Abstract
Human papillomavirus (HPV) virus-like particle (VLP) vaccines are highly effective at preventing viral infections and the development of precancerous lesions through the induction of high-titer neutralizing antibodies and strong cell-mediated immune responses. Women taking combined oral contraceptives (COCs), however, show large variabilities in the magnitudes of their antibody responses. The goal of the present study was to determine the effects of 17β-estradiol (E2) and progesterone (P4) alone and in combination on the cellular immune response to HPV type 16 (HPV-16) VLPs in vitro. Peripheral blood mononuclear cells (PBMCs) from healthy donor women were stimulated in vitro with HPV-16 VLPs (2.5 μg/ml) in the presence of E2 and P4 administered either alone or in combination; and lymphoproliferation, cytokine production, transcription factor expression, and steroid hormone receptor expression were analyzed. HPV-16 VLPs significantly increased the levels of lymphoproliferation, proinflammatory cytokine (gamma interferon [IFN-γ], interleukin-1β [IL-1β], IL-2, IL-6, IL-8, IL-12p70, IL-17, tumor necrosis factor alpha [TNF-α]) production, anti-inflammatory cytokine (IL-1ra, IL-10) production, and the expression of Erα and Erβ but decreased the levels of Foxp3 expression and production of transforming growth factor β (TGF-β). Exposure of PBMCs to E2 and P4 either alone or in combination significantly decreased the levels of lymphoproliferation and production of proinflammatory cytokines (IFN-γ, IL-12p70, TNF-α) but increased the levels of production of IL-10 and TGF-β and the expression of Foxp3 in response to HPV-16 VLPs. Treatment of cells with biologically relevant concentrations of sex steroid hormones suppressed the inflammatory response and enhanced the regulatory response to HPV-16 VLPs, which may have implications for predicting the long-term efficacy of HPV vaccines, adverse events, and cross-protection among women taking COCs.
Cervical cancer is the second most common cancer among women worldwide. The recent introduction of prophylactic vaccines against the human papillomavirus (HPV) are highly effective in reducing viral persistence and the development of precancerous cervical lesions (2). HPV vaccines utilize noninfectious fully assembled viral capsid proteins, termed virus-like particles (VLPs). Neutralizing antibodies are presumed to be the main effector molecules of vaccine-induced protection in vivo (42). Additionally, vaccination induces strong cell-mediated immune responses characterized by the production of pro- and anti-inflammatory cytokines and chemokines, including interleukin-1α (IL-1α), IL-1β, IL-2, gamma interferon (IFN-γ), IL-4, IL-6, IL-8, IL-10, and IP-10 in vitro (10). Cytokines, in particular, helper T-cell type 2 (Th2) responses, are required for antibody production and maintenance. Alterations of these cytokine signals could, therefore, affect antibody production over time, with downstream consequences on vaccine efficacy and adverse clinical outcomes occurring.
Combined oral contraceptives (COCs) that contain the steroid hormones 17β-estradiol (E2) and progesterone (P4) are associated with an increased risk for cervical cancer among HPV-infected women. The use of COCs for >5 years was associated with a nearly 3-fold increase in the risk of a diagnosis of cancer compared to the risk for age-matched controls in a large multicountry case-control study of premenopausal women >30 years of age (24). Women using COCs who receive the HPV vaccine also showed large interindividual variabilities in serum and cervical concentrations of anti-HPV type 16 (anti-HPV-16) antibodies following vaccination relative to those for nonhormonal contraceptive users (26). We hypothesize that hormonal modulation of immune cell function may cause variability in the immune response to the HPV vaccine, which translates into differences in vaccine immunogenicity, long-term efficacy, and HPV type-specific cross-protection.
E2 and P4 are immunomodulators with distinct effects on the pro- and anti-inflammatory immune responses in vitro and in vivo (18). E2 displays a dose-dependent bidirectional effect on cytokine production (as reviewed in reference 38). At low concentrations (concentrations less than 10−8 M), E2 increases the concentrations of TNF-α (22), IL-6 (19), IL-1β (29), and IFN-γ (1) in human peripheral blood mononuclear cells (PBMCs) stimulated with either lipopolysaccharide or phytohemagglutinin-L (PHA-L). Conversely, at high concentrations (concentrations greater than 10−6 M), E2 suppresses the synthesis of tumor necrosis factor alpha (TNF-α) (1) and IL-1β (29) and increases the levels of production of the anti-inflammatory cytokines IL-10 (16) and IL-1ra (33). High-dose E2 also increases the regulatory T-cell function (30) and the levels of IL-10-dependent total immunoglobulin production (16). P4 at both low and high concentrations suppresses both pro- and anti-inflammatory cytokine production (3), lymphoproliferation (3), and regulatory T-cell function (23). Additionally, PBMCs isolated from women on E2 alone or combined hormone replacement therapy (HRT) have higher concentrations of anti-inflammatory cytokines (e.g., IL-1ra) and Th2 cytokines (e.g., IL-6) than women not on HRT (34). Additionally, elevated levels of IL-10 are detected in the cervical secretions of adolescent women reporting current COC use (36). Lastly, the immunomodulatory effects of sex steroid hormones affect the incidence and severity of several autoimmune diseases (6) and sexually transmitted infections, such as those caused by chlamydia (17) and herpes simplex virus type 2 (13). The effects of sex hormones on the immune responses to HPV in women, however, have not been reported.
The goal of the current study was to assess the effects of E2 and P4, administered alone and in combination at doses relevant to COC use, on the inflammatory response to HPV-16 VLPs among healthy women. We hypothesized that P4, either alone or in combination with E2, would alter cytokine production toward an anti-inflammatory/regulatory phenotype in response to HPV-16 VLPs.
MATERIALS AND METHODS
Study population and recruitment.
Twelve healthy premenopausal women (average age, 35 years; age range, 23 to 42 years) who reported no hormonal contraception or replacement therapy use in the 3 months prior to recruitment were asked to donate 100 ml of heparinized whole blood. Sample collection was performed during the follicular phase of the menstrual cycle (i.e., within the first 7 days after the beginning of menstrual bleeding).
PBMCs were isolated by Ficoll-Hypaque density centrifugation. Briefly, whole blood was layered on top of Ficoll and centrifuged at 2,500 rpm for 30 min. After centrifugation, the plasma was separated and stored at −80°C. The PBMCs were washed, the cells were counted, and viability was determined by trypan blue exclusion. After the cells were counted, they were centrifuged and resuspended in 90% fetal bovine serum (FBS)-10% dimethyl sulfoxide at a final concentration of 25 × 106 cells/ml, aliquoted, and slowly frozen to −80°C overnight. The cells were cryopreserved in liquid nitrogen and were stored until they were cultured. The study protocol for blood collection and storage of PBMCs was approved by the Institutional Review Board of the Johns Hopkins Bloomberg School of Public Health.
Anti-HPV-16 antibody detection.
Frozen blood plasma samples collected at the time of PBMC isolation from each participant were tested for the presence of anti-HPV-16 IgG by a colorimetric sandwich enzyme-linked immunosorbent assay (ELISA), as described previously (41). Briefly, HPV-16 VLPs that contained the L1 and L2 proteins were absorbed onto 96-well PolySorp ELISA plates (Nunc, Naperville, IL) overnight at 4°C at a concentration of 0.4 to 0.5 μg/ml in phosphate-buffered saline (PBS; pH 7.2). The plates were then blocked at room temperature for 3 h with 0.5% (wt vol−1) polyvinyl alcohol (PVA; Sigma, St. Louis, MO) in PBS. After the plates were blocked, they were washed, the plasma samples were diluted 1:10 in 0.5% PVA, and the plates were incubated at 37°C for 1 h. After incubation, the plates were washed and incubated with horseradish peroxidase-conjugated goat anti-human IgG (gamma chain specific; Zymed, San Francisco, CA) diluted 1:4,000 in 0.5% PVA-0.025% Tween 20-0.8% (wt vol−1) polyvinylpyrrolidone (PVP; Sigma) in PBS at 37°C for 30 min. The plates were washed and incubated with 2,2′-azino-di-(3-ethylbednzthiazoline-6-sulfonate) hydrogen peroxide (ABTS; Kirkegaard & Perry, Gaithersburg, MD) for 20 min, the reaction was stopped by addition of 1% sodium dodecyl sulfate, and the absorbance was measured at 405 nm by use of a reference wavelength of 490 nm. Seropositivity was determined by comparing the optical density values of the study samples to those of the plasma samples previously collected from women known to be virgins (i.e., women presumed to be unexposed to HPV).
