Abstract
Arguably, one of the most common and consequential laboratory tests performed in the world is Mycobacterium tuberculosis susceptibility testing. M. tuberculosis resistance is defined by growth of ≥1% of a bacillary inoculum on the critical concentration of an antibiotic. The critical concentration was chosen based on inhibition of ≥95% of wild-type isolates. The critical concentration of isoniazid is either 0.2 or 1.0 mg/liter, that of rifampin is 1.0 mg/liter, that of pyrazinamide is 100 mg/liter, that of ethambutol is 5.0 mg/liter, and that of fluoroquinolones is 1.0 mg/liter. However, the relevance of these concentrations to microbiologic and clinical outcomes is unclear. Critical concentrations were identified using the ability to achieve the antibiotic area under the concentration-time curve/MIC ratio associated with ≥90% of maximal kill (EC90) of M. tuberculosis in ≥90% of patients. Population pharmacokinetic parameters and their variability encountered in tuberculosis patients were utilized in Monte Carlo simulations to determine the probability that particular daily doses of the drugs would achieve or exceed the EC90 in the epithelial lining fluid of 10,000 tuberculosis patients. Failure to achieve EC90 in ≥90% of patients at a particular MIC was defined as drug resistance. The critical concentrations of moxifloxacin and ethambutol remained unchanged, but a critical concentration of 50 mg/liter was identified for pyrazinamide, 0.0312 mg/liter and 0.125 mg/liter were defined for low- and high-level isoniazid resistance, respectively, and 0.0625 mg/liter was defined for rifampin. Thus, current critical concentrations of first-line antituberculosis drugs are overoptimistic and should be set lower. With the proposed breakpoints, the rates of multidrug-resistant tuberculosis could become 4-fold higher than currently assumed.
Tuberculosis (TB) is an epic disease of mankind. When Mycobacterium tuberculosis is isolated from patients, susceptibility testing is recommended, and treatment should be altered if there is drug resistance. The results are also utilized to monitor the emergence of drug-resistant strains (4, 11). The most widely used susceptibility tests classify isolates as either drug resistant or drug susceptible, based on their ability to grow in the presence of a “critical concentration” of the test drug. Based on a 1963 World Health Organization document, the critical concentration is defined as the lowest concentration of drug that inhibits ≥95% of wild-type strains of bacilli that have not been exposed to the drug but does not inhibit resistant strains isolated from patients not responding to therapy with the drug (11, 14). Drug resistance is said to be present when ≥1% of the M. tuberculosis population grows in the presence of the critical concentration (11, 14). Since 1963, the isoniazid critical concentrations have been set at 0.2 and 1.0 mg/liter, with that of rifampin at 1.0 mg/liter, that of pyrazinamide at 100 mg/liter, and those of ethambutol at 5 mg/liter and 7.5 mg/liter in Middlebrook medium (11, 14). The accuracy of these tests in predicting clinical failure is unclear. Moreover, population pharmacokinetics and antimicrobial pharmacokinetics-pharmacodynamics (PK/PD) have since been discovered and are now used to identify susceptibility breakpoints of many antibiotics (21, 23, 30, 58, 59, 62).
In all human beings, sexual reproduction imposes genetic diversity; genetic diversity leads to polymorphisms of xenobiotic metabolism enzymes and, ultimately, to pharmacokinetic variability. In addition, measures of physique, such as weight, also lead to variability. This means that concentrations achieved by a particular drug dose will vary from patient to patient. For an antibiotic dose to effectively kill M. tuberculosis, it must, by definition, achieve a certain threshold exposure above the MIC at the site of infection. Given that TB is a global pandemic and afflicts patients from a wide genetic and anthropometric base, there is expected to be wide pharmacokinetic variability and, therefore, variable rates of achievement of concentrations above the MIC. Ideally, dose-ranging studies that measure concentrations achieved in patients versus the MIC of patient bacillary isolates would need to be performed to establish the critical concentrations for large populations of patients. Isolates with MICs that cannot be achieved or exceeded in most patients would then be deemed resistant to the drug. However, such formal dose-ranging studies are unlikely to be performed for each individual anti-TB drug as monotherapy. Antimicrobial PK/PD-derived exposure targets and their integration with population pharmacokinetics in Monte Carlo simulations offer a rational approach to this problem (3, 23, 30, 62). In the current study, these methods were used to establish new critical concentrations of isoniazid, rifampin, pyrazinamide, ethambutol, and moxifloxacin.
MATERIALS AND METHODS
Outline.
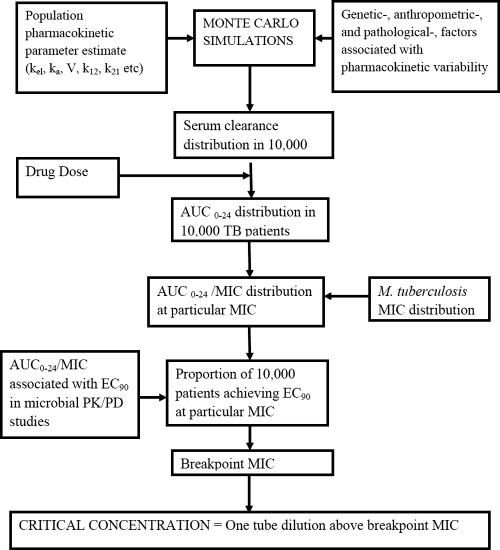
The steps taken to identify the critical concentrations of anti-TB drugs are shown in Fig. 1. Each of the steps is explained in the following paragraphs.
FIG. 1.
Outline of steps to identify critical concentrations.
Drug choice.
Rifampin, isoniazid, pyrazinamide, and ethambutol were chosen because they constitute the first-line anti-TB drugs used in most of the world. Moxifloxacin, which is undergoing phase III clinical studies (10, 15, 29, 63) and will likely be included in the list of first-line agents, was also examined.
Definitions.
The definitions used were a modification of those proposed by the Clinical and Laboratory Standards Institute (CLSI) (14). Susceptible isolates are those inhibited by those critical concentrations that can be achieved at the site of infection by standard doses of the drug. Intermediate susceptibility is encountered when isolates can be inhibited by concentrations achieved by a drug dose higher than the standard dose, if such a dose can be tolerated. Resistant isolates are not inhibited by concentrations achieved by any tolerable drug doses.
For bacterial pneumonias, as is the case for TB, effective drug exposures at the site of infection are best reflected by concentrations in epithelial lining fluid (ELF) (21, 22, 31, 35). The drug exposure pattern that best explains microbial kill of M. tuberculosis by rifampin, isoniazid, moxifloxacin, ethambutol, and pyrazinamide is the ratio of the area under the concentration-time curve from 0 to 24 h to the MIC (AUC0-24/MIC) (32-35, 38, 39, 56, 61). Thus, the magnitude of the AUC0-24/MIC ratio in ELF will best predict microbial kill, based on the inhibitory sigmoid Emax model. In this model, optimal kill is defined as ≥90% of maximal kill (EC90). This ability to achieve EC90 has been utilized to identify susceptibility breakpoints of antimicrobial agents used to treat other bacteria (8). Given that the MIC is the denominator in the AUC0-24/MIC index, as the MIC increases, the exposure ratio will decrease. An MIC is eventually reached where exposure falls below the EC90 in 10% or more of patients, and at this MIC the isolate is defined as resistant to the drug (8, 62).
