Abstract
Proteases have been found to play essential roles in many biological processes, including the pathogenesis of leishmaniasis. Most parasites rely on their intracellular and extracellular protease repertoire to invade and multiply in mammalian host cells. However, few studies have addressed serine proteases in Leishmania and their role in host pathogenesis. Here we report the intracellular distribution of a novel L. donovani secretory serine protease in the flagellar pocket, as determined by immunogold labeling. Flow cytometry and confocal immunofluorescence analysis revealed that the expression of the protease diminishes sequentially from virulent to attenuated strains of this species and is also highly associated with the metacyclic stage of L. donovani promastigotes. The level of internalization of parasites treated with the anti-115-kDa antibody into host macrophages was significantly reduced from that of non-antibody-treated parasites, suggesting that this serine protease probably plays a role in the infection process. In vivo studies confirmed that this serine protease is a potential vaccine candidate. Altogether, the 115-kDa serine protease might play vital roles in L. donovani pathogenesis and hence could be recognized as a potential candidate for drug design.
Leishmania species, belonging to the family Trypanosomatidae, are a group of protozoan pathogens that cause a spectrum of chronic diseases ranging from self-healing cutaneous lesions to a lethal visceral disorder. They represent a major health problem in tropical and subtropical areas around the world. Leishmania species have a relatively simple life cycle, with parasite stage differentiation regulated by environmental signals encountered in their different hosts. They are digenetic parasites: the flagellated promastigote forms, which can be derived in axenic culture, differentiate within the alimentary tracts of sandfly vectors from a replicating procyclic to a nonreplicating, infectious metacyclic stage, whereas obligately intracellular amastigotes live and replicate in mammalian mononuclear phagocytes, such as macrophages (1).
Recent advances in genomic analysis of several of the major global parasites have revealed key factors involved in the pathogenesis of parasite diseases. Among the major virulence factors identified are parasite-derived proteases. Parasite proteases play significant roles in the pathogenesis of parasitic diseases; the proteases are involved in the invasion of the host via parasite migration through tissue barriers, degradation of host proteins for their nutrition, immune evasion, and activation of inflammation (21). The migration of the parasite in host tissues is mediated by the release of proteolytic enzymes that can degrade the tissue barriers. Potent proteolytic enzymes of cysteine, serine, and metalloprotease classes have been identified in secretory products of many of the parasites (8, 19, 26, 27, 30). Serine proteases have been reported to have strong hydrolytic activity against a wide range of extracellular matrix (ECM) components and human plasma proteins; hence, they are thought to play vital roles in the host tissue invasion process (17). These proteases are found to localize in different cellular compartments. In trypanosomatids, the flagellar pocket, a specialized region formed by invagination of the plasma membrane, has been found to harbor a virulence-associated protease destined to be exported extracellularly (11, 31). However, the specific molecular function of the extracellular serine protease of the Indian strain of Leishmania donovani has not been elucidated yet.
Our previous study demonstrated that a 115-kDa serine protease is secreted by an Indian strain of L. donovani (8). In the present report, we have demonstrated the intracellular localization of this parasite-derived secreted serine protease and also its differential expression in virulent, avirulent, and attenuated strains of L. donovani as well as in different cell cycle stages of the parasite. A combination of in vitro and in vivo experiments suggests that the protease might play a role in host-parasite interactions and could be validated as a potential vaccine candidate.
MATERIALS AND METHODS
Culture of L. donovani and macrophages.
Leishmania donovani strain MHOM/IN/1983/AG83 was isolated from Indian patients with visceral leishmaniasis. The promastigotes used were at or near the stationary phase of growth in the 4th passage (4th P) (virulent) or 34th passage (34th P) (avirulent) of in vitro culture after transformation from liver- or spleen-derived amastigotes. Promastigotes were cultured at 22°C in medium 199 with Hanks' salt containing HEPES, l-glutamine, 10% heat-inactivated fetal calf serum (FCS), penicillin at 50 U ml−1, and streptomycin at 50 μg ml−1 (all from Gibco BRL/Life Technologies, Middlesex, United Kingdom) (24). Amastigotes of Leishmania donovani were isolated from the spleens of infected mice by homogenization of the organs, followed by Percoll gradient fractionation, as described elsewhere (6). The axenic amastigotes were cultured according to the method of Li et al. (20). Axenic amastigote cells were visualized under a light microscope to confirm transformation; these were harvested 4 days after initial differentiation, with at least one serial dilution. UR6 promastigotes of L. donovani were cultured on solid blood agar slants containing glucose, sodium chloride, peptone, beef extract, and rabbit blood supplemented with gentamicin (7). UR6 cells were harvested by scraping in 10 mM phosphate-buffered saline (PBS) (pH 7.2) from 3-day-old blood agar slants. For subsequent flow cytometry experiments, UR6 cells were maintained in liquid medium (medium 199) supplemented with 10% FCS. Metacyclic parasites of virulent and attenuated cells were isolated from 2- and 4-day-old cultures by the peanut agglutinin (PNA) agglutination method (29).
The murine macrophage cell line RAW 264.7 was cultured at 37°C under 5% CO2 in RPMI 1640 supplemented with 10% heat-inactivated FCS, 10 mM HEPES (pH 7.3), 100 U/ml penicillin, and 100 μg/ml streptomycin. For experiments, cells between passages 3 and 10 were seeded on sterile glass coverslips and were grown overnight.
