Abstract
Wild-type lipopolysaccharide (LPS) of Neisseria meningitidis normally contains six acyl chains. Penta-acylated LPS forms were generated through inactivation of the lpxL1 gene or through the expression of the Bordetella bronchiseptica pagL gene in N. meningitidis. The resulting LPS species, designated LpxL1 LPS and PagL LPS, respectively, display reduced endotoxic activity compared to wild-type LPS. Here, we determined the adjuvant potential of PagL LPS by comparison with the broadly used LpxL1 LPS. We also investigated the potential benefit for adjuvanticity of coincorporating these LPS species, together with the meningococcal opacity-associated protein OpaJ as a model antigen, in a liposomal delivery system. PagL LPS showed a higher endotoxic activity than LpxL1 LPS, and their incorporation into liposomes significantly reduced their endotoxic activity as determined by measuring the induction of interleukin-6 (IL-6) production in a murine macrophage cell line. To determine the adjuvant effect, BALB/c mice were immunized with OpaJ-containing liposomes and either free LPS or LPS coincorporated into the proteoliposomes. OpaJ-containing liposomes adjuvanted with AlPO4 or not adjuvanted at all were included as control groups. In the appropriate dose, PagL LPS showed a superior adjuvant effect compared with LpxL1 LPS, and for both LPS types, free LPS showed a higher adjuvant effect than when coincorporated into the liposomes, as evidenced by higher titers of IgG2a and IgG2b antibodies against OpaJ+ meningococci and higher bactericidal titers. In conclusion, PagL LPS is a better adjuvant than LpxL1 LPS, but coincorporation of either LPS into proteoliposomes did not improve their adjuvant activity.
Meningococcal disease is a systemic infection caused by Neisseria meningitidis, a common and usually harmless inhabitant of the human oropharynx. Invasive N. meningitidis strains can express one of six different polysaccharide capsules, on the basis of which they are classified as serogroup A, B, C, Y, W135, or X. Vaccines based on capsular polysaccharides confer protection against bacteria of the same serogroup, but, unfortunately, the serogroup B capsular polysaccharide cannot be used due to its poor immunogenicity in humans and the potential risk of autoimmunity (17). Subcapsular antigens, mainly outer membrane proteins (OMPs) (1, 11, 48), have been extensively examined as alternative candidates for the development of a vaccine against this serogroup.
Outer membrane vesicle (OMV)-based vaccines have been shown to be effective in defined geographical regions where specific bacterial clones dominate (29). Their effectiveness is dependent on the maintenance of the native conformation of the antigenic OMPs. A potential drawback is that OMVs contain a wide range of additional components, such as lipopolysaccharides (LPS), and antigenically variable proteins, such as PorA or PorB, which can modify the toxicity of the vaccine preparation and the immune response to the main vaccine antigens (43). An alternative strategy is the use of delivery systems that are able to maintain the native conformation of selected OMPs while at the same time having the advantage of more defined vaccine preparations. Liposome particles consist of phospholipid bilayers, which mimic the bacterial outer membrane conditions, and have been used successfully with incorporated OMPs or LPS structures in mucosal and parenteral immunization routes (4, 23, 31, 45).
The adjuvant potential of LPS has been broadly studied in vaccine development, but its general use is restricted because of unacceptable toxicity. Several strategies have been used to reduce its toxicity in vaccine formulations, such as its incorporation into liposomes or by structural modification of the lipid A moiety, which is the primary mediator of its biological effects. In particular, inactivation of the genes involved in lipid A acyloxyacylation has generated less toxic LPS derivatives that showed adjuvant potential (20, 21, 36). The lipid A moiety determines the activation of the main LPS receptor, Toll-like receptor 4 (TLR4) in complex with MD-2 (30, 42), resulting in induction of the expression of proinflammatory cytokines, such as interleukin-6 (IL-6). The LPS-binding protein (LBP), helped by the membrane-anchored CD14, facilitates LPS transfer to the TLR4 receptor located on the cell surface.
Wild-type meningococcal lipid A contains six fatty acyl chains attached to a diphosphorylated d-glucosamine disaccharide in a symmetrical distribution. LpxL1 LPS misses the 2′ secondary C12 acyl chain as a result of the inactivation of the lpxL1 gene (44). The adjuvant potential of LpxL1 LPS was analyzed in liposomal particles containing the meningococcal OMP PorA (3). The resulting proteoliposomes with incorporated LpxL1 LPS induced higher humoral and cellular anti-PorA responses in mice than when conventional adjuvants or monophosphoryl lipid A was used, demonstrating the adjuvant potential of these formulations. However, those studies did not specifically address the contribution of the incorporation of LpxL1 LPS into the liposomes versus its presence in the vaccine preparation. Another penta-acylated meningococcal LPS derivative was obtained through expression in N. meningitidis of the pagL gene of Bordetella bronchiseptica. PagL expression leads to lipid A deacylation at the 3 position, and the endotoxic activity of the resulting LPS, here referred to as PagL LPS, was reduced compared to that of wild-type LPS (20). The adjuvant activity of the PagL LPS has not been studied yet. The aims of the present study were (i) to compare the endotoxic and the adjuvant activities of meningococcal PagL and LpxL1 LPS and (ii) to investigate the influence of the coincorporation of LPS derivatives and a meningococcal opacity-associated (Opa) protein into liposomes on the resulting adjuvant effect. The investigation is based on the hypothesis that antigen and adjuvant codelivered in the same liposome particle may induce a more effective antigen presentation by cells of the immune system than when the adjuvant is added separately to the formulation (35).
MATERIALS AND METHODS
Bacterial strains and antibodies.
Escherichia coli strain BL21(DE3) containing pET11d-OpaJ129 was used for expression of recombinant OpaJ protein in inclusion bodies as described by de Jonge et al. (9). Phase variants of N. meningitidis strain H44/76 (B:15:P1.7,16) expressing either immunotype L3 or L8 LPS and either expressing OpaJ (OpaJ+) or not (OpaJ−) were selected by colony blotting with appropriate antibodies, which are described below. Derivatives of this strain (immunotype L8) carrying either plasmid pEN11-pagL (20) or an lpxL1::kanR mutation (44) have been described previously. The meningococcal strains were grown at 37°C on GC medium base (Difco) supplemented with IsoVitaleX (Becton Dickinson) in a humid atmosphere containing 5% CO2. Bacterial suspensions were heat inactivated for 30 min at 56°C.
Monoclonal antibodies (MAbs) 15-1.P5.5 (9) and MN5C11G (41), both of the IgG2a isotype, were used for the specific detection of OpaJ and PorA P1.16, respectively, and MAbs 4A8B2 and 43F8.10, both of the IgG3 isotype (unpublished data), were used for the specific detection of the oligosaccharide part of the LPS of immunotypes L3 and L8, respectively.
Purification and quantification of LPS and Opa protein.
