Abstract
Adherence to fibrinogen and fibronectin plays a crucial role in Staphylococcus aureus experimental endocarditis. Previous genetic studies have shown that infection and carriage isolates do not systematically differ in their virulence-related genes, including genes conferring adherence, such as clfA and fnbA. We set out to determine the range of adherence phenotypes in carriage isolates of S. aureus, to compare the adherence of these isolates to the adherence of infection isolates, and to determine the relationship between adherence and infectivity in a rat model of experimental endocarditis. A total of 133 healthy carriage isolates were screened for in vitro adherence to fibrinogen and fibronectin, and 30 isolates were randomly chosen for further investigation. These 30 isolates were compared to 30 infective endocarditis isolates and 30 blood culture isolates. The infectivities of the carriage isolates, which displayed either extremely low or high adherence to fibrinogen and fibronectin, were tested using a rat model of experimental endocarditis. The levels of adherence to both fibrinogen and fibronectin were very similar for isolates from healthy carriers and members of the two groups of infection isolates. All three groups of isolates showed a wide range of adherence to fibrinogen and fibronectin. Moreover, the carriage isolates that showed minimal adherence and the carriage isolates that showed strong adherence had the same infectivity in experimental endocarditis. Adherence was proven to be important for pathogenesis in experimental endocarditis, but even the least adherent carriage strains had the ability to induce infection. We discuss the roles of differential gene expression, human host factors, and gene redundancy in resolving this apparent paradox.
Staphylococcus aureus is a human commensal, but at the same time it is one of the most important bacterial pathogens that cause community-acquired and nosocomial infections. It can produce a wide variety of diseases, from benign skin infections, such as folliculitis or furunculosis, to life-threatening conditions, like osteomyelitis, septic arthritis, sepsis, pneumonia, and endocarditis (12). About 20% of humans carry S. aureus permanently in their noses, and another 60% are intermittent carriers (16). The association between S. aureus nasal carriage and staphylococcal disease has been reported for several decades (43, 44). More recently, it has been unambiguously shown that carriers have a higher risk of infection, at least when they are hospitalized (41, 45).
The pathogenicity of S. aureus involves a wide range of cell wall-associated adhesins and extracellular toxins. Surface adhesins, referred to as microbial surface components recognizing adhesive matrix molecules (MSCRAMMs), bind to the host extracellular matrix and thus promote tissue colonization and infection (30). The major MSCRAMMs involved in S. aureus pathogenesis are particular surface proteins that are covalently bound to the cell wall peptidoglycan via a conserved LPXTG motif (22). Genomic analyses indicated that the S. aureus genome contains up to 21 such LPXTG surface proteins (38). In addition to their multiplicity, these proteins often have redundant functions, as exemplified by clumping factors A and B (ClfA and ClfB), which bind fibrinogen (1, 27), and fibronectin-binding proteins A and B (FnBPA and FnBPB), which bind fibronectin (21), fibrinogen (42), and elastin (37).
It has been unambiguously demonstrated that in S. aureus ClfA and FnBPA are key pathogenicity factors, at least in infective endocarditis. This has been achieved by expressing these adhesins in bacteria lacking the rest of the S. aureus surface features (34). Using a variety of truncated and chimera constructs of these proteins, Que et al. observed that fibrinogen-binding domains were necessary and sufficient for colonization of damaged valves in experimental endocarditis, but not for persistence and invasion, whereas fibronectin-binding domains of FnBPA were unable to initiate infection but mediated aortic cell invasion and microbial persistence. Thus, the two adherence functions were necessary for progressive infection (35).
Despite the great effort to establish whether there are specific genetic determinants that distinguish carriage and invasive infection strains, the answer was largely negative (8, 25). Genetic studies revealed that many S. aureus wild-type strains lack some of the genes coding for LPXTG motif proteins, but there is no overall difference between carriage and infection isolates (19). However, fnbA and clfA are nearly always present in carriage and clinical isolates, confirming their pivotal role. Moreover, sequence analysis of functional regions of the two proteins has shown that there is a high degree of conservation in sporadic and epidemic isolates of S. aureus (17). On the other hand, the presence of the genes does not imply that there is efficient expression of a protein on the cell surface. It is entirely possible that either the carriage isolates express FnBPA and ClfA at a lower level or the adherence to the host matrix is less efficient. To our knowledge, a comparison of the adherence phenotypes of infection and carriage isolates has never been conducted.
