Abstract
Vibrio parahaemolyticus, one of the human-pathogenic vibrios, causes three major types of clinical illness: gastroenteritis, wound infections, and septicemia. Thermostable direct hemolysin (TDH) secreted by this bacterium has been considered a major virulence factor of gastroenteritis because it has biological activities, including cytotoxic and enterotoxic activities. Previous reports revealed that V. parahaemolyticus strain RIMD2210633, which contains tdh, has two sets of type III secretion system (T3SS) genes on chromosomes 1 and 2 (T3SS1 and T3SS2, respectively) and that T3SS1 is responsible for cytotoxicity and T3SS2 is involved in enterotoxicity, as well as in cytotoxic activity. However, the relative importance and contributions of TDH and the two T3SSs to V. parahaemolyticus pathogenicity are not well understood. In this study, we constructed mutant strains with nonfunctional T3SSs from the V. parahaemolyticus strain containing tdh, and then the pathogenicities of the wild-type and mutant strains were evaluated by assessing their cytotoxic activities against HeLa, Caco-2, and RAW 264 cells, their enterotoxic activities in rabbit ileal loops, and their lethality in a murine infection model. We demonstrated that T3SS1 was involved in cytotoxic activities against all cell lines used in this study, while T3SS2 and TDH had cytotoxic effects on a limited number of cell lines. T3SS2 was the major contributor to V. parahaemolyticus-induced enterotoxicity. Interestingly, we found that both T3SS1 and TDH played a significant role in lethal activity in a murine infection model. Our findings provide new indications that these virulence factors contribute to and orchestrate each distinct aspect of the pathogenicity of V. parahaemolyticus.
Vibrio parahaemolyticus is a Gram-negative halophilic bacterium that inhabits estuarine and coastal waters and can be isolated from seafood (6, 7, 9). It causes acute gastroenteritis in humans after they consume contaminated raw or undercooked seafood. Although this microorganism is better known for causing gastroenteritis, it also can cause wound infections and septicemia (5, 23, 36).
Most clinical isolates of V. parahaemolyticus from patients with diarrhea show β-hemolysis on Wagatsuma agar (37). This phenomenon is known as the Kanagawa phenomenon (KP) and is considered a good marker to distinguish between pathogenic and nonpathogenic V. parahaemolyticus strains (25, 37). Thermostable direct hemolysin (TDH), which is responsible for the KP (28, 43), has multiple biological activities, including hemolysis, enterotoxicity, cytotoxicity, and cardiotoxicity (11-13, 26, 29, 32, 34, 38). For this reason, TDH has been considered a major virulence factor of V. parahaemolyticus.
Whole-genome sequencing of a KP-positive V. parahaemolyticus strain revealed that this strain contains two sets of gene clusters for the type III secretion system (T3SS), T3SS1 and T3SS2, one on each of its two chromosomes (21). T3SS gene clusters have been detected in numerous Gram-negative animal and plant pathogens, where they enable delivery of virulence factor proteins (effectors) into the cytosol of eukaryotic cells (4, 8, 15). V. parahaemolyticus T3SS1 is reportedly involved in cytotoxic activity against a variety of cell lines (1, 2, 19, 31), while T3SS2 has been demonstrated to be involved in enterotoxicity in the rabbit ileal loop test, as well as in cytotoxic activity against Caco-2 and HCT-8 cells (18, 19, 33). However, these studies used a tdhAS deletion mutant strain of V. parahaemolyticus (POR-1) as the parent for construction of the T3SS deletion mutations to exclude any biological activities of TDH. It is therefore still not clear what the contribution of T3SSs to these activities is under conditions in which TDH is produced.
V. parahaemolyticus sometimes also causes wound infections and septicemia (5, 23, 36). Unlike the information for gastrointestinal illness, there is no epidemiological information that indicates a correlation between any known virulence factor of V. parahaemolyticus and septicemia. The results of an intraperitoneal challenge experiment indicated that the lethality in mice due to V. parahaemolyticus infection occurred irrespective of the production of TDH, suggesting that another virulence factor(s) besides TDH may contribute to V. parahaemolyticus-induced septicemia (10).
In this study, we constructed mutant strains with nonfunctional T3SSs from a V. parahaemolyticus strain possessing tdh to investigate the roles and contributions of the two T3SSs and TDH to the pathogenicity of V. parahaemolyticus (i.e., cytotoxic activity against several cell lines, enterotoxicity in the rabbit ileal loop test, and lethality in mice).
MATERIALS AND METHODS
Bacterial strains and plasmids.
V. parahaemolyticus RIMD2210633 (KP positive, serotype O3:K6) (27) was used for construction of deletion mutants and functional studies. All bacterial strains and plasmids used in this study are listed in Table 1.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description | Reference or source |
|---|---|---|
| V. parahaemolyticus strains | ||
| RIMD2210633 (WT) | Clinical isolate; KP positive; serotype O3:K6 | 27 |
| ΔvscN1 | T3SS1-deficient strain; vscN1 deletion mutant (deletion from nt −225 to 1068 of the gene) derived from WT | This study |
| ΔvscN2 | T3SS2-deficient strain; in-frame vscN2 deletion mutant (deletion from nt 160 to 978 of the gene) derived from WT | This study |
| ΔvscN1 ΔvscN2 | T3SS1- and T3SS2-deficient strain; vscN1 (deletion from nt −225 to 1068 of the gene) and vscN2 (deletion from nt 160 to 978 of the gene) double-deletion mutant derived from WT | This study |
| POR-1 | tdhAS null mutant strain; tdhAS deletion mutant derived from WT | 32 |
| POR-2 | TDH- and T3SS1-deficient strain; in-frame vcrD1 deletion mutant (deletion from nt 640 to 1731 of the gene) derived from POR-1 | 33 |
| POR-3 | TDH- and T3SS2-deficient strain; in-frame vcrD2 deletion mutant (deletion from nt 315 to 761 of the gene) derived from POR-1 | 33 |
| ΔvcrD1 ΔvcrD2 | TDH-, T3SS1-, and T3SS2-deficient strain; vcrD1 (deletion from nt 640 to 1731 of the gene) and vcrD2 (deletion from nt 315 to 761 of the gene) double-deletion mutant derived from POR-1 | 19 |
| E. coli strains | ||
| DH5α | F− φ80lacZΔM15 Δ(lacZYA-argF)U169 deoR recA1 endA1 hsdR17 phoA supE44 thi-1 gyrA96 relA1 λ− | Novagen |
| SM10 λpir | thi thr leu tonA lacY supE recA::RP4-2Tc::Mu λpir R6K | 24 |
| Plasmids | ||
| pT7bule | Multicopy (ColE1 ori) TA cloning vector, Ampr | Novagen |
| pYAK1 | R6K-ori suicide vector for gene replacement, Cmr | 17 |
| pYAK1-ΔvscN1 | Derivative of suicide vector pYAK1 for generating the vscN1 deletion mutant | 33 |
| pYAK-ΔvscN2 | Derivative of suicide vector pYAK1 for generating the vscN2 deletion mutant | 33 |
| pSA19Cm-MCS | Complement vector for V. parahaemolyticus, Cmr | 18 |
| pSACm-1 | pSA19Cm-MCS containing promoter of vopN1-vopD1 operon in EcoRI-BamHI site | 31 |
| pSA-tdhP | pSA19Cm-MCS containing promoter of tdhA in EcoRI-SmaI site | 18 |
| ptdhA | Derivative of pSACm-MCS containing tdhA loci | This study |
| pvcrD1 | Derivative of pSACm-1 containing vcrD1 loci | This study |
| pvscN2 | Derivative of pSA-tdhP containing vscN2 loci | This study |
Construction of the T3SS deletion mutants.
