Abstract
The principal objectives of this study were to develop autologous antigen-presenting cells (APCs) and to characterize the antigen-specific T-cell responses to the M and N proteins of porcine reproductive and respiratory syndrome virus (PRRSV) by using those APCs in outbred pigs. The orf6 and orf7 genes fused with porcine granulocyte-macrophage colony-stimulating factor (GM-CSF) were cloned into the mammalian expression vector to generate two plasmid DNAs, namely, pcDNA3.1-GM-CSF-PRRSV-M and pcDNA3.1-GM-CSF-PRRSV-N. Three of six pigs in two groups were repeatedly immunized with either plasmid DNA construct, and four pigs were used as controls. The recombinant M and N proteins fused with the protein transduction domain (PTD) of the human immunodeficiency virus type 1 transactivator of transcription protein were employed to generate major histocompatibility complex-matched autologous APCs from each pig. The levels of T-cell proliferation and gamma interferon (IFN-γ) synthesis were compared between pigs immunized with the two plasmid DNAs after stimulation of the peripheral blood mononuclear cells (PBMCs) of each pig with the autologous antigen-presenting dendritic cells and PBMCs. Higher levels of T-cell proliferation and IFN-γ synthesis were identified in PBMCs isolated from the pigs immunized with pcDNA3.1-GM-CSF-PRRSV-M than in those isolated from the pigs immunized with pcDNA3.1-GM-CSF-PRRSV-N. By way of contrast, serum antibodies were detected only in pigs immunized with pcDNA3.1-GM-CSF-PRRSV-N. However, no T-cell response or antibody production was detected in the control pigs. These results suggest that the M protein of PRRSV is a more potent T cell-stimulating antigen than the N protein. Nevertheless, it should be emphasized that the N protein substantially induces both cellular and humoral immune responses. The newly developed protocol for generating self APCs may prove effective in further efforts to characterize additional PRRSV proteins involved in the induction of cell-mediated immunity.
Porcine reproductive and respiratory syndrome (PRRS) virus (PRRSV) is an enveloped virus that belongs to the genus Arterivirus, the family Arteriviridae, and the order Nidovirales. PRRSV has a single-stranded positive-sense RNA genome composed of nine open reading frames (ORFs) (5). PRRSV is currently recognized as the pathogen responsible for the greatest economic losses in the swine industry worldwide. PRRSV induces late-term reproductive failure in sows and pneumonia in young pigs (30, 41). The establishment of persistent infection is one of the principal characteristics of PRRSV (42). The delayed induction of both T cell-mediated immunity and neutralizing antibodies is assumed to contribute to persistent PRRSV infection (8, 26). The control of PRRSV by vaccination is challenging because of the significant antigenic diversity known to exist among all strains of this RNA virus and the resultant insufficient generation of protective immunity toward heterologous PRRSV strains (18). The correlates of the protection indicators of broad reactive immunity attainable by vaccination have yet to be well-defined (22).
Serum neutralizing antibodies may be one of the factors contributing to protection against PRRSV (22). The GP5 and GP4 proteins are representative antigens that contribute to the induction of neutralizing antibodies (13, 27). However, it has become evident that T cell-mediated immunity is essential for effective protection against PRRSV (25, 47). The increased cellular immunity was significantly related to the reduced clinical symptoms in PRRSV-infected piglets and sows (17, 23). Analysis of the T-cell responses to the structural proteins of PRRSV indicated that the M protein may perform a major role in the induction of cellular immunity in infected pigs (2). When the M protein was fused with GP5, it induced stronger cell-mediated immunity compared to that achieved with the M protein alone (16, 46). By way of contrast, only a few papers have suggested that the N protein may have the capacity to induce cell-mediated immunity and to reduce the viral loads in infected animals (19, 29). In order to gain a better understanding of the nature of immunity to PRRSV, further comparative analyses of cell-mediated immunity against the M and N proteins of PRRSV (the PRRSV-M and PRRSV-N proteins, respectively) should be conducted.
Although a few lines of inbred miniature pigs which express the homologous major histocompatibility complex (MHC) molecules exist (6, 28), the majority of outbred conventional pigs harbor polymorphic MHC molecules on their cells. That is the primary reason that it has proven difficult to conduct a proper analysis of MHC-restricted T-cell immune responses in pigs immunized with vaccines or infected with the wild-type viruses. Therefore, the development of MHC-matched autologous antigen-presenting cells (APCs) is important for assessment of the T cell-mediated immune responses in pigs (20). The generation of autologous APCs with primary cells is also relatively difficult, owing to the insufficient transfection or expression efficacy of the viral genes delivered into them (3). The transactivator of transcription (TAT) protein of human immunodeficiency virus (HIV) type 1 (HIV-1) harbors a protein transduction domain (PTD) (12, 14). It has previously been determined that TAT-conjugated macromolecules can efficiently be delivered into a variety of mammalian cells (3, 31). APCs transduced with the TAT conjugated with viral antigens could induce T cell-specific immune responses to those antigens (32, 36, 38). The well-described ability of TAT fusion proteins to rapidly transduce proteins into many primary mammalian cells suggests that this property might be exploited to develop autologous APCs displaying viral antigens in outbred animals (3).
In the study described here, we immunized conventional pigs with two plasmid DNA constructs encoding the PRRSV M and N proteins that were fused with the porcine granulocyte-macrophage colony-stimulating factor (GM-CSF). This system was useful for analysis of the immune responses very specific to individual antigens in the vaccinated pigs. The property of TAT-mediated protein transduction was newly applied in an effort to generate MHC-matched and autologous primary APCs in pigs. The APCs were used to stimulate memory T-cell populations in peripheral blood mononuclear cells (PBMCs) isolated from the immunized pigs. Different features of the T cell-mediated immune responses induced by the M and N proteins of PRRSV and the application of the protocol to generate autologous APC are discussed herein.
MATERIALS AND METHODS
Animals.
A total of 10 conventionally reared 4-week-old pigs were obtained from a PRRS-free herd. Their PRRSV-negative status was confirmed prior to the inoculation of the plasmid DNAs by reverse transcriptase (RT) PCR and a serologic enzyme-linked immunosorbent assay (ELISA; IDEXX). All experiments were conducted under the guidelines of the Institutional Animal Care and Use Committee (IACUC) of Konkuk University, South Korea (permit number KU08020).
Cloning of ORFs 6 and 7 of PRRSV into cloning and expression vectors.
PRRSV strain LMY (GenBank accession number DQ473474), the representative PRRSV strain isolated in South Korea, was used to clone orf6 and orf7. It was provided by the National Veterinary Research Quarantine Service (NVRQS), Anyang, South Korea. Marc-145 cells were infected with 105.7 50% tissue culture infective doses/ml PRRSV. When complete cytopathic effects (CPEs) were apparent at day 5 after infection, viral RNA was purified from the cell culture supernatants by use of a QIAamp viral RNA minikit (Qiagen, Valencia, CA), in accordance with the manufacturer's instructions. The single-stranded cDNA was synthesized in a tube containing 20 μl of reaction reagents, including 5 μg of viral RNA, 1 μg of oligo(dT) primer, 4 μl of 5× buffer, 2 μl of 0.1 M dithiothreitol, 1 μl of 10 mM deoxynucleoside triphosphates, 1 μl of RNasin, and 1 μl of Moloney murine leukemia virus (MMLV) RT (4 units/μl; Qiagen) at 37°C for 1.5 h. The entire orf6 of PRRSV, including two restriction enzyme sites for EcoRI and EcoRV at the 5′ and 3′ ends, respectively, was amplified by PCR with primers PRRSV-M FP1 and PRRSV-M RP1 (Table 1) and the cDNA. Similarly, the entire orf7, including two restriction enzyme sites for EcoRI and EcoRV at the 5′ and 3′ ends, respectively, was amplified with primers PRRSV-N FP1 and PRRSV-N RP1 (Table 1) and the cDNA. In another PCR, the orf7 harboring two different restriction enzyme sites for XhoI and XbaI at the 5′ and 3′ ends, respectively, was amplified with primers PRRSV-N FP3 and PRRSV-N RP3 (Table 1) and the cDNA. The PCR conditions used for the amplification of orf6 and orf7 were as follows: 30 cycles of denaturation at 94°C for 30 s, annealing at 62°C for 30 s, and extension at 72°C for 40 s. The PCR products were cloned into the pCR2.1-TOPO vector (Invitrogen), in accordance with the manufacturer's instructions. The correct clones were selected following the identification of the insert DNA sequences. The selected clones were named pCR2.1-PRRSV-M1, pCR2.1-PRRSV-N1, and pCR2.1-PRRSV-N3. These cloned genes were used to generate the DNA vaccines and the Escherichia coli expression vector. The partial orf6 of PRRSV, which was composed of 270 bp (256 to 525 bp), excluding the 5′ transmembrane regions, was amplified by PCR with primers PRRSV-M FP2 and PRRSV-M RP2 (Table 1) and the cDNA. The intact 372-bp orf7 was also amplified by PCR with primers PRRSV-N FP2 and PRRSV-N RP2 (Table 1) and the cDNA. The primers utilized in these reactions did not contain the sequences for the restriction enzymes at their 5′ ends. The PCR conditions and cloning processes applied were identical to those used to clone the PRRSV M1 and PRRSV N1 genes. The PCR products were cloned into the E. coli expression vector pQE30-UA (Qiagen), in accordance with the manufacturer's instructions. The correct clones were selected after the identification of their insert DNA sequences. The selected clones were designated pQE30-UA-PRRSV-M2 and pQE30-UA-PRRSV-N2, respectively.