Pharmacokinetic-pharmacodynamic exposure targets.
All antimicrobial PK/PD studies that examined M. tuberculosis and first-line drugs were examined. All studies that utilized models that had a pharmacokinetic system and measured the drug concentrations achieved were chosen if they had a true dose-effect experiment or had clearly identified the PK/PD exposure associated with optimal kill. Eight studies, three performed in mice and five in the hollow-fiber system, fulfilled these criteria (32-35, 38, 39, 56, 61). For isoniazid, the EC90 was an AUC0-24/MIC ratio of 567 in one study and 707 in another (34, 39). The lower exposure was utilized in the current study. The EC90 was an AUC0-24/MIC ratio of 1,360 for rifampin (38), 209 for pyrazinamide at pH 5.8 (35), 56 for moxifloxacin (33), and 119 for ethambutol (61).
Monte Carlo simulations.
Population pharmacokinetic studies that employed compartmental model analysis were examined. The pharmacokinetic parameter estimates and the ELF-to-plasma ratios from the studies are shown in Table 1 (16-19, 31, 40, 41, 52, 53, 60, 64, 65, 68). These parameter estimates and their variances were incorporated into subroutine PRIOR of ADAPT 5 (25). ELF drug protein binding was assumed to be negligible (41). For a specified drug dose, the AUC0-24/MIC ratio will be affected most by variability in systemic clearance (SCL) and in the MIC, since AUC is directly proportional to dose/SCL. Given this situation, Monte Carlo simulations were performed to identify the AUC0-24 expected to be achieved in each of 10,000 virtual TB patients after administration of a specific dose. Both normal and log-normal distributions were examined, and the final distribution was chosen based on the best recapitulation of the original population pharmacokinetic data. At each MIC, the proportion of patients who achieved or exceeded the EC90 AUC0-24/MIC ratio was determined. Failure to achieve the EC90 at the particular MIC was defined as drug resistance. The highest MIC that allowed achievement of EC90 in ≥90% of patients was defined as the breakpoint MIC. The critical concentration, which would be used in Middlebrook medium to indicate drug resistance, was then set at 1 tube dilution higher than the breakpoint MIC, assuming twofold dilutions of drug concentrations.
TABLE 1.
Pharmacokinetic parameter estimates used as prior data in Monte Carlo simulationsa
| Drug and grouping | Ka (h−1) | SCL (liters h−1) | V (liters) | ELF/plasma ratio |
|---|---|---|---|---|
| Pyrazinamide | 3.56 ± 1.84 | 3.42 ± 0.79 | 29.20 ± 7.69 | 17.8 |
| Isoniazid | ||||
| Fast acetylators | 4.2 ± 5.64 | 49.96 ± 12.48 | 1.10 ± 0.31 | 2.0 |
| Slow acetylators | 6.6 ± 7.49 | 14.96 ± 3.29 | 0.89 ± 1.1 | 2.0 |
| Rifampin | ||||
| Preautoinduction | 2.57 ± 1.75 | 8.59 ± 2.36 | 41.53 ± 8.02 | 0.3 |
| Postautoinduction | 1.15 ± 0.05 | 19.2 ± 0.41 | 53.2 ± 1.07 | 0.3 |
| Ethambutol | 0.84 ± 0.51 | 2.17 ± 1.29 | 9.14 ± 10.47 | 1.0 |
| Moxifloxacin | 5.95 | 14.3 ± 0.78 | 62.7 ± 5.45 | 1.0 |
For pyrazinamide, SCL variability is also driven by weight (64). Two in three adults in the United States are either obese or overweight, and their weight distributions have been published. In one set of Monte Carlo simulations, the weight distribution of people in the United States was used (45, 50), such that SCL was increased by 0.545 liter h−1 for every 10-kg increase above 50 kg, based on the literature (64). In another simulation, the population of TB patients encountered in South Africa, who had a typical weight of 48 kg, was examined (64). Pyrazinamide doses of 2, 3, 4, and 5 g a day were examined. Doses higher than 2 g per day are not currently recommended because their toxicity is unknown. However, they may be associated with higher efficacy (35).
The relationship between N-acetyltransferase 2 (NAT-2) and isoniazid acetylator status is well known. Indeed, 88% of all between-patient SCL variability is due to the number of high-activity NAT-2*4 alleles in patients (42). Two sets of simulations were performed, the first with 100% fast-acetylator patients and the second in 100% slow-acetylator patients. The doses of 300, 600, and 900 mg recommended for the intensive and continuation phases of therapy were examined (6). For rifampin, SCL is most dramatically affected by autoinduction, and therefore two sets of simulations were performed for 600-, 900-, and 1,200-mg daily doses (6, 26, 31), with one using preautoinduction parameters and the second using postautoinduction parameters. For moxifloxacin and ethambutol, no consistent covariate associated with SCL has been identified. In the simulations, daily moxifloxacin doses of 400, 600, and 800 mg a day were examined (33). For ethambutol, the maximum recommended dose of ethambutol of 1,600 mg per day was examined in the simulations.
MIC range.
Rifampin, isoniazid, and ethambutol MIC distributions were obtained from graphs published by Yamane et al., who examined 1,217 clinical M. tuberculosis isolates by using a Middlebrook broth microdilution method that had 98 to 99% concordance with CLSI methods (66). The MIC distribution of moxifloxacin was based on a study by Rodriquez et al., who determined MICs for 243 clinical isolates of M. tuberculosis (54). For pyrazinamide, the MIC distribution was based on studies by Salfinger and Heifets (37, 55). Since pyrazinamide PK/PD studies were carried out in Middlebrook broth at pH 5.8 in our laboratory (35) and since the commercial Bactec Migit 960 PZA kit examines susceptibility in Middlebrook broth at pH 6.0 (by our measurement), the translation of MICs from 5.8 to 6.0 was made based on the Henderson-Hasselbalch relationship (67).
RESULTS
Moxifloxacin.
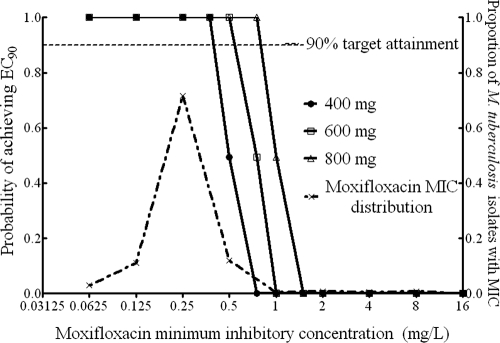
When 400 mg of moxifloxacin per day was administered, the breakpoint MIC was 0.5 mg/liter (Fig. 2). The critical concentration should thus be set at 1.0 mg/liter.