Identification and purification of the 115-kDa serine protease.
An extracellular serine protease with a molecular mass of 115 kDa from an Indian strain of Leishmania donovani has been reported (8). Briefly, the extracellular proteases secreted in the culture supernatant of early-passage (4th-passage) L. donovani were subjected to gelatin substrate gel electrophoresis and were treated with different classes of protease inhibitors. We noted that the activity of the 115-kDa protease was completely inhibited by the serine protease inhibitor aprotinin. The protease was then purified by ammonium sulfate precipitation and was subjected to aprotinin-agarose affinity chromatography, followed by continuous elution electrophoresis. The purified serine protease was further characterized (8).
Raising of antisera.
A polyclonal antibody against the 115-kDa extracellular protease was raised by subcutaneous injection of 80 μg of purified protease emulsified in Freund's complete adjuvant into a male rabbit. Three booster doses were administered at intervals of 2 weeks by injecting the purified protease emulsified in Freund's incomplete adjuvant. At 10 days after the fourth injection, blood was collected from the rabbit's ear, and the antiserum against the 115-kDa protease was separated (25). A mouse polyclonal antiserum was also raised against purified L. donovani gp63, which was purified according to the method described by Soteriadou et al. (32). A polyclonal antibody was purified from the antiserum by protein A agarose (Sigma Chemical Co., St. Louis, MO) affinity chromatography (14). The peak fractions were concentrated and kept frozen at −80°C.
Electrophoresis and immunoblotting.
Western blotting was carried out according to the method described by Towbin et al. (33) with some modifications. Briefly, cell-free cultured supernatants of virulent promastigotes and whole-cell lysates of virulent promastigotes, avirulent promastigotes, and attenuated UR6 cells were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (18) under nonreducing conditions and were transferred to nitrocellulose membranes (pore size, 0.45 μm). Membranes were incubated for 1 h with the anti-115-kDa polyclonal antiserum (dilution, 1: 500) in Tris-buffered saline (TBS) at 22°C, washed in TBS, and incubated with appropriate horseradish peroxidase (HRP)-conjugated secondary antibodies. Membranes were developed with 0.2 mM 4-chloro-1-naphthol.
Flow cytometric analysis.
The anti-115-kDa extracellular protease antibody was labeled with fluorescein isothiocyanate (FITC) by using the Pierce EZ-Label FITC protein labeling kit. Early-passage (4th-P), late-passage (34th-P), and UR6 cells and axenic amastigotes of virulent AG83 were harvested and stained with the FITC-labeled anti-115-kDa extracellular protease antibody. Procyclic and metacyclic promastigotes of early-passage virulent cells and attenuated cells from 3- and 4-day-old cultures were also isolated and stained with the FITC-labeled anti-115-kDa extracellular protease antibody. Briefly, the cells were washed three times in PBS and were then fixed in 1% paraformaldehyde. The fixed cells were taken up in a fluorescence-activated cell sorter (FACS) buffer (catalog no. 333138; Becton Dickinson) and were stained with the FITC-labeled anti-115-kDa protease (dilution, 1:100). The fluorescence of the fixed stained cells was detected on a FACSCalibur system (BD Biosciences), and the data were analyzed using CellQuest software.
Confocal immunofluorescence microscopy.
L. donovani promastigotes (early and late passages) and UR6 cells were centrifuged at 3,000 × g for 10 min, washed in PBS, and resuspended at a concentration of 107 cells/ml. Cells were applied to poly-l-lysine-coated glass coverslips and were fixed in PBS containing 4% paraformaldehyde for 30 min. Coverslips were rinsed once with PBS to remove the fixative and were incubated in a blocking solution consisting of PBS supplemented with 5% FCS, 0.1% Tween 20, and 0.1% Triton X-100 for 1 h at room temperature. To investigate the specificity of the anti-115-kDa antibody, an irrelevant antibody (anti-gp63) was tested. A primary antibody against L. donovani gp63 (raised in mouse) and a purified antibody against the L. donovani 115-kDa serine protease (raised in rabbit) were diluted 1:500 in blocking solution and were incubated separately or together with the promastigotes of L. donovani. After incubation of fixed parasites with the primary antibody for 1 h, coverslips were rinsed six times for 5 min with a PBS wash solution containing 0.1% Tween 20. Fixed cells were then incubated separately or together with an FITC-conjugated anti-rabbit secondary antibody at a 1:1,000 dilution and with a tetramethyl rhodamine isocyanate (TRITC)-conjugated anti-mouse secondary antibody at a 1:100 dilution in blocking buffer. The incubation and all subsequent steps were performed in the dark. Secondary antibodies were removed by soaking coverslips six times, for 5 min each time, in wash buffer. Cells were then washed in PBS and were observed under a laser scanning microscope (LSM 510; Carl Zeiss, Jena, Germany). The images were further processed using Adobe Photoshop, version 7.0 (Adobe Systems).
Immunogold labeling.
