Abstract
The bacterial phosphoenolpyruvate phosphotransferase system (PTS) is a highly conserved phosphotransfer cascade whose components modulate many cellular functions in response to carbohydrate availability. Here, we further elucidate PTS control of Vibrio cholerae carbohydrate transport and activation of biofilm formation on abiotic surfaces. We then define the role of the PTS in V. cholerae colonization of the adult germfree mouse intestine. We report that V. cholerae colonizes both the small and large intestines of the mouse in a distribution that does not change over the course of a month-long experiment. Because V. cholerae possesses many PTS-independent carbohydrate transporters, the PTS is not essential for bacterial growth in vitro. However, we find that the PTS is essential for colonization of the germfree adult mouse intestine and that this requirement is independent of PTS regulation of biofilm formation. Therefore, competition for PTS substrates may be a dominant force in the success of V. cholerae as an intestinal pathogen. Because the PTS plays a role in colonization of environmental surfaces and the mammalian intestine, we propose that it may be essential to successful transit of V. cholerae through its life cycle of pathogenesis and environmental persistence.
In water, in soil, and in the eukaryotic host, bacteria survive by monitoring their environment and continuously adjusting their nutrient uptake and catabolism machinery to mirror environmental availability. While the ability to maximize environmental nutrients may not be critical in nutrient-rich environments such as those found in the laboratory, in natural environments where nutrients are scarce and competition for these nutrients may be fierce, rapid and precise adaptation is the key to survival.
The bacterial phosphoenolpyruvate phosphotransferase system (PTS) is a multicomponent phosphotransfer cascade that coordinates transport and phosphorylation of selected carbohydrates through PTS-associated transporters. Because transport of PTS substrates rapidly depletes the PTS of phosphorylated intermediates, the phosphorylation states of PTS components serve as cytoplasmic reporters of environmental nutrient availability (5).
Phosphotransfer through the PTS is initiated when the cytoplasmic phosphoenolpyruvate-protein phosphotransferase, or enzyme I (EI), accepts a phosphate from phosphoenolpyruvate. EI is required for the transport of all PTS substrates. EI transfers its phosphate group to histidine protein (HPr), another cytoplasmic protein involved in the transport of most PTS substrates. HPr transfers its phosphate to any of a number of enzymes II, which are membrane-associated complexes that carry out the transport and phosphorylation of specific PTS substrates and comprise multiple subunits (EIIA, EIIB, EIIC, and sometimes EIID). A fructose-specific multidomain protein, FPr, which consists of an N-terminal EIIA-like domain, a central domain of unknown function, and a C-terminal HPr-like domain, is also present in many Gram-negative bacteria (15). In the transport of fructose by the Escherichia coli PTS, FPr is favored over HPr in the phosphotransfer cascade. Furthermore, a parallel PTS system is encoded by many bacterial genomes (29, 30). This system is termed the PTSNtr because of its chromosomal proximity to the genes encoding the nitrogen-specific sigma factor, σ54. The PTSNtr includes an EI homolog (EINtr), an HPr homolog (NPr), and an EIIA homolog (EIIANtr). Although there is some evidence of cross talk between the carbon-specific PTS and the PTSNtr (26), the latter system is thought to function primarily in regulation rather than transport.
Vibrio cholerae is a Gram-negative, halophilic bacterium that inhabits saline and freshwater aquatic environments (6). In these environments, V. cholerae is hypothesized to attach to abiotic surfaces by elaboration of an exopolysaccharide termed VPS in a process known as VPS-dependent biofilm formation (40). Many of the vps genes, which are required for VPS synthesis, are carried in two large operons consisting of vpsA to vpsK and vpsL to vpsQ (39).
Some strains of V. cholerae are also able to cause the human diarrheal disease cholera after ingestion in contaminated food or water. Disease requires the presence of the toxin-coregulated pilus, the primary intestinal colonization factor of V. cholerae (9, 35), as well as cholera toxin, which acts on the epithelial cells of the small intestine to produce an intense, osmotic diarrhea. Therefore, the ability to colonize the small intestine is an important component in V. cholerae virulence.
We hypothesized that to persist in all these environments, V. cholerae must sense and respond to preferred carbon sources and, therefore, that the regulatory and transport functions of the PTS might figure prominently in its success. Only two studies thus far have examined the transport and regulatory functions of V. cholerae PTS components. One of these documented a regulatory function for the phosphorylated form of EI in the repression of VPS-dependent biofilm formation on abiotic surfaces (12), while the other identified the chitobiose transport apparatus (1). In this work, we performed a mutagenesis-based study of the roles of V. cholerae PTS components in sugar transport and VPS-dependent biofilm formation. To evaluate the role of the PTS signal transduction cascade in the colonization of the adult mammalian intestine, we implemented and characterized an adult germfree mouse model of disease. We have shown that V. cholerae is present in the distal small intestine as well as the large intestine and have found that the requirement for the PTS in intestinal colonization is independent of VPS synthesis. These results suggest a role for the V. cholerae PTS in the colonization of environmental surfaces and the adult mammalian intestine. The PTS is found in almost all bacterial species and has been shown previously to play important roles in the surface attachment and virulence of many of these species (14, 16, 18, 27, 31, 33, 34). Because the PTS is also highly conserved, we suggest that it may be an excellent target for inhibition of colonization of both environmental surfaces and host epithelia by diverse bacteria.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
The bacterial strains and plasmids used in this study are listed in Table 1. V. cholerae strain PW249 was used for all mouse colonization experiments. V. cholerae strain PW357 was used for biofilm assays. Bacteria were cultivated in minimal medium (MM) alone or MM supplemented with a carbon source (Sigma), as specified below, at a 0.5% (wt/vol) concentration (12). Where noted, the medium was also supplemented with streptomycin (100 μg/ml), ampicillin (50 or 100 μg/ml), or l-arabinose (0.04%, wt/vol). Where indicated, 0.1 M phosphate-buffered saline (PBS) was used (pH 7.0).
