Abstract
Novel treatments for multidrug-resistant tuberculosis (MDR-TB), extensively drug-resistant tuberculosis (XDR-TB), or latent TB are needed urgently. Recently, we reported the formulation and characterization of the nitroimidazo-oxazine PA-824 for efficient aerosol delivery as dry powder porous particles and the subsequent disposition in guinea pigs after pulmonary administration. The objective of the present study was to evaluate the effects of these PA-824 therapeutic aerosols on the extent of TB infection in the low-inoculum aerosol infection guinea pig model. Four weeks after infection by the pulmonary route, animals received daily treatment for 4 weeks of either a high or a low dose of PA-824 dry powder aerosol. Animals received PA-824 cyclodextrin/lecithin suspensions orally as positive controls, and those receiving placebo particles or no treatment were negative controls. The lungs and spleens of animals receiving the high dose of inhaled PA-824 particles exhibited a lower degree of inflammation (indicated by wet tissue weights), bacterial burden, and tissue damage (indicated by histopathology) than those of untreated or placebo animals. Treatment with oral PA-824 cyclodextrin/lecithin suspension resulted in a more significant reduction in the bacterial burden of lungs and spleen, consistent with a dose that was larger than inhaled doses (eight times the inhaled low dose and four times the inhaled high dose). However, histopathological analysis revealed that the extent of tissue damage was comparable in groups receiving the oral or either inhaled dose. The present studies indicate the potential use of PA-824 dry powder aerosols in the treatment of TB.
Mortality rates attributed to tuberculosis (TB) worldwide are increasing due to the epidemic of HIV coinfection and the emergence of multidrug-resistant (MDR) and extensively drug-resistant (XDR) mycobacterial strains (20). There is an urgent need for new therapies with (i) activity against MDR/XDR and latent TB, (ii) shorter treatment duration, and (iii) safe and effective use in HIV-infected TB patients. PA-824, a leading compound in the 4-nitroimidazo-oxazine subclass, has shown promise in all those areas, as demonstrated by efficacy studies in the TB mouse model (11, 16-18, 24, 26). The mechanism of action of PA-824 appears to be the inhibition of the synthesis of protein and cell wall lipid after activation by the Mycobacterium tuberculosis F420 cofactor (13, 28). MIC values of PA-824 against M. tuberculosis range from 0.015 to 0.25 μg/ml for drug-sensitive strains and from 0.03 to 0.53 μg/ml for drug-resistant strains (9). In addition, this drug is active against nonreplicating anaerobic M. tuberculosis.
Pharmacokinetic (PK) studies in rats and mice indicate that when delivered by the oral route, PA-824 has excellent tissue penetration (23); however, its pharmacokinetics appears to be different when given to other species, including guinea pigs, rabbits, and humans (19, 25). The disposition, safety, and tolerability of single and multiple escalating doses of PA-824 were recently evaluated in two clinical studies with healthy volunteers (8, 9). After oral administration of tablets once daily, maximal PA-824 plasma concentrations of 3 to 3.8 μg/ml were reached at 4 to 5 h and steady state was reached after 5 to 6 days, with an average half-life of 16 to 20 h, varying by dose group. PA-824 appeared to be well tolerated with no effects on vital signs, but adverse effects, including headache, stomach discomfort, and clinically benign, reversible elevated serum creatinine levels, were observed at higher doses in these clinical studies (8, 9).
The goal of the work presented in this paper was to investigate the effectiveness of pulmonary administration of PA-824 in a low-dose infection guinea pig model of tuberculosis. PA-824 exhibits sparing solubility and requires formulation in exotic excipients for oral delivery at therapeutic doses. Local delivery in the absence of these excipients to the site of treatment would be beneficial pharmaceutically and therapeutically. Therefore, advantages of this approach might include elimination of potential adverse effects and formulation additives used to increase solubility and oral bioavailability. This would be achieved by delivering smaller total doses, with limited additives, directly to the primary site of infection, maximizing local concentrations while limiting systemic exposure.
The formulation and characterization of PA-824 in a dry powder porous particle form for efficient aerosol delivery was recently published (25). The disposition of PA-824 was determined after pulmonary administration of three escalating doses of this powder to guinea pigs and compared to that after intravenous (i.v.) and oral administration. Maximal PA-824 plasma concentrations of 2 to 4.6 μg/ml were reached 3 to 4 h after administering the powders by inhalation. Significantly longer half-lives and mean residence times were observed in animals dosed by the pulmonary route than after oral or i.v. administration. Moreover, while no PA-824 was detected in lung fluid after oral or i.v. administration, sustained levels of PA-824 were detected in the lung 32 h after pulmonary delivery of powders. Because of these encouraging findings, the present pharmacodynamic studies were conducted in the guinea pig model of TB to determine the efficacy of PA-824 powders delivered by the pulmonary route in the treatment of TB. Prior to dosing, studies were performed to determine aerodynamic properties of particles in a novel dosing chamber to determine a dosing regimen for the efficacy study.
