 to 70 years [median, 52 years]) with a working clinical diagnosis of suspected Lyme neuroborreliosis (erythema migrans within 4 months before the appearance of neurological symptoms and/or signs, but no pleocytosis). At the time of inclusion in the study (at initial examination), the median duration of neurological signs/symptoms was 10 (range, 2 to 90) days for patients with clinically evident Lyme neuroborreliosis and 21 (range, 2 to 90) days for patients with suspected Lyme neuroborreliosis (P = 0.1560). Erythema migrans was still present in 34/61 (55.7%) patients, comprising 9/34 (26.5%) patients with a working diagnosis of evident Lyme neuroborreliosis and 25/27 (92.6%) patients with suspected Lyme neuroborreliosis (P < 0.0001).
The control group comprised 32 adult patients with TBE. Patients with TBE (20 men and 12 women; ages, 19 to 78 years [median, 54 years]) had clinical signs/symptoms of meningoencephalitis, CSF pleocytosis, and serological confirmation of TBE virus infection demonstrated by the presence of specific serum IgM and IgG antibodies. At the time of inclusion in the study (time of initial examination), the median duration of the signs/symptoms was 14 (range, 2 to 25) days. We chose patients with TBE as a control group because of the accessibility of simultaneously obtained serum and CSF samples, and because no cross-reactivity in serological assays between borrelia and TBE virus infection has been described.
Patients presented at the Department of Infectious Diseases, University Medical Center Ljubljana, in the years 2006 to 2008. None of the persons included in the study reported recent treatment with antibiotics, and none had received a Lyme vaccine.
The study approach was approved by the Medical Ethics Committee of the Ministry of Health of the Republic of Slovenia (no. 35/08/06).
Methods.
Blood and CSF samples were taken from all the patients simultaneously. The second-generation Lyme Borreliosis IDEIA kit (DakoCytomation Denmark A/S, Glostrup, Denmark), in which purified native B. burgdorferi flagellin protein serves as the antigen, was used for the detection of specific antibodies in serum and CSF. The results of the enzyme immunoassay (EIA) are expressed as optical densities (OD) and were interpreted according to the manufacturer's instructions.
The samples were also tested with an indirect chemiluminescence immunoassay, the Liaison Borrelia IgM and IgG quantitative test (Diasorin, Saluggia, Italy). The magnetic particles used in the Borrelia Liaison test are coated with recombinant antigens: OspC of B. afzelii strain PKo for IgM antibody detection and VlsE of B. garinii strain PBi for detection of both IgM and IgG antibodies. The Liaison test is completely automated. Results are expressed in arbitrary units (AU) and are graded for serum antibodies (IgM and IgG, respectively) as negative (<18 and <10 AU/ml), equivocal (18 to 22 AU/ml and 10 to 15 AU/ml), or positive (>22 and >15 AU/ml). The grades for antibodies in CSF (IgM and IgG, respectively) are negative (<2.5 and <4.5 AU/ml), equivocal (2.5 to 3.5 AU/ml and 4.5 to 5.5 AU/ml), and positive (>3.5 and >5.5 AU/ml).
For the assessment of intrathecal synthesis of borrelial antibodies on the basis of Liaison test results, we used the approach described by Reiber and Peter (15). Briefly, we calculated CSF/serum concentration quotients for albumin (Qalb), total IgM (Qtotal IgM), total IgG (Qtotal IgG), specific borrelial IgM antibodies (QspecIgM), and specific borrelial IgG (QspecIgG). The antibody index was calculated as the ratio of the CSF/serum quotient of specific antibodies (Qspec) to the corresponding CSF/serum quotient of total immunoglobulins (Qtotal IgM, Qtotal IgG) (7). Antibody index values of >1.4 were interpreted as indicating intrathecal production of borrelial antibodies (14).
Intrathecal synthesis was also determined using the IDEIA Lyme Neuroborreliosis kit (DakoCytomation, Cambridgeshire, United Kingdom) with B. afzelii flagellar antigen. We followed the manufacturer's instructions for test performance and interpretation of the results. Accordingly, test results are expressed as an index where a value of ≥0.3 indicates intrathecal synthesis of specific borrelial antibodies.