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Genotype or description | Source or reference |
|---|---|---|
| E. coli SM10λpir | thi thr leu tonA lacY supE recA::RP4-2-Tc::Mu (λpirR6K) Kmr | 21 |
| V. cholerae strains | ||
| PW357 | MO10 lacZ::vpsLp→lacZ Smr | 8 |
| PW751 | MO10 lacZ::vpsLp→lacZ ΔPTS Smr | 12 |
| PW836 | MO10 lacZ::vpsLp→lacZ ΔEIIAGlc Smr | 12 |
| PW961 | MO10 lacZ::vpsLp→lacZ ΔEI Smr | 12 |
| PW964 | MO10 lacZ::vpsLp→lacZ ΔHPr Smr | 12 |
| MO10 lacZ::vpsLp→lacZ ΔFPr Smr | This study | |
| MO10 lacZ::vpsLp→lacZ ΔNPr Smr | This study | |
| PW965 | MO10 lacZ::vpsLp→lacZ ΔHPr ΔFPr Smr | This study |
| MO10 lacZ::vpsLp→lacZ ΔHPr ΔNPr Smr | This study | |
| MO10 lacZ::vpsLp→lacZ ΔPTS ΔFPr Smr | This study | |
| MO10 lacZ::vpsLp→lacZ ΔEIIANtr1 Smr | This study | |
| MO10 lacZ::vpsLp→lacZ ΔEIIANtr2 Smr | This study | |
| MO10 lacZ::vpsLp→lacZ VC0672::pGP704 Smr Apr | This study | |
| MO10 lacZ::vpsLp→lacZ VCA1045::pGP704 Smr Apr | This study | |
| MO10 lacZ::vpsLp→lacZ VCA0245::pGP704 Smr Apr | This study | |
| MO10 lacZ::vpsLp→lacZ VC1820::pGP704 Smr Apr | This study | |
| MO10 lacZ::vpsLp→lacZ VC1822::pGP704 Smr Apr | This study | |
| MO10 lacZ::vpsLp→lacZ VC1826::pGP704 Smr Apr | This study | |
| PW249 | MO10 | This study |
| MO10 ΔEI Smr | This study | |
| MO10 ΔEI ΔvpsL Smr | This study | |
| Plasmids for construction of deletions | ||
| pWM91 | oriR6K mobRP4 lacI pTac tnp mini-Tn10(Km) Kmr Apr | 20 |
| pWM91ΔPTS | pWM91 carrying an unmarked, in-frame deletion of the PTS operon | 12 |
| pWM91ΔEIIAGlc | pWM91 carrying an unmarked, in-frame deletion in VC0964 | 12 |
| pWM91ΔEI | pWM91 carrying an unmarked, in-frame deletion in VC0965 | 12 |
| pWM91ΔHPr | pWM91 carrying an unmarked, in-frame deletion in VC0966 | 12 |
| pWM91ΔNPr | pWM91 carrying an unmarked, in-frame deletion in VC2533 | This study |
| pWM91ΔFPr | pWM91 carrying an unmarked, in-frame deletion in VCA0518 | This study |
| pWM91ΔEIIANtr1 | pWM91 carrying an unmarked, in-frame deletion in VC2531 | This study |
| pWM91ΔEIIANtr2 | pWM91 carrying an unmarked, in-frame deletion in VC1824 | This study |
| Plasmids for construction of insertions | ||
| pGP704::VC1820 | pGP704 carrying an internal fragment of VC1820 | This study |
| pGP704::VC1822 | pGP704 carrying an internal fragment of VC1822 | This study |
| pGP704::VC1826 | pGP704 carrying an internal fragment of VC1826 | This study |
| pGP704::VCA1045 | pGP704 carrying an internal fragment of VCA1045 | This study |
| pGP704::VCA0245 | pGP704 carrying an internal fragment of VCA0245 | This study |
| pGP704::VC0672 | pGP704 carrying an internal fragment of VC0672 | This study |
| pGP704::VC1283 | pGP704 carrying an internal fragment of VC1283 | This study |
| Plasmids used in rescue experiments | ||
| pBAD-TOPO-EI | pBAD-TOPO carrying the coding sequence of VC0965 | 12 |
| pBAD-TOPO-HPr | pBAD-TOPO carrying the coding sequence of VC0966 | 12 |
| pBAD-TOPO-HPr(H15A) | pBAD-TOPO carrying a VC0966 variant encoding an H-to-A mutation at position 15 | This study |
| pBAD-TOPO-FPr | pBAD-TOPO carrying the coding sequence of VCA0518 | This study |
| pBAD-TOPO-FPr(H324A) | pBAD-TOPO carrying a VCA0518 variant encoding an H-to-A mutation at position 324 | This study |
| pBAD-TOPO-FPr(309-401) | pBAD-TOPO carrying a fragment of VCA0518 encoding positions 309 to 401 | This study |
Construction of insertion mutants.
An internal fragment of each of the following targeted genes (VC0672, VC1820, VC1822, VC1826, VCA1045, VCA0245, and VC1283) was amplified by PCR using the primer sets listed in Table 2, which all contained an EcoRI restriction site. The resulting amplification products were subcloned into the cloning vector pCR2.1-TOPO (Invitrogen). After sequence verification, the gene fragment was removed from the cloning vector by restriction digestion with EcoRI (Invitrogen) and purified using the QIAquik gel purification kit (Qiagen). The suicide vector pGP704 (21) was also digested with EcoRI and then treated with alkaline phosphatase. The digested suicide vector and gene fragment were ligated with T4 ligase (Invitrogen), and then E. coli strain SM10λpir was transformed with the construct. The suicide plasmid was integrated into the gene of interest by conjugation of SM10λpir with the relevant V. cholerae strain. Mutants were selected on Luria-Bertani (LB) agar plates supplemented with streptomycin and ampicillin and checked by PCR analysis.
TABLE 2.
Primers used in this study
| Primer function | Relevant gene or gene product | Primer name | Sequence |
|---|---|---|---|
| Gene deletion | FPr | LH55 | TGCTCCACCACAGCCATCACT |
| LH56 | TAACGAGCGGCCGCACATTCTTAACTCCTGTCTGCC | ||
| LH57 | TGCGGCCGCTCGTTAGGCTTAGGCGAAGGTTAAGG | ||
| LH58 | TCATGCTGCGCAGTTGGGCA | ||
| NPr | LH43 | CTGCTTGGCCCACTCAAAG | |
| LH44 | TAACGAGCGGCCGCACATAGGGGCTCCTAGGATTG | ||
| LH45 | TGCGGCCGCTCGTTAGAGTAAAGCTCCTCACTAGC | ||
| LH46 | GTGACACATCATCCGGCAAG | ||
| EIIANtr1 | LH121 | CTCAGTGACAGCAAGATCGCA | |
| LH122 | TAACGAGCGGCCGCAGGTGCAGTCCAATGAAAGTAC | ||
| LH123 | TGCGGCCGCTCGTTAAGCGATCAAGAGCTGTACAACATC | ||
| LH124 | GACTGAAACACCATCACAAGGTC | ||
| EIIANtr2 | LH49 | TCGCTAGAGTAACGAGCGAT | |
| LH50 | TAACGAGCGGCCGCACATCATGCCAAGGATCAGCG | ||
| LH51 | TGCGGCCGCTCGTTACTGATGTACGCGATGTCACGC | ||
| LH52 | TGTTGCTGCAAGCTCTCCTTCA | ||
| Gene insertion | VC1820 | LH87 | ATATGAATTCCTCATCAGTTAAATACTTTATGAGCC |
| LH88 | TATAGAATTCTTACATAAATATGGTGGTCTGCGCC | ||
| VC1822 | LH89 | ATATGAATTCCCCATCTGATCGAACCTGAAATC | |
| LH90 | TATAGAATTCTTACGGCTGGTAATCGGCATAATCCAT | ||
| VC1826 | LH91 | ATATGAATTCGCAAGCGAATTCCAAACAAGCCGT | |
| LH92 | TATAGAATTCTTAATCAGCCCTTTGCTTTCTGCTTGG | ||
| EINtr | LH71 | ATATGAATTCTTGCGCGCATGTCGGATGTTTATC | |
| LH72 | TATAGAATTCTTATCGATTCGTGCGCCATCAAGAGTA | ||
| VC1283 | LH192 | ATATGAATTCGATTCAGTTGGGCTTTGTCGGTA | |
| LH193 | TATAGAATTCTTACCTGTGCCAGAATTGTCGTCA | ||
| VCA0245 | LH151 | ATATGAATTCCAACTCAATCCAACTGCAAGCCAAAGCC | |
| LH152 | TATAGAATTCTTACTACGCGATGTGCGCTAATTCTTGTTGG | ||
| VCA1045 | LH105 | ATATGAATTCGGAGAACATTCACCTTGGCCTGAA | |
| Mutant rescue | FPr | LH61 | TTAGAACTCACTACACAAGATATTC |
| LH62 | ACCTTCGCCTAAGCCAGCATTGATC | ||
| FPr(309-401)a | LH195 | CGCGCTCATACGGCGACTTT | |
| HPr(H15A) | LH17 | AAAACGGCCTTGCCACTCGT | |
| LH18 | ACGAGTGGCAAGGCCGTTTT | ||
| FPr(H324A) | LH59 | AAATAGCCATGGTCTGGCCGCTCGTC | |
| LH60 | GACGAGCGGCCAGACCATGGCTATTT | ||
| Use in quantitative RT-PCR | EI | ACCGTTATCGAAGAGCAAGCCACT | |
| TCTGCGCAGTTTCAGAAGGCGTTA | |||
| EIIAGlc | GCGATCAAGCCTGCTGGTAACAAA | ||
| AAGCCTTCACCTTTCAGCTCAACC | |||
| HPr | TGTTCAAGCTGCAAACGCTAGGTC | ||
| GCAACTAGGTGCTCAACAGCTTCT | |||
| FPr | ATCACTGAGGAAACGATAGCCGCA | ||
| ACTTGACCATCGCCATCCAGGTTA | |||
| ClpX | AGAGTTCATTGGTCGTCTGCCTGT | ||
| AACAACGCCGCATACTGTTTGGTC |
FPr(309-401), fragment of FPr comprising positions 309 to 401.