Stimulants and antigens.
HPV-16 VLPs were prepared as described previously (39). Briefly, recombinant baculovirus containing the L1 open reading frame (ORF) of the HPV-16 genome was transfected into an Trichonusia ni (High Five) cell line. The VLPs were isolated and purified by cesium chloride and sucrose gradient centrifugation, followed by dialysis in PBS overnight. The VLPs were stored at a final concentration of 0.4 mg/ml in PBS at 4°C. The VLPs were added to the cell culture at a concentration of 2.5 μg/ml on the basis of the findings of previous studies (10).
The Limulus amebocyte lysate endotoxin assay was performed according to the manufacturer's protocol (Cambrex, East Rutherford, NJ) to confirm that there was no endotoxin contamination of the VLP preparation. Briefly, Limulus amebocyte lysate was added to the VLP preparation and the mixture was incubated for 10 min at 37°C. After incubation, the endotoxin concentration was visualized by use of a colorimetric reaction and was compared to the concentrations on a four-point standard curve. At a concentration of 0.4 mg/ml, the VLP preparation had <0.1 endotoxin units (EU)/ml.
Anti-CD3/CD28 antibodies were used as a positive control for T-cell stimulation in cell culture. The antibodies were purchased and stored at a concentration of 0.5 mg/ml at 4°C (BD Bioscience). Both antibodies were added to the cell culture at a final concentration of 1 μg/ml, on the basis of the results of dose-response experiments.
Hormones.
17β-Estradiol (E2) and 4-pregnen-3,20-dione (P4) (Steraloids Inc., Newport, RI) were resuspended at a concentration of 0.001 M in pure ethanol and were stored in single-use aliquots at −80°C prior to use. For use in cell culture, the hormones were diluted in cell culture medium to final concentrations of 10−6, 10−8, and 10−10 M. These concentrations reflect the serum hormone levels observed among women using COCs (8). The combined treatments contained E2 at a concentration of 10−8 M and P4 added at a concentration of either 10−6 M or 10−10 M, which reflect the concentrations used in different COC formulations. The final concentration of ethanol (0.0006%) in the cell culture was minimal. In a separate experiment, the cells were exposed to medium containing ethanol or no ethanol, and no effect of the ethanol on lymphoproliferation was observed (data not shown); thus, subsequent experiments involved the treatment of PBMCs with medium that did not contain ethanol.
PBMC cultures.
Cryopreserved PBMCs were gently thawed in a 37°C water bath, washed twice in RPMI 1640 without phenol red (MediaTech, Manassas, VA), and resuspended at a concentration of 2 × 106 cells/ml in RPMI 1640 without phenol red and 20% charcoal-dextran-stripped FBS (HyClone, Logan, UT). The PBMCs were cultured in 96-well culture plates at 37°C in RPMI 1640 without phenol red supplemented with 2 mM l-glutamine, 100 U penicillin-streptomycin, and 10% charcoal-dextran-stripped FBS. PBMCs stimulated with anti-CD3/CD28 were cultured for 72 h on the basis of the results of the time-series experiments. PBMCs stimulated with VLPs were cultured for 72 h on the basis of the findings of previous studies (10). The hormones and stimulants were added simultaneously at the beginning of cell culture. The cell supernatants were collected for cytokine measurement and were frozen at −80°C. The cell pellets were frozen at −80°C in Trizol (Invitrogen, Carlsbad, CA) and were used for analysis of the gene expression profiles.
Cytospin preparations and Diff-Quick staining.
Prior to culture, cryopreserved aliquots of PBMCs were thawed and resuspended in 1 ml of ice-cold PBS with 1% FBS and 1 mM EDTA. The cells were diluted to a concentration of 1 × 106 cells/ml. Diluted cell solutions were added to cytospin columns with a glass microscope slide, spun at 500 rpm for 5 min, dried on a heat block for 5 min, and then stained with Diff-Quick stain (Dade Behring Inc., Deerfield, IL) to differentiate the lymphocyte, monocyte, and neutrophil subpopulations. To confirm the cell morphology, 10 representative images of the cell types from two different PBMC samples were sent to two hematological pathologists. The numbers of lymphocytes, monocytes, and neutrophils from four representative fields at ×20 magnification were counted; and the amounts were expressed as a percentage of the total number of cells counted.
Lymphoproliferation assay.
Lymphoproliferation was measured by a nonradioactive assay according to the manufacturer's protocol (Promega, Madison, WI). Briefly, cell cultures were treated with a tetrazolium compound and an electron-coupling reagent and were incubated at 37°C for the last 4 h of the 72 h of incubation. Uptake and processing of these substances by lymphocytes produce a formazan salt that induces a colorimetric change that is measured by a spectrophotometer at 490 nm.
Cytotoxicity assay.
The cytotoxicity caused by hormone treatment of cells was assessed by a nonradioactive colorimetric assay that measures total lactate dehydrogenase (LDH) release from apoptotic cells into the supernatant (Promega). Briefly, frozen cell culture supernatants were thawed and aliquoted into 96-well cell culture plates. An electron-coupling reagent and tetrazolium salt were added, and the plates were incubated for 20 min. The reaction induces the production of a formazan that is correlated to the amount of LDH present in the supernatant. The amount of formazan was then estimated through a color change reaction measured by a spectrophotometer at 490 nm.
Cytokine measurements.
For experiments involving HPV-16 VLPs, the levels of the following cytokines were measured by a polystyrene bead-based multiplex assay, according to the manufacturer's protocol (Bio-Rad, Hercules, CA): IFN-γ, IL-1β, IL-1ra, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12p70, IL-17, and TNF-α. The plates were read with a Luminex instrument (Bio-Rad). This assay has a reported lower limit of detection of 1 to 3 pg/ml for each cytokine target and an intra-assay coefficient of variation <20%. The transforming growth factor β (TGF-β) concentrations in 7 of 12 participants were measured by ELISA, according to the manufacturer's protocol (R&D Systems, Minneapolis, MN). This assay has a reported lower limit of detection of 30 pg/ml and an intra-assay coefficient of variation of <10%.
RNA extraction and cDNA preparation.
PBMC pellets frozen in Trizol (Invitrogen) were thawed on ice and transferred to fastPrep homogenization tubes (MP Biomedicals, Salon, OH). After homogenization, the cells were incubated at room temperature for 5 min, treated with chloroform, and centrifuged; and the aqueous layer containing RNA was mixed with isopropyl alcohol and glycogen, according to the manufacturer's protocol (Invitrogen). The concentration of the extracted RNA was determined by spectrophotometric analysis (Nanodrop, Wilmington, DE). The RNA concentration and purity were standardized to 20 ng/μl for cDNA preparation. The purity of the extracted RNA was further assessed by confirming the presence of the 16S ribosomal subunit by using a microfluidic card-based gel detection system (Agilent, Santa Clara, CA).
cDNA was prepared by using 100 ng of input RNA. The RNA was treated with 1 U of DNase I (Invitrogen) to remove DNA contamination. After the DNase treatment, a random primer (100 ng/μl) and deoxynucleoside triphosphates (10 mM) were added to the RNA. Single-strand cDNA was prepared with Superscript II reverse transcriptase (200 U; Invitrogen) under the following reaction conditions: 42°C for 50 min and 70°C for 15 min.
Real-time PCR.