Here we determined the levels of adherence to fibrinogen and fibronectin of 133 carriage isolates. We compared these isolates to 30 infective endocarditis and 30 blood culture isolates. In addition, we compared the infectivities of isolates displaying extreme adherence phenotypes in a rat model of experimental endocarditis.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The carriage isolates used in this study were described in detail elsewhere (39). Briefly, 133 S. aureus isolates were collected from 406 healthy adults in western Switzerland in 2005 and 2006. The blood culture isolates originated from patients at a tertiary care hospital in western Switzerland, and they came from the same catchment area as the carriage isolates and were collected over the same time period. These isolates were consecutive isolates received by the clinical microbiology laboratory of the university hospital and thus represented both community and acquired episodes of bacteremia that were associated or not associated with intravenous catheter colonization. Infective endocarditis isolates were collected between January and December 1999 during a population-based study conducted prospectively in six regions of France (15). S. aureus was grown at 37°C without agitation in tryptic soy broth (Difco Laboratories, Detroit, MI). S. aureus NCTC 8325-4 was used as a control strain to monitor the overall quality of the assays. Strains DU5883 (FnBPA− FnBPB−), a mutant of NCTC8325-4, (13) and DU5852 (clfA−) (23), were used as negative controls for adherence to fibronectin and fibrinogen, respectively.
Bacterial adherence to solid-phase fibrinogen and fibronectin.
We modified a previously described staphylococcal adherence assay (24) to measure the ability of S. aureus to adhere to low levels of surface-adsorbed fibrinogen and fibronectin. Serial 2-fold dilutions of fibrinogen (Sigma) or fibronectin (Sigma) in phosphate-buffered saline (PBS) were placed in 96-well plates (Nunc-Immuno plates; MaxiSorp surface; Nalge Nunc International). PBS without a ligand was placed in the last well as a negative control. The plates were incubated at 4°C for 16 h. Then they were washed three times with PBS, and 200 μl of 2% bovine serum albumin (Fluka) in PBS was added to each well to block nonspecific sites. The plates were incubated for 1.5 h and then washed three times with PBS. Bacterial cultures were harvested in the mid-logarithmic phase of growth (optical density at 600 nm [OD600], 0.6). After centrifugation for 10 min at 3,000 rpm, cells were frozen. Shortly before the test, the cells were resuspended in PBS, and the concentration was adjusted to 5 × 109 CFU/ml. Portions (50 μl) of the cell suspension were applied to individual wells (2.5 × 108 CFU per well) and incubated for 2 h at 37°C. Then the wells were washed with PBS and fixed at 55°C for 30 to 45 min. Bound bacteria were detected by staining with crystal violet, and the OD570 was determined with an enzyme-linked immunosorbent assay plate reader.
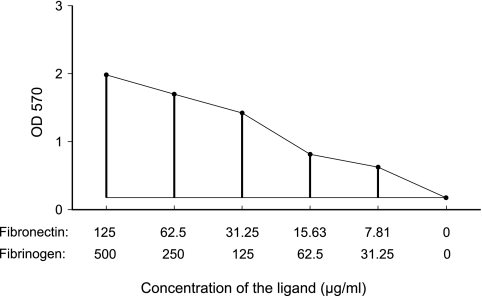
For the initial screen of 133 carriage isolates, the assay was carried out once with 11 ligand concentrations starting with same initial concentration. For all subsequent tests the measurements were repeated twice with five concentrations using independently grown bacterial cultures. The adherence score was calculated by subtracting the control value (no ligand) from the area under the curve. The optical densities for five substrate concentrations were added, and from the resulting value we subtracted the value for the well containing no ligand multiplied by 6 (Fig. 1).
FIG. 1.
Relationship between ligand concentration and adherence measured using optical density. The adherence value was calculated as an approximation of the area under the curve as follows. The optical density values for all concentrations were added, and the control value (which represented the optical density when no ligand was present) was multiplied by six and subtracted from the sum.
Rat model of infective endocarditis.
All animal experiments were carried out according to Swiss federal and cantonal regulations (authorization 879-6). Sterile aortic vegetations were produced in female Wistar rats as described previously (14). Groups of animals were inoculated with 103 or 104 CFU from cultures in the exponential growth phase. These inoculum sizes were used because clinical isolates have been shown to induce 50% and 90% experimental endocarditis in this model (5, 6, 26). Rats were sacrificed 24 h after inoculation, quantitative vegetation and spleen culture analyses were performed, and bacterial densities were expressed in log10 CFU/g. Median bacterial titers in the vegetations were compared by using the nonparametric Kruskal-Wallis test with Dunn's correction for multiple comparisons. The chi-square test with Yates correction was used to detect differences among infection rates. Differences were considered significant if the P value was <0.05, using two-tailed significance levels.
