Abstract
At present no completely effective treatments are available for Cryptosporidium parvum infections in humans and livestock. Based on previous data showing the neutralizing potential of a panel of monoclonal antibodies developed against C. parvum, and based on the fact that innate immune peptides and enzymes have anticryptosporidial activity, we engineered several of these antibodies into antibody-biocide fusion proteins. We hypothesized that the combination of high-affinity antibody targeting with innate immune molecule-mediated killing would result in a highly effective new antiprotozoal agent. To test this hypothesis, we expressed antibody-biocide fusion proteins in a mammalian cell culture system and used the resulting products for in vitro and in vivo efficacy experiments. Antibody-biocide fusion proteins efficiently bound to, and destroyed, C. parvum sporozoites in vitro through a membrane-disruptive mechanism. When antibody-biocide fusion proteins were administered orally to neonatal mice in a prophylactic model of cryptosporidiosis, the induction of infection was reduced by as much as 81% in the mucosal epithelium of the gut, as determined on the basis of histopathological scoring of infectious stages. Several versions of antibody fusion proteins that differed in antigen specificity and in the biocide used had strong inhibitory effects on the initiation of infection. The results lay the groundwork for the development of a new class of antimicrobials effective against Cryptosporidium.
Cryptosporidium parvum is a zoonotic apicomplexan parasite that primarily infects cattle (10). C. parvum causes an economically important diarrheal disease in calves and other livestock, especially in the first few weeks of life (8). At least two species of Cryptosporidium commonly infect humans: C. parvum (previously known as C. parvum type 2) and the human-specific species Cryptosporidium hominis (previously known as C. parvum type 1) (34). In otherwise healthy human hosts, the diarrhea produced by cryptosporidiosis is rarely fatal, but deaths may occur in immunocompromised individuals (6, 13, 17, 29). In tropical countries, diarrhea resulting from infection with Cryptosporidium spp. may result in chronically impaired childhood development (9, 14).
No consistently or completely effective therapeutic drugs exist for cryptosporidiosis in humans or livestock. Currently, management depends on supportive therapy and good hygiene (28). Nitazoxanide has been approved for use in humans, but Cryptosporidium lacks the specific enzyme target for this drug, and results are mixed (36). Recent trials of nitazoxanide in calves for the prevention or treatment of cryptosporidiosis have likewise proved disappointing, with no clinical or parasitologic efficacy observed (26). New approaches to therapy for cryptosporidiosis are therefore urgently needed (32).
Riggs et al. developed monoclonal antibody (MAb) 3E2, which binds to the circumsporozoite-like antigen (CSL), and showed that it could partially neutralize Cryptosporidium parvum in mice (24) and calves (19), albeit at pharmacologically high dosages. Nevertheless, the high cost of production of this antibody from the hybridoma renders its use in the field impracticable. Schaefer et al. then developed a library of monoclonal antibodies against functionally defined C. parvum sporozoite antigens and showed some to be effective at reducing infection in mice (25). A formulation of MAbs targeting three different neutralization-sensitive antigens provided significant additive efficacy over those of the individual MAbs, or combinations of two MAbs, when administered orally to mice.
Several workers have shown that antimicrobial peptides and certain enzymes are active against apicomplexan parasites (1, 11, 16, 30, 31, 35). In general, high doses were needed to effect neutralization. In a prior study, we explored the effects of a number of antimicrobial peptides and enzymes on the viability of C. parvum in vitro (7).
Based on these demonstrated effects of peptides and antibodies against parasites, we hypothesized that by using the high-affinity binding of antibodies, we could target antimicrobial peptides and enzymes (collectively termed “biocides” below) for delivery at the protozoal surface. We reasoned that this precision targeting would reduce the dosage needed and therefore would also reduce cost and potential host toxicity.
To do this, we needed to construct fusion proteins, comprising the variable and constant regions of antibodies that specifically bind to C. parvum fused to selected peptide biocides, in such a way that the functions of both parts were retained. We used the previously developed library of hybridomas as a source of immunoglobulin variable regions directed to a variety of epitopes on C. parvum sporozoites, and we selected antimicrobial peptides/enzymes as sources of biocides (7). By using genetic engineering to combine these elements, and a retrovector gene transfer system, we were able to express functional fusion proteins at high yields in mammalian cell culture. In addition, we modified the isotype and configuration of selected immunoglobulin molecules so as to change size and functionality. We then tested these fusion proteins for their efficacies in killing C. parvum in vitro and in reducing infection in neonatal mice that were concurrently challenged with C. parvum oocysts. Using a mouse model, this study demonstrates that significantly greater neutralization of C. parvum can be obtained by use of the fusion proteins than by use of an antibody or a biocide alone, thus offering a promising alternative approach to the control of C. parvum.
MATERIALS AND METHODS
Hybridomas.
Three hybridomas producing antibodies directed to different neutralization-sensitive antigens on Cryptosporidium parvum have been created previously (21, 22, 24, 25) (Table 1).
TABLE 1.
Characteristics of monoclonal antibodies and derived recombinant fusion proteins specific for three different epitopes on C. parvuma
| Antibody | Epitope specificity | Hybridoma isotype | Fusion protein component |
Molecular mass (kDa)b | ||
|---|---|---|---|---|---|---|
| MAb (recombinant isotype) | Peptide | Enzyme | ||||
| 4H9 | C. parvum sporozoites, GP25-500 | IgG1 | IgG1 | LL37 | 158 | |
| IgG1 | sPLA2 IIa | 177 | ||||
| IgG1 | 147 | |||||
| IgG2b | LL37 | 160 | ||||
| 3E2 | C. parvum sporozoites, CSL | IgM | IgM monomer | LL37 | 190 | |
| IgM halfmer | LL37 | 95 | ||||
| IgG1 | 147 | |||||
| IgG1 | LL37 | 158 | ||||
| N/Ac | 970 | |||||
| 18.44 | C. parvum sporozoites, CPS-500 | IgG3 | IgG1 | LL37 | 158 | |
| IgG1 | sPLA2 IIa | 177 | ||||
| N/A | 147 | |||||
| 166 | L. monocytogenes cell wall | IgG2b | IgG2b | LL37 | 159 | |
| IgG2b | sPLA2 IIa | 178 | ||||
| IgG2b | 148 | |||||
All light chains are of the kappa isotype.
Molecular mass of the immunoglobulin alone or of the fusion protein compromising the immunoglobulin plus the peptide or enzyme.
