Abstract
This study aimed to evaluate the use of protein A-peroxidase (horseradish peroxidase [HRPO]) in indirect enzyme-linked immunosorbent assays (iELISAs) and IgG avidity assays for serological distinction between Brucella abortus S19-vaccinated and -infected cows. Four groups were analyzed: GI, 41 nonvaccinated seropositive cows; GII, 79 S19-vaccinated heifers analyzed at 3 months postvaccination; GIII, 105 S19-vaccinated cows analyzed after 24 months of age; and GIV, 278 nonvaccinated seronegative cows. IgG levels and avidity to B. abortus smooth lipopolysaccharide (S-LPS) were determined using anti-bovine IgG-HRPO or protein A-HRPO conjugates. Similar levels of IgG anti-S-LPS were found with GI using both conjugates. Lower IgG levels were detected with GII, GIII, and GIV using protein A-HRPO. Both conjugates showed high performance in discriminating GI from GIII, with high sensitivity (Se; 97.6%) and specificity (Sp; 97.1%). Protein A-HRPO was better in distinguishing GI from GIV (Se, 97.6%; Sp, 94.6%) and GI from GII (Se, 80.5%; Sp, 94.9%). Protein A-HRPO excluded a higher number of positive samples with GII and GIV. IgG avidity showed that protein A-HRPO, but not anti-IgG-HRPO, was able to distinguish nonvaccinated from vaccinated cattle, showing a higher avidity index (AI) with GI than with GII, with 78% of serum samples in GII showing an AI of <50%. Therefore, the iELISA using B. abortus S-LPS antigen and protein A-HRPO conjugate for preferential detection of the IgG2 subclass was shown to be suitable for serological distinction between S19-vaccinated and -infected cows. Also, antibodies generated after vaccination showed lower avidity, suggesting a role for the IgG2 subclass as an antibody of higher-affinity maturation after infection, constituting an additional tool for differentiating vaccinated from infected cattle.
Brucellosis is a major zoonosis and has been considered an emerging or reemerging disease worldwide (11). Pathogens from the genus Brucella can infect a wide variety of mammals, causing abortion and infertility in domestic herds as well as debilitating illness in humans that may persist intermittently for years (13, 21).
Diagnosis of brucellosis in cattle caused by Brucella abortus is difficult to establish because of the variable time of incubation and the absence of clinical signs other than abortion (24). Bacteriological isolation of the microorganism confirms the diagnosis, and this result is taken as the gold standard against which other tests have to be compared (31). However, as the rate of isolation of B. abortus from blood or tissue cultures generally presents low sensitivity and the results are not available immediately because this procedure may be time consuming and processing a large number of samples is cumbersome, the diagnosis is based mainly on serological methods (5, 9). Accordingly, most conventional serological assays, such as the rose bengal test (RBT), serum agglutination test (SAT), and complement fixation test (CFT), use whole-cell preparations or lipopolysaccharide (LPS)-enriched fractions, which are often obtained from smooth (S) B. abortus strains, being rich in S-LPS, which induces a strong antibody response (5, 27). The classical serological techniques rely mainly on the detection of antibodies to this antigen fraction (4).
Enzyme-linked immunosorbent assays (ELISAs) have been evaluated for their diagnostic performance to detect serum antibodies to B. abortus in cattle, and such techniques offer several advantages over other tests, including that sera do not need to be heat inactivated as for CFT or pretreated with 2-mercaptoethanol (2ME) as for SAT/2ME; the reactivity is measured objectively, reducing subjective errors; and they are particularly advantageous in mass testing programs (24, 33, 34).
In many countries, control programs are based on different protocols of B. abortus S19 vaccination to reduce prevalence, followed by the diagnosis and elimination of reactors (1). S19 vaccine is antigenically similar to the virulent strain, and therefore, serodiagnosis by conventional tests does not permit precise differentiation of vaccinated from infected animals (10, 27). Consequently, other tests have been developed, including the competitive ELISA (cELISA) and fluorescence polarization assay (FPA), which have eliminated most reactions due to residual antibodies produced in response to S19 vaccination (27, 31). However, the use of highly specific reagents as monoclonal antibodies in cELISA or costly equipment in FPA has made these assays unfeasible for various laboratories throughout the world (30).
Immunoglobulin-binding proteins, such as protein A, protein G, or recombinant protein A/G labeled with horseradish peroxidase (HRPO), have been used as valuable tools in the ELISA for detection of anti-Brucella antibodies in various animal species (29, 30). While protein G reacts with both bovine IgG subclasses (IgG1 and IgG2), protein A reacts more specifically with the IgG2 subclass, whose levels increase with the intensity of antigen exposure in Brucella infection (22, 32). On the other hand, the avidity of specific IgG antibodies has been used often to discriminate acute from chronic infections for several diseases, such as toxoplasmosis (19) and neosporosis (3), as well as for human brucellosis (12, 15, 20), showing that antibodies with high avidity would be useful in excluding recent infection. However, there is currently no information on IgG avidity assays for bovine brucellosis, particularly with an emphasis on differentiating vaccinated from infected cattle.
The purpose of the present study was to evaluate the use of protein A-peroxidase as conjugate in indirect ELISAs and IgG avidity assays in order to establish a serological distinction between B. abortus S19-vaccinated and -infected cows.
MATERIALS AND METHODS
Bovine serum samples.