Approximately 107 early-passage promastigotes and amastigotes of L. donovani were fixed in 2% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.4) for 4 h at 4°C. Samples were dehydrated in ethanol and embedded in LR White resin. Ultrathin sections were cut and incubated with an anti-115-kDa extracellular serine protease antibody (1:20). The binding of the primary antibody was visualized by incubation with a gold (diameter, 10 nM)-conjugated secondary antibody (1:50). Samples were stained with uranyl acetate and with lead citrate and were finally investigated with an FEI Tecnai G2 transmission electron microscope (with the SIS Protem program) (15).
Effects of antisera on macrophage infectivity.
Parasites were incubated for 1 h with the anti-115-kDa antibody (dilution, 1:200) and were then washed three times with excess PBS to remove the unbound antibody completely. In parallel experiments, parasites were also treated with preimmune serum. Preimmune serum- or antibody-treated promastigotes and untreated promastigotes were used to infect cultures of adherent macrophages (RAW 264.7 cell line) on glass coverslips (1 × 106 macrophages/coverslip) in 0.5 ml of RPMI 1640 containing10% FCS at a ratio of 10 parasites per macrophage. The coverslips were kept at 37°C under a humidified 5% CO2 atmosphere. After sequential incubation for 24 h, 48 h, and 72 h, the cells were fixed in methanol and were stained with Giemsa stain for the determination of intracellular parasite numbers (2, 22). In a separate set of experiments, parasites pretreated with the anti-115-kDa antibody were incubated with the macrophages, which had previously been incubated only with the purified 115-kDa protease (1 μg/ml) (4), and the experiments proceeded as described above.
In vivo assay of L. donovani infectivity after immunization with the protease.
Groups of six female BALB/c mice, 4 to 6 weeks old, were vaccinated subcutaneously or intraperitoneally with 10 μg of the purified 115-kDa serine protease in Freund's complete adjuvant in a volume of 0.1 ml. Three booster doses were given in Friend's incomplete adjuvant at biweekly intervals with the same amount of protein. Control mice received the adjuvant alone. One month after the last booster, one set of mice was challenged intracardially with 5 × 105 virulent L. donovani promastigotes, and another set of mice was challenged with 5 × 108 parasites. Starting at 4 weeks postchallenge, the mice were sacrificed at different times up to 8 months, and parasite burdens in the spleen and liver were quantitatively evaluated (5) by microscopic observation of Giemsa-stained preparations of impression smears of the tissues.
RESULTS AND DISCUSSION
Immunoblotting.
A polyclonal antibody raised against the purified serine protease was employed in Western blot analysis of the whole-cell lysates of 4th-P, 34th-P, and UR6 promastigotes. The cell-free culture supernatant of the early-passage cell was analyzed by immunoblotting as well. It was observed in the experiment that a 115-kDa band appeared in all the lanes, but the intensities of the bands differed significantly (Fig. 1). The prominent band observed in Fig. 1, lane b, unambiguously suggested the intracellular appearance of the protease in virulent L. donovani promastigotes. Moreover, Western blotting for detection of the protease in whole-cell extracts of 34th-P L. donovani promastigotes (Fig. 1, lane c) and UR6 cells (lane d) produced a faint band, showing decreased amounts of the protease in avirulent and attenuated L. donovani parasites.
FIG. 1.

Western blot analysis of the 115-kDa protease of L. donovani. Cell-free cultured supernatants from early-passage promastigotes and whole-cell extracts from virulent, avirulent, and attenuated strains of L. donovani were loaded directly onto an SDS gel and used for immunoblot detection with the anti-115-kDa serine protease antibody. Lane a, purified protease (2 μg protein) from the culture supernatant of L. donovani; lane b, whole-cell extract of early-passage (4th-P) L. donovani promastigotes (100 μg protein); lane c, whole-cell extract of late-passage (34th-P) L. donovani promastigotes (100 μg protein); lane d, whole-cell extract of UR6 promastigotes (100 μg protein). Actin was used as a loading control to normalize the data.
Flow cytometric analysis.
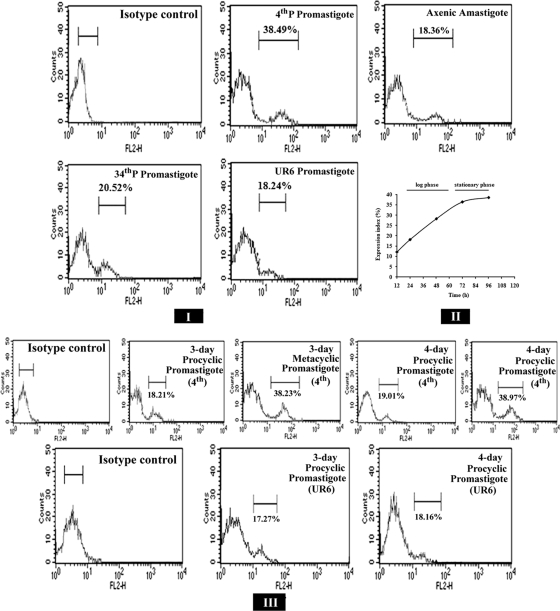
It has been reported that long-term culture in vitro leads to the loss of virulence by Leishmania donovani promastigotes (10). Our previous study identified the presence of a 115-kDa secretory serine protease in virulent L. donovani promastigotes (8). In the present study, flow cytometric analysis suggested a negative correlation between the level of expression of the 115-kDa protein and the number of times the parasites were cultivated in vitro, and hence a positive correlation with their virulence. As observed in Fig. 2I, the anti-protease antibody recognized 38.49% of the cells of the virulent 4th-P promastigotes and only 20.52% of the avirulent 34th-P promastigotes, suggesting reduced expression of the protein with an increase in serial cell passage number during in vitro cultivation, when the parasites also exhibit a relative loss of virulence. The anti-115-kDa antibody was found to recognize only 18.24% of the nonpathogenic attenuated UR6 promastigotes (Fig. 2I), as determined with reference to the number of control cells recognized, whereas the same antibody reacted with 18.36% of the total population of infective amastigotes. This lesser recognition of amastigotes, as well as of avirulent and attenuated promastigotes, might be due to the lower expression of the protease in these cells. These observations indicate that the 115-kDa serine protease is an exclusive secretory product of virulent L. donovani promastigotes, rather than of avirulent and attenuated promastigotes or infective amastigotes of virulent L. donovani. These results also demonstrate that a discrete population of cells is associated with abundant expression of 115-kDa protease.