Meningococcal PagL LPS and LpxL1 LPS were extracted from derivatives of strain H44/76 immunotype L8 carrying either plasmid pEN11-pagL or an lpxL1::kanR mutation, respectively, using the hot-phenol extraction method (47). Recombinant OpaJ isolated from E. coli inclusion bodies was purified by ion-exchange chromatography using an NaCl gradient to elute the protein and refolded in vitro as described previously (9). The purified protein was concentrated using a filtration procedure (Pall Filtron 1KD, New York, NY). Purified protein was aliquoted and stored at −20°C until use. The purity and identity of the isolated LPS and OpaJ protein were confirmed by electrophoretic and Western blotting techniques.
Concentrations of purified OpaJ and of OpaJ reconstituted into proteoliposomes were determined with the Pierce protein assay kit (Pierce, Rockford, IL). LPS concentrations in purified LPS preparations and in reconstituted (proteo)liposomes were determined by gas chromatographic quantification of fatty acids as described previously (2).
Electrophoretic and immunoblotting techniques.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Tricine-glycine-SDS-PAGE were performed using 12% or 16% (wt/vol) acrylamide gels (Bio-Rad Laboratories, Inc.). The folding of the OpaJ protein was determined by seminative SDS-PAGE (9). After electrophoresis, protein or LPS bands were visualized with Coomassie brilliant blue (Fluka, Buch, Switzerland) or LPS-specific silver staining (19), or they were transferred to nitrocellulose membranes. In dot-blotting assays, proteins were immobilized on the membrane at room temperature during 20 min and incubated with 500 μg of purified PagL LPS or LpxL1 LPS in phosphate-buffered saline, pH 7.4 (PBS), at room temperature for 1 h. The binding of LPS to the proteins on the membranes was detected with appropriately diluted MAb 43F8.10. All immunoblots were developed with goat anti-mouse IgG-alkaline phosphatase conjugate (Southern Biotech) and 5-bromo-4-chloro-3-indolylphosphate-nitroblue tetrazolium (BCIP-NTB) as the substrate.
Preparation of liposome suspensions.
Liposomes were prepared using the detergent dilution method described previously (3). Liposomes were made of dimyristoyl-phosphatidylcholine, dimyristoyl-phosphatidylglycerol, and cholesterol in a molar ratio of 8:2:2. All lipids were obtained from Sigma-Aldrich (St. Louis, MO). Appropriate amounts of each lipid were dissolved in chloroform-methanol (2:1, vol/vol) in a round-bottom flask, and a lipid film was obtained by solvent evaporation in a rotary evaporator under vacuum. The lipid film was solubilized in TBS (50 mM Tris-HCl, 150 mM NaCl, pH 8.4) containing LPS, folded OpaJ, or a mixture of LPS and folded OpaJ. The suspension was properly mixed in a rotary evaporator and incubated for 1 h on ice at 200 rpm. The resulting (proteo)liposomes were pelleted by ultracentrifugation (160,000 × g for 1 h) and filtered through 0.22-μm sterile filters to keep a homogeneous particle size among liposome preparations. An additional ultracentrifugation step was used to remove LPS not incorporated into the liposomes. For all liposome preparations, the liposome size was measured by dynamic light scattering (DLS), and its phospholipid content was analyzed as described by Rouser et al. (34) using sodium phosphate as the standard (Merck, Darmstadt, Germany). After liposome preparation, protein and LPS concentrations were determined as described above. The protein density of the proteoliposomes was expressed as the protein/phosphate (μg/μmol) ratio.
Flow cytometry analysis.
The incorporation and surface exposure of OpaJ and LPS in liposomes were determined by flow cytometry (FCM) using appropriate MAbs. All incubations were carried out for 1 h at 37°C with constant shaking; washes were performed by ultracentrifugation (160,000 × g for 1 h) and subsequent resuspension in TBS. Liposomes labeled with Neuro-Dio cell-labeling solution (Biotium, Inc., Hayward, CA) were washed twice and incubated with MAbs diluted in TBS containing 1% (wt/vol) bovine serum albumin. After washing, a goat anti-mouse IgG3 cyanine 5 conjugate and/or a goat anti-mouse IgG2a R-phycoerythrin conjugate (Southern Biotech, Birmingham, AL) was used to detect antibody binding, and the resulting fluorescence intensities of 104 labeled liposomes were measured in an FCM Calibur flow cytometer (Becton Dickinson, San Jose, CA). Neuro-Dio-labeled and unlabeled empty liposomes were used as negative controls. Unlabeled liposomes stained with each fluorochrome were included to check for overlapping of fluorescence. BD Cell Quest software (BD Biosciences, San Jose, CA) and MDI software (version 2.9; http://www.bio-soft.net/other.html) were used for data acquisition and analysis.
Immunization protocol.
Female BALB/c mice 6 to 8 weeks old and of approximately equal weight were used to analyze the immune response against liposome formulations. Groups of eight mice each were immunized subcutaneously on days 0 and 28 with 5 μg of OpaJ incorporated into liposomes adjuvanted or not with purified penta-acylated L8 LPS as follows: 1 μg of free LpxL1 LPS, 1 μg of LpxL1 LPS coincorporated into the proteoliposomes, 0.1 or 1 μg of free PagL LPS, or 0.1 or 1 μg of PagL LPS coincorporated into the proteoliposomes. Control groups were immunized with OpaJ-containing liposomes adjuvanted or not with 0.5 mg AlPO4. Mice were bled on day 42, and the sera obtained were stored at −20°C.
IL-6 determination.
The endotoxicities of the various preparations were tested by stimulation of the production of the proinflammatory cytokine IL-6 in the murine macrophage cell line J774A.1 (ATCC TIB-67; American Type Culture Collection, Manassas, VA) following the procedure described previously (20) with some modifications. In short, macrophages were seeded in 24-well microtiter plates (3 × 105 cells/well) in 400 μl of Iscove's modified Dulbecco's medium (IMDM) supplemented with 100 units/ml penicillin, 100 μg/ml streptomycin, 300 μg/ml l-glutamine, and 10% (vol/vol) fetal calf serum. All components were obtained from Gibco BRL. The cells were first incubated with serial dilutions of adjuvant preparations to test the working concentrations of each adjuvant. Subsequently, appropriate dilutions of each preparation were used to analyze the effect of adjuvant and antigen combinations. Buffers, empty liposomes, and nonstimulated cells were used as negative controls.
Cells were incubated for 16 to 18 h at 37°C in a humid atmosphere with 5% CO2, and the IL-6 concentrations in the supernatants were determined in an enzyme-linked immunosorbent assay (ELISA) against mouse IL-6 according to the manufacturer's instructions (BD Bioscience P, San Diego, CA).
ELISA.
The total IgG, IgG1, IgG2a, IgG2b, IgG3, and IgM titers were determined in whole-cell ELISAs using OpaJ+ or OpaJ− derivatives of N. meningitidis strain H44/76 of immunotype L3. Total IgG against purified LPS was determined in ELISAs using wild-type L3 LPS and LpxL1 and PagL LPS of immunotype L8. In short, flat-bottom 96-well microtiter plates were coated overnight at 37°C with 100 μl of a whole-cell suspension (A620 of 0.08 in PBS) or for 2 h at 37°C with 100 μl of 10 μg/ml purified LPS. Threefold serial dilutions were performed for each individual serum, and antibody binding was detected with horseradish peroxidase-conjugated goat anti-mouse total IgG, appropriate IgG subclasses, or IgM (Southern Biotech) at a 1:5,000 dilution. Binding of MAbs against PorA P1.16, L3 LPS, and L8 LPS was used as controls. The antibody titers of each serum were calculated as the reciprocal dilution that gave 50% of the maximal optical density at 450 nm.