Statistical analysis.
The difference in the proportion of positive tests between the two assays was analyzed using the two-tailed McNemar exact test or Fisher's exact test. P values of <0.05 were interpreted as statistically significant. For statistical analysis, borderline results were combined with positive results. Quantitative data were analyzed using the Mann-Whitney test. The statistical program SPSS Statistics, version 17.0, was used for the analyses.
RESULTS
Borrelial antibodies in serum and CSF.
Borrelial IgM and IgG antibodies were detected by at least one of the two assays in the sera of 22/34 (64.7%) and 28/34 (82.4%) patients with clinically evident Lyme neuroborreliosis, respectively. The corresponding results for antibodies in CSF were 22/34 (64.7%) and 20/34 (58.8%) (Tables 1 and 2). Antibodies were demonstrated more frequently with the Liaison test than with the IDEIA kit; nevertheless, the difference between the tests was significant only for the detection of IgM (21/34 versus 7/34; P < 0.0001) and IgG (20/34 versus 14/34; P = 0.0313) antibodies in CSF, not in serum (Tables 1 and 2). Among the patients with suspected Lyme neuroborreliosis, we detected borrelial IgM and IgG antibodies in the sera of 15/27 (55.6%) and 16/27 (59.3%) individuals, respectively, by at least one of the two methods. The corresponding results for CSF antibodies were 0/27 (0%) and 4/27 (14.8%) (Tables 1 and 2). Although the Liaison test seemed to be more sensitive than the IDEIA kit, a statistically significant difference was found only for the detection of serum IgM antibodies (12/27 versus 5/27; P = 0.0200) (Tables 1 and 2).
TABLE 1.
Comparison of the enzyme immunoassay and Liaison test systems for detection of borrelial IgM and IgG in the sera of patients with different clinical diagnoses
| Group and LIA resulta | No. (%) with the indicated EIA result |
|||||||
|---|---|---|---|---|---|---|---|---|
| IgM |
IgG |
|||||||
| Pos | Bord | Neg | All | Pos | Bord | Neg | All | |
| LNB | ||||||||
| Pos | 13 (38.2) | 1 (2.9) | 5 (14.7) | 19 (55.9) | 22 (64.7) | 0 | 3 (8.8) | 25 (73.5) |
| Bord | 1 (2.9) | 0 | 1 (2.9) | 2 (5.9) | 0 | 1 (2.9) | 1 (2.9) | 2 (5.8) |
| Neg | 0 | 1 (2.9) | 12 (35.3) | 13 (38.2) | 0 | 1 (2.9) | 6 (17.4) | 7 (20.6) |
| All | 14 (41.2) | 2 (5.9) | 18 (52.9) | 34 (100) | 22 (64.7) | 2 (5.9) | 10 (29.4) | 34 (100) |
| sLNB | ||||||||
| Pos | 4 (14.8) | 1 (3.7) | 7 (25.9) | 12 (44.4) | 11 (40.7) | 0 | 4 (14.8) | 15 (55.6) |
| Bord | 0 | 0 | 2 (7.4) | 2 (7.4) | 0 | 0 | 1 (3.7) | 1 (3.7) |
| Neg | 1 (3.7) | 0 | 12 (44.4) | 13 (48.1) | 0 | 0 | 11 (40.7) | 11 (40.7) |
| All | 5 (18.5) | 1 (3.7) | 21 (77.8) | 27 (100) | 11 (40.7) | 0 | 16 (59.3) | 27 (100) |
| TBE | ||||||||
| Pos | 0 | 0 | 4 (12.5) | 4 (12.5) | 12 (37.5) | 2 (6.3) | 1 (3.1) | 15 (46.9) |
| Bord | 0 | 0 | 2 (6.3) | 2 (6.3) | 0 | 0 | 1 (3.1) | 1 (3.1) |
| Neg | 0 | 1 (3.1) | 25 (78.1) | 26 (81.2) | 2 (6.3) | 0 | 14 (43.8) | 16 (50.0) |
| All | 0 | 1 (3.1) | 31 (96.9) | 32 (100) | 14 (43.8) | 2 (6.3) | 16 (50.0) | 32 (100) |
LIA, Liaison test; LNB, Lyme neuroborreliosis; sLNB, suspected Lyme neuroborreliosis; TBE, tick-borne encephalitis; Pos, positive; Bord, borderline; Neg, negative.