Serum samples of four groups of cattle were obtained as follows: GI, nonvaccinated seropositive cows aged over 24 months and originated from areas where Brucella is endemic or an outbreak is occurring (n = 41); GII, S19-vaccinated heifers between 3 and 8 months of age, originated from areas where Brucella is endemic, with serum samples collected at 3 months postvaccination (n = 79); GIII, S19-vaccinated cows between 3 and 8 months of age, originated from areas where Brucella is endemic, with serum samples collected after 24 months of age (n = 105); and GIV, nonvaccinated seronegative cows aged over 24 months and originated from areas where Brucella is nonendemic (n = 278). Areas where Brucella is endemic are located in the state of Minas Gerais, Brazil, while the areas where it is nonendemic or where an outbreak is occurring are located in the Santa Catarina state of Brazil, where brucellosis vaccination is not currently performed. The different groups were established under real field conditions. Thus, serum samples of GI were kindly provided by Laboratório Nacional Agropecuário (LANAGRO, Ministério da Agricultura, Pecuária e Abastecimento, Pedro Leopoldo, State of Minas Gerais, Brazil) and Companhia Integrada de Desenvolvimento Agrícola de Santa Catarina (CIDASC, São José, State of Santa Catarina, Brazil), and serum samples of GIV were provided by the latter only. Besides the epidemiological data, these groups were also selected based on positive (GI) and negative (GIV) results of classical diagnostic tests, such as the RBT and SAT/2ME. Serum samples from GII and GIII were provided by Cooperativa Agropecuária Ltda. Uberlândia (CALU, Uberlândia, State of Minas Gerais, Brazil). All serum samples were stored at −20°C until they were tested.
Brucella antigen extraction.
S-LPS from B. abortus S19 (Vallée S/A Produtos Veterinários, Montes Claros, MG, Brazil) was extracted by the hot phenol-water method (31). Briefly, 1 g of dry bacteria was treated with 30 ml 90% phenol and 30 ml distilled water at 66°C for 15 min with constant agitation. After centrifugation at 10,000 × g for 10 min at 4°C, the phenol layer was collected, filtered to remove cellular debris, and added to 80 ml cold methanol containing 1% methanol saturated with sodium acetate. After incubation for 2 h at 4°C, the resulting precipitate was recovered by centrifugation (10,000 × g for 10 min at 4°C) and treated with 15 ml distilled water overnight at 4°C with stirring. After centrifugation as described above, the supernatant was collected and stored at 4°C. The pellet was again resuspended in distilled water, stirred overnight, and centrifuged, and the supernatants were pooled (crude S-LPS). Next, 5% trichloroacetic acid was added to the crude S-LPS and incubated for 10 min at room temperature. The pellet was removed by centrifugation, and the translucent supernatant was dialyzed against distilled water (four changes) for 2 days at 4°C and subsequently freeze-dried. Stock solutions containing 1 mg/ml (wt/vol) were prepared in water and stored at −20°C.
Classical agglutination tests.
The RBT and SAT/2ME were performed as described previously (23, 31). The RBT buffered antigen (pH 3.63) and the SAT/2ME antigen were produced from B. abortus strain S1119-3 following standard procedures (6) and were kindly provided from the Instituto de Tecnologia do Paraná (TECPAR), Brazil. An agglutination titer of ≥1:25 for the SAT/2ME was considered a positive result for both vaccinated and nonvaccinated cows. For the RBT, any degree of agglutination was considered positive (23, 31).
iELISA.
An indirect ELISA (iELISA) was carried out to detect bovine IgG antibodies to B. abortus S-LPS as previously described (31), with minor modifications. Optimization of the reaction was established in preliminary experiments through block titration of the reagents, using positive (GI) and negative (GIV) control sera. Microtiter plates were coated with B. abortus S-LPS (100 μg/ml) in 0.06 M carbonate buffer (pH 9.6) for 18 h at 4°C. After the plates were washed four times in phosphate-buffered saline (PBS, pH 7.2) containing 0.05% Tween 20 (PBS-T), plates were blocked with 1% bovine serum albumin (BSA) in PBS-T (PBS-T-BSA) for 30 min at 37°C. Serum samples were diluted 1:50 in PBS-T containing 7.5 mM (each) EDTA and EGTA, added in duplicate, and incubated for 1 h at 37°C. Positive- and negative-control sera were included in each plate. Between each reaction step, the plates were washed four times with PBS-T to remove excess reagents using an automatic microtiter plate washer. Subsequently, peroxidase-labeled anti-bovine IgG (whole molecule, A5295; Sigma Chemical Co., St. Louis, MO) diluted 1:20,000 in PBS-T-BSA or protein A-peroxidase (Sigma Chemical Co.) diluted 1:2,000 in PBS-T-BSA was added and incubated for 1 h at 37°C. The enzymatic activity was measured by adding the substrate (0.03% H2O2 and 0.01 M 2,2′-azino-bis-3-ethyl-benzthiazoline sulfonic acid [ABTS] in 0.1 M phosphate-citrate buffer, pH 5.0). The optical density (OD) was read at 405 nm using a plate reader (Titertek Multiskan Plus; Flow Laboratories, McLean, VA). Antibody titers were expressed as previously described (29, 30) as the reactivity index (RI) relative to the mean OD of positive-control sera, according to the following formula: RI = (mean OD sample/mean OD positive control) × 100.
IgG avidity.
IgG avidity assays were performed based on a previous study working with serum antibodies from pig herds to Salmonella (35), with modifications. Microplates were coated and blocked as described for the iELISA. Positive serum samples were diluted (1:50, 1:100, 1:250, 1:500, 1:1,000, and 1:2,000) and added in quadruplicate. After incubation for 1 h at 37°C, duplicate wells were rinsed with 8 M urea in PBS-T, whereas the other duplicate wells were rinsed for 10 min with PBS-T only. Next, the wells were washed three times in PBS-T, and subsequent steps were performed as described above for the iELISA. Results were expressed as the avidity index (AI) and calculated for each dilution of the serum samples that had a positive result, according to the following formula: AI = (RI samples with urea/RI samples without urea) × 100. The average of the determinations of AIs obtained at different dilutions was calculated and expressed as the mean AI.
Statistical analysis.
Statistical analyses were performed using GraphPad Prism version 4.0 (GraphPad Software Inc., San Diego, CA). To determine the threshold RI value that could be considered a positive result in iELISAs, a two-graph receiver operating characteristic (TG-ROC) analysis was done (14). Sensitivity and specificity values with 95% confidence intervals (CI), as well as positive and negative likelihood ratios (LR+ and LR−, respectively), were also calculated (25). A comparison between antibody levels (RI) obtained by peroxidase-labeled protein A and anti-bovine IgG conjugates within each group was carried out by the Mann-Whitney test. Differences between percentages of positive serum samples obtained with the two conjugates were analyzed by the chi-square test. Differences between the avidity indexes of seropositive groups were analyzed by the Student t test. Values of P of <0.05 were considered statistically significant.