TABLE 1.
Vectors and primers used to clone viral and cellular genes
| Vector | Primer name | DNA sequence (5′ → 3′) | Product size (bp) |
|---|---|---|---|
| pCR2.1-TOPO | PRRSV-M FP1 | GAA TTC ATG GGG TCG TCC TTA GAT GACa | 537 |
| PRRSV-M RP1 | GAT ATC TTA TTT GGC ATA TTT AAC AAGb | ||
| pCR2.1-TOPO | PRRSV-N FP1 | GAA TTC ATG CCA AAT AAC AAC GGC AAG CAa | 384 |
| PRRSV-N RP1 | GAT ATC TCA GGC TGA GGG TGA TGC TGT Gb | ||
| pQE30-UA | PRRSV-M FP2 | TAC TCA GCC ATA GAA ACC | 270 |
| PRRSV-M RP2 | TTA TTT GGC ATA TTT AAC AAG | ||
| pQE30-UA | PRRSV-N FP2 | ATG CCA AAT AAC AAC GGC AAG CA | 372 |
| PRRSV-N RP2 | TCA GGC TGA GGG TGA TGC TGT G | ||
| pCR2.1-TOPO | PRRSV-N FP3 | CTC GAG ATG CCA AAT AAC AAC GGC AAG CAc | 384 |
| PRRSV-N RP3 | TCT AGA TCA GGC TGA GGG TGA TGC TGT Gd | ||
| pCR2.1-TOPO | TAT FP | GGC AGG AAG AAG CGG AGA CAG CGA CGA AGA CCT CCT CAA TGC | 42 |
| TAT RP | GCA TTG AGG AGG TCT TCG TCG CTG TCT CCG CTT CTT CCT GCC | ||
| pQE30 | TAT-PRRSV-M FP | GGA TCC GGC AGG AAG AAG CGG AGA CAG CGA CGA AGA CCTe | 324 |
| CCT CAA TGC TAC TCA GCC ATA GAA ACC | |||
| TAT-PRRSV-M RP | GTC GAC TTA TTT GGC ATA TTT AACf | ||
| pQE30-UA | TAT-PRRSV-N FP | GGC AGG AAG AAG CGG AGA CAG CGA | 414 |
| TAT-PRRSV-N RP | TCA GGC TGA GGG TGA TGC TGT G | ||
| pcDNA3.1 | GM-CSF FP | GCC GCC GCC ATG GGG CTG CAG AAC CTG CTT Cg | 447 |
| GM-CSF RP | GAA TTC CTT TTT GAC TGG CCC CCA GCA Aa |
Italics indicate the EcoRI restriction enzyme site.
Italics indicate the EcoRV restriction enzyme site.
Italics indicate the XhoI restriction enzyme site.
Italics indicate the XbaI restriction enzyme site.
Italics indicate the BamHI restriction enzyme site.
Italics indicate the SalI restriction enzyme site.
Italics indicate the Kozak sequence.
Cloning of TAT, TAT-PRRSV-M, and TAT-PRRSV-N genes into E. coli expression vector.
The HIV TAT gene was amplified directly without a template DNA with the primers TAT FP and TAT RP, which were composed of 42 nucleotides encoding 14 amino acids of PTD (Table 1). PCR was conducted for 30 cycles of denaturation at 94°C for 30 s and 30 s of annealing/extension at 72°C. The PCR product was cloned into the pCR2.1-TOPO vector, in accordance with the manufacturer's instructions. The clone was designated pCR2.1-TAT. The recombinant TAT-PRRSV-M gene was amplified with primers TAT-PRRSV-M FP, which was composed of the whole TAT sequence and a sequence of the orf6 region from 256 bp, and TAT-PRRSV-M RP (Table 1) and with pCR2.1-PRRSV-M1 as the template DNA. The PCR conditions were as follows: 30 cycles of denaturation at 94°C for 30 s, annealing at 48°C for 1 min, and extension at 72°C for 30 s. The PCR product was cloned into the pDrive vector (Qiagen). The clone was designated pDrive-TAT-PRRSV-M. The plasmid DNA was digested with the restriction enzymes BamHI and SalI (New England Biolabs). The DNA fragment was subcloned into the pQE30 vector (Qiagen), which had already been digested with BamHI and SalI. The cloned plasmid was designated pQE30-TAT-PRRSV-M. The orf7 gene of PRRSV was released from pCR2.1-PRRSV-N3 by digestion with XhoI and XbaI (New England Biolabs) and was ligated with the TAT gene in the pCR2.1-TAT vector, which had previously been digested with the XhoI and XbaI restriction enzymes. The selected clone was designated pCR2.1-TAT-PRRSV-N. The recombinant TAT-PRRSV-N gene was amplified with primers TAT-PRRSV-N FP and TAT-PRRSV-N RP (Table 1) and with pCR2.1-TAT-PRRSV-N as the template DNA under the PCR conditions identical to those employed for the amplification of the PRRSV-N gene, as described above. The PCR product was cloned directly into the E. coli protein expression vector pQE30-UA (Qiagen). The clone was designated pQE30-UA-TAT-PRRSV-N.
Expression of recombinant proteins in E. coli.
E. coli (M 15; Qiagen) colonies harboring the pQE30-UA-PRRSV-M, pQE30-UA-PRRSV-N, pQE30-TAT-PRRSV-M, and pQE30-UA-TAT-PRRSV-N clones were inoculated into 20 ml of LB broth containing 100 μg/ml of ampicillin (Sigma Aldrich, St. Louis, MO) and 25 μg/ml of kanamycin (Sigma Aldrich) and incubated for 12 h at 37°C. The bacterial cultures were then inoculated into 1,000 ml of fresh LB broth. When the optical density (OD) value (600 nm) of the bacterial cultures reached 0.6, isopropyl-β-d-thiogalactopyranoside (Gene All, South Korea) was added to a final concentration of 1 mM. After 4 h of culture, the bacterial cells were collected by centrifugation. The recombinant proteins were purified through a nickel column, in accordance with the manufacturer's protocols. The identities of purified PRRSV-M, PRRSV-N, TAT-PRRSV-M, and TAT-PRRSV-N were confirmed by Western blotting. The proteins blotted onto the nitrocellulose membranes (Whatman, United Kingdom) were exposed to porcine serum acquired from a PRRSV-infected pig. The membranes were then exposed to secondary antibody, horseradish peroxidase (HRP)-conjugated goat anti-porcine IgG (Southern Biotech). The protein-specific bands were identified by color development with 3,3′-diaminobenzidine (DAB; Pierce).
Generation of DCs.