SBA assay.
The serum bactericidal activity (SBA) of each individual serum was tested as described previously (25). Twofold serial dilutions of heat-inactivated serum (30 min at 56°C) were mixed with baby-rabbit complement (Pel-Freez Biologicals, Rogers, AR) at 20% and 250 CFU of strain H44/76 OpaJ+ immunotype L3 and incubated for 60 min at 37°C. Controls were used to test the complement activity and bacterial sensitivity to sera without complement. Titers were expressed as the final dilution giving at least 90% killing of the added bacteria. SBA titers lower than 10 were considered negative.
Statistical analysis.
The data obtained by DLS for individual liposome preparations were analyzed using the Mann-Whitney test. Before statistical analysis, antibody and bactericidal sera titers were log10 converted. For the ELISAs with purified LPS and for SBA, both the geometric mean and all individual mouse data for each group are shown. Data obtained by whole-cell ELISAs were expressed as mean log10 for eight independent sera. All data were considered for statistical comparison using one-way analysis of variance (ANOVA) and Graph Pad software. Statistically significant differences were marked at a P value of <0.05.
RESULTS
Liposome characterization.
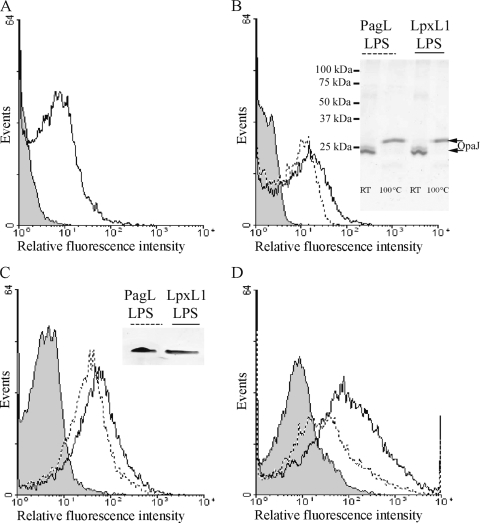
The N. meningitidis opacity proteins are OMPs involved in cellular adhesion and invasion. Methods for the in vitro refolding of recombinant OpaJ protein and its incorporation into liposomes have been described previously (10), making it a valuable model antigen for our purpose, i.e., to evaluate and compare the adjuvant effects of LpxL1 and PagL LPS derivatives in liposomes. Purified recombinant OpaJ protein was incorporated into liposomes, either separately or in combination with the LPS derivatives. To confirm the incorporation of both components into the liposome particles, immunodetection techniques were used with MAbs specific for OpaJ and LPS immunotype L8 oligosaccharide. Liposomes were detected in flow cytometry assays with a commercial lipid stain. Subsequently, OpaJ and PagL and LpxL1 LPS were detected in the liposomal particles with the MAbs, showing the expected antigen and adjuvant coincorporation and surface exposure (Fig. 1A, B, and C). In accordance with the FCM results, both molecules were also detected in liposomal suspensions by electrophoretic techniques (insets in Fig. 1B and C) and ELISAs (data not shown). Seminative SDS-PAGE analysis of these suspensions was used to investigate whether the folded conformation of OpaJ protein was retained in the liposomes; indeed, OpaJ showed heat-modifiable electrophoretic mobility in this assay (Fig. 1B, inset), as expected for the correctly folded protein (9). A decreased reactivity of the LPS-specific MAb was seen in the FCM assays upon coincubation with the OpaJ-specific MAb (Fig. 1D), suggesting a close proximity of OpaJ and LPS in the delivery system, further demonstrating their actual coincorporation into the same particles.
FIG. 1.
Incorporation of OpaJ protein and LPS derivatives into liposomes analyzed by flow cytometry and electrophoretic techniques. (A) Reactivity of anti-OpaJ MAb 15-1-P5.5 against OpaJ-containing liposomes measured by FCM analysis. (B) Reactivity of the anti-OpaJ MAb with liposomes containing LpxL1 LPS and OpaJ (continuous line) and with liposomes containing PagL LPS and OpaJ (dotted line). The inset shows the heat modifiability of OpaJ coincorporated with PagL or LpxL1 LPS into liposomes determined in seminative SDS-PAGE as a measure for its correct folding. Before electrophoresis, the samples were treated for 10 min in sample buffer with 0.1% SDS at room temperature (RT) or with 2% SDS at 100°C. The positions of molecular size markers are indicated at the left side of the membrane. (C) Reactivity of anti-L8 LPS MAb 43F8.10 with liposomes containing LpxL1 LPS and OpaJ (continuous line) and with liposomes containing PagL LPS and OpaJ (dotted line). The inset demonstrates the presence of LPS in the liposomes in an LPS-specific silver-stained Tricine-glycine-SDS-polyacrylamide gel. (D) Reactivity of the anti-L8 LPS MAb against liposomes containing PagL LPS and OpaJ in the presence (dotted line) or absence (continuous line) of the anti-OpaJ MAb. In all graphics, the reaction of antibodies with empty liposomes was included as negative control (gray-filled profile).
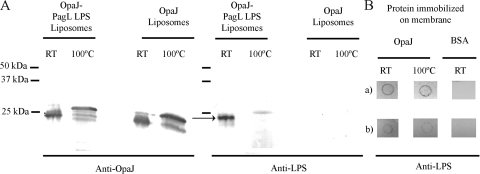
Complexes of OpaJ and LPS were also detected by Western blot analysis of the liposomes containing OpaJ and PagL LPS after seminative SDS-PAGE, which revealed the comigration of LPS with the folded conformation of OpaJ (Fig. 2A). This complex dissociated after heating of the sample at 100°C. Subsequently, dot-blotting assays were used to investigate whether the formation of these complexes required the coentrapping of both molecules into liposomes. To this end, OpaJ was immobilized on a membrane, which was subsequently incubated with PagL or LpxL1 LPS, and binding of LPS to the immobilized protein was detected with the anti-L8 LPS-specific MAb. Both types of LPS bound to membrane-immobilized OpaJ (Fig. 2B), indicating that the formation of these OpaJ-LPS complexes results from the inherent affinity between both components and does not require their coincorporation into liposomes. The results suggested a somewhat higher binding of OpaJ with PagL LPS than with LpxL1 LPS (Fig. 2B).
FIG. 2.