Construction of in-frame deletion mutants.
The in-frame deletion mutants were constructed as described previously (8). Briefly, for the construction of the FPr deletion (ΔFPr), NPr deletion (ΔNPr), EIIANtr1 deletion (ΔEIIANtr1), and EIIANtr2 deletion (ΔEIIANtr2) mutants, the primers listed in Table 2 were used to amplify genome sequences spanning an in-frame deletion in the gene of interest. These DNA fragments were joined by the technique of gene splicing by overlap extension (SOE) (11), cloned into pCR2.1-TOPO, and then subcloned into the suicide vector pWM91 by ligation after digestion with XhoI and SpeI. All other plasmids used in the construction of in-frame deletion mutants were available in our laboratory (12). Suicide plasmids were used to generate in-frame deletion mutants by double homologous recombination.
Construction of rescue plasmids.
Plasmids used for rescue experiments, which are listed in Table 1, were constructed as described previously (12, 37), using the primers listed in Table 2. Briefly, either the native sequence or a truncated version of the targeted gene, excluding the start and stop codons, was amplified by PCR. Point mutations were generated by amplifying two gene fragments using internal primers with overlapping sequences containing the desired mutation. The two fragments were joined by the SOE technique and cloned into pBAD-TOPO according to the protocol of the plasmid manufacturer (Invitrogen). The sequence of the inserted fragment was confirmed by amplification and sequence analysis. The rescue plasmids were then introduced into V. cholerae strains by electroporation, and transformants were selected on LB agar supplemented with ampicillin. A pBAD-TOPO vector containing either lacZ or an antisense fragment of VC0964 was used as a control plasmid for rescue experiments. Western blot analyses to confirm the expression of the protein from the cloned gene were performed as reported previously (12) with the following modifications. Strains were cultured in MM supplemented with pyruvate at 27°C until stationary phase was reached, and a V5 C-terminal epitope tag was used for visualization.
Quantitative analysis of total growth and biofilm formation.
Quantification of surface association was performed as described previously (13). Briefly, the strains were grown overnight on LB agar plates at 27°C. The following morning, the resulting colonies were used to inoculate borosilicate tubes filled with 300 μl of MM alone or MM supplemented with the carbon source indicated below. After incubation for 24 h at 27°C, the planktonic cell suspension was collected and the planktonic cell density was estimated by measuring the optical density at 655 nm (OD655) with a Benchmark Plus microplate spectrophotometer (Bio-Rad). To determine the number of surface-attached cells, a 300-μl volume of PBS and a small volume of 1-mm glass beads were added to the surface-attached cells remaining in the borosilicate tube, and these were dispersed by subjection to a vortex. The OD655 of the resulting cell suspension was measured. Total growth measurements are the sum of the OD655s recorded for planktonic and surface-associated cell suspensions.
PTS transport assay.
Fermentation assays were performed with pH indicator plates containing MM agar alone or MM agar supplemented with the carbon source (0.5%, wt/vol) indicated below, to which a mixture of the pH indicators thymol blue (0.06 g/liter; Sigma) and bromothymol blue (0.06 g/liter; Sigma) had been added. Test strains were first grown overnight in liquid MM at 27°C with shaking and then diluted 100-fold in PBS. Samples of 10 μl of each dilution were spotted onto pH indicator plates, and the plates were allowed to dry and then incubated at 27°C for 18 h.
Quantification of EI, glucose-specific EIIA (EIIAGlc), HPr, and FPr gene transcription.
Wild-type V. cholerae was cultured for 18 h in MM alone or MM supplemented with the carbon source (0.5%, wt/vol) indicated below. The cells were then pelleted by centrifugation, and total RNA was isolated using the RNeasy kit (Qiagen) and treated with RNase-free DNase I to remove contaminating DNA. Reverse transcription-PCR (RT-PCR) was performed using 1 to 2 ng of total RNA with the SuperScript III first-strand kit (Invitrogen). Subsequently, 15 ng of the resulting cDNA served as a template for quantitative PCR using iTaq SYBR green supermix with carboxy-X-rhodamine (ROX; Bio-Rad) and 5 pmol of the relevant primers (Table 2) in a 20-μl reaction mixture. The level of the clpX (VC1921) transcript was used to normalize all measurements. As controls, template-free reaction mixtures were included to confirm the absence of contaminating DNA or RNA. The experiments were conducted with a StepOnePlus PCR system (Applied Biosystems) using the following steps: (i) 95°C for 10 min, (ii) 40 cycles of denaturation for 15 s at 95°C and annealing and extension for 1 min at 60°C, and (iii) dissociation curve analysis from 60 to 90°C. Data were analyzed using StepOne software version 2.0 (Applied Biosystems). Measurements were performed in triplicate.
β-Galactosidase assays.
Strains were inoculated into MM supplemented with pyruvate to achieve an initial OD655 of approximately 0.05. Cultures were incubated at 27°C for 4 h until an OD655 of approximately 0.2 was reached. The final OD655 was subsequently used for normalization of the β-galactosidase measurements. One hundred microliters of each culture was moved into a white 96-well plate (Nunc). Three freeze-thaw cycles were performed, and then 100 μl of the Beta-Glo luminescent substrate (Promega) was added to each well. After incubation in the dark at room temperature for 30 min, luminescence was measured by an Infinite 200 spectrophotometer (Tecan). Three experimental replicates of each sample were included, and the experiment was repeated multiple times.
Intestinal colonization of germfree mice.
Colonization assays were performed using 3-week-old female Swiss Webster mice raised under germfree conditions (Taconic). Upon their arrival, mice were transferred into sterilized isolators maintained under positive pressure. Two mice were housed in each autoclaved cage, and the mice were given free access to autoclaved food and water. Only mice used for similar infections were housed in the same isolator. Mice were allowed 4 days for acclimatization, and then preinfection stools were collected to document the absence of microbes.
For intestinal colonization of mice, the V. cholerae strains indicated below were grown overnight in LB broth at 27°C with shaking. The following day, these cultures were diluted 1:10 in LB broth and grown for 4 h at 27°C with shaking. A sample from a wild-type V. cholerae culture either alone or combined with a sample from a ΔEI mutant culture or a ΔEI ΔvpsL mutant culture was diluted 1:20 in 200 ml of a sterile 27 mM saline solution in a sterilized water bottle. One milliliter of this solution was removed from the bottle and used for quantification of the initial inoculum size and, if relevant, for estimation of the ratio of wild-type V. cholerae bacteria to the ΔEI mutant bacteria. The mice were given free access to this bacterial suspension for 24 h, after which time the bottle containing the bacterial suspension was removed and replaced with a bottle containing sterile water. Prior to initiating these experiments, we verified that, in the saline solution used for V. cholerae infection of mice, the numbers of V. cholerae bacteria and the ratio of wild-type V. cholerae bacteria to the ΔEI mutant bacteria remained constant over a 24-h period.
At the times indicated in the figures, two stool pellets were collected from each mouse and stool pellets from cohoused mice were pooled, weighed, and dispersed into 1 ml of PBS by using a handheld disposable pellet mixer driven by a cordless motor (VWR). After being spun briefly to pellet debris, the stool suspensions were diluted in PBS and dilutions were plated onto LB agar to quantify total CFU per 100 mg of stool. Wild-type V. cholerae generates large colonies surrounded by a yellow halo on pH indicator plates supplemented with sucrose, while ΔEI mutants form smaller colonies with no halo due to their inability to transport and ferment sucrose. Therefore, where relevant, dilutions were also plated onto pH indicator plates supplemented with sucrose to determine the ratio of wild-type V. cholerae bacteria to ΔEI mutant bacteria.