Quantification of the mRNA transcripts of Foxp3, estrogen receptor (ER) α (Erα), estrogen receptor β (Erβ), progesterone receptor (PR; Pgr), glucocorticoid receptor (GR; Gcr), and beta-actin (βactin) was performed by real-time PCR. The primer and probe sequences were purchased as premixed reagents from Applied Biosystems (Foster City, CA). The PCRs were performed in a final volume of 25 μl containing 2× universal master mix without uracil N-glycosylase (Applied Biosystems), 20× primer-probe mixtures, and 1 μl of input cDNA. The thermocycling conditions were as follows: 50°C for 2 min, 95°C for 10 min, and 50 cycles of 95°C for 15 s and 60°C for 1 min. Relative expression values were generated by using the −ΔΔ method (21).
Statistical analyses.
Proliferation, cytokine production, and gene expression values were compared across the stimulation conditions and the hormone treatment groups by two-way analysis of variance (ANOVA). Cytokine values were log10 transformed. A stimulation index (SI) of proliferation, cytokine production, and gene expression was generated by normalizing the responses for PBMCs treated with hormones to those for PBMCs not treated with hormones (non-hormone-treated controls) under a matched stimulation condition: SI = (X with hormone + medium)/(X with medium), where X is the response.
Student's t test and the Wilcoxon rank-sum test were used to assess the differences in the immune cell responses within a specific hormone treatment group compared with those in the non-hormone-treated controls under a given stimulation condition. Differences were considered significant if P was <0.05.
Hierarchical cluster analysis was performed to explore the effects of hormones on global changes in the pro- and anti-inflammatory immune responses characterized by groups of cytokines that share similar phenotypes. Clustering was assessed by using the Euclidian minimal distance method of the SI values for each cytokine, and heat maps were generated (Partek, St. Louis, MO). Cytokines were classified as either pro- or anti-inflammatory a priori. Cytokines identified as proinflammatory included IFN-γ, IL-1β, IL-2, IL-6, IL-8, IL-12p70, IL-17, and TNF-α. Cytokines considered anti-inflammatory were IL-1ra, IL-4, IL-5, IL-10, and TGF-β. The validity of the observed clusters was evaluated on the basis of their recognized known relationship to pro- and anti-inflammatory immune responses.
Three of 12 women included in this study had detectable anti-HPV-16 antibodies. Exclusion of the data for these women from the analysis did not alter the effects of HPV-16 VLP or E2 and P4 on immune marker expression (data not shown).
RESULTS
Cellular composition of PBMC specimens from healthy women.
The cellular composition and diversity across PBMC isolates from our study population were evaluated by using cytospin preparations and nuclear and membrane staining of cryopreserved specimens prior to culture. The mean number of cells counted per participant was 52 ± 9 per visual field. The average proportions of lymphocytes, monocytes, and polymorphonuclear cells were 46% (95% confidence interval [CI], 37% to 54%), 16% (95% CI, 13% to 19%), and 39% (95% CI, 30% to 47%), respectively. The PBMC specimens used in this study were primarily composed of both lymphocytes and polymorphonuclear cells. There was no difference in cell composition by the age of the study participants sampled.
HPV-16 VLPs induce cellular proliferation.
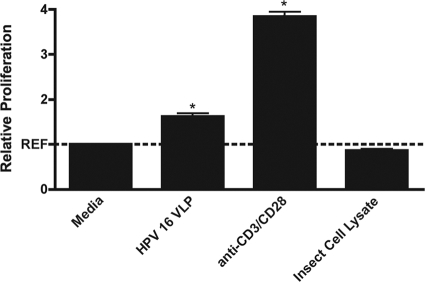
Treatment of PBMCs with HPV-16 VLPs alone induced a 61% ± 0.09% increase in the level of lymphoproliferation compared to that achieved by treatment of PBMCs with medium alone (P < 0.001). Given the high percentage of lymphocytes present in each PBMC cell culture, anti-CD3/CD28 was added to cultures as a positive control for T-cell stimulation. Treatment with this pan-T-cell stimulant alone caused a nearly 4-fold increase in the level of lymphoproliferation compared to that for cells treated with medium alone (P < 0.001). The insect cell lysate in which VLPs were generated was used as a negative control to control for the nonspecific stimulation caused by the lysate. Treatment of the PBMCs with cell lysate alone did not induce proliferation to a level above that for cells treated with medium alone (Fig. 1).
FIG. 1.
Mean relative lymphoproliferation (± standard error of the mean) in PBMCs stimulated with HPV-16 VLPs, anti-CD3/CD28, or insect cell lysate compared with that in cells treated with medium alone. *, P < 0.05, by Student's t test.
HPV-16 VLPs induce expression of hormone receptors.
Sex steroid hormones elicit their effect on human leukocyte populations by interacting with nuclear and membrane receptors (7, 38). Previous studies of isolated leukocyte populations in human PBMCs have identified both estrogen receptor isoforms (ERα and ERβ) as well as the glucocorticoid receptor (GR) on monocytes, T cells, and B cells from healthy women (27, 35). In order to determine whether HPV-16 VLPs potentiate hormone action in PBMCs, Erα, Erβ, Pgr, and Gcr mRNA transcripts were quantified. Erα, Erβ, and Gcr, but not Pgr, were detected in PBMCs. Stimulation with HPV-16 VLPs induced 10-fold and 11-fold increases in the levels of Erα and Erβ expression, respectively (P < 0.05), relative to the levels of expression in PBMCs exposed to medium alone (Fig. 2). Gcr expression was nonsignificantly upregulated by HPV-16 VLP stimulation compared to the level of upregulation in cells exposed to medium alone.
FIG. 2.
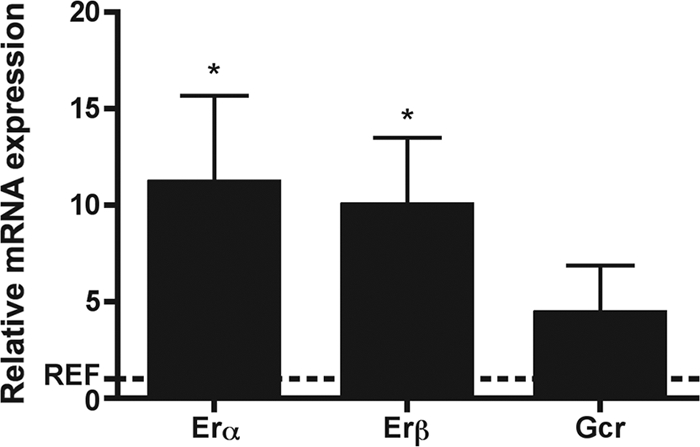
Mean relative levels of expression of Erα, Erβ, and Gcr (± standard error of the mean) in PBMCs stimulated with HPV-16 VLPs compared with that in cells treated with medium alone. *, P < 0.05, by the Wilcoxon rank-sum test.
HPV-16 VLP induces pro- and anti-inflammatory cytokine production.
HPV-16 VLPs induced the upregulation of IFN-γ, IL-1β, IL-1ra, IL-2, IL-6, IL-8, IL-10, IL-12p70, IL-17, and TNF-α (P < 0.05) in PBMCs compared with the level of regulation in cells treated with medium alone. Conversely, lower concentrations of TGF-β were observed among PBMCs stimulated with HPV-16 VLP compared to the concentrations observed among PBMCs treated with medium alone (P < 0.05) (Table 1).
TABLE 1.