Efficacy studies with oral doses of PA-824 have been conducted in guinea pigs (24) and mice (11, 16-18, 24, 26). The guinea pig model of TB more closely resembles the progression and pathogenesis of the disease in humans and guinea pigs can more readily be dosed by the pulmonary route with aerosol than mice; hence, efficacy studies in this species are believed to be more relevant to assessing the efficacy of PA-824 dry powder aerosols for TB treatment.
MATERIALS AND METHODS
Materials.
l-Leucine was obtained from Spectrum Chemicals & Laboratory Products (Gardena, CA), and the phospholipid 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) was from Genzyme Pharmaceuticals (Cambridge, MA). PA-824 was received from the Global Alliance for TB Drug Development (New York, NY). Acetonitrile, ethanol USP grade, and methanol were purchased from Pharmco Products Inc. (Brookfield, CT). Water from a Millipore Corp. (Billerica, MA) Milli-Q water purification system was used.
Manufacture of PA-824 and placebo particles.
Respirable drug-containing and placebo powders were prepared by spray drying. The PA-824 particles were manufactured from a 70% ethanol solution at 55°C with 75% (wt/wt) PA-824, 20% (wt/wt) l-leucine, and 5% (wt/wt) DPPC, and the placebo was a 70% ethanol solution containing 90% (wt/wt) l-leucine and 10% (wt/wt) DPPC. The dry powders were prepared using a Niro Mobile Minor spray dryer (Columbia, MD) with an inlet temperature of 107°C and feedstock flow rate of 60 ml/min, as detailed elsewhere (25).
Characterization of dry powders.
The spray-dried powders were characterized in triplicate for particle size (volume and aerodynamic distributions), morphology, and PA-824 content. The volume particle size distribution (volume median diameter) of the spray-dried powder was measured by laser diffraction using a HELOS system with a RODOS dry dispersing unit (Sympatec Inc., Lawrenceville, NJ) at an applied pressure of 200 kPa. The aerodynamic properties and particle distribution of the powder (fine particle fraction [FPF] less than 5.8 μm, mass median aerodynamic diameter [MMAD], and geometric standard deviation [GSD]) were determined with standard methodologies by using an eight-stage Andersen nonviable 1ACFM cascade impactor (ACI; Copley Scientific Ltd., Nottingham, United Kingdom) and hand-held, breath-activated, capsule-based dry powder inhaler device (Plastiape, Osnago, Italy). The morphology of the dry particles was evaluated using a 982 field emission scanning electron microscope (SEM; LEO, Carl Zeiss, Inc., Thornwood, NY) after coating powder samples with a platinum/palladium layer (208HR sputter coater; Cressington Scientific Instruments Inc., Watford, United Kingdom).
The PA-824 content of the spray-dried powder was determined by a reverse-phase high-performance liquid chromatography (HPLC) method using an Agilent Technologies 1100 series HPLC system (Palo Alto, CA). The mobile phase was run on a linear gradient from 20% acetonitrile and 80% water to 60% acetonitrile and 40% water over 30 min with 5 min of equilibration time. Analysis was performed on a 50-μl injection volume at a flow rate of 1.5 ml/min through an Agilent Zorbax Eclipse XDB-C18 column (4.6 by 150 mm), and absorbance was recorded at 330 nm. An Agilent Zorbax Eclipse XDB-C18 analytical guard column was also used.
Respiratory infection.
All animal procedures were approved by the University of North Carolina Chapel Hill Institutional Animal Care and Use Committee. Specific-pathogen-free male Dunkin-Hartley guinea pigs (Hilltop, Scottsdale, PA) weighing 369 ± 45 g were housed individually in a biosafety level 3 (BSL-3) containment area with a 12-h light/dark cycle. Animals were allowed free access to water and food at all times. Animals were infected via the respiratory route with a small inoculum (2 ×105 CFU/ml) of M. tuberculosis strain H37Rv. Animals were placed randomly in an exposure chamber, and aerosols were generated by pumping compressed air through a modified MRE type 3 jet Collison nebulizer (Waltham, MA) containing 5 ml of bacterial suspension. Validation of this procedure indicated that it results in the inhalation and retention of 10 to 15 viable, virulent organisms per guinea pig (12, 27). Animals remained untreated for 4 weeks following infection, when the bacterial burden is known to plateau (14). Body weights of each animal were recorded throughout the study, as were changes in behavior or any other signs of toxicity.
Determination of PA-824 inhaled dose.
A custom-designed dry powder dosing chamber (7) was used to deliver aerosol powders to the animals. The dosing chamber was initially loaded in separate studies with two amounts of PA-824 powders, referred to as high (360 mg) and low (180 mg) nominal doses, in order to achieve the actual dose inhaled by each animal. These initial loading doses of powder were sufficient to deliver doses to the animal based on (i) efficiency of delivery to the port of the dosing chamber and (ii) the proportion of this dose inhaled by the animal. The doses delivered to the port were measured experimentally, and the respirable dose was calculated. The efficiency of delivery to the port of the dosing chamber from which the animal inhaled was approximately 6% of the nominal doses (11.3 or 22.4 mg). The dose to the animal, derived from the respirable dose (known as fine particle fraction, or proportion of dose at the chamber port <3.2 μm in rodents) was approximately 20% (low dose) or 28% (high dose) (2.3 and 6.4 mg). Ultimately, measures of delivered dose were obtained experimentally from analysis of plasma PA-824 concentrations, which were assumed to reflect dose delivered to the lungs. Four healthy animals (no TB infection) for each dose were placed in the ports of the dry powder dosing chamber and allowed to inhale PA-824 aerosol. Particles were introduced into the dosing chamber in designated quantities at 5-min intervals. Animals in the high-dose group were exposed for 60 min, whereas animals in the low-dose group were treated for 30 min.
