Abstract
A Candida glabrata calcineurin mutant exhibited increased susceptibility to both azole antifungal and cell wall-damaging agents and was also attenuated in virulence. Although a mutant lacking the downstream transcription factor Crz1 displayed a cell wall-associated phenotype intermediate to that of the calcineurin mutant and was modestly attenuated in virulence, it did not show increased azole susceptibility. These results suggest that calcineurin regulates both Crz1-dependent and -independent pathways depending on the type of stress.
Infections caused by the opportunistic fungal pathogen Candida glabrata are often difficult to treat due in part to its intrinsic or rapidly acquired resistance to azole antifungals (25). Calcineurin, a serine-threonine-specific protein phosphatase (1), has attracted attention as a new target of antifungal therapy based on studies in several pathogenic fungi, including Candida albicans, Cryptococcus neoformans, and Aspergillus fumigatus (reviewed in reference 31). To date, very little is known about the calcineurin pathway in C. glabrata, although it has been reported that azole antifungals and calcineurin inhibitors have mild synergistic effects against C. glabrata wild-type strains (8, 15, 22). The transcription factor Crz1 is a downstream effector of calcineurin and is involved in azole tolerance in C. albicans (14, 23, 28); however, a Crz1 homolog in C. glabrata has yet to be characterized. Therefore, our objective was to evaluate the potential roles of calcineurin and its downstream target Crz1 in antifungal tolerance and virulence of C. glabrata through the characterization of mutant phenotypes.
Calcineurin is a heterodimer consisting of a catalytic A subunit and a Ca2+-binding regulatory B subunit, and the association between the two subunits is necessary for phosphatase activity (19). To genetically disrupt calcineurin, we completely deleted the CNB1 open reading frame (ORF) encoding the regulatory B subunit. C. glabrata orthologs of CNB1 and CRZ1 were identified in the genome database Génolevures (http://www.genolevures.org/). The primers and strains used in this study are listed in Tables 1 and 2, respectively. C. glabrata cells were propagated in minimal medium (0.7% yeast nitrogen base without amino acids, 2% dextrose) at 30°C, unless otherwise noted. Gene deletion was performed by a one-step PCR-based technique as described previously (13). Briefly, a 1-kb XhoI fragment containing C. glabrata HIS3 was excised from pCgACH (17) and inserted into pBluescript II SK(+) (Stratagene, La Jolla, CA) to yield pBSK-HIS. A deletion construct was amplified from pBSK-HIS with primers tagged with the 100-bp sequences homologous to the flanking regions of the target ORF. Transformation of C. glabrata was performed using the lithium acetate protocol (6). Both PCR and Southern blotting were performed to verify that the desired homologous recombination occurred at the target locus without ectopic integration. To construct a centromere-based plasmid containing a C. glabrata TRP1 marker, a 1,025-bp SacI-KpnI fragment containing the Saccharomyces cerevisiae PGK1 promoter, a polylinker, and the C. glabrata HIS3 3′ flanking region was excised from pGRB2.2 (12) and inserted into the corresponding site of pCgACT (17) to yield pCgACT-P. The entire ORFs of C. glabrata CNB1 and CRZ1 were amplified from the genomic DNA of CBS138 (10) and inserted into pCgACT-P to generate pCgACT-PNB and pCgACT-PRZ, respectively. The constructed plasmids were verified by sequencing before use. Complemented strains were made by transforming mutant strains with a plasmid construct containing the corresponding wild-type gene.
TABLE 1.