Mean cytokine concentrations of PBMCs stimulated with HPV-16 VLPs and medium alone
| Cytokine | Mean cytokine concn (±SEM ) |
P value | |
|---|---|---|---|
| Medium alone | HPV-16 VLPs | ||
| Proinflammatory cytokines | |||
| IFN-γ | 1.34 (0.33) | 2,167.7 (655.5) | 0.007 |
| IL-1β | 1.98 (0.98) | 1,250.8 (289.8) | 0.001 |
| IL-2 | 4.35 (0.90) | 10.5 (1.7) | 0.005 |
| IL-6 | 35 (24.6) | 16,577.4 (3,533.9) | 0.001 |
| IL-8 | 1,948.1 (847.4) | 158,965.1 (47,399.3) | 0.007 |
| IL-12p70 | 0.3 (0.03) | 42.6 (19.5) | 0.05 |
| IL-17 | 1.15 (0.15) | 29.7 (6.9) | 0.002 |
| TNF-α | 2.10 (0.44) | 1,984.9 (456.9) | 0.001 |
| Anti-inflammatory cytokines | |||
| IL-1ra | 116.9 (21.5) | 2,542.8 (585) | 0.002 |
| IL-5 | 0.25 (0.02) | 1.31 (0.29) | 0.08 |
| IL-10 | 1.14 (0.25) | 144.15 (40.9) | 0.005 |
| TGF-βa | 1,108.3 (103.4) | 768.2 (104.4) | 0.040 |
n = 7.
Sex hormones affect basal and HPV-16 VLP-induced proliferation.
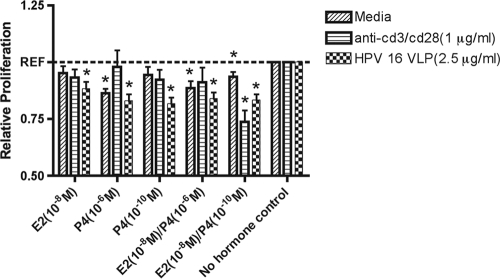
E2 and P4 reduced the levels of lymphoproliferation of PBMCs under basal (P < 0.001), HPV-16 VLP-stimulated (P < 0.001), and anti-CD3/CD28-stimulated (P < 0.005) conditions (Fig. 3). Among the cells stimulated with medium alone, the levels of proliferation of PBMCs were reduced 15%, 14%, and 8% in cells treated with P4 at 10−6 M (P < 0.001), E2 at 10−8 M plus P4 at 10−6 M (P < 0.005), or E2 at 10−8 M plus P4 at 10−10 M (P = 0.005), respectively, compared to the level for PBMCs exposed to medium alone. Under HPV-16 VLP-stimulated conditions, the proliferation of PBMCs was consistently reduced more than 20% in cells treated with P4 at 10−6 M (P < 0.001), P4 at 10−10 M (P < 0.001), or E2 at 10−8 M plus P4 at 10−10 M (P < 0.001) compared with the level for PBMCs stimulated with HPV-16 VLP alone. Among the cells stimulated with anti-CD3/CD28, the level of proliferation was reduced by more than 25% after treatment with E2 at 10−8 M plus P4 at 10−10 M (P < 0.001) compared to the level for PBMCs stimulated with anti-CD3/CD28 alone. To confirm that the reduced level of proliferation of PBMCs exposed to E2 and P4 was not due to the enhanced cytotoxicity caused by hormone treatment, a nonradioactive cytotoxicity assay was performed. No difference in cytotoxicity was observed before or after hormone treatment among cells treated with either HPV-16 VLP or medium alone (data not shown).
FIG. 3.
Mean relative lymphoproliferation (± standard error of the mean) in PBMCs treated with E2 or P4 alone or in combination compared with that in non-hormone-treated cells stimulated with either HPV-16 VLPs, anti-CD3/CD28, or medium alone. *, P < 0.05, by Student's t test.
Sex hormones shift pro- and anti-inflammatory cytokine responses to HPV-16 VLPs.
Treatment of PBMCs with E2 and P4 induced the differential regulation of pro- and anti-inflammatory cytokine production. Under basal conditions, treatment with P4 at 10−6 M increased the levels of production of IL-1β, IL-8, and IL-12p70 (P < 0.05) but decreased the level of IL-1ra production (P < 0.05). Treatment with E2 at 10−8 M plus P4 at 10−10 M increased the level of IL-12p70 production (P < 0.05), whereas treatment with E2 (10−8 M) alone decreased the level of production of TNF-α (P < 0.05) compared to that for non-hormone-treated PBMCs. Treatment of PBMCs with P4 (10−6 M) alone decreased the level of production of TGF-β compared to that for non-hormone-treated PBMCs (Table 2).
TABLE 2.
Mean relative basal cytokine production by PBMCs treated with E2 or P4, or both, compared with that by non-hormone-treated controls
| Cytokine | Mean relative basal level of cytokine production (±SEM) |
||||
|---|---|---|---|---|---|
| E2 (10−8 M) | P4 (10−6 M) | P4 (10−10 M) | E2 (10−8 M) + P4 (10−6 M) | E2 (10−8 M) + P2 (10−10 M) | |
| Proinflammatory markers | |||||
| IFN-γa | 1.16 (0.18) | 2.93 (1.66) | 2.15 (0.59) | 1.67 (0.40) | 1.08 (0.21) |
| IL-1β | 1.11 (0.25) | 3.90 (3.13) | 1.61 (0.46)b | 0.93 (0.11) | 2.69 (1.52) |
| IL-2 | 1.04 (0.19) | 0.93 (0.21) | 1.45 (0.50) | 0.96 (0.21) | 1.15 (0.15) |
| IL-6 | 1.17 (0.24) | 4.46 (3.68) | 1.58 (0.39) | 0.94 (0.13) | 4.62 (3.33) |
| IL-8 | 1.06 (0.21) | 1.33 (0.68) | 1.28 (0.22)b | 0.85 (0.11) | 1.76 (0.61) |
| IL-12 | 1.05 (0.09) | 1.29 (0.17) | 1.14 (0.08)b | 1.17 (0.08) | 1.10 (0.09)b |
| TNF-αc | 0.83 (0.41)b | 1.32 (0.68) | 0.92 (0.11) | 1.01 (0.19) | 1.56 (0.57) |
| Anti-inflammatory markers | |||||
| IL-1ra | 1.10 (0.15) | 0.88 (0.51) | 1.21 (0.15) | 0.94 (0.10) | 1.26 (0.19) |
| IL-10 | 3.74 (2.64) | 4.32 (2.68) | 2.33 (0.94) | 2.19 (1.03) | 3.99 (1.76) |
| TGF-βd | 0.98 (0.08) | 0.86 (0.04)b | 0.92 (0.07) | 0.94 (0.04) | 0.89 (0.05) |
The results for 5 of 12 participants were below the limit of detection.
P < 0.05.
The results for 2 of 12 participants were below the limit of detection.
n = 7.
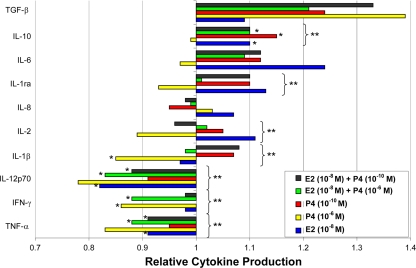
Following HPV-16 VLP stimulation, E2 and P4 administration alone or in combination caused a general reduction in the concentrations of IL-12p70 (P < 0.02), IFN-γ (P < 0.05), and TNF-α (P < 0.001) relative to those for non-hormone-treated PBMCs (Fig. 4). Conversely, hormone treatment resulted in higher concentrations of the anti-inflammatory cytokines IL-10 (P < 0.003) and IL-1ra (P < 0.03) in PBMCs compared with those in non-hormone-treated PBMCs stimulated with HPV-16 VLPs.
FIG. 4.
Mean relative cytokine production (± standard error of the mean) in PBMCs treated with E2 or P4 alone or in combination compared with that in non-hormone-treated cells stimulated with HPV-16 VLPs. The values presented on the x axis represent the relative difference (reference value = 1.0) in each cytokine concentration (y axis) due to hormone treatment compared to the cytokine concentration among PBMCs stimulated with HPV-16 VLPs but not treated with hormone. *, P < 0.05, by Student's t test, for comparison of the results for cells exposed to each individual hormone treatment and those for cells exposed to medium alone; **, P < 0.05, by two-way ANOVA, for comparison of the results for cells exposed to all hormone treatments and cells exposed to medium alone.