N/A, the monoclonal antibody was used in its native hybridoma-derived form as a control.
Since MAb 3E2 has been shown previously to have significant neutralizing efficacy against C. parvum infection in neonatal mice (24) and calves (19), we included it here as a positive control. As an isotype control antibody, we used MAb 166 (a kind gift from K. Ziegler, Emory University, Atlanta, GA), directed to Listeria monocytogenes (37). Hybridoma-derived MAb 166 and recombinant MAb 166 do not bind to C. parvum sporozoites, as determined by an immunofluorescence assay (IFA) (data not shown).
Assembly of genetic constructs.
Total RNA was isolated from hybridoma cells (RNeasy kit; Qiagen, Inc., Valencia, CA) and reverse transcribed into cDNA using oligo(dT) primers (AffinityScript cDNA synthesis kit; Stratagene, La Jolla, CA). Immunoglobulin variable-region genes were amplified from cDNA using degenerate upper primers semispecific for the signal peptide region combined with lower primers specific for the constant region (Novagen Ig-primer set; EMD Biosciences, San Diego, CA), and the PCR products obtained were cloned (Strataclone PCR cloning kit; Stratagene, La Jolla, CA). Multiple clones derived from the same PCR product were sequenced to test for PCR-derived mutations and the correct reading frame using Lasergene (DNAStar, Inc., Madison, WI) and were compared with sequences in GenBank to confirm that they were of mouse immunoglobulin origin. Immunoglobulin gene constant regions were extracted from hybridoma cDNA using primers (oligonucleotides obtained from Integrated DNA Technologies, Coralville, IA) to the known constant sequence of the murine IgG1 or IgG2b isotype. The (G4S)3 linker, including flanking regions compatible with the heavy-chain sequence at the 5′ end and with the biocide sequence at the 3′ end, was synthesized by Blue Heron Biotechnology (Bothell, WA). The gene for human phospholipase A2 (PLA2) group IIA was obtained from the ATCC gene collection (MGC-14516). The coding region for LL37, the active portion of human cathelicidin hCAP-18, was assembled by PCR amplification of three long overlapping oligomers that were based on GenBank accession no. NM_004345. The RNA export and stabilization element (RESE) is based on the woodchuck hepatitis virus RNA export element and enhances RNA export from the nucleus in the absence of RNA splicing (38).
To engineer the various IgM-based constructs, we first isolated the variable and constant regions from the 3E2 hybridoma cell line as described above and constructed a full-size IgM molecule for testing purposes. In the absence of the J chain, IgM spontaneously forms hexamers, which we confirmed by size analysis using polyacrylamide gel electrophoresis (PAGE). Once we confirmed the binding of the 3E2 hexamer to sporozoites by an IFA (described below), the 3E2 sequences were used to construct monomeric and halfmeric fusion proteins. The fusion proteins were constructed by eliminating two or three of the interchain disulfide bonds in the IgM heavy-chain genes. This was done as described by Wiersma and Shulman (33), by using site-directed mutagenesis PCR to introduce the requisite cysteine-to-serine codon changes into the nucleotide sequence: C337S, C414S, and C575S to make halfmers and C414S and C475S to make monomers.
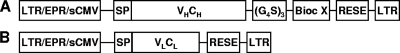
All elements were assembled in a series of overlap PCRs, and the final product containing flanking restriction endonuclease sites was cloned into a murine leukemia virus (MLV)-based replication-incompetent retroviral expression system (Pantropic retroviral vector system; Clontech, Mountain View, CA) modified to include the simian cytomegalovirus (CMV) promoter (GenBank accession no. U38308) (Fig. 1). Due to the very high rates of gene transfer into mammalian host cells achieved with the retroviral system, the Neor gene, used for selection, is not essential and was removed from the retrovector backbone.
FIG. 1.
Genetic constructs for making antibody fusions using the MLV-based retroviral vector. (A) Retroviral construct used to encode the antibody heavy chain fused to an antimicrobial. (B) Retroviral construct used to encode the antibody light chain. LTR, long terminal repeat; EPR, extended packaging region; sCMV, simian cytomegalovirus promoter; SP, signal peptide; VH, heavy-chain variable region; VL, light-chain variable region; CH, heavy-chain constant region; CL, light-chain constant region; RESE, RNA export and stabilization element; (G4S)3, glycine-serine linker; Bioc, biocide.
To confirm the production of correctly assembled recombinant antibody-biocide fusion products, PAGE was performed under both reducing and nonreducing conditions, followed by Western blotting using an affinity-purified goat anti-mouse IgG antibody or an anti-mouse IgM antibody (Bethyl Laboratories, Montgomery, TX).
Expression in cell culture.
The retroviral construct containing the gene of interest was cotransfected with a plasmid containing the gene for vesicular stomatitis virus glycoprotein into GP2-293 packaging cells (Pantropic retroviral expression system; Clontech, Mountain View, CA) to produce infectious replication-incompetent pseudotyped retrovector particles. These were harvested by centrifugation (75,000 × g) and resuspended for 2 h; then they were used to transduce CHO cells. The vector was removed and replaced with fresh SFM4 medium (HyClone, Logan, UT) after 16 h. Ten to 12 days after transduction, cell pools were analyzed by an enzyme-linked immunosorbent assay (ELISA) for the detection of recombinant products using heavy-chain capture and light-chain signal generation (Bethyl Laboratories, Montgomery, TX). Upon confirmation of the presence of correctly assembled immunoglobulins, individual cells were isolated in 96-well plates by limiting dilution. After 12 days, supernatants were reanalyzed, and the highest-producing clones were selected and expanded. Recombinant products were produced in standard tissue culture flasks or 500-ml Erlenmeyer flasks with agitation. Typically, cultures were harvested after 8 to 10 days of incubation; cells were removed by double centrifugation (400 × g for 10 min; 6,000 × g for 10 min); and supernatants were analyzed to determine the product concentration using ELISA codetection of immunoglobulin heavy and light chains. The recombinant products or hybridoma-derived MAbs used for these studies were prepared either from unprocessed cell culture supernatants or from supernatants concentrated as much as 3-fold using Amicon Centricon Plus-20 centrifugal filtration devices (Millipore, Billerica, MA) to provide equal protein concentrations.