Porcine monocyte-derived dendritic cells (DCs) were generated as described previously but with some modifications (15, 39). In brief, PBMCs were isolated from heparin-treated whole-blood cells of each pig by 30 min of centrifugation at 400 × g in the presence of Histopaque 1077 (Sigma Aldrich). The cells were adjusted to 2 × 106/ml and were cultured in minimal essential medium (Welgene, South Korea) supplemented with 10% fetal bovine serum, 10 ng/ml of porcine GM-CSF (Endogen), and 10 ng/ml of interleukin-4 (IL-4) (Endogen) for 16 h in a six-well plate. After the removal of unbound cells, the attached cells were cultured for 6 days in medium supplemented with GM-CSF and IL-4 in order to induce their differentiation into immature monocyte-derived DCs. The identity of the DCs was confirmed by fluorescent-activated cell sorter analysis (FACS) analysis with fluorescein isothiocyanate (FITC)-conjugated antibodies specific for porcine MHC class I (mouse anti-porcine swine leukocyte antigen I; Serotec), MHC class II (mouse anti-porcine swine leukocyte antigen II; Serotec), CD172a (mouse anti-porcine CD172a; Serotec), and a human cytotoxic T-lymphocyte antigen 4 fusion protein recognizing porcine CD80 and CD86 (human CD152 Ig-FITC fusion protein; Ancell). The maturation of DCs was induced by treating the immature DCs with tumor necrosis factor alpha (TNF-α; 250 U/ml; Serotec) for 16 h. The mature DCs were analyzed by FACS analysis, as described elsewhere (11).
Transduction of TAT-PRRSV-M and TAT-PRRSV-N recombinant proteins into mammalian cells.
The transduction of TAT-PRRSV-M and TAT-PRRSV-N recombinant proteins was verified by using three different types of porcine cells: PK15 cells, DCs, and PBMCs. The TAT-PRRSV-M and TAT-PRRSV-N proteins, adjusted to a final concentration of 500 μg/ml, were added to the cells for 7 h. After lysis of the protein-transduced cells, the cell lysates were analyzed by Western blotting with the same antibodies used to identify the recombinant PRRSV-M and PRRSV-N proteins.
Construction of mammalian expression vector DNAs.
Porcine PBMCs were cultured for 2 days in the presence of 5 μg/ml of concanavalin A (ConA; Sigma Aldrich). Total cellular RNA was extracted from the cells with an RNeasy minikit (Qiagen), in accordance with the manufacturer's instructions. After the synthesis of cDNA with oligo(dT) primer and cellular RNA by MMLV RT, the GM-CSF gene was amplified with primer GM-CSF FP harboring a Kozak sequence and primer GM-CSF RP (Table 1). PCR was conducted for 30 cycles of denaturation at 94°C for 30 s, annealing at 62°C for 30 s, and extension at 72°C for 30 s. The PCR product was ligated into the mammalian expression vector pcDNA3.1 (Invitrogen), in accordance with the manufacturer's procedures. The clone was designated pcDNA3.1-GM-CSF. DNA vaccines were constructed by conjugating the porcine GM-CSF gene with the PRRSV-M and PRRSV-N genes in the pcDNA3.1 vector. In brief, pCR2.1-PRRSV-M1 and pCR2.1-PRRSV-N1 were digested with the EcoRI and EcoRV restriction enzymes (New England Biolabs), and the DNA bands containing the PRRSV-M and PRRSV-N genes were purified. The PRRSV-M and PRRSV-N genes were ligated into pcDNA3.1-GM-CSF, which had been digested with EcoRI and EcoRV. The clones were designated pcDNA3.1-GM-CSF-PRRSV-M and pcDNA3.1-GM-CSF-PRRSV-N, respectively.
Expression of GM-CSF-PRRSV-M and GM-CSF-PRRSV-N genes and proteins in mammalian cells.
3T3 cells adjusted to 5 × 105/ml were cultured for 1 day in Dulbecco modified Eagle medium (DMEM) (Welgene, South Korea) supplemented with 10% fetal bovine serum without antibiotics in six-well plates. The transfection reagents were prepared in accordance with the manufacturer's instructions. In brief, 4.0 μg of plasmid DNA—either pcDNA3.1-GM-CSF-PRRSV-M or pcDNA3.1-GM-CSF-PRRSV-N DNA—was dissolved in 250 μl of DMEM, and the solution was then mixed with 10 μl of Lipofectamine 2000 reagent (Invitrogen) in 250 μl of DMEM without serum and antibiotics for 20 min at room temperature. The mixture of plasmid DNA and Lipofectamine was added to the cells, and the cells were incubated for 2 days. The total RNA fraction was then extracted from the cells with an RNeasy minikit. The remaining DNA was removed from the RNA by treating 1 μg of total RNA with 1 μl of DNase I (1 U/μl). After cDNA synthesis with the purified RNA as described above, PCR was conducted to detect the expression of the GM-CSF-PRRSV-M and GM-CSF-PRRSV-N genes with primer set GM-CSF FP and PRRSV-M RP1 and primer set GM-CSF FP and PRRSV-N RP1 (Table 1), respectively. The expression of the GM-CSF, PRRSV-M, and PRRSV-N proteins was identified by Western blotting. GM-CSF expression was identified with mouse anti-porcine GM-CSF antibody (R&D Systems) and goat anti-mouse IgG (Biosource). The expression of the PRRSV-M and PRRSV-N proteins was identified by using the same antibodies and methods used to detect the proteins expressed in E. coli.
Immunization of pigs with plasmid DNAs and determination of antibody titers.
A total of 10 pigs were employed in this study. Two immunization groups, composed of three pigs per group, were intramuscularly immunized with 500 μg of pcDNA3.1-GM-CSF-PRRSV-M and pcDNA3.1-GM-CSF-PRRSV-N, respectively. The plasmid DNAs were mixed with the adjuvant dimethyldioctadecylammonium bromide (DDA; Sigma Aldrich) prior to injection into the pigs, as described in the report of another study (37). Four pigs were injected with phosphate-buffered saline and utilized as a nonimmunized control group. The plasmid DNAs were administered a total of 8 times on days 0, 7, 14, 21, 28, 35, 100, and 193. Sera were collected from the immunized and control pigs prior to each injection. The PRRSV-specific antibody titers in the sera were determined by ELISA. In brief, 5 μg/ml of the recombinant PRRSV-M and PRRSV-N proteins were coated onto microwell plates overnight. The serum samples, diluted 50-fold, were added to the respective antigens and incubated for 2 h at 37°C. The 5,000-fold-diluted goat anti-porcine IgG-HRP was added to the plates. After 20 min of incubation at 37°C, tetramethylbenzidine substrate was added to develop the color and H2SO4 stop solution was added to the reaction mixture. The OD values at 450 nm were determined with an automatic ELISA reader (Tecan Sunrise, Switzerland).
Assessment of T-cell proliferation and cytokine production.
All experiments designed to evaluate T-cell proliferation and cytokine synthesis were conducted in 96-well flat-bottom plates (SPL, South Korea) with MHC-matched autologous APCs and effector T cells for each pig. T cell-containing PBMCs were prepared from nonimmunized and immunized pigs 14 days after the final vaccination. The PBMCs and monocyte-derived DCs were used as two different types of APCs to deliver PRRSV-M or PRRSV-N antigens to effector T cells. The PBMCs, which had been stimulated for 5 days with ConA (5 μg/ml), were treated with TAT-PRRSV-M or TAT-PRRSV-N protein to use them as the APCs. The DCs were used as APCs after the immature DCs were treated with TNF-α (250 U/ml) for 16 h to make them mature. The TAT-PRRSV-M or TAT-PRRSV-N protein (5 μg/ml) was also added to the immature DCs during the maturation period. Those antigen-presenting PBMCs and DCs were then treated for 1 h with mitomycin C (10 μg/ml; Sigma Aldrich). The APCs were added to effector T cells at effector T cell-to-APC (E/APC) ratios of 5:1 (5 × 106:1 × 106 cells/ml), and the cells were incubated for 3 days. Positive control cells were stimulated with 5 μg/ml of ConA and incubated under conditions identical to those employed for the APC-stimulated cells. T-cell proliferation was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays (Sigma Aldrich), in accordance with the manufacturer's instructions. In brief, 10 μl of MTT reagent (5 mg/ml) was added to each well of the plate, and the plate was incubated for 5 h in a cell culture incubator. One hundred microliters of stop solution (20% SDS in 50% dimethyl formamide) was added to each well, and the plate was incubated for 1 h at room temperature. The OD values of each well were determined with an ELISA reader at 540 nm. The amounts of porcine gamma interferon (IFN-γ), IL-6, and IL-10 in the cell culture supernatants collected from the antigen-stimulated T cells were determined with conventional ELISA kits (R&D Systems), in accordance with the manufacturer's instructions.