Binding of LpxL1 and PagL LPS to OpaJ protein. (A) Western blot patterns of liposomes containing OpaJ and PagL LPS or only OpaJ incubated with the anti-OpaJ MAb 15-1-P5.5 (left panel) or the anti-L8 LPS MAb 43F8.10 (right panel). Prior to SDS-PAGE, the samples were treated in sample buffer with 0.1% SDS at room temperature (RT) or with 2% SDS at 100°C. A complex of LPS and OpaJ is indicated in the right panel with an arrow. The positions of molecular size markers are indicated at the left side of the membranes. (B) Far-dot blots containing 15 μg of membrane-immobilized OpaJ that was denatured by heating for 30 min (100°C) or not (RT) prior to application on the membrane. The membranes were subsequently incubated with 500 μg of purified PagL LPS (a) or LpxL1 LPS (b) and then with the anti-L8 LPS-specific MAb 43F8.10. Bovine serum albumin (BSA) was used as a control for background binding.
After having established the correct incorporation of both adjuvants and antigen into the liposomes, physical and chemical properties of the liposomes were tested. DLS showed that the liposomes, which were filtered through 0.22-μm filters, ranged in size from 174.6 to 222.3 nm. The mean size was 214.0 ± 20.5 nm and the mean polydispersity index was 0.20 ± 0.03, in accordance with previous data (3). Mann-Whitney test analysis indicated no statistical differences between independent liposome preparations. The protein and phospholipid contents of the OpaJ-containing liposomes were determined, and thereby a protein/phosphate ratio (μg/μmol) of 93.36 ± 7.26 was found.
Endotoxicity activity.
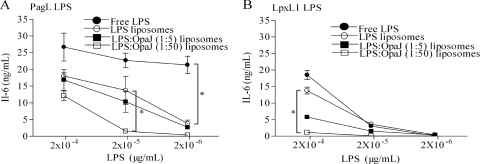
The biological activities of the different formulations were determined by measuring the production of the proinflammatory cytokine IL-6 in murine macrophages. Empty liposomes and OpaJ-containing liposomes with or without added AlPO4 were used as controls, and they were not found to induce detectable amounts of IL-6 under the conditions applied. Among the LPS-containing preparations, free PagL LPS was a stronger inducer of IL-6 production than LpxL1 LPS; however, smaller differences between both LPS types were found after their incorporation into liposomes (Fig. 3, compare panels A and B). Previously, others have observed that the toxicity of LPS decreases after its incorporation into liposomes (8, 15). In our analysis, we observed a similar effect, as both LPS derivatives showed a decreased capacity to stimulate IL-6 production when incorporated into liposomes. This decrease was more pronounced with PagL LPS than with LpxL1 LPS.
FIG. 3.
IL-6 induction in mouse macrophage cell line J774A.1 by PagL LPS (A) and LpxL1 LPS (B). Tested were free LPS, liposomes with incorporated LPS, and liposomes with coincorporated LPS and OpaJ at LPS/protein ratios of 1:5 and 1:50 (μmol/μg). The IL-6 concentration in the supernatant of cell cultures was quantified by ELISA. The data represent the averages and standard errors from three independent experiments. Statistically significant differences between liposome preparations are marked with one asterisk (P < 0.05).
We also analyzed whether the coincorporation of OpaJ with LPS into the liposomes affected toxicity. Interestingly, coincorporation of OpaJ indeed led to a further decrease in LPS activity in a dose-dependent way (Fig. 3). The addition of free OpaJ protein to LPS-containing liposomes or of OpaJ-containing liposomes to free LPS did not modulate the LPS activity (data not shown).
Immune response to liposome preparations.
The influence of the coincorporation of LPS derivatives into OpaJ-containing proteoliposomes on adjuvanticity was evaluated in BALB/c mice. Animals were injected with 1 μg of LPS and 5 μg of protein per dose for both LPS types as previously described (3, 10). However, PagL LPS has not been tested in animal experiments, and its optimal adjuvant dose has not been determined yet. Since PagL LPS showed a higher biological activity than LpxL1 LPS in the IL-6 induction assays (Fig. 3), we also included a 10-fold-lower dose of this LPS (0.1 μg) in the immunization scheme. To determine the immune response against OpaJ protein, we compared the reactivities of the sera against OpaJ+ and OpaJ− cells of N. meningitidis strain H44/76 in immunoassays. Since specific antibodies directed against immunotype L8 LPS were detected in some of the sera (described below), we selected variants of these strains expressing immunotype L3 LPS for these assays. Table 1 summarizes the complete characterization of the immune response in all immune sera. For Western blot analysis, sera from all animals within a group were pooled. All pooled sera showed specific IgG reactivity against OpaJ protein in Western blots of OpaJ+ cells, while no reactivity was found against the OpaJ− cells (Fig. 4A). This was also observed in whole-cell ELISAs (Table 1); however, with this method, considerable levels of IgG antibodies reacting with the OpaJ− cells were detected in the group that was immunized with proteoliposomes without adjuvant. LPS-adjuvanted proteoliposomes elicited generally higher IgG titers against OpaJ protein than nonadjuvanted or AlPO4-adjuvanted proteoliposomes, a clear indication of the adjuvant potential of both LPS types in vivo. However, no differences were observed between the groups that received the LPS incorporated into the proteoliposomes or as free adjuvant.
TABLE 1.
Characterization of the immune response elicited by OpaJ-containing liposomes adjuvanted with LpxL1 or PagL LPS as measured in ELISAs
| Group | LPS dose (μg) | Anti-OpaJ titera |
Nonspecific IgG titerb | Nonspecific IgM titerb | |||||
|---|---|---|---|---|---|---|---|---|---|
| IgG | IgG1 | IgG2a | IgG2b | IgG3 | IgM | ||||
| Not adjuvanted | 3.5 | 3.4 | 0.4 | 0.8 | 0.3 | 2.1 | 2.2 | 1.7 | |
| AlPO4 | 3.6 | 3.6 | 0.8 | 1.9 | 1.0 | 2.5 | 0.6 | 1.5 | |
| LpxL1 coincorporated | 1 | 4.0* | 4.3* | 2.0*§ | 2.7* | 1.8 | 0.6 | 0.6 | 0.6 |
| Free LpxL1 | 1 | 4.1* | 4.2 | 3.0*§ | 3.2* | 1.0 | 1.8 | 0.6 | 1.6 |
| PagL coincorporated | 1 | 3.8 | 3.4 | 2.7* | 2.9* | 1.3 | 0.6 | 0.3 | 0.6 |
| Free PagL | 1 | 3.8 | 4.1 | 3.2* | 3.1* | 1.3 | 2.3 | 0.6 | 1.7 |
| PagL coincorporated | 0.1 | 3.9 | 3.9 | 3.0* | 3.0*§ | 1.3 | 1.0 | 0.6 | 0.3 |
| Free PagL | 0.1 | 4.2* | 4.1 | 3.3* | 3.7*§ | 1.0 | 1.6 | 0.6 | 0.8 |
The titer of each anti-OpaJ Ig was determined by whole-cell ELISA in plates coated with OpaJ+ cells of N. meningitidis strain H44/76 of immunotype L3. Data are expressed as the mean log10 titer. The results for the different groups were compared by ANOVA with a confidence level of 95% when appropriate. *, statistically significant differences compared with the nonadjuvanted group (P < 0.05). §, statistically significant differences between groups adjuvanted with free LPS or LPS coincorporated with OpaJ into the liposomes (P < 0.05).