RESULTS
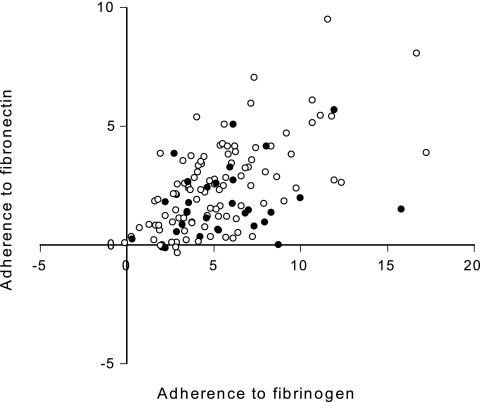
The initial screen of 133 carriage isolates originating from healthy carriers showed that there was great variation in adherence to fibrinogen and fibronectin (Fig. 2). The two adherence scores were significantly correlated (Spearman's rank correlation, 0.52; P < 0.0001).
FIG. 2.
Adherence to fibrinogen and fibronectin of 133 carriage isolates. Each circle represents an isolate (one assay). The axes indicate adherence scores as described in Material and Methods. The isolates indicated by filled circles were subsequently used for comparison with infection isolates.
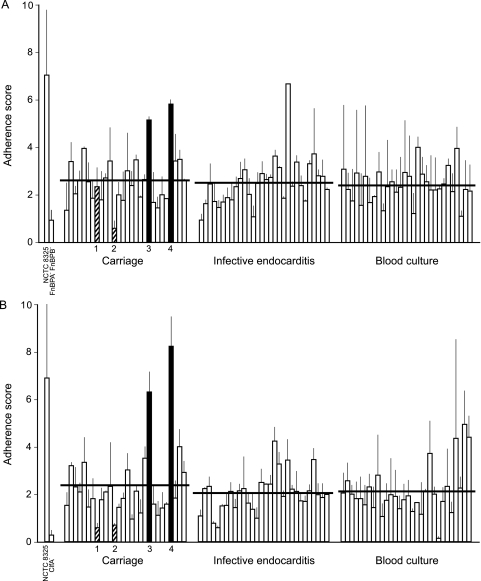
Twenty-eight carriage isolates were randomly chosen for further investigation. Similar to carriage isolates, both types of infection isolates displayed variability in adherence to fibronectin and fibrinogen (Fig. 3). The adherence to both ligands was the same for the three groups of isolates. However, individual strains differed significantly in adherence to fibrinogen (Kruskal-Wallis chi-square test, 123; df, 87; P = 0.006). The differences in adherence to fibronectin were not significant (Kruskal-Wallis chi-square test, 105; df, 87; P = 0.08). Strain NCTC 8325-4 exhibited relatively strong adherence to both substrates.
FIG. 3.
Adherence of S. aureus carriage and infection isolates to fibronectin (A) and fibrinogen (B). The bars and error bars indicate the average and standard deviation for each isolate (two assays per isolate). The average for each category is indicated by a horizontal line. Isolates which were used for testing in the experimental endocarditis model are indicated by filled bars (high adherence) and striped bars (low adherence) and numbers.
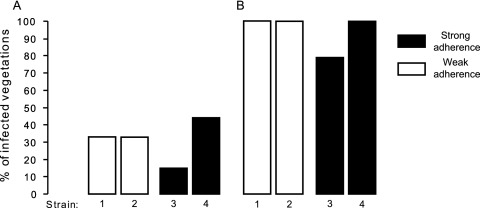
We chose four carriage isolates which exhibited very low (two isolates) and very high (two isolates) levels of adherence to fibrinogen and fibronectin to test infectivity in the rat model of experimental endocarditis. The abilities of all isolates to induce endocarditis were similar for both inoculum sizes tested (Fig. 4). In addition, all animals had 80 to 100% infected spleens and similar densities of bacteria in vegetations and spleens irrespective of the challenge strain (Table 1). There were no differences among the strains in any of the variables measured.
FIG. 4.
Incidence of endocarditis in rats challenged with 103 (A) or 104 (B) CFU of S. aureus carriage isolates exhibiting a low (strains 1 and 2) or high (strains 3 and 4) level of adherence to fibrinogen and fibronectin.
TABLE 1.
