Abstract
A human Campylobacter jejuni infection model provided controlled exposure to assess vaccine efficacy and investigate protective immunity for this important diarrheal pathogen. A well-characterized outbreak strain, C. jejuni 81-176, was investigated using a volunteer experimental infection model to evaluate the dose range and duration of protection. Healthy Campylobacter-seronegative adults received C. jejuni strain 81-176 via oral inoculation of 105, 107, or 109 CFU (5 adults/dose), which was followed by clinical and immunological monitoring. Based on dose range clinical outcomes, the 109-CFU dose (n = 31) was used to assess homologous protection at 28 to 49 days (short-term veterans [STV]; n = 8) or 1 year (long-term veterans [LTV]; n = 7) after primary infection. An illness dose effect was observed for naïve subjects (with lower doses, 40 to 60% of the subjects were ill; with the 109-CFU dose, 92% of the subjects were ill) along with complete protection for the STV group and attenuated illness for the LTV group (57%). Partial resistance to colonization was seen in STV (25% of the subjects were not infected; 3-log-lower maximum excretion level). Systemic and mucosal immune responses were robust in naïve subjects irrespective of the dose or the severity of illness. In contrast, in STV there was a lack of circulating antibody-secreting cells (ASC), reflecting the local mucosal effector responses. LTV exhibited comparable ASC responses to primary infection, and anamnestic fecal IgA responses likely contributed to self-resolving illness prior to antibiotic treatment. Campylobacter antigen-dependent production of gamma interferon by peripheral blood mononuclear cells was strongly associated with protection from illness, supporting the hypothesis that TH1 polarization has a primary role in acquired immunity to C. jejuni. This study revealed a C. jejuni dose-related increase in campylobacteriosis rates, evidence of complete short-term protection that waned with time, and immune response patterns associated with protection.
Campylobacter species, the most common of which is Campylobacter jejuni, are zoonotic food- and waterborne bacterial enteropathogens (1, 2, 52). The gastrointestinal tracts of animals used for food, such as chickens, are the reservoirs for these common organisms (1). Worldwide, C. jejuni is among the most frequent causes of diarrhea, including traveler's diarrhea, and the spectrum of illness ranges from mild watery diarrhea to febrile dysentery (6, 14, 16, 21). Evidence for acquired immunity against C. jejuni has been obtained from epidemiologic studies performed in developing countries that documented that there is a decline in the incidence of disease with increasing age that is accompanied by a shift in the illness-to-infection ratio for children between 2 and 5 years old, development of resistance to colonization, and a shorter excretion period during convalescence (12, 44, 45). Age-related increases in C. jejuni-specific serology coincide with acquisition of resistance to infection and illness (9, 12, 45). In addition, a lower incidence of Campylobacter-associated diarrhea was observed in infants whose mothers had colostral Campylobacter-specific secretory IgA antibodies in their breast milk (13).
Evidence for acquired immunity against C. jejuni has also been obtained from studies performed in industrialized countries. Reduced C. jejuni-associated diarrhea rates correlated with increased levels of Campylobacter-specific IgA antibody in chronic consumers of raw milk on dairy farms compared to individuals exposed to raw milk for the first time, as well as a lower risk of diarrhea for travelers to regions where C. jejuni is hyperendemic (10, 11, 51). Black and colleagues performed the initial study of an experimental C. jejuni infection in humans (5a, 7). A human Campylobacter infection model provided controlled exposure coupled with predefined endpoints to assess the efficacy of a candidate vaccine and to investigate pathogenesis and immunity. C. jejuni 81-176, a milk-borne outbreak strain (26), was one of the two strains investigated. This study documented the pathogenicity of C. jejuni; however, low and variable rates of attack (40 to 60%) without an illness dose response (106 to 109 CFU delivered in skim milk) were observed (5a, 7). Infection with or without illness induced serologic and intestinal antibody responses. Higher prechallenge C. jejuni-specific (acid-extracted protein) serologic and jejunal fluid IgA levels in noninfected subjects than in infected subjects were observed, as were increased levels of jejunal fluid IgA during rechallenge in subjects who remained well after a second exposure.
A rare (approximately 1 in 1,000 to 3,000 C. jejuni enteritis cases [30, 42]) but potentially life-threatening complication of Campylobacter infection is Guillain-Barré syndrome (GBS), a postinfectious polyneuropathy that is a leading cause of paralysis (32, 49). Research evidence supports the hypothesis that the C. jejuni-GBS association is due to molecular mimicry, where peripheral nerve gangliosides share epitopes with C. jejuni outer lipooligosaccharide (LOS) cores, leading to a misdirected and harmful immune response (17, 25, 53, 54). Prestudy characterization of the challenge strain revealed no evidence of ganglioside mimicry associated with GBS pathogenesis (12).
The campylobacteriosis clinical outcomes observed in the study of Black et al. were not sufficiently frequent or predictable based on the dose to support evaluation of vaccine efficacy. In the current study, two modifications were included, inoculum delivery with bicarbonate buffer and C. jejuni-specific serologic screening, based on post hoc analysis of data from the previous study (3). The change in the method of inoculum delivery was based on evidence obtained with a human Shigella infection model, which showed that 11/12 (92%) naïve subjects developed clinical illness when 1.4 × 103 CFU was delivered with bicarbonate buffer (2 g NaHCO3 in 150 ml distilled water), compared to attack rates of 50 to 60% (upper limits) with challenge doses between 5 × 103 and 1 × 108 CFU in skim milk in previous studies (27).
In this study we report a refined human C. jejuni 81-176 infection model which demonstrated that there was a dose-related increase in campylobacteriosis rates and provided evidence of complete short-term protection that waned with time and cell-mediated immune response patterns that were associated with protection. This work improves the model for future application and provides directions for additional refinements.
(This study was presented in part at the 10th International Congress of Immunology, New Delhi, India, 1998, and at the 10th International Workshop on Campylobacter, Helicobacter and Related Organisms, Baltimore, MD, 1999.)
MATERIALS AND METHODS
Study design.
This study included three stages: a dose range analysis (105, 107, and 109 CFU; 5 subjects/group), confirmation of the selected dose (109 CFU) with moderately severe (≥70%) target campylobacteriosis, and homologous challenge. Subjects were rechallenged 1 to 2 months (short-term veterans [STV]) or 1 year (long-term veterans [LTV]) after the first infection.
Participants and subject eligibility.
Healthy adults (ages, 18 to 55 years) were recruited from the greater Washington, DC, area and enrolled after informed consent was obtained. The criteria for exclusion included poor health; personal or family history of GBS or inflammatory arthritis; macrolide or fluoroquinolone allergy; commercial food handler; clinically significant abnormalities as determined by physical examination or basic laboratory screening (complete blood count, serum chemistry, urinalysis, HIV-1 enzyme-linked immunosorbent assay [ELISA], hepatitis B surface antigen, hepatitis C virus ELISA, HLA-B27, and serum pregnancy test); abnormal bowel habits; regular use of antidiarrheal or anticonstipation agent; antacid therapy; antibiotic use (7 days before admission); use of immunosuppressive drugs; and participation in research involving another investigational product. Prior exposure to Campylobacter, as determined by history or serology (C. jejuni glycine extract IgA ELISA absorbance at 405 nm of >0.5 at 1:1,000 dilution, based on a previous report [3]), was also a basis for exclusion.