FIG. 2.
Target exposure attainment by moxifloxacin.
Pyrazinamide.
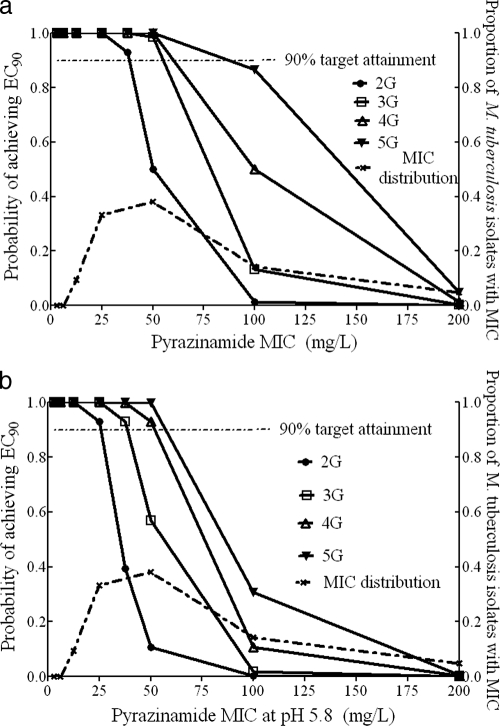
The target attainment for pyrazinamide, assuming a typical weight of 48 kg in all patients, is shown in Fig. 3a. Based on this, the breakpoint MIC in patients receiving the standard 2 g a day was 37.5 mg/liter at pH 5.8 or 50 mg/liter at pH 6.0. Similarly, the breakpoint MIC for 3 g and 4 g was 50 mg/liter at pH 6.0, while that of the highest dose of 5 g was 100 mg/liter. This is the most optimistic scenario. When the SCL sampling distribution was adjusted to take patient weight into consideration, the breakpoint MIC (pH 6.0) fell to 25 mg/liter for a daily dose of 2 g and to 50 mg/liter for the rest of the doses (Fig. 3b). The critical concentration should thus be set at 50 mg/liter at pH 6.0 for the standard dose and at 100 mg/liter if higher doses will be used in the future.
FIG. 3.
Target exposure attainment by pyrazinamide. (a) Patients with a typical weight of 48 kg. (b) Patient population with 66% of patients being overweight.
Isoniazid.
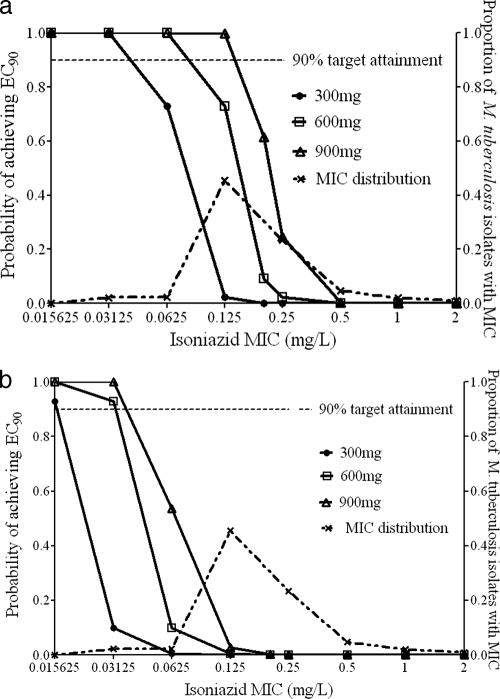
For slow acetylators, the breakpoint MIC in patients receiving 300 mg a day was 0.0312 mg/liter, but it was 0.125 mg/liter for 900 mg a day, as shown in Fig. 4a. However, a population of 100% slow acetylators represents the best-case scenario in terms of high enough isoniazid exposures. As can be seen in Fig. 4b, the breakpoint MICs fell to 0.0156 mg/liter for 300 mg a day and to 0.0312 mg/liter for the higher doses when 100% fast acetylators were examined. Summation of target attainment probabilities indicated that for patient populations in which ≥10% of TB patients are fast acetylators, the breakpoints revert to those for 100% fast acetylators in patients treated with 300 to 600 mg a day. However, if 900 mg a day is administered, isolates with an MIC of 0.0625 mg/liter can be killed effectively in a population with up to 20% fast acetylators. Based on these data, the critical concentration would be 0.0312 mg/liter for low-level resistance and 0.125 mg/liter for high-level resistance.
FIG. 4.
Target exposure attainment by isoniazid. (a) One hundred percent slow-acetylator population. (b) One hundred percent fast-acetylator population.
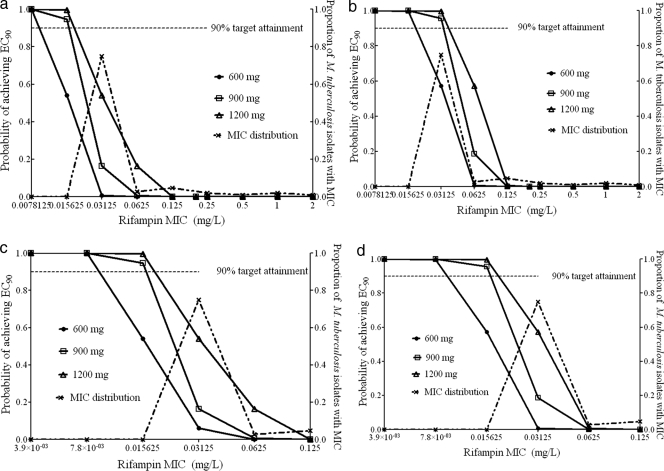
Rifampin.
For rifampin, the breakpoint MIC was only 0.0078 mg/liter for the standard dose and 0.0156 mg/liter for doses of 900 to 1,200 mg, using SCL values obtained prior to autoinduction (Fig. 5a). Given this dramatic difference from current susceptibility breakpoints, the target exposure was lowered to an AUC0-24/MIC ratio of 665. This target exposure was recently used by Goutelle et al., based on the fact that it is associated with a kill of 1 log10 CFU in mice, considered significant kill (31). With this lower exposure target, there was only a doubling of the breakpoint MICs, to 0.0156 and 0.0312 mg/liter prior to autoinduction (Fig. 5b). Use of SCL values obtained postinduction revealed even lower breakpoint MICs for the same dosing and target exposure circumstances, as shown in Fig. 5c and d. These data mean that in the most optimistic scenario, the rifampin critical concentration should be 0.0625 mg/liter, and even that only if rifampin doses higher than 600 mg are administered.
FIG. 5.
Target exposure attainment by rifampin. (a and b) Preautoinduction with EC90 target (a) and 1-log10 kill target (b). (c and d) Postautoinduction with EC90 target (c) and 1-log10 kill target (d).