Primers used in this study
| Primera | Target gene | Sequence (5′-3′)b |
|---|---|---|
| For gene deletion | ||
| CgCNB 100-F | CNB1 | GTATGTGATGCTTCTCACAGGGTTCAGACGGTTACATACCATCGCTTGAGAGTCATAGTAAATGTTCAGGTTCACGATTAAATCATGCTTTCTCTTTGATAATACGACTCACTATAGGGC |
| CgCNB 100-R | CNB1 | GCGAACTCTGAAATGTAGATCAAGGATTATTCTGTCCTTGAAATGGGTGTTGATGTCCCTCACTAGGAAAGACAACCACTTTACTATTGTAAGGGGTGACGCTCTAGAACTAGTGGATCC |
| CgCRZ 100-F | CRZ1 | GATAACGAGTTGGACGCCCTCTTTTGGAAGTCTGTTCTGGTTGCAGATGCTTATAGACCCTGGATCAAGCACTTCATTTCATTGGGATTACAGCTTTTCTAATACGACTCACTATAGGGC |
| CgCRZ 100-R | CRZ1 | CACAATCTTGATTCTGAAGAAAAAAATTTATCATTAAAAATACTGGAGGTTTGTGTTAATTTATTCCAAAGTAACACCCATCTCAGTTGCTTGAATATTCGCTCTAGAACTAGTGGATCC |
| For gene cloning | ||
| CgCNB1-F2-5P | CNB1 | ATCAAGGGAAATGGGAGC |
| CgCNB1-R1-5P | CNB1 | CGCCCTAAGTTACATCTCTCCTCG |
| CgCRZ1-F1-E | CRZ1 | CGGAATTCATGGGCGATAACGAAGAGGA |
| CgCRZ1-R1938-E | CRZ1 | CGGAATTCTTATTCCAAAGTAACACCCATCTCA |
“F” and “R” indicate forward and reverse primers, respectively.
Sequences homologous to flanking regions of the target ORF are shown in italics. Sequences shown in boldface are present in pBSK-HIS. Restriction sites are underlined.
TABLE 2.
Strains used in this study
| Strain | Genotype or description | Reference or source |
|---|---|---|
| CBS138 | Wild type | 10 |
| 2001T | Δtrp1 (a derivative of CBS138) | 16 |
| 2001HT | Δhis3 Δtrp1 (made from 2001T) | 16 |
| TG11 | 2001T containing pCgACT-P | This study |
| TG161 | Δcnb1::HIS3 Δtrp1 (made from 2001HT) | This study |
| TG162 | TG161 containing pCgACT-P | This study |
| TG163 | TG161 containing pCgACT-PNB | This study |
| TG171 | Δcrz1::HIS3 Δtrp1 (made from 2001HT) | This study |
| TG172 | TG171 containing pCgACT-P | This study |
| TG173 | TG171 containing pCgACT-PRZ | This study |
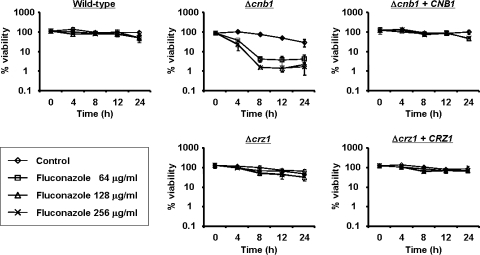
To examine the susceptibility of the generated mutants to antifungal agents, MIC assays were performed (Table 3) with a commercially prepared colorimetric microdilution panel (ASTY; Kyokuto Pharmaceutical Industrial Co., Ltd.) (24). Although increased azole susceptibility was observed in the Δcnb1 strain, the Δcrz1 strain displayed susceptibility levels similar to, or in some instances lower than, those of wild-type cells. The CNB1-complemented strain displayed recovered azole tolerance. Neither the Δcnb1 nor Δcrz1 strain had an effect on amphotericin B susceptibility. Next, we monitored the percent viability of each strain in the presence and absence of fluconazole as described previously (15). Although the antifungal activity of fluconazole is generally fungistatic, the drug was fungicidal for the Δcnb1 strain (Fig. 1). In contrast, the deletion of CRZ1 did not affect the antifungal activity of fluconazole. These results suggest that calcineurin is involved in azole tolerance via a Crz1-independent pathway in C. glabrata.
TABLE 3.