Characterization of C. jejuni strain.
C. jejuni 81-176 (Penner serotype 23/36) was isolated from a child with watery diarrhea during a milk-borne outbreak (26) and was used in a previous challenge study (7). An isolate recovered from a subject with diarrhea was used to prepare a master seed lot at the Walter Reed Army Institute of Research Pilot BioProduction Facility (preserved in 15% glycerol at −85°C). The challenge strain is susceptible to macrolides and fluoroquinolones.
Preparation and delivery of challenge inocula.
Challenge inocula were prepared from the master seed stock as previously described (7), except that Mueller-Hinton soft agar was used to select for highly motile C. jejuni cells, which were pooled and subcultured on Brucella agar (Difco, Detroit, MI). Confluent growth was harvested in cold phosphate-buffered saline (PBS), and the optical density at 625 nm (OD625) was adjusted to the appropriate target value (postinoculation number of CFU as determined by plate counting). A 5-ml aliquot was mixed with 150 ml of sterile water containing 2 g of sodium bicarbonate and then ingested with 90 min of fasting before and after inoculation.
Dose selection, randomization, and blinding procedures.
Subjects were randomly assigned to groups during the dose range double-blinded phase. After the dose range phase, the study was unblinded to allow selection of the dose used for subsequent studies.
Clinical outcome definitions.
Infection was defined as two consecutive C. jejuni-positive stool cultures ≥24 h after oral inoculation. Diarrhea was defined as ≥3 loose or liquid stools per 24-h period or ≥2 loose or liquid stools with a volume ≥300 ml in a 24-h period. The associated symptoms included in the clinical grading procedure were fever (≥38.1°C), abdominal cramps, nausea, vomiting, tenesmus, and dysentery (gross blood in two specimens), and the following scale was used: grade 0, not present; grade 1, mild, barely noticed; grade 2, moderate, easily noticed, with some change in normal activities; and grade 3, severe, unable to perform normal activities. Campylobacteriosis, the primary endpoint, was defined as documented infection and clinical illness that occurred prior to the first dose of antibiotic. The campylobacteriosis categories used were mild (diarrhea with no associated symptom greater than grade 1), moderate (any two of the following indications: diarrhea, fever, one or more grade 2 associated symptoms, and one grade 3 associated symptom), and severe (any two of the following indications: diarrhea with a total of >9 stools, dysentery, high fever [>38.6°C], and more than one grade 3 associated symptom).
Clinical monitoring and management.
Subjects were evaluated daily while they were in a restricted-access inpatient ward until they were cleared for discharge following antibiotic therapy and resolution of clinical illness. Any subject who developed diarrhea received appropriate oral rehydration (intravenous if needed). All subjects received azithromycin (500 mg orally once a day for 5 days) irrespective of the infection or illness status 5 days after inoculation (the antibiotic start day was extended to day 7 for the LTV group). The subjects who met criteria for severe campylobacteriosis were treated immediately, whereas the other subjects received treatment 72 h after the onset of illness. Weekly clinical visits occurred until day 28 postinoculation to assess evidence of relapse and postinfection sequelae.
Stool microbiology.
Routine stool bacteriology and parasitology studies were performed 1 week before admission. During the inpatient period, stools were evaluated to determine their volume, stool grade, visible and occult blood, and fecal leukocytes (once a day for diarrheal stools). At least one stool or rectal swab was cultured every day. The primary isolation media included Campylobacter blood agar (Campy-BAP; Remel), Butzler blood agar (B-BAP; Remel), and CAMPY-thioglycolate enrichment medium incubated at 42°C in a 5% O2-10% CO2 environment. After 48 h of incubation, selected Campylobacter colonies were confirmed by Gram staining, the oxidase reaction, and the Lior 5 serotype. Quantitative estimates were obtained by plating serial dilutions of 10% stool suspensions in PBS onto CAMPY-BAP. Plates with colony counts in excess of 300 colonies, at the highest dilution, were considered to contain 3 × 108 CFU/g.
C. jejuni immunology. (i) Sample collection.
Peripheral blood was collected for plasma separation by centrifugation and then stored at −30°C, and peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll density gradient centrifugation followed by cryopreservation (4, 5). Stool samples were kept on ice, and 2- to 4-g aliquots were frozen within 10 h after collection at −70°C before processing.
(ii) Serology.
Campylobacter glycine extract (GE)-specific serum antibodies were measured by ELISA using peroxidase-conjugated isotype-specific goat anti-human immunoglobulins as described elsewhere (5, 34). Endpoint titers were calculated by determining the reciprocal of the highest dilution that gave a net (antigen well − control well) absorbance of ≥0.15. Endpoint titers were loge transformed and expressed as geometric mean titers.
(iii) ASC.
Levels of antibody-secreting cells (ASC) were determined by using the enzyme-linked immunosorbent spot-forming assay described elsewhere (3). Nunc immunoplates were coated with 3 μg/ml of GE in pH 9.6 buffer. The PMBCs were washed and suspended at a concentration of 3.33 × 106 cells/ml in complete medium (CM) (RPMI containing 10% heat-inactivated fetal calf serum, 2 mM l-glutamine, and 50 μg/ml gentamicin). Following 4 h of incubation with PBMCs (3.33 × 105 PBMCs/well in triplicate), the secreted antibodies were detected with alkaline phosphatase-conjugated goat anti-human IgA (0.25 μg/ml; KPL, Gaithersburg, MD). Spots were visualized with nitroblue tetrazolium (10 μg/ml)-5-bromo-4-chloro-3-indolylphosphate (BCIP) (5 μg/ml) (Sigma Chemicals, MO) in 0.6% agarose and counted with a dissecting scope. A sample with ≥5 ASC/106 PBMCs was considered a responder sample.
(iv) Fecal IgA.
A 10% stool suspension was prepared in buffer containing protease inhibitors (3), incubated for 20 min at 4°C, and centrifuged, and the supernatant was frozen at −70°C. The total IgA content was determined by an ELISA using goat anti-human F(ab′)2 (1 μg/ml; Jackson Laboratories) as the capture antibody and isotype-specific horseradish peroxidase-conjugated goat anti-human IgA as the detecting antibody (22, 34). The Campylobacter-specific IgA content was determined by the ELISA described above, except that the incubation time was 3 h. Endpoint titers were determined and adjusted to 500 μg/ml of total IgA; samples with <50 μg/ml total IgA were excluded.
(v) IFN-γ assay.
PBMCs were washed and suspended at a concentration of 5 × 105 viable cells/ml of CM (RPMI containing 10% heat-inactivated human AB serum, 2 mM l-glutamine, 5 × 10−7 mM 2-mercaptoethanol, and 50 μg/ml gentamicin). Twenty microliters of medium alone or medium containing 105 formalin-killed C. jejuni 81-176 whole cells in addition to 200 μl of CM containing 105 PBMCs was added to wells of round-bottom 96-well tissue plates in duplicate. Following 72 h of incubation at 37°C in 5% CO2, supernatants were collected and stored at −70°C until they were assayed to determine the gamma interferon (IFN-γ) levels by a capture ELISA using paired monoclonal antibodies, and the levels were interpolated using known standards (Endogen, Woburn, MA).