The titer of each antiserum was determined by whole-cell ELISA in plates coated with OpaJ− cells of N. meningitidis strain H44/76 of immunotype L3. Data are expressed as the mean log10 titer.
FIG. 4.
Specific IgG responses in sera obtained after immunization of mice with OpaJ-containing liposomes using different adjuvants. (A) Western blots containing whole-cell lysates of OpaJ+ cells of N. meningitidis strain H44/76 with immunotype L3 probed with pooled sera of mice immunized with nonadjuvanted liposomes (lane 1) or liposomes adjuvanted with LpxL1 LPS coincorporated into the liposomes (lane 2), free LpxL1 LPS (lane 3), 1 μg of PagL LPS coincorporated into the liposomes (lane 4), 1 μg of free PagL LPS (lane 5), or nonimmune serum (lane 6). Lane 7 shows the reactivity of the sera of the mice immunized with proteoliposomes and 1 μg of free PagL LPS with a whole-cell lysate of Opa− cells. Prior to SDS-PAGE, the bacterial suspensions were heated in sample buffer with 2% SDS at 100°C. The positions of molecular size markers are indicated at the left. (B) Quantitative total IgG titers against purified L8 LPS measured in ELISAs. Sera from mice immunized with LpxL1 LPS-adjuvanted liposomes and from the control groups were tested against purified LpxL1 L8 LPS, and those from mice immunized with PagL LPS-adjuvanted liposomes were tested against purified PagL L8 LPS. The data show the geometric mean (horizontal line), individual serum titers, and number of responder mice/group. Statistically significant differences between serum groups are marked with one asterisk (P < 0.05). Similar results were obtained when the sera tested here against purified LpxL1 L8 LPS were tested against PagL LPS and vice versa (data not shown). No reactivity was detected with L3 LPS. Note that only LPS of immunotype L8 was used for the immunizations.
Determination of the subclass distribution of the anti-OpaJ antibodies measured in ELISAs using the Opa+ immunotype L3 cells showed significantly higher IgG2a and IgG2b levels in the mice that received the LPS-adjuvanted proteoliposomes than in the control groups (Table 1). LPS incorporated into the proteoliposomes resulted in relatively lower IgG2a and IgG2b titers than free LPS for both LPS types, and in some cases these differences were statistically significant (Table 1). In contrast, the LPS coincorporation tended to induce higher IgG3 antibody titers. IgG1 antibodies were induced in all groups and showed a variable distribution. In general, higher IgG1 amounts were found in the sera of the groups that received LPS as an adjuvant, where LpxL1 LPS in particular enhanced the induction of this isotype, independent of whether it was incorporated into the liposomes or not. The sera of the groups that received LPS as an adjuvant showed lower or similar IgM titers than the control sera; however, the IgM antibodies detected were not specifically directed against OpaJ, as evidenced by the comparison of the titers against the OpaJ+ and OpaJ− cells (Table 1).
Finally, ELISAs with purified PagL or LpxL1 immunotype L8 LPS and wild-type immunotype L3 LPS as immobilized antigens were used to determine the induction of LPS-specific antibodies. The incorporation of LPS into the liposomes increased the LPS-specific antibody response in the case of PagL LPS, whereas LpxL1 LPS was hardly immunogenic in any of the groups (Fig. 4B). No reaction against immunotype L3 wild-type LPS in any of the sera was detected (data not shown).
Functional antibody response.
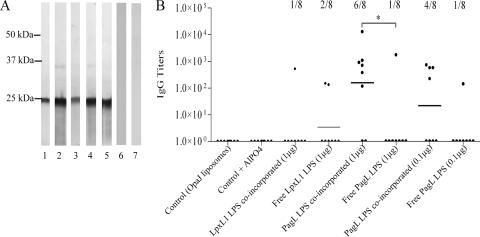
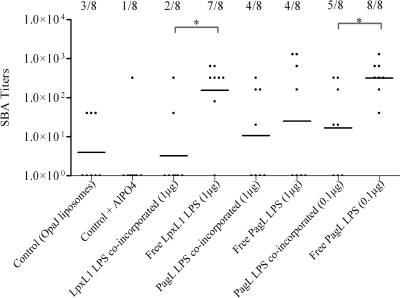
SBA assays were performed to determine the induction of functional antibodies. Both types of LPS increased the OpaJ-specific bactericidal activity of immune sera when administrated as free LPS with OpaJ-containing liposomes (Fig. 5). Control groups and groups that received LPS incorporated into the proteoliposomes showed a statistically significantly lower bactericidal activity than those that received free LPS as adjuvant (P < 0.05). In general, proteoliposomes adjuvanted with PagL LPS elicited more responder mice than those adjuvanted with LpxL1 LPS, reflecting the slightly higher biological activity of PagL LPS in vivo. To summarize, both LPS species showed a clear adjuvant effect, as evidenced by altered IgG2a,b/IgG1 ratios and higher bactericidal titers. However, coincorporation of LPS into liposomes significantly reduced its adjuvant effect.
FIG. 5.
Functional activity of sera obtained after immunization of mice with several OpaJ-containing liposome formulations tested in SBA assays using Opa+ cells of N. meningitidis strain H44/76 of immunotype L3. The data represent the geometric mean (horizontal line), the SBA titers of individual mice, and the number of responder mice/group. Results are given for the sera of mice immunized with OpaJ-containing liposomes alone (control), with OpaJ-containing liposomes adjuvanted with AlPO4 at 0.5 mg/ml (control + AlPO4), or with the different LPS species (with dose/mouse indicated in parentheses) incorporated or not into liposomes. Statistically significant differences between sera groups are marked with one asterisk (P < 0.05). SBA titers were considered positive at serum dilutions higher than 10.
DISCUSSION
LpxL1 LPS has been extensively studied in various delivery systems with meningococcal as well as other antigens (3, 13, 24, 44). Studies with the meningococcal PagL LPS were until now restricted to determine its endotoxic activity in vitro (20), whereas its adjuvant properties and its behavior in delivery systems are unknown.
In the present study, both LPS derivatives showed clear adjuvant capacity in liposomal vaccine formulations, in accordance, for LpxL1 LPS, with previous results obtained in our laboratory (10). PagL LPS displayed a significantly higher endotoxic activity than LpxL1 LPS (ca. 100-fold), which is accompanied by a higher adjuvant activity. Since both LPS types used in this study contain the same L8 oligosaccharide structure, their differential activity must reflect the different lipid A region. Incomplete lipid A variants generally induce a lower cellular response than wild-type LPS (39), and specifically, modifications in the phosphorylation pattern and the length or number of fatty acyl chains affect the endotoxic activity (28, 40). Both meningococcal LPS forms used in this study are penta-acylated, missing either the secondary C12 chain at the 2′ position (LpxL1 LPS) or the primary 3OH-C12 at the 3 position (PagL LPS). Since these LPS species display, nevertheless, differential activities, our results imply that not only the number but also the position of the acyl chains in the lipid A structure contributes to the cellular response to LPS.