Antifungal susceptibilities of C. glabrata strains
| Strain (genotype) | MIC (μg/ml)a |
||||
|---|---|---|---|---|---|
| FLC | MCZ | ITC | VRC | AMB | |
| TG11 (wild type) | 16 | 0.5 | 2 | 0.25 | 0.5 |
| TG162 (Δcnb1) | 4 | 0.125 | 0.5 | 0.125 | 0.5 |
| TG163 (Δcnb1 + CNB1) | 16 | 0.5 | 2 | 0.25 | 0.5 |
| TG172 (Δcrz1) | 32 | 1 | 1 | 0.5 | 0.5 |
| TG173 (Δcrz1 + CRZ1) | 16 | 0.5 | 1 | 0.25 | 0.5 |
FLC, fluconazole; MCZ, miconazole; ITC, itraconazole; VRC, voriconazole; AMB, amphotericin B.
FIG. 1.
Time-kill curves of C. glabrata wild-type and mutant strains exposed to fluconazole. Logarithmic-phase cells (5 × 105 CFU/ml) were incubated in minimal medium with agitation in the presence or absence of fluconazole at the indicated concentrations. The total number of cells was counted using a hemocytometer, and the number of viable cells was determined by plating the appropriate dilutions on yeast extract-peptone-dextrose (YPD) plates. The data are expressed as the percentages of viability and represent the means and standard deviations for three independent experiments.
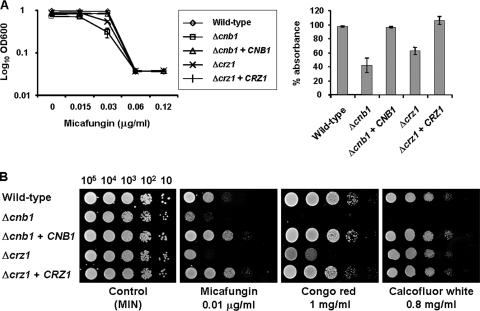
To examine cell wall-associated phenotypes in the Δcnb1 and Δcrz1 strains, we examined their susceptibilities to different types of cell wall-damaging agents, including micafungin (inhibitor of β-1,3-glucan synthesis), Congo red (inhibitor of chitin and β-glucan fiber formation), and calcofluor white (inhibitor of chitin polymer assembly), using a previously described method (15, 20, 26). Micafungin was kindly provided by Astellas (Tokyo, Japan) and dissolved in distilled water. Decreased micafungin tolerance was observed in the Δcnb1 and Δcrz1 strains compared to that in the wild-type control, and this was reversed in the reconstituted strains (Fig. 2). While the Δcnb1 strain showed decreased tolerance to both Congo red and calcofluor white, the Δcrz1 strain exhibited only moderately decreased tolerance to Congo red and was unaffected by calcofluor white exposure (Fig. 2B). These results suggest that the calcineurin-Crz1 pathway plays a role in the response to β-1,3-glucan defects and that calcineurin also regulates a Crz1-independent pathway(s) in response to impaired chitin construction in C. glabrata.
FIG. 2.
Susceptibilities of C. glabrata wild-type and mutant strains to cell wall-damaging agents. (A) Logarithmic-phase cells (2.5 × 103 CFU/ml) were incubated in minimal medium in either the presence or absence of micafungin, and the optical density at 600 nm (OD600) was measured after 24 h (left panel). The percentages of absorbance were calculated from the OD600 of each culture after 24 h of incubation in the presence of 0.03 μg/ml micafungin relative to those in the absence of micafungin (right panel). Data represent the means and standard deviations for three independent experiments. (B) Serial 10-fold dilutions of C. glabrata log-phase cells were spotted onto minimal medium (MIN) plates containing micafungin, Congo red, or calcofluor white at the indicated concentrations. Plates were incubated at 30°C for 48 h. All sensitivity tests were repeated at least three times. C. glabrata strains were as follows: wild type, strain 2001T containing an empty vector (strain TG11); Δcnb1, a Δcnb1 strain containing an empty vector (strain TG162); Δcnb1 + CNB1, a CNB1-complemented strain made with pCgACT-PNB (strain TG163); Δcrz1, a Δcrz1 strain containing an empty vector (strain TG172); and Δcrz1 + CRZ1, a CRZ1-complemented strain made with pCgACT-PRZ (strain TG173).