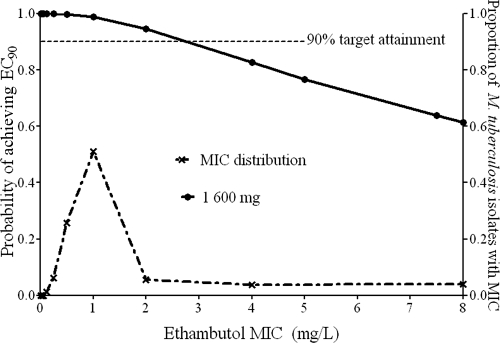
Ethambutol.
The ethambutol breakpoint MIC was 2.0 mg/liter at the highest dose of 1,600 mg a day (21 mg/kg/day), as shown in Fig. 6. Therefore, the critical concentration should be 4.0 mg/liter. However, exposures able to kill at the so-called high-level resistance level of 7.5 mg/liter could not be achieved in 1/3 of patients, so this breakpoint should be discarded.
FIG. 6.
Target exposure attainment by ethambutol.
DISCUSSION
In 1963, Canetti et al. wisely noted that every culture of wild M. tuberculosis would contain a small proportion of resistant bacilli (11). Restated using current language, every M. tuberculosis population is heterogeneous and contains a subpopulation of drug-resistant bacilli. They concluded that the difference between resistant and susceptible cultures was that the former had a larger proportion of resistant mutants, comprising at least 1% of the bacillary population. In order to capture this proportion of resistant mutants, certain “critical” drug concentrations were chosen. However, this approach is strictly a laboratory one; it is not designed to predict clinical outcomes. Effective drug concentrations that approximate the “critical concentrations” must be achieved in most TB patients if the laboratory test is to predict clinical outcome. Indeed, drug concentrations achieved in TB patients are important determinants of treatment response (13). Therefore, population pharmacokinetics, especially the wide variability encountered in actual patients, must also be taken into consideration. Finally, since the intent of anti-TB chemotherapy is to kill the tubercle bacillus, the relationship between drug concentration at the site of infection and microbial kill or antimicrobial PK/PD properties must of necessity also be part of determining the breakpoint (2, 21). When all these factors were taken into consideration, current critical concentrations for the three cornerstone anti-TB drugs were found to be overoptimistic for most TB patients treated with standard doses.
The most dramatic findings concerned isoniazid. Critical concentrations of 0.0312 mg/liter for low-level resistance and 0.125 mg/liter for high-level resistance were identified. The impact of the proposed change can be understood by examining the MIC distribution in Fig. 4, where the modal isoniazid MIC for clinical isolates is 0.125 mg/liter, so that with the proposed breakpoints the proportion of isolates with high-level resistance would change from 20% with current standards to ∼50% with the proposed critical concentrations. However, as can be seen from examining Fig. 4a, there are some circumstances, such as when slow acetylators are treated with higher doses, when concentrations achieved can kill organisms with an MIC of 0.125 mg/liter, or even of 0.2 mg/liter. This comports with clinical observations that some patients infected by isolates with so-called “low-level” isoniazid resistance will respond to high doses of isoniazid (48). Nevertheless, this is a limited circumstance. If the proportion of fast-acetylator TB patients is ≥10%, which is the case in most of the world, based on the prevalence of NAT-2 alleles (5, 24, 43), a concentration of 0.2 mg/liter would be overoptimistic as well, and the critical concentration for low-level resistance should be 0.0625 mg/liter. However, with the use of 900 mg a day, the critical concentration for high-level resistance is 0.125 mg/liter.
Rifampin results were as dramatic, with a critical concentration 16-fold lower than the current one of 1 mg/liter. Even when a lower AUC/MIC target exposure was used, the recalculated breakpoints were still low. Goutelle et al. recently performed population pharmacokinetic studies of patients with rifampin and examined the possibility that a rifampin dose of either 600 mg or 1,200 mg once daily could achieve the desired AUC0-24/MIC ratio with a 1.0-log10 CFU reduction in lung bacillary burden (31). Inspection of their graphs reveals that the breakpoint MICs would still be <0.01 mg/liter for doses up to 1,200 mg. Interestingly, even when they used the low AUC0-24/MIC ratio of 271 in plasma that is associated with a 1.0-log10 CFU reduction in mouse lung bacillary burden, the breakpoint value would increase only to 0.1 mg/liter. Thus, even though Goutelle et al. performed their study with a different set of patient pharmacokinetics, a different simulation program, and different assumptions from those used here, one would still reach the same conclusion of dramatically lower breakpoints. The consequence is that the proposed critical concentration of 0.0625 mg/liter means that 22% of the 1,217 clinical isolates in the data set used here would be considered rifampin resistant, compared to 13.4% with the standard critical concentration of 1.0 mg/liter.
Multidrug-resistant TB (MDR-TB) is defined as TB with simultaneous resistance to isoniazid and rifampin. With the proposed susceptibility breakpoints, the proportion of MDR-TB cases in most locales is expected to increase. As an illustration, based on multiplying resistance proportions as conditional probabilities, the frequency of MDR-TB would change from 2.4% with the old definitions to 10.0% with the new concentrations for the data set from Japan used here. The former estimate is very similar to the proportion of MDR-TB cases of 1.9% reported from Japan (4), suggesting that the latter estimate, derived from conditional probabilities and the proposed critical concentrations to estimate MDR-TB prevalence, may also be correct. It should be considered, however, that the rates will change based on the MIC distribution in each locale.
For pyrazinamide, the susceptibility breakpoints have been somewhat unclear, with some studies suggesting either 900 mg/liter or 1,200 mg/liter on agar, while commercial tests have employed a breakpoint of 100 mg/liter in Middlebrook broth (36). The current study suggests that the susceptibility breakpoint should be 50 mg/liter for a dose of 2 g a day, with 100 mg/liter applicable only to higher doses. Since the modal MIC is 50 mg/liter for the clinical isolates tested (Fig. 3), the effect of the modest change from 100 to 50 mg/liter dramatically changes the proportion of pyrazinamide-resistant isolates, from about 19% to 57%.
8-Methoxyfluoroquinolones such as moxifloxacin have benefited from extensive antimicrobial PK/PD work and population pharmacokinetic analysis during their development. Therefore, it is not surprising that the susceptibility breakpoint, which was first chosen for M. tuberculosis based on breakpoints in other bacterial species which had undergone extensive antimicrobial PK/PD work, corresponds to the findings reported in this paper. Similar to the case for moxifloxacin, ethambutol critical concentrations were also close to currently used standards. If one were to use the 2-fold dilution of drug utilized with most susceptibility tests, the level should be set at 4.0 mg/liter. However, this is unlikely to change the proportion of ethambutol-resistant isolates encountered in the clinic. On the other hand, the so-called high-level resistance breakpoint of 7.5 mg/liter could not be achieved by a full 34% of patients treated with the maximum recommended ethambutol dose, so this should be dropped.