Statistical analysis.
T-cell proliferation and cytokine production were measured at least three times, in triplicate, per pig. The significance of the difference in the experimental data between individual pigs in the vaccinated and the control groups was determined by Student's t test with the Instat (version 3.0) program (GraphPad Software, San Diego, CA).
RESULTS
Expression of TAT-conjugated PRRSV-M and PRRSV-N proteins.
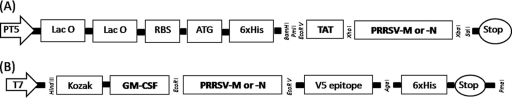
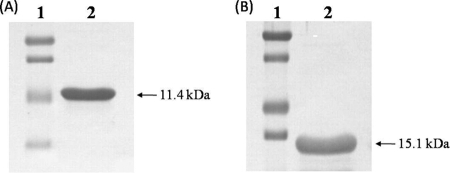
As the M protein of PRRSV has transmembrane regions that hamper protein expression in E. coli, we deleted the transmembrane regions at the 5′ end, which encompassed nucleotides 1 to 255 (amino acids 1 to 85). This deletion made possible the expression of the remaining PRRSV-M region in E. coli (data not shown). The fusion gene of the TAT and transmembrane region-deleted orf6 of PRRSV was cloned into the E. coli expression vector. By way of contrast, the entire N gene of PRRSV was cloned into the E. coli expression vector as a fused form with the TAT gene (Fig. 1A). The recombinant proteins of TAT-PRRSV-M and TAT-PRRSV-N were expressed in E. coli transformed with the pQE30-TAT-PRRSV-M and pQE30-UA-TAT-PRRSV-N vectors, respectively. The pure TAT-fused PRRSV-M and PRRSV-N proteins were identified by Western blotting to be 11.4- and 15.1-kDa proteins, respectively (Fig. 2A and B).
FIG. 1.
Cloning of viral genes into protein expression vectors. (A) The recombinant TAT-PRRSV-M and TAT-PRRSV-N genes were cloned into the pDrive and pCR2.1 cloning vectors, respectively. Once they were cloned into the cloning vectors, the insert DNAs were subcloned into the pQE30 and pQE30-UA E. coli expression vectors. (B) The porcine GM-CSF gene was cloned into the pcDNA3.1 mammalian protein expression vector. orf6 and orf7 of PRRSV cloned in the pCR2.1 vector were subcloned downstream of the GM-CSF gene.
FIG. 2.
Expression of the recombinant proteins in E. coli. The recombinant TAT-PRRSV-M (A) and TAT-PRRSV-N (B) proteins were expressed in E. coli after transformation with the pQE30-TAT-PRRSV-M and the pQE30-UA-TAT-PRRSV-N vectors, respectively. The identities of the products expressed were confirmed by Western blotting with porcine serum obtained from a PRRSV-infected pig, followed by Western blotting with HRP-conjugated goat anti-porcine IgG. The protein bands were identified by color development involving DAB. Lanes 1, standard protein markers.
Generation of DCs.
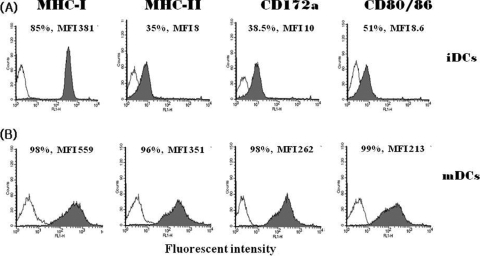
The immature monocyte-derived DCs were generated by culturing the PBMCs for 6 days in tissue culture medium supplemented with porcine GM-CSF and IL-4. The immature DCs expressed relatively low levels of the typical DC markers. The levels of expression of the DC markers in the immature DCs were 85% for MHC class I molecules, 35% for MHC class II molecules, 38.5% for CD172a molecules, and 51% for CD80/CD86 molecules (Fig. 3A). The immature DCs were induced to mature by treatment with TNF-α. Such treatment induced substantially higher levels of expression of all of the DC markers for which the immature DCs were assessed. The levels of expression of the DC markers in the mature DCs were 98% for MHC class I molecules, 96% for MHC class II molecules, 98% for CD172a molecules, and 99% for CD80/CD86 molecules (Fig. 3B). The highly enhanced expression of the MHC and costimulatory molecules in the mature DCs suggested that they might prove useful as APCs to stimulate antigen-specific T cells.
FIG. 3.
Generation of monocyte-derived DCs. The levels of expression of DC markers were identified in the immature DCs (A) and mature DCs (B). The immature DCs were generated from monocytes by culturing PBMCs with GM-CSF and IL-4 for 6 days. The immature DCs became mature DCs as the result of 1 day of treatment with TNF-α. The levels of expression of MHC class I, MHC class II, CD172a, and CD80/CD86 molecules were determined by FACS analysis. The expression of MHC class I, MHC class II, and CD172a molecules was determined by the use of an FITC-conjugated monoclonal antibody specific for each corresponding molecule. The expression of CD80/CD86 was determined with an FITC-conjugated human CD152Ig protein. The x and y axes in each panel indicate the cell count and fluorescent intensity, respectively.
Transduction of TAT-fused PRRSV-M and PRRSV-N proteins into porcine cells.
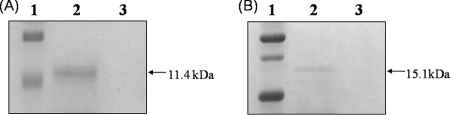
The TAT-conjugated PRRSV-M and PRRSV-N proteins were transduced into PK15 cells. After transduction, the TAT-PRRSV-M and TAT-PRRSV-N proteins in the cell lysates were shown by Western blotting to have the predicted sizes of 11.4 and 15.1 kDa, respectively (Fig. 4A and B). The TAT-conjugated proteins could also transduce the primary porcine PBMCs and DCs (data not shown). These results clearly demonstrate that the TAT-conjugated PRRSV-M and PRRSV-N proteins can enter the primary cells as well as the cells of the established cell line. The data also suggest that the MHC-matched autologous primary cells, such as DCs and PBMCs, transduced with the TAT-conjugated PRRSV-M and PRRSV-N proteins could be used as APCs to stimulate antigen-specific T cells.
FIG. 4.
Transduction of the TAT-conjugated PRRSV-M and PRRSV-N proteins. PK15 cells were transduced with the TAT-PRRSV-M and TAT-PRRSV-N proteins. The proteins in the cell lysates were identified by Western blotting. (A) Lane 1, standard protein marker; lane 2, the TAT-PRRSV-M-transduced cell lysate; lane 3, PRRSV-M-transduced cell lysate. (B) Lane 1, standard protein marker; lane 2, TAT-PRRSV-N-transduced cell lysate; lane 3, PRRSV-N-transduced cell lysate.
Expression of PRRSV-M and PRRSV-N proteins in mammalian cells.
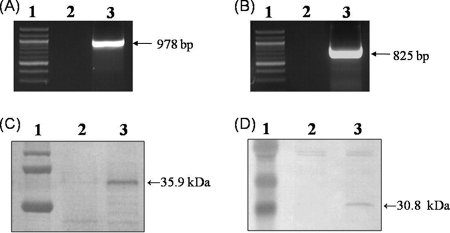
The entire PRRSV-M and PRRSV-N genes were subcloned downstream of the porcine GM-CSF gene in the mammalian expression vector pcDNA3.1 (Fig. 1B). The expression of the recombinant genes and proteins in 3T3 cells was identified after transfection with the pcDNA3.1-GM-CSF-PRRSV-M and pcDNA3.1-GM-CSF-PRRSV-N vectors by the RT-PCR and Western blotting methods, respectively. Expression of both the GM-CSF-fused PRRSV-M and PRRSV-N recombinant genes was identified. The recombinant GM-CSF-PRRSV-M and GM-CSF-PRRSV-N genes were identified as 978- and 825-bp RT-PCR products, respectively (Fig. 5A and B). The expression of both the PRRSV-M and PRRSV-N proteins fused with GM-CSF in the transfected cells was identified by Western blotting. The recombinant GM-CSF-PRRSV-M and GM-CSF-PRRSV-N proteins were found to be 35.9 and 30.8 kDa, respectively, by using the convalescent-phase serum from pigs infected with PRRSV (Fig. 5C and D). When the cell lysates were analyzed with the monoclonal antibody specific for porcine GM-CSF, protein products of the same size were detected (data not shown). These results show that the vectors constructed to express the PRRSV-M and PRRSV-N proteins as GM-CSF-fused recombinant proteins functioned properly in the mammalian cells. Therefore, we confirmed that the protein expression vectors constructed in this study could be used to immunize pigs.