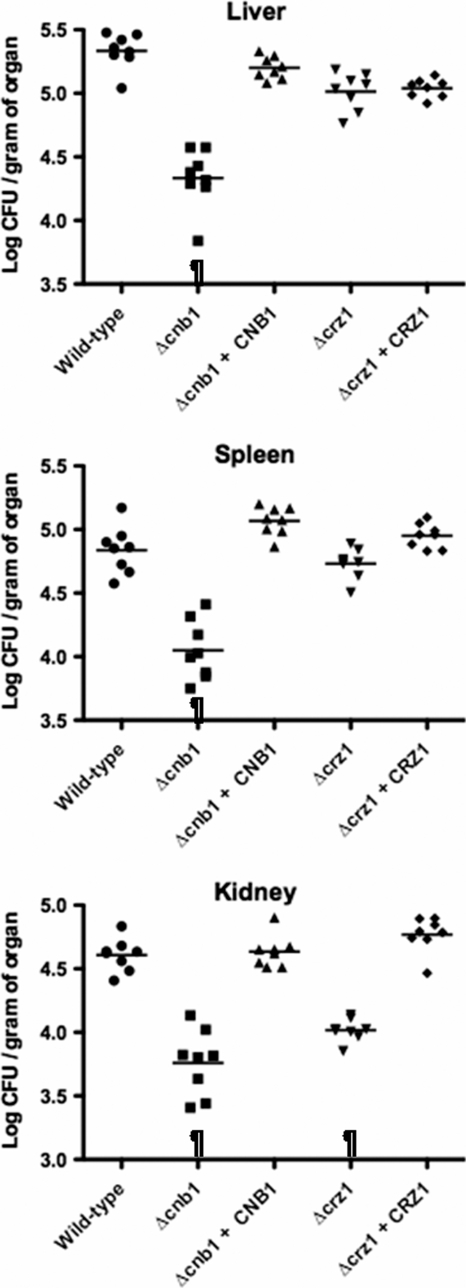
To date, the involvement of calcineurin and Crz1 in virulence has not been reported in C. glabrata. In contrast to the C. neoformans calcineurin mutant (21), deletion of either CNB1 or CRZ1 did not affect cell growth at 37°C in C. glabrata (data not shown), which is a necessary prerequisite for comparing virulence levels. We therefore performed a virulence assay using a murine model of disseminated candidiasis as described previously (5). Briefly, groups of 10 female, 8-week-old, BALB/c mice (Charles River Laboratories Japan, Inc., Japan) were infected via the lateral tail vein. The mice were euthanized 7 days after injection to determine the number of organ CFU. In this study, no mice died before euthanasia. Statistical analyses were performed using the Kruskal-Wallis test with Dunn's posttest for multiple comparisons. A P value of <0.05 was considered statistically significant. Mice infected with the Δcnb1 strain showed significantly reduced fungal burden in all examined organs compared to those infected with the wild-type control and CNB1-complemented strains (Fig. 3). Decreased numbers of CFU of the Δcrz1 strain were statistically significant in the kidney but not in the liver and spleen. The results from this assay indicate that the loss of calcineurin results in attenuated virulence while a deletion of CRZ1 causes only a partial reduction.
FIG. 3.
Virulence assay using a mouse model of disseminated candidiasis. Groups of 10 mice were intravenously inoculated with 8 × 107 cells for each C. glabrata strain. Three target organs (liver, spleen, and bilateral kidneys) were excised 7 days after injection. Appropriate dilutions of organ homogenates were plated, and the numbers of CFU were counted after 3 days of incubation at 30°C. Numbers of recovered CFU from each organ are indicated for individual mice in the scatter plots. The geometric mean is shown as a bar. Representative data of two independent experiments are shown. C. glabrata strains are as follows: wild type, strain TG11 (wild-type control) (filled circles); Δcnb1, TG162 (Δcnb1 strain containing an empty vector) (squares); Δcnb1 + CNB1, TG163 (CNB1-complemented strain made with pCgACT-PNB) (triangles); Δcrz1, TG172 (Δcrz1 strain containing an empty vector) (inverted triangles); Δcrz1 + CRZ1, TG173 (CRZ1-complemented strain made with pCgACT-PRZ) (diamonds). ¶, P < 0.05 (Kruskal-Wallis test with Dunn's posttest).
