Abstract
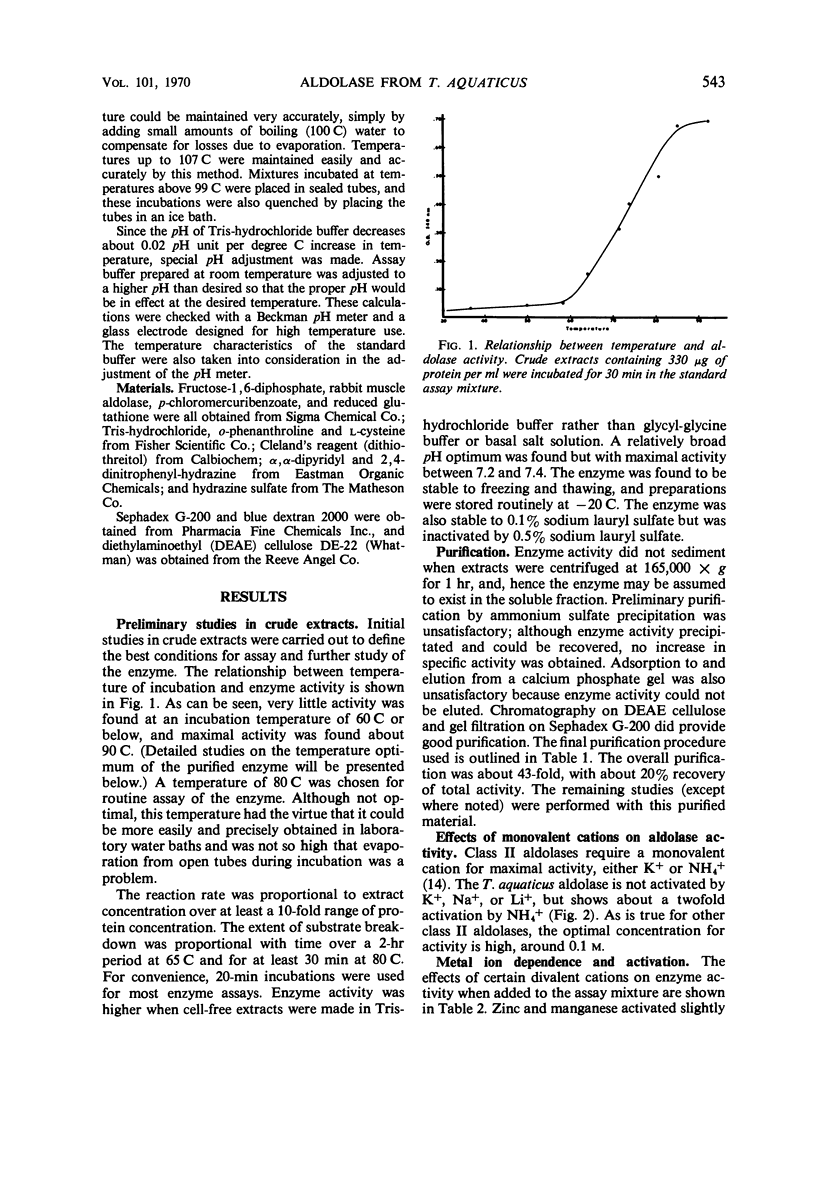
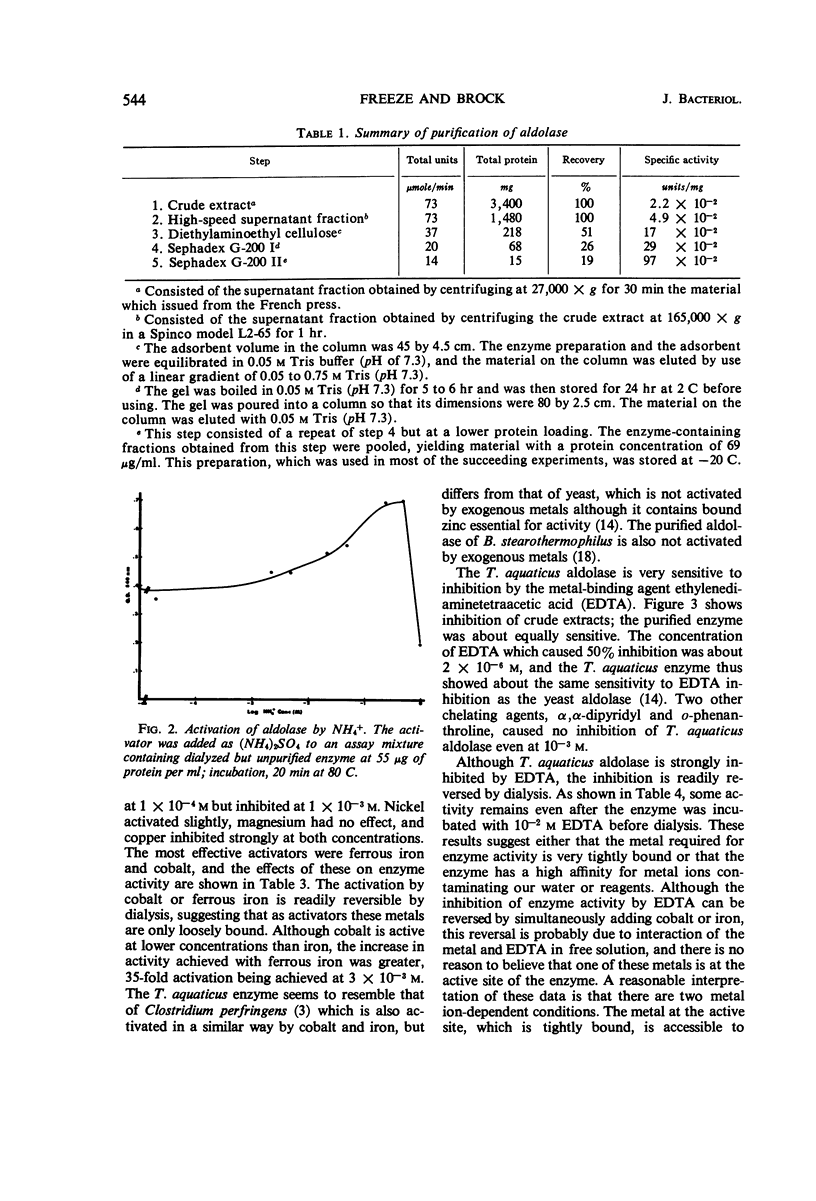
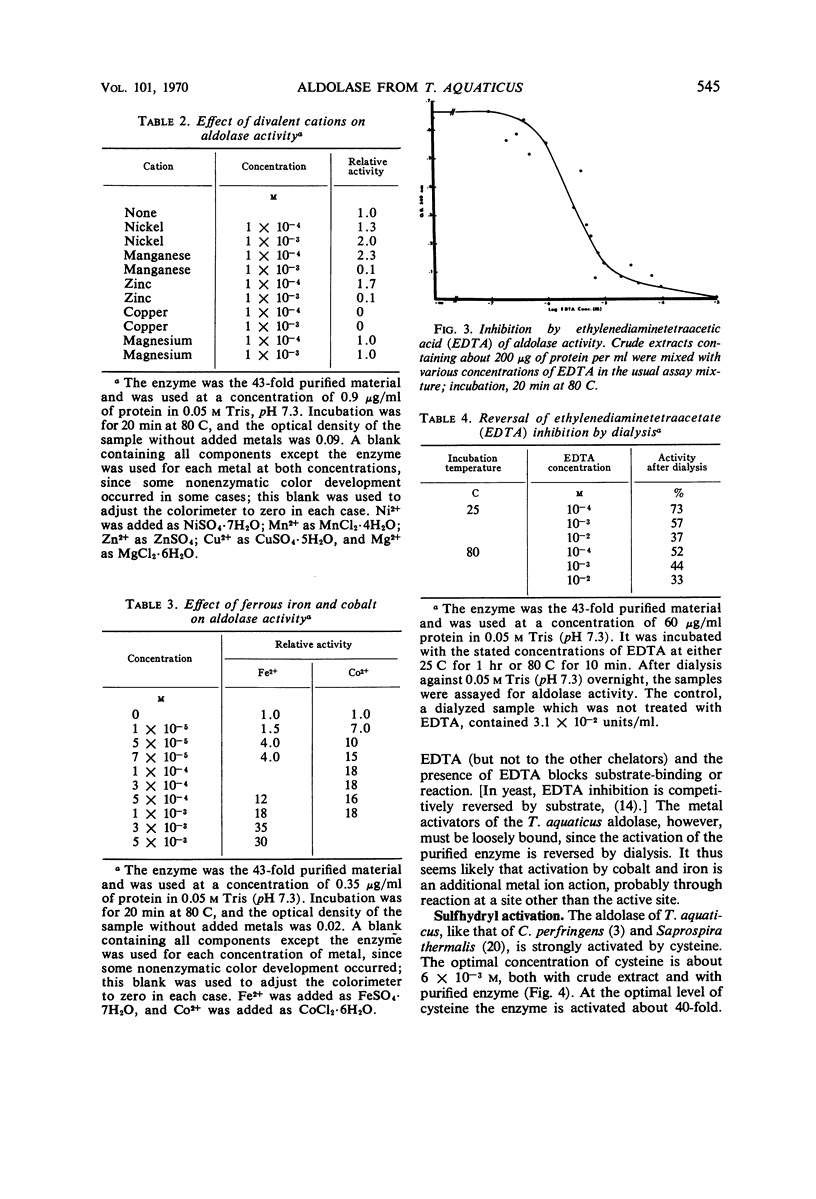
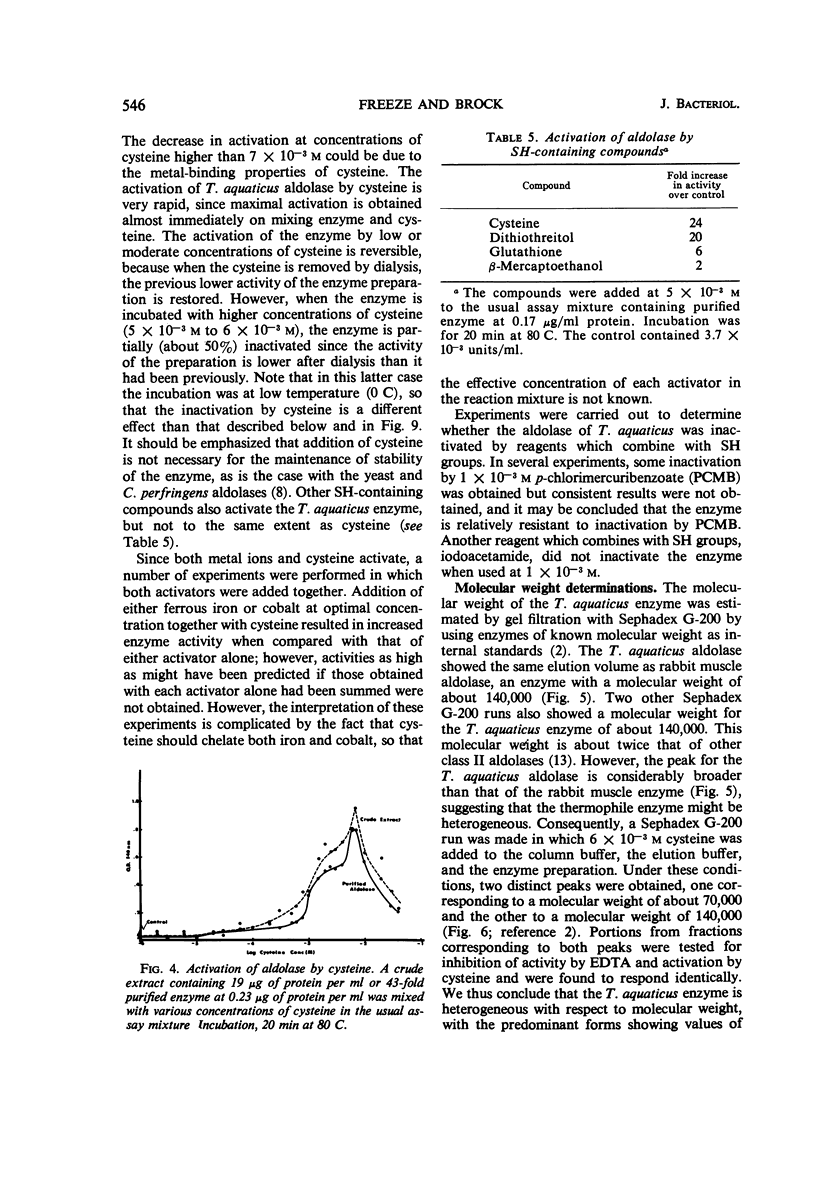
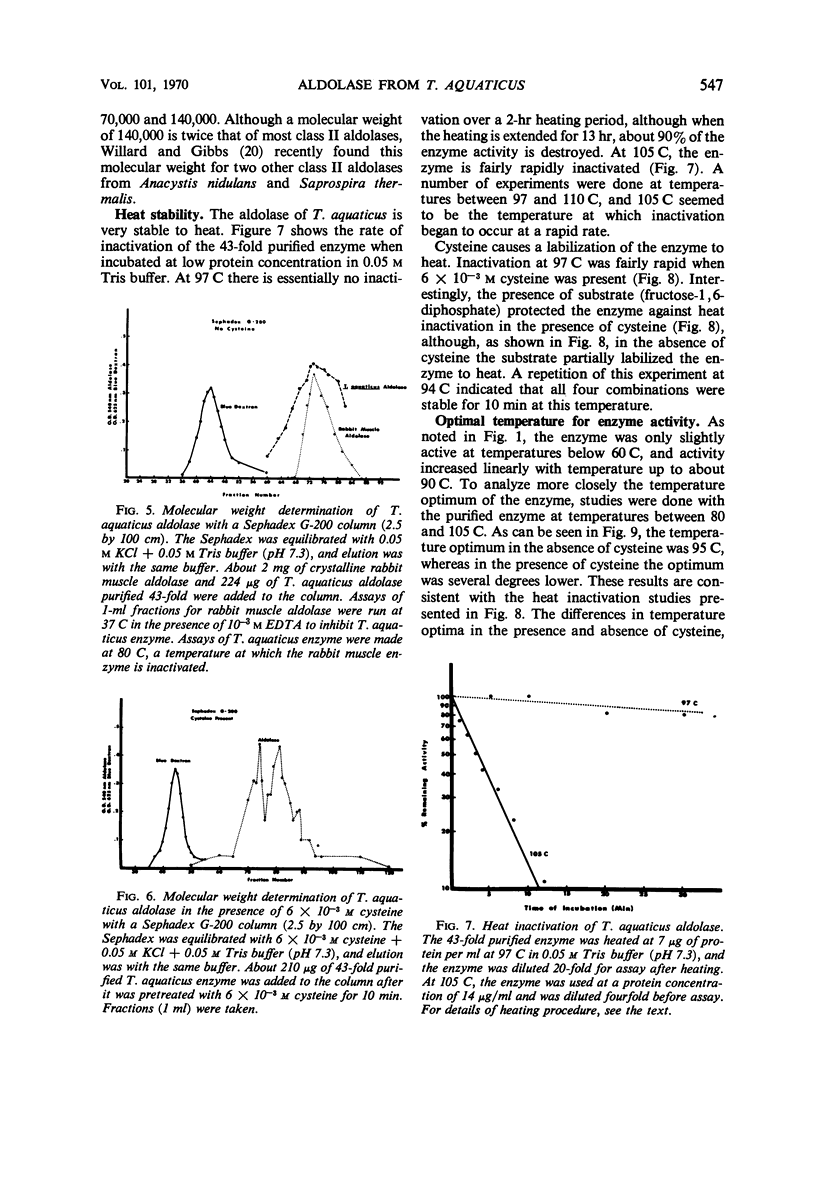
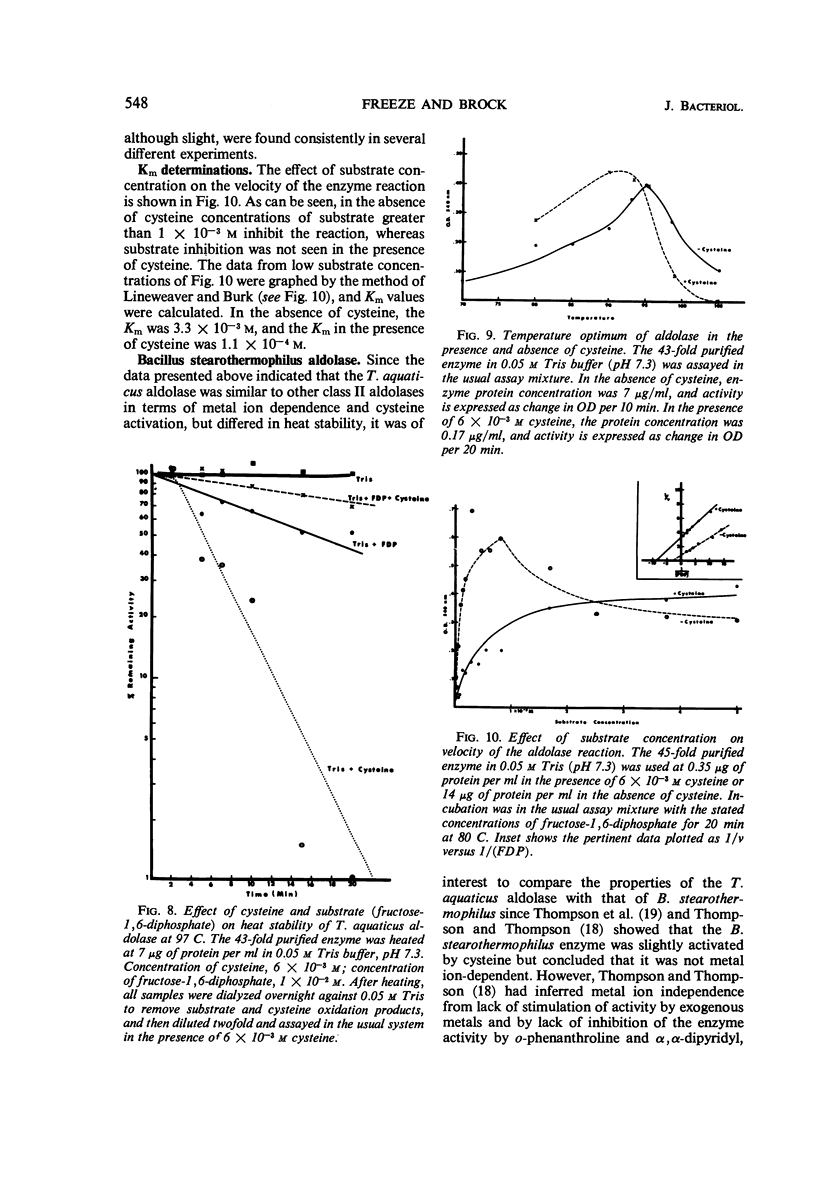
Data are presented on the purification and properties of the thermostable fructose-1,6-diphosphate aldolase of Thermus aquaticus, a nonsporulating, extreme thermophile. The enzyme shows little activity at temperatures below 60 C and optimal activity at about 95 C. The enzyme was purified 43-fold by diethylaminoethyl cellulose column chromatography and Sephadex G-200 gel filtration. The enzyme is activated by high concentrations of NH4+ and low concentrations of Fe2+ and Co2+ and is strongly inhibited by ethylenediaminetetraacetic acid (EDTA). The activation by Fe2+ and Co2+ and the inhibition by EDTA are both reversed by dialysis. The enzyme is greatly activated