Bacterial counts in infected spleens and vegetations
| Inoculum containing 103 CFU |
Inoculum containing 104 CFU |
|||||||
|---|---|---|---|---|---|---|---|---|
| Bacterial counts (log10 CFU/g) |
Bacterial counts (log10 CFU/g) |
|||||||
| Location | Adhesion | Strain | n | Median | Range | n | Median | Range |
| Spleens | Weak | 1 | 9 | 2.21 | 1.56-4.71 | 9 | 4.73 | 3.79-5.71 |
| 2 | 7 | 2.57 | 1.6-4.67 | 9 | 4.7 | 3.28-5.8 | ||
| Strong | 3 | 8 | 2.18 | 2.02-4.65 | 10 | 4.715 | 3.48-6.67 | |
| 4 | 9 | 3.65 | 2.91-4.53 | 10 | 5.73 | 4.72-6.78 | ||
| Vegetations | Weak | 1 | 3 | 6.32 | 4.59-7.76 | 10 | 9.05 | 4.64-9.66 |
| 2 | 3 | 7.90 | 7.68-7.98 | 8 | 8.59 | 7.96-9.29 | ||
| Strong | 3 | 1 | 6.28 | 8 | 7.58 | 5.25-9.39 | ||
| 4 | 4 | 8.47 | 4.41-8.84 | 9 | 8.50 | 4.23-9.63 | ||
DISCUSSION
No difference in adherence between infection and carriage isolates.
The present study showed that a prominent aspect of the S. aureus phenotype, adherence to fibrinogen and fibronectin, does not correlate with the source of isolates. The levels of adherence to fibrinogen and fibronectin, as measured in in vitro assay, were very similar for isolates obtained from healthy carriers and members of two different groups of infection isolates. Given the prominent role of adherence to fibrinogen and fibronectin conferred by ClfA and FnBPA, which was demonstrated in an experimental endocarditis study (35), stronger adherence could have been expected for infective endocarditis isolates than for carriage isolates. Our phenotypic observations complement the results of previous studies, which did not identify factors associated with increased virulence in S. aureus at the genetic level. It was demonstrated previously that all lineages (clonal complexes) of S. aureus were equally likely to cause an infection (8). Likewise, Lindsay et al. (19) were unable to identify any genes overrepresented in infection isolates, further confirming the lack of simple genetic determinants of staphylococcal infection. This suggested that most, if not all, natural isolates of S. aureus are capable of infection. We have not tested whether the clfA and fnbA genes are present in the isolates used in this study, because previous studies demonstrated that these genes were nearly universally present in human infection and carriage isolates (17, 31). However, a recent study reported a tendency toward specific features for methicillin-resistant S. aureus strains responsible for persistent bacteremia compared with the strains responsible for transient bacteremia (48). Persistent bacteremia isolates were phenotypically more adherent to fibrinogen, fibronectin, and endothelial cells in vitro. Nevertheless, although persistence was associated with a phenotypic and molecular signature, the two types of strains were equally able to induce endocarditis in rabbits, which suggests that a disease-inducing capacity is universal in S. aureus strains. Similarly, it has been shown that certain genotypes (clonal complexes) might be associated with more severe disease (10). However, these findings appear to be due, at least in part, to the association of certain genotypes, predominantly ST36, with methicillin resistance. While no specific genetic makeup correlated with invasive infection, specific genes are indeed related to specific disease syndromes, such as the genes encoding superantigens (e.g., toxic shock syndrome toxin and enterotoxins) or exfoliative toxins.
Human host factors are crucial for S. aureus infection. The most important risk factor is illness, as shown by the nearly 100-fold increase in the risk of invasive staphylococcal infection in humans who are ill, from 0.03% in the general population (or approximately 30 cases/100,000 people [18]) to 5.7% during hospitalization (80 cases/14,000 people [45]). Various medical conditions and interventions, such as diabetes, dialysis, surgery, and drug and alcohol abuse, have been identified as important risk factors (18). Nasal carriage was shown to increase the risk of staphylococcal bacteremia 3-fold but not the risk of death in hospitalized nonsurgical patients (45). Specific genetic predispositions do not seem to play a major role, with the exception of male gender and a few very rare inherited immune deficiencies. Nevertheless, whether host predisposition is a unique factor promoting S. aureus infection remains to be determined.
Wide range of adherence phenotypes but no correlation with infectivity.
A wide range of adherence phenotypes was observed for all types of isolates, including infective endocarditis isolates displaying very low adherence. In particular, the carriage isolates displaying very low adherence might be expected to show lower infectivity in experimental endocarditis. However, even these isolates were capable of inducing experimental valve infection. Human endocarditis isolates were tested previously on multiple occasions using this model, and they exhibited infectivities very similar to those reported here (4-7, 26). This was clearly shown by the minimum inoculum size necessary to infect 90% of the animals, which was 104 CFU for both the isolates used in the present study and endocarditis isolates tested in previous experiments.
Proven role of adherence in infection but no difference in infectivity between strongly and weakly adherent isolates.
