Abstract
Attenuated activity of echinocandin antifungals at high concentrations, known as the “paradoxical effect,” is a well-established phenomenon in Candida albicans and Aspergillus fumigatus. In the yeast C. albicans, upregulation of chitin biosynthesis via the protein kinase C (PKC), high-osmolarity glycerol response (HOG), and Ca2+/calcineurin signaling pathways is an important cell wall stress response that permits growth in the presence of high concentrations of echinocandins. However, nothing is known of the molecular mechanisms regulating the mold A. fumigatus and its paradoxical response to echinocandins. Here, we show that the laboratory strain of A. fumigatus and five of seven clinical A. fumigatus isolates tested display various magnitudes of paradoxical growth in response to caspofungin. Interestingly, none of the eight strains showed paradoxical growth in the presence of micafungin or anidulafungin. Treatment of the ΔcnaA and ΔcrzA strains, harboring gene deletions of the calcineurin A subunit and the calcineurin-dependent transcription factor, respectively, with high concentrations of caspofungin revealed that the A. fumigatus paradoxical effect is calcineurin pathway dependent. Exploring a molecular role for CnaA in the compensatory chitin biosynthetic response, we found that caspofungin treatment resulted in increased chitin synthase gene expression, leading to a calcineurin-dependent increase in chitin synthase activity. Taken together, our data suggest a mechanistic role for A. fumigatus calcineurin signaling in the chitin biosynthetic response observed during paradoxical growth in the presence of high-dose caspofungin treatment.
The echinocandin antifungals inhibit 1,3-β-d-glucan synthesis, and all three currently available echinocandins (caspofungin, anidulafungin, and micafungin) have activity against both yeasts and molds. However, in vitro and in vivo studies with this antifungal class have revealed an interesting “paradoxical effect” on growth, evidenced by a recurrent increase in the growth of the fungal organism at drug concentrations above a certain threshold (36).
The paradoxical effect was first described for caspofungin treatment of Candida albicans and was not found to be due to mutation or increased expression of glucan synthase activity (30, 32). Further studies showed that this paradoxical effect on growth occurred in many Candida species, appearing in both planktonic and biofilm cells (22). Early efforts to elucidate a mechanism revealed increased chitin content in the cell wall upon caspofungin treatment (31). Initial studies with the Saccharomyces cerevisiae FKS1 deletion mutant, a strain defective in the 1,3-β-d-glucan synthase, showed that chitin biosynthesis is upregulated in response to glucan depletion (14). Furthermore, at the molecular level, several signal transduction pathways have been implicated in the regulation of the C. albicans paradoxical effect, including protein kinase C (PKC), high-osmolarity glycerol response (HOG), and calcineurin signaling events (25, 34, 35). Although it is well described, the paradoxical effect is known to be a species- and echinocandin-specific occurrence among Candida spp. (5, 12).
The echinocandins possess fungistatic activity against the mold Aspergillus fumigatus; however, little is known about the molecular mechanisms controlling the paradoxical effect in this organism. In contrast to what was observed in the yeast studies, the first evidence of a paradoxical effect in A. fumigatus came from in vivo studies. Wiederhold et al. found that the pulmonary fungal burden was greater in a murine model of invasive pulmonary aspergillosis in animals receiving the highest doses of caspofungin (37). However, recent in vivo data suggest that the A. fumigatus paradoxical effect may be echinocandin specific (18), and an in vitro XTT-based assay revealed a paradoxical increase in Aspergillus metabolic activity that appears to be species, strain, and echinocandin specific (3, 4).
In A. fumigatus, pharmacologic inhibition of calcineurin or deletion of calcineurin pathway components in combination with caspofungin treatment has been shown to attenuate growth and enhance cell wall damage (15, 29). Previously, we showed that echinocandin treatment of A. fumigatus produces an increase in cell wall chitin content and that the calcineurin pathway plays an important role in this response (13). Here, we sought to investigate the paradoxical effect in our laboratory strain compared to that observed in multiple clinical isolates and to further understand the mechanistic linkage of the calcineurin pathway to the regulation of chitin biosynthesis in response to caspofungin treatment.
MATERIALS AND METHODS
A. fumigatus strains and cell wall assay conditions.
The strains used in this study are listed in Table 1. All strains were maintained on glucose minimal medium (GMM) as previously described (28). To analyze cell wall composition changes in response to echinocandin treatment, 1 × 107 conidia/ml from each strain were inoculated into GMM broth and incubated for 48 h at 37°C in the presence or absence of the indicated concentration of drug. Hyphae were harvested, and quantification of cell wall chitin and 1,3-β-d-glucan was performed as previously described (13, 16). To visualize the in vitro paradoxical effect, 5,000 conidia from each strain were inoculated onto GMM agar impregnated with ascending doses of each echinocandin (0, 0.5, 1, 2, 4, and 8 μg/ml) and allowed to grow for 48 h at 37°C. The radial growth rate was calculated as the change in colony diameter observed between 24 and 48 h and was expressed as mm/h. Caspofungin, anidulafungin, and micafungin were dissolved and stored as previously described (13). Trifluoperazine (TFP) and FK506 were dissolved in sterile distilled water at 100 mM and 5 mg/ml, respectively, and stored at −20°C until use. Chitinase (C6137; Sigma) was resuspended in potassium phosphate buffer (200 mM potassium phosphate, 2 mM calcium chloride), pH 6.0, at 1 U/ml immediately before use.
TABLE 1.
Strains used in this study
| Strain | Genetic background | Source or reference |
|---|---|---|
| Af293 | Wild type | |
| ΔcnaA strain | Af293.1 | 28 |
| ΔcrzA strain | Af293.1 | 10 |
| Af165.86 | Wild type; clinical isolate | DUMRU Strain Repository |
| Af153.90 | Wild type; clinical isolate | DUMRU Strain Repository |
| Af168.95 | Wild type; clinical isolate | DUMRU Strain Repository |
| Af182.99 | Wild type; clinical isolate | DUMRU Strain Repository |
| Af119.00 | Wild type; clinical isolate | DUMRU Strain Repository |
| Af175.03 | Wild type; clinical isolate | DUMRU Strain Repository |
| Af182.03 | Wild type; clinical isolate | DUMRU Strain Repository |
Identification of putative CDRE sites and real-time RT-PCR for chitin synthase gene expression analysis.