by cysteine and less so by other sulfhydryl compounds. Activation by cysteine is reversible by dialysis. The purified enzyme had a molecular weight as determined by Sephadex G-200 gel filtration of 140,000; after incubation of enzyme with cysteine, another molecular species was also found with a molecular weight of 70,000. The purified enzyme is stable at low protein concentrations to 97 C but is rapidly inactivated at 105 C. In cysteine the enzyme is more heat labile; heat inactivation in the presence of cysteine is prevented by substrate, although, in the absence of cysteine, substrate partially labilizes the enzyme to heat. The temperature optimum for enzyme activity is several degrees lower in the presence of cysteine than in its absence, and the Km is threefold lower. It is concluded that the T. aquaticus enzyme resembles some other aldolases of Rutter's class II, except for its extreme heat stability. The T. aquaticus enzyme is compared with that of Bacillus stearothermophilus, a moderate thermophile. Although the T. aquaticus enzyme is considerably more heat stable, the enzymes from the two thermophiles have many similarities. New data are presented which show that the B. stearothermophilus aldolase is metal ion-dependent, in disagreement with earlier reports.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amelunxen R., Lins M. Comparative thermostability of enzymes from Bacillus stearothermophilus and Bacillus cereus. Arch Biochem Biophys. 1968 Jun;125(3):765–769. doi: 10.1016/0003-9861(68)90512-2. [DOI] [PubMed] [Google Scholar]
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARD R. C., GUNSALUS I. C. Glucose metabolism of Clostridium perfringens: existence of metallo-aldolase. J Bacteriol. 1950 Mar;59(3):387–400. doi: 10.1128/jb.59.3.387-400.