Dissection of the mouse intestine, spatial distribution of V. cholerae bacteria, and microscopic analysis.
The mice in cage 1, which were colonized with V. cholerae alone, were sacrificed 6 days postinoculation. Mice in cages 4 and 5 were sacrificed 36 days postinoculation. While these mice were initially colonized with wild-type V. cholerae and a ΔEI ΔvpsL mutant, at the time of sacrifice, only wild-type V. cholerae remained in the intestines. After the mice were sacrificed, the entire intestine from the duodenum to the anus was removed.
To determine the spatial distribution of V. cholerae bacteria within the intestine, intestines were divided into five segments corresponding to the proximal small bowel, the middle small bowel, the distal small bowel, the cecum, and the large intestine. The intestinal contents contained within a 2- to 3-cm portion of each segment were collected, and the total CFU per 100 mg of intestinal contents were enumerated as described above.
For microscopic analysis, two intestines harvested from mice sacrificed at day 36 of colonization were placed directly in 10% neutral buffered formalin (BDH). After fixation for 2 h, one intestine was emptied of stool, rinsed, and then returned to the formalin. After 48 h of fixation, intestines were dehydrated, embedded in paraffin, and sectioned. Washed intestines were stained with hematoxylin and eosin. Intestines retaining their fecal contents were Gram stained by the method of Brown and Brenn (2). Slides were examined with the 63× oil objective lens of a Zeiss Axiophot microscope, and color micrographs were obtained with a Spot camera (Diagnostic Instruments, Inc.).
RESULTS
Identification of the sugar transport specificities of V. cholerae EI, HPr, and EIIA homologs.
There are 25 homologs of PTS components encoded within the V. cholerae genome. We first used mutational analysis in conjunction with a simple colorimetric, agar plate-based assay to assess the roles of the two EI homologs, three HPr homologs, and nine EIIA homologs in sugar transport. In this assay, bacteria are cultured on MM-based agar containing the sugar of interest and a pH indicator (indicator agar). Transport and fermentation of the sugar by the bacterial colony result in acidification of the surrounding medium, leading to a change in color from green to yellow. Therefore, a strain that is unable to metabolize a particular sugar will form a green colony on indicator agar containing that sugar (Fig. 1). Our results, which are summarized in Table 3, show that the V. cholerae PTS is solely responsible for the transport of N-acetylglucosamine, sucrose, trehalose, fructose, mannitol, and mannose. Glucose transport, however, is present in PTS mutants, suggesting the existence of an alternative transport system. Components of the PTSNtr did not show any specificity for the sugars tested.
FIG. 1.
Sugar specificities of PTS components as determined by an agar plate-based sugar fermentation assay. Wild-type (WT) V. cholerae and different mutants were assayed on MM agar plates containing a pH indicator and supplemented with N-acetylglucosamine (NAG) or other carbon sources as indicated. Medium acidification upon sugar fermentation leads to a yellow color. The strain key for agar plates is included below. The key is color coded as follows: blue, strains carrying mutations in EI homologs; red, strains carrying mutations in HPr homologs; and green, strains carrying mutations in EIIA homologs.
TABLE 3.
Summary of PTS component transport specificities
| Component | Gene | E. coli ortholog | Specificitya for: |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Glucose | NAG | Sucrose | Trehalose | Maltose | Fructose | Mannitol | Mannose | |||
| EI | VC0965 | EI | X | X | X | X | X | X | ||
| VC0672 | EINtr | |||||||||
| HPr | VC0966 | HPr | X | X | X | X | X | X | ||
| VCA0518 | FPr | X | ||||||||
| VC2533 | NPr | |||||||||
| EIIA | VC0964 | EIIAGlc | X | X | X | |||||
| VC1283 | ||||||||||
| VC1820 | ||||||||||
| VC1822 | ||||||||||
| VC1824 | EIIANtr2 | |||||||||
| VC1826 | X | |||||||||
| VC2531 | EIIANtr1 | |||||||||
| VCA0245 | ||||||||||
| VCA0518 | FPr | X | ||||||||
| VCA1045 | X | |||||||||
X denotes specificity for the indicated sugar. NAG, N-acetylglucosamine.
The V. cholerae FPr homolog encoded at locus VCA0518 is the primary HPr homolog utilized in fructose transport. However, we observed that the colonies formed by ΔFPr mutants eventually became yellow on indicator plates containing fructose. Additionally, the ΔHPr and ΔHPr ΔNPr mutant colonies developed a yellow color slightly more slowly than wild-type colonies, as evidenced in Fig. 1. Both these observations suggest that HPr participates in fructose transport.
We also identified the substrate specificities of a number of EIIA homologs. The transporters of N-acetylglucosamine, sucrose, and trehalose share an EIIAGlc subunit, while the EIIA encoded by VCA1045 participates in mannitol transport and the EIIA encoded by VC1826 participates in mannose transport.
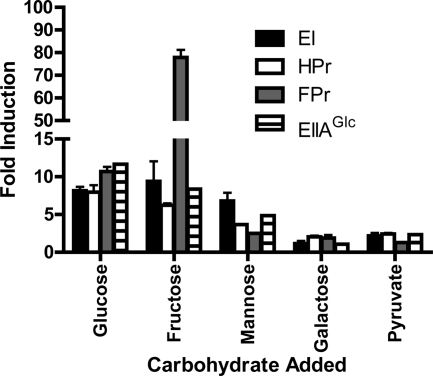
Previous microarray experiments indicated that transcription of the PTS component genes might be modulated by PTS substrate availability (22). To confirm this, we used quantitative RT-PCR to compare transcription patterns of EI, HPr, FPr, and EIIAGlc genes in MM alone and in MM supplemented with both PTS and non-PTS substrates. As shown in Fig. 2, transcription of PTS component genes was activated at least 5-fold by PTS substrates but not by non-PTS carbohydrates. Additional support for the identification of VCA0518 as the V. cholerae FPr gene was provided by the results demonstrating that the transcription of VCA0518 was activated approximately 80-fold in MM supplemented with fructose, in contrast to that of the HPr gene, which was activated only 5-fold.
FIG. 2.
PTS sugars activate PTS gene transcription. EI, EIIAGlc, HPr, and FPr transcript levels in wild-type V. cholerae grown in MM alone or MM supplemented with the indicated carbon sources were analyzed by quantitative RT-PCR. Three experimental replicates were performed. The data were analyzed using the ΔΔCT method for comparison to measurements from bacteria grown in MM alone. clpX was used as a standard.
Role of PTS components in V. cholerae accumulation on abiotic surfaces.
In freshwater environments, V. cholerae forms biofilms by elaborating an adhesive exopolysaccharide that has been termed VPS (13, 40). The phosphorylated form of EI has been implicated previously in the repression of VPS-dependent biofilm formation (12). Therefore, we questioned if one or more of the three V. cholerae HPr homologs might be involved in this regulatory pathway. We first compared patterns of biofilm formation by wild-type V. cholerae and mutants carrying deletions in one or more of the HPr homologs in MM supplemented with glucose. As shown in Fig. 3A, activation of biofilm formation by strains with a ΔHPr or ΔFPr mutant background was not observed. However, when both HPr and FPr were absent, biofilm formation increased approximately 2-fold compared to levels observed for a ΔEI mutant. In contrast, deletion of NPr in wild-type V. cholerae, a ΔHPr mutant background, or a ΔHPr ΔFPr mutant background had no additive effect on biofilm formation (Fig. 3 and data not shown), suggesting that cross talk between the two PTS pathways does not play a role in the regulation of biofilm formation.
FIG. 3.