Calcineurin-dependent response elements (CDREs) are promoter sequences that have been shown to bind to the calcineurin pathway transcription factor Crz1p in S. cerevisiae (23). These CDRE sequences were identified in each of the chitin synthase gene promoters through manual interpretation of the genomic sequence upstream of the gene coding region using the CDRE site consensus sequence GNGGC(G/T)CA (26, 38). For reverse transcription-PCR (RT-PCR) analysis, conidia from the wild-type (WT) and ΔcnaA strains were inoculated into GMM broth (1 × 107 conidia/ml) and incubated at 37°C for 48 h. After 48 h of growth, each culture was treated with caspofungin (4 μg/ml) alone or caspofungin (4 μg/ml) plus FK506 (200 ng/ml) for 90 min. Hyphae were then harvested by vacuum filtration, washed 5 times with sterile distilled water, and frozen under liquid nitrogen. Total RNA was extracted using Qiagen RNeasy columns (Qiagen, Inc.) and was treated with DNase I (Ambion). Approximately 500 ng of the RNA was subjected to first-strand cDNA synthesis (Bio-Line). Real-time PCR assays were performed with 20-μl reaction volumes that contained 1× iQ SYBR green master mix, 0.2 μM each primer, and 2 μl of a 1:5 dilution of the cDNA. In a separate experiment, GMM cultures of the wild-type and ΔcnaA strains were incubated as described above, followed by addition of caspofungin (4 μg/ml) for 4 h and incubation at 37°C at 250 rpm. RNA was extracted and RT-PCR performed as described above. The primers used for real-time PCR expression analysis are listed in Table 2. Real-time PCRs from each independent experiment were performed in triplicate, and the expression levels of all genes of interest were normalized to β-tubulin levels. Real-time PCRs were performed in biological triplicate and the data presented as the mean ± standard deviation (SD). The 2−ΔΔCt method of analysis was used to determine the changes in gene expression in each sample following treatment with caspofungin.
TABLE 2.
Oligonucleotides used in this study
| Gene | Primer sequence (5′-3′) |
|
|---|---|---|
| Forward | Reverse | |
| chsA | CTCGATCTTTGTCTCCTTGGG | TGTGATGTCGTGGGTGTTG |
| chsB | GCTCTCCACTGTCGGTCTCT | GGTCGTTGTTGATGGTGTTG |
| chsC | TTGCTGCGAGTTTGTATTCC | GCCAGTAGGATGCCAAAGAG |
| chsD | CAGAACACGATCCGAACAAC | GCTTCGCACCCAAGTAGAAC |
| chsE | TGGTGTTCGTTGACTTGCTC | TCATCCATCCAACCATTTCC |
| chsF | AACCTGCTTCTTCTGGGTGA | GAGCACGAGTTCCATGAGGT |
| chsG | AGGATGAGGGCAAAGAGGTT | AAGGCGTTGCTAAAGATCCA |
| chsEb | GCCATTACAGAAGCGATCCG | CCAACTCCTGGTCAATCTGG |
Preparation of membrane fractions.
A protocol for preparation of crude and microsomal membrane fractions was adapted from the method of Mellado et al. (21). Briefly, 1 × 107 conidia/ml from each strain were grown in GMM broth for 24 h at 37°C at 250 rpm. Caspofungin (4 μg/ml) alone or caspofungin (4 μg/ml) plus FK506 (200 ng/ml) was added to each culture and then incubated further for an additional 24 h. The resulting mycelia were harvested by vacuum filtration over sterile Miracloth (Calbiochem) and washed three times with sterile water. The wet mycelial mats were immediately stored on ice, followed by homogenization with a mortar and pestle under liquid nitrogen. The disrupted mycelia were then resuspended in 1 ml of extraction buffer (50 mM Tris-HCl [pH 7.5], 50 mM EDTA, and 1 mM phenylmethylsulfonyl fluoride [PMSF]) and centrifuged at 8,000 rpm at 4°C. Following centrifugation, the supernatants were removed and a portion was set aside as the crude extract. The remaining supernatant fraction was centrifuged at 100,000 × g for 40 min at 4°C. The pelleted membranes were then washed with 1 ml wash buffer (50 mM Tris-HCl [pH 7.5] and 5 mM magnesium acetate) and centrifuged as described above. After being washed, microsomal membrane fraction pellets were resuspended in 50 mM Tris-HCl [pH 7.5] plus 30% glycerol. Washed membrane fractions were solubilized by the addition of 1% digitonin and incubation at 30°C for 15 min. Solubilized membranes were stored at −80°C until further use. To quantify the total protein in solubilized membrane extracts, the Bradford assay was performed on each sample.
Chitin synthase activity assay.
A nonradioactive chitin synthase activity assay was adapted from the method of Lucero et al. (20). All components used in the assay were obtained and prepared as previously described (20). The assay is performed with a 96-well-plate format and is composed of 2 steps: preparation of the plate wells and performing of the assay.
(i) Preparation of 96-well plates (3595; Corning) coated with WGA.
Wheat germ agglutinin (WGA) stock solution (1 mg/ml WGA in 50 mM Tris-HCl, pH 7.5) was diluted to a concentration of 50 μg/ml in sterile distilled water. Next, 100 μl of the diluted WGA stock solution was added to each well and the plates were allowed to incubate at room temperature for 16 to 20 h. After incubation, the wells were emptied by vigorous shaking and washed 3 times by immersion in running distilled water, followed by vigorous shaking after each wash. After the wash, 30 μl of blocking buffer (20 mg/ml bovine serum albumin in 50 mM Tris-HCl, pH 7.5) was added to each well and the plates were incubated for 3 h at room temperature.
(ii) Performing the assay.
The plates were emptied of blocking buffer by vigorous shaking. Once the wells were emptied, 50 μl of 2× reaction mixture (32 mM Tris-HCl [pH 7.5], 4.3 mM magnesium acetate, 32 mM GlcNAc, 1.1 mM UDP-GlcNAc, and 2 μl of 2 mg/ml trypsin) was added to the appropriate wells. The total well volume was then brought to 100 μl/well by adding sterile distilled water and/or cell membrane fractions. Five micrograms of total protein was added to each well. The controls consisted of a 2× reaction mixture that contained no UDP-GlcNAc (for assaying the background absorbance) and a 2× reaction mixture containing no trypsin. A standard curve for chitin was prepared as previously described, using chitin dissolved in 1.7 M acetic acid (20). The plates were immediately sealed and incubated at room temperature for 90 min. After incubation, 20 μl of 50 mM EDTA was added to each well and the plates were slowly vortexed for 30 s. To detect the chitin produced during incubation and now adhered to the well, 100 μl of a 1-μg/ml WGA-horseradish peroxidase conjugate (diluted in blocking buffer) was added to each well and incubated at room temperature for 15 min. The plates were then emptied by vigorous shaking and washed 5 times by immersion in a large reservoir of running distilled water. After a wash to remove unbound chitin, 100 μl of tetramethylbenzidine (TMB) peroxidase reagent (32048; Thermo Scientific) was added to each well and the plates were immediately placed in a Multiskan Ascent plate reader. Absorbance values were obtained at 600 nm. Raw absorbance values were converted into chitin equivalents by comparison to the standard curve. Results are presented as the mean chitin concentration (μg/ml) detected in each well ± SD.
RESULTS
The A. fumigatus paradoxical effect is echinocandin and strain specific in an in vitro radial growth assay.
