Abstract
Daptomycin is a cyclic lipopeptide antibiotic approved for the treatment of skin and skin structure infections caused by Gram-positive pathogens and for that of bacteremia and right-sided endocarditis caused by Staphylococcus aureus. Daptomycin failed to meet noninferiority criteria for the treatment of community-acquired pneumonia, likely due to sequestration in pulmonary surfactant. Many analogues of daptomycin have been generated by combinatorial biosynthesis, but only two displayed improved activity in the presence of bovine surfactant, and neither was as active as daptomycin in vitro. In the present study, we generated hybrid molecules of the structurally related lipopeptide A54145 in Streptomyces fradiae and tested them for antibacterial activity in the presence of bovine surfactant. Hybrid A54145 nonribosomal peptide synthetase (NRPS) biosynthetic genes were constructed by genetic engineering and were expressed in combination with a deletion of the lptI methyltransferase gene, which is involved in the formation of the 3-methyl-glutamic acid (3mGlu) residue at position 12. Some of the compounds were very active against S. aureus and other Gram-positive pathogens; one compound was also highly active in the presence of bovine surfactant, had low acute toxicity, and showed some efficacy against Streptococcus pneumoniae in a mouse model of pulmonary infection.
Daptomycin (Fig. 1) is an acidic lipodepsipeptide antibiotic produced by a nonribosomal peptide synthetase (NRPS) mechanism in Streptomyces roseosporus (4, 6). It has a novel mechanism of action targeting Gram-positive pathogens (5, 6, 34, 41). Daptomycin is composed of a 13-member peptide cyclized to form a 10-member ring and a 3-member exocyclic tail, to which is attached a decanoic acid side chain to the N terminus of l-Trp1. S. roseosporus normally produces a mixture of lipopeptides; the predominant factors are designated A21978C1 to A21978C3, with anteiso-undecanoate, iso-dodecanoate, and anteiso-tridecanoate side chains, respectively. Daptomycin is produced commercially by S. roseosporus by feeding decanoic acid during fermentation (4, 6). These lipopeptides contain the nonproteinogenic amino acids l-ornithine (Orn), l-threo-3-methyl-glutamic acid (3mGlu), and l-kynurenine (Kyn), and they also contain three d-amino acids (Fig. 1).
FIG. 1.
Daptomycin and A54145. (A) Structures of daptomycin and A54145B1. (B) Linear structures of modular Dpt and Lpt nonribosomal peptide synthetases. Each module is composed of condensation (C), adenylation (A), thiolation (T), and optionally epimerase (E) domains, arranged as C-A-T (for l-amino acids) or C-A-T-E (for d-amino acids) and responsible for the activation and assembly of amino acid residues into the lipopeptides. The thioesterases (Te) at the ends of DptD and LptD are responsible for the cyclization and release of the final products.
Daptomycin has been approved for the treatment of complicated skin and skin structure infections caused by Gram-positive bacteria (1) and for bacteremia and right-sided endocarditis caused by Staphylococcus aureus, including methicillin-resistant S. aureus (MRSA) (16). However, daptomycin failed to meet noninferiority criteria in a clinical trial to treat community-acquired pneumonia (CAP) (37), even though it is very active against Streptococcus pneumoniae in vitro (6). Poor efficacy against CAP may be due to sequestration of daptomycin in surfactant in the lung alveolar space (40).
Attempts have been made to improve the activity of daptomycin in the presence of bovine surfactant by reprogramming lipopeptide biosynthesis to alter the amino acid sequence in the tridecapeptide by genetic engineering (32, 35, 36). Two compounds displayed reduced inhibition by surfactant (this report), but both had increased MICs against S. aureus in the absence of surfactant, and neither showed efficacy against CAP in a mouse model. The compound that was least inhibited by surfactant (CB-182,106) had Val13 substituted for Kyn13 (Table 1). The source of the NRPS module that catalyzes the incorporation of Val13 was the lptD gene from the A54145 biosynthetic pathway in Streptomyces fradiae (31).
TABLE 1.
Antibacterial activities of lipopeptides and inhibition by bovine surfactant
| Compounda | Amino acid at position: |
MIC (μg/ml) for S. aureus 42b |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 5 | 6 | 8 | 9 | 11 | 12 | 13 | − Surf | + Surf (1%) | Ratio (+/−) | |
| Daptomycin | d-Asn | Asp | Gly | Orn | d-Ala | Asp | d-Ser | 3mGlu | Kyn | 0.5 | 64 | 128 |
| CB-181,220 | d-Asn | Asp | Gly | Orn | d-Ala | Asp | d-Ser | 3mGlu | Kyn | 0.5 | 64 | 128 |
| CB-182,098 | d-Asn | Asp | Gly | Orn | d-Ala | Asp | d-Ser | 3mGlu | Trp | 1 | 32 | 32 |
| CB-182,107 | d-Asn | Asp | Gly | Orn | d-Ala | Asp | d-Ser | 3mGlu | Ile | 2 | 8 | 4 |
| CB-182,106 | d-Asn | Asp | Gly | Orn | d-Ala | Asp | d-Ser | 3mGlu | Val | 4 | 8 | 2 |
| CB-182,130 | d-Asn | Asp | Gly | Orn | d-Ala | Asp | d-Ser | Glu | Kyn | 8 | 16 | 2 |
| CB-182,166 | d-Asn | Asp | Gly | Orn | d-Ala | Asp | d-Ala | 3mGlu | Kyn | 1 | 16 | 16 |
| CB-182,290 | d-Asn | Asp | Gly | Orn | d-Ala | Asp | d-Asn | 3mGlu | Kyn | 1 | 16 | 16 |
| CB-182,123 | d-Asn | Asp | Gly | Orn | d-Ser | Asp | d-Ser | 3mGlu | Kyn | 1 | 32 | 32 |
| CB-182,296 | d-Asn | Asp | Gly | Orn | d-Lys | Asp | d-Asn | 3mGlu | Kyn | 1 | 32 | 32 |
| A54145E | d-Glu | hAsn | Sar | Ala | d-Lys | moAsp | d-Asn | 3mGlu | Ile | 1 | 32 | 32 |
| A54145D | d-Glu | hAsn | Sar | Ala | d-Lys | moAsp | d-Asn | Glu | Ile | 2 | 4 | 2 |
| CB-182,548 | d-Glu | hAsn | Sar | Ala | d-Lys | moAsp | d-Ala | 3mGlu | Ile | 1 | 16 | 16 |
| CB-182,332 | d-Glu | hAsn | Sar | Ala | d-Lys | moAsp | d-Ser | 3mGlu | Ile | 2 | 16 | 8 |
| CB-182,571 | d-Glu | hAsn | Sar | Ala | d-Ala | moAsp | d-Asn | 3mGlu | Ile | 1 | 32 | 32 |
| CB-182,549 | d-Glu | hAsn | Sar | Ala | d-Ser | moAsp | d-Asn | 3mGlu | Ile | 1 | 16 | 16 |
| CB-182,510 | d-Glu | hAsn | Sar | Ala | d-Asn | moAsp | d-Asn | 3mGlu | Ile | 8 | 64 | 8 |
| CB-182,363 | d-Glu | Asn | Sar | Ala | d-Lys | moAsp | d-Asn | 3mGlu | Ile | 2 | 16 | 8 |
| CB-182,575 | d-Asn | hAsn | Sar | Ala | d-Lys | moAsp | d-Asn | 3mGlu | Ile | 2 | 4 | 2 |
| CB-182,509 | d-Glu | hAsn | Sar | Ala | d-Lys | moAsp | d-Ala | Glu | Ile | 8 | 16 | 2 |
| CB-182,336 | d-Glu | hAsn | Sar | Ala | d-Lys | moAsp | d-Ser | Glu | Ile | 64 | 128 | 2 |
| CB-182,567 | d-Glu | hAsn | Sar | Ala | d-Ala | moAsp | d-Asn | Glu | Ile | 4 | 8 | 2 |
| CB-182,532 | d-Glu | hAsn | Sar | Ala | d-Ser | moAsp | d-Asn | Glu | Ile | 8 | 8 | 1 |
| CB-182,531 | d-Glu | hAsn | Sar | Ala | d-Asn | moAsp | d-Asn | Glu | Ile | 16 | 16 | 1 |
| CB-182,444 | d-Asn | hAsn | Sar | Ala | d-Lys | moAsp | d-Asn | Glu | Ile | 8 | 8 | 1 |
| CB-182,561 | d-Asn | Asp | Sar | Ala | d-Lys | moAsp | d-Asn | 3mGlu | Ile | 1 | 2 | 2 |
| CB-182,560 | d-Asn | Asp | Sar | Ala | d-Lys | moAsp | d-Asn | Glu | Ile | 8 | 16 | 2 |
Daptomycin has a decanoyl side chain. All other compounds have anteiso-undecanoyl side chains. Amino acid substitutions are shown in boldface. The compounds related to daptomycin (CB-181,220 to CB-182,296) were generated in previous studies (32, 35, 36). A54145E and A54145D are natural lipopeptide factors produced by S. fradiae (8, 9); the latter is highly enriched by fermentation of an lptI mutant in a medium supplemented with l-Ile (Alexander et al., submitted).