The differential activities of the LPS species may be attributed to different interactions of the LPS species with the TLR4/MD-2 complex itself or, alternatively, to different transfer of the LPS to this receptor. Lipid A deacylation alters the physicochemical characteristics of LPS when it is associated with membranes or in aqueous suspension (6, 20), whereas a possible effect on its accessibility to LBP was also proposed (20). During the preparation of the liposomes, PagL LPS was more easily incorporated than LpxL1 LPS (data not shown), suggesting different physicochemical properties, which might be the reason for their differential activity in vitro. These differences were reflected also in the animal response. In the immunizations, 1 μg/dose of free PagL LPS was less effective than 0.1 μg/dose of PagL LPS or 1 μg of LpxL1 LPS, as evidenced by lower bactericidal titers and fewer responding mice. This could be explained by toxic effects associated with this LPS concentration, a strong indication of higher activity of PagL LPS compared with LpxL1 LPS in vivo.
The subclass distribution of anti-OpaJ antibodies elicited by 0.1 μg/dose of free or coincorporated PagL LPS showed a stronger enhancement of the IgG2a and IgG2b subclasses compared with 1 μg/dose of free or coincorporated LpxL1 LPS (Table 1). Induction of IgG2a and IgG2b in mice is related to a Th1 response and efficient complement activation, while induction of only IgG1 antibodies reflects a Th2 type of response (16). The different isotype pattern is reflected in the SBA titers of the sera and the number of responding mice observed within the various groups (Fig. 5). Furthermore, only PagL LPS coincorporation into liposomes induced a significant antibody response to L8 LPS (Fig. 4B). In conclusion, we showed that PagL LPS is a stronger adjuvant in vivo than LpxL1 LPS and is therefore a promising alternative to LpxL1 LPS in vaccine formulations.
The strategy of codelivery of antigen and adjuvant within the same particle in order to enhance antigen presentation and processing by immune cells is pursued by several research groups (4, 8, 13, 24). However, demonstration of the relevant biological activity of the meningococcal LPS derivatives and evidence of their relative efficacy compared to other adjuvants have not been published. In the present study, coincorporation of the two LPS forms with OpaJ protein into liposomes did not improve their adjuvant capacity. This is in line with previous work by Mirlashari et al. (27) and Fisseha et al. (18), who reported a reduction of the endotoxic activity when meningococcal LPS was entrapped in OMVs or bacterial membranes. Similar results were found when Salmonella LPS was used to stimulate the macrophage cell line RAW 264.7 or peritoneal macrophages (14). Taken together, the data suggest that inclusion into liposomes interferes with the availability of LPS for activating its cellular receptors, probably due to a reduced accessibility of the LPS to LBP and CD14, which facilitate transfer to the TLR4/MD-2 receptor complex.
Other studies have shown that the endotoxicity of wild-type LPS can be reduced by its inclusion into liposomes, while its adjuvant potential and, consequently, improvement of the elicited immune response were retained (8, 12, 22, 32, 33). In contrast, the results of the immunization experiments in our study were in agreement with the in vitro data, as inclusion of LPS into liposomes reduced both its endotoxicity and its adjuvanticity. This discrepancy with previous work can be explained in two ways: (i) because LpxL1 LPS and PagL LPS already have reduced endotoxicity, their inclusion into liposomes will further reduce their activity, while the more active wild-type LPS retains a higher activity after its inclusion into liposomes, sufficient for its adjuvant capacities, and (ii) the possible adjuvant potential of free LPS was underestimated in the previous work, since only the immune response elicited to LPS was evaluated (8, 32). We found that liposome-entrapped PagL LPS elicited a significantly higher immune response against LPS than did free LPS (Fig. 4B). However, this result is not indicative of an optimal adjuvant effect, since the bactericidal antibody response elicited to OpaJ protein was significantly higher when free LPS was used (Fig. 5). Therefore, we conclude that the coincorporation of LPS derivatives with OMPs in liposomes does not improve their adjuvant capacity in vaccine formulations.
In contrast to our results with LPS, it has been demonstrated that the coincorporation of synthetic oligodeoxynucleotides expressing CpG motifs with protein antigens into liposomes enhances their adjuvant potential (4). A possible explanation for this discrepancy is in the different localization of the cellular receptors of these adjuvants. Bacterial DNA containing CpG motifs is recognized by TLR9, which is intracellularly expressed in antigen-presenting cells (26, 42, 46). Liposomes are internalized by endocytosis in antigen-presenting cells (14), and TLR ligands can be processed and subsequently recognized by TLR-expressing endosomes; this does not apply to the ligands of the cell surface-located TLR4 receptor. Thus, it seems that the efficacy of the coincorporation of adjuvant and antigen in liposomes is determined by the nature of the adjuvant used.
In this study, we also showed that associations of antigen and LPS in liposomes can influence the resulting LPS activity. Both LPS types studied bound to OpaJ (Fig. 2B) and OpaB (data not shown) and showed a reduced biological activity after coincorporation of increasing amounts of OpaJ with LPS into the liposomes (Fig. 3). Association of LPS to OMPs in the bacterial outer membrane was previously evidenced by a differential accessibility of MAbs to certain OMPs when different types of LPS were expressed (1, 5). This association is probably maintained by hydrophobic and charge interactions between LPS and specific protein domains (7). Since meningococcal LPS-deficient strains do not show drastic alterations in overall OMP composition (37, 38), LPS appears not to be essential for the biogenesis of OMPs, but the presence of these OMP/LPS complexes suggests a role in promoting the proper folding of OMPs. In liposomes, this association between LPS and OMPs such as OpaJ protein might reduce the availability of LPS to LBP and, thereby, the subsequent activation of its receptor (Fig. 3). Since the purpose of this work was the assessment of adjuvanticity, future studies should address the nature of the Opa-LPS associations shown here. Nevertheless, antigen-adjuvant associations in delivery systems and their biological implications in vaccine development should also be investigated for other adjuvants.
In summary, this study demonstrates that PagL LPS displays a more potent biological activity than LpxL1 LPS and that this LPS can be used in liposome formulations to induce high bactericidal titers against liposome-incorporated OpaJ protein. In addition, we found that while the inclusion of LpxL1 LPS and PagL LPS into liposomes reduced their inherent endotoxicity, it did not improve but rather reduced their adjuvant activity. Interestingly, the inclusion of PagL LPS into liposomes also elicited an LPS-directed immune response, which may also be relevant in meningococcal vaccine formulations.
Acknowledgments
This work was partially supported by a personal grant from the Dirección Xeral de Investigación e Desenvolvemento e Innovación from the Xunta de Galicia, Spain (IN809A 2006/164-0).
We thank Carlos Ferreirós and Claire Boog for supporting the grant application and Gideon F. Kersten for his help and advice in the preparation of liposomes.
Footnotes
Published ahead of print on 27 January 2010.