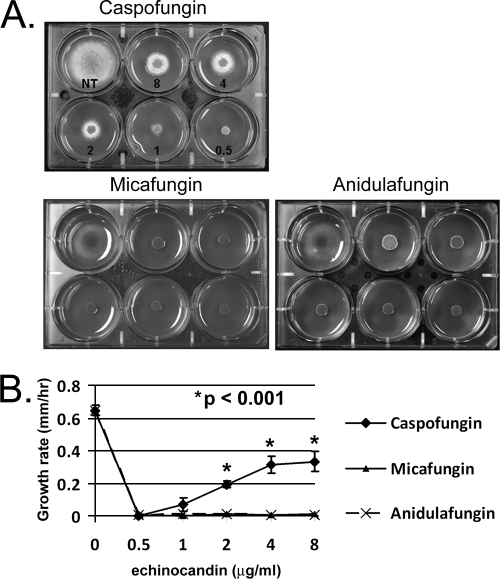
It is well documented that the paradoxical effect is an echinocandin- and species-specific response among Candida spp. (5, 12). The in vitro evidence supporting drug and strain specificity in A. fumigatus is derived from antifungal assays or a recently developed XTT-based assay utilizing metabolic activity as an end point (4). Here, we visualized the A. fumigatus paradoxical effect by employing the commercially available echinocandins (caspofungin, micafungin, and anidulafungin) in a radial growth assay employing GMM impregnated with a range of physiologically relevant drug concentrations (0.5 to 8 μg/ml). The wild-type laboratory stain (Af293) displayed a paradoxical increase in growth as the concentration of caspofungin increased (Fig. 1A and B). Af293 did not display the paradoxical increase in radial growth when inoculated at high concentrations of micafungin or anidulafungin, indicating an echinocandin-specific effect for this strain (Fig. 1A and B).
FIG. 1.
A. fumigatus exhibits paradoxical growth at high concentrations of caspofungin. (A) Comparison of A. fumigatus wild-type strain Af293 grown for 48 h at 37°C in the presence of 8, 4, 2, 1, and 0.5 μg/ml of caspofungin, micafungin, or anidulafungin. NT, no treatment. (B) Quantification of radial growth rates among wild-type cultures treated with caspofungin, micafungin, or anidulafungin. Results and error bars represent the means ± SD (mm/h) for biological triplicates.
In order to explore the additional possibility of a strain-specific paradoxical effect in A. fumigatus, we also analyzed the growth of seven additional A. fumigatus clinical isolates, from the Duke University Mycology Research Unit (DUMRU) Strain Repository, following treatment with caspofungin. Caspofungin was chosen for initial studies because the paradoxical effect was noted to occur in Af293. When they were grown in the presence of caspofungin (0.5 to 8 μg/ml), we noted the development of a paradoxical effect in five of the seven clinical isolates. Interestingly, the magnitudes of the paradoxical effect, as gauged by colony diameter, varied among these clinical isolates (data not shown). The remaining two clinical isolates displayed no paradoxical effect in the presence of caspofungin (data not shown). To test the echinocandin specificity among the clinical isolates, each isolate was then also treated with the same range (0.5 to 8 μg/ml) of micafungin and anidulafungin. No paradoxical increase in growth was noted to occur among the clinical isolates in response to treatment with either micafungin or anidulafungin. The A. fumigatus paradoxical effect is thus specific to caspofungin and to a subset of isolates, demonstrating both echinocandin and strain specificity.
The A. fumigatus paradoxical effect is controlled by the calcineurin signaling pathway.
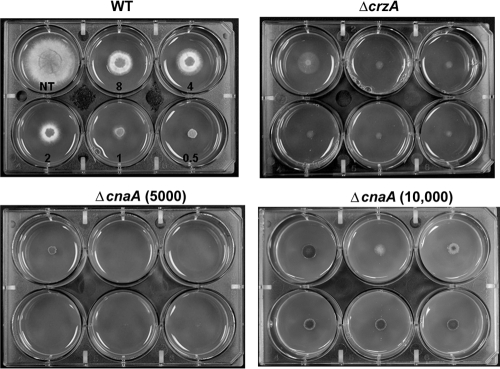
To explore the role of the calcineurin pathway, we tested the ΔcnaA mutant and ΔcrzA A. fumigatus strains, deleted for the calcineurin A catalytic subunit and the downstream calcineurin-responsive zinc finger transcription factor (10, 28), respectively, along with the wild-type parent strain, Af293. When grown on media containing increasing concentrations of caspofungin (0.5 to 8 μg/ml), Af293 displayed a paradoxical increase in radial growth as caspofungin treatment increased to 4 μg/ml (Fig. 2). No appreciable difference in colony diameter was noted to occur between 4 and 8 μg/ml caspofungin. In contrast, the ΔcnaA strain grew only slightly at 0.5 μg/ml caspofungin and showed no detectable growth at 1 μg/ml and greater (Fig. 2). Similar to the ΔcnaA strain, the ΔcrzA mutant displayed reduced growth at all caspofungin treatment levels, with no appreciable increase in colony diameter at higher levels of caspofungin (Fig. 2). The ΔcnaA and ΔcrzA mutants also displayed similar reductions in growth and lack of paradoxical effect when grown on micafungin or anidulafungin (data not shown).
FIG. 2.
The calcineurin pathway controls A. fumigatus paradoxical growth in response to caspofungin treatment. Comparison of wild-type (WT), ΔcnaA, and ΔcrzA strains grown for 48 h at 37°C in the presence of 8, 4, 2, 1, and 0.5 μg/ml of caspofungin. NT, no treatment. Growth of the ΔcnaA mutant was examined at two concentrations, 5,000 conidia (ΔcnaA 5,000) and 10,000 conidia (ΔcnaA 10,000) per inoculum, to clarify growth characteristics in response to caspofungin treatment.
Although the ΔcrzA mutant did not display increased growth at high caspofungin concentrations, it still showed some growth on caspofungin compared to the ΔcnaA mutant (Fig. 2). This led us to question whether the ΔcnaA mutant lacks a paradoxical effect simply because it cannot germinate at high caspofungin concentrations or because it has such limited radial outgrowth. To answer these questions, we first used a higher-level inoculum of the ΔcnaA mutant (10,000 conidia) and found that, similar to what was found for the ΔcrzA strain, limited growth with no paradoxical effect occurred at all concentrations of caspofungin tested (Fig. 2). These results indicated that the ΔcnaA mutant does not lack the paradoxical effect simply because the strain will not germinate in the presence of high caspofungin concentrations. Moreover, we found that the ΔrasA mutant, used in our earlier studies (13), displayed a paradoxical increase in growth at high caspofungin concentrations (data not shown). Since the ΔrasA mutant also displays severely restricted radial outgrowth on solid agar, limited radial outgrowth is likely not the reason for the lack of paradoxical growth in the calcineurin mutants. In addition, we did not find any increased sensitivity of the ΔcnaA and ΔcrzA mutants to the cell membrane antifungals amphotericin B and voriconazole, indicating that the effects seen are specific to the cell wall antifungal caspofungin (data not shown). Taken together, these data indicate that the lack of paradoxical growth seen in the calcineurin pathway mutants is an outcome directly related to cell wall stress induced via caspofungin treatment and calcineurin pathway mutation.