Our findings have several clinical implications, depending on the resources available for clinical care in an area. One approach would be to increase the doses of anti-TB drugs so that optimal concentrations can be achieved in TB lesions. Figures 2 to 5 demonstrate that this can be achieved, to a certain extent. Indeed, the need for higher doses for virtually all first-line anti-TB drugs has been demonstrated (7, 26, 28, 31, 33-35). Unfortunately, for most of the first-line drugs, the chasm between drug exposures needed to optimally kill bacilli and those achieved in patients is too large to be bridged by dose increases without serious toxicity for the TB patients. Thus, the more realistic alternative would be to identify truly drug-resistant isolates and then use an alternative agent to replace the drug. As an example, clinical trials have demonstrated that moxifloxacin can replace either isoniazid or ethambutol, with no increase in therapy duration (10, 15, 29, 63). Newer agents such as TMC207 can also be used for M. tuberculosis strains resistant to both isoniazid and rifampin (27). Unfortunately, this would place a financial burden on resource-poor countries, which may end up resorting to more-toxic second-line alternatives. Indeed, even the laboratory changes needed for the proposed susceptibility breakpoints could also impose hardship on these countries. However, the treatment of TB patients in such countries should still rely on application of correct scientific information, and help for these countries should be directed at improving microbiology laboratories and therapeutic capacity.
Finally, using current resistance breakpoints, clinical cure rates for “drug-susceptible” TB vary by region, from about 60% to 95%, under directly observed therapy programs. However, the true success rate, when one examines studies of long-term morbidity and mortality as well as recurrence rates, is likely lower (20, 51, 57). In addition, current therapy for TB consists of 3 or 4 of the first-line drugs, administered simultaneously, while susceptibility breakpoints apply to each of the particular drugs in the combination, not to the entire regimen. Thus, even when there is resistance to one drug, the other drugs may still be able to kill the resistant mutants. Indeed, even though it was suboptimal compared to the current regimen, when two-drug therapy was used in the past there was a relatively good clinical response (1, 9). It is also known that the efficacies of nonantagonistic drugs in combination therapy will be at least additive, effectively “reducing” the MIC (49). In addition, as can be seen in Fig. 2 to 6, based on between-patient pharmacokinetic variability, some patients will still attain high enough concentrations of drug even when infected by a “resistant” organism and will still respond. Thus, the success rates of current therapy are more a composite outcome due to multiple drugs and processes. Nevertheless, if an isolate is truly resistant to an antibiotic, it is inappropriate to continue giving the drug, since this incurs increased toxicity risk without a therapeutic benefit. Therefore, an alternative drug should be given.
There are limitations to this study. First, imprecision of pharmacokinetic parameter estimates and antimicrobial PK/PD indices could lead to wrong susceptibility breakpoints. This would be a concern especially given the dramatic changes proposed for isoniazid and rifampin. Fortunately, both isoniazid and rifampin have undergone many good population pharmacokinetic studies as well as antimicrobial PK/PD work from two different disease models by two independent groups, which all showed broadly similar parameters (31, 32, 34, 38, 39, 42, 53, 65). This means that the parameters used are accurate, and imprecision of parameter values would not account for the dramatic changes in susceptibility breakpoints. In addition, MacGowan and colleagues recently demonstrated, using moxifloxacin and 10 Staphylococcus aureus isolates, that there is actually a distribution in the AUC0-24/MIC ratios associated with optimal kill due to strain-by-strain variation (44). Incorporation of this variability led to identification of susceptibility breakpoints which were lower than those obtained from a single exposure estimate. If this is true for M. tuberculosis, then the critical concentrations would be even more dramatically lower than our calculations. Similarly, exposures associated with different measures of kill, such as EC95 and EC99, have been proposed by some for use in susceptibility breakpoint determination. These create a higher hurdle for the drug to achieve and would result in even more dramatically lower breakpoints than those proposed in this paper. Finally, it could be argued that the proposed breakpoints are merely preliminary. This could be conceded. It is envisioned that each of these concentrations will be examined in clinical isolates from large numbers of TB patients who have undergone treatment and either succeeded or failed therapy. However, the currently recommended standards are no less preliminary. In general, epidemiological cutoff values (such as the 95% cutoff point in MIC distribution) are best used as an epidemiological tool to detect changes in drug resistance rates but are poorly configured to detect resistance in each patient and to individualize therapy (23). Thus, it is not surprising that studies on how well the standard critical concentrations of isoniazid predict clinical outcomes such as relapse have been contradictory (12, 46, 47). It has been argued persuasively that breakpoints identified using the older, “standard” approaches, especially those that utilize measures of central tendency of drug concentration (e.g., mean peak concentration), may actually foster bacterial drug resistance development and are thus detrimental (23). On the other hand, based on the approach utilized in this paper, new anti-TB compounds currently being developed and in phase II studies will have the possibility of breakpoints being determined early in the process and then prospectively validated in subsequent phase III clinical trials.
Acknowledgments
Funding for this study was provided by an NIH Director New Innovator Award (NIGM/NIH 1 DP2 OD001886) and by NIAID/NIH grant R01AI079497.
Pharmacokinetic parameter and variance information for most drugs has been published, but extra information was provided by Charles Peloquin (pyrazinamide and ethambutol), Justin Wilkins, and Helen McIlleron (pyrazinamide). Jotam Pasipanodya made many important comments to improve the manuscript.
Footnotes
Published ahead of print on 19 January 2010.
REFERENCES
- 1.Allison, S. T.1956. Pyrazinamide-isoniazid in low dosage in treatment of pulmonary tuberculosis. Am. Rev. Tuberc. 74:400-409. [DOI] [PubMed] [Google Scholar]
- 2.Ambrose, P. G., S. M. Bhavnani, C. M. Rubino, A. Louie, T. Gumbo, A. Forrest, and G. L. Drusano.2007. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: it's not just for mice anymore. Clin. Infect. Dis. 44:79-86. [DOI] [PubMed] [Google Scholar]
- 3.Ambrose, P. G., A. K. Meagher, J. A. Passarell, S. A. Van Wart, B. B. Cirincione, S. M. Bhavnani, and E. Ellis-Grosse.2009. Application of patient population-derived pharmacokinetic-pharmacodynamic relationships to tigecycline breakpoint determination for staphylococci and streptococci. Diagn. Microbiol. Infect. Dis. 63:155-159. [DOI] [PubMed] [Google Scholar]
- 4.Anonymous.2009. Anti-tuberculosis drug resistance in the world. Fourth global report. WHO/IUATLD Global Project on Anti-Tuberculosis Drug Resistance Surveillance, 2000-2007. World Health Organization, Geneva, Switzerland.