(vi) CRP.
The C-reactive protein (CRP) content of plasma samples was determined using a commercial kit (Dade Behring, Marburg, Germany). Data are expressed below in micrograms of CRP per milliliter; samples containing <6 μg/ml of CRP were considered negative, and the concentrations in these samples were considered 3 μg/ml.
Statistical analysis.
Baseline characteristics and summary findings were compared using analysis of variance, Kruskal-Wallis tests, and chi-square tests, as appropriate. Confidence intervals (CI) were generated using a normal approximation to the binomial distribution. Times to events were evaluated using Kaplan-Meier analyses. All tests were two tailed, and P values of <0.05 were considered statistically significant. Statistical analyses were performed using SPSS for Windows (version 10.1).
RESULTS
Subject enrollment and baseline characteristics.
Following consent, subjects were enrolled; members of the naïve group received 105 CFU (n = 5), 107 CFU (n = 5), or 109 CFU (n = 36), and STV (n = 8) and LTV (n = 7) received 109 CFU. There were no differences in demographic characteristics among the groups. The median age of the naïve subjects was 30 years (interquartile range [IQR], 22.0 to 40.3 years), which was comparable to the median age of the veterans (32 years; IQR, 22.0 to 42.0 years); the majority of the subjects were males (70% and 80%, respectively).
Clinical outcomes.
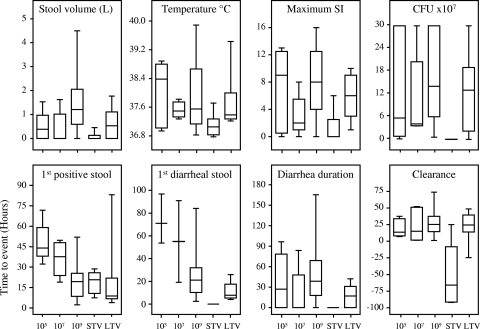
A dose-related increase in the incidence of campylobacteriosis of any severity was observed for naïve subjects (P = 0.006). No dose relationship was observed for associated symptoms or for meeting criteria for severe campylobacteriosis (Table 1); however, compared to the results obtained with lower doses, the total volume of diarrhea was 3-fold greater for the group that received 109 CFU (means, 462 ml and 1,469 ml; P = 0.01) (Fig. 1). The maximum illness severity index (SI), as shown in Fig. 1, provided a composite semiquantitative score that accounted for gastrointestinal and systemic features. The median scores were similar for the groups that received 105 and 109 CFU; however, the scores for 25% of the subjects in the group that received 109 CFU were greater than 12, compared to 10% of the subjects in the groups that received lower doses. Early treatment was more common in the group that received 109 CFU (61%, compared to 10% for the groups that received lower doses).
TABLE 1.
Clinical outcome of experimental C. jejuni 81-176 infection
| Clinical outcome | % of: |
||||
|---|---|---|---|---|---|
| Naïve subjects who receiveda: |
Veteransa |
||||
| 105 CFU (n = 5) | 107 CFU (n = 5) | 109 CFU (n = 36) | STV (n = 8) | LTV (n = 7) | |
| Infectionb | 100 | 100 | 100 | 75 | 100 |
| Campylobacteriosisc | |||||
| Met primary endpoint | 60 | 40 | 92 | 0d | 57 |
| Mild illness | 0 | 20 | 25 | 0 | 14 |
| Moderate illness | 20 | 0 | 22 | 0 | 29 |
| Severe illness | 40 | 20 | 45 | 0 | 14 |
| Associated symptoms | |||||
| Fever | 60 | 0 | 44 | 0 | 14 |
| Abdominal cramps | 60 | 60 | 83 | 25 | 86 |
| Nausea | 40 | 40 | 50 | 25 | 57 |
| Vomiting | 20 | 0 | 14 | 0 | 14 |
| Dysentery | 40 | 20 | 11 | 0 | 0 |
| Hemoccult | 60 | 40 | 56 | 0 | 43 |
| Fecal leukocytese | 75 | 67 | 31 | 25 | 14 |
Groups were defined based on exposure to C. jejuni 81-176, as follows: naïve subjects, one exposure to C. jejuni; and veterans (STV and LTV), two exposures to C. jejuni. The STV and LTV groups are described in Materials and Methods. For veterans the clinical outcomes after the initial exposure were as follows: for the STV group, one subject with mild campylobacteriosis, three subjects with moderate campylobacteriosis, and four subjects with severe campylobacteriosis; and for the LTV group, three subjects that did not meet the clinical endpoint (after the initial exposure, two subjects with moderate campylobacteriosis and one subject who was not ill) and four subjects with campylobacteriosis (after the initial exposure, one subject who was not ill and three subjects with severe campylobacteriosis).
Infection was defined as two consecutive stool cultures that were positive for C. jejuni at ≥24 h postinoculation.
The levels of campylobacteriosis were defined as follows (all required documentation of infection). Mild campylobacteriosis was defined as 3 to 9 loose or liquid stools per 24 h or ≥2 loose stools per 24 h consisting of ≥300 ml. Moderate campylobacteriosis was defined as two of the following: diarrhea, oral temperature of ≥38.1°C, and moderate or severe gastrointestinal signs or symptoms (abdominal cramps, nausea, vomiting, tenesmus, and gross blood in stools). Severe campylobacteriosis was defined as any two of the following plus infection: high fever (oral temperature, >38.6°C), diarrhea (>9 stools), >1 severe associated symptom, and dysenteric stools (gross blood in two specimens).
The campylobacteriosis attack rates were different for the veteran groups (P = 0.03, Fisher's exact test).
The numbers of subjects tested for fecal leukocytes were as follows: 4 subjects who received 105 CFU, 3 subjects who received 107 CFU, all naïve subjects who received 109 CFU, and all subjects in the veteran groups.
FIG. 1.
Selected clinical and microbiological outcomes of C. jejuni infection (upper panels) and times to clinical and microbiological events (lower panels). The groups studied were naïve subjects who were inoculated with 105, 107, and 109 CFU and STV and LTV who received 109 CFU. The box plots show medians, interquartile ranges, and minimum and maximum values. The first diarrheal stool was the first loose or liquid stool fulfilling the definition of diarrhea. Clearance data were based on the time of the last C. jejuni-positive stool culture (time zero was the time when the first antibiotic dose was given). The illness severity index (SI) was calculated using gastrointestinal symptoms (abdominal cramps, nausea, vomiting, tenesmus, and dysentery), systemic symptoms (chills and sweats, malaise, joint aches, loss of appetite, and headache), the maximum temperature, and diarrhea (highest value for stool frequency or volume over the illness episode). The composite score was calculated as follows: symptom score (0, no symptoms; 1, grade 1 symptoms; 2, grade 2 symptoms; 3, 1 or 2 grade 3 symptoms; 4, ≥3 grade 3 symptoms) plus the maximum temperature score (0, <38.1°C; 1, 38.1 to 38.3°C; 2, 38.4 to 38.6°C; 3, 38.7 to 38.8°C; 4, >38.8°C) plus the diarrhea score (0, no loose or liquid stools; 1, <3 stools or <500 ml; 2, 3 to 6 stools or 500 to 999 ml; 3, 7 to 9 stools or 1,000 to 1,499 ml; 4, >9 stools or >1,500 ml). The SI ranged from 0 to 16. The P values for comparisons of naïve subjects who received 109 CFU and subjects who received lower doses were as follows: for median incubation time, Breslow P = 0.0198 and log rank P = 0.0038; and for median time to infection, Breslow P = 0.0004 and log rank P = 0.0003.