To provide evidence that the role of the calcineurin pathway was not specific to our laboratory strain, the five clinical isolates identified in this study as displaying the paradoxical effect were cotreated with FK506, a pharmacological inhibitor of calcineurin, and caspofungin in our in vitro assay. Cotreatment of Af293 and the clinical isolates with these two compounds removed the paradoxical effect for all strains tested (data not shown). The paradoxical effect due to caspofungin is controlled by the calcineurin pathway, and this role for calcineurin is common among most wild-type isolates, despite the paradoxical effect being strain and echinocandin specific.
Caspofungin-mediated transcriptional upregulation of chitin synthase genes is controlled by the calcineurin pathway.
In a previous study, we showed that an intact calcineurin pathway is necessary for a compensatory increase in cell wall chitin in response to treatment with 1 μg/ml echinocandin (13). In C. albicans, the calcineurin pathway is required for upregulation of chitin synthase gene expression in response to cell wall damage (25). In yeast, calcineurin induces transcriptional changes through activation of Crz1p, which, in turn, binds to conserved calcineurin-dependent response element (CDRE) sequences in gene promoter regions, inducing transcription (38). By manual analysis of the genomic sequences upstream of the A. fumigatus chitin synthase genes, we identified at least one putative CDRE in each of the eight chitin synthase genes (Table 3). We hypothesized that the major role of CnaA in relation to the paradoxical effect is upregulation of chitin biosynthesis via transcriptional control of chitin synthase genes. To test this hypothesis, we examined the impact of caspofungin treatment and calcineurin signaling on transcriptional regulation of chitin synthase genes in A. fumigatus by employment of real-time PCR analysis.
TABLE 3.
Putative CDRE sequences in chitin synthase promoters
| Gene | Gene product | A. fumigatus genome locus | Putative CDRE site(s) |
|---|---|---|---|
| chsA | Chitin synthase A | Afu2g01870 | 1885-AGGCTC-1880; 1677-GAGGCGGT-1670 |
| chsB | Chitin synthase B | Afu4g04180 | 1330-AGCCTC-1324; 1522-GAGGCTGA-1514; 116-GAGGCTGA-109 |
| chsC | Chitin synthase C | Afu5g00760 | 1905-AGGCTG-1900; 1350-GAGGCGTCA-1343; 346-AGGCTG-341 |
| chsD | Chitin synthase D | Afu1g12600 | 1687-AGCCTC-1682 |
| chsE | Chitin synthase E | Afu2g13440 | 142-GAGGCGCT-135 |
| chsF | Chitin synthase F | Afu8g05630 | 1089-GAGGCT-1084 |
| chsG | Chitin synthase G | Afu3g14420 | 728-AGCCTC-723; 955-GAGGCGCT-948; 709-AGGCTC-704; 649-AGGCTC-644 |
| chsEb | Chitin synthase Eb | Afu2g13430 | 584-GAGGCTG-577; 722-CAGGCTA-715 |
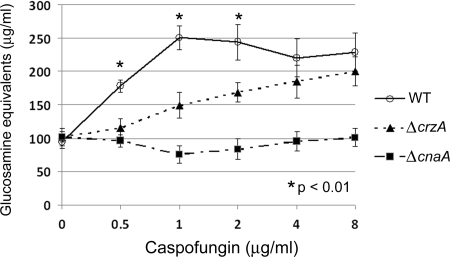
Treatment with caspofungin concentrations up to 1 μg/ml caused dose-dependent increases in total chitin content in the wild-type strain, confirming our earlier results (Fig. 3). However, treatment with higher concentrations of caspofungin (2, 4, and 8 μg/ml) did not result in further increases in chitin content. These data are consistent with studies employing C. glabrata, showing that higher concentrations of caspofungin did not lead to further increases in chitin content (9). The cell wall chitin content levels were statistically lower for the ΔcrzA and ΔcnaA strains than for the wild-type strain following treatment with caspofungin at 0.5, 1, and 2 μg/ml, and the chitin content levels remained statistically lower for the ΔcnaA strain at 4 and 8 μg/ml (Fig. 3). Compared to the level for the nontreated control, the chitin content levels in the ΔcrzA mutant were increased following treatment with 2 μg/ml (P < 0.05), 4 μg/ml (P < 0.005), and 8 μg/ml (P < 0.005) caspofungin. There was no change in chitin content in the ΔcnaA strain at any dose of caspofungin. In addition, the 1,3-β-d-glucan content levels were decreased for all strains after caspofungin treatment, with no significant difference in reduction levels between the strains (data not shown). These data suggest that caspofungin treatment results in increased chitin biosynthesis over a broad range of drug concentrations and that the calcineurin pathway is required for this response.
FIG. 3.
Chitin content increases in response to ascending doses of caspofungin. Analysis of total chitin content in the WT (○), ΔcnaA (▪), and ΔcrzA (▴) strains in response to treatment with 0.5, 1, 2, 4, and 8 μg/ml caspofungin. All analyses were performed in biological triplicate for statistical analysis. Results and error bars represent the means ± SD for glucosamine equivalents. NT, no treatment.
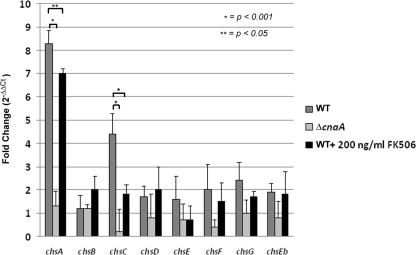
To explore roles for caspofungin treatment and calcineurin pathway activity in transcriptional regulation of chitin synthase genes, quantitative PCR was then used to measure the relative transcriptional levels of each of the eight A. fumigatus chitin synthase genes from the wild-type and ΔcnaA strains following treatment with caspofungin. Conidia from the wild-type and ΔcnaA strains were incubated in GMM liquid medium and cultures subsequently shocked with caspofungin (4 μg/ml) for 90 min. Compared with the ΔcnaA mutant strain, the wild-type strain displayed a statistically significant increase in expression of chsA (8.4-fold ± 0.5-fold increase) and chsC (4.9-fold ± 0.4-fold increase) upon caspofungin treatment (Fig. 4). Although not statistically significant, the expression levels of chsD, chsE, chsF, and chsG trended higher in the wild-type strain than in the ΔcnaA mutant (Fig. 4). Moreover, cotreatment of the wild-type strain with both caspofungin (4 μg/ml) and FK506 (200 ng/ml) resulted in diminished transcriptional responses to caspofungin treatment for both chsA (∼1.5-fold decrease compared to the level for caspofungin alone) and chsC (∼2.5-fold decrease compared to the level for caspofungin alone) (Fig. 4). These results indicated that caspofungin treatment leads to a calcineurin-dependent upregulation of at least two of the eight A. fumigatus chitin synthase genes, chsA and chsC. To examine the temporal nature of the chitin synthase transcriptional response, a second experiment was performed, in which the wild-type and ΔcnaA strains were grown in GMM for 48 h, followed by caspofungin (4 μg/ml) shock for 4 h. After the cultures were exposed for this increased time frame, real-time PCR analysis of each of the chitin synthase genes showed only minor differences (<2-fold change) in transcript levels, and these changes were independent of calcineurin (data not shown). These results suggest that the calcineurin-controlled transcriptional upregulation of chsA and chsC in response to caspofungin treatment is an early and transient event. In addition, these data suggest that the calcineurin pathway controls the transcription of these chitin synthases, leading to upregulation of chitin biosynthesis, presumably as a compensatory response to 1,3-β-d-glucan synthesis inhibition.