- 5.Bidstrup, C., P. H. Andersen, P. Skinhoj, and A. B. Andersen.2002. Tuberculous meningitis in a country with a low incidence of tuberculosis: still a serious disease and a diagnostic challenge. Scand. J. Infect. Dis. 34:811-814. [DOI] [PubMed] [Google Scholar]
- 6.Blumberg, H. M., W. J. Burman, R. E. Chaisson, C. L. Daley, S. C. Etkind, L. N. Friedman, P. Fujiwara, M. Grzemska, P. C. Hopewell, M. D. Iseman, R. M. Jasmer, V. Koppaka, R. I. Menzies, R. J. O'Brien, R. R. Reves, L. B. Reichman, P. M. Simone, J. R. Starke, and A. A. Vernon.2003. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am. J. Respir. Crit. Care Med. 167:603-662. [DOI] [PubMed] [Google Scholar]
- 7.Bock, N. N., T. R. Sterling, C. D. Hamilton, C. Pachucki, Y. C. Wang, D. S. Conwell, A. Mosher, M. Samuels, and A. Vernon.2002. A prospective, randomized, double-blind study of the tolerability of rifapentine 600, 900, and 1,200 mg plus isoniazid in the continuation phase of tuberculosis treatment. Am. J. Respir. Crit. Care Med. 165:1526-1530. [DOI] [PubMed] [Google Scholar]
- 8.Booker, B. M., P. F. Smith, A. Forrest, J. Bullock, P. Kelchlin, S. M. Bhavnani, R. N. Jones, and P. G. Ambrose.2005. Application of an in vitro infection model and simulation for reevaluation of fluoroquinolone breakpoints for Salmonella enterica serotype Typhi. Antimicrob. Agents Chemother. 49:1775-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.British Medical Research Council.1969. A controlled comparison of four regimens of streptomycin plus pyrazinamide in the retreatment of pulmonary tuberculosis. Tubercle 50:81-114. [DOI] [PubMed] [Google Scholar]
- 10.Burman, W. J., S. Goldberg, J. L. Johnson, G. Muzanye, M. Engle, A. W. Mosher, S. Choudhri, C. L. Daley, S. S. Munsiff, Z. Zhao, A. Vernon, and R. E. Chaisson.2006. Moxifloxacin versus ethambutol in the first 2 months of treatment for pulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 174:331-338. [DOI] [PubMed] [Google Scholar]
- 11.Canetti, G., S. Froman, J. Grosset, P. Hauduroy, M. Langerova, H. T. Mahler, G. Meissner, D. A. Mitchison, and L. Sula.1963. Mycobacteria: laboratory methods for testing drug sensitivity and resistance. Bull. W. H. O. 29:565-578. [PMC free article] [PubMed] [Google Scholar]
- 12.Cattamanchi, A., R. B. Dantes, J. Z. Metcalfe, L. G. Jarlsberg, J. Grinsdale, L. M. Kawamura, D. Osmond, P. C. Hopewell, and P. Nahid.2009. Clinical characteristics and treatment outcomes of patients with isoniazid-monoresistant tuberculosis. Clin. Infect. Dis. 48:179-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chideya, S., C. A. Winston, C. A. Peloquin, W. Z. Bradford, P. C. Hopewell, C. D. Wells, A. L. Reingold, T. A. Kenyon, T. L. Moeti, and J. W. Tappero.2009. Isoniazid, rifampin, ethambutol, and pyrazinamide pharmacokinetics and treatment outcomes among a predominantly HIV-infected cohort of adults with tuberculosis from Botswana. Clin. Infect. Dis. 48:1685-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clinical and Laboratory Standards Institute.2003. Susceptibility testing of mycobacteria, nocardiae, and other aerobic actinomycetes; approved standard. Clinical and Laboratory Standards Institute, Wayne, PA. [PubMed]
- 15.Conde, M. B., A. Efron, C. Loredo, G. R. De Souza, N. P. Graca, M. C. Cezar, M. Ram, M. A. Chaudhary, W. R. Bishai, A. L. Kritski, and R. E. Chaisson.2009. Moxifloxacin versus ethambutol in the initial treatment of tuberculosis: a double-blind, randomised, controlled phase II trial. Lancet 373:1183-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conte, J. E., Jr., J. A. Golden, S. Duncan, E. McKenna, and E. Zurlinden.1999. Intrapulmonary concentrations of pyrazinamide. Antimicrob. Agents Chemother. 43:1329-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conte, J. E., Jr., J. A. Golden, J. Kipps, E. T. Lin, and E. Zurlinden.2001. Effects of AIDS and gender on steady-state plasma and intrapulmonary ethambutol concentrations. Antimicrob. Agents Chemother. 45:2891-2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conte, J. E., J. A. Golden, J. E. Kipps, E. T. Lin, and E. Zurlinden.2004. Effect of sex and AIDS status on the plasma and intrapulmonary pharmacokinetics of rifampicin. Clin. Pharmacokinet. 43:395-404. [DOI] [PubMed] [Google Scholar]
- 19.Conte, J. E., Jr., J. A. Golden, M. McQuitty, J. Kipps, S. Duncan, E. McKenna, and E. Zurlinden.2002. Effects of gender, AIDS, and acetylator status on intrapulmonary concentrations of isoniazid. Antimicrob. Agents Chemother. 46:2358-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cox, H., Y. Kebede, S. Allamuratova, G. Ismailov, Z. Davletmuratova, G. Byrnes, C. Stone, S. Niemann, S. Rusch-Gerdes, L. Blok, and D. Doshetov.2006. Tuberculosis recurrence and mortality after successful treatment: impact of drug resistance. PLoS Med. 3:e384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Craig, W. A.2007. Pharmacodynamics of antimicrobials: general concepts and applications, p. 1-19. In C. H. Nightangle, P. G. Ambrose, G. L. Drusano, and T. Murakawa (ed.), Antimicrobial pharmacodynamics in theory and practice. Informa Healthcare USA, Inc., New York, NY.
- 22.Craig, W. A., J. Redington, and S. C. Ebert.1991. Pharmacodynamics of amikacin in vitro and in mouse thigh and lung infections. J. Antimicrob. Chemother. 27(Suppl. C):29-40. [DOI] [PubMed] [Google Scholar]
- 23.Dalhoff, A., P. G. Ambrose, and J. W. Mouton.2009. A long journey from minimum inhibitory concentration testing to clinically predictive breakpoints: deterministic and probabilistic approaches in deriving breakpoints. Infection 37:296-305. [DOI] [PubMed] [Google Scholar]
- 24.Dandara, C., C. M. Masimirembwa, A. Magimba, S. Kaaya, J. Sayi, D. K. Sommers, J. R. Snyman, and J. A. Hasler.2003. Arylamine N-acetyltransferase (NAT2) genotypes in Africans: the identification of a new allele with nucleotide changes 481C→T and 590G→A. Pharmacogenetics 13:55-58. [DOI] [PubMed] [Google Scholar]
- 25.D'Argenio, D. Z., A. Schumitzky, and X. Wang.2009. ADAPT 5 user's guide: pharmacokinetic/pharmacodynamic systems analysis software. Biomedical Simulations Resource, Los Angeles, CA.