FIG. 2.
Flow cytometric analysis of expression of the 115-kDa extracellular serine protease. (I) Fluorescence histograms showing the expression levels of the 115-kDa protease in 4th-P, 34th-P, and UR6 promastigotes and axenic amastigotes of L. donovani. (II) Expression of the 115-kDa protease of 4th-P promastigotes of L. donovani at different phases of growth. The expression index measures the percentage of cells of the total population recognized by the anti-115-kDa antibody. (III) Fluorescence histograms showing the expression levels of the 115-kDa protease at procyclic and metacyclic stages of virulent promastigotes and at procyclic stages of attenuated UR6 promastigotes at 72 h and 96 h of culture.
The growth of Leishmania promastigotes within the sandfly midgut or in in vitro culture is accompanied by differentiation of some of these organisms into metacyclic-stage promastigotes, which can be distinguished from noninfective procyclic promastigotes by their loss of agglutination by PNA lectin (29). The metacyclic stages of promastigotes are uniquely adapted to survival within vertebrate hosts. On the other hand, the relative loss of virulence of PNA-agglutinable (PNA+) procyclic promastigotes could suggest considerable differences in the metacyclogenetic potential of the parasites with different lengths of time in culture. Thus, both noninfective and infective stages of Leishmania promastigotes within the cultured populations are associated with certain specific developmental changes that could help this parasite to adapt to, and survive within, the vertebrate host. Differentiation of procyclic promastigotes into metacyclic promastigotes (metacyclogenesis) is known to be crucial for infectivity (3, 9). Infective-stage promastigotes are generated in vitro as organisms approach the stationary phase of growth (2, 29). To correlate these changes in the expression of the 115-kDa protease with metacyclogenesis, the expression of the protease was studied in promastigotes derived from 0 to 96 h of culture. Increased infectivity of virulent L. donovani promastigotes from early-log phase to stationary phase was observed with the increase in the percentage of PNA− promastigotes from 0 to 96 h (data not shown), and concurrently, the expression of the 115-kDa protease was found to be high, reaching a plateau at 96 h (Fig. 2II). da Silva and Sacks (9) have also shown that log-phase promastigotes represent a homogeneous population of noninfective parasites, whereas as many as 50% of the stationary-phase organisms appeared to be transformed into infective-stage promastigotes. To further confirm the association of the protease with virulence, the expression of the protease was studied in metacyclic (PNA−) and procyclic (PNA+) promastigotes isolated from 12-, 24-, 48-, 72-, and 96-h cultures. It was noted that the expression of the protease was strongly associated with metacyclic promastigotes. Representative results for the expression of the 115-kDa protease in metacyclic and procyclic promastigotes isolated from 72-h and 96-h cultures of virulent and attenuated strain are shown in Fig. 2III. The results of this study showed 38.23% (72 h) and 38.97% (96 h) recognition in metacyclic promastigotes of 4th-passage L. donovani by the anti-115-kDa protease antibody, compared to 18.21% (72 h) and 19.01% (96 h) in procyclic promastigotes of the same early-passage promastigotes. For attenuated UR6 promastigotes, all of which were found to be PNA+, the anti-115-kDa protease antibody was found to recognize 17.27% (72 h) and 18.16% (96 h) of cells of the total population (Fig. 2III).
During metacyclogenesis, several proteins with functions in protein synthesis, protein metabolism, and protein folding, etc., are downregulated because these promastigotes are in stationary phase and are mostly growth arrested (23). But Gull (13) found an increased abundance of isoforms of proteins involved in motility, including paraflagellar rod protein, α-tubulin, and β-tubulin, in metacyclic promastigotes, which are more active and motile. Similarly, in the present study, the increased expression and secretion of the 115-kDa protease in the infective metacyclic promastigotes could suggest an active role of this protease in the invasion process. Thus, the presence of the 115-kDa serine protease in the culture medium of virulent promastigotes of L. donovani could be a significant phenotypic marker of relative virulence displayed by the promastigotes obtained from culture.
Confocal microscopy.