REFERENCES
- 1.Arenas, J., A. Abel, S. Sánchez, J. Marzoa, S. Berrón, P. van der Ley, M. T. Criado, and C. M. Ferreiros. 2008. A cross-reactive neisserial antigen encoded by the NMB0035 locus shows high sequence conservation but variable surface accessibility. J. Med. Microbiol. 57:80-87. [DOI] [PubMed] [Google Scholar]
- 2.Arigita, C., W. Jiskoot, J. Westdijk, C. van Ingen, W. E. Hennink, D. J. A. Crommelin, and G. F. A. Kersten. 2004. Stability of mono- and trivalent meningococcal outer membrane vesicle vaccines. Vaccine 22:629-642. [DOI] [PubMed] [Google Scholar]
- 3.Arigita, C., T. Luijkx, W. Jiskoot, M. Poelen, W. E. Hennink, D. J. A. Crommelin, P. van der Ley, C. van Els, and G. F. A. Kersten. 2005. Well-defined and potent liposomal meningococcal B vaccines adjuvated with LPS derivatives. Vaccine 23:5091-5098. [DOI] [PubMed] [Google Scholar]
- 4.Badiee, A., M. R. Jaafari, A. Samiei, D. Soroush, and A. Khamesipour. 2008. Co-encapsulation of CpG ODN with rLmSTI1 in liposome enhances immune response and protection in immunized BALB/c mice against leishmaniasis. Clin. Vaccine Immunol. 15:668-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bowden, R. A., A. Cloeckaert, M. S. Zygmunt, S. Bernard, and G. Dubray. 1995. Surface exposure of outer membrane protein and lipopolysaccharide epitopes in Brucella species studied by enzyme-linked immunosorbent assay and flow cytometry. Infect. Immun. 63:3945-3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brandenburg, K., B. Lindner, A. Schromm, M. H. J. Koch, J. Bauer, A. Merkli, C. Zbaeren, J. G. Davies, and U. Seydel. 2000. Physicochemical characteristics of triacyl lipid A partial structure OM-174 in relation to biological activity. Eur. J. Biochem. 267:3370-3377. [DOI] [PubMed] [Google Scholar]
- 7.Braun, V., M. Braun, and H. Killmann. 2000. Iron transport in Escherichia coli. Crystal structure of FhuA, an outer membrane iron and antibiotic transporter. Adv. Exp. Med. Biol. 485:33-43. [DOI] [PubMed] [Google Scholar]
- 8.Chhibber, S., S. Wadhwa, and V. Yadav. 2004. Protective role of liposome incorporated lipopolysaccharide antigen of Klebsiella pneumoniae in a rat model of lobar pneumonia. Jpn. J. Infect. Dis. 57:150-155. [PubMed] [Google Scholar]
- 9.de Jonge, M. I., M. P. Bos, H. J. Hamstra, W. Jiskoot, P. van Ulsen, J. Tommassen, L. van Alphen, and P. van der Ley. 2002. Conformational analysis of opacity proteins from Neisseria meningitidis. Eur. J. Biochem. 269:5215-5223. [DOI] [PubMed] [Google Scholar]
- 10.de Jonge, M. I., H. J. Hamstra, W. Jiskoot, P. Roholl, N. A. Williams, J. Dankert, L. van Alphen, and P. van der Ley. 2004. Intranasal immunisation of mice with liposomes containing recombinant meningococcal OpaB and OpaJ proteins. Vaccine 22:4021-4028. [DOI] [PubMed] [Google Scholar]
- 11.Delgado, M., D. Yero, O. Niebla, S. Gonzalez, Y. Climent, Y. Perez, K. Cobas, E. Caballero, D. Garcia, and R. Pajon. 2007. Lipoprotein NMB0928 from Neisseria meningitidis serogroup B as a novel vaccine candidate. Vaccine 25:8420-8431. [DOI] [PubMed] [Google Scholar]
- 12.Desiderio, J. V., and S. G. Campbell. 1985. Immunization against experimental murine salmonellosis with liposome-associated O-antigen. Infect. Immun. 48:658-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Vries, J. J. C., L. Bungener, W. ter Veer, L. van Alphen, P. van der Ley, J. Wilschut, and A. Huckriede. 2009. Incorporation of LpxL1, a detoxified lipopolysaccharide adjuvant, in influenza H5N1 virosomes increases vaccine immunogenicity. Vaccine 27:947-955. [DOI] [PubMed] [Google Scholar]
- 14.Dijkstra, J., J. W. Larrick, J. L. Ryan, and F. C. Szoka. 1988. Incorporation of LPS in liposomes diminishes its ability to induce tumoricidal activity and tumor necrosis factor secretion in murine macrophages. J. Leukoc. Biol. 43:436-444. [DOI] [PubMed] [Google Scholar]
- 15.Dijkstra, J., J. W. Mellors, J. L. Ryan, and F. C. Szoka. 1987. Modulation of the biological activity of bacterial endotoxin by incorporation into liposomes. J. Immunol. 138:2663-2670. [PubMed] [Google Scholar]
- 16.Ey, P. L., G. J. Russell-Jones, and C. R. Jenkin. 1980. Isotypes of mouse IgG-1. Evidence for ‘non-complement-fixing’ IgG1 antibodies and characterization of their capacity to interfere with IgG2 sensitization of target red blood cells for lysis by complement. Mol. Immunol. 17:699-710. [DOI] [PubMed] [Google Scholar]
- 17.Finne, J., M. Leinonen, and P. H. Mäkelä. 1983. Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet ii:355-357. [DOI] [PubMed] [Google Scholar]
- 18.Fisseha, M., P. Chen, B. Brandt, T. Kijek, E. Moran, and W. Zollinger. 2005. Characterization of native outer membrane vesicles from lpxL mutant strains of Neisseria meningitidis for use in parenteral vaccination. Infect. Immun. 73:4070-4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fomsgaard, A., M. A. Freudenberg, and C. Galanos. 1990. Modification of the silver staining technique to detect lipopolysaccharide in polyacrylamide gels. J. Clin. Microbiol. 28:2627-2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geurtsen, J., L. Steeghs, H. J. Hamstra, J. Ten Hove, A. de Haan, B. Kuipers, J. Tommassen, and P. van der Ley. 2006. Expression of the lipopolysaccharide-modifying enzymes PagP and PagL modulates the endotoxic activity of Bordetella pertussis. Infect. Immun. 74:5574-5585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geurtsen, J., R. J. Vandebriel, E. R. Gremmer, B. Kuipers, J. Tommassen, and P. van der Ley. 2007. Consequences of the expression of lipopolysaccharide-modifying enzymes for the efficacy and reactogenicity of whole-cell pertussis vaccines. Microbes Infect. 9:1096-1103. [DOI] [PubMed] [Google Scholar]
- 22.Guzman, C. A., G. Molinari, M. W. Fountain, M. Rohde, K. N. Timmis, and M. J. Walker. 1993. Antibody responses in the serum and respiratory tract of mice following oral vaccination with liposomes coated with filamentous hemagglutinin and pertussis toxoid. Infect. Immun. 61:573-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Humphries, H. E., J. N. Williams, R. Blackstone, K. A. Jolley, H. M. Yuen, M. Christodoulides, and J. E. Heckels. 2006. Multivalent liposome-based vaccines containing different serosubtypes of PorA protein induce cross-protective bactericidal immune responses against Neisseria meningitidis. Vaccine 24:36-44. [DOI] [PubMed] [Google Scholar]