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock T. D., Freeze H. Thermus aquaticus gen. n. and sp. n., a nonsporulating extreme thermophile. J Bacteriol. 1969 Apr;98(1):289–297. doi: 10.1128/jb.98.1.289-297.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock T. D. Life at high temperatures. Evolutionary, ecological, and biochemical significance of organisms living in hot springs is discussed. Science. 1967 Nov;158(3804):1012–1019. doi: 10.1126/science.158.3804.1012. [DOI] [PubMed] [Google Scholar]
- Friedman S. M. Protein-synthesizing machinery of thermophilic bacteria. Bacteriol Rev. 1968 Mar;32(1):27–38. doi: 10.1128/br.32.1.27-38.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert D., Gordon H., Subrahmanyan V., Green D. E. Zymohexase: With an Addendum by E. C. Bate-Smith. Biochem J. 1940 Jul;34(7):1108–1123. doi: 10.1042/bj0341108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAGANNATHAN V., SINGH K., DAMODARAN M. Carbohydrate metabolism in citric acid fermentation. 4. Purification and properties of aldolase from Aspergillus niger. Biochem J. 1956 May;63(1):94–105. doi: 10.1042/bj0630094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOFFLER H. Protoplasmic differences between mesophiles and thermophiles. Bacteriol Rev. 1957 Dec;21(4):227–240. doi: 10.1128/br.21.4.227-240.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- RICHARDS O. C., RUTTER W. J. Preparation and properties of yeast aldolase. J Biol Chem. 1961 Dec;236:3177–3184. [PubMed] [Google Scholar]
- RUTTER W. J. EVOLUTION OF ALDOLASE. Fed Proc. 1964 Nov-Dec;23:1248–1257. [PubMed] [Google Scholar]
- Rutter W. J., Rajkumar T., Penhoet E., Kochman M., Valentine R. Aldolase variants: structure and physiological significance. Ann N Y Acad Sci. 1968 Jun 14;151(1):102–117. doi: 10.1111/j.1749-6632.1968.tb11881.x. [DOI] [PubMed] [Google Scholar]
- Singleton R., Jr, Kimmel J. R., Amelunxen R. E. The amino acid composition and other properties of thermostable glyceraldehyde 3-phosphate dehydrogenase from Bacillus stearothermophilus. J Biol Chem. 1969 Mar 25;244(6):1623–1630. [PubMed] [Google Scholar]
- THOMPSON P. J., THOMPSON T. L. Some characteristics of a purified heat-stable aldolase. J Bacteriol. 1962 Oct;84:694–700. doi: 10.1128/jb.84.4.694-700.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMPSON T. L., MILITZER W. E., GEORGI C. E. Partial denaturation of a bacterial aldolase without loss of activity. J Bacteriol. 1958 Oct;76(4):337–341. doi: 10.1128/jb.76.4.337-341.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard J. M., Gibbs M. Purification and characterization of the fructose diphosphate aldolases from Anacystis is nidulans and Saprospira thermalis. Biochim Biophys Acta. 1968 Feb 5;151(2):438–448. doi: 10.1016/0005-2744(68)90112-5. [DOI] [PubMed] [Google Scholar]