Blood samples (0.35 ml) were collected after dispersion of the last portion of particles in the chamber at 0, 0.08, 0.25, 0.5, 1, 2, 3, 4, 5, 6, 8, 12, and 24 h, and plasma was separated and analyzed for PA-824 concentration as described previously (25).
Preparation of PA-824 suspension for oral delivery.
In order to overcome the poor solubility of PA-824, it was prepared in a cyclodextrin/lecithin formulation as previously described (11, 24, 26). Briefly, a known amount of PA-824 was added to 1 ml of a 10% solution of hydroxypropyl-β-cyclodextrin (Sigma-Aldrich, Co., St. Louis, MO), and the mixture was stirred gently for 24 h at room temperature (22 to 25°C). This suspension was then sonicated for 10 min (Sonic Dismembrator probe; Fisher Scientific, Pittsburgh, PA). Frozen lecithin (Avanti Polar Lipids, Inc., Alabaster, AL) was added while stirring at room temperature. Lastly, the suspension was cooled in an ice bath and sonicated intermittently for 15 min to maintain the suspension temperature below 50°C.
Treatments.
After infection was established, animals were randomly assigned to five experimental groups that received the following treatments: inhaled PA-824 particles at low and high doses loaded into the dosing chamber (180 and 360 mg, respectively), inhaled placebo particles (180 mg), oral PA-824 suspension (40 mg/kg), and the untreated controls. For treatment, the respective doses of PA-824 and placebo particles were loaded at intervals into the dosing chamber and administered to the animals by passive inhalation as described above. All animals were treated daily for 4 weeks.
Necropsy.
After 4 weeks of daily treatment (8 weeks after infection), animals were sacrificed and necropsy was performed as follows. The chest and peritoneal cavities were opened and the lungs, spleen, and liver were inspected to determine the number of superficial lesions, and their wet weights were recorded. The caudal left lung lobe and residual spleen tissue were placed in 10% neutral buffered formalin for histopathological evaluation. For bacteriology, the caudal right lung lobe and approximately three-quarters of the spleen tissue were homogenized separately using Teflon-glass homogenizers. The homogenates were diluted with sterile saline, and aliquots for each tissue were inoculated onto duplicate M7H10 agar plates (Hardy Diagnostics, Santa Maria, CA). The plates were incubated at 37°C for 3 weeks. Visible CFU were counted, and data are expressed as the base 10 logarithm (log).
Histopathology.
Formalin-fixed lung and spleen tissues were trimmed, embedded in paraffin wax, and sectioned at 5 μm. For lungs, midsaggital sections were taken from the right middle lobes. For spleens, a single cross-section was taken for processing. The sections were mounted on glass slides, and microscopic examinations were conducted with hematoxylin-eosin-stained slides from each block. Microscopic examinations were conducted by a pathologist (Charles River Pathology Associates, Durham, NC) who was blinded with respect to the treatment received by the animals.
Statistical analyses.
Data were analyzed with the analysis of variance (ANOVA) and least-squares significant differences multiple comparison method. A probability level of 5% (P < 0.05) was considered to be statistically significant.
RESULTS
Particle manufacture and characterization.
Drug-containing particles with a load of approximately 75% (wt/wt) PA-824 were prepared by spray drying. The resulting dry powder formulation consisted of thin-walled porous particle structures. The median volume diameter of the particles was 3.95 ± 0.01 μm, and the powder had desirable aerosol properties for human pulmonary delivery as indicated by an MMAD of 4.45 ± 0.10 μm, GSD of 1.43 ± 0.07, and FPF of 57.6 ± 3.6%. The PA-824 content of the powder was 75.7 ± 0.7% of the total mass as estimated by HPLC with UV detection.
Placebo particles had a similar thin-walled structure. The median volume diameter of the particles was 6.48 ± 0.03 μm, and the aerodynamic particle size distribution was characterized as follows: MMAD of 4.73 ± 0.02 μm, GSD of 1.52 ± 0.01, and FPF of 61.2 ± 1.4%.
Determination of PA-824 inhaled dose.
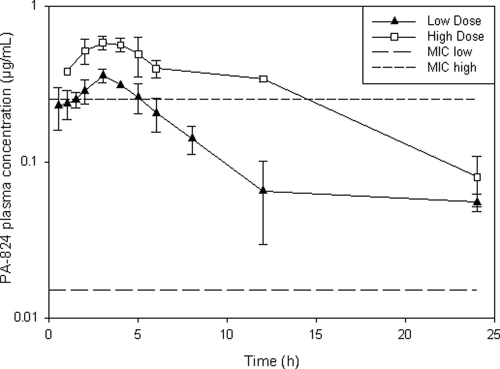
PA-824 plasma concentrations versus time curves are shown in Fig. 1. Considering the upper limit in the MIC reported by Tyagi et al. (0.015 to 0.25 μg/ml) (26) for drug-sensitive M. tuberculosis, including the H37Rv strain used in the present study, animals inhaling the low and high doses of PA-824 particles would have plasma drug levels above the MIC for 5 h and >12 h, respectively, whereas considering the lower MIC value, PA-824 plasma concentrations would be above the MIC for more than 24 h after both doses.