- 26.Diacon, A. H., R. F. Patientia, A. Venter, P. D. van Helden, P. J. Smith, H. McIlleron, J. S. Maritz, and P. R. Donald.2007. Early bactericidal activity of high-dose rifampin in patients with pulmonary tuberculosis evidenced by positive sputum smears. Antimicrob. Agents Chemother. 51:2994-2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diacon, A. H., A. Pym, M. Grobusch, R. Patientia, R. Rustomjee, L. Page-Shipp, C. Pistorius, R. Krause, M. Bogoshi, G. Churchyard, A. Venter, J. Allen, J. C. Palomino, T. De Marez, R. P. van Heeswijk, N. Lounis, P. Meyvisch, J. Verbeeck, W. Parys, K. de Beule, K. Andries, and D. F. Mc Neeley.2009. The diarylquinoline TMC207 for multidrug-resistant tuberculosis. N. Engl. J. Med. 360:2397-2405. [DOI] [PubMed] [Google Scholar]
- 28.Donald, P. R., D. Maher, J. S. Maritz, and S. Qazi.2006. Ethambutol dosage for the treatment of children: literature review and recommendations. Int. J. Tuberc. Lung Dis. 10:1318-1330. [PubMed] [Google Scholar]
- 29.Dorman, S. E., J. L. Johnson, S. Goldberg, G. Muzanye, N. Padayatchi, L. Bozeman, C. M. Heilig, J. Bernardo, S. Choudhri, J. H. Grosset, E. Guy, P. Guyadeen, M. C. Leus, G. Maltas, D. Menzies, E. L. Nuermberger, M. Villarino, A. Vernon, and R. E. Chaisson.2009. Substitution of moxifloxacin for isoniazid during intensive phase treatment of pulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 180:273-280. [DOI] [PubMed] [Google Scholar]
- 30.Drusano, G. L., S. L. Preston, C. Hardalo, R. Hare, C. Banfield, D. Andes, O. Vesga, and W. A. Craig.2001. Use of preclinical data for selection of a phase II/III dose for evernimicin and identification of a preclinical MIC breakpoint. Antimicrob. Agents Chemother. 45:13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goutelle, S., L. Bourguignon, P. H. Maire, G. M. Van, J. E. Conte, Jr., and R. W. Jelliffe.2009. Population modeling and Monte Carlo simulation study of the pharmacokinetics and antituberculosis pharmacodynamics of rifampin in lungs. Antimicrob. Agents Chemother. 53:2974-2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gumbo, T., A. Louie, M. R. Deziel, W. Liu, L. M. Parsons, M. Salfinger, and G. L. Drusano.2007. Concentration-dependent Mycobacterium tuberculosis killing and prevention of resistance by rifampin. Antimicrob. Agents Chemother. 51:3781-3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gumbo, T., A. Louie, M. R. Deziel, L. M. Parsons, M. Salfinger, and G. L. Drusano.2004. Selection of a moxifloxacin dose that suppresses drug resistance in Mycobacterium tuberculosis, by use of an in vitro pharmacodynamic infection model and mathematical modeling. J. Infect. Dis. 190:1642-1651. [DOI] [PubMed] [Google Scholar]
- 34.Gumbo, T., A. Louie, W. Liu, D. Brown, P. G. Ambrose, S. M. Bhavnani, and G. L. Drusano.2007. Isoniazid bactericidal activity and resistance emergence: integrating pharmacodynamics and pharmacogenomics to predict efficacy in different ethnic populations. Antimicrob. Agents Chemother. 51:2329-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gumbo, T., C. S. W. Siyambalapitiyage Dona, C. Meek, and R. Leff.2009. Pharmacokinetics-pharmacodynamics of pyrazinamide in a novel in vitro model of tuberculosis for sterilizing effect: a paradigm for faster assessment of new antituberculosis drugs. Antimicrob. Agents Chemother. 53:3197-3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heifets, L., and T. Sanchez.2000. New agar medium for testing susceptibility of Mycobacterium tuberculosis to pyrazinamide. J. Clin. Microbiol. 38:1498-1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heifets, L. B.1991. Antituberculosis drugs: antimicrobial activity in vitro, p. 13-57. In L. B. Heifets (ed.), Drug susceptibility in the chemotherapy of mycobacterial infections. CRC Press, Boca Raton, FL.
- 38.Jayaram, R., S. Gaonkar, P. Kaur, B. L. Suresh, B. N. Mahesh, R. Jayashree, V. Nandi, S. Bharat, R. K. Shandil, E. Kantharaj, and V. Balasubramanian.2003. Pharmacokinetics-pharmacodynamics of rifampin in an aerosol infection model of tuberculosis. Antimicrob. Agents Chemother. 47:2118-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jayaram, R., R. K. Shandil, S. Gaonkar, P. Kaur, B. L. Suresh, B. N. Mahesh, R. Jayashree, V. Nandi, S. Bharath, E. Kantharaj, and V. Balasubramanian.2004. Isoniazid pharmacokinetics-pharmacodynamics in an aerosol infection model of tuberculosis. Antimicrob. Agents Chemother. 48:2951-2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Katiyar, S. K., S. Bihari, and S. Prakash.2008. Low-dose inhaled versus standard dose oral form of anti-tubercular drugs: concentrations in bronchial epithelial lining fluid, alveolar macrophage and serum. J. Postgrad. Med. 54:245-246. [DOI] [PubMed] [Google Scholar]
- 41.Kiem, S., and J. J. Schentag.2008. Interpretation of antibiotic concentration ratios measured in epithelial lining fluid. Antimicrob. Agents Chemother. 52:24-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kinzig-Schippers, M., D. Tomalik-Scharte, A. Jetter, B. Scheidel, V. Jakob, M. Rodamer, I. Cascorbi, O. Doroshyenko, F. Sorgel, and U. Fuhr.2005. Should we use N-acetyltransferase type 2 genotyping to personalize isoniazid doses? Antimicrob. Agents Chemother. 49:1733-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin, H. J., C. Y. Han, B. K. Lin, and S. Hardy.1994. Ethnic distribution of slow acetylator mutations in the polymorphic N-acetyltransferase (NAT2) gene. Pharmacogenetics 4:125-134. [DOI] [PubMed] [Google Scholar]
- 44.MacGowan, A. P., R. Reynolds, A. R. Noel, and K. E. Bowker.2009. Bacterial strain-to-strain variation in pharmacodynamic index magnitude, a hitherto unconsidered factor in establishing antibiotic clinical breakpoints. Antimicrob. Agents Chemother. 53:5181-5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McDowell, M. A., C. D. Fryar, R. Hirsch, and C. L. Ogden.2005. Anthropometric reference data for children and adults: U.S. population, 1999-2002. Number 361. National Center for Health Statistics, Hyattsville, MD. [PubMed]