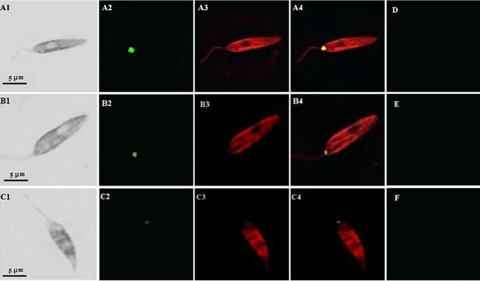
We used confocal immunofluorescence microscopy to compare the relative intensities and hence the relative expression levels of the 115-kDa protease in the virulent and the nonpathogenic attenuated promastigotes of an Indian strain of L. donovani. Cells were first incubated in the presence of a primary antibody against the serine protease; thereafter, they were probed with a fluorescence-conjugated secondary antibody, and intensities were visualized by confocal immunofluorescence microscopy. In order to confirm the localization and the specificity of recognition of the 115-kDa anti-protease antibody, confocal microscopy was also performed with an antibody against Leishmania gp63. In the immunofluorescence assay, the anti-115-kDa antibody was found to react in the cell body, where labeling was restricted to the posterior region of the promastigotes. By comparison of the relative intensities of the immunolabeled protease (Fig. 3), it appears that the relative quantity of the 115-kDa protein is directly proportional to the virulence of the parasite. This is in agreement with the fact that early-passage, virulent promastigotes (Fig. 3A2) showed significantly more-intense labeling than late-passage, avirulent promastigotes (Fig. 3B2), a finding consistent with the in vitro flow cytometric expression pattern. Similarly, the intensity of expression of the protease in the nonpathogenic attenuated UR6 cells (Fig. 3C2) was extremely low. Figure 3A3, B3, and C3 (red channel) are immunoconfocal images of parasites treated with the anti-gp63 antibody. The images of the green and red channels were merged into single images (Fig. 3A4, B4, and C4), where yellow fluorescence represents the colocalization of the red and green fluorescence signals. The phase-contrast images of the same fields are shown in Fig. 3A1, B1, and C1. No fluorescence was detected in parallel preparations probed with the preimmune serum (Fig. 3D, E, and F). These results corroborate our findings that the increased level of the 115-kDa serine protease in early-passage, virulent parasites over that in late-passage, avirulent parasites was quite significant. Hence, this direct relationship of parasite virulence to protease expression suggests that the protease might play some role in host infection. Work is in progress in our laboratory to determine the exact role of this protease in pathogenesis.
FIG. 3.
Immunolocalization and differential expression of the 115-kDa serine protease in L. donovani. Early-passage (A1 to A4), late-passage (B1 to B4), and UR6 (C1 to C4) promastigotes of L. donovani were fixed and stained with an anti-115-kDa antibody and an anti-gp63 antibody as described in Materials and Methods. The immunofluorescence images of the promastigotes labeled for the 115-kDa serine protease are shown in the green channel (A2, B2, and C2), and those labeled for gp63 are shown in the red channel (A3, B3, and C3). Merged images (yellow channel) are shown in panels A4, B4, and C4. The corresponding phase-contrast images are shown on the left (A1, B1, and C1). No fluorescence was detected in similar preparations reacted with the preimmune serum (D, E, and F).
Immunoelectron microscopy.
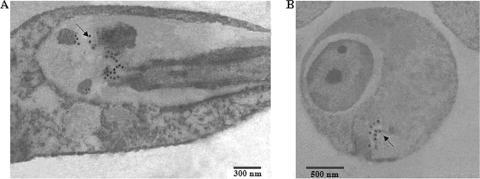
To gain insight into the precise cellular distribution of the serine protease, we employed an immunogold electron microscopy technique. Electron micrographs of thin sections of amastigotes and early-passage promastigotes revealed that most of the labeling seems to be associated with the flagellar pocket region at both stages of the parasite (Fig. 4). This finding clearly indicates that the 115-kDa protease is distributed throughout the flagellar pocket region, a compartment in protozoan architecture that is formed by an invagination of the plasma membrane where the flagellum emerges from the cell body. In recent years, it has been well known that, in contrast to mammalian cells, in trypanosomes the flagellar pocket membrane plays a pivotal role in protein trafficking between the interior and the exterior of the cell (27). Our findings elaborate the idea of the protease being transported outside the cell. Previous studies have also indicated the localization and exocytosis of a serine protease in Leishmania amazonensis through the flagellar pocket (31). The cellular location could be implicated in determining the physiological role of the protease and might be taken into account for future drug design strategies, although the exact mechanism of vesicle fusion, which might be involved in the transport process, still remains uncertain, and more-detailed study of the nature of this compartment needs to be performed.
FIG. 4.
Intracellular localization of the 115-kDa serine protease of L. donovani by immunogold electron microscopy. Promastigotes (A) and amastigotes (B) of L. donovani were labeled with an anti-115-kDa serine protease antibody prior to detection with a gold-conjugated secondary antibody. Electron microscopy images show the presence of gold particles in the flagellar pocket regions of the parasites (arrows).
Effect of antisera on infectivity for macrophages.