− Surf, without surfactant; + Surf (1%), in the presence of 1% surfactant; ratio (+/−), ratio of the MIC in the presence of surfactant to the MIC in the absence of surfactant.
A54145 is a complex of cyclic lipopeptide antibiotics with activity against Gram-positive pathogens (4, 6, 8, 12). A54145 factors have a number of features in common with daptomycin (Fig. 1), including the following. (i) Both are composed of tridecapeptides that cyclize to form 10-member rings. (ii) Both require Ca2+ ions for antibacterial activity, and both have Asp (or methoxy-Asp [moAsp9] for A54145) residues at positions 7 and 9 in the peptide that forms part of the DXDG Ca2+-binding motif (4, 20). (iii) Both have achiral amino acids (Gly or sarcosine [Sar]) at positions 5 and 10 and d-amino acids at positions 2, 8, and 11. (iv) Both initiate biosynthesis by coupling long-chain fatty acids to the N-terminal Trp1, and both cyclize the 10-member ring by forming an ester bond between the carboxy group of the terminal amino acid and the hydroxyl group of Thr4. (v) Both have the rare amino acid 3mGlu12. However, in spite of the similarities, A54145 differs from daptomycin in amino acids at eight positions (Fig. 1). In addition to these differences, some of the A54145 factors have Val13 substituted for Ile13, and others have Glu12 substituted for 3mGlu12 (6, 8, 9). The four natural peptide structures are coupled to three predominant fatty acid side chains, iso-decanoyl, n-decanoyl, and anteiso-undecanoyl, thus generating a small combinatorial set of related compounds.
A54145E, which contains anteiso-undecanoate, 3mGlu12, and Ile13 and is enriched by supplementation of the fermentation medium with Ile (4, 9), is the most potent antibiotic but displays the highest level of acute toxicity of the A54145 factors (12). The antibacterial activity of A54145E was inhibited 32-fold by 1% bovine surfactant, whereas that of daptomycin was inhibited 128-fold (Table 1). We initiated further studies by isolating several of the A54145 factors, and we showed that A54145D, which was 2-fold less active than A54145E in the absence of surfactant, was inhibited only 2-fold by bovine surfactant. This observation prompted more in-depth studies to explore the structure-activity relationships (SAR) around A54145 by modifying the tridecapeptide by combinatorial biosynthesis. Recent studies have shown that the producer of A54145, S. fradiae, can be genetically manipulated, and an ectopic trans-complementation system has been established to facilitate the reprogramming of the A54145 biosynthetic pathway (D. Alexander, J. Rock, X. He, V. Miao, P. Brian, and R. H. Baltz, submitted for publication). In the present study, we generated several A54145 analogs by modifying the A54145 NRPS using segments of the daptomycin NRPS genes. We identified hybrid molecules that displayed good antibacterial activity in the presence of bovine surfactant. One compound showed low toxicity and weak antibacterial activity against S. pneumoniae in a mouse pneumonia model.
MATERIALS AND METHODS
Strains, plasmids, media, and fermentation conditions.
Key strains and plasmids are listed in Table 2. S. aureus, S. pneumoniae, Enterococcus faecalis, and Enterococcus faecium strains were grown in Mueller-Hinton broth supplemented with 50 mg/liter CaCl2 (MHBc) at 37°C with agitation at 200 rpm as described previously (29). Prior to MIC testing, individual colonies were isolated by streaking bacteria from frozen glycerol stocks onto tryptic soy agar fortified with 5% sheep blood (TSAB; bioMérieux, Lombard, IL) and were grown at 37°C for ∼24 h. S. pneumoniae was incubated at 37°C in TSAB with 5% CO2. Escherichia coli was grown in LB liquid or agar medium with appropriate selection antibiotics (36). S. fradiae strains were grown for 24 h in CSM broth (25) containing the appropriate antibiotics to grow a starter culture. A 4% inoculum of the starter culture was transferred to 125-ml baffled flasks containing 25 ml of A355 seed medium (30) and was grown at 30°C for 24 h at 200 rpm. A 4% inoculum of the seed culture was transferred to 250-ml baffled flasks containing 50 ml of DSF production medium (9) which was modified by the addition of 0.79% (wt/vol) l-Ile. Production cultures were grown at 30°C for 6 days at 200 rpm. S. fradiae strains were grown for 48 h in Trypticase soy broth for the starter cultures and 48 h in A355 seed medium for seed cultures. Fermentation broths were harvested by centrifugation at 24,000 × g for 30 min at 4°C.
TABLE 2.
Strains and plasmids
| Strain or plasmid | Relevant characteristic(s) | Source or reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| BAS849 | ΔlamB106 imp-4213 | 39 |
| BW25113 | laclqrrnBT14 ΔlacZWJ16hsdR514 | 13 |
| ML22 | DH10B::pUZ8002 | 11 |
| E. faecalis | ||
| 201 | CLSI test strain | ATCC 49452 |
| 312 | E. faecalis 201 mutant with reduced susceptibility to Dap | Cubist strain collection |
| E. faecium | ||
| 14 | CLSI test strain | ATCC 6569 |
| 384 | E. faecium 14 mutant with reduced susceptibility to Dap | Cubist strain collection |
| S. aureus | ||
| 42 | CLSI test strain | ATCC 29213 |
| 339 | CLSI clinical MRSA isolate | ATCC 43300 |
| MW2 | MRSA; genomic sequence available | 2 |
| 1616 | MW2 with reduced susceptibility to Dap | 17 |
| 1695 | MW2 with reduced susceptibility to Dap | 17 |
| S. pneumoniae 402 | CLSI test strain | ATCC 6303 |
| S. fradiae | ||
| XH25 | rpsL; producer of A54145 | Alexander et al., submitted |
| DA613 | XH25 ΔlptI::tsr | Alexander et al., submitted |
| DA740 | XH25 ΔlptBCD | Alexander et al., submitted |
| DA901 | XH25 ΔlptBCD ΔlptI::tsr | Alexander et al., submitted |
| DA1187 | XH25 ΔlptEFABCDGHJKLMNPI | Alexander et al., submitted |
| XH1000 | DA1187::pKN55 | This study |
| KN707 | DA901::pKN56 | This study |
| KN681 | DA740::pKN56 | This study |
| KN715 | DA901::pKN57 | This study |
| KN689 | DA740::pKN57 | This study |
| KN723 | DA901::pKN58 | This study |
| KN697 | DA740::pKN58 | This study |
| KN728 | DA901::pKN59 | This study |
| KN701 | DA740::pKN59 | This study |
| KN730 | DA901::pKN60 | This study |
| KN705 | DA740::pKN60 | This study |
| KN649 | DA1187::pKN61 | This study |
| KN665 | XH1000::pKN61 | This study |
| KN661 | DA1187::pKN63 | This study |
| KN677 | XH1000::pKN63 | This study |
| XH1003 | DA1187::pXH11 | This study |
| XH1035 | XH1000::pXH11 | This study |
| Plasmids | ||
| pDA2048 | pECBAC1-derived; oriT att/intφC31Amr::ermEp*lptBCDGH | Alexander et al., submitted |
| pDA2054 | pECBAC1-derived; oriT att/intφC31Amr::lptEFABCDGHJKLMNP | Alexander et al., submitted |
| pRT802 | Kanratt/intφBT1 | 19 |
| pKN54 | pRT802::ermEp* | This study |
| pKN55 | pKN54::dptIJ | This study |
| pKN56 | pDA2048 ΔD-Lys8-CAT::D-Ala-CAT | This study |
| pKN57 | pDA2048 ΔD-Lys8-CAT::D-Ser-CAT | This study |
| pKN58 | pDA2048 ΔD-Lys8-CAT::D-Asn-CAT | This study |
| pKN59 | pDA2048 ΔD-Asn11-CAT::D-Ala-CAT | This study |
| pKN60 | pDA2048 ΔD-Asn11-CAT::D-Ser-CAT | This study |
| pKN61 | pDA2054 ΔD-Glu2-D-Lys8::D-Asn2-D-Ala8 | This study |
| pKN63 | pDA2054 ΔD-Glu2-Thr4::D-Asn2-D-Thr4 | This study |
| pXH11 | pDA2054 ΔD-Glu2-CAT::D-Asn2-CAT | This study |
Antibacterial activities of lipopeptides.