- 46.Menzies, D., A. Benedetti, A. Paydar, I. Martin, S. Royce, M. Pai, A. Vernon, C. Lienhardt, and W. Burman.2009. Effect of duration and intermittency of rifampin on tuberculosis treatment outcomes: a systematic review and meta-analysis. PLoS Med. 6:e1000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mitchison, D. A., and A. J. Nunn.1986. Influence of initial drug resistance on the response to short-course chemotherapy of pulmonary tuberculosis. Am. Rev. Respir. Dis. 133:423-430. [DOI] [PubMed] [Google Scholar]
- 48.Moulding, T. S.1981. Should isoniazid be used in retreatment of tuberculosis despite acquired isoniazid resistance? Am. Rev. Respir. Dis. 123:262-264. [DOI] [PubMed] [Google Scholar]
- 49.Mouton, J. W., M. L. van Ogtrop, D. Andes, and W. A. Craig.1999. Use of pharmacodynamic indices to predict efficacy of combination therapy in vivo. Antimicrob. Agents Chemother. 43:2473-2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ogden, C. L., M. D. Carroll, L. R. Curtin, M. A. McDowell, C. J. Tabak, and K. M. Flegal.2006. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA 295:1549-1555. [DOI] [PubMed] [Google Scholar]
- 51.Pasipanodya, J. G., T. L. Miller, M. Vecino, G. Munguia, R. Garmon, S. Bae, G. Drewyer, and S. E. Weis.2007. Pulmonary impairment after tuberculosis. Chest 131:1817-1824. [DOI] [PubMed] [Google Scholar]
- 52.Peloquin, C. A., D. J. Hadad, L. P. Molino, M. Palaci, W. H. Boom, R. Dietze, and J. L. Johnson.2008. Population pharmacokinetics of levofloxacin, gatifloxacin, and moxifloxacin in adults with pulmonary tuberculosis. Antimicrob. Agents Chemother. 52:852-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peloquin, C. A., G. S. Jaresko, C. L. Yong, A. C. Keung, A. E. Bulpitt, and R. W. Jelliffe.1997. Population pharmacokinetic modeling of isoniazid, rifampin, and pyrazinamide. Antimicrob. Agents Chemother. 41:2670-2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rodriguez, J. C., M. Ruiz, M. Lopez, and G. Royo.2002. In vitro activity of moxifloxacin, levofloxacin, gatifloxacin and linezolid against Mycobacterium tuberculosis. Int. J. Antimicrob. Agents 20:464-467. [DOI] [PubMed] [Google Scholar]
- 55.Salfinger, M., and L. B. Heifets.1988. Determination of pyrazinamide MICs for Mycobacterium tuberculosis at different pHs by the radiometric method. Antimicrob. Agents Chemother. 32:1002-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shandil, R. K., R. Jayaram, P. Kaur, S. Gaonkar, B. L. Suresh, B. N. Mahesh, R. Jayashree, V. Nandi, S. Bharath, and V. Balasubramanian.2007. Moxifloxacin, ofloxacin, sparfloxacin, and ciprofloxacin against Mycobacterium tuberculosis: evaluation of in vitro and pharmacodynamic indices that best predict in vivo efficacy. Antimicrob. Agents Chemother. 51:576-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shaw, J. E., J. G. Pasipanodya, and T. Gumbo. Meningeal tuberculosis has very poor long term mortality despite standard therapy. Medicine (Baltimore), in press. [DOI] [PubMed]
- 58.Sheiner, L. B., B. Rosenberg, and V. V. Marathe.1977. Estimation of population characteristics of pharmacokinetic parameters from routine clinical data. J. Pharmacokinet. Biopharm. 5:445-479. [DOI] [PubMed] [Google Scholar]
- 59.Sheiner, L. B., B. Rosenberg, and K. L. Melmon.1972. Modelling of individual pharmacokinetics for computer-aided drug dosage. Comput. Biomed. Res. 5:411-459. [DOI] [PubMed] [Google Scholar]
- 60.Simon, N., E. Sampol, J. Albanese, C. Martin, P. Arvis, S. Urien, B. Lacarelle, and B. Bruguerolle.2003. Population pharmacokinetics of moxifloxacin in plasma and bronchial secretions in patients with severe bronchopneumonia. Clin. Pharmacol. Ther. 74:353-363. [DOI] [PubMed] [Google Scholar]
- 61.Srivastava, S., S. Musuka, C. Sherman, C. Meek, R. Leff, and T. Gumbo. Efflux pump derived multiple drug resistance to ethambutol monotherapy in Mycobacterium tuberculosis and ethambutol pharmacokinetics-pharmacodynamics. J. Infect. Dis., in press. [DOI] [PMC free article] [PubMed]
- 62.Turnidge, J., and D. L. Paterson.2007. Setting and revising antibacterial susceptibility breakpoints. Clin. Microbiol. Rev. 20:391-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang, J. Y., J. T. Wang, T. H. Tsai, C. L. Hsu, C. J. Yu, P. R. Hsueh, L. N. Lee, and P. C. Yang.2010. Adding moxifloxacin is associated with a shorter time to culture conversion in pulmonary tuberculosis. Int. J. Tuberc. Lung Dis. 14:65-71. [PubMed] [Google Scholar]
- 64.Wilkins, J. J., G. Langdon, H. McIlleron, G. C. Pillai, P. J. Smith, and U. S. Simonsson.2006. Variability in the population pharmacokinetics of pyrazinamide in South African tuberculosis patients. Eur. J. Clin. Pharmacol. 62:727-735. [DOI] [PubMed] [Google Scholar]
- 65.Wilkins, J. J., R. M. Savic, M. O. Karlsson, G. Langdon, H. McIlleron, G. Pillai, P. J. Smith, and U. S. Simonsson.2008. Population pharmacokinetics of rifampin in pulmonary tuberculosis patients, including a semimechanistic model to describe variable absorption. Antimicrob. Agents Chemother. 52:2138-2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yamane, N., S. Ichiyama, S. Kawahara, Y. Iinuma, H. Saitoh, M. Shimojima, H. Udagawa, and I. Nakasone.1999. Multicenter evaluation of broth microdilution test, BrothMIC MTB, to determine minimum inhibitory concentrations (MICs) of antimicrobial agents for Mycobacterium tuberculosis—evaluation of interlaboratory precision and interpretive compatibility with agar proportion method. Jpn. J. Clin. Pathol. 47:754-766. [PubMed] [Google Scholar]
- 67.Zhang, Y., S. Permar, and Z. Sun.2002. Conditions that may affect the results of susceptibility testing of Mycobacterium tuberculosis to pyrazinamide. J. Med. Microbiol. 51:42-49. [DOI] [PubMed] [Google Scholar]
- 68.Ziglam, H. M., D. R. Baldwin, I. Daniels, J. M. Andrew, and R. G. Finch.2002. Rifampicin concentrations in bronchial mucosa, epithelial lining fluid, alveolar macrophages and serum following a single 600 mg oral dose in patients undergoing fibre-optic bronchoscopy. J. Antimicrob. Chemother. 50:1011-1015. [DOI] [PubMed] [Google Scholar]