The recombinant products expressed are referred to in this paper by the variable-region source-recombinant isotype-biocide (for instance, 4H9-G1-LL37 [where G1 stands for IgG1]). Recombinant products lacking a fused biocide are referred to by the variable-region source-recombinant isotype (for instance, 4H9-G1). Hybridoma-derived MAbs are referred to by the hybridoma name followed by “MAb” (for instance, the 3E2 MAb).
Cryptosporidium parvum oocyst source.
The C. parvum Iowa isolate (12) has been maintained since 1988 by propagation in newborn Cryptosporidium-free Holstein bull calves (21, 23), which were the source of oocysts for all experiments. Oocysts were isolated from calf feces by sucrose density gradient centrifugation and were stored in 2.5% KCr2O7 (4°C) (2, 23). For challenge of neonatal mice, oocysts were used within 30 days of isolation and were disinfected with 1% peracetic acid immediately prior to administration (20). To obtain isolated sporozoites for use in vitro, oocysts were first treated with hypochlorite prior to excystation (37°C, 0.15% [wt/vol] taurocholate, 1 h); then they were passed through a sterile polycarbonate filter (pore size, 2.0 μm; Poretics, Livermore, CA), and used immediately (23, 25). Oocyst excystation was determined immediately prior to mouse administration, or was determined in order to obtain isolated sporozoites, and always exceeded 90%.
Assays for binding of recombinant products to sporozoites and in vitro assessment of viability.
For immunofluorescence assays to assess binding, excysted sporozoites were aliquoted onto Teflon-coated multiwell glass slides, air dried, and gently heat fixed. Individual wells were incubated (30 min, 37°C) with either concentration-matched recombinant fusion products, a recombinant antibody, an isotype-matched control MAb of irrelevant specificity, or a CHO cell supernatant control; then they were washed with phosphate-buffered saline (PBS), incubated with fluorescein-conjugated, affinity-purified goat anti-mouse IgM/IgG/IgA (Kirkegaard & Perry, Gaithersburg, MD), washed, and finally examined by epifluorescence microscopy.
To quantify the parasiticidal activities of recombinant products, sporozoite viability after in vitro incubation with individual products was assessed using fluorescein diacetate (FDA) and propidium iodide (PI) as previously described (3, 7). In brief, freshly excysted sporozoites were incubated (15 min, 37°C) in CHO medium containing individual recombinant products (50 μg/ml) or in spent CHO cell medium. Heat-killed (20 s, 100°C) sporozoites were used as an internal control. FDA (final concentration, 8 μg/ml) and PI (final concentration, 3 μg/ml) were added to the sporozoite preparations, which were incubated further (30 min, 21°C) and then examined in fluid-phase wet mounts by epifluorescence microscopy. A minimum of 100 sporozoites were counted for each preparation to determine the percentage of reduction in viability, calculated as [(mean viability of CHO medium-treated sporozoites − mean viability of recombinant-product-treated sporozoites)/mean viability of CHO medium-treated sporozoites] × 100. The mean values for test and control preparations were examined for significant differences using JMP software and analysis of variance (ANOVA) (SAS, Cary, NC).
Evaluation of recombinant products for efficacy in vivo.
Groups of 10 8-day-old specific-pathogen-free (SPF) ICR mice were administered, by gastric intubation, 5 × 104 oocysts (50 times the 50% mouse infective dose [MID50]) (23) concurrently with recombinant antibody fusion proteins or combinations of individual MAbs and biocides (100 μl; concentration range, 10 to 100 μg/ml). At 3 h and every 12 h thereafter, mice received additional treatments (100 μl; concentration range, 10 to 100 μg/ml) by gastric intubation for a total of nine treatments. Cimetidine (10 mg/kg of body weight) was included with all treatments. Groups of 10 8-day-old control mice were treated identically with a CHO cell culture supernatant or the recombinant antibody alone. After euthanasia at 92 to 94 h postinfection (p.i.), the jejunum, ileum, cecum, and colon were collected, coded, and examined histologically by the same investigator, without knowledge of the treatment group, for C. parvum stages in the mucosal epithelium. Scores of 0 (no infection), 1 (<33% of the mucosa infected), 2 (33 to 66% of the mucosa infected), or 3 (>66% of the mucosa infected) were assigned to longitudinal sections representing the entire length of (i) the terminal jejunum, (ii) the ileum, (iii) the cecum, and (iv) the colon; then these scores were summed to obtain an infection score (0 to 12) for each mouse (21, 24). The percentage of reduction of infection was calculated as (mean infection score with the control − mean infection score with the product)/mean infection score with the control × 100. The control treatment in all in vivo experiments was the CHO cell culture supernatant.
Experimental results were analyzed in JMP, version 8 (SAS Institute, Cary, NC), by ANOVA. Differences between means were tested with a Tukey-Kramer honestly significant difference (HSD) test, with an alpha value of 0.05 indicating significance.
All mice were maintained in biosafety level 2 (BSL-2) biocontainment at the University of Arizona and in accordance with the PHS Guide for the Care and Use of Laboratory Animals (14a).
Nucleotide sequence accession numbers.
Sequences coding for the variable regions of MAbs 18.44, 4H9, and 3E2 have been deposited in GenBank under accession numbers GU126674 to GU126679.
RESULTS
Production of recombinant antibodies and antibody-biocide fusions.
Recombinant fusion proteins comprising monoclonal antibodies and biocides were assembled using the basic retroviral constructs shown in Fig. 1. The use of the retroviral system allowed us to generate stable cell lines for all the recombinant products shown in Table 1 in a short time. Cell supernatant-containing products were tested in the in vitro assay and the neonatal mouse model. All products showed the expected sizes on Western blots for either heavy chains alone, heavy-chain-biocide fusion proteins, or kappa light chains (data not shown). The binding specificities of the recombinant 4H9-, 3E2-, and 18.44-derived products for C. parvum sporozoites, and the lack of binding of recombinant 166-derived products, were confirmed by immunofluorescence assays (data not shown). Table 1 shows the recombinant products and control antibodies tested in this study. These products were generated consecutively. To conserve resources and prevent redundancy, not every possible combination of isotype and biocide was produced a priori; rather, the decisions as to which combinations to produce were based on data obtained from prior fusions.
Direct effects of antibody-biocide fusion proteins on sporozoite viability.