This is the first report characterizing the phenotypes of C. glabrata CNB1 and CRZ1 mutants, and it has identified both similarities and differences with findings for other fungi. For example, the observed C. glabrata Δcnb1 strain phenotype, which is characterized by an increased susceptibility to azoles and cell wall-damaging agents as well as decreased virulence, is consistent with previous findings for other pathogenic fungi, such as C. albicans (2-4, 27), C. neoformans (11, 18, 21), and A. fumigatus (9, 30). To date, an ortholog of Crz1 in C. neoformans has not been identified and a mutant phenotype associated with azole susceptibility in A. fumigatus has yet to be reported; thus, the full importance of this transcriptional factor is not clear for these fungi. Although the virulence of a Δcrz1 mutant is highly attenuated in A. fumigatus (7, 29), this mutation has little effect on virulence in both C. albicans (14, 23) and C. glabrata (Fig. 3). In contrast to that in C. albicans (14, 23, 28), the loss of Crz1 did not result in increased azole susceptibility in C. glabrata. In addition, the C. glabrata Δcrz1 strain exhibited increased susceptibility to micafungin and Congo red but not to calcofluor white. Taken together, these results indicate that calcineurin-mediated Crz1 regulation is dependent upon the type of stress and that the regulatory mechanisms vary among fungal species. Further characterization of these mutant phenotypes will help to discover a novel and conserved calcineurin target in pathogenic fungi.
Acknowledgments
We thank Hironobu Nakayama for providing C. glabrata strains 2001T and 2001HT and plasmids pCgACH and pCgACT and Brendan Cormack for providing pGRB2.2.
This research was partially supported by a Grant-in-Aid for Scientific Research (no. 19790324 to T.M. and no. 21390305 to S.K.) from the Japanese Ministry of Education, Culture, Sports, Science and Technology, a grant from the Global Centers of Excellence Programs, Nagasaki University, and grants from the Ministry of Health, Labor and Welfare (H20-nanchi-ippan-035, H20-shinko-ippan-012, and H20-shinko-ippan-015 to Y.M.).
Footnotes
Published ahead of print on 25 January 2010.
REFERENCES
- 1.Aramburu, J., A. Rao, and C. B. Klee.2000. Calcineurin: from structure to function. Curr. Top. Cell. Regul. 36:237-295. [DOI] [PubMed] [Google Scholar]
- 2.Bader, T., B. Bodendorfer, K. Schroppel, and J. Morschhauser.2003. Calcineurin is essential for virulence in Candida albicans. Infect. Immun. 71:5344-5354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bader, T., K. Schroppel, S. Bentink, N. Agabian, G. Kohler, and J. Morschhauser.2006. Role of calcineurin in stress resistance, morphogenesis, and virulence of a Candida albicans wild-type strain. Infect. Immun. 74:4366-4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blankenship, J. R., F. L. Wormley, M. K. Boyce, W. A. Schell, S. G. Filler, J. R. Perfect, and J. Heitman.2003. Calcineurin is essential for Candida albicans survival in serum and virulence. Eukaryot. Cell 2:422-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, K. H., T. Miyazaki, H. F. Tsai, and J. E. Bennett.2007. The bZip transcription factor Cgap1p is involved in multidrug resistance and required for activation of multidrug transporter gene CgFLR1 in Candida glabrata. Gene 386:63-72. [DOI] [PubMed] [Google Scholar]