Although adherence to fibrinogen and fibronectin has been proven to be important for pathogenesis in experimental endocarditis, even the least adherent carriage strains were able to induce infection in this model. Several explanations can be offered for this apparent paradox. The first explanation is the possible inability of the in vivo endocarditis model to detect relevant differences. It is always questionable to use specific models, in this case experimental endocarditis, to examine general characterisitcs, such as infectivity. Moreover, induction of valve infection proceeds via at least two steps; it starts with tissue colonization, which may be reversible if the bacterium is susceptible (and accessible) to host defense mechanisms, and this is followed by invasion and persistence, when the microbes settle further, invade local tissues, and spread to distant sites. These two steps may involve different virulence factors that might be regulated differently to achieve optimal infectivity (see below). The present study addressed primarily induction of infection (with relatively early sacrifice 24 h after inoculation), because this is the unavoidable primum movens for further invasion. Moreover, it attempted to correlate adherence to fibrinogen and fibronectin with the capacity to promote experimental endocarditis, because the adhesins mediating these phenotypes were specifically associated with valve infection in previous studies (26, 32, 35), whereas other adhesins were not (46). Therefore, the system model is pertinent to the questions asked.
The second putative explanation is adhesin redundancy. Indeed, our observations are reminiscent of the situation where knockout mutations of major adherence-promoting genes had very modest effects on infectivity in experimental endocarditis, despite marked decreases in adherence to the specific ligands (9, 26). S. aureus possesses a wide array of virulence factors, including up to 21 LPXTG cell wall-anchored adhesins (38). Moreover, many isolates contain only some of these factors but are still infectious (19), suggesting that there is great functional overlap among the factors. This issue was recently studied by expressing 18 of the 21 S. aureus LPXTG proteins in surrogate lactococci and testing the recombinant organisms to determine their capacities to induce experimental endocarditis (46). ClfA and FnBPA significantly increased the ability of the recombinants to produce experimental valve infection, but other proteins (including Cna, SdrC, SdrD, SdrE, and Pls) had a marginal effect, suggesting that they might cooperate to cause infection as well. Hence, it is likely that an isolate of S. aureus needs only some of these proteins to display the full functionality necessary for an infection.
The third hypothesis concerns differential gene expression in various milieus. The difference between infection isolates and carriage isolates might be manifested only during actual infection and not under standard in vitro conditions. A seminal example is microbial crowding and quorum sensing via agr (accessory gene regulator), which promotes expression of surface adhesins during the exponential phase of growth and production of exoproteins in the stationary phase (28, 36). Apart from cell density, agr is highly dependent on environmental factors (49). For example, standard adherence experiments, including our experiments, are conducted under static conditions, whereas flow conditions were shown to have a notable effect on adherence phenotypes (40). There has been increasing interest in how the regulation via agr works in vivo (33), and remarkable differences in regulation, and consequently in the expression of virulence factors, between experimental and human infection and in vitro conditions have been found (2, 11, 20, 47). In particular, a recent study showed that the differences between in vitro and in vivo conditions involve the regulation of FnBPA by both the global regulon genes saeRS and the sigB gene (3). Moreover, many other regulators, which often interact with agr, have been described (e.g., sarA, sarR, sarS, sarT, sarV, sarU, sarY, rot, and alternative sigma factors) (for reviews, see references 2 and 33.
Because of the complexity of gene regulation in S. aureus, which is not fully understood yet, the differences in regulation, and consequently the differences in expression of virulence factors, undoubtedly present among natural isolates of S. aureus cannot be captured at the genetic level. Although in vitro profiles of adherence (in this case adherence to fibrinogen and fibronectin) did not correlate with in vivo infection in humans and experimental endocarditis in rats, the laboratory tests that have been performed cannot capture differential gene expression at the infection site. Hence, appraising the importance of adhesin expression for infection requires direct measurement of the adhesins present in infected tissues compared to the adhesins present under in vitro conditions. Such measurement requires more specific molecular approaches, such as in situ hybridization or proteomic techniques (29).
Acknowledgments
This work was supported by grant 3200B0-113854 to P.M. and by Marie Heim Vögtlin grant PMPDA-106195 to O.S., both from the Swiss National Science Foundation.
We thank Marlyse Giddey, Claudio Maldonaldo, and Delphine Morisset for outstanding technical support.
Editor: A. J. Bäumler
Footnotes
Published ahead of print on 11 January 2010.