FIG. 4.
Calcineurin controls transcriptional upregulation of chsA and chsC in response to caspofungin treatment. Transcriptional profile of the A. fumigatus chitin synthase genes in response to treatment with caspofungin (4 μg/ml) alone for the WT (dark gray bars) and ΔcnaA (light gray bars) strains or cotreatment with caspofungin (4 μg/ml) and FK506 (200 ng/ml) for the WT (black bars). Results are presented as the mean fold change (2−ΔΔCt) ± SD. All experiments were performed in biological triplicate for statistical analysis.
Calcineurin regulates increased chitin synthase activity in response to caspofungin.
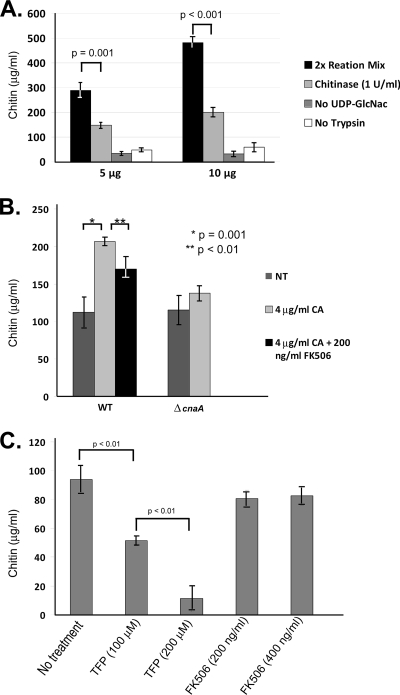
To verify that the calcineurin-dependent upregulation of chitin synthase gene products resulted in increased chitin synthase activity, we adapted a nonradioactive chitin synthase activity assay originally developed for S. cerevisiae for use with A. fumigatus (20). The S. cerevisiae chitin synthase activity values obtained using this nonradioactive assay were found to be slightly higher than, yet in similar proportion to, the results achieved with the conventional radioactive assay (20). This assay utilizes WGA-coated wells to capture chitin produced during incubation of cell membrane extracts in a reaction buffer containing the essential components for chitin synthesis. When employed in this assay, microsomal membrane extracts from the wild-type organism displayed chitin synthase activity levels that were dependent on the amount of protein added to the reaction mixture (5 μg total protein represents 289 ± 31.0 μg/ml chitin produced in 90 min; 10 μg total protein represents 481 ± 40.4 μg/ml chitin produced in 90 min) (Fig. 5A). In addition, detection of chitin synthesis in this assay was dependent on the presence of the chitin biosynthesis substrate UDP-GlcNAc and the presence of trypsin (for chitin synthase enzyme activation), as previously described (20) (Fig. 5A). To verify that neo-synthesized chitin is responsible for changes in signal output, chitinase (1 U/ml) was added to sample wells after the chitin synthesis reaction and allowed to incubate. This step was performed prior to detection of the end product chitin. While the entire signal could not be degraded, sample wells treated with chitinase showed significantly decreased chitin levels (∼54%) compared to those for the untreated samples (Fig. 5A). Therefore, only 54% of the original signal output could be contributed to neo-synthesized chitin, and all further chitin synthase activity values were normalized to this level. Taken together, these data confirmed that the nonradioactive assay is a reliable method for in vitro detection of chitin synthase activity in A. fumigatus.
FIG. 5.
Chitin synthase activity increases in response to caspofungin in a calcineurin-dependent manner. (A) The reproducibility of the nonradioactive chitin synthase assay was verified by analysis of total chitin synthase activity in increasing amounts of starting protein (5 and 10 μg). Results were calculated as stated in Materials and Methods and are presented as the mean ± SD for total chitin (μg/ml) produced during the 90-min incubation. Total chitin synthesis in complete reaction mixture (black bars), total chitin remaining after a 5-h treatment with chitinase (1 U/ml) at room temperature (light gray bars), chitin synthesis in reaction mixture lacking UDP-GlcNAc substrate (dark gray bars), and chitin synthesis in reaction mixture lacking trypsin for activation (white bars) are shown. Experiments were performed in biological triplicate for statistical analysis. (B) Comparison of the total chitin synthase activity of the wild-type (WT) and ΔcnaA strains after exposure to either 4 μg/ml caspofungin alone (gray bars) to that of the WT strain after cotreatment with 4 μg/ml caspofungin and 200 nM FK506 (black bar). Results and error bars represent the means ± SD for chitin (μg/ml). (C) Comparison of total chitin synthase activity in WT microsomal membrane extracts pretreated with TFP (100 or 200 μM) or FK506 (200 or 400 μg/ml) for 30 min at room temperature. Results and error bars represent the means ± SD for chitin (μg/ml). All experiments were performed in biological triplicate for statistical analysis.
Microsomal membrane extracts from wild-type and ΔcnaA strains treated with caspofungin (4 μg/ml) alone, as well as wild-type cultures cotreated with caspofungin (4 μg/ml) and FK506 (200 ng/ml), were tested in this assay to detect changes in total chitin synthase activity. The wild-type strain displayed a significant increase in chitin synthase activity when treated with caspofungin (206 ± 5.8 μg/ml chitin), compared to the level for the nontreated samples (112 ± 20.7 μg/ml chitin) (Fig. 5B). These findings correlated with the increase in total chitin content. Membrane fractions from untreated samples of the ΔcnaA strain displayed no significant change in chitin synthase activity (WT, 112 ± 20.7 μg/ml chitin; ΔcnaA, 115 ± 20.1 μg/ml chitin) and only a slight, statistically nonsignificant increase (137 ± 10.7 μg/ml chitin) after caspofungin treatment (Fig. 5B). Caspofungin treatment caused an increase in total chitin synthase activity that is dependent on the presence of calcineurin.
In Neurospora crassa, inhibition of calmodulin, a calcineurin pathway component, inhibits regeneration of protoplast cell walls and produces a dramatic decrease in chitin synthase activity in vitro (33). To see if components of the A. fumigatus calcineurin pathway could be pharmacologically inhibited in microsomal extracts to directly affect chitin synthase enzyme activity, FK506, a specific inhibitor of calcineurin activity, and trifluoperazine (TFP), a calmodulin inhibitor, were employed. Pretreatment of microsomal extracts from Af293 for 30 min with TFP (100 or 200 μM) resulted in a dose-dependent decrease in chitin synthase activity in the microsomal compartment (Fig. 5C). Microsomal extracts pretreated with FK506 (200 or 400 μg/ml) consistently displayed slight decreases in chitin synthase activity at each concentration (81 ± 6.2 μg/ml chitin or 83 ± 6.5 μg/ml chitin, respectively), but these changes were not found to be statistically significant or dose dependent (Fig. 5C). Interestingly, pretreatment of membranes with increasing concentrations of nikkomycin Z (1, 2, or 4 μg/ml), a specific inhibitor of chitin synthase activity, produced decreases in chitin synthase enzyme activity that mirrored the outcomes obtained with FK506 treatment. At all concentrations of nikkomycin Z, total chitin synthase enzyme activity was consistently decreased compared to that observed for nontreated extracts, but these changes were not statistically significant (data not shown).