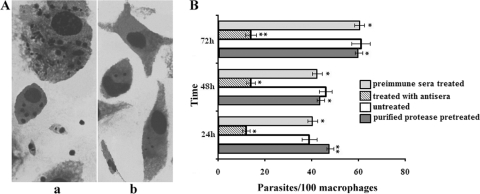
We examined the effect of the anti-115-kDa extracellular protease antibody on in vitro invasion of macrophages. Early-passage promastigotes were incubated with the antibody against the 115-kDa protease. Promastigote internalization was inhibited by treatment of the L. donovani promastigotes with the antibody (Fig. 5). Microscopic analysis showed the conspicuous presence of numerous intracellular parasites in the host macrophages when promastigotes were not treated with the antibody, but the numbers of antibody-treated parasites in macrophages were significantly reduced (Fig. 5A). Thus, one can speculate that the parasites did not mediate internalization when treated with the antibody. Figure 5B shows that the numbers of invading parasites diminished after incubation of the antibody-treated parasites with macrophages for different times (up to 72 h), whereas the number of invading control parasites (either left untreated or treated with preimmune serum) showed successive increases as the incubation time increased. We might speculate that the antibody interacts with the extracellular protease and that this interaction somehow interferes with the invasion of macrophages by parasites. Pretreatment of macrophages with the purified 115-kDa enzyme prior to invasion permitted efficient invasion by parasites treated with the anti-115-kDa antibody (Fig. 5B). Importantly, the purified protease treatment appeared to be efficient in the restoration of parasite invasion. These results indicate the involvement of the protease in target cell invasion.
FIG. 5.
Effect of an antibody against the 115-kDa serine protease on host cell invasion by early-passage L. donovani promastigotes. (A) Microscopic images of macrophages incubated with non-antibody-treated (a) or antibody-treated (b) parasites. (B) Parasites were treated with the antibody for 1 h, after which unbound antibodies were washed out, and parasites were incubated with macrophages for 24 h, 48 h, or 72 h. In another set of experiments, macrophages were pretreated with the purified protease and were then used in the macrophage invasion assay. The internalized parasites were counted after staining with Giemsa stain. Results of corresponding control experiments in which parasites were not treated with the antibody or were treated with preimmune serum are also shown. Reversal of invasion inhibition was observed for macrophages pretreated with the purified 115-kDa serine protease. Results are means ± standard deviations for four independent experiments. Asterisks indicate significance levels (*, P < 0.05; **, P < 0.01).
In vivo assay of L. donovani infectivity after immunization with the protease.
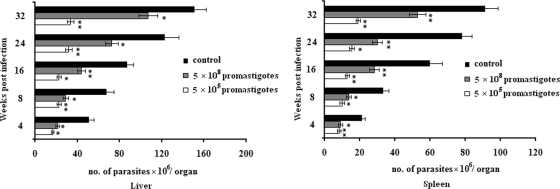
To evaluate the efficacy of the 115-kDa serine protease as a potential protective antigen, female BALB/c mice were vaccinated with the protease together with Freund's complete adjuvant, subcutaneously or intraperitoneally, and were boosted three times at 2-week intervals. Four weeks after the final injection, the mice were challenged with 5 × 105 or 5 × 108 virulent L. donovani promastigotes. The mice were sacrificed 1 month following parasite challenge, and the parasite burdens in spleens and livers were estimated by counting the parasite numbers in those tissues. Mice immunized with the 115-kDa protease exhibited significant protection, as observed by dramatically lower parasite burdens than those for mice receiving Freund's complete adjuvant alone. Mice challenged with 5 × 105 parasites showed no discernible infection in either the spleen or the liver for as long as 8 months, but infection developed after 4 months in mice challenged with 5 × 108 parasites (Fig. 6). Thus, vaccination against this protease is partially effective in eliciting a protective immune response. The mechanism of protection is currently unknown, and work is in progress to find out the basis of this protection. It could be due to an increase in the Th1/Th2 ratio, since several workers have reported that protective immunity in response to vaccination is associated with an increased Th1 response (12, 28). Immunomodulatory cytokines might also be involved in eliciting the immune protection (12, 28).
FIG. 6.
Evaluation of protection against visceral leishmaniasis in BALB/c mice after immunization with the purified 115-kDa serine protease. At different time points postimmunization, parasite burdens in the livers and spleens of mice challenged with 5 × 105 or 5 × 108 virulent L. donovani promastigotes were quantified in Giemsa-stained preparations of impression smears. The results are expressed as the number of parasites per organ. Each experiment was performed at least three times, and the results are means ± standard deviations. Asterisks indicate significance levels (*, P < 0.05; **, P < 0.01).
The localization of the 115-kDa protease inside the flagellar pocket, a specialized region of the plasma membrane involved in endocytosis and exocytosis, strongly suggests that this protease can be released into the extracellular medium by utilizing this vesicular compartment as the secretory pathway. On the other hand, the evidence that the 115-kDa secreted protease has strong proteolytic activity against extracellular matrix proteins, such as collagen and fibronectin, as demonstrated in our previous work (8), suggests that the protease is a superior agent for host tissue invasion. Our experimental data revealed that the relative abundance of the extracellular serine protease is greater in early-passage promastigotes than in late-passage and attenuated promastigotes. Therefore, this differential expression might play a crucial role in the life cycle of Leishmania donovani, and the molecular mechanisms that regulate the appearance of this protease in virulent, avirulent, and attenuated states should be further investigated. Previous reports have indicated that virulence for different Leishmania species is associated with different parameters, such as the length of in vitro culture (16), stationary versus log growth phase (17), and the expression of the major surface glycoprotein (34). We would infer from our findings that relative expression of the 115-kDa protease should be included in this list.