REFERENCES
- 1.Boden, M. K., and J. I. Flock. 1989. Fibrinogen binding protein clumping factor from Staphylococcus aureus. Infect. Immun. 57:2358-2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheung, A. L., A. S. Bayer, G. Y. Zhang, H. Gresham, and Y. Q. Xiong. 2004. Regulation of virulence determinants in vitro and in vivo in Staphylococcus aureus. FEMS Immunol. Med. Microbiol. 40:1-9. [DOI] [PubMed] [Google Scholar]
- 3.Cheung, A. L., S. J. Yang, A. S. Bayer, and Y. Q. Xiong. 2009. Disparity in the in vitro versus in vivo regulation of fibronectin binding proteins by 2 global regulators, saeRS and sigB, in Staphylococcus aureus. J. Infect. Dis. 200:1371-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Entenza, J. M., H. Drugeon, M. P. Glauser, and P. Moreillon. 1995. Treatment of experimental endocarditis due to erythromycin-susceptible or -resistant methicillin-resistant Staphylococcus aureus with RP 59500. Antimicrob. Agents Chemother. 39:1419-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Entenza, J. M., T. J. Foster, D. N. Eidhin, P. Vaudaux, P. Francioli, and P. Moreillon. 2000. Contribution of clumping factor B to pathogenesis of experimental endocarditis due to Staphylococcus aureus. Infect. Immun. 68:5443-5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Entenza, J. M., P. Moreillon, M. M. Senn, J. Kormanec, P. M. Dunman, B. Berger-Bachi, S. Projan, and M. Bischoff. 2005. Role of σB in the expression of Staphylococcus aureus cell wall adhesins ClfA and FnbA and contribution to infectivity in a rat model of experimental endocarditis. Infect. Immun. 73:990-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Entenza, J. M., J. Vouillamoz, M. P. Glauser, and P. Moreillon. 1997. Levofloxacin versus ciprofloxacin, flucloxacillin, or vancomycin for treatment of experimental endocarditis due to methicillin-susceptible or -resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 41:1662-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feil, E. J., J. E. Cooper, H. Grundmann, D. A. Robinson, M. C. Enright, T. Berendt, S. J. Peacock, J. M. Smith, M. Murphy, B. G. Spratt, C. E. Moore, and N. P. J. Day. 2003. How clonal is Staphylococcus aureus? J. Bacteriol. 185:3307-3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flock, J. I., S. A. Hienz, A. Heimdahl, and T. Schennings. 1996. Reconsideration of the role of fibronectin binding in endocarditis caused by Staphylococcus aureus. Infect. Immun. 64:1876-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fowler, V. G., C. L. Nelson, L. M. McIntyre, B. N. Kreiswirth, A. Monk, G. L. Archer, J. Federspiel, S. Naidich, B. Remortel, T. Rude, P. Brown, L. B. Reller, G. R. Corey, and S. R. Gill. 2007. Potential associations between hematogenous complications and bacterial genotype in Staphylococcus aureus infection. J. Infect. Dis. 196:738-747. [DOI] [PubMed] [Google Scholar]
- 11.Goerke, C., S. Campana, M. G. Bayer, G. Doring, K. Botzenhart, and C. Wolz. 2000. Direct quantitative transcript analysis of the agr regulon of Staphylococcus aureus during human infection in comparison to the expression profile in vitro. Infect. Immun. 68:1304-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gordon, R. J., and F. D. Lowy. 2008. Pathogenesis of methicillin-resistant Staphylococcus aureus infection. Clin. Infect. Dis. 46:S350-S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greene, C., D. McDevitt, P. Francois, P. E. Vaudaux, D. P. Lew, and T. J. Foster. 1995. Adhesion properties of mutants of Staphylococcus aureus defective in fibronectin-binding proteins and studies on the expression of fnb genes. Mol. Microbiol. 17:1143-1152. [DOI] [PubMed] [Google Scholar]
- 14.Heraief, E., M. P. Glauser, and L. R. Freedman. 1982. Natural history of aortic-valve endocarditis in rats. Infect. Immun. 37:127-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoen, B., F. Alla, C. Selton-Suty, I. Beguinot, A. Bouvet, S. Briancon, J. P. Casalta, N. Danchin, F. Delahaye, J. Etienne, V. Le Moing, C. Leport, J. L. Mainardi, R. Ruimy, and F. Vandenesch. 2002. Changing profile of infective endocarditis: results of a 1-year survey in France. JAMA 288:75-81. [DOI] [PubMed] [Google Scholar]