HPr and FPr repress biofilm-associated growth and vps gene transcription. The total growth and biofilm-associated growth of and vps transcription in wild-type (WT) V. cholerae and various PTS mutants were quantified. (A) Biofilm-associated and total growth in MM supplemented with glucose. (B) Biofilm-associated and total growth in MM supplemented with pyruvate. (C) β-Galactosidase activities of strains carrying a chromosomal vps-lacZ fusion at the lacZ site in MM supplemented with pyruvate. Error bars indicate the standard deviations of results from at least three experimental replicates. Measurements for the indicated V. cholerae mutants were compared to those for wild-type V. cholerae by using the t test of statistical significance. Biofilm measurements that are significantly different from wild-type biofilm measurements are marked with an asterisk (P < 0.0005). β-Galactosidase measurements for all mutants were significantly different from that for the wild-type strain (P < 0.01). Furthermore, the β-galactosidase activity of the ΔEI mutant was significantly different from that of the ΔHPr ΔFPr mutant (P = 0.002).
In MM supplemented with glucose, the V. cholerae PTS fulfills both transport and regulatory functions. To assess the contribution of PTS regulation alone to V. cholerae biofilm formation, we repeated these assays with MM supplemented with pyruvate, a carbohydrate that is not transported by the PTS. The differences in biofilm formation between wild-type and mutant V. cholerae strains in MM supplemented with pyruvate were much greater than those in MM supplemented with glucose, while differences in total growth were much less (Fig. 3B). Therefore, subsequent biofilm experiments were conducted with MM supplemented with pyruvate.
For E. coli, the addition of non-PTS substrates briefly increases the ratio of pyruvate to phosphoenolpyruvate within the cell, resulting in dephosphorylation of PTS components (10). Because dephosphorylation of the PTS in V. cholerae activates biofilm formation (12), the addition of pyruvate to the growth medium would be predicted to derepress biofilm formation briefly. However, over the course of our 24-h-long experiment, this brief effect of pyruvate does not contribute significantly to the observed biofilm phenotype.
To confirm that biofilm formation in MM supplemented with pyruvate was regulated by the PTS at the transcriptional level, we used a reporter strain containing a chromosomal vpsL-lacZ fusion to measure the activation of vps transcription by the PTS. As shown in Fig. 3C, vps transcription was increased approximately 30-fold in the ΔEI mutant background and approximately 10-fold in the ΔHPr ΔFPr mutant background compared to that in the wild-type background. In some experimental replicates, vps transcription was elevated in the ΔHPr and ΔFPr mutant backgrounds as well. The difference in activation of the vps genes in the ΔEI and ΔHPr ΔFPr mutant backgrounds was unexpected and suggests that additional pathways influence the regulation of vps transcription by EI, HPr, and FPr.
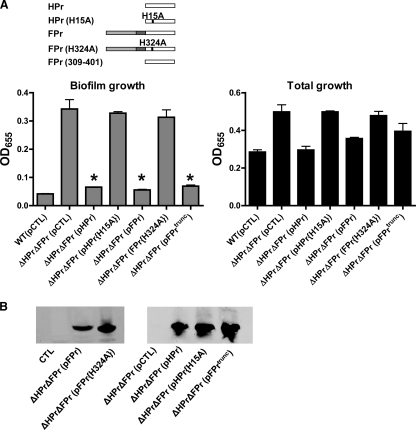
To determine whether HPr and FPr have redundant functions in the regulation of biofilm formation, we restored a wild-type allele for either HPr or FPr to a ΔHPr ΔFPr double mutant and compared biofilm formation by these rescued strains with that by the parental strain. As shown in Fig. 4A, the wild-type alleles for both HPr and FPr were able to complement the ΔHPr ΔFPr double mutant. However, when the phosphorylated histidine of either HPr or FPr was mutated to alanine, rescue was no longer observed in spite of adequate expression of the mutant protein (Fig. 4B). This finding supports the conclusion that only the phosphorylated forms of HPr and FPr take part in the repression of biofilm formation. FPr is composed of an N-terminal domain homologous to an EIIA component and a C-terminal domain homologous to HPr. We measured biofilm formation by the ΔHPr ΔFPr double mutant rescued with the C-terminal domain of FPr alone and found that this domain was sufficient to rescue repression of biofilm formation (Fig. 4A).
FIG. 4.
HPr and FPr must be phosphorylated to repress biofilm-associated growth. (A) Quantification of total and biofilm-associated growth of wild-type (WT) V. cholerae harboring a pBAD expression vector carrying a control sequence (pCTL) or of a ΔHPr ΔFPr mutant harboring a pBAD expression vector carrying either a control sequence (pCTL), the wild-type HPr gene (pHPr), a sequence encoding an unphosphorylatable form of HPr (pHPr{H15A}), the wild-type FPr gene (pFPr), a sequence encoding an unphosphorylatable form of FPr (FPr{H324A}), or a sequence encoding the C-terminal HPr-like domain of FPr including residues 309 to 401 (pFPrtrunc) in MM supplemented with pyruvate. Protein expression was induced with 0.04% l-arabinose. Schematic representations of the rescue constructs provided in trans are illustrated above the data. Error bars indicate the standard deviations of results from three experimental replicates. Asterisks indicate measurements significantly different from the measurement for the ΔHPr ΔFPr (pCTL) strain (P < 0.0014). (B) Western blots demonstrating expression of the relevant protein from the rescue plasmid. A V5 epitope tag was used for visualization.
We previously reported that V. cholerae biofilm formation is repressed by the phosphorylated form of EI. Given our observation that HPr and FPr must also be phosphorylated to repress biofilm formation, we hypothesized that EI was upstream of HPr and FPr in this signal transduction pathway. We reasoned that if this was the case, a wild-type EI allele would be unable to reverse the phenotype of a ΔEI mutant if both HPr and FPr were absent. To study this issue, we restored a wild-type EI allele in trans to a ΔPTS mutant (lacking EI, HPr, and EIIAGlc) or a ΔPTS ΔFPr mutant. As shown in Fig. 5, in spite of adequate expression of the transgenes, a wild-type EI allele was able to rescue the ΔPTS mutant only if FPr was present. All these data suggest that EI is in the same regulatory pathway as HPr and FPr.
FIG. 5.
HPr and FPr are downstream of EI in the pathways regulating biofilm growth. (A) Quantification of total and biofilm-associated growth of a ΔPTS mutant and a ΔPTS ΔFPr mutant harboring a pBAD expression vector carrying either a control sequence (pCTL) or the wild-type gene encoding EI (pEI) in MM supplemented with pyruvate. Protein expression was induced with 0.04% l-arabinose. Error bars indicate the standard deviations of results from three experimental replicates. The asterisk indicates a measurement significantly different from that for the ΔPTS (pCTL) mutant (P = 0.0019). (B) Western blot demonstrating expression of the relevant protein from the rescue plasmid. A V5 epitope tag was used for visualization. WT, wild type.
Because the most likely next element in this signal transduction cascade is an EIIA component, we compared patterns of biofilm formation by a series of mutants with each EIIA component deleted singly and biofilm formation by wild-type V. cholerae. However, biofilm formation was not consistently activated by deletion of any of the EIIA homologs (data not shown).
An adult germfree mouse model of V. cholerae infection.
The suckling mouse model of infection has been used extensively in defining the primary virulence factors of V. cholerae, and EI has been identified previously in a signature-tagged mutagenesis screen for V. cholerae colonization factors in the infant mouse (4, 19, 36). However, we were interested in the role of the PTS in adult mammals, whose intestinal environment is quite different from that in suckling mammals. For this reason, we examined the role of EI in an adult infection model. Because wild-type V. cholerae is rapidly cleared from the intestines of conventionally raised adult mice (3, 25), we used the adult germfree mouse model (32) with the goal of studying the role of the PTS in the colonization of the adult mammalian intestine. Three-week-old gnotobiotic mice were given access for 24 h to a dilute saline solution inoculated with V. cholerae. To document colonization, V. cholerae CFU in the stool were monitored. As shown in Fig. 6A, the numbers of V. cholerae CFU in the intestine reached a plateau after 72 h, and these numbers were maintained for the remainder of the experiment. To examine the longitudinal distribution of V. cholerae bacteria within the intestine, we harvested intestines at 6 and 36 days postinoculation, divided each intestine into segments, and quantified V. cholerae bacteria in the proximal small intestine (segment 1), the middle small intestine (segment 2), and the distal small intestine (segment 3), as well as in the cecum (segment 4) and large intestine (segment 5). As shown in Fig. 6B, the number of bacteria in the proximal small intestine was quite variable but generally much lower than those found in the middle and distal regions of the small intestine. Large numbers of bacteria were found in the cecum and large intestine. Furthermore, the distributions and numbers of V. cholerae bacteria in the intestine at 6 and 36 days postinoculation were similar.