In summary, our work suggests the intracellular distribution of a novel type of serine protease, capable of degrading the ECM, in the flagellar pocket region of L. donovani. The expression of this protease at relatively high levels in early-passage promastigotes compared to levels in late-passage and attenuated stages suggests that the 115-kDa serine protease could contribute to parasite invasion and virulence. Taken together, these observations could help us to acquire important knowledge about the mode of action of this protease, at least in this particular species of Leishmania, and this protease can be considered as a potential target for antileishmanial drug design, to help eradicate this devastating disease affecting millions around the world.
Acknowledgments
Thanks are due to Amritlal Mandal (Department of Physiology, University of Arizona, Tucson, AZ) and S. Roy (Infectious Diseases and Immunology Division, IICB, Kolkata, India) for help with our research.
Financial assistance from the Indian Council of Medical Research (New Delhi, India) and the Council of Scientific and Industrial Research (New Delhi, India) is acknowledged.
Footnotes
Published ahead of print on 27 January 2010.
REFERENCES
- 1.Alexander, J., and D. G. Russel. 1992. The interaction of Leishmania species and macrophages. Adv. Parasitol. 31:175-254. [DOI] [PubMed] [Google Scholar]
- 2.Atayde, V. D., M. Cortez, R. Souza, J. F. da Silveira, and N. Yoshida. 2007. Expression and cellular localization of molecules of the gp82 family in Trypanosoma cruzi metacyclic trypomastigotes. Infect. Immun. 75:3264-3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bates, P. A. 1994. Complete developmental cycle of Leishmania mexicana in axenic culture. Parasitology 108:1-9. [DOI] [PubMed] [Google Scholar]
- 4.Breton, C. B., T. Blisnick, H. Jouin, J. C. Barale, T. Rabilloud, G. Langsley, and L. H. P. da Silva. 1992. Plasmodium chabaudi p68 serine protease activity required for merozoite entry into mouse erythrocytes. Proc. Natl. Acad. Sci. U. S. A. 89:9647-9651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breton, M., M. J. Tremblay, M. Ouellette, and B. Papadopoulou. 2005. Live nonpathogenic parasitic vector as a candidate vaccine against visceral leishmaniasis. Infect. Immun. 73:6372-6382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Channon, J. Y., M. B. Roberts, and J. M. Blackwell. 1984. A study of the differential respiratory burst activity elicited by promastigotes and amastigotes of Leishmania donovani in murine resident peritoneal macrophages. Immunology 53:345-355. [PMC free article] [PubMed] [Google Scholar]
- 7.Chaudhuri, G., T. K. Chatterjee, and A. B. Banerjee. 1982. Growth factor requirements for in vitro growth of Leishmania donovani. Indian J. Med. Res. 76:157-163. [PubMed] [Google Scholar]
- 8.Choudhury, R., S. K. Bhaumik, T. De, and T. Chakraborti. 2009. Identification, purification, and characterization of a secretory serine protease in an Indian strain of Leishmania donovani. Mol. Cell. Biochem. 320:1-14. [DOI] [PubMed] [Google Scholar]
- 9.da Silva, R., and D. L. Sacks. 1987. Metacyclogenesis is a major determinant of Leishmania promastigote virulence and attenuation. Infect. Immun. 55:2802-2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De, T., and S. Roy. 1999. Infectivity and attenuation of Leishmania donovani promastigotes: association of galactosyl transferase with loss of parasite virulence. J. Parasitol. 85:54-59. [PubMed] [Google Scholar]
- 11.Duboise, S. M., M. A. Vannier-Santos, D. Costa-Pinto, L. Rivas, A. A. Pan, Y. Traub-Cseko, W. De Souza, and D. McMahon-Pratt. 1994. The biosynthesis, processing and immunolocalization of Leishmania pifanoi amastigote cysteine proteases. Mol. Biochem. Parasitol. 68:119-132. [DOI] [PubMed] [Google Scholar]
- 12.Ferreira, J. H. L., L. G. Gentil, S. S. Dias, C. E. C. Fedeli, S. Katz, and C. L. Barbiéri. 2008. Immunization with the cysteine proteinase Ldccys1 gene from Leishmania (Leishmania) chagasi and the recombinant Ldccys1 protein elicits protective immune responses in a murine model of visceral leishmaniasis. Vaccine 26:677-685. [DOI] [PubMed] [Google Scholar]
- 13.Gull, K. 1999. The cytoskeleton of trypanosomatid parasites. Annu. Rev. Microbiol. 53:629-655. [DOI] [PubMed] [Google Scholar]
- 14.Harlow, E., and D. Lane. 1988. Antibodies: a laboratory manual, p.617-618. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 15.Jiménez-Jiménez, C., J. Carrero-Lérida, M. Sealey-Cardona, L. M. Ruiz Pérez, J. A. Urbina, and D. González Pacanowska. 2008. Delta24(25)-sterol methenyltransferase: intracellular localization and azasterol sensitivity in Leishmania major promastigotes overexpressing the enzyme. Mol. Biochem. Parasitol. 160:52-59. [DOI] [PubMed] [Google Scholar]