DISCUSSION
Paradoxical growth of Aspergillus spp. and Candida spp. in the presence of high echinocandin concentrations is a well-documented phenomenon with potentially important clinical implications. Here, we show that the paradoxical effect of A. fumigatus is specific to the echinocandin and the clinical strains. These data match findings by Antachopolis et al. showing that the paradoxical effect of Aspergillus spp., as measured by metabolic output, is species, strain, and echinocandin specific (4). Our data also show that, among the A. fumigatus isolates tested, a paradoxical increase in growth at high echinocandin concentrations is seen only in response to treatment with caspofungin. These findings are also in accordance with previously published results indicating that the paradoxical effect on growth with Aspergillus species is more commonly seen with caspofungin than with micafungin or anidulafungin (3). However, it is interesting to note that increasing the doses of these drugs does not further inhibit the growth of the wild-type organism. That is, the wild-type strain is equally inhibited by treatment with 0.5 μg/ml and 8 μg/ml micafungin or anidulafungin. Moreover, we previously reported that chitin biosynthesis in the wild type is upregulated in response to treatment with any one of the echinocandins, not just caspofungin, and that the ΔcnaA mutant is equally sensitive to each of these cell wall antifungals (13). Since paradoxical growth recovery was seen only in the wild-type strain with caspofungin treatment and high concentrations of caspofungin treatment reduced growth in the ΔcnaA mutant (Fig. 2), calcineurin-mediated upregulation of chitin biosynthesis in response to echinocandin treatment may be important for survival whereas another mechanism is employed to permit radial growth recovery in the presence of caspofungin. In further support of this hypothesis, attempts to use CaCl2-supplemented medium to induce calcineurin activity were not successful in overcoming the lack of paradoxical growth in the wild-type organism treated with anidulafungin or micafungin (data not shown).
Although the paradoxical increases in growth obtained at high concentrations of caspofungin are well documented for C. albicans and A. fumigatus, significant work toward delineation of the controlling mechanisms has been performed only for C. albicans. Nothing is known of the molecular mechanism controlling the paradoxical effect in Aspergillus species. The previously published work strongly suggests that increased chitin biosynthesis is an essential component of the cell wall stress response in C. albicans and S. cerevisiae (14, 25, 31, 34). The filamentous fungi contain more chitin synthase genes than yeast species, and their functional interplay and redundancy during growth and stress are of interest (8). We previously showed that the calcineurin pathway is required for a compensatory chitin content increase in response to echinocandin treatment (13), implying that a similar stress response may exist in filamentous fungi. Here, we show that chitin biosynthesis, as measured by total chitin content, is increased over a range of ascending doses of caspofungin and that functional CnaA is important for this response. We hypothesized that CnaA is essential to the transcriptional and/or posttranslational control of chitin synthases in A. fumigatus.
Initial work with the ΔcnaA strain in our laboratory suggested that calcineurin did not play a major role in the regulation of basal transcriptional levels of chitin synthase genes (10). However, our data presented here indicate that regulation of transcriptional levels of chitin synthase genes in response to caspofungin is controlled, at least in part, by the calcineurin pathway. This is supported by the lack of upregulation of chsA and chsC in the ΔcnaA mutant and the decrease in upregulation induced by addition of FK506, a specific calcineurin inhibitor. Calcineurin pathway proteins have been shown to regulate chitin synthase expression levels in other fungal species, including C. albicans (17, 25), Magnaporthe oryzae (7), and Aspergillus nidulans (27). Lenardon et al. recently published a study dissecting the promoter sequences of the two class I chitin synthases in C. albicans (17). Their study showed that putative CDRE promoter elements, comprising a sequence known to be associated with binding to calcineurin-dependent effector proteins, were not utilized for chitin synthase transcriptional regulation when cells were stressed with CaCl2, calcofluor white, or sorbitol. Instead, the calcineurin pathway was found to control chitin synthase gene expression in response to cell wall damage through an alternative, indirect mechanism. Whether the A. fumigatus CnaA protein directly activates its downstream transcription factor, CrzA, to induce increased transcription of chsA and chsC via the putative CDRE sites, identified in this study, is a focus of future research.
We cannot rule out the possibility that the A. fumigatus calcineurin pathway may also play roles in the transcriptional regulation of other gene subsets essential to survival in the presence of caspofungin. In support of this, we show that the CrzA transcription factor is necessary for development of the paradoxical effect but that phenotypes derived from deletion of the crzA gene are not as severe as those gained from cnaA mutation. Several studies published on the transcriptional response to caspofungin in S. cerevisiae, C. albicans, and A. niger found that a variety of gene families are upregulated upon treatment. These functional categories include carbohydrate and lipid metabolism genes, protein synthesis genes, and even a smaller subset of genes involved in energy generation (1, 19, 24). Interestingly, Chamilos et al. have shown that caspofungin susceptibility in strains of C. parapsilosis with high caspofungin MICs can be enhanced by simultaneous inhibition of cellular respiration (6). This study could suggest that regulation of mitochondrial respiratory pathways may be an important cellular response to caspofungin treatment. Since calcineurin has been implicated in mitochondrial dynamics in mammalian neuronal cells (2), it is tempting to speculate about a similar link between mitochondrial respiration and CnaA function in A. fumigatus.
To verify that the calcineurin-dependent transcriptional upregulation of chsA and chsC produced increased chitin synthase activity, we showed for the first time that a nonradioactive-chitin-synthase-activity assay was adaptable for reliable detection of total chitin synthase activity in A. fumigatus. Treatment of A. fumigatus with caspofungin (4 μg/ml) caused a calcineurin-dependent increase in chitin synthase activity with the use of this assay. In addition, cotreatment of the wild-type strain with caspofungin and FK506 produced less chitin synthase activity than caspofungin treatment alone, indicating that calcineurin activity is important for this potentially protective outcome in A. fumigatus.
The regulation of chitin synthase activity by Ca2+ and calcineurin pathway components has been explored for a limited number of fungal species. For example, calmodulin, a regulator of calcineurin signaling, has been implicated in the proper activation of chitin synthase activity in Neurospora crassa (33). In addition, upregulation of chitin synthase activity in response to osmotic pressure can be attenuated by blockade of Ca2+ channels in the dimorphic fungus Benjaminiella poitrasii (11). Our in vitro data suggest that activity of calcineurin and calmodulin is important for wild-type levels of chitin synthase activity in A. fumigatus. Treatment of A. fumigatus Af293 microsomal extracts with the anticalmodulin agent TFP (200 μM) resulted in almost complete inhibition of chitin synthase activity. Interestingly, inhibition of calcineurin with FK506 at 20 times the concentration typically used in vivo resulted in only a minimal decrease in chitin synthase activity. Since treatment of the wild-type strain with FK506 in culture led to decreased chitin synthase gene expression and chitin synthase activity, FK506 is likely not as efficient at inhibiting calcineurin activity in the microsomal extract. Combined with the results derived from treatment of A. fumigatus hyphae with FK506 in culture, our in vitro data suggest that both calmodulin and calcineurin play direct and indirect roles in induction of chitin synthase enzyme activity in response to caspofungin.