FIG. 6.
V. cholerae persists in the distal portion of the adult germfree mouse intestine for 1 month. (A) Enumeration of V. cholerae CFU in stool pellets over time. Mice were infected by having free access to V. cholerae-inoculated saline solution for 24 h. At each indicated time point, two stool pellets were collected from each mouse. Stool pellets from cohoused mice were pooled. The samples were weighed and homogenized in 1 ml of PBS. Serial dilutions of this suspension were spread onto LB agar plates, and the resulting colonies were enumerated. Data were normalized with respect to stool weight. This experiment included 10 mice housed 2 to a cage. Cage 1 mice were monitored for 6 days, mice in cages 2 and 3 were monitored for 13 days, and cage 4 and 5 mice were monitored for 23 days. (B) Quantification of V. cholerae bacteria in the proximal small intestine (1), the middle small intestine (2), the distal small intestine (3), the cecum (4), and the large intestine (5) at 6 and 36 days postinoculation. Measurements at 6 days are shown in blue, while measurements at 36 days are shown in red. Bars represent the geometric means of measurements. Numbers of CFU in all segments of the small intestine were statistically significantly different from those in the large intestine (P = 0.0286). (C) Micrographs of different hematoxylin- and eosin-stained portions of the small intestine of a V. cholerae-infected, previously germfree mouse. The intestine was fixed in neutral buffered formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin. Bar, 10 μm.
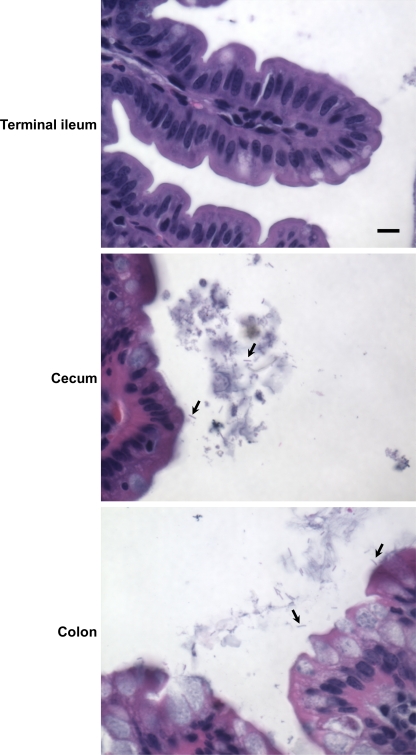
In humans, V. cholerae causes a noninflammatory diarrhea that preserves the architecture of the intestinal epithelium. To determine if this was the case in a germfree mouse model, at 36 days postinoculation, sections of the mouse intestine were briefly fixed, emptied of fecal contents, more thoroughly fixed, sectioned, and stained with hematoxylin and eosin. Micrographs of segments of the intestine with the highest burdens of V. cholerae, the terminal ileum, cecum, and colon, are shown in Fig. 6C. Examination of several sections suggested that the integrity and overall structure of the epithelial cells of these segments were largely preserved. We did not identify bacteria in these sections, suggesting that V. cholerae is easily dissociated from the intestinal epithelium in this model. To determine if a close association between V. cholerae and the epithelium was visible in the intact germfree mouse intestine, sections of mouse intestine along with the fecal contents were fixed, sectioned, Gram stained, and examined by microscopy. As shown in Fig. 7, no bacteria were observed in sections of the terminal ileum. In the cecum and colon, many rod-shaped, lightly stained bacteria were observed. Some bacteria were associated with the intestinal mucus, but most were found within the intestinal contents. Therefore, we conclude that colonization is not wholly dependent on attachment to the intestinal surface.
FIG. 7.
V. cholerae is found primarily in the lumen of the large intestine rather than on the epithelial surface. Micrographs of Gram-stained sections of the small intestine of a previously germfree, V. cholerae-infected mouse are shown. Bacteria are indicated by arrows. Bar, 10 μm.
PTS-dependent carbohydrate transport but not biofilm formation is required for colonization of the germfree mouse intestine.
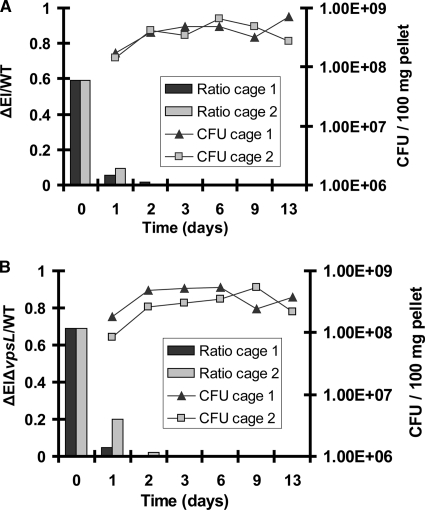
We hypothesized that survival in the intestinal lumen might require one or more of the functions of the PTS. To test whether the PTS in general was required for colonization of the mouse intestine, we performed a competition experiment with wild-type V. cholerae and a ΔEI mutant. As shown in Fig. 8A, within 3 days of inoculation of mice with approximately equal numbers of wild-type and mutant V. cholerae bacteria, the ΔEI mutant was no longer detectable in the fecal pellets. Furthermore, at the termination of the experiment, the ΔEI mutant was not detected in any segment of the small or large intestine (data not shown).
FIG. 8.
ΔEI and ΔEI Δvps mutants have a competitive disadvantage in the germfree mouse intestine. Exponentially grown wild-type (WT) V. cholerae bacteria were mixed at an approximately 1:1 ratio with either ΔEI mutant bacteria (A) or ΔEI ΔvpsL mutant bacteria (B). The resulting Vibrio suspensions were diluted 20-fold in saline and used to inoculate four mice housed in two cages. On the indicated days, fecal pellets were collected and plated onto LB agar to determine total numbers of CFU. Samples were also plated onto sucrose pH indicator agar to determine the mutant/WT ratio. The WT strain, which is able to ferment sucrose, forms large yellow colonies on this medium, and the ΔEI and ΔEI ΔvpsL mutants, which are unable to transport sucrose, form small green colonies.
Although the vps genes are expressed in the human intestine (7, 17), they have never been shown to play a role in colonization. In fact, in the infant mouse model, VPS synthesis has been shown to interfere with colonization (38). To determine if the colonization defect of the ΔEI mutant was a result of its inability to repress VPS synthesis, we also analyzed competition between wild-type V. cholerae and a ΔEI ΔvpsL double mutant. The double mutant contains an in-frame deletion in the vpsL-vpsQ operon. As shown in Fig. 8B, a colonization defect similar to that of the ΔEI mutant was observed for the ΔEI ΔvpsL double mutant.
DISCUSSION
The PTS is a multicomponent phosphotransfer cascade that coordinates the availability of high-energy carbon sources with many aspects of bacterial cellular physiology. Here, we have further elucidated the roles of the V. cholerae PTS in the transport of a variety of sugars and in biofilm formation. Furthermore, we have evaluated these functions in the colonization of the germfree mouse intestine.