MICs were determined by growing cells in MHBc with or without 1% bovine pulmonary surfactant (Survanta; Abbott Laboratories, Columbus, OH) as described previously (29, 40). Assays were performed in accordance with Clinical and Laboratory Standards Institute (CLSI) methodologies. Cultures were incubated at 37°C with rotation (200 rpm), except for S. pneumoniae, which was incubated at 37°C under 5% CO2 without aeration. For the testing of MICs for S. pneumoniae, MHBc was supplemented with 5% lysed horse blood (Hemostat Laboratories, Dixon, CA).
In vivo efficacy and toxicity testing was performed using CD-1 female mice inoculated intranasally with S. pneumoniae (5 × 106 CFU in 0.1 ml per mouse). At 1 and 4 h postinoculation, 10 control mice received two subcutaneous (s.c.) injections of 0.01 M saline phosphate buffer, pH 7.4, and 5 mice per group received daptomycin, CB-182,561, or vancomycin. Mouse lungs were harvested at ∼24 h postinoculation, homogenized in 4 ml distilled water, serially diluted, and plated onto colistin-nalidixic acid agar (CNA) medium containing 5% sheep blood (18).
Extraction and purification of lipopeptides.
A production culture (4 liters) was centrifuged to remove biomass, and supernatant broth was loaded onto an open glass column (60 by 500 mm) packed with 300 ml preconditioned Diaion HP20 resin in water. The column was first eluted with 1.0 liter of water, followed sequentially by 10%, 20%, 30%, 40%, and 50% isopropanol (1.0 liter each). The eluates were monitored by high-performance liquid chromatography (HPLC) analysis, and the fractions containing the target compounds were concentrated by rotary evaporation and lyophilization to afford the crude material, which was further subjected to a 350-ml Sephadex LH-20 column and was eluted with a mixture of methanol (MeOH) and H2O (1:1). Fractions containing target components were collected to yield lyophilized powders. Final purification was achieved by semipreparative HPLC using a Waters SymmetryPrep C8 column (19 by 300 mm; particle size, 7 μm) at a flow rate of 20 ml/min. For hydrophilic compounds, solvent B was linearly changed from 30% to 45% over 35 min, and for hydrophobic compounds, solvent B was linearly changed from 30% to 58% over 25 min.
Chromatography was conducted on Diaion HP20 resin (Itochu Chemicals America Inc., NY) and Sephadex LH-20 (Pharmacia Biotech, Uppsala, Sweden) columns. Analytical HPLC was performed at ambient temperature using a Waters Alliance 2690 HPLC system and a 996 photodiode array detector (Waters, Milford, MA). Semipreparative HPLC was performed on a Varian system equipped with two model SD-1 PrepStar solvent delivery modules, a PDA ProStar detector, a ProStar injector, and a model 701 fraction collector (Varian, Lake Forest, CA).
HPLC analysis.
The product isolation process was monitored, and the target compounds were analyzed, by a Waters HPLC system with a Waters Symmetry C8 column (4.6 by 250 mm; particle size, 5 μm) with a Waters Guard C8 cartridge. Mobile solvent systems included acetonitrile buffered with 0.01% trifluoroacetic acid (solvent B) and water buffered with 0.01% trifluoroacetic acid (solvent A), and the flow rate was kept at 1.5 ml/min. For hydrophilic compounds, solvent B was changed linearly from 30% to 45% over 14 min, and for hydrophobic compounds, solvent B was changed linearly from 30% to 90% over 14 min.
Characterization of lipopeptides by LC-MS and LC-MS-MS.
Liquid chromatography-mass spectrometry (LC-MS) analysis of lipopeptides was performed as previously described (30, 32). LC-tandem MS (LC-MS-MS) analysis of selected compounds 1 to 3 and the linearized hydrolysates 1a to 3a was carried out as described previously (21, 35).
Construction of recombinant plasmids.
The cloned A54145 biosynthetic gene cluster (31; Alexander et al., submitted) was modified by module exchanges using λ Red-mediated recombination essentially as described previously (36) with the primers described in Table S1 in the supplemental material. Briefly, multiple domains and modules of A54145 NRPS genes were deleted between the T-C1 and T-E1 or the T-C1 and E-C1 linker sites by recombinational exchange with a Genr gene. The Genr gene was then removed by NheI/PmeI digestion and was replaced by fragments cloned by the gap repair technique from daptomycin NRPS genes (36). Recombinant plasmids were introduced into S. fradiae mutants (Table 2) by conjugation from E. coli ML22 and site-specific insertion into the chromosomal φC31 attB site (Alexander et al., submitted). The hybrid genes were expressed from the ermEp* promoter along with the other NRPS genes expressed from the native chromosomal locus or from the φBT1 attB site in S. fradiae (Alexander et al., submitted). To express the dptI gene encoding the methyltransferase from the daptomycin biosynthetic pathway, pKN54, which carries the ermEp* promoter and φBT1 attP, was constructed by cloning the 1.8-kb BglII/SmaI fragment from pHM11a, containing ermEp* and a transcriptional terminator (33), at the BamHI/EcoRV sites of pRT802, which encodes the φBT1 integration system (19). The recombinant containing pKN54 was grown in LB agar medium with kanamycin (50 μg/ml). The PCR fragment coding for dptIJ was amplified as described previously (35) and was cloned at NdeI/HindIII sites on pKN54 to generate pKN55.
Replacement of single residues at position 2, 8, or 11.
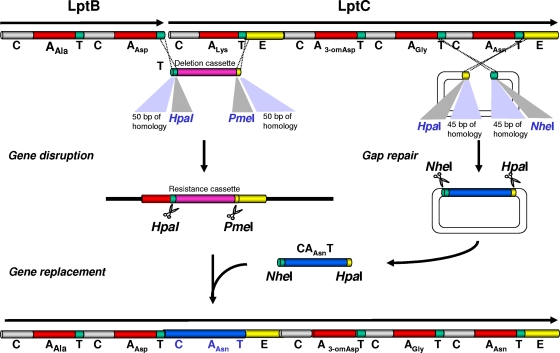
To replace the amino acid residue at d-Asn11 of the A54145 core, pDA2048 containing the lptBCDGH genes was modified as follows. The DNA fragment encoding the d-Asn11 module was replaced by the Genr gene, amplified by using primers lpt-Del-Asn11-B and lpt-Del-Asn11-CAT, by λ Red-mediated recombination. The Genr gene was then removed and replaced by a DNA fragment coding for d-Ala8 or d-Ser11 from dptBC. These almost identical fragments were cloned by the gap repair technique (36) using the same pair of primers (dpt-Ala/Ser-B-P13 and dpt-Ala/Ser-CAT-P14-II). Similarly, the condensation-adenylation-thiolation (C-A-T) multidomains of the d-Lys8 module were deleted by using primers lpt-Del-Lys8-B-Nhe and lpt-Del-Lys8-CAT-II and were replaced by the cloned d-Ala or d-Ser domains. The constructs were made to result in the deletion of the stop codon of lptB and the fusion of lptB and lptC, creating a new T-C linker (Fig. 2). The following hybrid plasmids were generated: pKN56 (lptBCD::CAAla8T), pKN57 (lptBCD::CASer8T), pKN58 (lptBCD::CAAsn8T), pKN59 (lptBCD::CAAla11T), and pKN60 (lptBCD::CASer11T).