FIG. 1.
Plasma concentration versus time curves (log scale) after passive inhalation of PA-824 particles in the dry powder dispersion chamber.
The fraction of PA-824 powder delivered to the systemic circulation by aerosol, which was calculated by the ratio of the area under the time-concentation curve (AUC) after aerosol administration versus the AUC after powder intratracheal insufflation (25), was determined to be 6.7% for the low dose and 13.5% for the high dose. Thus, the estimated dose absorbed by the animals receiving the low dose was 4.8 mg/kg and those receiving the high dose was 9.7 mg/kg, as calculated using the ratio of the AUC for aerosol and i.v. administration and the bioavailability (25). It is worth noting that this absorbed dose does not take into account drug that may have remained in the airways, not absorbed, or absorbed in the lung tissue.
Efficacy studies.
No significant changes in behavior or symptoms of toxicity were observed in the animals during the 4-week treatment period.
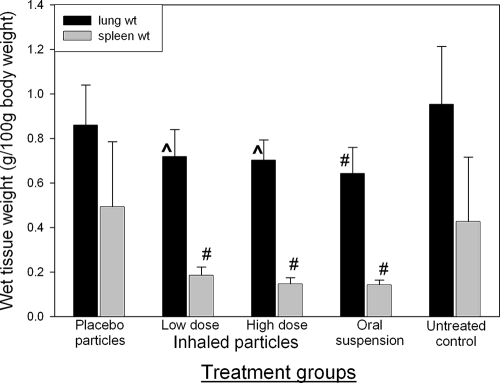
Wet tissue weight has been used as an indirect marker of inflammation (2-4, 10, 15). In the present studies, wet lung and spleen weights were used as indicators of the degree of inflammation of each organ. Increased organ weights are indicative of the extent of inflammation due to TB infection in that organ. Figure 2 shows the organ weights corrected for body weight at the time of necropsy. The wet lung weights of animals receiving high and low doses of inhaled PA-824 particles were significantly lower than those of untreated controls. The wet lung weight in animals receiving the oral suspension of PA-824 was significantly lower than that of untreated controls and animals receiving placebo particles. There was no significant difference between the lung weights in animals receiving inhaled and oral treatments, suggesting a comparable effect of both formulations on lung inflammation.
FIG. 2.
Lung and spleen wet weights of TB-infected guinea pigs receiving the different treatments daily for 4 weeks (average ± standard deviation; n = 6). ^, significantly lower than untreated control (P = 0.0048); #, significantly lower than untreated control and placebo (P = 0.001).
Likewise, the wet spleen weights in animals receiving either oral or inhaled treatment were significantly lower than those in untreated or placebo animals. There was no significant difference between the spleen weight in animals receiving inhaled and oral treatments.
The number of viable bacteria per ml (CFU/ml) of organ homogenate in lungs and spleens of animals receiving the different daily treatments is shown in Fig. 3. Bacterial burden in treated animals (inhaled and oral) was significantly lower than in untreated and placebo controls. The number of bacteria in the lungs of animals receiving 40 mg/kg of PA-824 suspension orally was significantly lower (1.6 ± 1.3 CFU/ml) than all other groups (untreated, placebo, and PA-824 aerosol treated; P < 0.0001). As for the bacterial burden in the lungs of animals receiving the inhaled treatments, those receiving the high dose of PA-824 particles displayed a significantly lower burden (3.4 ± 0.4 CFU/ml; P = 0.0084) than those in untreated animals (4.2 ± 0.3 CFU/ml) and in those receiving placebo particles (4.6 ± 0.5 CFU/ml). However, the number of bacteria in the lungs of animals receiving the low dose of inhaled PA-824 particles (3.8 ± 0.3 CFU/ml) was significantly lower (P = 0.0074) than in those receiving placebo particles (4.6 ± 0.5 CFU/ml).
FIG. 3.
Number of viable bacteria (CFU/ml) at necropsy in lung and spleen tissues of TB-infected guinea pigs receiving the different treatments daily for 4 weeks (average ± standard deviation; n = 6). +, significantly lower than any other group (P < 0.0001); $, significantly lower than low-dose group, untreated, control, or placebo (P = 0.0027); #, significantly lower than untreated control or placebo (P = 0.0084); *, significantly lower than placebo (P = 0.0074).