The vscN1 deletion (nucleotides [nt] −225 to 1068) and in-frame vscN2 deletion (nt 160 to 978) strains of V. parahaemolyticus RIMD2210633 were constructed using deletion vectors (33) (Table 1) by introducing homologous recombination as previously described (33). Briefly, deletion vectors, which contained the sacB gene conferring sensitivity to sucrose, were introduced into Escherichia coli SM10 λpir and transferred to V. parahaemolyticus strain RIMD2210633 by conjugation. The mutant strains were selected using resistance to 10% sucrose and sensitivity to chloramphenicol.
Complementation of deleted genes in the mutant strains.
Complementation of deleted genes was carried out follows. The DNA region including a deleted gene was amplified by PCR using the following primers: for complementation of tdhA, tdhA-comp-F (5′-GGATCCGGCCATGTTACCGCTTGAGG-3′) and tdhA-comp-R (5′-GTCGACGCCTTATCGCTTGGTCGATAGCTGG-3′); for complementation of vcrD1, vcrD1-comp-F (5′-GGATCCACAACTGGACCAGCGTTACCT-3′) and vcrD1-comp-R (5′-GTCGACCGTCACTTCGATCCCCGTTTG-3′); and for complementation of vscN2, vscN2-comp-F (5′-GGATCCTTCATTTCAGGTGATTGAATAATGCTCAAG-3′) and vscN2-comp-R (5′-GTCGACGCTTCCTTGCTTGCTTTTCTATCC-3′). Each amplified fragment was cloned, and its sequence was ascertained. The insert was excised by restriction digestion with BamHI and SalI (at the sites underlined above) and cloned into the appropriate vector as described in Table 1. Each plasmid was introduced into V. parahaemolyticus mutant strains by electroporation (1.5 kV, 100 Ω, 25 μF).
Cytotoxicity assays.
The cytotoxic assays were performed as described previously (19). Briefly, eukaryotic cells (HeLa, Caco-2, and RAW 264 cells) were infected at a multiplicity of infection (MOI) of 10. After infection, the release of lactate dehydrogenase (LDH) into the medium was quantified with a CytoTox96 kit (Promega, Madison, WI) used according to the manufacturer's instructions. To evaluate the neutralizing effect of anti-TDH antibodies (39) on cytotoxicity, bacterial suspensions were mixed with anti-TDH antibodies before infection.
Rabbit ileal loop test.
The rabbit ileal loop test was performed as previously described, with slight modifications, and six rabbits were used in each experiment (33). The isogenic mutant strains of V. parahaemolyticus (109 CFU/loop) or purified TDH (150 μg/loop) was injected into the ligated ileal loop of a rabbit, which was followed by measurement of the fluid accumulation in each loop at 18 h after injection. To evaluate the effect of anti-TDH serum on enterotoxicity, the serum was mixed with a bacterial suspension or purified TDH before injection. Fluid accumulation (FA) ratios were calculated by determining the amount of accumulated fluid (in milliliters) per centimeter of ligated rabbit small intestine.
Murine infection model.
Bacterial suspensions (108 CFU) were inoculated intraperitoneally into female C3H/HeN mice that were 4 to 5 weeks old, after which we examined the symptoms and calculated the numbers of mice killed at specified times. All animal experiments were performed using an experimental protocol approved by the Ethics Review Committee for Animal Experimentation of the Research Institute for Microbial Diseases (Osaka University, Osaka, Japan).
Western blot analysis.
Secreted proteins were prepared as described previously (18). Samples used for Western blot analysis were separated by SDS-PAGE. Transferred membranes (Millipore, Bedford, MA) were probed with anti-VopD1 (33), anti-VopD2 polyclonal antibody (18), or anti-TDH monoclonal antibody (39) and then with horseradish peroxidase-conjugated goat anti-rabbit antibody (ZYMED) or rabbit anti-mouse antibody (ZYMED). The blots were developed with an ECL Western blot kit (Amersham Biosciences, Piscataway, NY).
RPLA assay.
The reverse passive latex agglutination (RPLA) assay was performed according to the manufacturer's instructions (Denka Seiken Co. Ltd.).
Statistical analysis.
Student's t tests assuming unequal variances were used for statistical analyses (P values of <0.05 were considered statistically significant). An analysis of the murine survival ratio was performed with Kaplan-Meier and log rank tests (P values of <0.01 were considered statistically significant).
RESULTS
TDH is secreted in a T3SS-independent manner.