FIG. 2.
Construction of hybrid NRPS using λ Red-mediated recombination. In this example, domains of the d-Lys8 module were deleted and replaced by a selectable marker, which was in turn replaced by the corresponding domains of the d-Asn11 module cloned by the gap repair technique (36). The process results in the fusion of LptB and LptC and the elimination of the LptB stop codon.
The C-A-T tridomain of the d-Glu2 condensation-adenylation-thiolation-epimerase (C-A-T-E) module located in plasmid pDA2054, which encodes the entire A54145 gene cluster from lptE through lptP but lacks lptI, was deleted and replaced by a Genr gene amplified by using primers lpt-Del-Glu2-B and lpt-Del-Glu2-CAT; the Genr gene was then replaced by the cloned domains of d-Ser or the A54145 NRPS d-Asn11 cloned by using primers lpt-Asn11-B-B-P13 and lpt-Asn11-CAT-P14.
Replacement of multiple amino acids.
The DNA fragments coding for modules 2 to 4 or modules 2 to 8 on pDA2054 were deleted by exchanging the DNA segments with the Genr gene, amplified by using primers lpt-Del-Glu2-B and lpt-Del-Thr4-CAT or primers lpt-Del-Glu2-B and lpt-Del-Lys8-CATE2-II. The Genr gene was then removed and replaced by a fragment from daptomycin NRPS coding for modules 2 to 4 (cloned by using primers dpt-Asn2-pick-B and dpt-Thr4-pick-CAT) or modules 2 to 8 (cloned by using primers dpt-Asn2-pick-B and dpt-Ala8-CATE2-P14-II) to generate pKN61 or pKN63, respectively.
Combinatorial biosynthesis.
The plasmids containing single or multiple module exchanges on pDA2048 (for the exchange of module 8 or 11) or pDA2054 (for the exchange of module 2 and multimodules 2 to 4 or 2 to 8) were expressed in S. fradiae mutants lacking the corresponding regions (DA740 [ΔlptBCD] or XH1003 [ΔlptEFABCDGHJKLMNP]) (Table 2) or in mutants lacking both of these regions and lptI (DA901 [ΔlptBCD ΔlptI::tsr] or DA1187 [ΔlptEFABCDGHJKLMNPI]). Recombinant strains derived from these mutants were used to produce multiple novel hybrid compounds.
RESULTS
Inhibition of lipopeptide antibiotic activity by bovine surfactant.
The antibacterial activities of a number of daptomycin analogs generated by combinatorial biosynthesis (32, 35, 36) were determined in the presence or absence of 1% bovine pulmonary surfactant (Table 1). The antibacterial activities of most of the compounds were inhibited 16- to 64-fold by surfactant. The antibacterial activity of CB-182,130, containing Glu12 substituted for 3mGlu12, was inhibited only 2-fold by surfactant, but it was 16-fold less active than daptomycin in the absence of surfactant. Hybrid compounds containing Ile13 or Val13 were less inhibited by bovine surfactant (about 4-fold and 2-fold, respectively), but they were 4-fold and 8-fold less active against S. aureus than daptomycin, respectively, in the absence of surfactant (Table 1).
Two of the natural A54145 factors (D and E) were also tested for antibacterial activity. A545145E, which has 3mGlu12 and is much more toxic than daptomycin (12), had a MIC of 1 μg/ml in the absence of surfactant but was inhibited 32-fold by surfactant (Table 1). A54145D, which has Glu12 and is substantially less toxic than A54145E, had MICs of 4 and 2 μg/ml with and without surfactant, respectively. Thus, A54145D had an improved MIC profile overall relative to that of CB-182,130, the daptomycin analog containing Glu12. We therefore initiated modifications of A54145 NRPS genes with the goal of generating new lipopeptide analogs with antibacterial activity profiles and low toxicity similar to those of daptomycin, but with increased antibacterial activity in the presence of bovine surfactant.
Genetic engineering of the A54145 NRPS.
The λ Red-mediated recombination system (Fig. 2) was utilized to exchange single or multiple modules or multidomains in bacterial artificial chromosome (BAC) vectors containing different sets of A54145 biosynthetic genes (Table 2), using several splicing sites located in the interdomain regions similar to those used to generate novel derivatives of daptomycin (14, 36). Exchanges made at position 8 resulted in the elimination of the stop codon of lptB to generate fused lptBC hybrid genes. Exchanges at position 8 or 11 in the fused lptBC gene were carried out in plasmid pDA2048, and the plasmids were introduced into strains DA740 (ΔlptBCD) and DA901 (ΔlptBCD lptI::tsr) at the φC31 attB site to generate novel compounds containing 3mGlu and Glu, respectively, at position 12. Exchanges at positions 2, 2 and 3, or 2 to 8 were made in plasmid pDA2054, which was then introduced into strain DA1187 at the φC31 attB site to produce novel compounds containing Glu12. The same changes were coupled with 3mGlu12 by introducing plasmid pKN55 containing the dptIJ genes (from the daptomycin pathway) into the φBT1 attB site in the recombinants. Sixteen hybrid A54145 analogs were produced in shake flask fermentation media containing Ile to enrich for compounds containing anteiso-undecanoate side chains and Ile13 to simplify the analysis, and the predicted compounds were detected by LC-MS. The yields of the compounds containing the predicted amino acid changes and anteiso-undecanoate side chains are shown in Table 3. The hybrid compounds containing one to three changes were produced at 3 to 48 mg/liter, while the compounds with five or six amino acid substitutions were produced at ∼1 mg/liter. The best producers were those with substitutions of d-Ser11 at position 8 or 11 (>20 mg/liter), whereas compounds with substitutions of d-Ala at position 8 or 11 produced about 5-fold-lower yields.
TABLE 3.
Lipopeptide yields from recombinant S. fradiae strains
| Strain | Compounda | Amino acid change(s) | Mass ion (m/z) | Yield (mg/liter) |
|---|---|---|---|---|
| DA613 | A54145D | Glu12 | 1,657.8 | 300 |
| XH1035 | CB-182,575 | d-Asn2 | 1,656.8 | 15.0 |
| XH1003 | CB-182,444 | d-Asn2 Glu12 | 1,643.7 | 9.0 |
| KN665 | CB-182,561 | d-Asn2 Asp3 | 1,642.8 | 11.5 |
| KN649 | CB-182,560 | d-Asn2 Asp3 Glu12 | 1,628.7 | 5.7 |
| KN686 | CB-182,571 | d-Ala8 | 1,615.0 | 3.4 |
| KN711 | CB-182,567 | d-Ala8 Glu12 | 1,600.0 | 5.1 |
| KN691 | CB-182,549 | d-Ser8 | 1,631.0 | 26.6 |
| KN716 | CB-182,532 | d-Ser8 Glu12 | 1,616.0 | 21.8 |
| KN698 | CB-182,510 | d-Asn8 | 1,658.0 | 10.3 |
| KN724 | CB-182,531 | d-Asn8 Glu12 | 1,644.0 | 7.4 |
| KN701 | CB-182,548 | d-Ala11 | 1,629.8 | 8.3 |
| KN727 | CB-182,509 | d-Ala11 Glu12 | 1,615.8 | 7.0 |
| DA1351 | CB-182,332 | d-Ser11 | 1,645.8 | 47.6 |
| DA1380 | CB-182,336 | d-Ser11 Glu12 | 1,631.7 | 29.9 |
| KN677 | NA | d-Asn2 Asp3 Gly5 Orn6d-Ala8 | 1,614.7 | 0.9 |
| KN662 | NA | d-Asn2 Asp3 Gly5 Orn6d-Ala8 Glu12 | 1,600.8 | 0.6 |
NA, not available.