With the same dose, 92% of the naïve subjects, none of the STV, and 57% of the LTV met the campylobacteriosis endpoint (Table 1). STV were not completely asymptomatic (median SI, 0; IQR, 0 to 2.75) (Fig. 1). LTV had reduced attack rates compared to the naïve subjects who received 109 CFU (57% and 92%, respectively; P = 0.045). However, the median SIs (6 [IQR, 3 to 9] and 8 [IQR, 4 to 12.75], respectively) were not statistically significantly different (P = 0.24). The reduced severity in LTV was evident because there was less fever and severe campylobacteriosis (14% of the subjects for both, compared with 44%). The median duration of diarrhea after the first antibiotic dose for LTV was −43 h (diarrhea resolved >1.5 days prior to antibiotic treatment), compared to 10 h after the first dose for naïve subjects.
The time to onset of diarrhea was significantly shorter for the group that received 109 CFU than for the groups that received the lower doses; the median incubation times were 21.0 h (95% confidence interval [CI], 16.9 to 25.5 h) and 71.1 h (95% CI, 33.8 to 108.3 h), respectively (Fig. 1). Similar incubation times were observed for naïve subjects and long-term veterans. The range of duration of diarrhea was wider for the naïve group that received 109 CFU, primarily due to earlier onset and slower recovery.
Higher rates of hemoccult positivity and fecal leukocytes were observed for naïve subjects who received the lower doses (Table 1). Compared to LTV (43%) and the naïve subjects who received the lower doses, STV had no detectable positive hemoccult. Fecal leukocytes were uncommon in the veteran groups. The peripheral blood median white blood cell counts (2 days postinoculation), determined only for naïve subjects and STV, were within the normal range; however, leukocytosis (>10,000 cells/mm3) occurred in 20% of the subjects who received 105 and 109 CFU (highest level, 17,000 cells/mm3 in subjects who received 109 CFU). The median peak CRP values were similar (24 to 48) irrespective of the dose for the naïve groups, and these levels were higher than those for either veteran group (3 to 6).
Microbiology results.
All naïve and LTV subjects became infected, whereas 2 of 8 STV did not become infected (Table 1). The median concentration for STV (2.0 × 105 CFU/g; IQR, 3 × 102 to 5 × 105 CFU/g) was also 3 logs lower than that for LTV (1.0 × 108 CFU/g; IQR, 2.0 × 107 to 2.0 × 108 CFU/g) or that for naïve subjects (1.3 × 108 CFU/g; IQR, 5.0 × 107 to 3.0 × 108 CFU/g) who received 109 CFU (P < 0.0001) (Fig. 1). Not including data for STV, the median level of bacteria excreted was lower for asymptomatic subjects than for symptomatic subjects (4.0 × 107 CFU/g [IQR, 6.0 × 106 to 1.1 × 108 CFU/g] and 2.0 × 108 CFU/g [IQR, 6.2 × 107 to 3.0 × 108 CFU/g], respectively) (P = 0.001) (data not shown).
The median time to infection was shorter for naïve subjects who received 109 CFU (18.9 h; 95% CI, 9.8 to 28.0 h) than for naïve subjects who received lower doses (43.8 h; 95% CI, 34.0 to 53.7 h) (Fig. 1). The time to infection for veterans was similar to the time to infection for naïve subjects who received 109 CFU (for STV, 20.3 h [95% CI, 11.4 to 29.2 h; for LTV, 8.9 h [95% CI, 4.5 to 13.3 h). The time to clearance (Fig. 1) was dependent on antibiotic usage, with the exception of the time to clearance for short-term veterans, who cleared their infections prior to antibiotic treatment.
Immunological responses.
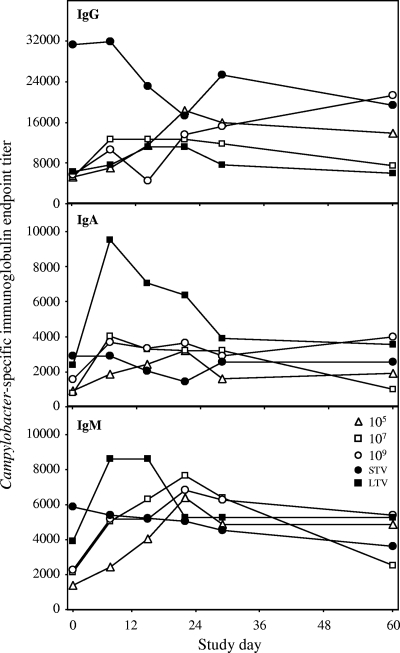
A detailed immunological analysis of acquired mucosal and systemic humoral and cellular immunity to Campylobacter was conducted. Increasing doses of C. jejuni (over a 4-log range) were not significantly associated with higher levels of Campylobacter-specific serum immunoglobulins in naïve subjects. In addition to the levels, the kinetics of serum responses and the responder rates were indistinguishable for the different doses (Fig. 2) (78 to 80% for IgM, 75 to 100% for IgA, and 60 to 64% for IgG). In naïve subjects, the level of IgG increased gradually and remained above the baseline level during the study period (except for the group that received 107 CFU). Compared to the level of IgG, the level of serum IgA or IgM was lower and tended to decline from study day 21 to study day 60. Overall, veterans had higher baseline serum antibody levels than naïve subjects; however, only IgG levels were significantly higher in STV (P = 0.003). Following rechallenge, no increase in antibody levels in STV was detected; however, LTV exhibited early increased IgM levels (peak at day 10, compared to day 21 for naïve subjects) and increased IgA levels (P = 0.02).
FIG. 2.
Serologic responses following experimental infection with C. jejuni. The groups studied were naïve subjects who received inocula containing 105, 107, and 109 CFU and STV and LTV who received 109 CFU. Data were obtained for IgG, IgA, and IgM.
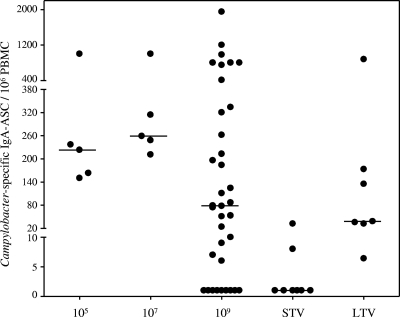
The level of antigen-specific ASC in circulation was determined as a surrogate marker for mucosal immune activation. After inoculation Campylobacter-specific IgA-specific ASC were detected in all naïve subjects and LTV, and the maximum median concentrations were 220, 260, 281, and 135 ASC/106 PBMCs for the naïve groups that received 105, 107, and 109 CFU and the LTV group, respectively (Fig. 3). In contrast, only 2 of 8 STV had detectable levels of IgA-specific ASC (8 and 32 ASC/106 PBMCs).
FIG. 3.