In conclusion, the work presented here supports a mechanistic role for calcineurin in the development of the paradoxical effect of A. fumigatus. This role likely involves the calmodulin-mediated activation of CnaA in response to cell wall damage following caspofungin treatment. Activated CnaA can then dephosphorylate its transcription factor, CrzA, which, in turn, induces transcription of chsA and chsC. These chitin synthases may be responsible for the chitin biosynthetic response to 1,3-β-d-glucan synthase inhibition. Although our data support this model, we cannot rule out the possibility that calcineurin may posttranslationally control other proteins important for chitin biosynthesis, including the chitin synthases themselves, via dephosphorylation. In fact, the increased chitin content observed in the ΔcrzA mutant at high caspofungin concentrations supports the possibility of an alternative mechanism for CnaA function in chitin biosynthesis (Fig. 3). Our data also suggest a conserved role for the calcineurin pathway in chitin biosynthesis regulation in response to cell wall damage in both yeasts and filamentous fungi alike. Our preliminary results indicate the need for further studies elucidating the specific molecular mechanisms of the calcineurin pathway, including possible calcineurin-independent calmodulin mechanisms, in response to echinocandin treatment. A deeper understanding of these mechanisms could lead to even greater therapeutic benefits from echinocandin treatment.
Acknowledgments
This work was supported by a K08 award (A1061149) to W.J.S., a Basic Science Faculty Development grant from the American Society for Transplantation, a Children's Miracle Network grant, an Astellas Investigator-Initiated Trial Award, and the Molecular Mycology and Pathogenesis Training Program grant at Duke University (5T32-AI052080) to J.R.F.
Footnotes
Published ahead of print on 1 February 2010.
REFERENCES
- 1.Agarwal, A. K., P. D. Rogers, S. R. Baerson, M. R. Jacob, K. S. Barker, J. D. Cleary, L. A. Walker, D. G. Nagle, and A. M. Clark.2003. Genome-wide expression profiling of the response to polyene, pyrimidine, azole, and echinocandin antifungal agents in Saccharomyces cerevisiae. J. Biol. Chem. 278:34998-35015. [DOI] [PubMed] [Google Scholar]
- 2.Ankarcrona, M., J. M. Dypbukt, S. Orrenius, and P. Nicotera.1996. Calcineurin and mitochondrial function in glutamate-induced neuronal cell death. FEBS Lett. 394:321-324. [DOI] [PubMed] [Google Scholar]
- 3.Antachopoulos, C., J. Meletiadis, T. Sein, E. Roilides, and T. J. Walsh.2008. Comparative in vitro pharmacodynamics of caspofungin, micafungin, and anidulafungin against germinated and nongerminated Aspergillus conidia. Antimicrob. Agents Chemother. 52:321-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antachopoulos, C., J. Meletiadis, T. Sein, E. Roilides, and T. J. Walsh.2007. Concentration-dependent effects of caspofungin on the metabolic activity of Aspergillus species. Antimicrob. Agents Chemother. 51:881-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chamilos, G., R. E. Lewis, N. Albert, and D. P. Kontoyiannis.2007. Paradoxical effect of echinocandins across Candida species in vitro: evidence for echinocandin-specific and Candida species-related differences. Antimicrob. Agents Chemother. 51:2257-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chamilos, G., R. E. Lewis, and D. P. Kontoyiannis.2006. Inhibition of Candida parapsilosis mitochondrial respiratory pathways enhances susceptibility to caspofungin. Antimicrob. Agents Chemother. 50:744-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi, J., Y. Kim, S. Kim, J. Park, and Y.-H. Lee.2009. MoCRZ1, a gene encoding a calcineurin-responsive transcription factor, regulates fungal growth and pathogenicity of Magneporthe oryzae. Fungal Genet. Biol. 46:243-254. [DOI] [PubMed] [Google Scholar]
- 8.Choquer, M., M. Boccara, I. R. Gonçalves, M.-C. Soulié, and A. Vidal-Cros.2004. Survey of the Botrytis cinerea chitin synthase multigenic family through the analysis of six euascomycetes genomes. Eur. J. Biochem. 271:2153-2164. [DOI] [PubMed] [Google Scholar]
- 9.Cota, J. M., J. L. Grabinski, R. L. Talbert, D. S. Burgess, P. D. Rogers, T. D. Edlind, and N. P. Wiederhold.2008. Increases in SLT2 expression and chitin content are associated with incomplete killing of Candida glabrata by caspofungin. Antimicrob. Agents Chemother. 52:1144-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cramer, R. A., Jr., B. Z. Perfect, N. Pinchai, S. Park, D. S. Perlin, Y. G. Asfaw, J. Heitman, J. R. Perfect, and W. J. Steinbach.2008. The calcineurin target CrzA regulates conidial germination, hyphal growth and pathogenesis of Aspergillus fumigatus. Eukaryot. Cell 7:1085-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deshpande, M. V., R. O'Donnell, and G. W. Gooday.1997. Regulation of chitin synthase activity in the dimorphic fungus Benjaminiella poitrasii by external osmotic pressure. FEMS Microbiol. Lett. 152:327-332. [DOI] [PubMed] [Google Scholar]
- 12.Fleischhacker, M., C. Radecke, B. Schulz, and M. Ruhnke.2008. Paradoxical growth effects of the echinocandins caspofungin and micafungin, but not of anidulafungin, on clinical isolates of Candida albicans and C. dubliniensis. Eur. J. Clin. Microbiol. Infect. Dis. 27:127-131. [DOI] [PubMed] [Google Scholar]
- 13.Fortwendel, J. R., P. R. Juvvadi, N. Pinchai, B. Z. Perfect, J. A. Alspaugh, J. R. Perfect, and W. J. Steinbach.2009. Differential effects of inhibiting chitin and 1,3-{beta}-D-glucan synthesis in Ras and calcineurin mutants of Aspergillus fumigatus. Antimicrob. Agents Chemother. 53:476-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Rodriguez, L. J., J. A. Trilla, C. Castro, M. H. Valdivieso, A. Duran, and C. Roncero.2000. Characterization of the chitin biosynthesis process as a compensatory mechanism in the fks1 mutant of Saccharomyces cerevisiae. FEBS Lett. 478:84-88. [DOI] [PubMed] [Google Scholar]
- 15.Kontoyiannis, D., R. Lewis, N. Osherov, N. Albert, and G. May.2003. Combination of caspofungin with inhibitors of the calcineurin pathway attenuates growth in vitro in Aspergillus species. J. Antimicrob. Chemother. 51:313-316. [DOI] [PubMed] [Google Scholar]