We have identified the V. cholerae PTS transport systems for mannose and mannitol. VC1826 encodes the mannose permease of V. cholerae, an EII with fused A, B, and C domains. VC1826 is in a putative operon with VC1827, which encodes a mannose-6-phosphate isomerase homolog. However, the V. cholerae mannose permease is not closely related to that of E. coli, which contains an additional membrane-associated D domain (28). Instead, the V. cholerae mannose permease is most similar to the fructose-specific family of permeases. The mannitol permease of V. cholerae, which is encoded by VCA1045, or mtlA, is also composed of fused A, B, and C domains, and the gene is in a putative operon with mtlD, encoding a mannitol-1-phosphate 5-dehydrogenase, and mtlR, the mannitol operon repressor. In contrast to the V. cholerae mannose permease, the mannitol permease is highly conserved with respect to the mannitol transport systems of other organisms.
Our previous experiments demonstrated that the phosphorylated form of EI specifically represses the transcription of the vps genes and the growth of biofilm-associated cells in MM supplemented with glucose (12). In this work, we have shown that this signal transduction pathway is active in MM supplemented with the non-PTS substrate pyruvate, establishing that substrate transport is not a prerequisite for PTS regulation of biofilm formation. Furthermore, we have shown that HPr and FPr also participate in this signal transduction cascade in a redundant fashion, most likely by receiving a phosphate from EI. We were unable to identify a single EIIA component downstream of HPr and FPr in this signaling cascade. Alternative signaling mechanisms include direct interaction between HPr-P and FPr-P and a downstream component, phosphotransfer from HPr or FPr to a non-PTS component, and phosphotransfer from HPr and FPr to more than one EIIA component. These possibilities are currently under investigation.
The suckling mouse model of infection has been used extensively in defining the primary virulence factors of V. cholerae (4, 36). Several adult mouse models of disease have also been developed (3, 23, 24, 32). There are several poorly understood differences between the suckling and adult mouse models of disease. In the adult conventionally raised mouse, colonization of the small intestine is not robust and drops precipitously after inoculation, it is independent of the toxin-coregulated pilus, and it does not result in secretion of fluid into the intestinal lumen. Therefore, for the study of virulence, the suckling mouse is a better model.
However, for the study of the nutritional requirements of the bacterium within the intestine, we propose that the adult mouse may be an informative and complementary model. The intestinal environment in the suckling mammal is quite different from that in older mammals in at least two ways. First, a suckling mammal ingests only breast milk. Second, while digestion takes place intracellularly in the neonatal intestine after pinocytosis of liquid nutrients, in older mammals, enzymes anchored to the intestinal brush border are responsible for digestion of proteins and carbohydrates within the intestinal lumen. Therefore, while EI has been identified previously as an intestinal colonization factor in a signature-tagged mutagenesis screen carried out with infant mice (19), we considered it important to characterize V. cholerae colonization of the adult germfree mouse intestine in more depth and then test the importance of EI in this environment. We found that V. cholerae was able to colonize both the small and large intestines of germfree mice. Although the level of colonization of the small intestine was approximately three orders of magnitude lower than that of the large intestine, this distribution did not change over the course of several weeks. This finding suggests that, while commensal flora destabilizes V. cholerae colonization of the mouse intestine, it may not be responsible for the poor colonization of the small intestine that is observed in conventionally raised adult mice.
We then tested the role of the PTS in colonization of the adult mouse intestine. We documented rapid intestinal clearance of a ΔEI mutant in the presence of wild-type V. cholerae. Thus, as observed for the conventionally raised infant mouse, the PTS is required for colonization of the adult germfree mouse intestine. VPS-dependent biofilm formation interferes with V. cholerae colonization of the suckling mouse intestine (38). Therefore, we questioned whether VPS-dependent biofilm formation might play a role in the colonization defect of the ΔEI mutant. However, a ΔEI ΔvpsL mutant had an equally severe colonization defect. This finding suggests that increased VPS synthesis is not a dominant factor in the colonization defect of the ΔEI mutant, and we hypothesize that carbohydrate transport is most likely the critical function of EI in intestinal colonization. However, additional, unstudied regulatory functions of EI may also play a role.
While best known for its role in carbohydrate transport, the PTS is truly a multicomponent signal transduction cascade that integrates wide-ranging aspects of bacterial cellular physiology with environmental nutrient availability. Here, we have documented the critical roles of a subset of V. cholerae PTS components in nutrient scavenging, biofilm formation, and colonization of the mammalian intestine. We hypothesize that the role of the PTS in sensing, integrating, and responding to nutritional cues is essential for colonization of both aquatic and host environments; thus, the PTS safely shepherds V. cholerae through the environmental and intestinal stages in its life cycle. Furthermore, based on the widespread conservation of PTS components in both Gram-negative and Gram-positive species but not in eukaryotes, we suggest that inhibitors of the PTS will find widespread applications in both environmental and medical control of bacterial colonization.
Acknowledgments
Preparation of samples for histological analysis was performed by the Dana Farber/Harvard Cancer Center rodent histopathology core. Micrographs were obtained in the Harvard Digestive Diseases Center imaging core. We thank the ARCH staff and Gustavo Esquivel in particular for advice and assistance in performing germfree mouse experiments. We also thank Cristin Berkey, Alexandra Purdy, and Simon Dove for helpful suggestions and careful reading of the manuscript.
This work was supported by NIH grant AI50082 to P.I.W.
Editor: S. M. Payne
Footnotes
Published ahead of print on 1 February 2010.
REFERENCES
- 1.Berg, T., S. Schild, and J. Reidl. 2007. Regulation of the chitobiose-phosphotransferase system in Vibrio cholerae. Arch. Microbiol. 187:433-439. [DOI] [PubMed] [Google Scholar]
- 2.Brown, J. H., and L. Brenn. 1931. A method for the differential staining of Gram-positive and Gram-negative bacteria in tissue sections. Bull. Johns Hopkins Hosp. 48:69-73. [Google Scholar]
- 3.Butterton, J. R., E. T. Ryan, R. A. Shahin, and S. B. Calderwood. 1996. Development of a germfree mouse model of Vibrio cholerae infection. Infect. Immun. 64:4373-4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiang, S. L., and J. J. Mekalanos. 1998. Use of signature-tagged transposon mutagenesis to identify Vibrio cholerae genes critical for colonization. Mol. Microbiol. 27:797-805. [DOI] [PubMed] [Google Scholar]
- 5.Deutscher, J., C. Francke, and P. W. Postma. 2006. How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria. Microbiol. Mol. Biol. Rev. 70:939-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faruque, S. M., and G. B. Nair. 2002. Molecular ecology of toxigenic Vibrio cholerae. Microbiol. Immunol. 46:59-66. [DOI] [PubMed] [Google Scholar]