- 6.Cormack, B. P., and S. Falkow.1999. Efficient homologous and illegitimate recombination in the opportunistic yeast pathogen Candida glabrata. Genetics 151:979-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cramer, R. A., Jr., B. Z. Perfect, N. Pinchai, S. Park, D. S. Perlin, Y. G. Asfaw, J. Heitman, J. R. Perfect, and W. J. Steinbach.2008. Calcineurin target CrzA regulates conidial germination, hyphal growth, and pathogenesis of Aspergillus fumigatus. Eukaryot. Cell 7:1085-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cruz, M. C., A. L. Goldstein, J. R. Blankenship, M. Del Poeta, D. Davis, M. E. Cardenas, J. R. Perfect, J. H. McCusker, and J. Heitman.2002. Calcineurin is essential for survival during membrane stress in Candida albicans. EMBO J. 21:546-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.da Silva Ferreira, M. E., T. Heinekamp, A. Hartl, A. A. Brakhage, C. P. Semighini, S. D. Harris, M. Savoldi, P. F. de Gouvea, M. H. de Souza Goldman, and G. H. Goldman.2007. Functional characterization of the Aspergillus fumigatus calcineurin. Fungal Genet. Biol. 44:219-230. [DOI] [PubMed] [Google Scholar]
- 10.Dujon, B., D. Sherman, G. Fischer, P. Durrens, S. Casaregola, I. Lafontaine, J. De Montigny, C. Marck, C. Neuveglise, E. Talla, N. Goffard, L. Frangeul, M. Aigle, V. Anthouard, A. Babour, V. Barbe, S. Barnay, S. Blanchin, J. M. Beckerich, E. Beyne, C. Bleykasten, A. Boisrame, J. Boyer, L. Cattolico, F. Confanioleri, A. De Daruvar, L. Despons, E. Fabre, C. Fairhead, H. Ferry-Dumazet, A. Groppi, F. Hantraye, C. Hennequin, N. Jauniaux, P. Joyet, R. Kachouri, A. Kerrest, R. Koszul, M. Lemaire, I. Lesur, L. Ma, H. Muller, J. M. Nicaud, M. Nikolski, S. Oztas, O. Ozier-Kalogeropoulos, S. Pellenz, S. Potier, G. F. Richard, M. L. Straub, A. Suleau, D. Swennen, F. Tekaia, M. Wesolowski-Louvel, E. Westhof, B. Wirth, M. Zeniou-Meyer, I. Zivanovic, M. Bolotin-Fukuhara, A. Thierry, C. Bouchier, B. Caudron, C. Scarpelli, C. Gaillardin, J. Weissenbach, P. Wincker, and J. L. Souciet.2004. Genome evolution in yeasts. Nature 430:35-44. [DOI] [PubMed] [Google Scholar]
- 11.Fox, D. S., M. C. Cruz, R. A. Sia, H. Ke, G. M. Cox, M. E. Cardenas, and J. Heitman.2001. Calcineurin regulatory subunit is essential for virulence and mediates interactions with FKBP12-FK506 in Cryptococcus neoformans. Mol. Microbiol. 39:835-849. [DOI] [PubMed] [Google Scholar]
- 12.Frieman, M. B., J. M. McCaffery, and B. P. Cormack.2002. Modular domain structure in the Candida glabrata adhesin Epa1p, a beta1,6 glucan-cross-linked cell wall protein. Mol. Microbiol. 46:479-492. [DOI] [PubMed] [Google Scholar]
- 13.Gola, S., R. Martin, A. Walther, A. Dunkler, and J. Wendland.2003. New modules for PCR-based gene targeting in Candida albicans: rapid and efficient gene targeting using 100 bp of flanking homology region. Yeast 20:1339-1347. [DOI] [PubMed] [Google Scholar]
- 14.Karababa, M., E. Valentino, G. Pardini, A. T. Coste, J. Bille, and D. Sanglard.2006. CRZ1, a target of the calcineurin pathway in Candida albicans. Mol. Microbiol. 59:1429-1451. [DOI] [PubMed] [Google Scholar]
- 15.Kaur, R., I. Castano, and B. P. Cormack.2004. Functional genomic analysis of fluconazole susceptibility in the pathogenic yeast Candida glabrata: roles of calcium signaling and mitochondria. Antimicrob. Agents Chemother. 48:1600-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kitada, K., E. Yamaguchi, and M. Arisawa.1995. Cloning of the Candida glabrata TRP1 and HIS3 genes, and construction of their disruptant strains by sequential integrative transformation. Gene 165:203-206. [DOI] [PubMed] [Google Scholar]
- 17.Kitada, K., E. Yamaguchi, and M. Arisawa.1996. Isolation of a Candida glabrata centromere and its use in construction of plasmid vectors. Gene 175:105-108. [DOI] [PubMed] [Google Scholar]