TABLE 2.
Comparison of the enzyme immunoassay and Liaison test systems for detection of borrelial IgM and IgG in the CSF of patients with different clinical diagnoses
| Group and LIA resulta | No. (%) with the indicated EIA result |
|||||||
|---|---|---|---|---|---|---|---|---|
| IgM |
IgG |
|||||||
| Pos | Bord | Neg | All | Pos | Bord | Neg | All | |
| LNB | ||||||||
| Pos | 7 (20.6) | 0 | 14 (41.2) | 21 (61.8) | 14 (41.2) | 0 | 6 (17.6) | 20 (58.8) |
| Bord | 0 | 0 | 1 (2.9) | 1 (2.9) | 0 | 0 | 0 | 0 |
| Neg | 0 | 0 | 12 (35.3) | 12 (35.3) | 0 | 0 | 14 (41.2) | 14 (41.2) |
| All | 7 (20.6) | 0 | 27 (79.4) | 34 (100) | 14 (41.2) | 0 | 20 (58.8) | 34 (100) |
| sLNB | ||||||||
| Pos | 0 | 0 | 0 | 0 | 0 | 0 | 4 (14.8) | 4 (14.8) |
| Bord | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Neg | 0 | 0 | 27 (100) | 27 (100) | 0 | 0 | 23 (85.2) | 23 (85.2) |
| All | 0 | 0 | 27 (100) | 27 (100) | 0 | 0 | 27 (100) | 27 (100) |
| TBE | ||||||||
| Pos | 0 | 0 | 4 (12.5) | 4 (12.5) | 1 (3.1) | 0 | 7 (21.9) | 8 (25) |
| Bord | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Neg | 0 | 0 | 28 (87.5) | 28 (87.5) | 0 | 0 | 24 (75) | 24 (75) |
| All | 0 | 0 | 32 (100) | 32 (100) | 1 (3.1) | 0 | 31 (96.9) | 32 (100) |
LIA, Liaison test; LNB, Lyme neuroborreliosis; sLNB, suspected Lyme neuroborreliosis; TBE, tick-borne encephalitis; Pos, positive; Bord, borderline; Neg, negative.
Among the patients with TBE, borrelial IgM and IgG antibodies were demonstrated in the sera of 7/32 (21.9%) and 18/32 (56.3%) individuals, respectively, by at least one of the two methods. The corresponding results for antibodies in CSF were 4/32 (12.5%) and 8/32 (25%) (Tables 1 and 2). Also in this group, the Liaison test detected specific antibodies more often than the IDEIA kit, although a significant difference between the assays was found only for IgG antibodies in CSF (8/32 versus 1/32; P = 0.0160) (Tables 1 and 2).
The results of the two tests for the detection of IgM and IgG antibodies were in complete agreement in 140/186 (75.3%) and 153/186 (82.3%) samples, respectively.
Intrathecal synthesis of borrelial antibodies.
Intrathecal synthesis of borrelial antibodies was detected in 27/93 (29.0%) patients, comprising 19/34 (55.9%) patients with clinically evident Lyme neuroborreliosis, 2/27 (7.4%) patients with suspected Lyme neuroborreliosis, and 6/32 (18.8%) patients with TBE.
Among all 27 patients with intrathecal production of borrelial antibodies, both IgM and IgG synthesis was found in 16 (59.3%); synthesis of IgM alone was found in 5 (17.9%); and synthesis of IgG alone was found in 6 (21.4%). The corresponding results for 19 patients with clinically evident Lyme neuroborreliosis and intrathecal production of borrelial antibodies were 15 (78.9%), 2 (10.5%), and 2 (10.5%).
In 9/21 patients with intrathecal production of borrelial IgM antibodies and in 1/22 patients with intrathecal synthesis of borrelial IgG antibodies, the corresponding specific antibodies were not demonstrated in serum.