RESULTS
iELISAs using peroxidase-labeled anti-bovine IgG or protein A conjugates.
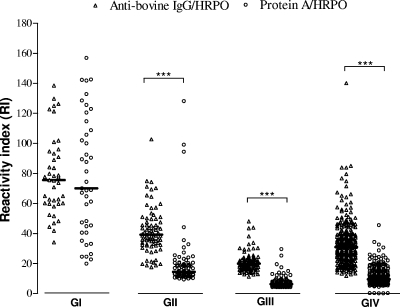
Levels of IgG antibodies to the B. abortus S-LPS-enriched fraction were determined by iELISAs using anti-bovine IgG or protein A labeled with HRPO as detection conjugates in four groups of bovine serum samples. As shown in Fig. 1, RI values obtained when using anti-bovine IgG-HRPO were significantly higher than those obtained using protein A-HRPO with the GII, GIII, and GIV groups (P < 0.0001), but no significant difference was found with the GI group for both conjugates.
FIG. 1.
Levels of IgG antibodies to Brucella abortus S-LPS-enriched fraction determined by iELISA, expressed as the reactivity index (RI), using anti-bovine IgG-HRPO or protein A-HRPO as conjugates among the different groups of bovine serum samples. GI, positive (nonvaccinated seropositive cows originated from areas where Brucella is endemic or where an outbreak is occurring, n = 40); GII, recently vaccinated (S19-vaccinated heifers between 3 and 8 months of age, originated from areas where Brucella is endemic, and serum samples were collected at 3 months postvaccination, n = 79); GIII, tardily vaccinated (S19-vaccinated cows between 3 and 8 months of age, originated from areas where Brucella is endemic, and serum samples were collected after 24 months of age, n = 105); GIV, negative (nonvaccinated seronegative cows originated from areas where Brucella is nonendemic, n = 278). The horizontal bars represent mean RI values. ***, P < 0.0001, Mann-Whitney test.
TG-ROC analysis.
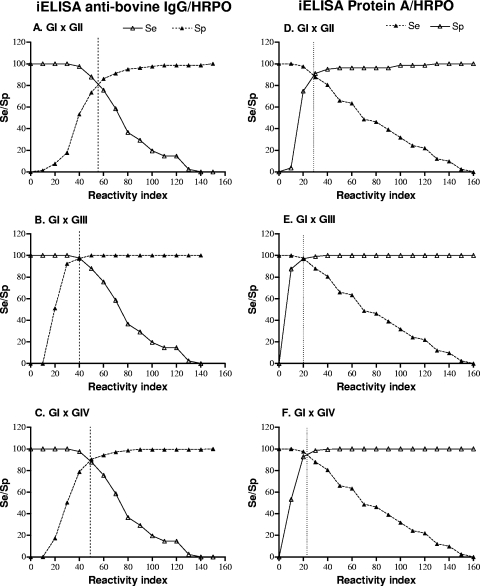
The threshold RI value for presumed positive and negative serum samples in iELISAs was selected by calculating the relative sensitivity (Se) and specificity (Sp) for each RI value using TG-ROC analysis, in which the intersection point of the two curves (Se and Sp) indicates the RI value at which equivalent maximum Se and Sp values can be achieved. Thus, TG-ROC curves were constructed in order to discriminate GI positive cows from GII vaccinated heifers (Fig. 2A and D), from GIII vaccinated cows (Fig. 2B and E), or from GIV seronegative animals (Fig. 2C and F) in iELISAs using anti-bovine IgG-HRPO or protein A-HRPO conjugates.
FIG. 2.
TG-ROC analysis of iELISA results for detection of IgG antibodies to Brucella abortus S-LPS-enriched fraction using anti-bovine IgG-HRPO or protein A-HRPO as conjugates. Sensitivity (Se) and specificity (Sp) of iELISAs were determined for each RI value in order to discriminate positive serum samples from recently vaccinated serum samples (GI × GII) (A and D), from tardily vaccinated serum samples (GI × GIII) (B and E), or from negative serum samples (GI × GIV) (C and F). The intersection point of the curves (Se and Sp) indicates the RI value at which equivalent maximum sensitivity and specificity can be achieved.
The cutoff values for RI that had the maximum combined Se and Sp, as well as likelihood ratios (LR+ and LR−) calculated for each condition, are demonstrated in Table 1. It was observed that iELISAs using either anti-bovine IgG-HRPO or protein A-HRPO showed the highest Se (97.6%) and Sp (97.1%) in discriminating GI from GIII animals, resulting in the highest LR+ (33.7) and lowest LR− (0.025) values. In contrast, when analyzing groups GI and GIV, it was found that the iELISA using protein A-HRPO showed higher Se (97.6%) and Sp (94.6%) values and noticeably higher LR+ (18.1) and lower LR− (0.025) values than the iELISA using anti-bovine IgG-HRPO. In addition, when groups GI and GII were analyzed, a higher Sp (94.9%) was obtained with protein A-HRPO than with anti-bovine IgG-HRPO (82.3%) conjugate, resulting in a significantly higher LR+ (15.8 versus 4.7).
TABLE 1.
Threshold values (cutoff) relative to iELISA for IgG antibodies to Brucella abortus in bovine serum samples from different groups
| iELISA | Groupsa | Cutoff (RI)d | Sensitivity (95% CI) | Specificity (95% CI) | LR+e | LR−e |
|---|---|---|---|---|---|---|
| Anti-IgG-HRPOb | GI × GII | 56 | 82.9 (71.4-94.4) | 82.3 (73.9-90.7) | 4.7 | 0.274 |
| GI × GIII | 40 | 97.6 (92.8-102.3) | 97.1 (93.9-100.3) | 33.7 | 0.025 | |
| GI × GIV | 49 | 87.8 (77.8-97.8) | 89.2 (85.6-92.9) | 8.1 | 0.137 | |
| Protein A-HRPOc | GI × GII | 38 | 80.5 (68.4-92.6) | 94.9 (90.1-99.8) | 15.8 | 0.205 |
| GI × GIII | 20 | 97.6 (92.8-102.3) | 97.1 (94.0-100.3) | 33.7 | 0.025 | |
| GI × GIV | 22 | 97.6 (92.8-102.3) | 94.6 (91.9-97.3) | 18.1 | 0.025 |
GI, nonvaccinated seropositive cows originated from areas where Brucella is endemic or an outbreak is occurring (n = 41); GII, S19-vaccinated heifers between 3 and 8 months of age, originated from areas where Brucella is endemic, and serum samples were analyzed at 3 months postvaccination (n = 79); GIII, S19-vaccinated cows between 3 and 8 months of age, originated from areas where Brucella is endemic, and serum samples were analyzed after 24 months of age (n = 105); GIV, nonvaccinated seronegative cows originated from areas where Brucella is nonendemic (n = 278).