- 16.Kluytmans, J., A. van Belkum, and H. Verbrugh. 1997. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin. Microbiol. Rev. 10:505-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuhn, G., P. Francioli, and D. S. Blanc. 2006. Evidence for clonal evolution among highly polymorphic genes in methicillin-resistant Staphylococcus aureus. J. Bacteriol. 188:169-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laupland, K. B., D. L. Church, M. Mucenski, L. R. Sutherland, and H. D. Davies. 2003. Population-based study of the epidemiology of and the risk factors for invasive Staphylococcus aureus infections. J. Infect. Dis. 187:1452-1459. [DOI] [PubMed] [Google Scholar]
- 19.Lindsay, J. A., C. E. Moore, N. P. Day, S. J. Peacock, A. A. Witney, R. A. Stabler, S. E. Husain, P. D. Butcher, and J. Hinds. 2006. Microarrays reveal that each of the ten dominant lineages of Staphylococcus aureus has a unique combination of surface-associated and regulatory genes. J. Bacteriol. 188:669-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loughman, J. A., S. A. Fritz, G. A. Storch, and D. A. Hunstad. 2009. Virulence gene expression in human community acquired Staphylococcus aureus infection. J. Infect. Dis. 199:294-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Massey, R. C., M. N. Kantzanou, T. Fowler, N. P. J. Day, K. Schofield, E. R. Wann, A. R. Berendt, M. Hook, and S. J. Peacock. 2001. Fibronectin-binding protein A of Staphylococcus aureus has multiple, substituting, binding regions that mediate adherence to fibronectin and invasion of endothelial cells. Cell. Microbiol. 3:839-851. [DOI] [PubMed] [Google Scholar]
- 22.Mazmanian, S. K., G. Liu, T. T. Hung, and O. Schneewind. 1999. Staphylococcus aureus sortase, an enzyme that anchors surface proteins to the cell wall. Science 285:760-763. [DOI] [PubMed] [Google Scholar]
- 23.McDevitt, D., P. Francois, P. Vaudaux, and T. J. Foster. 1994. Molecular characterization of the clumping cactor (fibrinogen receptor) of Staphylococcus aureus. Mol. Microbiol. 11:237-248. [DOI] [PubMed] [Google Scholar]
- 24.McDevitt, D., P. Vaudaux, and T. J. Foster. 1992. Genetic evidence that bound coagulase of Staphylococcus aureus is not clumping factor. Infect. Immun. 60:1514-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melles, D. C., R. F. J. Gorkink, H. A. M. Boelens, S. V. Snijders, J. K. Peeters, M. J. Moorhouse, P. J. van der Spek, W. B. van Leeuwen, G. Simons, H. A. Verbrugh, and A. van Belkum. 2004. Natural population dynamics and expansion of pathogenic clones of Staphylococcus aureus. J. Clin. Invest. 114:1732-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moreillon, P., J. M. Entenza, P. Francioli, D. McDevitt, T. J. Foster, P. Francois, and P. Vaudaux. 1995. Role of Staphylococcus aureus coagulase and clumping factor in pathogenesis of experimental endocarditis. Infect. Immun. 63:4738-4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ni Eidhin, D., S. Perkins, P. Francois, P. Vaudaux, M. Hook, and T. J. Foster. 1998. Clumping factor B (ClfB), a new surface-located fibrinogen-binding adhesin of Staphylococcus aureus. Mol. Microbiol. 30:245-257. [DOI] [PubMed] [Google Scholar]
- 28.Novick, R. P., H. F. Ross, S. J. Projan, J. Kornblum, B. Kreiswirth, and S. Moghazeh. 1993. Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO J. 12:3967-3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Panchaud, A., E. Widmer, M. Kussmann, P. Moreillon, and M. Affolter. 2007. Mass spectrometry as a rapid and powerful alternative to antibodies for detecting LPXTG wall-associated proteins of Staphylococcus aureus. Int. J. Mass Spectrom. 268:234-243. [Google Scholar]
- 30.Patti, J. M., B. L. Allen, M. J. McGavin, and M. Hook. 1994. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu. Rev. Microbiol. 48:585-617. [DOI] [PubMed] [Google Scholar]
- 31.Peacock, S. J., C. E. Moore, A. Justice, M. Kantzanou, L. Story, K. Mackie, G. O'Neil, and N. P. J. Day. 2002. Virulent combinations of adhesin and toxin genes in natural populations of Staphylococcus aureus. Infect. Immun. 70:4987-4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piroth, L., Y. A. Que, E. Widmer, A. Panchaud, S. Piu, J. M. Entenza, and P. Moreillon. 2008. The fibrinogen- and fibronectin-binding domains of Staphylococcus aureus fibronectin-binding protein A synergistically promote endothelial invasion and experimental endocarditis. Infect. Immun. 76:3824-3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pragman, A. A., and P. M. Schlievert. 2004. Virulence regulation in Staphylococcus aureus: the need for in vivo analysis of virulence factor regulation. FEMS Immunol. Med. Microbiol. 42:147-154. [DOI] [PubMed] [Google Scholar]