IgA-specific antibody-secreting cell (ASC) responses following C. jejuni infection. The groups studied were naïve subjects who received inocula containing 105, 107, and 109 CFU and STV and LTV who received 109 CFU. The response shown are the maximal responses between 6 and 9 days postinoculation. The horizontal lines indicate medians. No ASCs were detected before challenge in any group (data not shown).
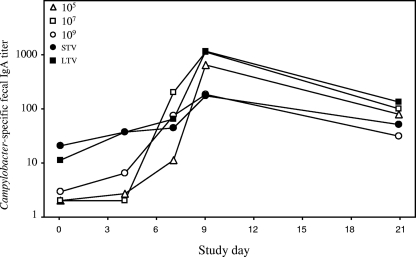
The amount of Campylobacter-specific fecal IgA in stools collected before and after infection was evaluated as a direct measure of the intestinal antibody response (Fig. 4). Compared to the levels for naïve subjects, the baseline fecal IgA levels were higher for the veteran groups (P < 0.0001), and detectable levels (endpoint titers, >1.5) were found in 20%, 75%, and 67% of the naïve subjects, STV, and LTV, respectively (P = 0.001). Irrespective of the dose, robust fecal IgA responses were observed in 86 to 100% of naïve subjects; these responses peaked between days 7 and 9 and remained elevated. The responder rates, as well as the maximum titers postchallenge, were higher for LTV than for STV (responder rates, 83 and 63%, respectively; median endpoint titers for STV, 471 and 184, respectively; P = 0.014). A difference in the kinetics of the fecal IgA response determined by the responder rates was noted. At day 4, 24% of the naïve subjects, 38% of the STV, and 67% of the LTV exhibited a ≥4-fold increase in the fecal IgA level compared with the baseline level.
FIG. 4.
Fecal IgA responses following experimental C. jejuni infection. The groups studied were naïve subjects who received inocula containing 105, 107, and 109 CFU and STV and LTV who received 109 CFU.
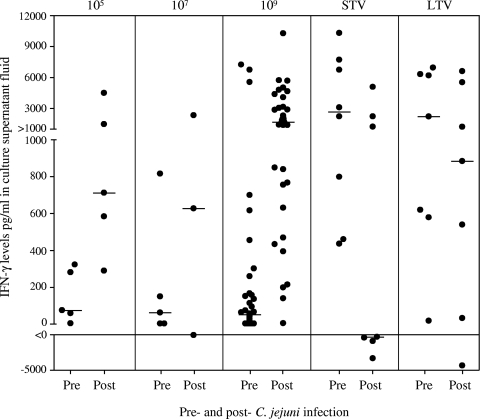
The in vitro induction of IFN-γ by Campylobacter-stimulated PBMCs was measured as an indicator of cellular immunity. Although the baseline median levels of IFN-γ in naïve subjects were similar (60 pg/ml; IQR, 2.5 to 212 pg/ml), the individual levels varied widely (Fig. 5). In contrast to the humoral responses, dose-dependent increases in the IFN-γ level and the frequency of responders (≥4-fold increase) were observed (medians for the groups that received <109 CFU, 669 pg/ml and 63% responders; medians for the group that received 109 CFU, 1,495 pg/ml and 86% responders). Compared to the baseline levels for the naïve subjects, the baseline levels were higher for veterans (P < 0.0001). The median levels postinfection for the STV, LTV, and naïve groups that received 109 CFU were 2,951, 3,229, and 8,54 pg/ml, respectively (P = 0.037). There was a wide spectrum of maximum changes, which tended toward higher values for the naïve subjects (negative change, 3%; 1 to 1,000 pg/ml, 37%; >1,000 pg/ml, 60%) than for the STV subjects (negative change, 57%; 1 to 1,000 pg/ml, none; >1,000 pg/ml, 43%) and the LTV subjects (negative change, 14%; 1 to 1,000 pg/ml, 43%; >1,000 pg/ml, 43%) (P < 0.0001).
FIG. 5.
In vitro IFN-γ responses following C. jejuni infection. The groups studied were naïve subjects who received inocula containing 105, 107, and 109 CFU and STV and LTV who received 109 CFU. The preinoculation values (Pre) were determined on the day of inoculation. The postinoculation values (Post) are the maximum differences between the values obtained either 28 or 60 days postinoculation and the preinoculation values.
The naïve subjects who received 109 CFU of C. jejuni and the veteran groups were used to assess the association between immunological parameters and disease outcome following challenge (Table 2). ASC and serologic levels at the time of challenge were not associated with the clinical outcome. Prechallenge Campylobacter-specific fecal IgA levels were not associated with disease outcome; however, the subsequent response in the naïve subjects with severe disease was lower than the response in the naïve subjects with disease that was not severe (33- and 378-fold increases, respectively; P = 0.004). Prechallenge in vitro production of IFN-γ correlated with disease outcome. The subjects who remained asymptomatic irrespective of exposure history had higher IFN-γ levels (5,378, 2,465, and 6,003 pg/ml in naïve subjects, STV, and LTV, respectively) at the time of challenge than the individuals who had severe disease (49 and 17 pg/ml in naïve subjects and LTV, respectively; P < 0.0001). Two of the three naïve subjects and three LTV subjects who were protected from illness had IFN-γ levels of >2,000 pg/ml; one LTV who met severe illness criteria had the lowest level (17 pg/ml). A prechallenge IFN-γ level of ≥400 pg/ml was observed for the upper 15% of the subjects. This threshold level could be used to predict the likelihood of subsequent illness; the incidence of campylobacteriosis was 74% among subjects with prechallenge IFN-γ levels of <400 pg/ml, and the incidence of illness was 17% among subjects with prechallenge IFN-γ levels of ≥400 pg/ml (P < 0.0001). Further, when the analysis was restricted to LTV and naïve subjects, the results demonstrated that the an IFN-γ level of ≥400 pg/ml was associated with protection (55% compared with 17%) (P = 0.009).
TABLE 2.
Association of clinical outcome and immune responses for experimental C. jejuni 81-176 infection (only for subjects who received 109 CFU)a
| Campylobacteriosis outcome | Serum IgAb |
Serum IgGb |
IgA-specific ASC postinoculation count (ASC/106 PBMCs)c | Fecal IgAd |
IFN-γ concn (pg/ml)e |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Preinoculation titer | Increase (fold) | Preinoculation titer | Increase (fold) | Preinoculation titer | Maximum postinoculation titer | Preinoculation | Maximum change | ||
| Naïve subjects | ||||||||||
| No illness | 3 | 1,274 | 8 (2-8) | 4,024 | 8 (2-8) | 898 (10-898) | 8 | 1,785 (374-1,785) | 5,378 | 2,724 (213-2,724) |
| Mild illness | 9 | 1,366 | 4 (2-20) | 8,434 | 4 (3-6) | 107 (65-837) | 3 | 728 (141-1,808) | 31 | 804 (179-1,437) |
| Moderate illness | 8 | 1,236 | 6 (2-14) | 7,245 | 4 (2-14) | 327 (119-1,033) | 2 | 871 (268-2,186) | 54 | 3,605 (441-4,770) |
| Severe illness | 16 | 1,356 | 4 (2-14) | 6,124 | 4 (2-16) | 252 (86-750) | 6 | 265 (60-620) | 49 | 1,737 (1,212-2,724) |
| Veterans | ||||||||||
| STV with no illness | 8 | 2,922 | 1 (1-2) | 41,357 | 1 (1-2) | 2 (0-5) | 21 | 185 (78-281) | 2,465 | 2,059 (1,054-4,915) |
| LTV | ||||||||||
| No illness | 3 | 2,018 | 8 (2-8) | 5,486 | 4 (1-4) | 72 (32-880) | 6 | 706 (340-1,141) | 6,003 | 183 (32-1,043) |
| Mild illness | 1 | 6,634 | 2 | 32,860 | 4 | 32 | 14 | 471 | 6,768 | 880 |
| Moderate illness | 2 | 1,636, 3,294 | 16; 4 | 13,360, 3,294 | 1, 4 | 6, 135 | 7, ns | 451, 2,113 | 620, 578 | 5,378, 6,439 |
| Severe illness | 1 | 1,636 | 7 | 1,636 | 4 | 174 | 18 | 22 | 17 | 539 |
See footnotes to Table 1 for definitions of clinical outcomes.