Among the PBMCs stimulated with HPV-16 VLPs, P4 alone at a concentration of 10−6 M significantly downregulated the production of IL-1β (SI = 0.85 ± 0.04; P < 0.001), IFN-γ (SI = 0.86 ± 0.03; P < 0.005), TNF-α (SI = 0.83 ± 0.03; P < 0.001), and IL-12p70 (SI = 0.78 ± 0.05 P = 0.001) compared with that for non-hormone-treated PBMCs (Fig. 4). IL-10 concentrations were increased among PBMCs treated with E2 at 10−8 M (SI = 1.10 ± 0.03; P < 0.05), P4 at 10−10 M (SI = 1.15 ± 0.02; P < 0.001), or E2 at 10−8 M plus P4 at 10−10 M (SI = 1.10 ± 0.03; P < 0.05) compared with those for non-hormone-treated PBMCs stimulated with HPV-16 VLPs. TGF-β concentrations were increased among PBMCs treated with P4 at 10−10 M (SI = 1.24 ± 0.09; P < 0.05). Among the HPV-16 VLP-stimulated PBMCs, cells treated with E2 at 10−8 M, E2 at 10−8 M plus P4 at 10−6 M, or E2 at 10−8 M plus P4 at 10−10 M had lower concentrations of TNF-α (for E2 at 10−8 M, SI = 0.91 ± 0.02 and P < 0.01; for E2 at 10−8 M plus P4 at 10−6 M, SI = 0.88 ± 0.02 and P < 0.001; for E2 at 10−8 M plus P4 at 10−10 M, SI = 0.91 ± 0.03 and P < 0.01) and IL-12p70 (for E2 at 10−8 M, SI = 0.82 ± 0.02 and P < 0.01; for E2 at 10−8 M plus P4 at 10−6 M, SI = 0.83 ± 0.04 and P < 0.01; for E2 at 10−8 M plus P4 at 10−10 M, SI = 0.88 ± 0.04 and P < 0.01). Lastly, among the PBMCs stimulated with HPV-16 VLPs, cells treated with E2 at 10−8 M plus P4 at 10−6 M had lower concentrations of IFN-γ (SI = 0.88 ± 0.03; P < 0.01) than non-hormone-treated PBMCs.
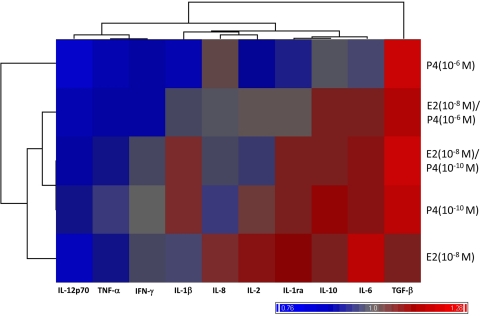
Correlation analyses were used to determine the presence of global shifts in the immune response to HPV-16 VLPs elicited by hormone treatment. Hierarchical cluster analysis by use of the Euclidean minimum-distance-method algorithm was performed on the group cytokine responses among PBMCs treated with hormones and non-hormone-treated PBMCs stimulated with HPV-16 VLPs. This analytic method yielded two primary clusters (Fig. 5). One cluster contained the cytokines IL-12p70, TNF-α, and IFN-γ, which were produced at lower concentrations in cells treated with E2 and P4 relative to the concentrations produced in non-hormone-treated PBMCs. The second cluster contained IL-10, IL-1ra, IL-2, IL-1β, and IL-6, which were generally produced at higher concentrations in E2- and P4-treated PBMCs relative to the concentrations produced in non-hormone-treated PBMCs, as well as IL-1β, IL-8, and IL-2, which were differentially up or downregulated on the basis of the hormone concentration. Lastly, the TGF-β responses were defined in their own cluster separate from the two clusters described above. Interestingly, the cluster that contained the anti-inflammatory cytokines IL-10 and IL-1ra also contained the proinflammatory cytokines IL-1β and IL-6, as well as IL-2, which is a marker of T-cell activation. Hierarchical cluster modeling identified a distinct shift in the immune response toward an anti-inflammatory dominant phenotype among PBMCs exposed to HPV-16 VLPs and treated with E2 and P4 compared to that achieved with non-hormone-treated PBMCs.
FIG. 5.
Hierarchical clustering of mean relative levels of cytokine production in PBMCs treated with E2 or P4 alone or in combination compared with those in non-hormone-treated cells stimulated with HPV-16 VLPs. Red shading denotes higher concentrations of a cytokine in response to a specified hormone treatment compared to the concentration of that cytokine produced by PBMCs stimulated with HPV-16 VLPs but not treated with any hormone (value > 1.0). Blue shading denotes lower concentrations of a cytokine in response to a specified hormone treatment compared to the concentration of that cytokine produced by PBMCs stimulated with HPV-16 VLPs but not treated with any hormone (value < 1.0). Gray shading denotes no change in the concentration of a cytokine in response to a specified hormone treatment compared to the concentration of that cytokine produced by PBMCs stimulated with HPV-16 VLPs but not treated with any hormone (value = 1.0). The similarities of these relative changes were correlated to one another by using the Euclidean minimum-distance-clustering algorithm, with the results being demonstrated in the column and row dendrograms.
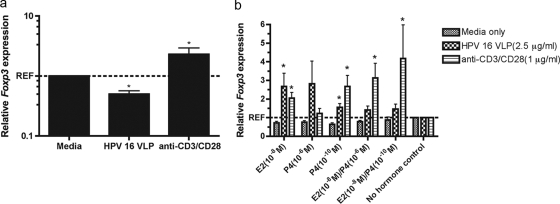
HPV-16 VLPs reduce Foxp3 expression, which is reversed by exposure to E2 and P4.
The increase in the levels of IL-10 and TGF-β production and the decrease in the levels of IFN-γ and IL-12p70 production observed by cluster analysis as well as the clustering of the IL-2 responses with IL-10 and TGF-β as opposed to IFN-γ and IL-12p70 is suggestive of an increase in the regulatory responses of the host to HPV-16 VLPs. To assess this hypothesis, the level of Foxp3 expression was measured in PBMCs. Stimulation with HPV-16 VLPs alone resulted in a 50% reduction in the level of Foxp3 expression compared to the level of expression in PBMCs exposed to medium alone (P < 0.05). Conversely, stimulation of cells with anti-CD3/CD28 alone caused a 2-fold increase in the level of Foxp3 expression relative to the level of expression in cells exposed to medium alone (P < 0.05) (Fig. 6a). Treatment of PBMCs with E2 or P4 either alone or in combination increased the level of Foxp3 expression in both HPV-16 VLP-stimulated (P < 0.001) and anti-CD3/CD28-stimulated (P < 0.003) cells (Fig. 6b). Among the cells stimulated with HPV-16 VLPs, treatment with E2 at 10−8 M (P < 0.05) or P4 at 10−10 M (P < 0.05) restored the level of Foxp3 expression to at or above the basal levels compared to the levels for non-hormone-treated PBMCs. Among the cells stimulated with anti-CD3/CD28, treatment with E2 at 10−8 M (P < 0.05), P4 at 10−10 M (P < 0.05), E2 at 10−8 M plus P4 at 10−6 M (P < 0.05), or E2 at 10−8 M plus P4 at 10−10 M (P < 0.05) increased the level of Foxp3 expression over 2-fold compared to that for non-hormone-treated PBMCs.
FIG. 6.
(a) Mean relative levels of expression of Foxp3 (± standard error of the mean) in PBMCs stimulated with HPV-16 VLPs or anti-CD3/CD28 compared with that in cells treated with medium alone. (b) Mean relative levels of expression of Foxp3 (± standard error of the mean) in PBMCs treated with E2 and P4 compared with that in non-hormone-treated cells stimulated with HVP 16 VLPs, anti-CD3/CD28, or medium alone. *, P < 0.05, by the Wilcoxon rank-sum test.