- 34.Que, Y. A., J. A. Haefliger, P. Francioli, and P. Moreillon. 2000. Expression of Staphylococcus aureus clumping factor A in Lactococcus lactis subsp. cremoris using a new shuttle vector. Infect. Immun. 68:3516-3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Que, Y. A., J. A. Haefliger, L. Piroth, P. Francois, E. Widmer, J. M. Entenza, B. Sinha, M. Herrmann, P. Francioli, P. Vaudaux, and P. Moreillon. 2005. Fibrinogen and fibronectin binding cooperate for valve infection and invasion in Staphylococcus aureus experimental endocarditis. J. Exp. Med. 201:1627-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Recsei, P., B. Kreiswirth, M. Oreilly, P. Schlievert, A. Gruss, and R. P. Novick. 1986. Regulation of exoprotein gene expression in Staphylococcus aureus by agr. Mol. Gen. Genet. 202:58-61. [DOI] [PubMed] [Google Scholar]
- 37.Roche, F. M., R. Downer, F. Keane, P. Speziale, P. W. Park, and T. J. Foster. 2004. The N-terminal A domain of fibronectin-binding proteins A and B promotes adhesion of Staphylococcus aureus to elastin. J. Biol. Chem. 279:38433-38440. [DOI] [PubMed] [Google Scholar]
- 38.Roche, F. M., R. Massey, S. J. Peacock, N. P. J. Day, L. Visai, P. Speziale, A. Lam, M. Pallen, and T. J. Foster. 2003. Characterization of novel LPXTG-containing proteins of Staphylococcus aureus identified from genome sequences. Microbiology 149:643-654. [DOI] [PubMed] [Google Scholar]
- 39.Sakwinska, O., G. Kuhn, C. Balmelli, P. Francioli, M. Giddey, V. Perreten, A. Riesen, F. Zysset, D. S. Blanc, and P. Moreillon. 2009. Genetic diversity and ecological success of Staphylococcus aureus strains colonizing humans. Appl. Environ. Microbiol. 75:175-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shenkman, B., E. Rubinstein, A. L. Cheung, G. E. Brill, R. Dardik, I. Tamarin, N. Savion, and D. Varon. 2001. Adherence properties of Staphylococcus aureus under static and flow conditions: roles of agr and sar loci, platelets, and plasma ligands. Infect. Immun. 69:4473-4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.von Eiff, C., K. Becker, K. Machka, H. Stammer, and G. Peters. 2001. Nasal carriage as a source of Staphylococcus aureus bacteremia. N. Engl. J. Med. 344:11-16. [DOI] [PubMed] [Google Scholar]
- 42.Wann, E. R., S. Gurusiddappa, and M. Hook. 2000. The fibronectin-binding MSCRAMM FnbpA of Staphylococcus aureus is a bifunctional protein that also binds to fibrinogen. J. Biol. Chem. 275:13863-13871. [DOI] [PubMed] [Google Scholar]
- 43.Weinstein, H. J. 1959. The relation between nasal-staphylococcal-carrier state and the incidence of postoperative complications. N. Engl. J. Med. 260:1303-1308. [DOI] [PubMed] [Google Scholar]
- 44.Wertheim, H. F. L., D. C. Melles, M. C. Vos, W. van Leeuwen, A. van Belkum, H. A. Verbrugh, and J. L. Nouwen. 2005. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect. Dis. 5:751-762. [DOI] [PubMed] [Google Scholar]
- 45.Wertheim, H. F. L., M. C. Vos, A. Ott, A. van Belkum, A. Voss, J. Kluytmans, P. H. J. van Keulen, C. Vandenbroucke-Grauls, M. H. M. Meester, and H. A. Verbrugh. 2004. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet 364:703-705. [DOI] [PubMed] [Google Scholar]
- 46.Widmer, E., Y. A. Que, J. M. Entenza, and P. Moreillon. 2006. New concepts in the pathophysiology of infective endocarditis. Curr. Infect. Dis. Rep. 8:271-279. [DOI] [PubMed] [Google Scholar]
- 47.Xiong, Y. Q., A. S. Bayer, M. R. Yeaman, W. van Wamel, A. C. Manna, and A. L. Cheung. 2004. Impacts of sarA and agr in Staphylococcus aureus strain Newman on fibronectin-binding protein A gene expression and fibronectin adherence capacity in vitro and in experimental infective endocarditis. Infect. Immun. 72:1832-1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xiong, Y. Q., V. G. Fowler, M. R. Yeaman, F. Perdreau-Remington, B. N. Kreiswirth, and A. S. Bayer. 2009. Phenotypic and genotypic characteristics of persistent methicillin-resistant Staphylococcus aureus bacteremia in vitro and in an experimental endocarditis model. J. Infect. Dis. 199:201-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yarwood, J. M., and P. M. Schlievert. 2003. Quorum sensing in Staphylococcus infections. J. Clin. Invest. 112:1620-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]