iELISA using anti-bovine IgG labeled with peroxidase (HRPO) as conjugate.
iELISA using protein A-HRPO as conjugate.
Reactivity index (RI) = (mean OD sample/mean OD positive control) × 100.
LR+, positive likelihood ratio; LR−, negative likelihood ratio. The figures in bold indicate significant LR values.
Analysis of positive samples related to different cutoff values.
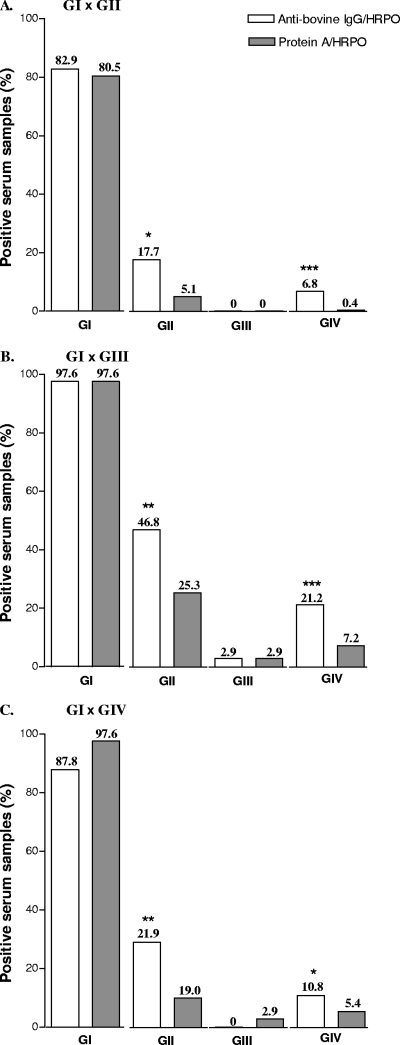
The percentages of positive bovine serum samples obtained by iELISAs using the anti-bovine IgG-HRPO or protein A-HRPO conjugates were calculated according to different cutoff values previously established (Fig. 3). The percentage of positive serum samples in both the GII and GIV groups obtained when using anti-bovine IgG-HRPO was significantly higher than that with protein A-HRPO for the three cutoff conditions analyzed (P < 0.05) (Fig. 3). On the other hand, no significant difference was found between both conjugates for GI and GIII cows, with high rates of positive samples in GI (>80%) and low positive rates in GIII (<3%) under all conditions tested. These results showed that iELISAs for detection of IgG to B. abortus S-LPS were able to discriminate nonvaccinated seropositive cows from vaccinated or seronegative animals using either anti-bovine IgG or protein A as detection reagents, although the protein A-HRPO conjugate showed better performance in identifying as negative GIV animals (92.8% to 99.6%) and GII vaccinated heifers (74.7% to 94.9%).
FIG. 3.
Percentage of positive bovine serum samples obtained by the iELISA for detection of IgG antibodies to Brucella abortus S-LPS-enriched fraction using anti-bovine IgG-HRPO or protein A-HRPO as conjugates, according to different cutoff values established in order to discriminate positive serum samples from recently vaccinated serum samples (GI × GII) (A), from tardily vaccinated serum samples (GI × GIII) (B), or from negative serum samples (GI × GIV) (C). Bars represent mean percentage. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (χ2 test).
IgG avidity.
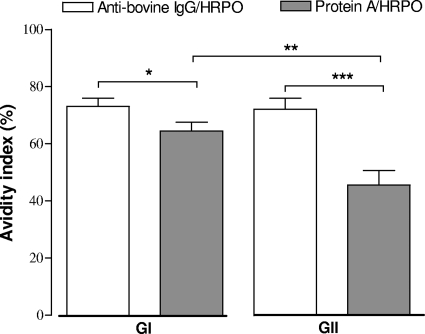
The avidity analysis of IgG anti-B. abortus S-LPS was performed by an iELISA with seropositive samples of GI and GII only, using the anti-bovine IgG-HRPO (cutoff, RI = 49) or protein A-HRPO (cutoff, RI = 22) detection conjugates. As shown in Fig. 4, the avidity index (mean AI, 45.5%) obtained with protein A-HRPO was significantly lower than that for anti-bovine IgG-HRPO (mean AI, 72.0%) (P = 0.0004) in the GII group, whereas a slight but significant difference was observed with the GI group (mean AI, 73.1 versus 64.4; P = 0.0423). When comparing the groups for the same conjugate, there was a significant difference in the AI only for protein A-HRPO, with a higher AI for the GI (mean AI, 64.4%) group than for the GII (mean AI, 45.4%) group (P = 0.0087). When avidity ranges were analyzed, GII heifers showed a predominance of samples (77.8%) with low avidity (AI < 50%) when protein A-HRPO was used, while the majority of samples (93.7%) with high avidity (AI > 50%) was found when using the anti-bovine IgG-HRPO conjugate (P < 0.05) (Table 2).
FIG. 4.
Mean avidity index (%) obtained by iELISAs for determination of IgG avidity to Brucella abortus S-LPS-enriched fraction using anti-bovine IgG-HRPO or protein A-HRPO as conjugates in positive serum samples from GI (positive) and GII (recently vaccinated) groups. Serum samples were considered positive for cutoff values established at an RI of >49 (anti-bovine IgG-HRPO) and an RI of >22 (protein A-HRPO). Data are represented as mean ± standard error of mean. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (Student t test).