DISCUSSION
The goal of the study described here was to assess the effects of treatment with E2 and P4 either alone or in combination on the in vitro immune responses to HPV-16 VLPs in PBMCs isolated from healthy donor women. In agreement with the findings of previous studies, stimulation of PBMCs with HPV-16 VLPs alone induced a significant increase in the levels of lymphoproliferation (28) and proinflammatory cytokine (IFN-γ, IL-1β, IL-2, IL-6, IL-8, IL-12p70, TNF-a) and anti-inflammatory cytokine (IL-1ra, IL-10) production (10). Additionally, this study demonstrated that HPV-16 VLPs significantly increased the level of production of IL-17. Increases in the level of IL-17, combined with increases in the level of IL-6, which is considered both a proinflammatory cytokine and a Th2 cytokine, is suggestive of the presence of CD4+ T cells of the Th17 lineage. Th17 T cells are associated with localized tissue inflammation, and their role in vaccine-induced immunity or HPV infection and related disease is currently unknown. HPV-16 VLPs are recognized and stimulate the enhanced expression of major histocompatibility complex classes I and II, as well as CD80/CD86 (20), on macrophages (9), dendritic cells (DCs) (20), and B cells (44) cultured in vitro. Additionally, HPV-16 VLPs interact with pattern recognition receptors, including Toll-like receptor 4, present on monocytes and DCs to elicit a strong inflammatory cytokine response, a type 1 interferon response (43), and B-cell differentiation (44) through an Myd88-dependent signaling pathway (43). Therefore, the robustness of the observed cytokine responses to HPV VLPs could be a result of the combined induction of both the innate and adaptive immune pathways.
In this study, HPV-16 VLPs downregulated the expression of Foxp3, which is the primary transcriptional regulator of CD4+ CD25+ regulatory T cells (4). A previous microarray study that assessed gene expression patterns in PBMCs stimulated with HPV-16 VLPs identified increases in the levels of expression of genes associated with regulation (11). Additionally, HPV-16 VLPs reduced the level of production of TGF-β, which is a cytokine produced by regulatory T cells and which functions to control effector T-cell activity and function (14). Interestingly, Foxp3 expression is inhibited by high levels of both IL-6 and IL-17, which are principle components of the Th17 T-cell lineage (45) and which were shown to be produced in response to HPV-16 VLPs. Therefore, the suppression of Foxp3 and TGF-β in our study in response to HPV-16 VLPs alone could be a result of the differentiation of T cells toward a Th17 phenotype. Furthermore, treatment of PBMCs with E2 (10−8 M) and P4 (10−10 M) increased the level of Foxp3 expression, and treatment with P4 (10−10 M) alone increased the level of TGF-β production. E2 at physiological and superphysiological concentrations can modulate the regulatory T-cell function and Foxp3 expression in either direction (23, 30). Conversely, P4 at elevated concentrations has been shown to reduce only the regulatory T-cell function and Foxp3 expression (23). Overall, these data suggest that HPV-16 VLPs alone suppress regulatory T-cell activity in favor of an inflammatory phenotype, whereas sex steroid hormones abrogate this effect to enhance a regulatory phenotype in PBMCs.
Concomitantly with the observed enhancement of a regulatory phenotype, treatment of PBMCs with E2 and P4 decreased the levels of lymphoproliferation and proinflammatory cytokine (IFN-γ, IL-12p70, TNF-α) production and increased the level of IL-10 production. The levels of IFN-γ and TNF-α have previously been shown to be lower in PBMCs isolated from HRT users than in those from non-HRT users (15, 31) and are suppressed by regulatory T-cell action (45). Additionally, IL-10 production is increased in PHA-L-stimulated PBMCs from healthy donors treated in vitro with E2 at concentrations representative of those in COCs (34). IL-10 is a recognized anti-inflammatory cytokine that is produced by many cell types, including regulatory T cells. The cytospin data collected in this study reveal that lymphocytes make up a majority of the cells in the PBMC samples used. Hierarchical cluster analysis of cytokine production in response to hormone treatment revealed a higher degree of similarity of the IL-2 and IL-10 responses than of the IL-2 and IFN-γ responses. This observation, in conjunction with the higher level of production of TGF-β and the increased expression of Foxp3 among hormone-treated PBMCs, suggests an enrichment of regulatory T-cell activity relative to the activities of CD4+ helper T cells and CD8+ cytotoxic effector T cells. Further studies examining the specific effects of E2 and P4 on isolated subsets of regulatory T cells as well as the effects of these hormone-treated regulatory T cells on CD4+ T-cell differentiation and CD8+ cytotoxic T-cell effector functions are warranted to follow up on these observations.
HPV-16 VLPs enhance the ability of PBMCs to respond to sex steroid hormones, particularly estrogens, through increases in ER mRNA levels. These hormone receptors are part of the nucleus-bound receptor family and are expressed in CD4+ and CD8+ T cells and monocytes (27). Conversely, PR mRNA was not detected in PBMCs, which is in accordance with the findings of previous work (35). GR mRNA, which binds to P4, was detected in PBMCs but was unaffected by HPV-16 VLP stimulation. While HPV-16 VLPs could potentiate the effects of E2 on lymphocyte and monocyte populations in vitro, it is important to consider that sex steroid hormones can also signal through non-nucleus-bound receptors or through receptor-independent mechanisms, such as G-protein signaling and Map kinases (38). Future work will measure these alternative signaling pathways.
The hormonally induced shifts in the immune response to HPV-16 VLPs observed have implications regarding both the natural history of infection and responses to HPV vaccination. In the context of a natural HPV infection, the presence of regulatory T cells is associated with precancerous cervical lesions and cervical cancer (37, 40). Additionally, the reduced level of proliferation, the reduced levels of IFN-γ and IL-2 production, and the higher levels of synthesis of IL-10 observed in vitro when human PBMCs are stimulated with either HPV-16 VLPs or the E6 and E7 early gene products are associated with an increased risk of viral persistence (12) and the development of precancerous lesions (5, 25). Therefore, the observed increase in cervical cancer risk associated with COC use could partially be explained by these hormone-induced immunological shifts. However, given that HPV infections are typically benign and rarely cause disease, it is important to note that it is still unclear which antigen target is most readily identified and acted upon by the host across the natural history of infection.
Immunogenicity studies of anti-HPV antibody production after HPV VLP vaccination among COC and non-COC users report that over the course of a 28-day pill regimen, COC users have more stable anti-HPV antibody titers than non-COC users, who have antibodies titers that fluctuate across the stages of their menstrual cycle (26). Therefore, it is unclear how the hormone-mediated enhancement of markers such as TGF-β, Foxp3, and IL-10 observed in the present study explains the in vivo observations. It is important to note, however, that while the anti-HPV antibody titers are relatively stable within individuals over time, high degrees of variability were observed across individuals. Therefore, the enhanced production of regulatory and anti-inflammatory immune markers may contribute to the heterogeneity of the antibody responses of COC users. IL-10 also can mediate 17β-estradiol-induced increases in total IgM and IgG in PBMCs taken from healthy women (16). The enhancement of key regulatory and anti-inflammatory markers may increase the level of antibody production. Unfortunately, we were unable to directly measure total immunoglobulin levels in our study. Furthermore, the effects of exposure to sex steroid hormones in vitro versus those in vivo on the immune responses to HPV-16 VLPs may be different. 17β-Estradiol exhibits a biphasic dose-response relationship with certain proinflammatory markers, such as TNF-α, IL-1β, and IFN-γ, in which higher doses suppress production and lower doses increase production in vitro (38). While the concentrations of E2 and P4 used in this study were selected on the basis of the estimated concentrations in serum among COC users, it is unclear how well they correlate with the concentrations among COC users in the prior immunogenicity studies.
The sizes of the effects of each individual cytokine in response to hormone treatment observed in the current study were small, leading to questions of the biological relevance of these findings, particularly in the context of vaccination and vaccine trials. The small effects observed among a number of cytokines sharing a similar immune pathway, however, may significantly alter clinical outcomes, such as long-term efficacy, adverse events following vaccination (32), and, in the case of HPV, the induction of cross-protection against other oncogenic HPV types.