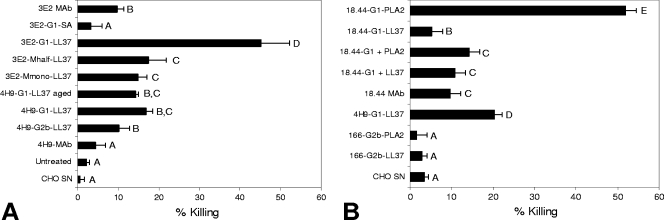
An in vitro viability assay was performed using different versions of antibody-biocide fusion proteins comprising antibodies with 3E2 (anti-CSL), 4H9 (anti-GP25-200), and 18.44 (anti-CPS-500) specificities plus the LL37 or PLA2 biocide (Fig. 2). This assessment showed that antibody-biocide fusion proteins targeting any one of these three antigens on the sporozoite surface mediate significantly higher efficacy at killing sporozoites in vitro than their stand-alone antibody counterparts. Since the IgM-based fusion protein 3E2-M-LL37, which adopts a hexamer configuration, presented technical difficulties for expression and purification, we designed smaller versions comprising an IgM monomer (two heavy chains plus two light chains) or an IgM halfmer (one heavy chain plus one light chain) fused to LL37. The 3E2-based fusions showed significantly higher efficacy at killing sporozoites in vitro than the hybridoma-derived 3E2 MAb (Fig. 2A).
FIG. 2.
In vitro killing of C. parvum sporozoites by fusion proteins, monoclonal antibodies, and combinations of antibody and biocide. Sporozoites were excysted and tested for viability (>98%) before being exposed to different compounds for 30 min at 37°C in PBS buffer. Sporozoite viability or death was determined by fluorescein diacetate or propidium iodide staining, respectively, and data were collected by epifluorescence microscopy. (A) Each component was used at 50 μg/ml except for 3E2-G1-LL37 (1.5 μg/ml). (B) Each component was used at 25 μg/ml. PLA2 and LL37 were used at equimolar concentrations. CHO SN, spent CHO cell medium; Untreated, untreated sporozoites in PBS. “MAb” indicates the use of a native hybridoma-derived antibody as a control. Means ± standard errors of the means and results of ANOVA for triplicate wells are shown. Bars not followed by the same letter are significantly different (alpha = 0.05).
Moreover, we also wanted to test if an isotype switch from IgM to IgG1 would maintain or enhance the efficacy of the 3E2 fusions. Surprisingly, the 3E2-G1-LL37 fusion showed outstanding efficacy in vitro. Unfortunately, this construct was not expressed well as a recombinant molecule, and we were unable to produce quantities sufficient to perform in vivo testing. We subjected 4H9-G1-LL37 to long-term storage at 4°C over a period of 3 months in order to evaluate stability. Storage resulted in only a 2.6% loss of activity, indicating good stability under refrigeration temperatures. The 4H9-G1-PLA2 fusion was also tested in this series but did not show any direct effect on the viability of C. parvum sporozoites in vitro (data not shown).
Figure 2B shows the effects on sporozoite viability of the 18.44 MAb, alone and in combination with PLA2 or LL37, compared to the corresponding 18.44 MAb-biocide fusions. The 18.44-G3 MAb itself exhibited a low rate of sporozoite killing, which increased slightly by simultaneous application of the MAb with recombinant PLA2 as individual, nonfused molecules. However, when 18.44 was expressed as IgG1 with PLA2 as a C-terminal fusion, the resulting 18.44-G1-PLA2 fusion protein showed an approximately 3.5-fold increase in activity over that of 18.44 and PLA2 tested in combination. The 51% reduction in sporozoite viability achieved by 18.44-G1-PLA2 is one of the strongest reductions we have detected in the in vitro assay. The 18.44-G1-LL37 fusion protein exhibited lower activity than the 18.44-G1-PLA2 fusion protein, indicating that a given epitope on the surface of C. parvum may differ in its suitability for mediating the targeted delivery of different biocides. The outcome of this experiment provided the initial indication that antibody-biocide fusions have a direct impact on sporozoite survival in vitro, greater than that of the corresponding stand-alone antibodies.
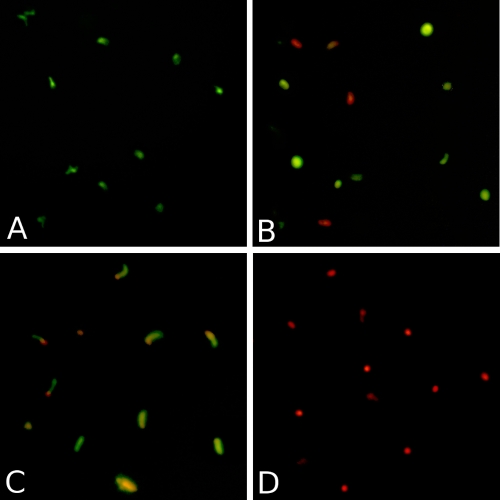
To evaluate morphological effects, sporozoites were exposed to various fusion proteins for 30 min and were then analyzed by immunofluorescence. Figure 3A shows a representative result for sporozoites exposed to either the CHO cell supernatant, monoclonal antibodies, or the 4H9-G1-PLA2 fusion protein, none of which showed any direct killing activity. Figure 3B is representative of the activities shown by all LL37 fusion proteins tested. The observation of spherical shapes and the increased number of dead cells are indicative of the degenerative process associated with disturbance of the osmoregulatory system in the sporozoite. The rates of killing of sporozoites by the fusion proteins differed considerably depending on the components used.
FIG. 3.
Fluorescence photomicrographs showing the effects of various fusion proteins and monoclonal antibody controls. (A) Representative image of C. parvum sporozoites after a 30-min exposure to either PBS, the CHO cell supernatant, 4H9-G1, 4H9-G2b, the 18.44 MAb, 4H9-G1-PLA2, 3E2-G1, or the 3E2 MAb. (B) Representative image of C. parvum sporozoites after a 30-min exposure to either 4H9-G2b-LL37, 4H9-G1-LL37, 3E2-G1-LL37, 3E2-Mhalf-LL37, or 3E2-Mmono-LL37. (C) C. parvum sporozoites after exposure to 18.44-G1-PLA2 for 30 min. (D) Heat-killed sporozoites. Viability or cell death was determined by the addition of fluorescein diacetate (green) (live cells) and propidium iodide (red) (dead cells). Each component was used at 25 μg/ml.