With the IDEIA kit, intrathecal synthesis of borrelial IgM and IgG antibodies was demonstrated in 11/34 (32.4%) and 13/34 (38.2%) patients with clinically evident Lyme neuroborreliosis, in 0/26 and 1/26 (3.8%) patients with suspected Lyme neuroborreliosis, and in 1/32 (3.1%) and 2/32 (6.3%) patients with TBE, respectively (Table 3). By use of the results from the Liaison test, calculated as described above, intrathecal synthesis of borrelial IgM and IgG antibodies was detected in 17/34 (50.0%) and 16/34 (47.0%) patients, respectively, with clinically evident Lyme neuroborreliosis, in 0/26 and 2/26 (7.7%) patients with suspected Lyme neuroborreliosis, and in 4/32 (12.5%) and 3/32 (9.4%) patients with TBE (Table 3). Although calculations of intrathecal antibody production on the basis of serum and CSF antibodies determined using the Liaison test gave positive results more often than the corresponding findings from the IDEIA kit, a statistically significant difference between the two tests was found only for intrathecal synthesis of IgM antibodies in patients with clinically evident Lyme neuroborreliosis (17/34 versus 11/34; P = 0.0315).
TABLE 3.
Comparison of intrathecal synthesis of borrelial antibodies determined by two distinct methods in patients with different clinical diagnoses
| Group and result by the Liaison testa | No. (%) with the indicated result by the Dako neuroborreliosis test |
|||||
|---|---|---|---|---|---|---|
| IgM |
IgG |
|||||
| Pos | Neg | All | Pos | Neg | All | |
| LNB | ||||||
| Pos | 11 (32.4) | 6 (17.6) | 17 (50) | 12 (35.3) | 4 (11.8) | 16 (47.1) |
| Neg | 0 | 17 (50) | 17 (50) | 1 (2.9) | 17 (50) | 18 (52.9) |
| All | 11 (32.4) | 23 (67.6) | 34 (100) | 13 (38.2) | 21 (61.8) | 34 (100) |
| sLNB | ||||||
| Pos | 0 | 0 | 0 | 1 (3.7) | 1 (3.7) | 2 (7.4) |
| Neg | 0 | 27 (100) | 27 (100) | 0 | 25 (92.6) | 25 (92.6) |
| All | 0 | 27 (100) | 27 (100) | 1 (3.7) | 26 (96.3) | 27 (100) |
| TBE | ||||||
| Pos | 1 (3.1) | 3 (9.4) | 4 (12.5) | 2 (6.2) | 1 (3.1) | 3 (9.3) |
| Neg | 0 | 28 (87.5) | 28 (87.5) | 0 | 29 (90.6) | 29 (90.6) |
| All | 1 (3.1) | 31 (96.9) | 32 (100) | 2 (6.2) | 30 (93.7) | 32 (100) |
The results of the Liaison test were calculated according to the method of Reiber and Lange (14). LNB, Lyme neuroborreliosis; sLNB, suspected Lyme neuroborreliosis; TBE, tick-borne encephalitis; Pos, positive; Neg, negative.
The levels of agreement of the results of the two tests for intrathecal antibody production were 83/93 (89.2%) for IgM and 86/93 (92.5%) for IgG. Of 27 specimens positive for synthesis of intrathecal borrelial IgM and/or IgG antibodies, 16 (59.3%) were positive by both tests, 11 (39.3%) were positive by the Liaison test only, and none were positive solely by the IDEIA kit. The corresponding findings for intrathecal synthesis of specific IgM antibodies were 12 (54.5%), 9 (45.5%), and 0, and those for intrathecal synthesis of specific borrelial IgG antibodies were 15 (68.2%), 6 (27.3%), and 0.
Among patients with clinically evident Lyme neuroborreliosis, the frequency of detection of intrathecal synthesis of borrelial IgM and/or IgG antibodies by at least one of the two tests or by an individual test was significantly higher than that for patients with suspected Lyme neuroborreliosis (19/34 versus 2/27 patients had at least one positive test result by the IDEIA kit and/or the Liaison test [P = <0.0001]; 14/34 versus 1/27 patients were positive by the IDEIA kit [P = 0.0007]; and 18/34 versus 2/27 patients were positive by the Liaison test [P = 0.0002]). The frequency of detection of intrathecal synthesis of borrelial antibodies for patients with clinically evident Lyme neuroborreliosis was also significantly higher than that for patients with TBE (19/34 versus 7/32 patients had at least one positive test result [P = 0.0060]; 14/34 versus 3/32 patients were positive by the IDEIA kit [P = 0.0044]; and 18/34 versus 6/32 patients were positive by the Liaison test [P = 0.0252]).