This study demonstrates the combined effects of the reproductive hormones E2 and P4 on the in vitro responses to HPV-16 VLPs. The introduction of a highly effective prophylactic subunit vaccine against HPV-16 offers a great potential for reducing the burden of cervical cancer. The disruption of vaccine-induced immunity could, however, have far-reaching implications in reducing the protective effect of the vaccine, thereby limiting its long-term effectiveness. Additional in vivo and in vitro studies with women pre- and postvaccination are needed to assess the effects of exogenous reproductive hormones on host immunity to vaccination, with a specific emphasis on efficacy, cross-protection, and adverse events in vivo being needed.
Acknowledgments
We thank Anne Jedlicka at the Johns Hopkins Bloomberg School of Public Health Genomics Core Facility for technical assistance with RNA isolation and hierarchical cluster analysis, Raphael Viscidi and his laboratory at the Johns Hopkins School of Medicine Department of Neurovirology and Pediatrics for performing serological analysis for HPV-16 antibody detection, and Ernst Spannhake and Brian Schofield at the Johns Hopkins Bloomberg School of Public Health Department of Environmental Health Science for their help with the cytospin analysis.
This research was paid for in part by a predoctoral training program in STI research (NIAID grant T32-AI050056-08), the Johns Hopkins Bloomberg School of Public Health Department of Epidemiology Doctoral Student Fund, and the W. Harry Feinstone Endowment.
Footnotes
Published ahead of print on 3 February 2010.
REFERENCES
- 1.Agarwal, S. K., and G. D. Marshall, Jr. 1999. Perimenstrual alterations in type-1/type-2 cytokine balance of normal women. Ann. Allergy Asthma Immunol. 83:222-228. [DOI] [PubMed] [Google Scholar]
- 2.Ames, A., and P. Gravitt. 2007. Human papillomavirus vaccine update. Curr. Infect. Dis. Rep. 9:151-158. [DOI] [PubMed] [Google Scholar]
- 3.Beagley, K. W., and C. M. Gockel. 2003. Regulation of innate and adaptive immunity by the female sex hormones oestradiol and progesterone. FEMS Immunol. Med. Microbiol. 38:13-22. [DOI] [PubMed] [Google Scholar]
- 4.Buckner, J. H., and S. F. Ziegler. 2008. Functional analysis of FOXP3. Ann. N. Y. Acad. Sci. 1143:151-169. [DOI] [PubMed] [Google Scholar]
- 5.Clerici, M., M. Merola, E. Ferrario, D. Trabattoni, M. L. Villa, B. Stefanon, D. J. Venzon, G. M. Shearer, G. De Palo, and E. Clerici. 1997. Cytokine production patterns in cervical intraepithelial neoplasia: association with human papillomavirus infection. J. Natl. Cancer Inst. 89:245-250. [DOI] [PubMed] [Google Scholar]
- 6.Cutolo, M., S. Capellino, A. Sulli, B. Serioli, M. E. Secchi, B. Villaggio, and R. H. Straub. 2006. Estrogens and autoimmune diseases. Ann. N. Y. Acad. Sci. 1089:538-547. [DOI] [PubMed] [Google Scholar]
- 7.Da Silva, J. A. 1999. Sex hormones and glucocorticoids: interactions with the immune system. Ann. N. Y. Acad. Sci. 876:102-117. [DOI] [PubMed] [Google Scholar]
- 8.Endrikat, J., H. Blode, C. Gerlinger, P. Rosenbaum, and W. Kuhnz. 2002. A pharmacokinetic study with a low-dose oral contraceptive containing 20 microg ethinylestradiol plus 100 microg levonorgestrel. Eur. J. Contracept. Reprod. Health Care 7:79-90. [PubMed] [Google Scholar]
- 9.Fausch, S. C., D. M. Da Silva, M. P. Rudolf, and W. M. Kast. 2002. Human papillomavirus virus-like particles do not activate Langerhans cells: a possible immune escape mechanism used by human papillomaviruses. J. Immunol. 169:3242-3249. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Pineres, A., A. Hildesheim, L. Dodd, T. J. Kemp, M. Williams, C. Harro, D. R. Lowy, J. T. Schiller, and L. A. Pinto. 2007. Cytokine and chemokine profiles following vaccination with human papillomavirus type 16 L1 virus-like particles. Clin. Vaccine Immunol. 14:984-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia-Pineres, A. J., A. Hildesheim, L. Dodd, T. J. Kemp, J. Yang, B. Fullmer, C. Harro, D. R. Lowy, R. A. Lempicki, and L. A. Pinto. 2009. Gene expression patterns induced by HPV-16 L1 virus-like particles in leukocytes from vaccine recipients. J. Immunol. 182:1706-1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia-Pineres, A. J., A. Hildesheim, R. Herrero, M. Trivett, M. Williams, I. Atmetlla, M. Ramirez, M. Villegas, M. Schiffman, A. C. Rodriguez, R. D. Burk, M. Hildesheim, E. Freer, J. Bonilla, C. Bratti, J. A. Berzofsky, and L. A. Pinto. 2006. Persistent human papillomavirus infection is associated with a generalized decrease in immune responsiveness in older women. Cancer Res. 66:11070-11076. [DOI] [PubMed] [Google Scholar]
- 13.Gillgrass, A. E., S. A. Fernandez, K. L. Rosenthal, and C. Kaushic. 2005. Estradiol regulates susceptibility following primary exposure to genital herpes simplex virus type 2, while progesterone induces inflammation. J. Virol. 79:3107-3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joosten, S. A., and T. H. Ottenhoff. 2008. Human CD4 and CD8 regulatory T cells in infectious diseases and vaccination. Hum. Immunol. 69:760-770. [DOI] [PubMed] [Google Scholar]
- 15.Kamada, M., M. Irahara, M. Maegawa, Y. Ohmoto, K. Murata, T. Yasui, S. Yamano, and T. Aono. 2001. Transient increase in the levels of T-helper 1 cytokines in postmenopausal women and the effects of hormone replacement therapy. Gynecol. Obstet. Invest. 52:82-88. [DOI] [PubMed] [Google Scholar]
- 16.Kanda, N., and K. Tamaki. 1999. Estrogen enhances immunoglobulin production by human PBMCs. J. Allergy Clin. Immunol. 103:282-288. [DOI] [PubMed] [Google Scholar]
- 17.Kaushic, C., F. Zhou, A. D. Murdin, and C. R. Wira. 2000. Effects of estradiol and progesterone on susceptibility and early immune responses to Chlamydia trachomatis infection in the female reproductive tract. Infect. Immun. 68:4207-4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klein, S. L. 2000. The effects of hormones on sex differences in infection: from genes to behavior. Neurosci. Biobehav. Rev. 24:627-638. [DOI] [PubMed] [Google Scholar]
- 19.Konecna, L., M. S. Yan, L. E. Miller, J. Scholmerich, W. Falk, and R. H. Straub. 2000. Modulation of IL-6 production during the menstrual cycle in vivo and in vitro. Brain Behav. Immun. 14:49-61. [DOI] [PubMed] [Google Scholar]
- 20.Lenz, P., P. M. Day, Y. Y. Pang, S. A. Frye, P. N. Jensen, D. R. Lowy, and J. T. Schiller. 2001. Papillomavirus-like particles induce acute activation of dendritic cells. J. Immunol. 166:5346-5355. [DOI] [PubMed] [Google Scholar]
- 21.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−delta delta C(T)) method. Methods (San Diego, Calif.) 25:402-408. [DOI] [PubMed] [Google Scholar]
- 22.Loy, R. A., J. A. Loukides, and M. L. Polan. 1992. Ovarian steroids modulate human monocyte tumor necrosis factor alpha messenger ribonucleic acid levels in cultured human peripheral monocytes. Fertil. Steril. 58:733-739. [DOI] [PubMed] [Google Scholar]