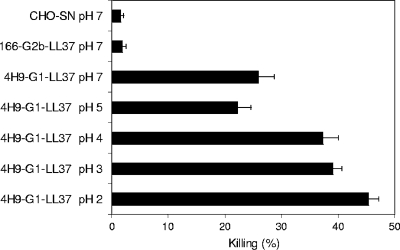
In anticipation of oral administration of the fusion protein products, we examined the effect of pH on the stability of 4H9-G1-LL37. After incubation at various pHs (PBS at pH 2, 3, 4, 5, or 7) at 37°C for 90 min, the fusion protein samples were neutralized to pH 7 and tested in vitro for their direct effects on sporozoite viability. There was no decrease in the effectiveness of the fusion protein following low-pH treatment (Fig. 4). Interestingly, the efficacy of 4H9-G1-LL37 almost doubled after exposure to pH 2. Since our goal in this experiment was simply to determine whether the pH values to which the fusion proteins would be exposed during gastrointestinal transit would be deleterious, the chemical mechanism for the observed enhancement of activity upon exposure to low pHs is the subject of ongoing studies.
FIG. 4.
In vitro killing of C. parvum sporozoites with low-pH-treated 4H9-G1-LL37. The 4H9-G1-LL37 fusion protein was exposed to various pH conditions for 90 min at 37°C before being neutralized to pH 7. Sporozoites were then exposed to the fusion proteins for 30 min at 37°C, followed by viability staining and microscopic analysis. Each bar represents a separate pH treatment for 4H9-G1-LL37. Neutral-pH-treated fusion protein 166-G2b-LL37 and the CHO cell supernatant (SN) were used as controls. Data are means ± standard errors of the means.
Fusion proteins inhibit the initiation of C. parvum infection in neonatal mice.
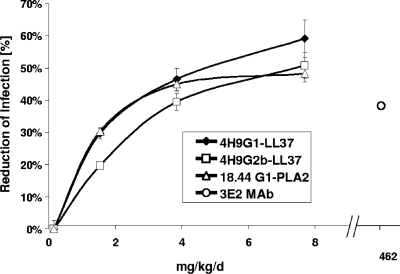
Cell culture-derived supernatants containing the recombinant fusion protein 4H9-G1-LL37, 4H9-G2b-LL37, or 18.44-G1-PLA2 or the 3E2 MAb as a control were orally administered to neonatal mice concomitantly with the oocyst challenge. Increasing dosages of fusion proteins led to greater prophylactic effects on infection, as determined by intestinal section scoring to quantify intracellular C. parvum stages (Fig. 5). The dose-response patterns of 4H9 fusion proteins in both the IgG1 and IgG2b formats were similar. A similar dose-response pattern was also observed with the 18.44-G1-PLA2 fusion protein. The efficacies of all three fusion proteins leveled off as the dose was increased to 7.7 mg/kg/day. The CHO cell supernatant (spent medium) controls had no significant effect on infection levels (data not shown). The positive control, the 3E2 MAb IgM pentamer, had to be used at a dosage of 462 mg/kg/day to induce a 37% reduction in the initiation of infection (lower dosages resulted in insignificant reductions in the initiation of infection [data not shown]). No adverse effects of the treatments were observed based on clinical appearance, growth, and suckling response. Further, no evidence of host toxicity was observed based on histopathological evaluation of intestinal sections for morphological changes.
FIG. 5.
Dose-dependent efficacies of different fusion proteins given orally against C. parvum infection in neonatal mice. Groups of 10 neonatal mice were challenged with C. parvum and were treated twice a day for 5 days with fusion proteins at either 1.5, 3.8, or 7.7 mg/kg/day. Intracellular C. parvum stages in the gut epithelium were quantified by microscopic analysis of gut longitudinal sections. As a positive control, the hybridoma-derived 3E2 MAb was included at 462 mg/kg/day. Mice treated with the CHO cell culture supernatant were considered 100% infected. Data are means ± standard errors of the means.
The data presented above clearly show that multiple recombinant fusion proteins differing either in their binding specificity or in their biocide component can be highly efficacious at reducing C. parvum infection in neonatal mice. Furthermore, the 4H9 and 18.44 fusions have significantly greater neutralizing activity than the hybridoma antibody 3E2, which was used as a comparator, showing efficacy at doses that are 60-fold lower.
Antibody-biocide fusion proteins are more effective than the sum of their parts at reducing the initiation of infection in the neonatal mouse model.
The in vitro data presented in Fig. 2B showed that selected antibody-biocide fusion proteins are significantly more effective at killing sporozoites than an equimolar matched mixture of single antibody and single biocide molecules. To further extend these observations, we compared the efficacy of an antibody plus a biocide administered as separate molecules with that of a fusion protein consisting of the two molecules against a C. parvum oocyst challenge in neonatal mice. The effectiveness of the fusion molecules was significantly greater than that of individual MAb and biocide components given as separate molecules in equimolar amounts (Table 2). The 4H9-biocide fusion molecules reduced intestinal infection by 74 to 81%, whereas the reduction achieved with a combination of 4H9-G1 plus PLA2 was 31%, and that with 4H9-G1 plus LL37 was 23%. The 4H9-G1-LL37, 4H9-G2b-LL37, and 4H9-G1-PLA2 fusion proteins were approximately equivalent in efficacy.
TABLE 2.
Efficacies of fusion proteins compared to those of their components against infection in neonatal mice
| Treatmenta | Dose (mg/kg/day) |
No. of mice | Mean infection score (SEb) | % Infection reduction | ANOVA resultc | |
|---|---|---|---|---|---|---|
| MAb/fusion | Biocide | |||||
| CHO supernatant (negative control) | NAd | NA | 10 | 6.40 (0.34) | 0 | A, B |
| 4H9-G1-LL37 | 9.7 | 9 | 1.67 (0.36) | 74 | D | |
| 4H9-G2b-LL37 | 8.0 | 9 | 1.22 (0.36) | 81 | D | |
| 4H9-G1-PLA2 | 13.5 | 9 | 1.67 (0.36) | 74 | D | |
| 4H9-G1 + LL37 | 9.7 | 0.2 | 10 | 4.90 (0.34) | 23 | B, C |
| 4H9-G1 + rPLA2 | 13.5 | 1.0 | 9 | 4.44 (0.36) | 31 | C |
| 3E2 MAb | 46.5 | 9 | 5.44 (0.36) | 15 | A, B, C | |
| 465.0 | 10 | 1.50 (0.34) | 77 | D | ||
Mice were inoculated with 5 × 104 purified oocysts by gastric intubation and were treated orally with recombinant and control products at 3 h and every 12 h thereafter for a total of 9 treatments. The dose of each biocide given in combination (+) with an antibody was adjusted to be equimolar to that of the antibody. Differences in treatment doses among different molecules were related to product availability and are of no relevance. rPLA2, recombinant PLA2.