TABLE 2.
IgG avidity ranges for Brucella abortus obtained in iELISA using anti-bovine IgG or protein A labeled with peroxidase (HRPO) in two groups of bovine serum samples
| Avidity rangea | No. of serum samples (%)b |
|
|---|---|---|
| Anti-bovine IgG-HRPO | Protein A-HRPO | |
| GI | ||
| Low avidity (AI <50%) | 4 (10) | 10 (25) |
| High avidity (AI >50%) | 36 (90) | 30 (75) |
| GII | ||
| Low avidity (AI <50%) | 1 (6.3) | 7 (77.8)* |
| High avidity (AI >50%) | 15 (93.7)* | 2 (22.2) |
GI, nonvaccinated seropositive cows originated from areas where Brucella is endemic or an outbreak is occurring (n = 40); GII, S19-vaccinated heifers between 3 and 8 months of age, originated from areas where Brucella is endemic, and serum samples were analyzed at 3 months postvaccination (n = 79).
*, significant differences between anti-bovine IgG-HRPO and protein A-HRPO as determined by the χ2 test with Yates correction (P < 0.05).
DISCUSSION
Bovine brucellosis has been controlled by programs that are based on B. abortus S19 vaccination using different schemes followed by serological diagnosis and elimination of seropositive animals (1). Thus, diagnostic procedures for brucellosis are required to differentiate antibody responses after vaccination from natural infection, and they should be specific, sensitive, and able to detect all stages of infection. Currently, no such test exists (24).
ELISAs have been evaluated as alternative techniques for this purpose, but the attempt to standardize primary binding assays has generally not been successful due to the wide variety of protocols used by individual laboratories and additional troubles in standardizing reagents, such as the anti-immunoglobulin conjugates required by the ELISA and the polyclonal anti-mouse antibodies required by the cELISA as detection reagents (28). In this context, ELISAs using affinity conjugates as protein A or G labeled with peroxidase have been shown to be useful in allowing a serological distinction of different IgG antibody isotypes, which can be important in characterizing the profile of Brucella infection (22, 32).
In the present study, we compared iELISAs using peroxidase-labeled anti-bovine IgG or protein A as conjugates for detection of the levels of IgG antibodies, as well as their avidity to B. abortus S-LPS in different groups of bovine serum samples. Initially, we observed that the levels of IgG to B. abortus S-LPS that were detected with both the GII and GIII vaccinated groups when using anti-bovine IgG-HRPO were higher than those with protein A-HRPO. On the other hand, the levels of IgG to B. abortus S-LPS that were found in the positive group (GI) for both conjugates were similar. Also, when using anti-bovine IgG-HRPO conjugate, a high index of reactivity was found for the GIV group. These results indicate that protein A-HRPO conjugate was able to establish a better serological distinction between vaccinated and nonvaccinated groups, as well as to improve the characterization of the seronegative animals.
We determined by TG-ROC analysis the threshold of RI values that had the maximum combined Se and Sp in order to discriminate seropositive nonvaccinated cows from vaccinated heifers (GI from GII) or from vaccinated cows (GI from GIII) or from seronegative animals (GI from GIV) in iELISAs using the two conjugates. It was observed that both iELISAs showed high performance in the discrimination of GI positive from GIII vaccinated cows, as demonstrated by the high values of Se (97.6%) and Sp (97.1%) and by significantly high LR+ and low LR− values, as a result of the high likelihood of infection for a positive sample and low likelihood of infection for a negative one. In contrast, the iELISA using protein A-HRPO showed better performance in distinguishing GI positive from GIV negative animals (RI = 22), with high Se (97.6%) and Sp (94.6%) and significant LR+ and LR− values compared to those for the anti-bovine IgG-HRPO conjugate (RI = 49). In addition, in the attempt to discriminate seropositive nonvaccinated cows from vaccinated heifers (GI from GII), the Sp (94.9%) obtained with protein A-HRPO was higher than that with anti-bovine IgG-HRPO (82.3%), although both conjugates showed similarly low Se (80 to 83%) values. These data resulted in LR+ values for protein A-HRPO higher than those for anti-bovine IgG-HRPO, indicating that the likelihood of infection is high for a positive result obtained with protein A-HRPO, reflecting better discrimination between positive nonvaccinated cows and vaccinated heifers.