- 23.Mjosberg, J., J. Svensson, E. Johansson, L. Hellstrom, R. Casas, M. C. Jenmalm, R. Boij, L. Matthiesen, J. I. Jonsson, and J. Ernerudh. 2009. Systemic reduction of functionally suppressive CD4dimCD25highFoxp3+ Tregs in human second trimester pregnancy is induced by progesterone and 17beta-estradiol. J. Immunol. 183:759-769. [DOI] [PubMed] [Google Scholar]
- 24.Moreno, V., F. X. Bosch, N. Munoz, C. J. Meijer, K. V. Shah, J. M. Walboomers, R. Herrero, and S. Franceschi. 2002. Effect of oral contraceptives on risk of cervical cancer in women with human papillomavirus infection: the IARC multicentric case-control study. Lancet 359:1085-1092. [DOI] [PubMed] [Google Scholar]
- 25.Nakagawa, M., D. P. Stites, S. Farhat, A. Judd, A. B. Moscicki, A. J. Canchola, J. F. Hilton, and J. M. Palefsky. 1996. T-cell proliferative response to human papillomavirus type 16 peptides: relationship to cervical intraepithelial neoplasia. Clin. Diagn. Lab. Immunol. 3:205-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nardelli-Haefliger, D., D. Wirthner, J. T. Schiller, D. R. Lowy, A. Hildesheim, F. Ponci, and P. De Grandi. 2003. Specific antibody levels at the cervix during the menstrual cycle of women vaccinated with human papillomavirus 16 virus-like particles. J. Natl. Cancer Inst. 95:1128-1137. [DOI] [PubMed] [Google Scholar]
- 27.Phiel, K. L., R. A. Henderson, S. J. Adelman, and M. M. Elloso. 2005. Differential estrogen receptor gene expression in human peripheral blood mononuclear cell populations. Immunol. Lett. 97:107-113. [DOI] [PubMed] [Google Scholar]
- 28.Pinto, L. A., J. Edwards, P. E. Castle, C. D. Harro, D. R. Lowy, J. T. Schiller, D. Wallace, W. Kopp, J. W. Adelsberger, M. W. Baseler, J. A. Berzofsky, and A. Hildesheim. 2003. Cellular immune responses to human papillomavirus (HPV)-16 L1 in healthy volunteers immunized with recombinant HPV-16 L1 virus-like particles. J. Infect. Dis. 188:327-338. [DOI] [PubMed] [Google Scholar]
- 29.Polan, M. L., J. Loukides, P. Nelson, S. Carding, M. Diamond, A. Walsh, and K. Bottomly. 1989. Progesterone and estradiol modulate interleukin-1 beta messenger ribonucleic acid levels in cultured human peripheral monocytes. J. Clin. Endocrinol. Metab. 69:1200-1206. [DOI] [PubMed] [Google Scholar]
- 30.Prieto, G. A., and Y. Rosenstein. 2006. Oestradiol potentiates the suppressive function of human CD4 CD25 regulatory T cells by promoting their proliferation. Immunology 118:58-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Puder, J. J., P. U. Freda, R. S. Goland, and S. L. Wardlaw. 2001. Estrogen modulates the hypothalamic-pituitary-adrenal and inflammatory cytokine responses to endotoxin in women. J. Clin. Endocrinol. Metab. 86:2403-2408. [DOI] [PubMed] [Google Scholar]
- 32.Querec, T. D., R. S. Akondy, E. K. Lee, W. Cao, H. I. Nakaya, D. Teuwen, A. Pirani, K. Gernert, J. Deng, B. Marzolf, K. Kennedy, H. Wu, S. Bennouna, H. Oluoch, J. Miller, R. Z. Vencio, M. Mulligan, A. Aderem, R. Ahmed, and B. Pulendran. 2009. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat. Immunol. 10:116-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rogers, A., and R. Eastell. 2001. The effect of 17beta-estradiol on production of cytokines in cultures of peripheral blood. Bone 29:30-34. [DOI] [PubMed] [Google Scholar]
- 34.Rogers, A., and R. Eastell. 1998. Effects of estrogen therapy of postmenopausal women on cytokines measured in peripheral blood. J. Bone Mineral Res. 13:1577-1586. [DOI] [PubMed] [Google Scholar]
- 35.Schust, D. J., D. J. Anderson, and J. A. Hill. 1996. Progesterone-induced immunosuppression is not mediated through the progesterone receptor. Hum. Reprod. 11:980-985. [DOI] [PubMed] [Google Scholar]
- 36.Scott, M. E., Y. Ma, S. Farhat, S. Shiboski, and A. B. Moscicki. 2006. Covariates of cervical cytokine mRNA expression by real-time PCR in adolescents and young women: effects of Chlamydia trachomatis infection, hormonal contraception, and smoking. J. Clin. Immunol. 26:222-232. [DOI] [PubMed] [Google Scholar]
- 37.Scott, M. E., Y. Ma, L. Kuzmich, and A. B. Moscicki. 2009. Diminished IFN-gamma and IL-10 and elevated Foxp3 mRNA expression in the cervix are associated with CIN 2 or 3. Int. J. Cancer 124:1379-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Straub, R. H. 2007. The complex role of estrogens in inflammation. Endocr. Rev. 28:521-574. [DOI] [PubMed] [Google Scholar]
- 39.Studentsov, Y. Y., M. Schiffman, H. D. Strickler, G. Y. Ho, Y. Y. Pang, J. Schiller, R. Herrero, and R. D. Burk. 2002. Enhanced enzyme-linked immunosorbent assay for detection of antibodies to virus-like particles of human papillomavirus. J. Clin. Microbiol. 40:1755-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van der Burg, S. H., S. J. Piersma, A. de Jong, J. M. van der Hulst, K. M. Kwappenberg, M. van den Hende, M. J. Welters, J. J. Van Rood, G. J. Fleuren, C. J. Melief, G. G. Kenter, and R. Offringa. 2007. Association of cervical cancer with the presence of CD4+ regulatory T cells specific for human papillomavirus antigens. Proc. Natl. Acad. Sci. U. S. A. 104:12087-12092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang, S. S., M. Schiffman, T. S. Shields, R. Herrero, A. Hildesheim, M. C. Bratti, M. E. Sherman, A. C. Rodriguez, P. E. Castle, J. Morales, M. Alfaro, T. Wright, S. Chen, B. Clayman, R. D. Burk, and R. P. Viscidi. 2003. Seroprevalence of human papillomavirus-16, -18, -31, and -45 in a population-based cohort of 10000 women in Costa Rica. Br. J. Cancer 89:1248-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wheeler, C. M., O. M. Bautista, J. E. Tomassini, M. Nelson, C. A. Sattler, and E. Barr. 2008. Safety and immunogenicity of co-administered quadrivalent human papillomavirus (HPV)-6/11/16/18 L1 virus-like particle (VLP) and hepatitis B (HBV) vaccines. Vaccine 26:686-696. [DOI] [PubMed] [Google Scholar]
- 43.Yang, R., F. M. Murillo, H. Cui, R. Blosser, S. Uematsu, K. Takeda, S. Akira, R. P. Viscidi, and R. B. Roden. 2004. Papillomavirus-like particles stimulate murine bone marrow-derived dendritic cells to produce alpha interferon and Th1 immune responses via MyD88. J. Virol. 78:11152-11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang, R., F. M. Murillo, M. J. Delannoy, R. L. Blosser, W. H. T. Yutzy, S. Uematsu, K. Takeda, S. Akira, R. P. Viscidi, and R. B. Roden. 2005. B lymphocyte activation by human papillomavirus-like particles directly induces Ig class switch recombination via TLR4-MyD88. J. Immunol. 174:7912-7919. [DOI] [PubMed] [Google Scholar]
- 45.Ziegler, S. F., and J. H. Buckner. 2009. FOXP3 and the regulation of Treg/Th17 differentiation. Microbes Infect. 11:594-598. [DOI] [PMC free article] [PubMed] [Google Scholar]