Using a pooled estimate of error variance.
Treatments not connected by the same letter are significantly different (alpha = 0.05).
NA, not applicable.
Antibody-biocide fusion proteins directed to different epitopes have a synergistic effect.
Various fusion proteins were created using the variable region of anti-CSL MAb 3E2 (Table 1). The effects of fusion proteins consisting of the 3E2 IgM monomer or the 3E2 IgM halfmer fused with LL37 were compared to those of the 4H9-biocide fusion molecules in the neonatal mouse model. When the 3E2-IgM monomer-LL37 fusion protein was given to mice at a dose of 7.7 mg/kg/day, a significant reduction in the initiation of infection over that with the control treatment was observed, demonstrating that the activity of antibody fusion proteins in vivo can be mediated through different surface-exposed epitopes. This observation further broadens the number of potential targets on the sporozoite surface for fusion protein-mediated neutralization. The most effective molecule of the series was the 3E2-IgM halfmer-LL37 fusion protein, which reduced infection by 82% at a relatively low dose. We have previously observed that combinations of MAbs targeting different neutralization-sensitive antigens can provide significant additive efficacy over that of the individual MAbs (25). Therefore, we next evaluated the effect of a combined fusion protein treatment targeting two distinct epitopes. When 4H9-G1-LL37 and 3E2-Mmono-LL37 were given together, each at half the dose used individually (3.8 mg/kg/day), a significant increase in efficacy over that of 3E2-Mmono-LL37 used alone at the 7.7-mg/kg/day dose was observed, indicating a synergistic effect (Table 3).
TABLE 3.
Testing of 3E2 IgM variants as single or combinatorial treatments in the neonatal mouse model
| Treatmenta | Dose (mg/kg/day) | Mean infection score (SEb) | % Infection reduction | ANOVA resultc |
|---|---|---|---|---|
| CHO SN | NAd | 8.65 (0.28) | 0 | A |
| 3E2-G1 | 7.7 | 5.4 (0.28) | 38 | B |
| 3E2-Mmono-LL37 | 7.7 | 4.5 (0.28) | 48 | B, C, D |
| 4H9-G1-LL37 | 7.7 | 3.7 (0.28) | 57 | D, E |
| 4H9-G1-LL37 + 3E2-Mmono-LL37 | 3.8 + 3.8 | 2.9 (0.28) | 66 | E, F |
| 3E2-Mhalf-LL37 | 2.5 | 1.6 (0.28) | 82 | F |
Mice were inoculated with 5 × 104 purified oocysts and were treated orally with a recombinant monoclonal antibody or antibody fusion protein at the dose indicated. For combinatorial treatments, each fusion protein was used at half the dose. SN, supernatant.
Using a pooled estimate of error variance.
Treatments not connected by same letter are significantly different (alpha = 0.05).
NA, not applicable.
DISCUSSION
In an effort to address the urgent need for therapeutics effective against Cryptosporidium parvum, we created a number of recombinant antibody-peptide and antibody-enzyme fusion proteins and evaluated their efficacies by both in vitro and in vivo assays. These recombinant molecules were constructed using genes derived from the hybridoma libraries developed by Riggs et al. and Schaefer et al. (21, 24, 25) and genes for human secretory phospholipase IIA and human cathelicidin antimicrobial peptide LL37. By using a retroviral expression system without the need for selectable markers, CHO production cell lines were created for each antibody and antibody-fusion protein tested. Overall, the expression levels of the fusion proteins in the CHO system were comparable to those of the monoclonal antibodies, indicating that these molecules offer promise as novel therapeutic entities in that they can be produced effectively in established expression systems. The recombinant proteins were tested as concentrated cell culture supernatants for their abilities to kill sporozoites in vitro and to reduce the initiation of C. parvum infection in a neonatal mouse model.
When sporozoites were exposed directly to recombinant antibodies lacking a fused biocide, only a minimal effect on viability was observed. In contrast, when the same recombinant antibody was fused to LL37, a strong antisporozoite effect was seen, leading to a rapid loss, within minutes, of osmotic equilibrium and sporozoite viability. Similarly, a fusion protein comprising 18.44-G1 and PLA2 showed significantly enhanced activity over 18.44-G1 and PLA2 used as separate molecules in vitro.
Exposure of 4H9-G1-LL37 to a low pH did not reduce the activity of the fusion protein; rather, it increased the antisporozoite activity. A possible explanation may lie in the observation of Johansson et al. (15), who have shown that at a low pH (pH 2 to 3), the LL37 “′uncoils,” which could potentially release inhibiting components bound to it. The same workers also showed that upon neutralization of the pH, the LL37 regains its α-helical conformation. In other animal studies, now in progress, we have observed that antibody-LL37 peptide fusion proteins are resistant to degradation during passage through the stomach and maintain binding specificity in the absence of antacid treatment (data not shown), findings consistent with the data presented in Fig. 4.
When administered orally to neonatal mice, coincident with a C. parvum oocyst challenge, the fusion proteins showed dose-dependent responses in their abilities to prophylactically reduce infection. The fusion products 4H9-G1-LL37, 4H9-G2b-LL37, and 4H9-G1-PLA2 each had an effect equivalent to that of the 3E2 MAb, but when given to mice at a 60-fold-lower dosage than that of 3E2. Heretofore, the 3E2 MAb has been considered the neutralizing monoclonal antibody standard for comparison for passive immunization against C. parvum (24). When selected antibodies and biocides are administered as single molecules, each at a concentration equimolar to that of the corresponding fusion protein, they have a significantly lower neutralizing effect than the fusion protein. The binding of the high-affinity antibody allows the delivery of a low, but effective, dose of the antimicrobial peptide LL37 or the PLA2 enzyme to the parasite surface.