Similarly, the number of bacteria in the spleens of animals receiving 40 mg/kg of PA-824 suspension orally (2.0 ± 1.6 CFU/ml) was significantly lower (P < 0.0001) than for all other groups (untreated, placebo, and PA-824 aerosol treated). However, the spleens of animals receiving the high dose of inhaled PA-824 showed lower bacterial burdens (4.1 ± 0.4 CFU/ml) than any other treatment (P = 0.0027) with the exception of oral PA-824. Spleens of animals receiving the low dose of inhaled PA-824 particles had a significantly lower bacterial burden (4.3 ± 0.3 CFU/ml) than those of animals receiving placebo particles (5.0 ± 0.5 CFU/ml) or untreated controls (4.6 ± 0.2 CFU/ml).
Histopathology.
Tables 1 and 2 summarize the histopathological analysis in infected guinea pig tissues after the different treatments. Levels of lung infection, as indicated by tissue damage, were mostly comparable among groups receiving oral and inhaled treatments (Table 1). The lungs of animals receiving oral or inhaled treatments had a significantly smaller proportion of lung lobes (<5%) affected by medium granulomas, compared with more than 30% of lung lobes affected by medium granulomas in animals receiving placebo particles or untreated controls (Fig. 4). However, although fewer granulomas were observed in treated animals, more of these exhibited caseous necrosis than in the negative control group, in which animals had more tissue involvement, presumably with higher numbers of granulomas, but fewer exhibited caseous necrosis.
TABLE 1.
Histopathological findings in infected guinea pig lung tissues after the different treatments
| Treatment | % of lung lobe with granulomasa | Granuloma size | % of granulomas with caseous necrosis | Amt of necrosis in affected granulomas |
|---|---|---|---|---|
| Placebo | 30 | Medium | 10-30 | Marked |
| Low dose | 5 | Medium | 20-50 | Mild/moderate |
| High dose | 5 | Medium/large | 60-90 | Mild/moderate |
| Oral | 2 | Medium | 60-70 | Mild |
| Untreated control | 30-40 | Medium | 10-20 | Moderate |
The percent affected is based on a visual estimate of the lung lobe without enumeration of the granulomas or measurements of the total parenchymal area.
TABLE 2.
Histopathological findings in infected guinea pig spleen tissues after the different treatmentsa
| Treatment | % White pulp with granulomas | Avg size of granulomas | Incidence of caseous necrosis in granulomas | Severity of caseous necrosis in granulomas | % Granulomas with necrosis |
|---|---|---|---|---|---|
| Placebo | 60-65 | Medium | Moderate | Minimal | 5-30 |
| Low dose | 40-50 | Medium | None | NAb | NA |
| High dose | 0-5 | Tiny | None | NA | NA |
| Oral | 0-60 | Small | Rare | Minimal | NA |
| Untreated control | 30-80 | Medium | Occasional | Minimal | 20 |
Each cross-section of spleen contained approximately 25 to 35 profiles of lymphoid white pulp, depending on the overall size of the spleen. The percent affected is the estimated percentage of white pulp profiles that contained granulomas.
NA, not applicable.
FIG. 4.
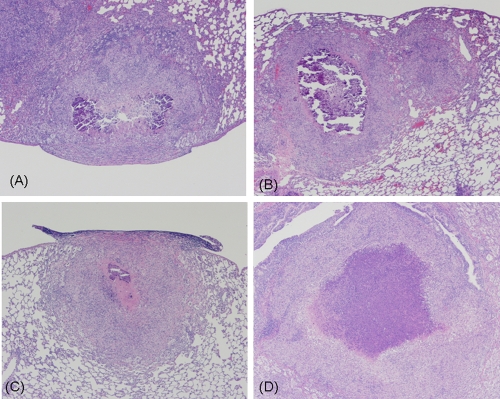
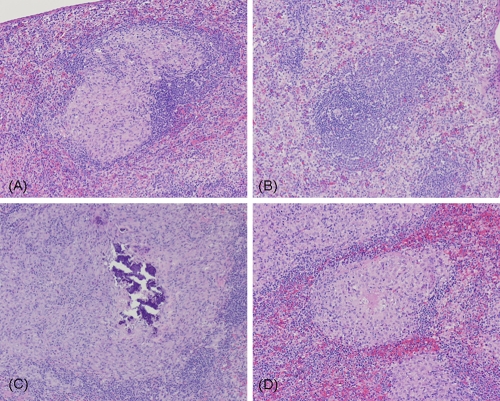
Lung histopathology of infected guinea pigs receiving the different treatments. Magnification, ×4. (A) Low dose of inhaled PA-824 particles; (B) high dose of inhaled PA-824 particles; (C) oral suspension of PA-824; (D) untreated controls. Note the open airways and smaller granulomas in panels B and C, contrasting with the large surface of lung tissue affected with large granulomas in panel D.
Histopathological evaluation of spleen tissues revealed that only <5% of the white pulp (lymphoid tissue arranged around the arteries of the spleen) was affected by tiny granulomas in the spleens of animals receiving the high dose of inhaled PA-824 particles and none of them exhibited caseous necrosis (Fig. 5B). Likewise, a smaller percentage of white pulp was affected by small granulomas in the spleens of animals receiving the oral PA-824 suspension (Fig. 5C), and some of these granulomas exhibited minimal caseous necrosis. In contrast, untreated animals and those treated with placebo particles had a high percentage (>60%) of white pulp affected by medium granulomas (Table 2; Fig. 5D).