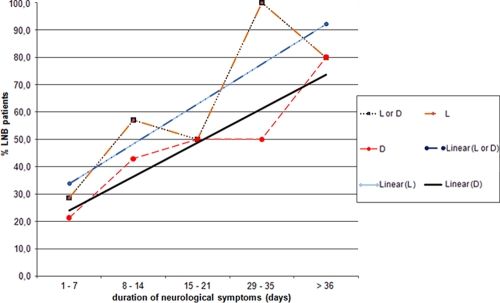
Figure 1 shows the relation between the duration of neurological symptoms and the detection of intrathecal synthesis of specific borrelial antibodies in patients with clinically evident Lyme neuroborreliosis. As the duration of neurological symptoms increases, the proportion of patients with detectable intrathecal synthesis of antibodies also increases.
FIG. 1.
Relation between duration of neurological symptoms and intrathecal synthesis as determined by a particular test in patients with clinically evident Lyme neuroborreliosis (LNB). L, positive intrathecal synthesis based on Liaison test results and calculated according to the method of Reiber and Lange (14); D, positive intrathecal synthesis determined by the Dako IDEIA Lyme Neuroborreliosis kit; L or D, positive intrathecal synthesis determined with at least one of the two tests; Linear, linear regression.
DISCUSSION
The immune response to infection with a microorganism depends on several factors, such as the quantity and characteristics of the infectious agent, the duration of the infection (disease), and the ability of the host to respond to the infection. It is well known that in borrelial infection the antibody immune response is slow (IgM antibodies usually appear 3 to 6 weeks after the beginning of infection, and IgG antibodies usually appear 6 weeks to 3 months after onset) (6) and that seropositivity increases with the duration of the disease (23). This is also true for early Lyme neuroborreliosis, which typically appears up to several weeks after infection, and thus, this pattern of immune response pertains to the patients included in the present study. However, demonstration of the immune response depends not only on the interaction between the microorganism and the host but also on the method used for detection of the response. Methods for routine antibody detection, such as immunofluorescence assays, enzyme-linked immunosorbent assays (ELISA), chemiluminescence immunoassays, and immunoblotting, differ in their abilities to detect borrelial antibodies and also in the manner in which the results are presented.
The aim of the present study was to analyze and compare the humoral immune responses in the serum and CSF samples of patients with Lyme neuroborreliosis, with special attention to intrathecal synthesis of borrelial antibodies. For that purpose, we chose two different serological tests and included three distinct, clinically well defined groups of patients: those with clinically evident Lyme neuroborreliosis, those with clinically suspected Lyme neuroborreliosis, and those with TBE.
With the IDEIA kit (flagellin antigen) and the Liaison test (OspC and VlsE antigens for IgM, VlsE for IgG), the IgM seropositivities of patients with clinically evident Lyme neuroborreliosis were 41.2% and 55.9%, respectively, and their IgG seropositivities were 64.7% and 73.5% (Table 1). These findings are mostly in line with other reports on the presence of borrelial antibodies in the sera of patients with Lyme neuroborreliosis, as determined by assays using the internal fragment of p41 as the antigen (IgM antibodies were detected in 49 to 67.9% of patients with Lyme neuroborreliosis and IgG in 34 to 76.6%) (1, 9) and by tests based on OspC as the antigen (47 to 53% seropositivity for IgM antibodies) (1, 9), but positivity was less than that in assays utilizing the VlsE antigen (73% for IgM and 100% for IgG) (1, 11, 21).
In our study, IgM antibodies were detected in the CSF samples of 20.6% and 61.8% of patients with clinically evident Lyme neuroborreliosis by the IDEIA kit and the Liaison test, respectively; the corresponding results for IgG antibodies were 41.2% and 58.8% (Table 2). Previous studies have reported sensitivities of 61% and 75% with the VlsE C6 ELISA and the VIDAS ELISA (determination of IgM and IgG) (16, 22).