Isolation and structure elucidation.
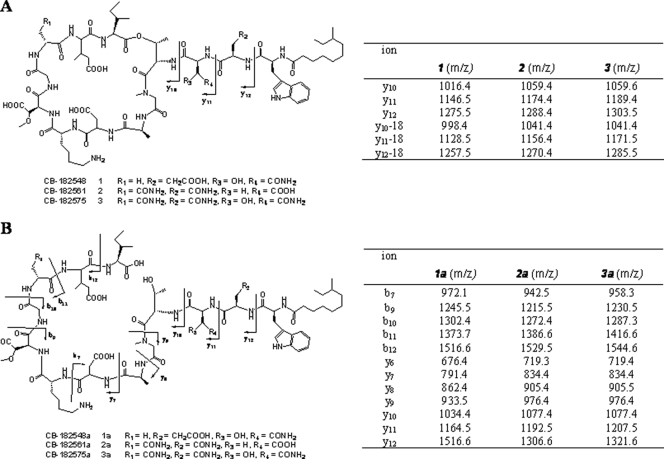
Fermentation broths from recombinants were harvested, and lipopeptides were isolated and purified as described in Materials and Methods. Each of the S. fradiae recombinants produced novel lipopeptides with ions of the predicted masses. Three of the compounds, CB-182,548 (compound 1), CB-182,561 (compound 2), and CB-182,575 (compound 3), were analyzed in detail. The structures of compounds 1 to 3 were supported by their high-resolution MS (HR-MS) data at m/z 1,629.8014 (Δ 0.12 ppm), 1,642.7971 (Δ 0.43 ppm), and 1,657.8096 (Δ 1.39 ppm) [M + H]+, respectively. The amino acid sequences of compounds 1 to 3 were determined by analysis of MS-MS data both of the parent compounds (Fig. 3A) and of the linear hydrolysates 1a to 3a (Fig. 3B). As summarized in Fig. 3A, the MS-MS spectra of compounds 1 to 3 provided limited but distinct product ions y10 to y12 along with their corresponding water loss peaks. The three fragments assigned, y12, y11, and y10, confirmed the side chain amino acid sequences of compounds 1 to 3 as anteiso-undecanoyl-Trp-Glu-hydroxy-Asn (hAsn), anteiso-undecanoyl-Trp-Asn-Asp, and anteiso-undecanoyl-Trp-Asn-hAsn, respectively (substituted amino acids are shown in boldface). Furthermore, the amino acid sequences of compounds 1 to 3 were determined by the analysis of the MS-MS data of compounds 1a to 3a (m/z 1,647.8, 1,660.8, and 1,675.8 [M + H]+, respectively), which were produced by hydrolysis of compounds 1 to 3 with lithium hydroxide. As shown in Fig. 3B, the experimental values of yn and bn agreed with their respective theoretical fragment ions. Therefore, the amino acid sequences of compounds 1 to 3 were strongly supported by linear hydrolysates 1a to 3a as anteiso-undecanoyl-Trp-Glu-hAsn-Thr-Sar-Ala-Asp-Lys-mAsp-Gly-Ala-3mGlu-Ile, anteiso-undecanoyl-Trp-Asn-Asp-Thr-Sar-Ala-Asp-Lys-mAsp-Gly-Asn-3mGlu-Ile, and anteiso-undecanoyl-Trp-Asn-hAsn-Thr-Sar-Ala-Asp-Lys-mAsp-Gly-Asn-3mGlu-Ile, respectively.
FIG. 3.
(A) Chemical structures of compounds 1 to 3 and product ions y10 to y12 of their LC-MS-MS spectra. (B) Chemical structures of linear hydrolysates 1a to 3a with MS-MS fragmentation patterns and the corresponding product ions yn and bn.
Antibacterial activities of A54145 analogs in the presence of surfactant.
A54145D and other Glu12-containing analogs were generally less sensitive to inhibition by bovine surfactant than the more-potent 3mGlu12-containing compounds (Table 1). Unlike A54145E, the 3mGlu12-containing analogs with substitutions of d-Asn2 for d-Glu2 (CB-182,575), or d-Asn2 Asp3 for d-Glu2 hAsn3 (CB-182,561), were inhibited only 2-fold by 1% surfactant.
Antibacterial spectra of novel A54145 derivatives.
The antibacterial activities of the A54145 derivatives against S. aureus 42, other Gram-positive pathogens with different susceptibilities to daptomycin, and an E. coli imp-4213 mutant, with an outer membrane more permeable to bulky antibiotics than those of typical E. coli strains, are presented in Table 4. Replacement of d-Asn11 by d-Ala or d-Ser did not alter antibacterial activity significantly. The only positively charged residue, d-Lys8, was replaced by d-Ala or d-Ser, but not by d-Asn, with no loss of antibacterial activity. However, none of these analogs showed substantially improved antibacterial activities in the presence of bovine surfactant. Replacement of d-Glu2 by d-Asn (CB-182,575), which reduced the acidic charge on the lipopeptide, also reduced activities against S. aureus and other Gram-positive bacteria about 2- to 4-fold from those of A54145E (Tables 1 and 4). However, double replacement of d-Glu2 and hAsn3 by d-Asn2 and Asp3 (CB-182,561), which moved the acidic charge from position 2 to position 3, resulted in an antibacterial activity and spectrum similar to those of daptomycin and A54145E. For all of the compounds, replacement of 3mGlu12 with Glu12 generally reduced the antibacterial activity against S. aureus but also reduced the level of inhibition by surfactant.
TABLE 4.
Antibacterial spectra of hybrid lipopeptides
| Straina | MIC (μg/ml) |
|||||
|---|---|---|---|---|---|---|
| Daptomycin | A54145E | CB-182,575 | CB-182,561 | CB-182,549 | CB-182,548 | |
| S. aureus 42 (MSSA, Daps) | 1 | 0.5 | 2 | 1 | 1 | 1 |
| S. aureus 399 (MRSA, Daps) | 0.5 | 2 | 2 | 0.5 | 1 | 1 |
| S. aureus MW2 (MRSA, Daps) | 1 | 1 | 4 | 1 | 0.5 | 1 |
| S. aureus 1695 (Dapr) | 4 | 4 | 16 | 4 | 4 | 8 |
| S. aureus 1616 (Dapr) | 16 | 16 | 32 | 16 | 8 | 16 |
| E. faecium 14 (Daps) | 2 | 4 | 8 | 4 | 4 | 4 |
| E. faecium 384 (Dapr) | 32 | 16 | 32 | 16 | 32 | 32 |
| E. faecalis 201 (Daps) | 2 | 4 | 16 | 4 | 4 | 4 |
| E. faecalis 312 (Dapr) | 128 | 128 | 128 | 64 | 128 | 128 |
| S. pneumoniae 402 (Daps) | 0.25 | 0.25 | 2 | 1 | 0.25 | 0.5 |
| E. coli BAS849 (imp) | 128 | 16 | 64 | 16 | 16 | 16 |
Daps, daptomycin susceptible; Dapr, daptomycin resistant; MSSA, methicillin-susceptible S. aureus.
From a mechanistic point of view, we were also interested in seeing if any of the compounds had any activity against E. coli. A54145E and three of the A54145 derivatives had MICs of 16 μg/ml against E. coli imp-4213, whereas daptomycin had a MIC of 128 μg/ml (Table 4), suggesting that these compounds may differ in the mechanism of action (see Discussion). Nonetheless, none of the compounds showed improved activity against daptomycin-resistant S. aureus or S. faecalis strains.