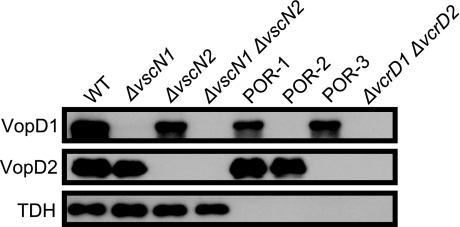
In most previous studies examining the cytotoxicity and enterotoxicity of V. parahaemolyticus T3SSs, mutant strains derived from a tdhAS gene deletion mutant of V. parahaemolyticus (POR-1) were used in order to exclude the possibility of involvement of TDH in the phenotypes (1-3, 18, 19, 31, 33). It is therefore still not clear to what extents each of the T3SSs and TDH contribute to the cytotoxic and enterotoxic activities induced by wild-type V. parahaemolyticus. To address this issue, we constructed mutant strains with nonfunctional T3SSs from a V. parahaemolyticus wild-type strain (WT) possessing tdh. We used these strains, together with previously constructed mutant strains (Table 1), for subsequent studies. First, the capacities of these strains to secrete TDH, as well as T3SS1- and T3SS2-dependent proteins, were assessed by Western blotting. As shown in Fig. 1, VopD1, which is a T3SS1-secreted protein (33), was not detected in the supernatants of nonfunctional T3SS1 mutant strains derived from the WT strain (the ΔvscN1 and ΔvscN1 ΔvscN2 strains) or from the tdhAS gene mutant strain POR-1 (the POR-2 and ΔvcrD1 ΔvcrD2 strains) (Fig. 1, top panel). Similarly, VopD2, which is a T3SS2-secreted protein (18), was not detected in the supernatants of the T3SS2-deficient mutant strains derived from the WT strain (the ΔvscN2 and ΔvscN1 ΔvscN2 strains) or from the tdhAS gene mutant strain POR-1 (the POR-3 and ΔvcrD1 ΔvcrD2 strains) (Fig. 1, middle panel). Unlike secretion of the VopD1 and VopD2 proteins, secretion of TDH was not affected by the presence of T3SSs (Fig. 1, bottom panel). In addition, RPLA measurement with a KP reverse passive latex agglutination kit (Denka Seiken Co. Ltd.) (42) revealed that there were not significant differences in the amounts of TDH secreted regardless of the presence of the T3SSs (data not shown). These results suggest that neither the T3SS1 apparatus nor the T3SS2 apparatus is involved in TDH secretion.
FIG. 1.
TDH is secreted in a T3SS-independent manner, as shown by immunoblot analysis of bacterial supernatants from isogenic derivatives of the V. parahaemolyticus RIMD2210633 strain grown for 6 h in LB broth containing 0.5% NaCl. The lanes contained wild-type strain RIMD2210633 (WT), a T3SS1-deficient strain derived from the WT strain (the ΔvscN1 strain), a T3SS2-deficient strain derived from the WT strain (the ΔvscN2 strain), a T3SS1- and T3SS2-deficient strain derived from the WT strain (the ΔvscN1 ΔvscN2 strain), a tdhAS mutant strain derived from the WT strain (POR-1), a T3SS1-deficient strain derived from POR-1 (POR-2), a T3SS2-deficient strain derived from POR-1 (POR-3), and a T3SS1- and T3SS2-deficient strain derived from POR-1 (the ΔvcrD1 ΔvcrD2 strain). Blots were probed with anti-VopD1 (top panel), anti-VopD2 polyclonal antibodies (middle panel), and anti-TDH monoclonal antibodies (bottom panel).
T3SS1- and T3SS2-dependent cytotoxic effects.
The cytotoxic activities of the tdh and T3SS deletion mutant strains were evaluated in terms of the release of cytosolic lactate dehydrogenase (LDH) from cultured cells. As shown in Fig. 2A, when HeLa cells were infected with the T3SS1-deficient mutant strains derived from the WT strain (the ΔvscN1 and ΔvscN1 ΔvscN2 strains) or from the tdhAS deletion mutant strain POR-1 (the POR-2 and ΔvcrD1 ΔvcrD2 strains), their cytotoxicities were dramatically less than the corresponding cytotoxicities resulting from WT and POR-1 infection, respectively. There was not a significant difference in cytotoxicity between the strains expressing TDH and the strains not expressing TDH (that is, between the WT and POR-1 strains, between the ΔvscN1 and POR-2 strains, between the ΔvscN2 and POR-3 strains, and between the ΔvscN1 ΔvscN2 and ΔvcrD1 ΔvcrD2 strains). Thus, T3SS1, but not T3SS2 and TDH, was a major contributor to the cytotoxic activity against HeLa cells observed. The cytotoxicity of POR-2 was fully restored by in trans complementation with the vcrD1 (pvcrD1) gene, but not by in trans complementation with the tdh (ptdhA) gene (Fig. 2B). In the same way, when Caco-2 cells were infected with T3SS1-deficient strains derived from the WT strain (the ΔvscN1 strain) and from the POR-1 strain (strain POR-2), the cytotoxicities were significantly reduced compared with the corresponding cytotoxic activities observed in WT and POR-1 infections, respectively (Fig. 2C). However, the T3SS1-deficient strains showed partial cytotoxicity, and this cytotoxicity was dramatically reduced when the cells were infected with both T3SS1- and T3SS2 deficient strains derived from the WT strain (the ΔvscN1 ΔvscN2 strain) and from the POR-1 strain with tdhAS deleted (the ΔvcrD1 ΔvcrD2 strain). The cytotoxicities of the ΔvcrD1 ΔvcrD2 and ΔvscN1 ΔvscN2 strains were fully restored by in trans complementation with the vcrD1 (pvcrD1) and vcsN2 (pvscN2) genes, respectively (Fig. 2D). As determined for HeLa cells, no participation of TDH in cytotoxic activity against Caco-2 cells was observed. These results indicate that T3SS1 exhibits cytotoxic activity against both cell lines, whereas T3SS2 exhibits cytotoxic activity only against Caco-2 cells, as previously reported (19). Moreover, TDH secreted by bacteria had little effect on the cytotoxic activity against these cell lines.
FIG. 2.
T3SS1- and T3SS2-dependent cytotoxic activity. HeLa cells (A and B) and Caco-2 cells (C and D) were infected with the RIMD2210633 (WT) strain or the isogenic mutants indicated at a multiplicity of infection (MOI) of 10. At 4.5 h (HeLa cells) and 6 h (Caco-2 cells) after infection, cytotoxic activity was evaluated by determining the amount of LDH released (relative to the amount of LDH released from uninfected cells treated with detergent, which was defined as 100%). The mutants tested were the ΔvscN1 strain (T3SS1 deficient), the ΔvscN2 strain (T3SS2 deficient), the ΔvscN1 ΔvscN2 strain (T3SS1 and T3SS2 deficient), POR-1 (tdhAS mutant), POR-2 (tdhAS and T3SS1 deficient), POR-3 (tdhAS and T3SS2 deficient), the ΔvcrD1 ΔvcrD2 strain (tdhAS, T3SS1, and T3SS2 deficient), POR-2/pvcrD1 (POR-2 complemented with vcrD1), POR-2/ptdhA (POR-2 complemented with tdhA), the ΔvcrD1 ΔvcrD2/pvcrD1 strain (the ΔvcrD1 ΔvcrD2 strain complemented with vcrD1), and the ΔvscN1 ΔvscN2/pvscN2 strain (the ΔvscN1 ΔvscN2 strain complemented with vscN2). The error bars indicate standard deviations for results from triplicate (A and C) or quadruplicate (B and D) independent experiments. The asterisks indicate that results were significantly different from the results obtained with the WT strain (*, P < 0.05; **, P < 0.01).