- 16.Lehmann, P. F., and L. O. White.1975. Chitin assay used to demonstrate renal localization and cortisone-enhanced growth of Aspergillus fumigatus mycelium in mice. Infect. Immun. 12:987-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lenardon, M. D., I. Lesiak, C. A. Munro, and N. A. R. Gow.2009. Dissection of the Candida albicans class I chitin synthase promoters. Mol. Genet. Genomics 281:459-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewis, R. E., N. D. Albert, and D. P. Kontoyiannis.2008. Comparison of the dose-dependent activity and paradoxical effect of caspofungin and micafungin in a neutropenic murine model of invasive pulmonary aspergillosis. J. Antimicrob. Chemother. 61:1140-1144. [DOI] [PubMed] [Google Scholar]
- 19.Liu, T. T., R. E. B. Lee, K. S. Barker, R. E. Lee, L. Wei, R. Homayouni, and P. D. Rogers.2005. Genome-wide expression profiling of the response to azole, polyene, echinocandin, and pyrimidine antifungal agents in Candida albicans. Antimicrob. Agents Chemother. 49:2226-2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lucero, H. A., M. J. Kuranda, and D. A. Bulik.2002. A nonradioactive, high throughput assay for chitin synthase activity. Anal. Biochem. 305:97-105. [DOI] [PubMed] [Google Scholar]
- 21.Mellado, E., G. Dubreucq, P. Mol, J. Sarfati, S. Paris, M. Diaquin, D. W. Holden, J. L. Rodriguez-Tudela, and J. P. Latge.2003. Cell wall biogenesis in a double chitin synthase mutant (chsG−/chsE−) of Aspergillus fumigatus. Fungal Genet. Biol. 38:98-109. [DOI] [PubMed] [Google Scholar]
- 22.Melo, A. S., A. L. Colombo, and B. A. Arthington-Skaggs.2007. Paradoxical growth effect of caspofungin observed on biofilms and planktonic cells of five different Candida species. Antimicrob. Agents Chemother. 51:3081-3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mendizabal, I., A. Pascual-Ahuir, R. Serrano, and I. F. de Larrinoa.2001. Promoter sequences regulated by the calcineurin-activated transcription factor Crz1 in the yeast ENA1 gene. Mol. Genet. Genomics 265:801-811. [DOI] [PubMed] [Google Scholar]
- 24.Meyer, V., R. A. Damveld, M. Arentshorst, U. Stahl, C. A. M. J. J. van den Hondel, and A. F. J. Ram.2007. Survival in the Presence of Antifungals: genome-wide expression profiling of Aspergillus niger in response to sublethal concentrations of caspofungin and fenpropimorph. J. Biol. Chem. 282:32935-32948. [DOI] [PubMed] [Google Scholar]
- 25.Munro, C. A., S. Selvaggini, I. de Bruijn, L. Walker, M. D. Lenardon, B. Gerssen, S. Milne, A. J. Brown, and N. A. Gow.2007. The PKC, HOG and Ca2+ signalling pathways co-ordinately regulate chitin synthesis in Candida albicans. Mol. Microbiol. 63:1399-1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruiz, A., R. Serrano, and J. Arino.2008. Direct regulation of genes involved in glucose utilization by the calcium/calcineurin pathway. J. Biol. Chem. 283:13923-13933. [DOI] [PubMed] [Google Scholar]
- 27.Spielvogel, A., H. Findon, H. N. Arst, L. Araujo-Bazan, P. Hernandez-Ortiz, U. Stahl, V. Meyer, and E. A. Espeso.2008. Two zinc finger transcription factors, CrzA and SltA, are involved in cation homeostasis and detoxification in Aspergillus nidulans. Biochem. J. 414:419-429. [DOI] [PubMed] [Google Scholar]
- 28.Steinbach, W. J., R. A. Cramer, Jr., B. Z. Perfect, Y. G. Asfaw, T. C. Sauer, L. K. Najvar, W. R. Kirkpatrick, T. F. Patterson, D. K. Benjamin, Jr., J. Heitman, and J. R. Perfect.2006. Calcineurin controls growth, morphology, and pathogenicity in Aspergillus fumigatus. Eukaryot. Cell 5:1091-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steinbach, W. J., R. A. Cramer, Jr., B. Z. Perfect, C. Henn, K. Nielsen, J. Heitman, and J. R. Perfect.2007. Calcineurin inhibition or mutation enhances cell wall inhibitors against Aspergillus fumigatus. Antimicrob. Agents Chemother. 51:2979-2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stevens, D. A., M. Espiritu, and R. Parmar.2004. Paradoxical effect of caspofungin: reduced activity against Candida albicans at high drug concentrations. Antimicrob. Agents Chemother. 48:3407-3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stevens, D. A., M. Ichinomiya, Y. Koshi, and H. Horiuchi.2006. Escape of Candida from caspofungin inhibition at concentrations above the MIC (paradoxical effect) accomplished by increased cell wall chitin; evidence for beta-1,6-glucan synthesis inhibition by caspofungin. Antimicrob. Agents Chemother. 50:3160-3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stevens, D. A., T. C. White, D. S. Perlin, and C. P. Selitrennikoff.2005. Studies of the paradoxical effect of caspofungin at high drug concentrations. Diagn. Microbiol. Infect. Dis. 51:173-178. [DOI] [PubMed] [Google Scholar]
- 33.Suresh, K., and C. Subramanyam.1997. A putative role for calmodulin in the activation of Neurospora crassa chitin synthase. FEMS Microbiol. Lett. 150:95-100. [DOI] [PubMed] [Google Scholar]
- 34.Walker, L. A., C. A. Munro, I. de Bruijn, M. D. Lenardon, A. McKinnon, and N. A. Gow.2008. Stimulation of chitin synthesis rescues Candida albicans from echinocandins. PLoS Pathog. 4:e1000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wiederhold, N., D. Kontoyiannis, R. Prince, and R. Lewis.2005. Attenuation of the activity of caspofungin at high concentrations against Candida albicans: possible role of cell wall integrity and calcineurin pathways. Antimicrob. Agents Chemother. 49:5146-5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wiederhold, N. P.2007. Attenuation of echinocandin activity at elevated concentrations: a review of the paradoxical effect. Curr. Opin. Infect. Dis. 20:574-578. [DOI] [PubMed] [Google Scholar]
- 37.Wiederhold, N. P., D. P. Kontoyiannis, J. Chi, R. A. Prince, V. H. Tam, and R. E. Lewis.2004. Pharmacodynamics of caspofungin in a murine model of invasive pulmonary aspergillosis: evidence of concentration-dependent activity. J. Infect. Dis. 190:1464-1471. [DOI] [PubMed] [Google Scholar]
- 38.Yoshimoto, H., K. Saltsman, A. P. Gasch, H. X. Li, N. Ogawa, D. Botstein, P. O. Brown, and M. S. Cyert.2002. Genome-wide analysis of gene expression regulated by the calcineurin/Crz1p signaling pathway in Saccharomyces cerevisiae. J. Biol. Chem. 277:31079-31088. [DOI] [PubMed] [Google Scholar]