FIG. 5.
Identification of the recombinant genes and proteins expressed in mammalian cells. The mammalian expression vectors encoding the GM-CSF-PRRSV-M and GM-CSF-PRRSV-N proteins were transfected into 3T3 cells. After 2 days, RT-PCR and Western blotting were conducted to identify the expression of the recombinant genes and proteins in the cells. (A) RT-PCR for the detection of the recombinant GM-CSF-PRRSV-M gene. Lane 1, standard DNA marker; lane 2, cells transfected with the empty vector; lane 3, cells transfected with the pcDNA3.1-GM-CSF-PRRSV-M vector. (B) RT-PCR for the detection of the recombinant GM-CSF-PRRSV-N gene. Lane 1, standard DNA marker; lane 2, cells transfected with empty vector; lane 3, cells transfected with the pcDNA3.1-GM-CSF-PRRSV-N vector. (C) Western blotting for the detection of the recombinant GM-CSF-PRRSV-M protein. Lane 1, standard protein marker; lane 2, cells transfected with the empty vector; lane 3, cells transfected with the pcDNA3.1-GM-CSF-PRRSV-M vector. (D) Western blotting for the detection of the recombinant GM-CSF-PRRSV-N protein. Lane 1, standard protein marker; lane 2, cells transfected with the empty vector; lane 3, cells transfected with the pcDNA3.1-GM-CSF-PRRSV-N vector.
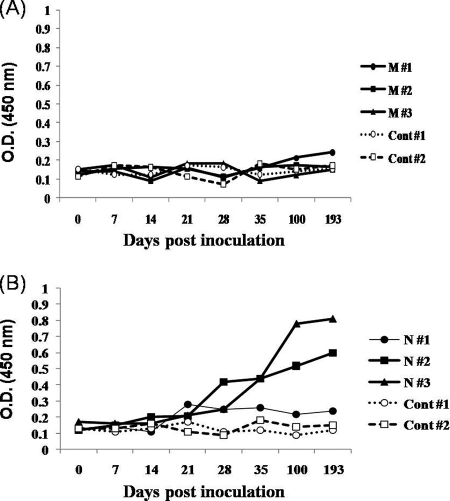
Antibody responses in pigs immunized with plasmids.
Antibody titers to the M and N proteins of PRRSV in the vaccinated and control pigs were determined by ELISA. The recombinant M and N proteins expressed from E. coli transformed with the pQE30-UA-PRRSV-M2 and pQE30-UA-PRRSV-N2 vectors were used as coating antigens in the ELISA. Three pigs immunized with pcDNA3.1-GM-CSF-PRRSV-M did not generate PRRSV-M protein-specific antibody (Fig. 6A). Until the pigs were immunized a total of 8 times, the antibody levels did not differ significantly from those noted in the control pigs. However, the other three pigs immunized with pcDNA3.1-GM-CSF-PRRSV-N generated PRRSV-N protein-specific antibody (Fig. 6B). After the fourth immunization with the corresponding plasmid DNA, the production of antibody to the N antigen was apparent in the immunized pigs. Thereafter, the level of antibody production increased steadily after the boosting injection of the plasmid. The antibody titers measured in the two immunized pigs were at least 4 to 5 times higher than those identified in the control pigs. On the other hand, the antibody level measured in one immunized pig was not higher than the levels measured in the other pigs. We also determined the antibody levels in the pigs that were immunized with the plasmid expressing PRRSV-M with the whole viral antigens. However, we did not observe significant increases in the production of antibodies to the whole viral antigens (data not shown). This result confirms that the PRRSV-M protein alone would not harbor the capacity to induce antibody. By way of contrast, the PRRSV-N protein seemed to function properly as a potent immunogenic antigen to B cells in pigs.
FIG. 6.
Antibody levels in the immunized pigs. Pigs were immunized with plasmid DNAs, and the levels antibodies to the PRRSV-M and PRRSV-N proteins were determined by ELISA. (A) Levels of antibody to the PRRSV-M protein in pigs immunized with pcDNA3.1-GM-CSF-PRRSV-M. Three pigs immunized with M protein (pigs M#1 to M#3, respectively) and two nonimmunized pigs (control pigs 1 and 2 [Cont#1 and Cont #2, respectively]) were examined. (B) Levels of antibody to PRRSV-N protein in pigs immunized with pcDNA3.1-GM-CSF-PRRSV-N. Three immunized pigs (N#1 to N#3, respectively) and two nonimmunized pigs (controls 1 and 2 [Cont#1 and Cont #2, respectively]) were assessed.
T-cell proliferation.
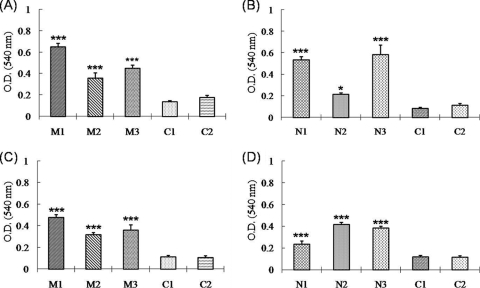
Autologous porcine PBMCs and DCs were used as APCs to stimulate antigen-specific memory T cells. The PBMCs and DCs were transduced with either TAT-conjugated PRRSV-M or PRRSV-N protein prior to coculture with PBMCs acquired from immunized and nonimmunized pigs. In preliminary experiments, we determined T-cell proliferation at a variety of E/APC ratios, such as 0.5:1 (5 × 105:1 × 106 cells/ml), 1:1 (1 × 106:1 × 106 cells/ml), and 5:1 (5 × 106 :1 × 106 cells/ml). The effector T cells obtained from the pigs immunized with either of the DNA vaccines evidenced significant proliferation compared to that observed for the effector T cells from the control pigs only at E/APC ratios of 5:1. Therefore, the optimal E/APC ratio was determined to be 5:1 (data not shown). Similarly, the optimal concentration of the TAT-conjugated M and N proteins was determined to be 5 μg/ml (data not shown). When the PBMCs treated with TAT-conjugated PRRSV-M protein were used as APCs, significant T-cell proliferation responses were noted in the PBMCs obtained from the three pigs immunized with pcDNA3.1-GM-CSF-PRRSV-M after 3 days of antigenic stimulation (Fig. 7A). PRRSV-N-specific T-cell proliferation was also noted after 3 days of stimulation in the PBMCs of the pigs immunized with pcDNA3.1-GM-CSF-PRRSV-N (Fig. 7B). The overall T-cell proliferation responses induced by PRRSV-M appeared to be higher than those induced by PRRSV-N. When T-cell proliferation was determined by using the antigen-transduced DCs as APCs, the responses in the immunized pigs were noted to be similar to those observed with the PBMCs (Fig. 7C and D). However, no significant T-cell proliferation was noted in the nonimmunized control pigs, regardless of which APCs were utilized. These data verify that the T-cell proliferation identified in PBMCs that originated from the immunized pigs was an antigen-specific response.
FIG. 7.
T-cell proliferation by antigen stimulation. T cell-containing PBMCs obtained from the immunized pigs and nonimmunized pigs (control pigs C1 and C2) were cocultured with APCs for 3 days at effector cell-to-APC ratios of 5:1. PBMCs and DCs transduced with TAT-conjugated PRRSV-M or PRRSV-N protein were used as APCs. Effector T cells from pigs immunized with the pcDNA3.1-GM-CSF-PRRSV-M (pigs M1 to M3) were stimulated by PBMCs (A) and DCs (C). Effector T cells from pigs immunized with pcDNA3.1-GM-CSF-PRRSV-N (pigs N1 to N3) were stimulated by PBMCs (B) and DCs (D). T-cell proliferation was determined by the MTT assay. Statistical significance was determined by Student's t test: *, P < 0.05; ***, P < 0.001.
Cytokine production from stimulated T cells.