T3SS1- and TDH-dependent cytotoxic activities against RAW 264 cells.
We next examined the cytotoxic activities against cells of the nonepithelial, macrophage-like cell line RAW 264. When RAW 264 cells were infected with the T3SS1-deficient strain derived from the WT strain (the ΔvscN1 strain), as well as with the T3SS1- and T3SS2-deficient strain derived from the WT strain (the ΔvscN1 ΔvscN2 strain), the cytotoxicities were apparently reduced compared with the cytotoxicity of the WT strain, although the difference was not statistically significant (Fig. 3A). However, cytotoxicity was almost absent when the cells were infected with the T3SS1-deficient strain derived from the tdhAS mutant strain POR-1 (POR-2) or with the T3SS1- and T3SS2-deficient strain derived from POR-1 (the ΔvcrD1 ΔvcrD2 strain) (Fig. 3A). The cytotoxicity of the ΔvcrD1 ΔvcrD2 strain was fully restored by in trans complementation with the vcrD1 (pvcrD1) and tdh (ptdhA) genes (Fig. 3B). Thus, not only T3SS1-dependent cytotoxic activity but also TDH-dependent cytotoxic activity against RAW 264 cells was observed. The latter activity was confirmed by using both a TDH-neutralizing monoclonal antibody (MAb 1-24) and anti-TDH serum (Fig. 3C). MAb 1-24 is known to be capable of neutralizing hemolysis induced by purified TDH by inhibiting it in the postbinding process (39). The cytotoxic activities observed with the T3SS1-deficient strain derived from the WT strain (the ΔvscN1 strain) and with the T3SS1- and T3SS2-deficient strain derived from the WT (the ΔvscN1 ΔvscN2 strain) were neutralized by both MAb 1-24 and anti-TDH serum. These results strongly indicate that not only T3SS1 but also TDH secreted by bacteria is responsible for the cytotoxic activity against RAW 264 cells.
FIG. 3.
T3SS1- and TDH-dependent cytotoxic activity against RAW 264 cells. (A and B) RAW 264 cells were infected with the RIMD2210633 (WT) strain or the isogenic mutants indicated at a multiplicity of infection (MOI) of 10. At 3 h after infection, cytotoxic activity was evaluated by determining the amount of LDH released (relative to the amount of LDH released from uninfected cells treated with detergent, which was defined as 100%). The mutants tested were the ΔvscN1 strain (T3SS1 deficient), the ΔvscN2 strain (T3SS2 deficient), the ΔvscN1 ΔvscN2 strain (T3SS1 and T3SS2 deficient), POR-1 (tdhAS mutant), POR-2 (tdhAS and T3SS1 deficient), POR-3 (tdhAS and T3SS2 deficient), the ΔvcrD1 ΔvcrD2 strain (tdhAS, T3SS1, and T3SS2 deficient), POR-2/pvcrD1 (POR-2 complemented with vcrD1), and POR-2/ptdhA (POR-2 complemented with tdhA). (C) RAW 264 cells with anti-TDH neutralizing monoclonal antibody (MAb 1-24), preimmne serum, or anti-TDH serum were infected with the ΔvscN1 and ΔvscN1 ΔvscN2 strains. Three hours after infection, cytotoxic activity was evaluated by determining the amount of LDH released. (D) Various concentrations of purified TDH (0.001 to 100 μg/ml) were used to challenge HeLa, Caco-2, and RAW 264 cells for 1 h. Cytotoxic activity was assayed by measuring the total amount of cellular LDH released into the culture supernatant. The error bars indicate standard deviations for results from triplicate (A, C, and D) or quadruplicate (B) independent experiments. Two asterisks indicate that the results are significantly different from the results obtained with the WT strain (P < 0.01).
RAW 264 cells are very sensitive to TDH.
As shown above, TDH secreted by bacteria could induce cytotoxic activity only against RAW 264 cells in the present study. We therefore evaluated the sensitivities of the cell lines to purified TDH. As shown in Fig. 3D, the cytotoxic activity of purified TDH against RAW 264 cells was saturated at a concentration of 1 μg/ml. For HeLa and Caco-2 cells, on the other hand, the cytotoxic activities of TDH at a concentration of 100 μg/ml were only 19.2% ± 8.7% and 15.4% ± 7.3%, respectively. These results show that RAW 264 cells were much more sensitive to purified TDH than HeLa and Caco-2 cells were.
T3SS2 is necessary for enterotoxicity.
It was thought until recently that TDH is the major contributor to enterotoxicity since purified TDH exhibits enterotoxic activity (12, 26, 38). Recent studies using TDH-deficient strains, however, showed that T3SS2 is also involved in enterotoxicity (18, 33). However, the relative contributions of these two virulence factors to enterotoxicity have not been examined yet. To address this issue, we used the rabbit ileal loop test to evaluate the enterotoxic activities of T3SS-deficient strains derived from the WT strain or from the strain with tdhAS deleted. As shown in Fig. 4A, although the enterotoxic activities of a tdh-deficient strain (POR-1) and a tdh- and T3SS1-deficient strain (POR-2) appeared to be slightly reduced compared with that of the WT or ΔvscN1 strain, there was no significant difference between these strains. However, the levels of enterotoxic activity of T3SS2-deficient mutant strains, as well as those of the T3SS1- and T3SS2-deficient strains derived from the WT strain (the ΔvscN2 and ΔvscN1 ΔvscN2 strains) and from POR-1 (the POR-3 and ΔvcrD1 ΔvcrD2 strains), were the same as the level of enterotoxic activity of the noninfected control (0.5% NaCl in LB broth). No difference in enterotoxicity due to the presence of T3SS1 was observed. The decrease in the enterotoxicity of the ΔvscN2 strain was restored by transcomplementation with the vscN2 gene (pvscN2) at the same level that is present in the WT strain (Fig. 4B). These findings strongly suggest that T3SS2, but not TDH and T3SS1, is a major contributor to V. parahaemolyticus-induced enterotoxicity in the present rabbit model.
FIG. 4.