- 24.Jain, V., R. Sahu, S. Misra-Bhattacharya, S. P. Vyas, and D. Kohli. 2008. Enhancement of T-helper type I immune responses against hepatitis B surface antigen by LPS derivatives adjuvanted liposomes delivery system. J. Drug Target 16:706-715. [DOI] [PubMed] [Google Scholar]
- 25.Luijkx, T. A., H. H. van Dijken, H. J. Hamstra, B. Kuipers, P. van der Ley, L. van Alphen, and G. van den Dobbelsteen. 2003. Relative immunogenicity of PorA subtypes in a multivalent Neisseria meningitidis vaccine is not dependent on presentation form. Infect. Immun. 71:6367-6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCluskie, M. J., R. D. Weeratna, and H. L. Davis. 2001. The potential of oligodeoxynucleotides as mucosal and parenteral adjuvants. Vaccine 19:2657-2660. [DOI] [PubMed] [Google Scholar]
- 27.Mirlashari, M. R., and T. Lyberg. 2003. Expression and involvement of Toll-like receptors (TLR)2, TLR4, and CD14 in monocyte TNF-alpha production induced by lipopolysaccharides from Neisseria meningitidis. Med. Sci. Monit. 9:BR316-BR324. [PubMed] [Google Scholar]
- 28.Nikaido, H. 2003. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 67:593-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oster, P., D. Lennon, J. O'Hallahan, K. Mulholland, S. Reid, and D. Martin. 2005. MeNZB: a safe and highly immunogenic tailor-made vaccine against the New Zealand Neisseria meningitidis serogroup B disease epidemic strain. Vaccine 23:2191-2196. [DOI] [PubMed] [Google Scholar]
- 30.Palsson-McDermott, E. M., and L. A. O'Neill. 2004. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology 113:153-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel, G. B., H. Zhou, A. Ponce, and W. Chen. 2007. Mucosal and systemic immune responses by intranasal immunization using archaeal lipid-adjuvanted vaccines. Vaccine 25:8622-8636. [DOI] [PubMed] [Google Scholar]
- 32.Petrov, A. B., B. F. Semenov, Y. P. Vartanyan, M. M. Zakirov, V. P. Torchilin, V. S. Trubetskoy, N. V. Koshkina, V. L. L'Vov, I. K. Verner, I. V. Lopyrev, and B. A. Dmitriev. 1992. Toxicity and immunogenicity of Neisseria meningitidis lipopolysaccharide incorporated into liposomes. Infect. Immun. 60:3897-3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richards, R. L., M. Rao, N. M. Wassef, G. M. Glenn, S. W. Rothwell, and C. R. Alving. 1998. Liposomes containing lipid A serve as an adjuvant for induction of antibody and cytotoxic T-cell responses against RTS,S malaria antigen. Infect. Immun. 66:2859-2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rouser, G., S. Fkeischer, and A. Yamamoto. 1970. Two dimensional thin layer chromatographic separation of polar lipids and determination of phospholipids by phosphorous analysis of spots. Lipids 5:494-496. [DOI] [PubMed] [Google Scholar]
- 35.Singh, M., A. Chakrapani, and D. O'Hagan. 2007. Nanoparticles and microparticles as vaccine-delivery systems. Expert Rev. Vaccines 6:797-808. [DOI] [PubMed] [Google Scholar]
- 36.Steeghs, L., M. Berns, J. Ten Hove, A. de Jong, P. Roholl, L. van Alphen, J. Tommassen, and P. van der Ley. 2002. Expression of foreign LpxA acyltransferases in Neisseria meningitidis results in modified lipid A with reduced toxicity and retained adjuvant activity. Cell. Microbiol. 4:599-611. [DOI] [PubMed] [Google Scholar]
- 37.Steeghs, L., H. de Cock, E. Evers, B. Zomer, J. Tommassen, and P. van der Ley. 2001. Outer membrane composition of a lipopolysaccharide-deficient Neisseria meningitidis mutant. EMBO J. 20:6937-6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steeghs, L., R. den Hartog, A. den Boer, B. Zomer, P. Roholl, and P. van der Ley. 1998. Meningitis bacterium is viable without endotoxin. Nature 392:449-450. [DOI] [PubMed] [Google Scholar]
- 39.Steeghs, L., B. Kuipers, H. J. Hamstra, G. Kersten, L. van Alphen, and P. van der Ley. 1999. Immunogenicity of outer membrane proteins in a lipopolysaccharide-deficient mutant of Neisseria meningitidis: influence of adjuvants on the immune response. Infect. Immun. 67:4988-4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takada, H., and S. Kotani. 1989. Structural requirements of lipid A for endotoxicity and other biological activities. Crit. Rev. Microbiol. 16:477-523. [DOI] [PubMed] [Google Scholar]
- 41.Toropainen, M., L. Saarinen, P. van der Ley, B. Kuipers, and H. Kayhty. 2001. Murine monoclonal antibodies to PorA of Neisseria meningitidis show reduced protective activity in vivo against B:15:P1.7,16 subtype variants in an infant rat infection model. Microb. Pathog. 30:139-148. [DOI] [PubMed] [Google Scholar]
- 42.Trinchieri, G., and A. Sher. 2007. Cooperation of Toll-like receptor signals in innate immune defence. Nat. Rev. Immunol. 7:179-190. [DOI] [PubMed] [Google Scholar]
- 43.van der Ley, P., and L. Steeghs. 2003. Lessons from an LPS-deficient Neisseria meningitidis mutant. J. Endotoxin Res. 9:124-128. [DOI] [PubMed] [Google Scholar]
- 44.van der Ley, P., L. Steeghs, H. J. Hamstra, J. Ten Hove, B. Zomer, and L. van Alphen. 2001. Modification of lipid A biosynthesis in Neisseria meningitidis lpxL mutants: influence on lipopolysaccharide structure, toxicity, and adjuvant activity. Infect. Immun. 69:5981-5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Venkatesan, N., and S. P. Vyas. 2000. Polysaccharide coated liposomes for oral immunization—development and characterization. Int. J. Pharm. 203:169-177. [DOI] [PubMed] [Google Scholar]
- 46.Weeratna, R. D., C. L. Brazolot Millan, M. J. McCluskie, and H. L. Davis. 2001. CpG ODN can re-direct the Th bias of established Th2 immune responses in adult and young mice. FEMS Immunol. Med. Microbiol. 32:65-71. [DOI] [PubMed] [Google Scholar]
- 47.Westphal, O., and K. Jann. 1965. Bacterial lipopolysaccharide extraction with phenol-water and further applications of the procedure. Methods Carbohydr. Chem. 5:83-91. [Google Scholar]
- 48.Zhu, D., V. Barniak, Y. Zhang, B. Green, and G. Zlotnick. 2006. Intranasal immunization of mice with recombinant lipidated P2086 protein reduces nasal colonization of group B Neisseria meningitidis. Vaccine 24:5420-5425. [DOI] [PubMed] [Google Scholar]