Because of its relative insensitivity to surfactant inhibition relative to the other A54145 derivatives (Table 1), and its superior antimicrobial activity relative to CB-182,575 (Table 4), CB-182,561 was selected for in vivo testing in mice.
Antibacterial activity of CB-182,561 in a mouse model of pneumonia.
Vancomycin was efficacious as a control antibiotic in a mouse model of S. pneumoniae pneumonia. S. pneumoniae counts (CFU) were reduced >10-, 103-, and 108-fold at doses of 6.25, 12.5, and 25 mg/kg of body weight, respectively (Table 5). CB-182,561 reduced the bacterial CFU about 3-fold at 25 and 50 mg/kg, whereas daptomycin showed no activity at this dose (not shown). CB-182,561 treatment produced no toxicity at the 12.5- and 25-mg/kg doses. However, at a 50-mg/kg dose, nonlethal toxicity was observed about 17 h after the second dose, including hind-limb paralysis and weakness of forelegs. The results of a separate experiment showed that A54145E was lethal to 5 out of 5 mice within 1 day at 25 mg/kg.
TABLE 5.
In vivo efficacies of CB-182,561 and vancomycin against S. pneumoniae lung infections in CD-1 female mice
| Compound and dose (mg kg−1) | Mean CFU/ml (SD) | Fraction of control surviving |
|---|---|---|
| Nonea | 2.69 × 108 (6.7 × 107) | 1.0 |
| CB-182,561 | ||
| 12.5 | 1.33 × 108 (7.1 × 107) | 0.49 |
| 25 | 9.36 × 107 (5.1 × 107) | 0.35 |
| 50 | 9.13 × 107 (7.0 × 107) | 0.34 |
| Vancomycin | ||
| 6.25 | 1.81 × 107 (6.9 × 106) | 0.07 |
| 12.5 | 2.85 × 105 (3.1 × 105) | 1.06 × 10−3 |
| 25 | 9.0 × 100 (0.0 × 100) | 3.35 × 10−8 |
As a control, 10 ml/kg of 0.01 M saline phosphate buffer, pH 7.4, was used.
DISCUSSION
Daptomycin is an important antibiotic for the treatment of infections caused by Gram-positive pathogens, including MRSA, but it failed to meet noninferiority criteria in clinical trials for CAP. The reason for the failure appears to involve sequestration of daptomycin by pulmonary surfactant (40), a mixture of phospholipids and proteins that facilitates the exchange of oxygen in the alveolar space (38). Surfactant appears to compete for the binding and insertion of daptomycin, which normally is inserted into bacterial membranes as a Ca2+-bound cation-like peptide (23, 26, 42). Daptomycin is preferentially inserted into acidic phospholipid membranes enriched for phosphatidylglycerol (PG), which is localized at the cell division septum in low-G+C-content Gram-positive bacteria (22).
A number of derivatives of daptomycin were generated by combinatorial biosynthesis (3) using an ectopic trans-complementation system that expressed the NRPS genes from as many as three different chromosomal loci (7, 11, 14, 32, 35, 36). This methodology provided a means of modifying the core peptide, which was not readily amenable to modification by medicinal chemical approaches (6). Several very active derivatives of daptomycin were generated by this approach, but only two compounds, with amino acid substitutions of Ile13 or Val13, had substantially improved antibacterial activity in the presence of bovine surfactant. These compounds were less active than daptomycin in the absence of surfactant and therefore were not candidates for further development. The improved activity of these compounds in the presence of bovine surfactant, however, prompted an evaluation of the activities of A54145 factors and derivatives generated by genetic engineering.
The A54145 biosynthetic gene cluster was cloned and sequenced (31), and molecular genetic methods were developed to genetically engineer the A54145 pathway (Alexander et al., submitted). The NRPS gene organization of A54145 is somewhat similar to that of daptomycin. Both dptA and lptA encode five modules that participate in the coupling of the long-chain fatty acids and the first five amino acids, and dptD and lptD encode dimodules for the coupling of the last two amino acids and for ring closure. The two pathways differ in that the six-module dptBC gene has a counterpart of two genes, lptB (two modules) and lptC (four modules). Unlike most NRPS proteins, which have interprotein docking domains to ensure proper protein-protein interactions to facilitate the sequential coupling of amino acids, lptB and lptC do not encode docking peptides (31). Analysis of the A54145 NRPS proteins identified only three NRPS subunits (43), leading to the suggestion that the translation of lptB and lptC may involve translational frameshifting at the overlapping lptA stop codon and the lptB start codon to generate a fused LptBC protein (4). Since this conjecture is not proven, it seemed prudent to avoid any complications in the genetic engineering of multidomain exchanges at position 8 (the first module of lptC); thus, we constructed fused, recombinant lptBC genes by deleting the stop codon of lptB and aligning the two genes in the same reading frame. All of the gene fusions yielded active protein that properly assembled novel derivatives of A54145 at yields ranging from 3.4 to 26.6 mg/liter. To our knowledge, this is the first example of the use of gene fusions for the genetic engineering of NRPSs.
On another technical note, we demonstrated that the DptI methyltransferase can be substituted for the LptI enzyme to produce some A54145 derivatives containing 3mGlu12. DptI, LptI, and GlmT from the calcium-dependent antibiotic (CDA) pathway (24) are novel enzymes that methylate α-ketoglutarate to form (3R)-3-methyl-2-oxoglutarate, a substrate for transamination to form 3mGlu (28). In previous studies it was shown that DptI functions efficiently to insert 3mGlu during the biosynthesis of daptomycin analogs by chimeric NRPS enzymes containing LptD or CdaPS3 substituted for DptD (32) and that GlmT can substitute for DptI in A21978C production (35). The present study, therefore, suggests that all three enzymes may be interchangeable. This may be important for some genetic constructions that include 3mGlu, since DptI is very efficient at providing 3mGlu, whereas strains expressing LptI produce lipopeptide mixtures containing as much as 50% Glu12, and even higher percentages early in the fermentation (8, 9). The lptI gene has one rare TTA codon, and therefore, its mRNA may not be translated in early stages of fermentation (10). In contrast, dptI has no TTA codons and therefore is not under the translational control of the bldA tRNA.
Previous work (32, 36; Alexander et al., submitted) and the work presented here indicate that the antibacterial activity of hybrid lipopeptides related to daptomycin or A54145 can be modified by changing the amino acid sequence of the tridecapeptide. In addition, the interaction with 1% bovine surfactant can also be drastically modified, with levels of inhibition ranging from no inhibition to 128-fold inhibition, as observed with daptomycin (Table 1). The ability to eliminate the inhibition imparted by pulmonary surfactant, while maintaining the excellent antibacterial activity and low toxicity of daptomycin, would potentially provide a clinical candidate with extended indications, including S. pneumoniae pneumonia. Two derivatives of daptomycin with changes at position 13 derived from the A54145 pathway had 8-fold-lower MICs than daptomycin in 1% surfactant but 4- and 8-fold-higher MICs in the absence of surfactant. A54145E, which, like daptomycin, contains 3mGlu12, has antibacterial activities and an antibacterial spectrum similar to those of daptomycin but is less inhibited by surfactant (32-fold versus 128-fold). However, it is much more toxic than daptomycin (12). A54145D, which contains Glu12, displays a 2-fold-higher MIC than A54145E against S. aureus but is much less toxic and is 8-fold more active in 1% surfactant. The Glu12-containing counterpart in the daptomycin series was 16-fold less active than daptomycin in the absence of surfactant and only 4-fold more active in the presence of surfactant (Table 1). These data indicated that both positions 12 and 13 strongly influence the degree of surfactant inhibition and that the methyl group of 3mGlu12 is a major contributor.
The work presented here has shown that a very active derivative of A54145D with good activity in the presence of surfactant can be obtained by substituting the amino acids from positions 2 and 3 from daptomycin (d-Asn and Asp for d-Glu and hAsn). In this case, the charged amino acid was shifted from position 2 to position 3. The best compound had some antibacterial activity in a mouse model of S. pneumoniae lung infection; however, it was substantially less active than vancomycin. There may be one or more reasons for this relatively poor in vivo efficacy. A54145 derivatives with improved activity in the presence of surfactant may still be sequestered by surfactant, albeit to a lower extent, or the pharmacokinetic profile and tissue penetration may not be sufficient for better in vivo efficacy.