T3SS2 is necessary for enterotoxicity. (A and B) Enterotoxicity induced by V. parahaemolyticus as evaluated by fluid accumulation in the rabbit ileal loop test. V. parahaemolyticus RIMD2210633 (109 CFU), the isogenic mutants indicated (109 CFU), and LB broth containing 0.5% NaCl (noninfected control) were injected into ligated rabbit ileal loops. The mutants tested were the ΔvscN1 strain (T3SS1 deficient), the ΔvscN2 strain (T3SS2 deficient), the ΔvscN1 ΔvscN2 strain (T3SS1 and T3SS2 deficient), POR-1 (tdhAS mutant), POR-2 (tdhAS and T3SS1 deficient), POR-3 (tdhAS and T3SS2 deficient), the ΔvcrD1 ΔvcrD2 strain (tdhAS, T3SS1, and T3SS2 deficient), and the ΔvscN2/pvscN2 strain (the ΔvscN2 strain complemented with vscN2). Eighteen hours after injection, the fluid accumulation (FA) ratio in each loop was determined. FA is the amount of accumulated fluid (in milliliters) per centimeter of ligated rabbit small intestine. The error bars indicate standard deviations for the results from triplicate experiments. Two asterisks indicate that the results are significantly different from the results obtained with the WT strain (P < 0.01).
Effect of anti-TDH serum on enterotoxicity induced by V. parahaemolyticus.
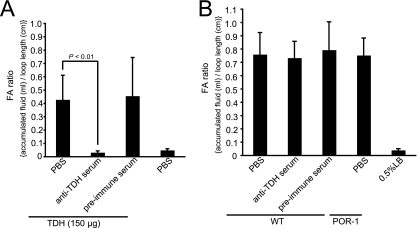
The effect of anti-TDH serum on purified-TDH-induced or V. parahaemolyticus-induced enterotoxicity was also examined. The fluid accumulation was pronounced as a result of injection of purified TDH, but it was completely inhibited by the anti-TDH serum (Fig. 5A). Although this anti-TDH serum could also neutralize the cytotoxicity caused by TDH secreted by bacteria observed in RAW 264 cells (Fig. 3C), the enterotoxicity induced by the WT strain was not inhibited at all (Fig. 5B). These results indicate that TDH secreted by bacteria is not responsible for the enterotoxicity caused by V. parahaemolyticus infection.
FIG. 5.
Effect of anti-TDH polyclonal serum on the accumulation of fluid in ileal loops induced by purified TDH or V. parahaemolyticus. (A) Purified TDH was mixed with either PBS, anti-TDH serum, or preimmune serum, after which the mixture was injected into a ligated rabbit ileal loop. PBS alone was used as a negative control without TDH. (B) A suspension of wild-type V. parahaemolyticus was mixed with either PBS, anti-TDH serum, or preimmune serum, after which the mixture was injected into a ligated rabbit ileal loop. POR-1 (tdhAS deficient) was mixed with PBS, and 0.5% LB (LB broth containing 0.5% NaCl) was used as a noninfected control. Eighteen hours after infection, the FA ratio in each loop was determined. The error bars indicate the standard deviations for the results from triplicate experiments.
TDH and T3SS1 are responsible for lethality in mice.
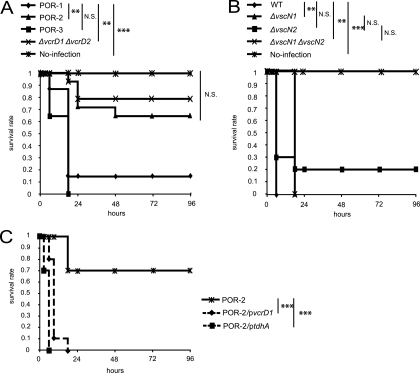
Although V. parahaemolyticus can cause not only gastroenteritis but also wound infections and septicemia, there is little experimental or epidemiological information about these effects. A previous study found that the difference between the lethality in mice induced by intraperitoneal infection with a KP-positive V. parahaemolyticus strain and the lethality in mice induced by intraperitoneal infection with a KP-negative V. parahaemolyticus strain was not significant, suggesting that another virulence factor(s) besides TDH could be involved in these pathogenic activities (10). We therefore examined the possible involvement of TDH and T3SSs in a murine infection model. The murine infection model is used as an animal sepsis model in studies of Vibrio vulnificus, which causes primary septicemia (41). To determine whether TDH is responsible for lethality in mice, the 50% lethal dose (LD50) of the WT strain was compared with that of tdhAS-deficient strain POR-1, and no significant difference in LD50 was observed between the WT and POR-1 strains (7.0 logs and 7.6 logs, respectively); the LD50s obtained were in the same range as the LD50s reported previously (10). Next, the lethal effects of T3SS-deficient strains derived from POR-1 were determined. Surprisingly, when T3SS1-deficient mutant strains, such as POR-2 (tdhAS- and T3SS1-deficient strain) and the ΔvcrD1 ΔvcrD2 strain (tdhAS-, T3SS1-, and T3SS2-deficient strain), were used, the lethality in mice was significantly reduced compared with that of the parent strain POR-1 and the tdhAS- and T3SS2-deficient strain POR-3 (Fig. 6A), while no contribution of T3SS2 to this lethal activity was observed (P values were not statistically significant as determined by a log rank test). We next examined whether T3SS1 is the sole virulence factor of V. parahaemolyticus in this murine infection model by using T3SS-deficient mutants derived from the TDH-expressing strain (WT). Unexpectedly, all mutant strains showed lethal activity against the mice in spite of the absence of T3SSs (Fig. 6B). Moreover, when mice were infected intraperitoneally with POR-2/pvcrD1 or POR-2/ptdhA, lethality was restored (Fig. 6C). This indicates that TDH and T3SS1 may have additive effects on virulence against mice, suggesting that, in addition to T3SS1, TDH may also be responsible for lethality in mice infected with V. parahaemolyticus.
FIG. 6.
TDH and T3SS1 are responsible for mouse lethality. (A) C3H/HeN female mice (n = 15) were infected intraperitoneally with tdhAS-deficient strain POR-1 and T3SS-deficient mutant strains derived from POR-1. The strains used were POR-1 (tdhAS mutant), POR-2 (tdhAS and T3SS1 deficient), POR-3 (tdhAS and T3SS2 deficient), and the ΔvcrD1 ΔvcrD2 strain (tdhAS, T3SS1, and T3SS2 deficient); a no-infection control was also included. (B) CH3/HeN female mice (n = 10) were infected intraperitoneally with WT and T3SS-deficient mutant strains derived from the WT strain. The strains used were the WT strain, the ΔvscN1 strain (T3SS1 deficient), the ΔvscN2 strain (T3SS2 deficient), and the ΔvscN1 ΔvscN2 strain (T3SS1 and T3SS2 deficient); a no-infection control was also included. (C) C3H/HeN female mice (n = 10) were infected intraperitoneally with POR-2, POR-2/pvcrD1 (POR-2 complemented with vcrD1), and POR-2/ptdhA (POR-2 complemented with tdhA). The lethality in mice was recorded at the times indicated. The murine survival rate was analyzed with Kaplan-Meier and log rank tests (**, P < 0.01; ***, P < 0.001; N.S., not significant).