The preinoculation titers are geometric means, and the increases are median increases compared with the baseline; the values in parentheses are IQR. For the veteran groups the values for moderate illness are values for two subjects, and the values for mild and severe illness values are values for a single subject.
The values are the maximum median numbers of IgA-specific ASC/106 PBMC; the values in parentheses are IQR.
The preinoculation titers are geometric mean titers. The maximum postinoculation titers are the maximum median titers; the values in parentheses are IQR.
The preinoculation concentrations are medians, and the maximum changes are the median changes from the baseline; the values in parentheses are IQR. The values for the naïve subjects with mild illness were based on data for eight subjects.
DISCUSSION
These studies confirmed and extended previous work by Black et al. that established a C. jejuni infection model in humans (5a, 7). As in the previous study, C. jejuni strain 81-176 exhibited 100% infectivity across the range of doses and a clinical spectrum of illness, consistent with clinical observations (8), ranging from mild watery diarrhea to dysentery with fever and abdominal cramps. No dysentery was observed in a milk-borne outbreak caused by this strain (26). Methodological changes, including inoculation with bicarbonate buffer and serologic screening, led to a dose response that included 92% campylobacteriosis at the highest dose (1 × 109 CFU), compared to a maximum of 60% illness in the previous challenge study. A dose response was observed during the outbreak based on the number of glasses of raw milk ingested (no milk, 0/20 subjects who were ill; 1 glass of milk, 35% ill subjects; ≥2 glasses of milk, 60% ill subjects) (26). Dose-response modeling to reconcile outbreak and experimental infection data supported the hypothesis that a very low dose (50% infectious dose, ≤102 CFU) is capable of causing infection and illness (47).
The incubation period for the 109-CFU dose (21 h) was less than the incubation period for lower doses (71 h), the incubation period determined in a previous study (53 h) (7), the incubation period determined in an investigation of an outbreak (68 h; range, 24 to 128 h) (26), and the incubation period for campylobacteriosis in general (14). Subjects who received the highest dose exhibited more severe clinical symptoms than subjects who received lower doses, as measured by higher total stool volume, and 25% of these subjects were in the highest severity index quartile.
Short-term protection from illness was observed, confirming a previous observation (7). In addition, 25% of the subjects were not infected, and the infected subjects exhibited 3-log-lower maximum excretion than naïve subjects or LTV, providing direct evidence that acquired immunity leads to resistance to colonization in addition to protection from illness not observed in the previous challenge study. Another important new observation is that partial protection at 1 year was shown by lower attack rates and milder disease with no effect on the infection status or level of colonization. Epidemiological evidence from developing countries (29, 40, 44, 45) and industrialized countries (5a) supports the hypothesis that protective immunity is likely acquired after repeated exposure to various strains. The change from illness to infection in early childhood followed by asymptomatic colonization (15, 16, 38, 43), the shorter excretion period in higher-incidence regions (24, 44), the lower incidence after repeated consumption of raw milk (11), and the emergence of less common serotypes in elderly populations (31) suggest that protection is long lasting, in contrast to the waning homologous protection observed at 1 year in this study, which may have been due to less background exposure to C. jejuni. There is limited comparative data from challenge studies to assess the duration of protection; however, infection-derived protective immunity to Vibrio cholerae persisted for 3 years (28).
The rates of robust systemic and mucosal immune responses observed after infection in virtually all naïve subjects were comparable to or higher than the rates observed after natural exposure to C. jejuni (5, 21, 35, 41, 48). Epidemiological evidence also supports the conclusion that the serum antibody level is associated with protection, as previously discussed; however, this association was not observed for challenges of veterans (although all STV had sustained high serum antibody levels following primary infection). The mucosal immune response patterns were different for different veterans, providing insight into effector responses that prevent and/or attenuate illness. In the protected STV the intestinal IgA levels were marginally boosted without circulating ASCs, likely reflecting effective local mucosal effector responses (23, 50). In contrast, LTV, who exhibited an attenuated and self-resolving illness, exhibited ASC responses comparable to those observed in subjects with primary infection but, most importantly, more rapid anamnestic fecal IgA responses (day 4) and greater IgA and IgM serologic responses than naïve subjects, likely contributing to rapid recovery from illness. In addition to distinct patterns, the model provides important evidence that C. jejuni-specific cell-mediated immune (CMI) responses correlate with protection, with in vitro IFN-γ levels of ≥400 pg/ml prechallenge yielding a relative risk of campylobacteriosis of 0.36 (95% CI, 0.24 to 0.55). In vitro data (20, 21), animal model data (39) and clinical observations, colitis biopsy data (37), and a higher risk in HIV/AIDS patients (33, 46) support the hypothesis that CMI has a primary role in C. jejuni clearance with TH1 polarization.
Major considerations in a Campylobacter challenge study include safety, management of acute illness, and the potential for postinfection sequelae. GBS is a rare disease, and C. jejuni infection is the most common antecedent event (14). Given the severity of this illness, strain 81-176 was characterized to rule out the possibility of ganglioside mimicry before the study proceeded (36). Following the study, further genetic analysis of the 81-176 LOS core revealed ganglioside mimics (18). Thus, strain 81-176 may pose a previously unrecognized risk for subjects. Campylobacter strains that lack mimicry have been described, and use of these strains in the model would further mitigate risk. Additional revision of the challenge model is needed to obtain attack rates comparable to those seen with 109 CFU using lower doses and to target moderate to severe clinical illness and a more typical illness onset time. Addition of cellular memory response screening has the potential to improve selection of naïve subjects, resulting in a more uniform and predictable attack rate for evaluation of vaccine efficacy. As recently described in an excellent review (19), appropriate interpretation of epidemiological and risk assessment studies depends on an improved understanding of the interplay and dynamics of protective immunity in campylobacteriosis at both the individual and population levels. This work increased our understanding of the duration of homologous protection in campylobacteriosis and provided further information on adaptive immune responses associated with protection.
Acknowledgments
We thank the technicians and staffs of the Naval Medical Research Center, Silver Spring, MD, and the U.S. Army Medical Research Institute of Infectious Diseases, Frederick, MD, for their assistance with this study.
This work was supported by Work Unit no. 643807A.849.D.A0002.