FIG. 5.
Spleen histopathology of infected guinea pigs receiving the different treatments. Magnification, ×10. (A) Low dose of inhaled PA-824 particles; (B) high dose of inhaled PA-824 particles; (C) oral suspension of PA-824; (D) untreated controls. Note the absence of granulomas in panels B and C, contrasting with the large surface of white pulp affected with large granulomas in panel D.
DISCUSSION
The aim of the present study was to evaluate the efficacy of PA-824 dry powder aerosols in the treatment of TB. PA-824, typically administered orally, demonstrates great promise in treating both active and latent tuberculosis, with the potential of treating MDR-TB. We postulated that by delivering drug-containing powders directly to the lungs (i.e., the initial site of TB infection and reactivation of the disease), high concentrations could be achieved to treat the bacteria locally, thus minimizing systemic exposure.
We have recently shown the potential for PA-824 to be formulated into a stable dry powder porous particle form for delivery by the pulmonary route via simple dry powder inhalers (25). The porous particle system is well suited for efficient delivery to the lungs (1, 5, 6, 21). This high drug load formulation has been shown to maintain physical, aerodynamic, and chemical stability at room temperature for 6 months and under refrigerated conditions for more than 1 year. This room temperature stability may reduce the need for a cold chain for storage and distribution, while short-term stability at elevated temperatures may allow for storage excursions under extreme conditions.
Treatment with the different PA-824 formulations and routes of administration (oral suspension and inhaled particles) appeared to be well tolerated by TB-infected animals, based on observations of the animals during the period of the study. Inhalation treatment with PA-824 particles appeared to reduce manifestations of disease in the lungs and spleens of guinea pigs. Animals receiving low and high doses of inhaled PA-824 aerosols showed significantly less inflammation (as indicated by wet tissue weights), a lower number of viable bacteria, and less tissue damage (as shown by histopathological analysis) than untreated animals or those inhaling placebo particles. Furthermore, bacterial burden appeared to be even lower in the spleens of animals that inhaled the high dose of PA-824 particles than in those that inhaled the low dose, and tissue damage was observed to a lesser extent in those animals as well. Notably, the percentage of white pulp affected by granulomas in spleen of animals inhaling the high dose appeared to be lower than that in animals receiving the oral PA-824 suspension, as revealed by histopathology (Table 2; Fig. 5), even though these orally treated animals exhibited lower bacterial burdens in the spleen and lung.
The greater reductions in bacterial burden observed in the lungs and spleens of animals receiving oral treatment are consistent with the larger dose of 40 mg/kg, compared to the delivered dose based on circulating concentrations after powder inhalation of 4.8 (low) and 9.7 (high) mg/kg. Hence, it is possible that these lower doses may not have resulted in plasma levels of PA-824 above the MIC for a sufficient time to have an enduring effect. It is also possible that the cyclodextrin/lecithin suspension may have aided in the penetration of PA-824 in tissues, thus contributing to the efficacy of the oral treatment.
The efficacy of oral doses of PA-824 in the treatment of TB has been previously evaluated in guinea pigs and mice as monotherapy and in combination with other drugs. In the first efficacy studies published by Stover et al. (24), a daily oral dose of 100 mg/kg of PA-824 reduced the bacterial burden in the lungs of guinea pigs by 1.5 logs and in mice by 3 logs. Tyagi et al. (26) reported a 4-log reduction in the bacterial burden of the lungs of mice after 8 weeks of daily 100-mg/kg doses of PA-824, which had a comparable effect to that of 25 mg/kg of isoniazid. At the same dose, Lenaerts et al. (11) achieved a 2-log reduction in lung bacterial burden after 2 weeks of treatment, a 3.5-log reduction after 6 weeks, and a 4-log reduction after 12 weeks of daily treatment. The difference in the results obtained by Tyagi et al. (26) and Lanaerts et al. (11) may lie in the M. tuberculosis strain employed to infect the mice. The study by Tyagi et al. employed strain H37Rv, whereas Lanaerts et al. employed the Erdman strain.
PA-824 appears to have greater efficacy in TB therapies when employed in combination treatments. While 2 months of treatment with PA-824 resulted in only a 2-log reduction in the bacterial burden of the lungs of mice, a combination of rifampin, pyrazinamide, and PA-824 decreased this bacterial burden by 6 logs (16). Other studies (18) also showed that a 6-month treatment with moxifloxacin and PA-824 completely sterilized the spleens of infected mice and that the combination of moxifloxaxin, pyrazinamide, and PA-824 cured mice more rapidly than the combination of rifampin, isoniazid, and pyrazinamide, shortening the treatment by at least 1 month (17).
The reductions in bacterial burden observed in the lungs and spleens of animals receiving oral treatment in the present study are similar to those observed by Stover et al. (24) in guinea pigs treated for 30 days, confirming the efficacy of oral PA-824 in the relevant guinea pig model of TB. The reduction in bacterial burden in the spleens of guinea pigs after oral treatment is similar to that reported for mice infected with M. tuberculosis Erdman strain after 2 weeks of treatment (11). However, in all other mouse studies employing monotherapy, the reductions in lung bacterial burdens have been greater (>1 log) than the one observed in the present study, which is likely to be the result of longer treatment times (>30 days) and different drug disposition in mice versus guinea pigs (16, 25).