DISCUSSION
In this study, our aim was to determine the roles of TDH and T3SSs in the three distinct aspects of the pathogenicity (cytotoxicity, enterotoxicity, and septicemia) of V. parahaemolyticus infection.
First, using an immunoblotting assay, we found that TDH-, T3SS1- and T3SS2-dependent proteins were secreted separately via their own secretion systems (Fig. 1). Our previous studies demonstrated that secretion of the T3SS1 and T3SS2 effector proteins is correlated with the translocation of these proteins into host cells (19, 33). Although TDH possesses a putative signal peptide sequence which is essential for secretion by the sec secretory pathway (30, 35) (i.e., a type II secretion system), it was possible that TDH is also secreted via the T3SS2 apparatus because the tdh genes are located proximate to the T3SS2 region in chromosome 2 (21). In the present study, we could not obtain positive evidence showing that TDH is secreted via the T3SSs (Fig. 1).
Our investigation demonstrated that T3SS1 is the dominant contributor to V. parahaemolyticus cytotoxicity, while T3SS2 and TDH can induce cytotoxic activity against only a limited number of cell lines (Fig. 2 and 3), even though there are no apparent differences in adherence to the host cells among mutant strains (data not shown). TDH-dependent cytotoxic activity was observed with RAW 264 cells (Fig. 3A) and was completely inhibited by addition of TDH-neutralizing monoclonal antibody MAb 1-24 and anti-TDH serum (Fig. 3B). In addition, we showed that RAW 264 cells are more sensitive than other cell lines to purified TDH (Fig. 3C), which suggests that this is the most likely reason that TDH-dependent cytotoxicity is observed only with RAW 264 cells.
Analysis and determination of virulence factors involved in enterotoxicity are important for elucidating the pathogenicity of V. parahaemolyticus, since the most common symptom of infection by this bacterium is gastroenteritis. We showed that there was no difference in enterotoxicity due to the presence of TDH or T3SS1 under our experimental conditions (Fig. 4). Furthermore, anti-TDH serum could not suppress the fluid accumulation caused by wild-type V. parahaemolyticus (Fig. 5B), even though the same concentration of the anti-TDH serum could inhibit bacterially secreted TDH-dependent cytotoxicity observed with RAW 264 cells (Fig. 3C), as well as purified TDH-induced enterotoxicity (Fig. 5A). These results agree with those of a previous study, which suggested that accumulation of fluid induced by V. parahaemolyticus is not directly related to TDH but may be caused by another factor(s) (14). It is possible that the predicted but as-yet-unknown factor(s) responsible for V. parahaemolyticus-induced enterotoxicity is an effector(s) injected by T3SS2. In contrast to our results, it was reported previously that the tdhAS deletion mutant strain did not exhibit enterotoxicity or exhibited partially reduced enterotoxicity in the rabbit ileal loop test (29, 32). Although the reason for this difference is not clear, it may be due to experimental differences in (i) bacterial strains (RIMD2210633 was used the present study, while in a previous study [29] AQ3815 was used), (ii) the number of bacteria used for infection (we used 109 cells/loop, while previous studies used 108 cells/loop [29, 32]), and (iii) growth media (we used LB broth supplemented with 0.5% NaCl, and other investigators used brain heart infusion broth supplemented with 2% NaCl [29] or LB broth supplemented with 3% NaCl [32]). We believe that especially the difference in the salt concentrations of the culture media may be the cause of the difference since T3SS2 genes tend to be expressed in media with low salt concentrations (unpublished data). It stands to the reason that the conditions of T3SS2 gene expression were not taken into consideration in previous studies since T3SS2 of V. parahaemolyticus had not been identified at that time. The culture conditions used in our study can evaluate enterotoxic activity more accurately since they allow expression of not only TDH but also both T3SSs (Fig. 1 to 3). We therefore concluded that T3SS2 is the dominant contributor to the enterotoxicity of V. parahaemolyticus. Although there have been several reports about T3SS2 effectors (19, 20, 40), the relationship between these effectors and T3SS2-dependent enterotoxicity remains unknown. Future studies need to explore whether any of these effectors are involved in T3SS2-dependent enterotoxicity.
Although it is known that V. parahaemolyticus can cause wound infections and septicemia, there is little experimental or epidemiological information about the symptoms, except for one previous study which reported that the difference in mouse lethality between KP-positive and KP-negative strains was not significant (10). In our study, we demonstrated the importance of T3SS1 and TDH in virulence in the murine infection model. As shown in Fig. 6A, we found that a TDH null mutant strain (POR-1) could have a lethal effect on mice when they were infected intraperitoneally and that T3SS1 made a major contribution to the lethal activity. These results can account for the previously reported finding that the difference in mouse lethality between KP-positive and KP-negative strains was not significant, because T3SS1-related genes were conserved in all V. parahaemolyticus strains regardless of whether they were KP positive or KP negative (16, 22). Although there was no significant difference in LD50 between WT and the tdhAS deletion mutant strain POR-1 (7.0 logs and 7.6 logs, respectively), TDH seemed to be partially involved in lethality. All mice died when they were infected by V. parahaemolyticus containing tdhAS regardless of the presence of T3SSs (Fig. 6B). In addition, we showed that both TDH and T3SS1 contributed to lethality in mice by performing a complementation study (Fig. 6C). The findings suggest that TDH and T3SS1 have additive effects on virulence for mice. In the case of V. vulnificus, a previous study determined that a high level of cytotoxic activity against macrophages is responsible for lethality in mice (41). Because TDH and T3SS1 contribute to cytotoxic activity against macrophage-like cell line RAW 264 (Fig. 3), this may be the reason why TDH and T3SS1, but not T3SS2, are responsible for lethality in mice. Although there have been no epidemiological studies examining whether V parahaemolyticus strains isolated from wound infections and septicemia patients are KP positive or KP negative, the prevalence of the T3SS1 genes in V. parahaemolyticus strains makes it reasonable to assume that not only KP-positive strains but also KP-negative strains are isolated from patients with such infections. Since there is no clinical evidence, genotype analysis of strains isolated from patients with wound infections and septicemia may be needed.
In this study, we determined the roles of TDH and T3SSs in the pathogenicity of V. parahaemolyticus. It is interesting that each virulence factor appears to have a specific role in a distinct aspect of each pathogenic mechanism. Although the question of which effector protein(s) is responsible for T3SS2-dependent enterotoxicity and which effector protein(s) is responsible for T3SS1-dependent mouse lethality remains to be answered, we expect that our findings will be a stepping stone toward understanding the pathogenic mechanism of V. parahaemolyticus.