When analyzing the percentages of positive bovine serum samples obtained for all groups with both conjugates according to different cutoff (RI) values previously established for all conditions tested, the use of protein A-HRPO enabled the detection of a percentage of positive samples in vaccinated heifers (GII) and seronegative animals (GIV) lower than that for the anti-bovine IgG-HRPO conjugate, reinforcing its efficiency in excluding antibodies generated after vaccination and distinguishing these groups serologically. In seropositive nonvaccinated (GI) and vaccinated (GIII) cows, however, both conjugates had similar performances. In addition, it is possible that the residual percentages of the positivity rates found with animals from GIV may be due to cross-reactivity, due mainly to the fact that the antigen preparation used in the present work was constituted by B. abortus S-LPS. It was already demonstrated in the literature that a number of bacteria induce antibody responses that cause false-positive reactions in tests for brucellosis, mostly when using B. abortus S-LPS (28-30, 32). As the serum samples of group IV came from animals with epidemiological characteristics well defined, i.e., unvaccinated animals from a brucellosis-free region, the possibility of infection or residual vaccination interference in the results obtained in the present work is low. Several studies have demonstrated the high sensitivity of the iELISA for B. abortus S-LPS using anti-immunoglobulin enzyme conjugates, but a low ability to discriminate S19-vaccinated animals (2, 27, 31, 33). There is a single study in the literature using radiolabeled protein A for detection of bovine antibody to B. abortus and more specifically for detection of bovine IgG2 subclass antibody to enhance assay specificity (22). In this context, in our study, the use of peroxidase-labeled protein A as a better alternative to radiolabeled protein A enabled the detection predominantly of the IgG2 isotype in seropositive nonvaccinated cows (GI), correlating this IgG isotype profile with an increased level of antigen exposure that is commonly seen with Brucella infection (22, 32). In addition, it is worthy to note that serum samples from GII vaccinated heifers were obtained 90 days after vaccination, whereas antibodies generated after vaccination have achieved the peak of production. Thus, the low detection of the IgG2 isotype in this vaccinated group and the subsequent definition of these GII serum samples as negative reinforce the ability of this affinity conjugate to discriminate nonvaccinated from vaccinated cattle. Therefore, the measurement of B. abortus-specific IgG2 antibody levels may be a valuable tool in identifying Brucella-infected cattle. On the other hand, it should be emphasized that vaccinated animals may undergo subsequent exposure to virulent Brucella strains when they are living in areas where Brucella is endemic, thus making it difficult to analyze the boosting effects of exposure upon vaccinal responses, and these issues may be unavoidable in field studies. In our study, however, we found a relatively low proportion of residual antibodies, likely the IgG2 isotype, detected by protein A-HRPO in both the GII and GIII vaccinated groups, suggesting a low probability that exposure to virulent Brucella strains has a boosting effect upon vaccinal responses. Recently, the use of a universal detection reagent, the chimeric protein A-protein G immunoglobulin receptors (PAG) labeled with peroxidase, has been standardized in the iELISA using S-LPS for the assessment of antibodies against Brucella spp. in serum samples from various species of domestic animals, including cattle, sheep, goats, and pigs (30). Although the PAG conjugate is available from several commercial sources and despite its added advantage over the anti-immunoglobulin conjugate, in addition to the fact that S-LPS can also be standardized, iELISAs using this universal reagent were able to exclude partially antibodies in vaccinated cattle, since vaccinate specificity was around 50% (29). The use of monoclonal antibodies that do not react with PAG has been standardized in a second generation of the cELISA for detection of bovine antibody to B. abortus, thus eliminating reagent variables as the requirement for polyclonal anti-mouse enzyme conjugate for detection. In this new cELISA, a slightly lower exclusion of S19-vaccinated animals was found, with vaccinate specificity of 53.8% (28, 29). In our study using the iELISA with protein A-HRPO, we found a high rate of exclusion of antibodies generated after vaccination, ranging from 75% to 95% for vaccinated heifers, with serum samples collected and analyzed at the peak of antibody response (GII group), during which serum samples gave positive results in conventional serological tests (data not shown). An additional advantage of this iELISA using protein A-HRPO is the possibility of distinguishing IgG subclasses, which is different from using the PAG conjugate, which binds to both the bovine IgG1 and IgG2 subclasses, in addition to the fact that the format of the iELISA eliminates the use of the monoclonal antibody specific for a common epitope of S-LPS derived from B. abortus required by the cELISA, which may not be available for most laboratories.
IgG avidity assays have been developed for several pathogens for which it is important to discriminate between recently acquired infection and those obtained in the more distant past (7, 8, 26). It has been proposed that in persistently infected animals antibodies will develop increased affinity to specific antigens of the pathogen over time (16). However, little is known about the affinity maturation developed in cattle, particularly to differentiate vaccinated from infected animals. In the present study, we investigated the value of the B. abortus-specific IgG avidity assay to discriminate seropositive nonvaccinated from vaccinated cattle in iELISAs using either anti-bovine IgG-HRPO or protein A-HRPO as detection conjugates. The use of protein A-HRPO, but not of the anti-immunoglobulin conjugate, was able to distinguish the two groups as determined by a higher mean AI in seropositive nonvaccinated cows and a lower mean AI in vaccinated heifers, with a predominance of serum samples with high IgG avidity in the former group and low IgG avidity in the latter one. Although there are no data in the literature on IgG avidity assays with bovine brucellosis, our results reinforce the hypothesis that lower-affinity antibodies may be present in Brucella-vaccinated cattle (30). However, our study is the first that has used avidity assays for characterizing B. abortus-specific IgG avidity in bovine serum samples, suggesting a role for the IgG2 subclass as an antibody of higher-affinity maturation after infection. It is worthy to note that animals from the GI group are almost exclusively chronically infected ones, considering their serological profiles determined by SAT/2-ME. These results are in agreement with the high avidity of specific IgG antibodies found in this group. In humans with a history of Malta fever, however, elevated IgG avidity would be indicative of immune memory, not current infection, whereas patients with antibody titers at the diagnostic borderline and low avidity would be indicative of primary infections (12). Thus, in human brucellosis, IgG antibodies with high avidity indices would be useful in excluding recent infection (20). It is noteworthy to mention, though, that there is no current vaccination for human brucellosis. Previous studies have demonstrated that lipopolysaccharides (LPS) from Salmonella enterica serovar Dublin stimulate predominantly a T-helper type 1 immune response, which enhances bovine IgG2 production, and that the mean peak ratio of IgG2/IgG1 in infected cattle was significantly greater than in those vaccinated (18). Interestingly, serum samples from vaccinated animals also gave low IgG2 titers to heterologous Salmonella LPS (17). On the basis of our results of B. abortus-specific IgG avidity assays using different conjugates, we can suggest that specific IgG1 and IgG2 antibodies have different affinity profiles and that the IgG2/IgG1 ratio is significantly greater in infections than in vaccinations. Thus, avidity assays may be used as additional tools to improve the serological distinction between infected and vaccinated cattle. In addition, the use of isotype-specific secondary antibodies in future studies with an emphasis on the kinetics of specific antibody avidity could be useful in more accurately defining isotype profiles in vaccinated-alone, infected-alone, and vaccinated/infected animals in controlled experimental trials. Altogether, the iELISA using B. abortus S-LPS as an antigen and protein A-HRPO as a detection conjugate was shown to be a potentially useful tool for better differentiating S19 antibody responses generated after vaccination and B. abortus infection due to a preferential detection of the IgG2 isotype, a valuable marker of Brucella infection. Also, the B. abortus-specific IgG avidity iELISA using protein A-HRPO demonstrated that antibodies generated after vaccination showed lower avidity, representing an additional technique to establish a serological distinction between vaccinated and infected cattle. This is particularly important in low-prevalence areas where S19 vaccination is traditionally used in heifers or in high-prevalence regions where adult cows are being revaccinated with reduced doses of S19 as part of eradication programs for bovine brucellosis. Therefore, the development of easy, inexpensive, and available protocols for serological diagnosis of brucellosis can allow more efficient and cost-effective detection, control, and elimination of infected reactors from livestock.