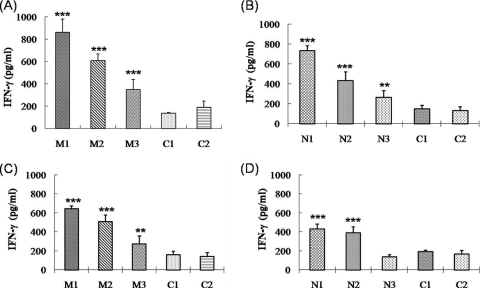
The cytokine production patterns were analyzed in the PBMCs obtained from the immunized and nonimmunized pigs by stimulating them with the autologous APCs transduced with the TAT-PRRSV-M or the TAT-PRRSV-N protein. The concentrations of IFN-γ, IL-6, and IL-10 were determined with cell culture medium samples obtained from the antigen-stimulated effector cells by ELISA. The T cells in the PBMCs obtained from pigs immunized with pcDNA3.1-GM-CSF-PRRSV-M produced more IFN-γ than the T cells in the PBMCs obtained from pigs immunized with pcDNA3.1-GM-CSF-PRRSV-N after in vitro stimulation with autologous PBMCs expressing PRRSV-M and PRRSV-N, respectively (Fig. 8A and B). The mean concentrations of IFN-γ produced from the two T-cell populations were 605 and 478 pg/ml, respectively. Similar results were noted for the T cells of PBMCs obtained from the pigs immunized with the two DNA vaccines, under conditions in which they were stimulated with DCs expressing the PRRSV-M and PRRSV-N antigens, respectively (Fig. 8C and D). However, in the case of the other cytokines, such as IL-6 and IL-10, their yields did not differ significantly from those generated in the T cells of the nonimmunized pigs. Their levels of IL-6 and IL-10 were consistently below 100 pg/ml (data not shown). These results would appear to indicate that the PRRSV-M protein has a more profound T-cell immunogenic effect than the PRRSV-N protein in immunized pigs.
FIG. 8.
IFN-γ production from T cells. T cell-containing PBMCs were obtained from immunized pigs and nonimmunized pigs (control pigs C1 and C2). They were stimulated for 3 days by APCs at an effector cell-to-APC ratio of 5:1. PBMCs and DCs transduced with TAT-conjugated PRRSV-M or PRRSV-N proteins were used as APCs. Effector T cells from pigs immunized with the pcDNA3.1-GM-CSF-PRRSV-M (pigs M1 to M3) were stimulated by PBMCs (A) and DCs (C). Effector T cells from pigs immunized with pcDNA3.1-GM-CSF-PRRSV-N (pigs N1 to N3) were stimulated by PBMCs (B) and DCs (D). The amounts of IFN-γ in the cell culture supernatants were determined by ELISA. Statistical significance was determined by Student t test: **, P < 0.01; ***, P < 0.001.
DISCUSSION
In this study, we compared the different immune responses induced in conventional pigs that were systematically immunized with plasmid DNAs encoding the GM-CSF-fused M and N proteins of PRRSV. We confirmed that the M protein has a profound capacity to induce cell-mediated immunity and that this ability of the M protein is significantly greater than that of the N protein. The methodology presented herein represents a practical approach that can be utilized for the primary scanning of other structural or nonstructural proteins of PRRSV, allowing the identification of the components principally involved in cell-mediated immunity. Since GM-CSF has previously been demonstrated to have potent adjuvant activity (33, 34, 45), we fused the porcine GM-CSF gene with the PRRSV-M and PRRSV-N genes in two plasmid DNAs to enhance the immune responses to the M and N proteins in the immunized pigs. We did not address the adjuvant effect of GM-CSF but focused principally on the distinct cellular and humoral immune responses generated by the M and N proteins commonly conjugated with GM-CSF. Despite the intrinsic difficulty presented by DNA immunization, which in our case required a series of repeated immunizations to develop a measurable cell-mediated response, this approach allowed us to compare the immunogenic characteristics of two immunogens. During the preparation of the manuscript, we referred to a recently published report showing that GM-CSF could augment the humoral and cellular immune responses to GP3 and GP5 of PRRSV in vaccinated pigs (40).
We also utilized the intact protein delivery capacity of the HIV-1 TAT gene to generate autologous APCs. The recombinant PRRSV-M and PRRSV-N proteins fused with the HIV-1 TAT gene were successfully translocated into primary porcine cells, such as PBMCs and DCs. When the porcine PBMCs and DCs that were transduced with the TAT-conjugated PRRSV-M or PRRSV-N protein were cocultured with PBMCs, they induced T-cell proliferation. This phenomenon was identified only in pigs immunized with the plasmids expressing PRRSV-M and PRRSV-N and not in the nonimmunized pigs. These results clearly indicate that the memory T cells generated in the pigs immunized with the PRRSV-M and PRRSV-N proteins could recognize the PRRSV-M and PRRSV-N antigens presented by the MHC-matched autologous APCs. Therefore, we anticipate that this newly developed protocol for the generation of MHC-matched autologous APCs should facilitate the analysis of T-cell responses in outbred experimental pigs after infection with various viruses or vaccines.
The principal characteristics of PRRSV infection are persistence and the deterred clearance of virus owing to the delay of IFN-γ and neutralizing antibody production in infected pigs (22, 26, 42). The T cell-mediated immune responses represented by IFN-γ are important in the control of PRRSV infections (1, 23). The study established with the PRRSV-modified live vaccines has also suggested that cell-mediated immune responses are the primary protective mechanism (47). Therefore, it is important to determine which PRRSV proteins are involved in the induction of IFN-γ. In a previously published report, the PRRSV-M protein induced the strongest T-cell responses, whereas the PRRSV-N protein induced a rather weak T cell-stimulating capacity (2). Such features of the PRRSV M and N structural proteins have been confirmed in this study. Under in vitro conditions, the stimulation of T cells with the APCs expressing the PRRSV-M antigen always generated significantly higher yields of IFN-γ than those detected with PRRSV-N. On the other hand, it was also suggested that the PRRSV-N protein-mediated cellular immune responses might prove relevant to efforts in the control of viral replication (29, 43). Therefore, more careful evaluations may be required to determine the T cell-mediated immune responses to the PRRSV-N protein. The recent identification of immunodominant T-cell epitopes in the N protein implies that the N protein also performs an important function in cellular immunity to PRRSV (9).
Another feature of immune responses in PRRSV-infected pigs is the increased production of IL-10, a typical suppressor of Th1-type immune responses (8, 35). The increased level of IL-10 expression would be expected to exert a suppressive effect on the expression of IFN-γ and TNF-α, both of which evidence antiviral activities (4). By way of contrast, the reduction of the level of IL-10 production in PRRSV-infected pigs was associated with viral clearance (17). It was also reported that pigs infected in utero evidenced enhanced expression of IL-6 and IL-10 mRNAs from their PBMCs (10). Therefore, these two cytokines mediate the suppression of the immune responses necessary for viral clearance in the PRRSV-infected pigs. We measured the levels of production of IL-6 and IL-10 in order to determine which cytokines are induced by the PRRSV-M and PRRSV-N proteins. However, the levels of IL-6 and IL-10 production were rather low in both the immunized and the control pigs. Therefore, the individual PRRSV-M and PRRSV-N proteins do not appear to induce T cells to generate these cytokines compared to the induction ability of whole virus.
Antibodies to the major structural proteins of PRRSV (GP5, M, and N) are generally detected within 1 to 2 weeks postinfection (21, 44). Although antibodies to N protein are the most abundant, they generally do not have neutralizing activity. Instead, antibodies against GP5 function as the most effective neutralizing antibodies (13, 44). In this study, we confirmed that the PRRSV-N protein functions as a good immunogen for the earlier and higher level of induction of antibodies compared to the time of antibody induction and the level of antibody induced by the M protein. The antibodies to the N protein, however, did not neutralize the virus (21, 44). Our laboratory results also confirmed that the N-specific antibody had no neutralizing effect (data not shown). It is known that two major B-cell epitopes are located at the C-terminal region of the PRRSV-M protein (7). In this study, we utilized the plasmid DNA encompassing the B-cell epitope region of the PRRSV-M gene to immunize the experimental pigs. However, we detected no M protein-specific antibodies in the DNA-immunized pigs. This sort of insufficient or absent antibody production was occasionally detected in the pigs immunized with plasmid DNAs expressing the M protein (19). M and GP5 exist as heterodimers linked by disulfide bonds in the virus particles and infected cells (24). Recombinant vaccinia virus coexpressing the GP5 and M proteins generated more antibodies to the M protein in infected mice. However, no antibodies were detected when the mice were infected only with M protein-expressing vaccinia virus (46). Therefore, we suggest that the M protein expressed by plasmid DNA in our case was not sufficient, in either its function or its conformation, to induce antibody in the pigs without interacting with GP5.