Acknowledgments
We thank R. Dryselius for critical reading of the manuscript.
This study was supported by Grants-in-Aid for Scientific Research on Priority Areas Applied Genomics and Matrix of Infection Phenomena and for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Editor: S. M. Payne
Footnotes
Published ahead of print on 19 January 2010.
REFERENCES
- 1.Bhattacharjee, R. N., K. S. Park, Y. Kumagai, K. Okada, M. Yamamoto, S. Uematsu, K. Matsui, H. Kumar, T. Kawai, T. Iida, T. Honda, O. Takeuchi, and S. Akira. 2006. VP1686, a Vibrio type III secretion protein, induces Toll-like receptor-independent apoptosis in macrophages through NFκ inhibition. J. Biol. Chem. 281:36897-36904. [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharjee, R. N., K. S. Park, K. Okada, Y. Kumagai, S. Uematsu, O. Takeuchi, S. Akira, T. Iida, and T. Honda. 2005. Microarray analysis identifies apoptosis regulatory gene expression in HCT116 cells infected with thermostable direct hemolysin-deletion mutant of Vibrio parahaemolyticus. Biochem. Biophys. Res. Commun. 335:328-334. [DOI] [PubMed] [Google Scholar]
- 3.Burdette, D. L., M. L. Yarbrough, A. Orvedahl, C. J. Gilpin, and K. Orth. 2008. Vibrio parahaemolyticus orchestrates a multifaceted host cell infection by induction of autophagy, cell rounding, and then cell lysis. Proc. Natl. Acad. Sci. U. S. A. 105:12497-12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cornelis, G. R., and F. Van Gijsegem. 2000. Assembly and function of type III secretory systems. Annu. Rev. Microbiol. 54:735-774. [DOI] [PubMed] [Google Scholar]
- 5.Daniels, N. A., L. MacKinnon, R. Bishop, S. Altekruse, B. Ray, R. M. Hammond, S. Thompson, S. Wilson, N. H. Bean, P. M. Griffin, and L. Slutsker. 2000. Vibrio parahaemolyticus infections in the United States, 1973-1998. J. Infect. Dis. 181:1661-1666. [DOI] [PubMed] [Google Scholar]
- 6.Deepanjali, A., H. S. Kumar, I. Karunasagar, and I. Karunasagar. 2005. Seasonal variation in abundance of total and pathogenic Vibrio parahaemolyticus bacteria in oysters along the southwest coast of India. Appl. Environ. Microbiol. 71:3575-3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DePaola, A., J. L. Nordstrom, J. C. Bowers, J. G. Wells, and D. W. Cook. 2003. Seasonal abundance of total and pathogenic Vibrio parahaemolyticus in Alabama oysters. Appl. Environ. Microbiol. 69:1521-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Espinosa, A., and J. R. Alfano. 2004. Disabling surveillance: bacterial type III secretion system effectors that suppress innate immunity. Cell. Microbiol. 6:1027-1040. [DOI] [PubMed] [Google Scholar]
- 9.Hara-Kudo, Y., K. Sugiyama, M. Nishibuchi, A. Chowdhury, J. Yatsuyanagi, Y. Ohtomo, A. Saito, H. Nagano, T. Nishina, H. Nakagawa, H. Konuma, M. Miyahara, and S. Kumagai. 2003. Prevalence of pandemic thermostable direct hemolysin-producing Vibrio parahaemolyticus O3:K6 in seafood and the coastal environment in Japan. Appl. Environ. Microbiol. 69:3883-3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoashi, K., K. Ogata, H. Taniguchi, H. Yamashita, K. Tsuji, Y. Mizuguchi, and N. Ohtomo. 1990. Pathogenesis of Vibrio parahaemolyticus: intraperitoneal and orogastric challenge experiments in mice. Microbiol. Immunol. 34:355-366. [DOI] [PubMed] [Google Scholar]
- 11.Honda, T., K. Goshima, Y. Takeda, Y. Sugino, and T. Miwatani. 1976. Demonstration of the cardiotoxicity of the thermostable direct hemolysin (lethal toxin) produced by Vibrio parahaemolyticus. Infect. Immun. 13:163-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Honda, T., Y. X. Ni, A. Hata, M. Yoh, T. Miwatani, T. Okamoto, K. Goshima, H. Takakura, S. Tsunasawa, and F. Sakiyama. 1990. Properties of a hemolysin related to the thermostable direct hemolysin produced by a Kanagawa phenomenon negative, clinical isolate of Vibrio parahaemolyticus. Can. J. Microbiol. 36:395-399. [DOI] [PubMed] [Google Scholar]
- 13.Honda, T., Y. X. Ni, and T. Miwatani. 1988. Purification and characterization of a hemolysin produced by a clinical isolate of Kanagawa phenomenon-negative Vibrio parahaemolyticus and related to the thermostable direct hemolysin. Infect. Immun. 56:961-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Honda, T., Y. Takeda, T. Miwatani, and N. Nakahara. 1983. Failure of antisera to thermostable direct hemolysin and cholera enterotoxin to prevent accumulation of fluid caused by Vibrio parahaemolyticus. J. Infect. Dis. 147:779. [DOI] [PubMed] [Google Scholar]
- 15.Hueck, C. J. 1998. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol. Mol. Biol. Rev. 62:379-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Izutsu, K., K. Kurokawa, K. Tashiro, S. Kuhara, T. Hayashi, T. Honda, and T. Iida. 2008. Comparative genomic analysis using microarray demonstrates a strong correlation between the presence of the 80-kilobase pathogenicity island and pathogenicity in Kanagawa phenomenon-positive Vibrio parahaemolyticus strains. Infect. Immun. 76:1016-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kodama, T., Y. Akeda, G. Kono, A. Takahashi, K. Imura, T. Iida, and T. Honda. 2002. The EspB protein of enterohaemorrhagic Escherichia coli interacts directly with alpha-catenin. Cell. Microbiol. 4:213-222. [DOI] [PubMed] [Google Scholar]
- 18.Kodama, T., H. Hiyoshi, K. Gotoh, Y. Akeda, S. Matsuda, K. S. Park, V. V. Cantarelli, T. Iida, and T. Honda. 2008. Identification of two translocon proteins of Vibrio parahaemolyticus type III secretion system 2. Infect. Immun. 76:4282-4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kodama, T., M. Rokuda, K. S. Park, V. V. Cantarelli, S. Matsuda, T. Iida, and T. Honda. 2007. Identification and characterization of VopT, a novel ADP-ribosyltransferase effector protein secreted via the Vibrio parahaemolyticus type III secretion system 2. Cell. Microbiol. 9:2598-2609. [DOI] [PubMed] [Google Scholar]