Acknowledgments
We thank Paulo Martins Soares Filho from the Laboratório Nacional Agropecuário (LANAGRO - Ministério da Agricultura, Pecuária e Abastecimento, Pedro Leopoldo, State of Minas Gerais, Brazil) and Rodney Carvalho de Oliveira from the Companhia Integrada de Desenvolvimento Agrícola de Santa Catarina (CIDASC-São José, State of Santa Catarina, Brazil) for kindly providing reference serum samples.
Financial support for this project was provided by Brazilian research agencies (CNPq and FAPEMIG).
Footnotes
Published ahead of print on 10 February 2010.
REFERENCES
- 1.Abalos, P., J. Daffner, and L. Pinochet. 2000. Evaluation of three Brucella soluble antigens used in an indirect ELISA to discriminate S19 vaccinated from naturally infected cattle. Vet. Microbiol. 7:161-167. [DOI] [PubMed] [Google Scholar]
- 2.Abalos, P., L. Ibarra, L. Pinochet, F. Navia, and X. Boisier. 1996. Residual anti-Brucella abortus strain 19 antibodies detected in adult cattle by two indirect-ELISA tests. Vet. Rec. 138:140. [DOI] [PubMed] [Google Scholar]
- 3.Aguado-Martinez, A., G. Alvarez-Garcia, I. Arnaiz-Seco, E. Innes, and L. M. Ortega-Mora. 2005. Use of avidity enzyme-linked immunosorbent assay and avidity Western blot to discriminate between acute and chronic Neospora caninum infection in cattle. J. Vet. Diagn. Invest. 17:442-450. [DOI] [PubMed] [Google Scholar]
- 4.Al Dahouk, S., H. Tomaso, K. Nöckler, H. Neubauer, and D. H. Frangoulidis. 2003. Laboratory-based diagnosis of brucellosis: a review of the literature. Part II: serological tests for brucellosis. Clin. Lab. 49:577-589. [PubMed] [Google Scholar]
- 5.Al Dahouk, S., K. Nöckler, H. C. Scholz, H. Tomaso, R. Bogumil, and H. Neubauer. 2006. Immunoproteomic characterization of Brucella abortus 1119-3 preparations used for the serodiagnosis of Brucella infections. J. Immunol. Methods 309:34-47. [DOI] [PubMed] [Google Scholar]
- 6.Alton, G. G., L. M. Jones, R. D. Angus, and J. M. Verger. 1988. Techniques for the brucellosis laboratory. Institut National de la Recherche Agronomique, Paris, France.
- 7.Béla, S. R., D. A. Oliveira Silva, J. P. Cunha-Júnior, C. P. Pirovani, F. A. Chaves-Borges, F. Reis de Carvalho, T. Carrijo de Oliveira, and J. R. Mineo. 2008. Use of SAG2A recombinant Toxoplasma gondii surface antigen as a diagnostic marker for human acute toxoplasmosis: analysis of titers and avidity of IgG and IgG1 antibodies. Diagn. Microbiol. Infect. Dis. 62:245-254. [DOI] [PubMed] [Google Scholar]
- 8.Björkman, C., K. Näslund, S. Stenlund, S. W. Maley, D. Buxton, and A. Uggla. 1999. An IgG avidity ELISA to discriminate between recent and chronic Neospora caninum infection. J. Vet. Diagn. Invest. 11:41-44. [DOI] [PubMed] [Google Scholar]
- 9.Cassataro, J., K. Pasquevich, L. Bruno, J. C. Wallach, C. A. Fossati, and P. C. Baldi. 2004. Antibody reactivity to Omp31 from Brucella melitensis in human and animal infections by smooth and rough brucellae. Clin. Diagn. Lab. Immunol. 11:111-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cloeckaert, A., V. Weynants, J. Godfroid, J. M. Verger, M. Grayon, and M. Zygmumnt. 1998. O-Polysaccharide epitopic heterogeneity at the surface of Brucella spp. studied by enzyme-linked immunosorbent assay and flow cytometry. Clin. Diagn. Lab. Immunol. 5:862-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corbel, M. J. 1997. Brucellosis: an overview. Emerg. Infect. Dis. 3:213-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de los Ángeles Mantecón, M., M. P. Gutiérrez, M. del Pilar Zarzosa, L. Fernández-Lago, J. de Dios Colmenero, N. Vizcaíno, M. A. Bratos, A. Almaraz, A. Cubero, M. F. Muñoz, A. Rodríguez Torres, and A. Orduña. 2008. Influence of brucellosis history on serological diagnosis and evolution of patients with acute brucellosis. J. Infect. 57:397-403. [DOI] [PubMed] [Google Scholar]
- 13.Golding, B., D. E. Scott, O. Scharf, L. Y. Huang, M. Zaitseva, C. Lapham, N. Eller, and H. Golding. 2001. Immunity and protection against Brucella abortus. Microbes Infect. 3:43-48. [DOI] [PubMed] [Google Scholar]
- 14.Greiner, M., D. Sohr, and P. Göbel. 1995. A modified ROC analysis for the selection of cut-off values and the definition of intermediate results of serodiagnostic tests. J. Immunol. Methods 185:123-132. [DOI] [PubMed] [Google Scholar]
- 15.Gutierrez, J., and C. Maroto. 1996. Are IgG antibody avidity assays useful in the diagnosis of infectious diseases? A review. Microbios 87:113-121. [PubMed] [Google Scholar]
- 16.Hansen, K. R., L. R. Nielsen, and P. Lind. 2006. Use of IgG avidity ELISA to differentiate acute from persistent infection with Salmonella Dublin in cattle. J. Appl. Microbiol. 100:144-152. [DOI] [PubMed] [Google Scholar]