In conclusion, use of the plasmid DNA approach and autologous APCs was shown to be useful for the characterization of the PRRSV structural proteins that induce cell-mediated immunity. The PRRSV-M protein served as a potent antigen for T cell-mediated immune responses but proved insufficient to elicit antibodies by itself. By way of contrast, the PRRSV-N protein generated a high level of antibody and also some degree of T-cell response. The identification of T-cell epitopes on the PRRSV-M protein may provide us with some valuable information and help us to gain a better understanding of immunity to PRRSV.
Acknowledgments
This work was supported by a South Korea Research Foundation grant funded by the South Korean Government (MOEHRD) (grant KRF-2006-331-E00353).
Footnotes
Published ahead of print on 3 February 2010.
REFERENCES
- 1.Bautista, E. M., and T. W. Molitor. 1999. IFN gamma inhibits porcine reproductive and respiratory syndrome virus replication in macrophages. Arch. Virol. 144:1191-1200. [DOI] [PubMed] [Google Scholar]
- 2.Bautista, E. M., P. Suarez, and T. W. Molitor. 1999. T cell responses to the structural polypeptides of porcine reproductive and respiratory syndrome virus. Arch. Virol. 144:117-134. [DOI] [PubMed] [Google Scholar]
- 3.Becker-Hapak, M., S. S. McAllister, and S. F. Dowdy. 2001. TAT-mediated protein transduction into mammalian cells. Methods 24:247-256. [DOI] [PubMed] [Google Scholar]
- 4.Charerntantanakul, W., R. Platt, and J. A. Roth. 2006. Effects of porcine reproductive and respiratory syndrome virus-infected antigen-presenting cells on T cell activation and antiviral cytokine production. Viral Immunol. 19:646-661. [DOI] [PubMed] [Google Scholar]
- 5.Conzelmann, K. K., N. Visser, P. Van Woensel, and H. J. Thiel. 1993. Molecular characterization of porcine reproductive and respiratory syndrome virus, a member of the arterivirus group. Virology 193:329-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Bruin, M. G., E. M. van Rooij, J. J. Voermans, Y. E. de Visser, A. T. Bianchi, and T. G. Kimman. 1997. Establishment and characterization of porcine cytolytic cell lines and clones. Vet. Immunol. Immunopathol. 59:337-347. [DOI] [PubMed] [Google Scholar]
- 7.de Lima, M., A. K. Pattnaik, E. F. Flores, and F. A. Osorio. 2006. Serologic marker candidates identified among B-cell linear epitopes of Nsp2 and structural proteins of a North American strain of porcine reproductive and respiratory syndrome virus. Virology 353:410-421. [DOI] [PubMed] [Google Scholar]
- 8.Diaz, I., L. Darwich, G. Pappaterra, J. Pujols, and E. Mateu. 2005. Immune responses of pigs after experimental infection with a European strain of porcine reproductive and respiratory syndrome virus. J. Gen. Virol. 86:1943-1951. [DOI] [PubMed] [Google Scholar]
- 9.Diaz, I., J. Pujols, L. Ganges, M. Gimeno, L. Darwich, M. Domingo, and E. Mateu. 2009. In silico prediction and ex vivo evaluation of potential T-cell epitopes in glycoproteins 4 and 5 and nucleocapsid protein of genotype-I (European) of porcine reproductive and respiratory syndrome virus. Vaccine 27:5603-5611. [DOI] [PubMed] [Google Scholar]
- 10.Feng, W. H., M. B. Tompkins, J. S. Xu, H. X. Zhang, and M. B. McCaw. 2003. Analysis of constitutive cytokine expression by pigs infected in-utero with porcine reproductive and respiratory syndrome virus. Vet. Immunol. Immunopathol. 94:35-45. [DOI] [PubMed] [Google Scholar]
- 11.Flores-Mendoza, L., E. Silva-Campa, M. Resendiz, F. A. Osorio, and J. Hernandez. 2008. Porcine reproductive and respiratory syndrome virus infects mature porcine dendritic cells and up-regulates interleukin-10 production. Clin. Vaccine Immunol. 15:720-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frankel, A. D., and C. O. Pabo. 1988. Cellular uptake of the Tat protein from human immunodeficiency virus. Cell 55:1189-1193. [DOI] [PubMed] [Google Scholar]
- 13.Gonin, P., B. Pirzadeh, C. A. Gagnon, and S. Dea. 1999. Seroneutralization of porcine reproductive and respiratory syndrome virus correlates with antibody response to the GP5 major envelope glycoprotein. J. Vet. Diagn. Invest. 11:20-26. [DOI] [PubMed] [Google Scholar]
- 14.Green, M., and P. M. Loewenstein. 1988. Autonomous functional domains of chemically synthesized human immunodeficiency virus Tat trans-activator protein. Cell 55:1179-1188. [DOI] [PubMed] [Google Scholar]
- 15.Guzylack-Piriou, L., S. Piersma, K. McCullough, and A. Summerfield. 2006. Role of natural interferon-producing cells and T lymphocytes in porcine monocyte-derived dendritic cell maturation. Immunology 118:78-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang, W., P. Jiang, Y. Li, J. Tang, X. Wang, and S. Ma. 2006. Recombinant adenovirus expressing GP5 and M fusion proteins of porcine reproductive and respiratory syndrome virus induce both humoral and cell-mediated immune responses in mice. Vet. Immunol. Immunopathol. 113:169-180. [DOI] [PubMed] [Google Scholar]
- 17.Johnsen, C. K., A. Botner, S. Kamstrup, P. Lind, and J. Nielsen. 2002. Cytokine mRNA profiles in bronchoalveolar cells of piglets experimentally infected in utero with porcine reproductive and respiratory syndrome virus: association of sustained expression of IFN-gamma and IL-10 after viral clearance. Viral Immunol. 15:549-556. [DOI] [PubMed] [Google Scholar]
- 18.Kimman, T. G., L. A. Cornelissen, R. J. Moormann, J. M. Rebel, and N. Stockhofe-Zurwieden. 2009. Challenges for porcine reproductive and respiratory syndrome virus (PRRSV) vaccinology. Vaccine 27:3704-3718. [DOI] [PubMed] [Google Scholar]
- 19.Kwang, J., F. Zuckermann, G. Ross, S. Yang, F. Osorio, W. Liu, and S. Low. 1999. Antibody and cellular immune responses of swine following immunisation with plasmid DNA encoding the PRRS virus ORF's 4, 5, 6 and 7. Res. Vet. Sci. 67:199-201. [DOI] [PubMed] [Google Scholar]
- 20.Laval, F., R. Paillot, S. Bollard, L. Fischer, J. C. Audonnet, C. Andreoni, and V. Juillard. 2002. Quantitative analysis of the antigen-specific IFNgamma+ T cell-mediated immune response in conventional outbred pigs: kinetics and duration of the DNA-induced IFNgamma+ CD8+ T cell response. Vet. Immunol. Immunopathol. 90:191-201. [DOI] [PubMed] [Google Scholar]
- 21.Loemba, H. D., S. Mounir, H. Mardassi, D. Archambault, and S. Dea. 1996. Kinetics of humoral immune response to the major structural proteins of the porcine reproductive and respiratory syndrome virus. Arch. Virol. 141:751-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopez, O. J., and F. A. Osorio. 2004. Role of neutralizing antibodies in PRRSV protective immunity. Vet. Immunol. Immunopathol. 102:155-163. [DOI] [PubMed] [Google Scholar]
- 23.Lowe, J. E., R. Husmann, L. D. Firkins, F. A. Zuckermann, and T. L. Goldberg. 2005. Correlation of cell-mediated immunity against porcine reproductive and respiratory syndrome virus with protection against reproductive failure in sows during outbreaks of porcine reproductive and respiratory syndrome in commercial herds. J. Am. Vet. Med. Assoc. 226:1707-1711. [DOI] [PubMed] [Google Scholar]