We detected IgM and IgG antibodies in sera and CSF more frequently with the Liaison test than with the IDEIA kit, but the difference between the two tests was significant only for the detection of IgM and IgG antibodies in the CSF of patients with clinically evident Lyme neuroborreliosis and for the detection of borrelial IgG antibodies in the CSF of patients with TBE (Tables 1 and 2). The differences between the two assays in the detection of specific antibodies could be a consequence of methodological distinctions, including dissimilarities of test antigens, and/or peculiarities in the interpretation of the test results. Differences could also have been due to different sensitivities and/or distinct specificities of the two tests. Flagellin protein (IDEIA) is a highly immunogenic antigen and elicits an early and strong immune response but is prone to cross-reactivity (13). In the Liaison test, OspC is one of the most immunodominant antigens in the IgM immune response, whereas the VlsE protein elicits a strong IgG response (1, 18).
The intrathecal synthesis of specific antibodies is a well-recognized diagnostic marker for Lyme neuroborreliosis and is an essential criterion for the diagnosis of Lyme neuroborreliosis in Europe (3, 19, 20). In the present study, two distinct methods were used for determination of the intrathecal synthesis of borrelial antibodies. Synthesis of borrelial IgM or IgG was detected by at least one of the methods in 19/34 (55.9%) patients with clinically evident Lyme neuroborreliosis, fewer than we had expected. Previous studies have reported higher positivity rates: the sensitivity of the Lyme Neuroborreliosis IDEIA kit was reported to be 79% (12), and the sensitivity of an ELISA using a whole-cell antigen of B. afzelii PKo was reported to be 75 to 80% (2, 16). We were unable to find corresponding information for the Liaison test (OspC and VlsE for IgM antibody detection; VlsE for IgG detection). The rather short duration of neurologic signs/symptoms in the patients studied could explain the relatively low proportion of those with intrathecal production of borrelial antibodies and could possibly explain the high ratio of specific IgM synthesis. Nevertheless, our study corroborated previous findings that the proportion of patients with established intrathecal synthesis of borrelial antibodies increases with the duration of neurological symptoms (Fig. 1). The levels of agreement of results of the two tests for intrathecal antibody detection were 83/93 (89.2%) for IgM and 86/93 (92.5%) for IgG. Among 27 specimens positive for intrathecal synthesis of borrelial IgM and/or IgG antibody, 17 (60.7%) were positive by both tests, 10 (37.0%) were positive with the Liaison test alone, and none were positive solely with the IDEIA. These findings could be due to the better specificity of the IDEIA approach or the higher sensitivity of the Liaison test.
In this study, we demonstrated a specific borrelial humoral immune response in the majority of patients with Lyme neuroborreliosis and also in several patients with TBE, who had been chosen to represent a control group. The latter finding suggests limited specificity. However, as indicated by reports on cases of concomitant Lyme borreliosis and TBE (5) and by the demonstration of borrelial genetic material in the CSF of several patients with TBE (4), patients with tick-transmitted illnesses, such as TBE, may not be an ideal control group. It was not unexpected that intrathecal synthesis of borrelial antibodies was detected in patients with clinically evident Lyme neuroborreliosis and in 2/27 (7.4%) patients with suspected Lyme neuroborreliosis. These two patients each had a positive IgG index, established for one by both tests and for the other only by the Liaison test (Table 3); in both patients, the duration of neurological symptoms was more than 5 weeks. However, the demonstration of intrathecal synthesis of specific borrelial antibodies in as many as 6/32 (18.8%) patients with TBE was rather unexpected. In 3 of these 6 patients who fulfilled criteria for TBE, only intrathecal borrelial IgM antibody synthesis was demonstrated, and for 2 of these 3, the presence of specific IgM antibodies in serum was not ascertained. In 2/6 patients, only intrathecal borrelial IgG antibody production was found, while in 1 of these 6 patients, intrathecal borrelial IgM and IgG antibody synthesis was established. The durations of symptoms (median, 12 days; range, 4 to 25 days) were similar among these six potentially coinfected patients and were also similar to the durations of neurologic symptoms in patients with clinically evident Lyme neuroborreliosis (median, 10 days; range, 2 to 90 days). Furthermore, only one of four TBE patients with intrathecal production of specific IgM antibody had the synthesis demonstrated by both tests; the other three were positive only by the Liaison test; and of three patients with a positive IgG index, two were positive by both approaches and one solely by the Liaison test. These findings suggest limited specificity of IgM testing and, to a lesser degree, also of IgG testing, but they could have also been the result of coinfection with B. burgdorferi sensu lato and TBE virus. For a reliable distinction between these two possibilities, which would have practical clinical consequences, appropriate control groups, consisting of persons not exposed to ticks and of patients with certain diseases (such as syphilis, infectious mononucleosis, or neurological diseases [for example, multiple sclerosis or Guillain-Barré syndrome]) would be required to assess the specificity of the tests.