The work presented here and elsewhere (11, 14, 32, 35, 36) has demonstrated that combinatorial biosynthesis can work for complex peptides such as daptomycin and A54145, enabling the generation of novel chemical structures not feasible at a similar scale by medicinal chemical approaches. It also showed that complex sets of traits that are likely attributable to subtle differences in partitioning into lipids of different makeups (bacterial membranes for antibacterial activity, mammalian membranes for toxicity, and surfactant for pulmonary efficacy) can be modified and optimized by making specific amino acid substitutions. Only a small sampling of tridecapeptide structure space has been tested, so it may be possible to further improve the properties of this important class of compounds. Additional combinations of changes can be envisioned by using modules that specify different amino acids present in other 10-member cyclic peptides distantly related to daptomycin, such as CDA, friulimicin, and amphomycin (4, 6). It has been shown in chemoenzymatic studies, for instance, that the A54145-excised T-thioesterase (TE) di-domain can catalyze both macrolactonization and macrolactamization (27), opening up many possibilities for producing cyclic peptides related to the known cyclic depsipeptides, daptomycin and A54145.
Finally, several of the A54145 derivatives were about 8-fold more active against E. coli imp-4213 than daptomycin (Table 4). E. coli imp-4213, which has a defective outer membrane, is very susceptible to vancomycin (MIC, 0.8 μg/ml [15]), which is normally prevented from reaching its target in E. coli because it does not penetrate the outer membrane. Daptomycin, on the other hand, has a MIC of 128 μg/ml (Table 4). It has been suggested that the poor activity of daptomycin against E. coli imp-4213 is a reflection of the lack of a major target for daptomycin action in E. coli (and other Gram-negative bacteria), possibly the YycG histidine kinase, which is required for viability in low-G+C-content Gram-positive bacteria but is not present in Gram-negative bacteria (5). The MIC of 16 μg/ml against the E. coli imp mutant observed with A54145 derivatives may suggest that they have a different antibacterial target (or partial target) that is present in Gram-positive and Gram-negative bacteria. Alternatively, they may partition into E. coli membranes more efficiently than daptomycin and disrupt membrane functions. Further work is needed to expand the number of hybrid compounds for further testing and to explore the possible differences in the mechanism of action between A54145 derivatives and daptomycin.
Supplementary Material
Acknowledgments
We thank T. C. Li, J. Rock, N. Cotroneo, K. Townsend, A. Mahamoon, and E. Solum for technical assistance.
This work was supported by Cubist Pharmaceuticals and in part by an NIH SBIR grant (2 R44 GM068173-08).
Footnotes
Published ahead of print on 19 January 2010.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Arbeit, R. D., D. Maki, F. P. Tally, E. Campanaro, and B. I. Eisenstein.2004. The safety and efficacy of daptomycin for the treatment of complicated skin and skin-structure infections. Clin. Infect. Dis. 38:1673-1681. [DOI] [PubMed] [Google Scholar]
- 2.Baba, T., F. Takeuchi, M. Kurada, H. Yuzawa, K. Aoki, A. Oguchi, Y. Nagai, N. Iwama, K. Asano, T. Naimi, H. Kuroda, L. Cui, K. Yamamoto, and K. Hiramatsu.2002. Genome and virulence determinants of high virulence community-acquired MRSA. Lancet 359:1819-1827. [DOI] [PubMed] [Google Scholar]
- 3.Baltz, R. H.2006. Molecular engineering approaches to peptide, polyketide and other antibiotics. Nat. Biotechnol. 24:1533-1540. [DOI] [PubMed] [Google Scholar]
- 4.Baltz, R. H.2008. Biosynthesis and genetic engineering of lipopeptide antibiotics related to daptomycin. Curr. Top. Med. Chem. 8:618-638. [DOI] [PubMed] [Google Scholar]
- 5.Baltz, R. H.2009. Daptomycin: mechanisms of action and resistance, and biosynthetic engineering. Curr. Opin. Chem. Biol. 13:144-151. [DOI] [PubMed] [Google Scholar]
- 6.Baltz, R. H., V. Miao, and S. K. Wrigley.2005. Natural products to drugs: daptomycin and related lipopeptide antibiotics. Nat. Prod. Rep. 22:717-741. [DOI] [PubMed] [Google Scholar]
- 7.Baltz, R. H., P. Brian, V. Miao, and S. K. Wrigley.2006. Combinatorial biosynthesis of lipopeptide antibiotics in Streptomyces roseosporus. J. Ind. Microbiol. Biotechnol. 33:66-74. [DOI] [PubMed] [Google Scholar]
- 8.Boeck, L. D., H. R. Papiska, R. W. Wetzel, J. S. Mynderse, D. S. Fukuda, F. P. Mertz, and D. M. Berry.1990. A54145, a new lipopeptide antibiotic complex: discovery, taxonomy, fermentation and HPLC. J. Antibiot. 43:587-593. [DOI] [PubMed] [Google Scholar]
- 9.Boeck, L. D., and R. W. Wetzel.1990. A54145, a new lipopeptide antibiotic complex: factor control through precursor directed biosynthesis. J. Antibiot. 43:607-615. [DOI] [PubMed] [Google Scholar]
- 10.Chater, K. F., and G. Chandra.2008. The use of the rare UUA codon to define “expression space” for genes involved in secondary metabolism, development and environmental adaptation in streptomycetes. J. Microbiol. 46:1-11. [DOI] [PubMed] [Google Scholar]
- 11.Coëffet-Le Gal, M.-F., L. Thurson, P. Rich, V. Miao, and R. H. Baltz.2006. Complementation of daptomycin dptA and dptD deletion mutations in-trans and production of hybrid lipopeptide antibiotics. Microbiology 152:2993-3001. [DOI] [PubMed] [Google Scholar]
- 12.Counter, F. T., N. E. Allen, D. S. Fukuda, J. N. Hobbs, J. Ott, P. W. Ensminger, J. S. Mynderse, D. A. Preston, and C. Y. Wu.1990. A54145, a new lipopeptide antibiotic complex: microbiological evaluation. J. Antibiot. 43:616-622. [DOI] [PubMed] [Google Scholar]
- 13.Datsenko, K. A., and B. Wanner.2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doekel, S., M.-F. Coëffet-Le Gal, J.-Q. Gu, M. Chu, R. H. Baltz, and P. Brian.2008. Non-ribosomal peptide synthetase module fusions to produce derivatives of daptomycin in Streptomyces roseosporus. Microbiology 154:2872-2880. [DOI] [PubMed] [Google Scholar]
- 15.Eggert, U. S., N. Ruiz, B. V. Falcone, A. A. Branstrom, R. C. Goldman, T. J. Silhavy, and D. Kahne.2001. Genetic basis for activity differences between vancomycin and glycolipid derivatives of vancomycin. Science 294:361-364. [DOI] [PubMed] [Google Scholar]
- 16.Fowler, V. G., H. W. Boucher, G. R. Corey, E. Abrutyn, A. W. Karchmer, M. E. Rupp, D. P. Levine, H. F. Chambers, F. P. Tally, G. A. Vigliani, C. H. Cabell, A. S. Link, I. DeMeyer, G. S. Filler, M. Zervos, P. Cook, J. Parsonnet, J. M. Bernstein, C. S. Price, G. N. Forrest, G. Fätkenheure, M. Gareca, S. J. Rehm, H. R. Brodt, A. Tice, S. E. Cosgrove, and the S. aureus Endocarditis and Bacteremia Study Group.2006. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N. Engl. J. Med. 355:653-655. [DOI] [PubMed] [Google Scholar]