The modest effect of inhaled PA-824 on bacterial burden in the present study may be due to the magnitude of the dose delivered to the animals (one-quarter and one-eighth of the oral dose). The amount of PA-824 powder actually deposited in the lungs of animals is limited by the physical characteristics of the drug particles and by the efficiency of delivery, including the length of time that an animal can be placed in the port of the dosing chamber to inhale the powder and the cutoff diameter in the nose of guinea pigs being significantly smaller than that for humans (22). Nevertheless, these limitations may not be an issue in patient therapy, considering the greater capacity for powder delivery compared to guinea pigs. An appropriate inhaler would be required to deliver therapeutic doses of powder. The likelihood of success with the combination of inhaled PA-824 therapy with another oral or inhaled drug(s) is good. In order to take advantage of synergistic drug effects on TB bacteria it may be feasible to design an effective therapy consisting of inhaled PA-824 with one or two more drugs, possibly moxifloxacin, which seems to have a better synergistic effect than with pyrazinamide, ethambutol, or ethionamide (17, 18).
Acknowledgments
This work was supported by a grant from the National Institutes of Health, NIAID, under grant number 5 U01 61336, and a National Science Foundation Graduate Research Fellowship.
We acknowledge the Global Alliance for TB Drug Development for PA-824 material used in this study and Melvin Spigelman and Doris Rouse for their assistance.
Footnotes
Published ahead of print on 19 January 2010.
REFERENCES
- 1.Bates, D. V., B. R. Fish, T. F. Hatch, T. T. Mercer, and P. E. Morrow.1966. Deposition and retention models for internal dosimetry of the human respiratory. Task Group on Lung Dynamics. Health Physics 12:173-207. [PubMed] [Google Scholar]
- 2.Demidov, S. V., V. G. Myasnikov, E. F. Chernushenko, and L. S. Osipova.1991. Effects of thymus preparations and antituberculous drugs on immunological reactivity and the course of tuberculous process in experimental animals. Problemy Tuberkuleza 12:52-54. [PubMed] [Google Scholar]
- 3.Devi, T. I., R. P. Gupta, R. K. Dhawan, V. V. S. Murti, B. Ramamurthy, T. Ramakrishnan, and T. A. Venkitasubramanian.1988. Chemotherapy of experimental tuberculosis with N-(2-naphthyl)glycine hydrazide dihydrochloride. Part I. Acute toxity and effect on wet weight, dry weight, glycogen and total lipids of organs. Indian J. Clin. Biochem. 3:37-44. [Google Scholar]
- 4.Drandarska, I., V. Kussovski, S. Nikolaeva, and N. Markova.2005. Combined immunomodulating effects of BCG and Lentinan after intranasal application in guinea pigs. Int. Immunopharmacol. 5:795-803. [DOI] [PubMed] [Google Scholar]
- 5.Edwards, D. A., J. Hanes, G. Caponetti, J. Hrkach, A. Ben-Jebria, M. L. Eskew, J. Mintzes, D. Deaver, N. Lotan, and R. Langer.1997. Large porous particles for pulmonary drug delivery. Science 276:1868-1872. [DOI] [PubMed] [Google Scholar]
- 6.Fiegel, J., L. Garcia-Contreras, M. Thomas, J. VerBerkmoes, K. Elbert, A. Hickey, and D. Edwards.2008. Preparation and in vivo evaluation of a dry powder for inhalation of capreomycin. Pharm. Res. 25:805-811. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Contreras, L., J. Fiegel, M. J. Telko, K. Elbert, A. Hawi, M. Thomas, J. VerBerkmoes, W. A. Germishuizen, P. B. Fourie, A. J. Hickey, and D. Edwards.2007. Inhaled large porous particles of capreomycin for treatment of tuberculosis in a guinea pig model. Antimicrob. Agents Chemother. 51:2830-2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ginsberg, A. M., M. W. Laurenzi, D. J. Rouse, K. D. Whitney, and M. K. Spigelman.2009. Assessment of the effects of the nitroimidazo-oxazine, PA-824, on renal function in healthy subjects. Antimicrob. Agents Chemother. 53:3726-3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ginsberg, A. M., M. W. Laurenzi, D. J. Rouse, K. D. Whitney, and M. K. Spigelman.2009. Safety, tolerability, and pharmacokinetics of PA-824 in healthy subjects. Antimicrob. Agents Chemother. 53:3720-3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang, S., D. Zhu, Y. Jiang, X. Luo, and Q. Chen.2005. Therapeutic effect of eukaryotic expression plasmid psIL-12 on murine infection with Mycobacterium tuberculosis. Zhonghua Yi Xue Za Zhi 24:373-376. (In Chinese.) [Google Scholar]
- 11.Lenaerts, A. J., V. Gruppo, K. S. Marietta, C. M. Johnson, D. K. Driscoll, N. M. Tompkins, J. D. Rose, R. C. Reynolds, and I. M. Orme.2005. Preclinical testing of the nitroimidazopyran PA-824 for activity against Mycobacterium tuberculosis in a series of in vitro and in vivo models. Antimicrob. Agents Chemother. 49:2294-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mainali, E. S., and D. N. McMurray.1998. Protein deficiency induces alterations in the distribution of T-cell subsets in experimental pulmonary tuberculosis. Infect. Immun. 66:927-931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manjunatha, U. H., H. Boshoff, C. S. Dowd, L. Zhang, T. J. Albert, J. E. Norton, L. Daniels, T. Dick, S. S. Pang, and C. E. Barry.2006. Identification of a nitroimidazo-oxazine-specific protein involved in PA-824 resistance in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U. S. A. 103:431-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McMurray, D. N.1994. Guinea pig model of tuberculosis, p. 135-148. In B. R. Bloom (ed.), Tuberculosis: pathogenesis, protection, and control. ASM Press, Washington, DC.