- 24.Mardassi, H., B. Massie, and S. Dea. 1996. Intracellular synthesis, processing, and transport of proteins encoded by ORFs 5 to 7 of porcine reproductive and respiratory syndrome virus. Virology 221:98-112. [DOI] [PubMed] [Google Scholar]
- 25.Martelli, P., S. Gozio, L. Ferrari, S. Rosina, E. De Angelis, C. Quintavalla, E. Bottarelli, and P. Borghetti. 2009. Efficacy of a modified live porcine reproductive and respiratory syndrome virus (PRRSV) vaccine in pigs naturally exposed to a heterologous European (Italian cluster) field strain: clinical protection and cell-mediated immunity. Vaccine 27:3788-3799. [DOI] [PubMed] [Google Scholar]
- 26.Meier, W. A., J. Galeota, F. A. Osorio, R. J. Husmann, W. M. Schnitzlein, and F. A. Zuckermann. 2003. Gradual development of the interferon-gamma response of swine to porcine reproductive and respiratory syndrome virus infection or vaccination. Virology 309:18-31. [DOI] [PubMed] [Google Scholar]
- 27.Meulenberg, J. J., A. P. van Nieuwstadt, A. van Essen-Zandbergen, and J. P. Langeveld. 1997. Posttranslational processing and identification of a neutralization domain of the GP4 protein encoded by ORF4 of Lelystad virus. J. Virol. 71:6061-6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mezrich, J. D., G. W. Haller, J. S. Arn, S. L. Houser, J. C. Madsen, and D. H. Sachs. 2003. Histocompatible miniature swine: an inbred large-animal model. Transplantation 75:904-907. [DOI] [PubMed] [Google Scholar]
- 29.Rompato, G., E. Ling, Z. Chen, H. Van Kruiningen, and A. E. Garmendia. 2006. Positive inductive effect of IL-2 on virus-specific cellular responses elicited by a PRRSV-ORF7 DNA vaccine in swine. Vet. Immunol. Immunopathol. 109:151-160. [DOI] [PubMed] [Google Scholar]
- 30.Rossow, K. D. 1998. Porcine reproductive and respiratory syndrome. Vet. Pathol. 35:1-20. [DOI] [PubMed] [Google Scholar]
- 31.Schwarze, S. R., A. Ho, A. Vocero-Akbani, and S. F. Dowdy. 1999. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science 285:1569-1572. [DOI] [PubMed] [Google Scholar]
- 32.Shibagaki, N., and M. C. Udey. 2002. Dendritic cells transduced with protein antigens induce cytotoxic lymphocytes and elicit antitumor immunity. J. Immunol. 168:2393-2401. [DOI] [PubMed] [Google Scholar]
- 33.Somasundaram, C., H. Takamatsu, C. Andreoni, J. C. Audonnet, L. Fischer, F. Lefevre, and B. Charley. 1999. Enhanced protective response and immuno-adjuvant effects of porcine GM-CSF on DNA vaccination of pigs against Aujeszky's disease virus. Vet. Immunol. Immunopathol. 70:277-287. [DOI] [PubMed] [Google Scholar]
- 34.Sun, X., L. M. Hodge, H. P. Jones, L. Tabor, and J. W. Simecka. 2002. Co-expression of granulocyte-macrophage colony-stimulating factor with antigen enhances humoral and tumor immunity after DNA vaccination. Vaccine 20:1466-1474. [DOI] [PubMed] [Google Scholar]
- 35.Suradhat, S., R. Thanawongnuwech, and Y. Poovorawan. 2003. Upregulation of IL-10 gene expression in porcine peripheral blood mononuclear cells by porcine reproductive and respiratory syndrome virus. J. Gen. Virol. 84:453-459. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka, Y., S. F. Dowdy, D. C. Linehan, T. J. Eberlein, and P. S. Goedegebuure. 2003. Induction of antigen-specific CTL by recombinant HIV trans-activating fusion protein-pulsed human monocyte-derived dendritic cells. J. Immunol. 170:1291-1298. [DOI] [PubMed] [Google Scholar]
- 37.van Rooij, E. M., H. L. Glansbeek, L. A. Hilgers, E. G. te Lintelo, Y. E. de Visser, W. J. Boersma, B. L. Haagmans, and A. T. Bianchi. 2002. Protective antiviral immune responses to pseudorabies virus induced by DNA vaccination using dimethyldioctadecylammonium bromide as an adjuvant. J. Virol. 76:10540-10545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Viehl, C. T., Y. Tanaka, T. Chen, D. M. Frey, A. Tran, T. P. Fleming, T. J. Eberlein, and P. S. Goedegebuure. 2005. Tat mammaglobin fusion protein transduced dendritic cells stimulate mammaglobin-specific CD4 and CD8 T cells. Breast Cancer Res. Treat. 91:271-278. [DOI] [PubMed] [Google Scholar]
- 39.Wang, X., M. Eaton, M. Mayer, H. Li, D. He, E. Nelson, and J. Christopher-Hennings. 2007. Porcine reproductive and respiratory syndrome virus productively infects monocyte-derived dendritic cells and compromises their antigen-presenting ability. Arch. Virol. 152:289-303. [DOI] [PubMed] [Google Scholar]
- 40.Wang, X., J. Li, P. Jiang, Y. Li, B. Zeshan, and J. Cao. 2009. GM-CSF fused with GP3 and GP5 of porcine reproductive and respiratory syndrome virus increased the immune responses and protective efficacy against virulent PRRSV challenge. Virus Res. 143:24-32. [DOI] [PubMed] [Google Scholar]
- 41.Wensvoort, G. 1993. Lelystad virus and the porcine epidemic abortion and respiratory syndrome. Vet. Res. 24:117-124. [PubMed] [Google Scholar]
- 42.Wills, R. W., A. R. Doster, J. A. Galeota, J. H. Sur, and F. A. Osorio. 2003. Duration of infection and proportion of pigs persistently infected with porcine reproductive and respiratory syndrome virus. J. Clin. Microbiol. 41:58-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xue, Q., Y. G. Zhao, Y. J. Zhou, H. J. Qiu, Y. F. Wang, D. L. Wu, Z. J. Tian, and G. Z. Tong. 2004. Immune responses of swine following DNA immunization with plasmids encoding porcine reproductive and respiratory syndrome virus ORFs 5 and 7, and porcine IL-2 and IFNgamma. Vet. Immunol. Immunopathol. 102:291-298. [DOI] [PubMed] [Google Scholar]
- 44.Yoon, K. J., J. J. Zimmerman, S. L. Swenson, M. J. McGinley, K. A. Eernisse, A. Brevik, L. L. Rhinehart, M. L. Frey, H. T. Hill, and K. B. Platt. 1995. Characterization of the humoral immune response to porcine reproductive and respiratory syndrome (PRRS) virus infection. J. Vet. Diagn. Invest. 7:305-312. [DOI] [PubMed] [Google Scholar]
- 45.Zhang, X., M. Divangahi, P. Ngai, M. Santosuosso, J. Millar, A. Zganiacz, J. Wang, J. Bramson, and Z. Xing. 2007. Intramuscular immunization with a monogenic plasmid DNA tuberculosis vaccine: enhanced immunogenicity by electroporation and co-expression of GM-CSF transgene. Vaccine 25:1342-1352. [DOI] [PubMed] [Google Scholar]
- 46.Zheng, Q., D. Chen, P. Li, Z. Bi, R. Cao, B. Zhou, and P. Chen. 2007. Co-expressing GP5 and M proteins under different promoters in recombinant modified vaccinia virus Ankara (rMVA)-based vaccine vector enhanced the humoral and cellular immune responses of porcine reproductive and respiratory syndrome virus (PRRSV). Virus Genes 35:585-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zuckermann, F. A., E. A. Garcia, I. D. Luque, J. Christopher-Hennings, A. Doster, M. Brito, and F. Osorio. 2007. Assessment of the efficacy of commercial porcine reproductive and respiratory syndrome virus (PRRSV) vaccines based on measurement of serologic response, frequency of gamma-IFN-producing cells and virological parameters of protection upon challenge. Vet. Microbiol. 123:69-85. [DOI] [PubMed] [Google Scholar]