- 16.Katakura, K., and A. Kobayashi. 1985. Enhancement of infectivity of Leishmania donovani promastigotes by serial mouse passages. J. Parasitol. 71:393-394. [PubMed] [Google Scholar]
- 17.Kong, H. H., T. H. Kim, and D. I. Chung. 2000. Purification and characterization of a secretory serine proteinase of Acanthamoeba healyi isolated from GAE. J. Parasitol. 86:12-17. [DOI] [PubMed] [Google Scholar]
- 18.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of the bacteriophage T4. Nature (London) 227:680-685. [DOI] [PubMed] [Google Scholar]
- 19.Law, R. H., P. M. Smooker, J. A. Irving, D. Piedrafita, R. Ponting, N. J. Kennedy, J. C. Whisstock, R. N. Pike, and T. W. Spithill. 2003. Cloning and expression of the major secreted cathepsin B-like protein from juvenile Fasciola hepatica and analysis of immunogenicity following liver fluke infection. Infect. Immun. 71:6921-6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li, Q., Y. Zhao, B. Ni, C. Yao, Y. Zhou, W. Xu, Z. Wang, and Z. Qiao. 2008. Comparison of the expression profiles of promastigotes and axenic amastigotes in Leishmania donovani using serial analysis of gene expression. Parasitol. Res. 103:821-828. [DOI] [PubMed] [Google Scholar]
- 21.McKerrow, J. H., C. Caffrey, B. Kelly, P. Loke, and M. Sajid. 2006. Proteases in parasitic diseases. Annu. Rev. Pathol. 1:497-536. [DOI] [PubMed] [Google Scholar]
- 22.Misra, S., T. Sanyal, D. Sarkar, P. K. Bhattacharya, and D. K. Ghosh. 1989. Evaluation of antileishmanial activity of trans-aconitic acid. Biochem. Med. Metab. Biol. 42:171-178. [DOI] [PubMed] [Google Scholar]
- 23.Mojtahedi, Z., J. Clos, and E. Kamali-Sarvestani. 2008. Leishmania major: identification of developmentally regulated proteins in procyclic and metacyclic promastigotes. Exp. Parasitol. 119:422-429. [DOI] [PubMed] [Google Scholar]
- 24.Mottram, J. C., A. E. Souza, J. E. Hutchison, R. Carter, M. J. Frame, and G. H. Coombs. 1996. Evidence from disruption of the lmcpb gene array of Leishmania mexicana that cysteine proteinases are virulence factors. Proc. Natl. Acad. Sci. U. S. A. 93:6008-6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mukherjee, S., A. Ukil, and P. K. Das. 2007. Immunomodulatory peptide from cystatin, a natural cysteine protease inhibitor, against leishmaniasis as a model macrophage disease. Antimicrob. Agents Chemother. 51:1700-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okenu, D. M., K. N. Opara, R. I. Nwuba, and M. Nwagwu. 1999. Purification and characterisation of an extracellularly released protease of Trypanosoma brucei. Parasitol. Res. 85:424-428. [DOI] [PubMed] [Google Scholar]
- 27.Overath, P., Y. D. Stierhof, and M. Wiese. 1997. Endocytosis and secretion in trypanosomatid parasites—tumultuous traffic in a pocket. Trends Cell Biol. 7:27-33. [DOI] [PubMed] [Google Scholar]
- 28.Rafati, S., A. A. Baba, M. Bakhshayesh, and M. Vafa. 2000. Vaccination of BALB/c mice with Leishmania major amastigote-specific cysteine proteinase. Clin. Exp. Immunol. 120:134-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sacks, D. L., and P. V. Perkins. 1984. Identification of an infective stage of Leishmania promastigotes. Science 223:1417-1419. [DOI] [PubMed] [Google Scholar]
- 30.Silva-Lopez, R. E., M. G. Coelho, and S. G. De Simone. 2005. Characterization of an extracellular serine protease of Leishmania (Leishmania) amazonensis. Parasitology 131:85-96. [DOI] [PubMed] [Google Scholar]
- 31.Silva-Lopez, R. E., J. A. Morgado-Díaz, C. R. Alves, S. Côrte-Real, and S. G. De Simone. 2004. Subcellular localization of an extracellular serine protease in Leishmania (Leishmania) amazonensis. Parasitol. Res. 93:328-331. [DOI] [PubMed] [Google Scholar]
- 32.Soteriadou, K. P., A. K. Tzinia, E. Panou-Pamonis, V. Tsikaris, M. Sakarellos-Daitsiotis, C. Sakarellos, Y. Papapoulou, and R. Matsas. 1996. Antigenicity and conformational analysis of the Zn2+-binding sites of two Zn2+-metalloproteases: Leishmania gp63 and mammalian endopeptidase-24.11. Biochem. J. 313:455-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. U. S. A. 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson, M. E., K. K. Hardin, and J. E. Donelson. 1989. Expression of the major surface glycoprotein of Leishmania donovani chagasi in virulent and attenuated promastigotes. J. Immunol. 143:678-684. [PubMed] [Google Scholar]