- 17.House, J. K., P. S. Bradford, and K. Darin. 2001. Serological distinction of bovine Salmonella carriers from vaccinated and acutely infected cows. J. Vet. Diagn. Invest. 13:483-488. [DOI] [PubMed] [Google Scholar]
- 18.House, J. K., P. S. Bradford, K. O'Connell, and D. C. VanMetre. 2001. Isotype-specific antibody responses of cattle to Salmonella Dublin lipopolysaccharide and porin following Salmonella Dublin vaccination and acute and chronic infection. J. Vet. Diagn. Invest. 13:213-218. [DOI] [PubMed] [Google Scholar]
- 19.Korhonen, M. H., J. Brunstein, H. Haario, A. Katnikov, R. Rescaldani, and K. Hedman. 1999. A new method with general diagnostic utility for the calculation of immunoglobulin G avidity. Clin. Diagn. Lab. Immunol. 6:725-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kutlu, S. S., A. Celikbas, O. Ergönül, M. Kutlu, S. Aksaray, E. Güvener, and B. Dokuzoguz. 2003. The value of the immunoglobulin G avidity test for the serological diagnosis of brucellosis. Mikrobiyol. Bul. 37:261-267. [PubMed] [Google Scholar]
- 21.Lapaque, N., I. Moriyón, E. Moreno, and J. P. Gorvel. 2005. Brucella lipopolysaccharide acts as a virulence factor. Curr. Opin. Microbiol. 8:60-66. [DOI] [PubMed] [Google Scholar]
- 22.Lawman, M. J. P., D. R. Ball, E. M. Hoffmann, L. E. Desjardin, and M. D. P. Boyle. 1986. Production of Brucella abortus-specific protein A-reactive antibodies (IgG2) in infected and vaccinated cattle. Vet. Microbiol. 12:43-53. [DOI] [PubMed] [Google Scholar]
- 23.MAPA. 2005. Manual Técnico do Programa Nacional de Controle e Erradicação da Brucelose e Tuberculose-PNCEBT. Ministério da Agricultura, Pecuária e Abastecimento, Secretaria de Defesa Agropecuária, Departamento de Defesa Animal, Brasília, Brasil.
- 24.McGiven, J. A., J. D. Tucker, L. L. Perrett, J. A. Stack, S. D. Brew, and A. P. MacMillan. 2003. Validation of FPA and cELISA for the detection of antibodies to Brucella abortus in cattle sera and comparison to SAT, CFT, and iELISA. J. Immunol. Methods 278:171-178. [DOI] [PubMed] [Google Scholar]
- 25.Mineo, J. R., D. A. O. Silva, M. C. Sopelete, G. S. Leal, L. H. G. Vidigal, L. E. R. Tápia, and M. I. Bacchin. 2005. Pesquisa na área biomédica: do planejamento à publicação. EDUFU, Uberlândia, Brazil.
- 26.Montoya, J. G., and O. Liesenfeld. 2004. Toxoplasmosis. Lancet 363:1965-1976. [DOI] [PubMed] [Google Scholar]
- 27.Nielsen, K. 2002. Diagnosis of brucellosis by serology. Vet. Microbiol. 90:447-459. [DOI] [PubMed] [Google Scholar]
- 28.Nielsen, K., P. Smith, W. L. Yu, C. Elmgren, G. Halbert, P. Nicoletti, B. Perez, S. Conde, L. Samartino, A. Nicola, R. Bermudez, and T. Renteria. 2008. Validation of a second generation competitive enzyme immunoassay (CELISA) for the diagnosis of brucellosis in various species of domestic animals. Vet. Immunol. Immunopathol. 125:246-250. [DOI] [PubMed] [Google Scholar]
- 29.Nielsen, K., P. Smith, W. L. Yu, C. Elmgren, P. Nicoletti, B. Perez, R. Bermudez, and T. Renteria. 2007. Second generation competitive enzyme immunoassay for detection of bovine antibody to Brucella abortus. Vet. Microbiol. 124:173-177. [DOI] [PubMed] [Google Scholar]
- 30.Nielsen, K., P. Smith, W. Yu, P. Nicoletti, P. Elzer, A. Vigliocco, P. Silva, R. Bermudez, T. Renteria, F. Moreno, A. Ruiz, C. Massengill, Q. Muenks, K. Kenny, T. Tollersrud, L. Samartino, S. Conde, G. D. Benitez, D. Gall, B. Perez, and X. Rojas. 2004. Enzyme immunoassay for the diagnosis of brucellosis: chimeric Protein A-Protein G as a common enzyme labeled detection reagent for sera for different animal species. Vet. Microbiol. 101:123-129. [DOI] [PubMed] [Google Scholar]
- 31.Office International des Épizooties (OIE). 2000. Chapter 2.3.1, Manual of standards for diagnostic tests and vaccines for terrestrial animals, 4th ed. Office International des Épizooties, Paris, France. http://www.oie.int. Accessed 27 September 2004.
- 32.Saegerman, C., L. De Waele, D. Gilson, J. Godfroid, P. Thiange, P. Michel, B. Limbourg, T. K.-O. Vo, J. Limet, J.-J. Letesson, and D. Berkvens. 2004. Evaluation of three serum i-ELISAs using monoclonal antibodies and protein G as peroxidase conjugate for the diagnosis of bovine brucellosis. Vet. Microbiol. 100:91-105. [DOI] [PubMed] [Google Scholar]
- 33.Samartino, L., D. Gall, R. Gregoret, and K. Nielsen. 1999. Validation of enzyme-linked immunosorbent assays for the diagnosis of bovine brucellosis. Vet. Microbiol. 70:193-200. [DOI] [PubMed] [Google Scholar]
- 34.Uzal, F. A., A. E. Carrasco, K. Nielsen, S. Echaide, and S. Cabrera. 1996. An ELISA using a monoclonal anti-IgG1 enzyme conjugate for the diagnosis of bovine brucellosis. Vet. Microbiol. 52:175-180. [DOI] [PubMed] [Google Scholar]
- 35.Wiuff, C., B. M. Thorberg, A. Engvall, and P. Lind. 2002. Immunochemical analyses of serum antibodies from pig herds in a Salmonella nonendemic region. Vet. Microbiol. 85:69-82. [DOI] [PubMed] [Google Scholar]