Overall, our in vivo mouse model data show a stronger effect of antibody-biocide fusion proteins on C. parvum infection (up to 82% prophylactic reduction of intestinal cell infection) than the in vitro viability assay (up to 50% killing of sporozoites). Since this has been seen consistently in a large number of experiments outside the scope of this paper, it is quite possible that the measurement of “killing” by the in vitro assay underestimates the actual damage that the molecules have done to the organism. It is possible that neutrophils, monocytes, and macrophages are stimulated through interaction with the Fc portion of the immunoglobulin and potentially by the LL37 peptide (5, 27) in immunologically sensitive locations such as Peyer's patches. Such interactions could lead to a more generalized immune stimulation that might beneficially impact the clearance of sporozoites, thus complementing the direct killing effect of the fusion proteins.
The most active anticryptosporidial fusion protein we examined was 3E2-Mhalf-LL37, which comprises only one IgM heavy chain fused to LL37 and one light chain. The smaller size of this molecule (94.7 kDa) may allow greater access to the known repetitive CSL epitope recognized by the 3E2 MAb and may thereby facilitate the interaction of LL37 with the parasite membrane, ultimately resulting in a higher level of killing. It is also conceivable that the pair of biocides on the C terminus of the protein interfere with one another and that a single molecule can be more effective.
The concept of using antibody targeting to deliver an antimicrobial payload has been explored for other organisms. Baral et al. (4) demonstrated the efficacy of nanobody fusion targeting of ApoL to trypanosomes, and Fischer and colleagues (18) have shown the efficacy of using antibody fusion proteins targeting Fusarium spp. Our results provide further evidence that the ability to build and express recombinant protein molecules combining elements from the innate and adaptive immune responses offers a novel approach to the combinatorial design of antimicrobials. For the first time we have presented an orally delivered recombinant protein immunotherapeutic that is effective at reducing Cryptosporidium parvum infection. Subsequent studies will examine whether this approach is also effective in a treatment model and whether it can bring about reductions in clinical disease in hosts naturally infected by C. parvum.
Acknowledgments
This study was supported by National Institutes of Health SBIR grant R44AI56944.
Footnotes
Published ahead of print on 19 January 2010.
REFERENCES
- 1.Aley, S. B., M. Zimmerman, M. Hetsko, M. E. Selsted, and F. D. Gillin.1994. Killing of Giardia lamblia by cryptdins and cationic neutrophil peptides. Infect. Immun. 62:5397-5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arrowood, M. J., and K. Donaldson.1996. Improved purification methods for calf-derived Cryptosporidium parvum oocysts using discontinuous sucrose and cesium chloride gradients. J. Eukaryot. Microbiol. 43:89S. [DOI] [PubMed] [Google Scholar]
- 3.Arrowood, M. J., J. M. Jaynes, and M. C. Healey.1991. In vitro activities of lytic peptides against the sporozoites of Cryptosporidium parvum. Antimicrob. Agents Chemother. 35:224-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baral, T. N., S. Magez, B. Stijlemans, K. Conrath, B. Vanhollebeke, E. Pays, S. Muyldermans, and P. De Baetselier.2006. Experimental therapy of African trypanosomiasis with a nanobody-conjugated human trypanolytic factor. Nat. Med. 12:580-584. [DOI] [PubMed] [Google Scholar]
- 5.Bowdish, D. M., D. J. Davidson, Y. E. Lau, K. Lee, M. G. Scott, and R. E. Hancock.2005. Impact of LL-37 on anti-infective immunity. J. Leukoc. Biol. 77:451-459. [DOI] [PubMed] [Google Scholar]
- 6.Cama, V. A., J. M. Ross, S. Crawford, V. Kawai, R. Chavez-Valdez, D. Vargas, A. Vivar, E. Ticona, M. Navincopa, J. Williamson, Y. Ortega, R. H. Gilman, C. Bern, and L. Xiao.2007. Differences in clinical manifestations among Cryptosporidium species and subtypes in HIV-infected persons. J. Infect. Dis. 196:684-691. [DOI] [PubMed] [Google Scholar]
- 7.Carryn, S., D. A. Schaefer, M. Imboden, E. J. Homan, R. D. Bremel, and M. W. Riggs.2004. Phospholipases and cationic peptides neutralize Cryptosporidium parvum sporozoite infectivity by either parasiticidal or non-parasiticidal mechanisms. Int. J. Antimicrob. Agents 24(Suppl. 2):S117. [DOI] [PubMed] [Google Scholar]
- 8.de Graaf, D. C., E. Vanopdenbosch, L. M. Ortega-Mora, H. Abbassi, and J. E. Peeters.1999. A review of the importance of cryptosporidiosis in farm animals. Int. J. Parasitol. 29:1269-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dillingham, R., A. Lima, and R. Guerrant.2002. Cryptosporidiosis: epidemiology and impact. Microbes Infect. 4:1059. [DOI] [PubMed] [Google Scholar]
- 10.Fayer, R.2008. General biology, p. 10-11. In R. Fayer and L. Xiao (ed.), Cryptosporidium and cryptosporidiosis, 2nd ed. CRC Press, Boca Raton, FL.
- 11.Giacometti, A., O. Cirioni, M. S. Del Prete, B. Skerlavaj, R. Circo, M. Zanetti, and G. Scalise.2003. In vitro effect on Cryptosporidium parvum of short-term exposure to cathelicidin peptides. J. Antimicrob. Chemother. 51:843-847. [DOI] [PubMed] [Google Scholar]
- 12.Heine, J., J. F. Pohlenz, H. W. Moon, and G. N. Woode.1984. Enteric lesions and diarrhea in gnotobiotic calves monoinfected with Cryptosporidium species. J. Infect. Dis. 150:768-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hong, D. K., C. J. Wong, and K. Gutierrez.2007. Severe cryptosporidiosis in a seven-year-old renal transplant recipient: case report and review of the literature. Pediatr. Transplant. 11:94-100. [DOI] [PubMed] [Google Scholar]
- 14.Huang, D. B., C. Chappell, and P. C. Okhuysen.2004. Cryptosporidiosis in children. Semin. Pediatr. Infect. Dis. 15:253-259. [DOI] [PubMed] [Google Scholar]
- 14a.Institute of Laboratory Animal Resources.1985. Guide for the care and use of laboratory animals. Public Health Service, Bethesda, MD.