- 20.Liverman, A. D., H. C. Cheng, J. E. Trosky, D. W. Leung, M. L. Yarbrough, D. L. Burdette, M. K. Rosen, and K. Orth. 2007. Arp2/3-independent assembly of actin by Vibrio type III effector VopL. Proc. Natl. Acad. Sci. U. S. A. 104:17117-17122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Makino, K., K. Oshima, K. Kurokawa, K. Yokoyama, T. Uda, K. Tagomori, Y. Iijima, M. Najima, M. Nakano, A. Yamashita, Y. Kubota, S. Kimura, T. Yasunaga, T. Honda, H. Shinagawa, M. Hattori, and T. Iida. 2003. Genome sequence of Vibrio parahaemolyticus: a pathogenic mechanism distinct from that of V. cholerae. Lancet 361:743-749. [DOI] [PubMed] [Google Scholar]
- 22.Meador, C. E., M. M. Parsons, C. A. Bopp, P. Gerner-Smidt, J. A. Painter, and G. J. Vora. 2007. Virulence gene- and pandemic group-specific marker profiling of clinical Vibrio parahaemolyticus isolates. J. Clin. Microbiol. 45:1133-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mertens, A., J. Nagler, W. Hansen, and E. Gepts-Friedenreich. 1979. Halophilic, lactose-positive Vibrio in a case of fatal septicemia. J. Clin. Microbiol. 9:233-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller, V. L., and J. J. Mekalanos. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 170:2575-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyamoto, Y., T. Kato, Y. Obara, S. Akiyama, K. Takizawa, and S. Yamai. 1969. In vitro hemolytic characteristic of Vibrio parahaemolyticus: its close correlation with human pathogenicity. J. Bacteriol. 100:1147-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyamoto, Y., Y. Obara, T. Nikkawa, S. Yamai, T. Kato, Y. Yamada, and M. Ohashi. 1980. Simplified purification and biophysicochemical characteristics of Kanagawa phenomenon-associated hemolysin of Vibrio parahaemolyticus. Infect. Immun. 28:567-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nasu, H., T. Iida, T. Sugahara, Y. Yamaichi, K. S. Park, K. Yokoyama, K. Makino, H. Shinagawa, and T. Honda. 2000. A filamentous phage associated with recent pandemic Vibrio parahaemolyticus O3:K6 strains. J. Clin. Microbiol. 38:2156-2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niikawa, T., Y. Obara, S. Yamai, and Y. Miyamoto. 1972. Purification of a hemolysin from Vibrio parahaemolyticus. Jpn. J. Med. Sci. Biol. 25:197-200. [PubMed] [Google Scholar]
- 29.Nishibuchi, M., A. Fasano, R. G. Russell, and J. B. Kaper. 1992. Enterotoxigenicity of Vibrio parahaemolyticus with and without genes encoding thermostable direct hemolysin. Infect. Immun. 60:3539-3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishibuchi, M., and J. B. Kaper. 1985. Nucleotide sequence of the thermostable direct hemolysin gene of Vibrio parahaemolyticus. J. Bacteriol. 162:558-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ono, T., K. S. Park, M. Ueta, T. Iida, and T. Honda. 2006. Identification of proteins secreted via Vibrio parahaemolyticus type III secretion system 1. Infect. Immun. 74:1032-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park, K. S., T. Ono, M. Rokuda, M. H. Jang, T. Iida, and T. Honda. 2004. Cytotoxicity and enterotoxicity of the thermostable direct hemolysin-deletion mutants of Vibrio parahaemolyticus. Microbiol. Immunol. 48:313-318. [DOI] [PubMed] [Google Scholar]
- 33.Park, K. S., T. Ono, M. Rokuda, M. H. Jang, K. Okada, T. Iida, and T. Honda. 2004. Functional characterization of two type III secretion systems of Vibrio parahaemolyticus. Infect. Immun. 72:6659-6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raimondi, F., J. P. Kao, C. Fiorentini, A. Fabbri, G. Donelli, N. Gasparini, A. Rubino, and A. Fasano. 2000. Enterotoxicity and cytotoxicity of Vibrio parahaemolyticus thermostable direct hemolysin in in vitro systems. Infect. Immun. 68:3180-3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rusch, S. L., and D. A. Kendall. 2007. Interactions that drive Sec-dependent bacterial protein transport. Biochemistry 46:9665-9673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ryan, W. J. 1976. Marine vibrios associated with superficial septic lesions. J. Clin. Pathol. 29:1014-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sakazaki, R., K. Tamura, T. Kato, Y. Obara, and S. Yamai. 1968. Studies on the enteropathogenic, facultatively halophilic bacterium, Vibrio parahaemolyticus. 3. Enteropathogenicity. Jpn. J. Med. Sci. Biol. 21:325-331. [DOI] [PubMed] [Google Scholar]
- 38.Sakurai, J., A. Matsuzaki, and T. Miwatani. 1973. Purification and characterization of thermostable direct hemolysin of Vibrio parahaemolyticus. Infect. Immun. 8:775-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang, G., T. Iida, K. Yamamoto, and T. Honda. 1997. Analysis of functional domains of Vibrio parahaemolyticus thermostable direct hemolysin using monoclonal antibodies. FEMS Microbiol. Lett. 150:289-296. [DOI] [PubMed] [Google Scholar]
- 40.Trosky, J. E., S. Mukherjee, D. L. Burdette, M. Roberts, L. McCarter, R. M. Siegel, and K. Orth. 2004. Inhibition of MAPK signaling pathways by VopA from Vibrio parahaemolyticus. J. Biol. Chem. 279:51953-51957. [DOI] [PubMed] [Google Scholar]
- 41.Tsuchiya, T., E. Mitsuo, N. Hayashi, Y. Hikita, H. Nakao, S. Yamamoto, K. Miyamoto, and H. Tsujibo. 2007. Vibrio vulnificus damages macrophages during the early phase of infection. Infect. Immun. 75:4592-4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoh, M., N. Kawakami, Y. Funakoshi, K. Okada, and T. Honda. 1995. Evaluation of two assay kits for thermostable direct hemolysin (TDH) as an indicator of TDH-related hemolysin (TRH) produced by Vibrio parahaemolyticus. Microbiol. Immunol. 39:157-159. [DOI] [PubMed] [Google Scholar]
- 43.Zen-Yoji, H., H. Hitokoto, S. Morozumi, and R. A. Le Clair. 1971. Purification and characterization of a hemolysin produced by Vibrio parahaemolyticus. J. Infect. Dis. 123:665-667. [DOI] [PubMed] [Google Scholar]