This study was approved by the scientific and ethical review committees of the U.S. Army Medical Research Institute of Infectious Diseases (protocol A-7707) and complied with all federal regulations governing the protection of human subjects.
The views expressed in this paper are our views and do not necessarily reflect the official policy or position of the Department of Navy, the Department of Defense, or the U.S. Government. D.R.T. is an employee of the U.S. Government, and this work was prepared as part of his official duties.
Editor: B. A. McCormick
Footnotes
Published ahead of print on 19 January 2010.
REFERENCES
- 1.Ailes, E., L. Demma, S. Hurd, J. Hatch, T. F. Jones, D. Vugia, A. Cronquist, M. Tobin-D'Angelo, K. Larson, E. Laine, K. Edge, S. Zansky, and E. Scallan. 2008. Continued decline in the incidence of Campylobacter infections, FoodNet 1996-2006. Foodborne Pathog. Dis. 5:329-337. [DOI] [PubMed] [Google Scholar]
- 2.Altekruse, S. F., D. L. Swerdlow, and N. J. Stern. 1998. Microbial food borne pathogens. Campylobacter jejuni. Vet. Clin. N. Am. 14:31-40. [PubMed] [Google Scholar]
- 3.Baqar, S., L. A. Applebee, T. C. Gilliland, Jr., L. H. Lee, C. K. Porter, and P. Guerry. 2008. Immunogenicity and protective efficacy of recombinant Campylobacter jejuni flagellum-secreted proteins in mice. Infect. Immun. 76:3170-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baqar, S., A. A. Nour El Din, D. A. Scott, A. L. Bourgeois, A. S. Mourad, M. T. Kleinosky, M. J. Oplinger, and J. R. Murphy. 1997. Standardization of measurement of immunoglobulin-secreting cells in human peripheral circulation. Clin. Diagn. Lab. Immunol. 4:375-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baqar, S., B. Rice, L. Lee, A. L. Bourgeois, A. N. El Din, D. R. Tribble, G. P. Heresi, A. S. Mourad, and J. R. Murphy. 2001. Campylobacter jejuni enteritis. Clin. Infect. Dis. 33:901-905. [DOI] [PubMed] [Google Scholar]
- 5a.Black, R., D. Perlman, M. Clements, M. Levine, and M. Blaser. 1992. Human volunteer studies with Campylobacter jejuni, p. 207-215. In I. Nachamkin, M. Blaser, and L. Tompkins (ed.), Campylobacter jejuni: current status and future trends. American Society for Microbiology, Washington, DC.
- 6.Black, R. E. 1990. Epidemiology of travelers' diarrhea and relative importance of various pathogens. Rev. Infect. Dis. 12(Suppl. 1):S73-S79. [DOI] [PubMed] [Google Scholar]
- 7.Black, R. E., M. M. Levine, M. L. Clements, T. P. Hughes, and M. J. Blaser. 1988. Experimental Campylobacter jejuni infection in humans. J. Infect. Dis. 157:472-479. [DOI] [PubMed] [Google Scholar]
- 8.Blaser, M. J., I. D. Berkowitz, F. M. LaForce, J. Cravens, L. B. Reller, and W. L. Wang. 1979. Campylobacter enteritis: clinical and epidemiologic features. Ann. Intern. Med. 91:179-185. [DOI] [PubMed] [Google Scholar]
- 9.Blaser, M. J., R. E. Black, D. J. Duncan, and J. Amer. 1985. Campylobacter jejuni-specific serum antibodies are elevated in healthy Bangladeshi children. J. Clin. Microbiol. 21:164-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blaser, M. J., D. J. Duncan, M. T. Osterholm, G. R. Istre, and W. L. Wang. 1983. Serologic study of two clusters of infection due to Campylobacter jejuni. J. Infect. Dis. 147:820-823. [DOI] [PubMed] [Google Scholar]
- 11.Blaser, M. J., E. Sazie, and L. P. Williams, Jr. 1987. The influence of immunity on raw milk-associated Campylobacter infection. JAMA 257:43-46. [PubMed] [Google Scholar]
- 12.Blaser, M. J., D. N. Taylor, and P. Echeverria. 1986. Immune response to Campylobacter jejuni in a rural community in Thailand. J. Infect. Dis. 153:249-254. [DOI] [PubMed] [Google Scholar]
- 13.Blaser, M. J., D. N. Taylor, and R. A. Feldman. 1983. Epidemiology of Campylobacter jejuni infections. Epidemiol. Rev. 5:157-176. [DOI] [PubMed] [Google Scholar]
- 14.Butzler, J. P. 2004. Campylobacter, from obscurity to celebrity. Clin. Microbiol. Infect. 10:868-876. [DOI] [PubMed] [Google Scholar]
- 15.Calva, J. J., G. M. Ruiz-Palacios, A. B. Lopez-Vidal, A. Ramos, and R. Bojalil. 1988. Cohort study of intestinal infection with campylobacter in Mexican children. Lancet i:503-506. [DOI] [PubMed] [Google Scholar]
- 16.Coker, A. O., R. D. Isokpehi, B. N. Thomas, K. O. Amisu, and C. L. Obi. 2002. Human campylobacteriosis in developing countries. Emerg. Infect. Dis. 8:237-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Godschalk, P. C., M. L. Kuijf, J. Li, F. St Michael, C. W. Ang, B. C. Jacobs, M. F. Karwaski, D. Brochu, A. Moterassed, H. P. Endtz, A. van Belkum, and M. Gilbert. 2007. Structural characterization of Campylobacter jejuni lipooligosaccharide outer cores associated with Guillain-Barré and Miller Fisher syndromes. Infect. Immun. 75:1245-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guerry, P., C. M. Szymanski, M. M. Prendergast, T. E. Hickey, C. P. Ewing, D. L. Pattarini, and A. P. Moran. 2002. Phase variation of Campylobacter jejuni 81-176 lipooligosaccharide affects ganglioside mimicry and invasiveness in vitro. Infect. Immun. 70:787-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Havelaar, A. H., W. van Pelt, C. W. Ang, J. A. Wagenaar, J. P. van Putten, U. Gross, and D. G. Newell. 2009. Immunity to Campylobacter: its role in risk assessment and epidemiology. Crit. Rev. Microbiol. 35:1-22. [DOI] [PubMed] [Google Scholar]
- 20.Hu, L., M. D. Bray, M. Osorio, and D. J. Kopecko. 2006. Campylobacter jejuni induces maturation and cytokine production in human dendritic cells. Infect. Immun. 74:2697-2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janssen, R., K. A. Krogfelt, S. A. Cawthraw, W. van Pelt, J. A. Wagenaar, and R. J. Owen. 2008. Host-pathogen interactions in Campylobacter infections: the host perspective. Clin. Microbiol. Rev. 21:505-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones, F. R., S. Baqar, A. Gozalo, G. Nunez, N. Espinoza, S. M. Reyes, M. Salazar, R. Meza, C. K. Porter, and S. E. Walz. 2006. New World monkey Aotus nancymae as a model for Campylobacter jejuni infection and immunity. Infect. Immun. 74:790-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kantele, A., J. M. Kantele, H. Arvilommi, and P. H. Makela. 1991. Active immunity is seen as a reduction in the cell response to oral live vaccine. Vaccine 9:428-431. [DOI] [PubMed] [Google Scholar]