- 15.Johansson, J., G. H. Gudmundsson, M. E. Rottenberg, K. D. Berndt, and B. Agerberth.1998. Conformation-dependent antibacterial activity of the naturally occurring human peptide LL-37. J. Biol. Chem. 273:3718-3724. [DOI] [PubMed] [Google Scholar]
- 16.McGwire, B. S., C. L. Olson, B. F. Tack, and D. M. Engman.2003. Killing of African trypanosomes by antimicrobial peptides. J. Infect. Dis. 188:146-152. [DOI] [PubMed] [Google Scholar]
- 17.Okhuysen, P. C., and A. C. White, Jr.1999. Parasitic infections of the intestines. Curr. Opin. Infect. Dis. 12:467-472. [DOI] [PubMed] [Google Scholar]
- 18.Peschen, D., H. P. Li, R. Fischer, F. Kreuzaler, and Y. C. Liao.2004. Fusion proteins comprising a Fusarium-specific antibody linked to antifungal peptides protect plants against a fungal pathogen. Nat. Biotechnol. 22:732-738. [DOI] [PubMed] [Google Scholar]
- 19.Riggs, M. W., D. A. Schaefer, and S. Carryn.2004. Control of bovine cryptosporidiosis using antibody-based strategies to disrupt parasite ligand function and target delivery of parasiticidal agents, p. 162. In Proceedings of the 85th Conference of Research Workers in Animal Diseases. Conference of Research Workers in Animal Diseases, Fort Collins, CO.
- 20.Riggs, M. W., V. A. Cama, H. L. Leary, Jr., and C. R. Sterling.1994. Bovine antibody against Cryptosporidium parvum elicits a circumsporozoite precipitate-like reaction and has immunotherapeutic effect against persistent cryptosporidiosis in SCID mice. Infect. Immun. 62:1927-1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riggs, M. W., T. C. McGuire, P. H. Mason, and L. E. Perryman.1989. Neutralization-sensitive epitopes are exposed on the surface of infectious Cryptosporidium parvum sporozoites. J. Immunol. 143:1340-1345. [PubMed] [Google Scholar]
- 22.Riggs, M. W., M. R. McNeil, L. E. Perryman, A. L. Stone, M. S. Scherman, and R. M. O'Connor.1999. Cryptosporidium parvum sporozoite pellicle antigen recognized by a neutralizing monoclonal antibody is a beta-mannosylated glycolipid. Infect. Immun. 67:1317-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riggs, M. W., and L. E. Perryman.1987. Infectivity and neutralization of Cryptosporidium parvum sporozoites. Infect. Immun. 55:2081-2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riggs, M. W., A. L. Stone, P. A. Yount, R. C. Langer, M. J. Arrowood, and D. L. Bentley.1997. Protective monoclonal antibody defines a circumsporozoite-like glycoprotein exoantigen of Cryptosporidium parvum sporozoites and merozoites. J. Immunol. 158:1787-1795. [PubMed] [Google Scholar]
- 25.Schaefer, D. A., B. A. Auerbach-Dixon, and M. W. Riggs.2000. Characterization and formulation of multiple epitope-specific neutralizing monoclonal antibodies for passive immunization against cryptosporidiosis. Infect. Immun. 68:2608-2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schnyder, M., L. Kohler, A. Hemphill, and P. Deplazes.2009. Prophylactic and therapeutic efficacy of nitazoxanide against Cryptosporidium parvum in experimentally challenged neonatal calves. Vet. Parasitol. 160:149-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sørensen, O., K. Arnljots, J. B. Cowland, D. F. Bainton, and N. Borregaard.1997. The human antibacterial cathelicidin, hCAP-18, is synthesized in myelocytes and metamyelocytes and localized to specific granules in neutrophils. Blood 90:2796-2803. [PubMed] [Google Scholar]
- 28.Stockdale, H. D., J. A. Spencer, and B. L. Blagburn.2008. Prophylaxis and chemotherapy, p. 28-35. In R. Fayer and L. Xiao (ed.), Cryptosporidium and cryptosporidiosis, 2nd ed. CRC Press, Boca Raton, FL.
- 29.Sulzyc-Bielicka, V., W. Kuzna-Grygiel, L. Kolodziejczyk, D. Bielicki, J. Kladny, M. Stepien-Korzonek, and B. Telatynska-Smieszek.2007. Cryptosporidiosis in patients with colorectal cancer. J. Parasitol. 93:722-724. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka, T., Y. Omata, A. Saito, K. Shimazaki, K. Yamauchi, M. Takase, K. Kawase, K. Igarashi, and N. Suzuki.1995. Toxoplasma gondii: parasiticidal effects of bovine lactoferricin against parasites. Exp. Parasitol. 81:614-617. [DOI] [PubMed] [Google Scholar]
- 31.Tarver, A. P., D. P. Clark, G. Diamond, J. P. Russell, H. Erdjument-Bromage, P. Tempst, K. S. Cohen, D. E. Jones, R. W. Sweeney, M. Wines, S. Hwang, and C. L. Bevins.1998. Enteric beta-defensin: molecular cloning and characterization of a gene with inducible intestinal epithelial cell expression associated with Cryptosporidium parvum infection. Infect. Immun. 66:1045-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tzipori, S., and G. Widmer.2008. A hundred-year retrospective on cryptosporidiosis. Trends Parasitol. 24:184-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiersma, E. J., and M. J. Shulman.1995. Assembly of IgM. Role of disulfide bonding and noncovalent interactions. J. Immunol. 154:5265-5272. [PubMed] [Google Scholar]
- 34.Xiao, L., and U. M. Ryan.2004. Cryptosporidiosis: an update in molecular epidemiology. Curr. Opin. Infect. Dis. 17:483-490. [DOI] [PubMed] [Google Scholar]
- 35.Zaalouk, T. K., M. Bajaj-Elliott, J. T. George, and V. McDonald.2004. Differential regulation of beta-defensin gene expression during Cryptosporidium parvum infection. Infect. Immun. 72:2772-2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu, G.2008. Biochemistry, p. 69-71. In R. Fayer and L. Xiao (ed.), Cryptosporidium and cryptosporidiosis, 2nd ed. CRC Press, Boca Raton, FL.
- 37.Ziegler, H. K., and C. A. Orlin.1984. Analysis of Listeria monocytogenes antigens with monoclonal antibodies. Clin. Invest. Med. 7:239-242. [PubMed] [Google Scholar]
- 38.Zufferey, R., J. E. Donello, D. Trono, and T. J. Hope.1999. Woodchuck hepatitis virus posttranscriptional regulatory element enhances expression of transgenes delivered by retroviral vectors. J. Virol. 73:2886-2892. [DOI] [PMC free article] [PubMed] [Google Scholar]