- 7.Hang, L., M. John, M. Asaduzzaman, E. A. Bridges, C. Vanderspurt, T. J. Kirn, R. K. Taylor, J. D. Hillman, A. Progulske-Fox, M. Handfield, E. T. Ryan, and S. B. Calderwood. 2003. Use of in vivo-induced antigen technology (IVIAT) to identify genes uniquely expressed during human infection with Vibrio cholerae. Proc. Natl. Acad. Sci. U. S. A. 100:8508-8513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haugo, A. J., and P. I. Watnick. 2002. Vibrio cholerae CytR is a repressor of biofilm development. Mol. Microbiol. 45:471-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herrington, D. A., R. H. Hall, G. Losonsky, J. J. Mekalanos, R. K. Taylor, and M. M. Levine. 1988. Toxin, toxin-coregulated pili, and the toxR regulon are essential for Vibrio cholerae pathogenesis in humans. J. Exp. Med. 168:1487-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hogema, B. M., J. C. Arents, R. Bader, K. Eijkemans, H. Yoshida, H. Takahashi, H. Aiba, and P. W. Postma. 1998. Inducer exclusion in Escherichia coli by non-PTS substrates: the role of the PEP to pyruvate ratio in determining the phosphorylation state of enzyme IIAGlc. Mol. Microbiol. 30:487-498. [DOI] [PubMed] [Google Scholar]
- 11.Horton, R. M., H. D. Hunt, S. N. Ho, J. K. Pullen, and L. R. Pease. 1989. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene 77:61-68. [DOI] [PubMed] [Google Scholar]
- 12.Houot, L., and P. I. Watnick. 2008. A novel role for enzyme I of the Vibrio cholerae phosphoenolpyruvate phosphotransferase system in regulation of growth in a biofilm. J. Bacteriol. 190:311-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kierek, K., and P. I. Watnick. 2003. Environmental determinants of Vibrio cholerae biofilm development. Appl. Environ. Microbiol. 69:5079-5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kok, M., G. Bron, G. Erni, and S. Mukhija. 2003. Effect of enzyme I of the bacterial phosphoenolpyruvate: sugar phosphotransferase system (PTS) on virulence in a murine model. Microbiology 149:2645-2652. [DOI] [PubMed] [Google Scholar]
- 15.Kornberg, H. 1986. The roles of HPr and FPr in the utilization of fructose by Escherichia coli. FEBS Lett. 194:12-15. [DOI] [PubMed] [Google Scholar]
- 16.Larsen, M. H., B. H. Kallipolitis, J. K. Christiansen, J. E. Olsen, and H. Ingmer. 2006. The response regulator ResD modulates virulence gene expression in response to carbohydrates in Listeria monocytogenes. Mol. Microbiol. 61:1622-1635. [DOI] [PubMed] [Google Scholar]
- 17.Lombardo, M. J., J. Michalski, H. Martinez-Wilson, C. Morin, T. Hilton, C. G. Osorio, J. P. Nataro, C. O. Tacket, A. Camilli, and J. B. Kaper. 2007. An in vivo expression technology screen for Vibrio cholerae genes expressed in human volunteers. Proc. Natl. Acad. Sci. U. S. A. 104:18229-18234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loo, C. Y., K. Mitrakul, I. B. Voss, C. V. Hughes, and N. Ganeshkumar. 2003. Involvement of an inducible fructose phosphotransferase operon in Streptococcus gordonii biofilm formation. J. Bacteriol. 185:6241-6254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merrell, D. S., D. L. Hava, and A. Camilli. 2002. Identification of novel factors involved in colonization and acid tolerance of Vibrio cholerae. Mol. Microbiol. 43:1471-1491. [DOI] [PubMed] [Google Scholar]
- 20.Metcalf, W. W., W. Jiang, L. L. Daniels, S. K. Kim, A. Haldimann, and B. L. Wanner. 1996. Conditionally replicative and conjugative plasmids carrying lacZα for cloning, mutagenesis, and allele replacement in bacteria. Plasmid 35:1-13. [DOI] [PubMed] [Google Scholar]
- 21.Miller, V. L., and J. J. Mekalanos. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 170:2575-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moorthy, S., and P. I. Watnick. 2005. Identification of novel stage-specific genetic requirements through whole genome transcription profiling of Vibrio cholerae biofilm development. Mol. Microbiol. 57:1623-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nygren, E., B. L. Li, J. Holmgren, and S. R. Attridge. 2009. Establishment of an adult mouse model for direct evaluation of the efficacy of vaccines against Vibrio cholerae. Infect. Immun. 77:3475-3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olivier, V., J. Queen, and K. J. Satchell. 2009. Successful small intestine colonization of adult mice by Vibrio cholerae requires ketamine anesthesia and accessory toxins. PLoS One 4:e7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olivier, V., N. H. Salzman, and K. J. Satchell. 2007. Prolonged colonization of mice by Vibrio cholerae El Tor O1 depends on accessory toxins. Infect. Immun. 75:5043-5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfluger, K., and V. de Lorenzo. 2008. Evidence of in vivo cross talk between the nitrogen-related and fructose-related branches of the carbohydrate phosphotransferase system of Pseudomonas putida. J. Bacteriol. 190:3374-3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poncet, S., E. Milohanic, A. Maze, J. N. Abdallah, F. Ake, M. Larribe, A. E. Deghmane, M. K. Taha, M. Dozot, X. De Bolle, J. J. Letesson, and J. Deutscher. 2009. Correlations between carbon metabolism and virulence in bacteria. Contrib. Microbiol. 16:88-102. [DOI] [PubMed] [Google Scholar]
- 28.Postma, P. W., J. W. Lengeler, and G. R. Jacobson. 1993. Phosphoenolpyruvate:carbohydrate phosphotransferase systems of bacteria. Microbiol. Rev. 57:543-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rabus, R., J. Reizer, I. Paulsen, and M. H. Saier, Jr. 1999. Enzyme I(Ntr) from Escherichia coli. A novel enzyme of the phosphoenolpyruvate-dependent phosphotransferase system exhibiting strict specificity for its phosphoryl acceptor, NPr. J. Biol. Chem. 274:26185-26191. [DOI] [PubMed] [Google Scholar]
- 30.Reizer, J., A. Reizer, M. J. Lagrou, K. R. Folger, C. K. Stover, and M. H. Saier, Jr. 1999. Novel phosphotransferase systems revealed by bacterial genome analysis: the complete repertoire of pts genes in Pseudomonas aeruginosa. J. Mol. Microbiol. Biotechnol. 1:289-293. [PubMed] [Google Scholar]
- 31.Rouquet, G., G. Porcheron, C. Barra, M. Reperant, N. K. Chanteloup, C. Schouler, and P. Gilot. 2009. A metabolic operon in extraintestinal pathogenic Escherichia coli promotes fitness under stressful conditions and invasion of eukaryotic cells. J. Bacteriol. 191:4427-4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sack, R. B., and C. E. Miller. 1969. Progressive changes of vibrio serotypes in germ-free mice infected with Vibrio cholerae. J. Bacteriol. 99:688-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shemesh, M., A. Tam, and D. Steinberg. 2007. Expression of biofilm-associated genes of Streptococcus mutans in response to glucose and sucrose. J. Med. Microbiol. 56:1528-1535. [DOI] [PubMed] [Google Scholar]
- 34.Stulke, J. 2007. Regulation of virulence in Bacillus anthracis: the phosphotransferase system transmits the signals. Mol. Microbiol. 63:626-628. [DOI] [PubMed] [Google Scholar]
- 35.Taylor, R. K., V. L. Miller, D. B. Furlong, and J. J. Mekalanos. 1986. Identification of a pilus colonization factor that is coordinately regulated with cholera toxin. Ann. Sclavo Collana Monogr. 3:51-61. [PubMed] [Google Scholar]
- 36.Thelin, K. H., and R. K. Taylor. 1996. Toxin-coregulated pilus, but not mannose-sensitive hemagglutinin, is required for colonization by Vibrio cholerae O1 El Tor biotype and O139 strains. Infect. Immun. 64:2853-2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Dellen, K. L., L. Houot, and P. I. Watnick. 2008. Genetic analysis of Vibrio cholerae monolayer formation reveals a key role for ΔΨ in the transition to permanent attachment. J. Bacteriol. 190:8185-8196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watnick, P. I., C. M. Lauriano, K. E. Klose, L. Croal, and R. Kolter. 2001. Absence of a flagellum leads to altered colony morphology, biofilm development, and virulence in V. cholerae O139. Mol. Microbiol. 39:223-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yildiz, F. H., N. A. Dolganov, and G. K. Schoolnik. 2001. VpsR, a member of the response regulators of the two-component regulatory systems, is required for expression of vps biosynthesis genes and EPS(ETr)-associated phenotypes in Vibrio cholerae O1 El Tor. J. Bacteriol. 183:1716-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yildiz, F. H., and G. K. Schoolnik. 1999. Vibrio cholerae O1 El Tor: identification of a gene cluster required for the rugose colony type, exopolysaccharide production, chlorine resistance, and biofilm formation. Proc. Natl. Acad. Sci. U. S. A. 96:4028-4033. [DOI] [PMC free article] [PubMed] [Google Scholar]