- 15.Niu, X., C. Liu, Y. Li, M. Li, and H. Wang.1998. Therapeutic action of Luolining capsules on tuberculotic guinea pigs. Zhongguo Yaoke Daxue Xuebao 29:142-144. [Google Scholar]
- 16.Nuermberger, E., I. Rosenthal, S. Tyagi, K. N. Williams, D. Almeida, C. A. Peloquin, W. R. Bishai, and J. H. Grosset.2006. Combination chemotherapy with the nitroimidazopyran PA-824 and first-line drugs in a murine model of tuberculosis. Antimicrob. Agents Chemother. 50:2621-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nuermberger, E., S. Tyagi, R. Tasneen, K. N. Williams, D. Almeida, I. Rosenthal, and J. H. Grosset.2008. Powerful bactericidal and sterilizing activity of a regimen containing PA-824, moxifloxacin, and pyrazinamide in a murine model of tuberculosis. Antimicrob. Agents Chemother. 52:1522-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nuermberger, E., S. Tyagi, K. N. Williams, I. Rosenthal, W. R. Bishai, and J. H. Grosset.2005. Rifapentine, moxifloxacin, or DNA vaccine improves treatment of latent tuberculosis in a mouse model. Am. J. Respir. Crit. Care Med. 172:1452-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Brien, R. J., and M. K. Spigelman.2005. New drugs for tuberculosis: current status and future prospects. Clin. Chest Med. 26:327-340. [DOI] [PubMed] [Google Scholar]
- 20.Sacchettini, J. C., E. J. Rubin, and J. S. Freundlich.2008. Drugs versus bugs: in pursuit of the persistent predator Mycobacterium tuberculosis. Nat. Rev. Microbiol. 6:41-52. [DOI] [PubMed] [Google Scholar]
- 21.Sacks, L. V., S. Pendle, D. Orlovic, M. Andre, M. Popara, G. Moore, L. Thonell, and S. Hurwitz.2001. Adjunctive salvage therapy with inhaled aminoglycosides for patients with persistent smear-positive pulmonary tuberculosis. Clin. Infect. Dis. 32:44-49. [DOI] [PubMed] [Google Scholar]
- 22.Snipes, M. B.1989. Long-term retention and clearance of particles inhaled by mammalian species. Crit. Rev. Toxicol. 20:175-211. [DOI] [PubMed] [Google Scholar]
- 23.Spigelman, M. K.2007. New tuberculosis therapeutics: a growing pipeline. J. Infect. Dis. 196:S28−S34. [DOI] [PubMed] [Google Scholar]
- 24.Stover, C. K., P. Warrener, D. R. VanDevanter, D. R. Sherman, T. M. Arain, M. H. Langhorne, S. W. Anderson, J. A. Towell, Y. Yuan, D. N. McMurray, B. N. Kreiswirth, C. E. Barry, and W. R. Baker.2000. A small-molecule nitroimidazopyran drug candidate for the treatment of tuberculosis. Nature 405:962. [DOI] [PubMed] [Google Scholar]
- 25.Sung, J. C., L. Garcia-Contreras, J. L. Verberkmoes, C. A. Peloquin, K. J. Elbert, A. J. Hickey, and D. A. Edwards.2009. Dry powder nitroimidazopyran antibiotic PA-824 aerosol for inhalation. Antimicrob. Agents Chemother. 53:1338-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tyagi, S., E. Nuermberger, T. Yoshimatsu, K. Williams, I. Rosenthal, N. Lounis, W. Bishai, and J. Grosset.2005. Bactericidal activity of the nitroimidazopyran PA-824 in a murine model of tuberculosis. Antimicrob. Agents Chemother. 49:2289-2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wiegeshaus, E. H., D. N. McMurray, A. A. Grover, G. E. Harding, and D. W. Smith.1970. Host-parasite relationships in experimental airborne tuberculosis. 3. Relevance of microbial enumeration to acquired resistance in guinea pigs. Am. Rev. Respir. Dis. 102:422-428. [DOI] [PubMed] [Google Scholar]
- 28.Williams, K. J., and K. Duncan.2007. Current strategies for identifying and validating targets for new treatment-shortening drugs for TB. Curr. Mol. Med. 7:297-307. [DOI] [PubMed] [Google Scholar]