In summary, assessment of the humoral immune response in the sera and CSF of patients with early Lyme neuroborreliosis confirmed previous findings on the relationship between the duration of illness and the proportion of patients with detectable responses. Analysis and comparison of two approaches for the evaluation of the immune response revealed several distinctions in sensitivity, including differences in the detection of intrathecal synthesis of borrelial antibodies.
Footnotes
Published ahead of print on 17 February 2010.
REFERENCES
- 1.Aguero-Rosenfeld, M. E., G. Wang, I. Schwartz, and G. P. Wormser. 2005. Diagnosis of Lyme borreliosis. Clin. Microbiol. Rev. 18:484-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blanc, F., B. Jaulhac, M. Fleury, J. de Seze, S. J. de Martino, V. Remy, G. Blaison, Y. Hansmann, D. Christmann, and C. Tranchant. 2007. Relevance of the antibody index to diagnose Lyme neuroborreliosis among seropositive patients. Neurology 69:953-958. [DOI] [PubMed] [Google Scholar]
- 3.Brouqui, P., F. Bacellar, G. Baranton, R. J. Birtles, A. Bjoersdorff, J. R. Blanco, G. Caruso, M. Cinco, P. E. Fournier, E. Francavilla, M. Jensenius, J. Kazar, H. Laferl, A. Lakos, S. Lotric Furlan, M. Maurin, J. A. Oteo, P. Parola, C. Perez-Eid, O. Peter, D. Postic, D. Raoult, A. Tellez, Y. Tselentis, and B. Wilske. 2004. Guidelines for the diagnosis of tick-borne bacterial diseases in Europe. Clin. Microbiol. Infect. 10:1108-1132. [DOI] [PubMed] [Google Scholar]
- 4.Cerar, T., K. Ogrinc, J. Cimperman, S. Lotric-Furlan, F. Strle, and E. Ruzic-Sabljic. 2008. Validation of cultivation and PCR methods for diagnosis of Lyme neuroborreliosis. J. Clin. Microbiol. 46:3375-3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cimperman, J., V. Maraspin, S. Lotric-Furlan, E. Ruzic-Sabljic, T. Avsic-Zupanc, and F. Strle. 2002. Double infection with tick borne encephalitis virus and Borrelia burgdorferi sensu lato. Wien. Klin. Wochenschr. 114:620-622. [PubMed] [Google Scholar]
- 6.Dressler, F., J. A. Whalen, B. N. Reinhardt, and A. C. Steere. 1993. Western blotting in the serodiagnosis of Lyme disease. J. Infect. Dis. 167:392-400. [DOI] [PubMed] [Google Scholar]
- 7.Felgenhauer, K. 1992. Barrier concepts and CSF analysis. J. Neurol. 239:59-60. [DOI] [PubMed] [Google Scholar]
- 8.Hammers-Berggren, S., K. Hansen, A. M. Lebech, and M. Karlsson. 1993. Borrelia burgdorferi-specific intrathecal antibody production in neuroborreliosis: a follow-up study. Neurology 43:169-175. [DOI] [PubMed] [Google Scholar]
- 9.Kaiser, R., and S. Rauer. 1999. Advantage of recombinant borrelial proteins for serodiagnosis of neuroborreliosis. J. Med. Microbiol. 48:5-10. [DOI] [PubMed] [Google Scholar]
- 10.Kaiser, R., and S. Rauer. 1998. Analysis of the intrathecal immune response in neuroborreliosis to a sonicate antigen and three recombinant antigens of Borrelia burgdorferi sensu stricto. Eur. J. Clin. Microbiol. Infect. Dis. 17:159-166. [DOI] [PubMed] [Google Scholar]