- 18.Kraus, P. R., D. S. Fox, G. M. Cox, and J. Heitman.2003. The Cryptococcus neoformans MAP kinase Mpk1 regulates cell integrity in response to antifungal drugs and loss of calcineurin function. Mol. Microbiol. 48:1377-1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kraus, P. R., and J. Heitman.2003. Coping with stress: calmodulin and calcineurin in model and pathogenic fungi. Biochem. Biophys. Res. Commun. 311:1151-1157. [DOI] [PubMed] [Google Scholar]
- 20.Miyazaki, T., H. F. Tsai, and J. E. Bennett.2006. Kre29p is a novel nuclear protein involved in DNA repair and mitotic fidelity in Candida glabrata. Curr. Genet. 50:11-22. [DOI] [PubMed] [Google Scholar]
- 21.Odom, A., S. Muir, E. Lim, D. L. Toffaletti, J. Perfect, and J. Heitman.1997. Calcineurin is required for virulence of Cryptococcus neoformans. EMBO J. 16:2576-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Onyewu, C., J. R. Blankenship, M. Del Poeta, and J. Heitman.2003. Ergosterol biosynthesis inhibitors become fungicidal when combined with calcineurin inhibitors against Candida albicans, Candida glabrata, and Candida krusei. Antimicrob. Agents Chemother. 47:956-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Onyewu, C., F. L. Wormley, Jr., J. R. Perfect, and J. Heitman.2004. The calcineurin target, Crz1, functions in azole tolerance but is not required for virulence of Candida albicans. Infect. Immun. 72:7330-7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pfaller, M. A., S. Arikan, M. Lozano-Chiu, Y. Chen, S. Coffman, S. A. Messer, R. Rennie, C. Sand, T. Heffner, J. H. Rex, J. Wang, and N. Yamane.1998. Clinical evaluation of the ASTY colorimetric microdilution panel for antifungal susceptibility testing. J. Clin. Microbiol. 36:2609-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pfaller, M. A., and D. J. Diekema.2007. Epidemiology of invasive candidiasis: a persistent public health problem. Clin. Microbiol. Rev. 20:133-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ram, A. F., and F. M. Klis.2006. Identification of fungal cell wall mutants using susceptibility assays based on Calcofluor white and Congo red. Nat. Protoc. 1:2253-2256. [DOI] [PubMed] [Google Scholar]
- 27.Sanglard, D., F. Ischer, O. Marchetti, J. Entenza, and J. Bille.2003. Calcineurin A of Candida albicans: involvement in antifungal tolerance, cell morphogenesis and virulence. Mol. Microbiol. 48:959-976. [DOI] [PubMed] [Google Scholar]
- 28.Santos, M., and I. F. de Larrinoa.2005. Functional characterization of the Candida albicans CRZ1 gene encoding a calcineurin-regulated transcription factor. Curr. Genet. 48:88-100. [DOI] [PubMed] [Google Scholar]
- 29.Soriani, F. M., I. Malavazi, M. E. da Silva Ferreira, M. Savoldi, M. R. Von Zeska Kress, M. H. de Souza Goldman, O. Loss, E. Bignell, and G. H. Goldman.2008. Functional characterization of the Aspergillus fumigatus CRZ1 homologue, CrzA. Mol. Microbiol. 67:1274-1291. [DOI] [PubMed] [Google Scholar]
- 30.Steinbach, W. J., R. A. Cramer, Jr., B. Z. Perfect, Y. G. Asfaw, T. C. Sauer, L. K. Najvar, W. R. Kirkpatrick, T. F. Patterson, D. K. Benjamin, Jr., J. Heitman, and J. R. Perfect.2006. Calcineurin controls growth, morphology, and pathogenicity in Aspergillus fumigatus. Eukaryot. Cell 5:1091-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steinbach, W. J., J. L. Reedy, R. A. Cramer, Jr., J. R. Perfect, and J. Heitman.2007. Harnessing calcineurin as a novel anti-infective agent against invasive fungal infections. Nat. Rev. Microbiol. 5:418-430. [DOI] [PubMed] [Google Scholar]