- 17.Friedman, L., J. D. Alder, and J. A. Silverman.2006. Genetic changes that correlate with reduced susceptibility to daptomycin in Staphylococcus aureus. Antimicrob. Agents Chemother. 50:2137-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fung, J. C., B. Lucia, E. Clark, M. Berman, J. Goldstein, and R. D. D'Amato.1982. Primary culture media for routine urine processing. J. Clin. Microbiol. 16:632-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gregory, M. A., R. Till, and M. C. M. Smith.2003. Integration site for Streptomyces phage φBT1 and development of site-specific integrating vectors. J. Bacteriol. 185:5320-5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grünewald, J., S. A. Sieber, C. Mahlert, U. Linne, and M. A. Marahiel.2004. Synthesis and derivatization of daptomycin: a chemoenzymatic route to acidic lipopeptide antibiotics. J. Am. Chem. Soc. 126:17025-17031. [DOI] [PubMed] [Google Scholar]
- 21.Gu, J.-Q., K. T. Nguyen, C. Gandhi, V. Rajgarhia, R. H. Baltz, P. Brian, and M. Chu.2007. Structural characterization of daptomycin analogues A21978C1-3(d-Asn11) produced by a recombinant Streptomyces roseosporus strain. J. Nat. Prod. 70:233-240. [DOI] [PubMed] [Google Scholar]
- 22.Hachmann, A.-B., E. R. Angert, and J. D. Helmann.2009. Genetic analysis of factors affecting susceptibility of Bacillus subtilis to daptomycin. Antimicrob. Agents Chemother. 53:1598-1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ho, S. W., D. Jung, J. R. Calhoun, J. D. Lear, M. Okon, W. R. P. Scott, R. E. W. Hancock, and S. K. Straus.2008. Effect of divalent cations on the structure of the antibiotic daptomycin. Eur. Biophys. J. 37:421-433. [DOI] [PubMed] [Google Scholar]
- 24.Hojati, Z., C. Milne, B. Harvey, L. Gordon, M. Borg, F. Flett, B. Wilkinson, P. J. Sidebottom, B. A. M. Rudd, M. A. Hayes, C. P. Smith, and J. Micklefield.2002. Structure, biosynthetic origin, and engineered biosynthesis of calcium-dependent antibiotics from Streptomyces coelicolor. Chem. Biol. 9:1175-1187. [DOI] [PubMed] [Google Scholar]
- 25.Hosted, T. J., and R. H. Baltz.1996. Mutants of Streptomyces roseosporus that express enhanced recombination within partially homologous genes. Microbiology 142:2803-2813. [DOI] [PubMed] [Google Scholar]
- 26.Jung, D., J. P. Powers, S. K. Straus, and R. E. W. Hancock.2008. Lipid-specific binding of the calcium-dependent antibiotic daptomycin leads to changes in lipid polymorphism of model membranes. Chem. Phys. Lipids 154:120-128. [DOI] [PubMed] [Google Scholar]
- 27.Kopp, F., J. Grünewald, C. Mahlert, and M. A. Marahiel.2006. Chemoenzymatic design for acidic lipopeptide hybrids: new insights into the structure-activity relationships of daptomycin and A54145. Biochemistry 45:10474-10481. [DOI] [PubMed] [Google Scholar]
- 28.Mahlert, C., F. Kopp, J. Thirlway, J. Micklefield, and M. A. Marahiel.2007. Stereospecific enzymatic transformation of α-ketoglutarate to (2S,3R)-3-methyl glutamate during acidic lipopeptide biosynthesis. J. Am. Chem. Soc. 129:12011-12018. [DOI] [PubMed] [Google Scholar]
- 29.Mascio, C. T. M., J. D. Alder, and J. A. Silverman.2007. Bactericidal action of daptomycin against stationary-phase and nondividing Staphylococcus aureus cells. Antimicrob. Agents Chemother. 51:4255-4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miao, V., M.-F. Coëffet-Le Gal, P. Brian, R. Brost, J. Penn, A. Whiting, S. Martin, R. Ford, I. Parr, M. Bouchard, C. J. Silva, S. K. Wrigley, and R. H. Baltz.2005. Daptomycin biosynthesis in Streptomyces roseosporus: cloning and analysis of the gene cluster and revision of peptide stereochemistry. Microbiology 151:1507-1523. [DOI] [PubMed] [Google Scholar]
- 31.Miao, V., R. Brost, J. Chapple, K. She, M.-F. Coëffet-Le Gal, and R. H. Baltz.2006. The lipopeptide antibiotic A54145 biosynthetic gene cluster from Streptomyces roseosporus. J. Ind. Microbiol. Biotechnol. 33:129-140. [DOI] [PubMed] [Google Scholar]
- 32.Miao, V., M.-F. Coëffet-Le Gal, K. Nguyen, P. Brian, J. Penn, A. Whiting, J. Steele, D. Kau, S. Martin, R. Ford, T. Gibson, M. Bouchard, S. K. Wrigley, and R. H. Baltz.2006. Genetic engineering in Streptomyces roseoporus to produce hybrid lipopeptide antibiotics. Chem. Biol. 13:269-276. [DOI] [PubMed] [Google Scholar]
- 33.Motamedi, H., A. Shafiee, and S. J. Cai.1995. Integrative vectors for heterologous expression in Streptomyces spp. Gene 160:25-31. [DOI] [PubMed] [Google Scholar]
- 34.Muthaiyan, A., J. A. Silverman, R. K. Jayaswal, and B. J. Wilkerson.2008. Transcriptional profiling reveals that daptomycin induces the Staphylococcus aureus cell wall stress stimulon and genes responsive to membrane depolarization. Antimicrob. Agents Chemother. 52:980-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nguyen, K., D. Kau, J.-Q. Gu, P. Brian, S. K. Wrigley, R. H. Baltz, and V. Miao.2006. A glutamic acid 3-methyltransferase encoded by an accessory gene locus important for daptomycin biosynthesis in Streptomyces roseosporus. Mol. Microbiol. 61:1294-1307. [DOI] [PubMed] [Google Scholar]
- 36.Nguyen, K., D. Ritz, J.-Q. Gu, D. Alexander, M. Chu, V. Miao, P. Brian, and R. H. Baltz.2006. Combinatorial biosynthesis of lipopeptide antibiotics related to daptomycin. Proc. Natl. Acad. Sci. U. S. A. 103:17462-17467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pertel, P. E., P. Bernardo, C. Fogarty, P. Matthews, R. Northland, M. Benvenuto, G. M. Thorne, S. A. Luperchio, R. D. Arbeit, and J. Alder.2008. Effects of prior effective therapy on the efficacy of daptomycin and ceftriaxone for the treatment of community-acquired pneumonia. Clin. Infect. Dis. 46:1142-1151. [DOI] [PubMed] [Google Scholar]
- 38.Pryhuber, G. S.1998. Regulation and function of pulmonary surfactant protein B. Mol. Genet. Metab. 64:217-228. [DOI] [PubMed] [Google Scholar]
- 39.Sampson, B. A., R. Misra, and S. A. Benson.1989. Identification and characterization of a new gene of Escherichia coli K-12 involved in outer membrane permeability. Genetics 122:491-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silverman, J. A., L. I. Morton, A. D. Van Praagh, T. Li, and J. Alder.2005. Inhibition of daptomycin by pulmonary surfactant: in vitro modeling and clinical impact. J. Infect. Dis. 191:2149-2152. [DOI] [PubMed] [Google Scholar]
- 41.Silverman, J. A., N. G. Perlmutter, and H. M. Shapiro.2003. Correlation of daptomycin bactericidal activity and membrane depolarization in Staphylococcus aureus. Antimicrob. Agents Chemother. 47:2538-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Straus, S. K., and R. E. W. Hancock.2006. Mode of action of the new antibiotic for Gram-positive pathogens daptomycin: comparison with cationic antimicrobial peptides and lipopeptides. Biochim. Biophys. Acta 1758:1215-1223. [DOI] [PubMed] [Google Scholar]
- 43.Wessels, P., H. von Döhren, and H. Kleinkauf.1996. Biosynthesis of the acylpeptidolactones of the daptomycin type. A comparative analysis of peptide synthetases forming A21978C and A54145. Eur. J. Biochem. 242:665-673. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.