- 11.Ledue, T. B., M. F. Collins, J. Young, and M. E. Schriefer. 2008. Evaluation of the recombinant VlsE-based Liaison chemiluminescence immunoassay for detection of Borrelia burgdorferi and diagnosis of Lyme disease. Clin. Vaccine Immunol. 15:1796-1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ljøstad, U., T. Skarpaas, and A. Mygland. 2007. Clinical usefulness of intrathecal antibody testing in acute Lyme neuroborreliosis. Eur. J. Neurol. 14:873-876. [DOI] [PubMed] [Google Scholar]
- 13.Luft, B. J., J. J. Dunn, R. J. Dattwyler, G. Gorgone, P. D. Gorevic, and W. H. Schubach. 1993. Cross-reactive antigenic domains of the flagellin protein of Borrelia burgdorferi. Res. Microbiol. 144:251-257. [DOI] [PubMed] [Google Scholar]
- 14.Reiber, H., and P. Lange. 1991. Quantification of virus-specific antibodies in cerebrospinal fluid and serum: sensitive and specific detection of antibody synthesis in brain. Clin. Chem. 37:1153-1160. [PubMed] [Google Scholar]
- 15.Reiber, H., and J. B. Peter. 2001. Cerebrospinal fluid analysis: disease-related data patterns and evaluation programs. J. Neurol. Sci. 184:101-122. [DOI] [PubMed] [Google Scholar]
- 16.Roux, F., E. Boyer, B. Jaulhac, E. Dernis, F. Closs-Prophette, and X. Puechal. 2007. Lyme meningoradiculitis: prospective evaluation of biological diagnosis methods. Eur. J. Clin. Microbiol. Infect. Dis. 26:685-693. [DOI] [PubMed] [Google Scholar]
- 17.Ruzić-Sabljić, E., V. Maraspin, S. Lotric-Furlan, T. Jurca, M. Logar, A. Pikelj-Pecnik, and F. Strle. 2002. Characterization of Borrelia burgdorferi sensu lato strains isolated from human material in Slovenia. Wien. Klin. Wochenschr. 114:544-550. [PubMed] [Google Scholar]
- 18.Schulte-Spechtel, U., G. Lehnert, G. Liegl, V. Fingerle, C. Heimerl, B. J. Johnson, and B. Wilske. 2003. Significant improvement of the recombinant Borrelia-specific immunoglobulin G immunoblot test by addition of VlsE and a DbpA homologue derived from Borrelia garinii for diagnosis of early neuroborreliosis. J. Clin. Microbiol. 41:1299-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stanek, G., S. O'Connell, M. Cimmino, E. Aberer, W. Kristoferitsch, M. Granstrom, E. Guy, and J. Gray. 1996. European Union Concerted Action on Risk Assessment in Lyme Borreliosis: clinical case definitions for Lyme borreliosis. Wien. Klin. Wochenschr. 108:741-747. [PubMed] [Google Scholar]
- 20.Stanek, G., and F. Strle. 2003. Lyme borreliosis. Lancet 362:1639-1647. [DOI] [PubMed] [Google Scholar]
- 21.Steere, A. C., G. McHugh, N. Damle, and V. K. Sikand. 2008. Prospective study of serologic tests for Lyme disease. Clin. Infect. Dis. 47:188-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vermeersch, P., S. Resseler, E. Nackers, and K. Lagrou. 2009. The C6 Lyme antibody test has low sensitivity for antibody detection in cerebrospinal fluid. Diagn. Microbiol. Infect. Dis. 64:347-349. [DOI] [PubMed] [Google Scholar]
- 23.Wilske, B., V. Fingerle, and U. Schulte-Spechtel. 2007. Microbiological and serological diagnosis of Lyme borreliosis. FEMS Immunol. Med. Microbiol. 49:13-21. [DOI] [PubMed] [Google Scholar]