- 24.Kapperud, G., J. Lassen, S. M. Ostroff, and S. Aasen. 1992. Clinical features of sporadic Campylobacter infections in Norway. Scand. J. Infect. Dis. 24:741-749. [DOI] [PubMed] [Google Scholar]
- 25.Koga, M., M. Gilbert, M. Takahashi, J. Li, S. Koike, K. Hirata, and N. Yuki. 2006. Comprehensive analysis of bacterial risk factors for the development of Guillain-Barré syndrome after Campylobacter jejuni enteritis. J. Infect. Dis. 193:547-555. [DOI] [PubMed] [Google Scholar]
- 26.Korlath, J. A., M. T. Osterholm, L. A. Judy, J. C. Forfang, and R. A. Robinson. 1985. A point-source outbreak of campylobacteriosis associated with consumption of raw milk. J. Infect. Dis. 152:592-596. [DOI] [PubMed] [Google Scholar]
- 27.Kotloff, K. L., J. P. Nataro, G. A. Losonsky, S. S. Wasserman, T. L. Hale, D. N. Taylor, J. C. Sadoff, and M. M. Levine. 1995. A modified Shigella volunteer challenge model in which the inoculum is administered with bicarbonate buffer: clinical experience and implications for Shigella infectivity. Vaccine 13:1488-1494. [DOI] [PubMed] [Google Scholar]
- 28.Levine, M. M., R. E. Black, M. L. Clements, L. Cisneros, D. R. Nalin, and C. R. Young. 1981. Duration of infection-derived immunity to cholera. J. Infect. Dis. 143:818-820. [DOI] [PubMed] [Google Scholar]
- 29.Martin, P. M., J. Mathiot, J. Ipero, M. Kirimat, A. J. Georges, and M. C. Georges-Courbot. 1989. Immune response to Campylobacter jejuni and Campylobacter coli in a cohort of children from birth to 2 years of age. Infect. Immun. 57:2542-2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCarthy, N., and J. Giesecke. 2001. Incidence of Guillain-Barré syndrome following infection with Campylobacter jejuni. Am. J. Epidemiol. 153:610-614. [DOI] [PubMed] [Google Scholar]
- 31.Miller, G., G. M. Dunn, T. M. Reid, I. D. Ogden, and N. J. Strachan. 2005. Does age acquired immunity confer selective protection to common serotypes of Campylobacter jejuni? BMC Infect. Dis. 5:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nachamkin, I. 2001. Campylobacter enteritis and the Guillain-Barré syndrome. Curr. Infect. Dis. Rep. 3:116-122. [DOI] [PubMed] [Google Scholar]
- 33.Perlman, D. M., N. M. Ampel, R. B. Schifman, D. L. Cohn, C. M. Patton, M. L. Aguirre, W. L. Wang, and M. J. Blaser. 1988. Persistent Campylobacter jejuni infections in patients infected with the human immunodeficiency virus (HIV). Ann. Intern. Med. 108:540-546. [DOI] [PubMed] [Google Scholar]
- 34.Porter, C. K., H. El Mohammady, S. Baqar, D. M. Rockabrand, S. D. Putnam, D. R. Tribble, M. S. Riddle, R. W. Frenck, P. Rozmajzl, E. Kilbane, A. Fox, R. Ruck, M. Lim, Y. J. Johnston, E. Murphy, and J. W. Sanders. 2008. Case series study of traveler's diarrhea in U.S. military personnel at Incirlik Air Base, Turkey. Clin. Vaccine Immunol. 15:1884-1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Porter, C. K., M. S. Riddle, D. R. Tribble, S. D. Putnam, D. M. Rockabrand, R. W. Frenck, P. Rozmajzl, E. Kilbane, A. Fox, R. Ruck, M. Lim, J. Johnston, E. Murphy, and J. W. Sanders. 9 November 2009. The epidemiology of travelers' diarrhea in Incirlik, Turkey: a region with a predominance of heat-stabile toxin producing enterotoxigenic Escherichia coli. Diagn. Microbiol. Infect. Dis. doi: 10.1016/j.diagmicrobio.2009.10.002. [DOI] [PubMed]
- 36.Prendergast, M. M., D. R. Tribble, S. Baqar, D. A. Scott, J. A. Ferris, R. I. Walker, and A. P. Moran. 2004. In vivo phase variation and serologic response to lipooligosaccharide of Campylobacter jejuni in experimental human infection. Infect. Immun. 72:916-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pulimood, A. B., B. S. Ramakrishna, A. B. Rita, P. Srinivasan, V. Mohan, S. Gupta, B. Perakath, G. Kang, G. Chandy, and K. A. Balasubramanian. 2008. Early activation of mucosal dendritic cells and macrophages in acute Campylobacter colitis and cholera: an in vivo study. J. Gastroenterol. Hepatol. 23:752-758. [DOI] [PubMed] [Google Scholar]
- 38.Rao, M. R., A. B. Naficy, S. J. Savarino, R. Abu-Elyazeed, T. F. Wierzba, L. F. Peruski, I. Abdel-Messih, R. Frenck, and J. D. Clemens. 2001. Pathogenicity and convalescent excretion of Campylobacter in rural Egyptian children. Am. J. Epidemiol. 154:166-173. [DOI] [PubMed] [Google Scholar]
- 39.Rathinam, V. A., K. A. Hoag, and L. S. Mansfield. 2008. Dendritic cells from C57BL/6 mice undergo activation and induce Th1-effector cell responses against Campylobacter jejuni. Microbes Infect. 10:1316-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruiz-Palacios, G. M., J. J. Calva, L. K. Pickering, Y. Lopez-Vidal, P. Volkow, H. Pezzarossi, and M. S. West. 1990. Protection of breast-fed infants against Campylobacter diarrhea by antibodies in human milk. J. Pediatr. 116:707-713. [DOI] [PubMed] [Google Scholar]
- 41.Strid, M. A., J. Engberg, L. B. Larsen, K. Begtrup, K. Molbak, and K. A. Krogfelt. 2001. Antibody responses to Campylobacter infections determined by an enzyme-linked immunosorbent assay: 2-year follow-up study of 210 patients. Clin. Diagn. Lab. Immunol. 8:314-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tam, C. C., L. C. Rodrigues, I. Petersen, A. Islam, A. Hayward, and S. J. O'Brien. 2006. Incidence of Guillain-Barré syndrome among patients with Campylobacter infection: a general practice research database study. J. Infect. Dis. 194:95-97. [DOI] [PubMed] [Google Scholar]
- 43.Taylor, D. 1992. Campylobacter infections in developing countries, p. 20-30. In I. Nachamkin, M. Blaser, and L. Tompkins (ed.), Campylobacter jejuni: current status and future trends. American Society for Microbiology. Washington, DC.
- 44.Taylor, D. N., P. Echeverria, C. Pitarangsi, J. Seriwatana, L. Bodhidatta, and M. J. Blaser. 1988. Influence of strain characteristics and immunity on the epidemiology of Campylobacter infections in Thailand. J. Clin. Microbiol. 26:863-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taylor, D. N., D. M. Perlman, P. D. Echeverria, U. Lexomboon, and M. J. Blaser. 1993. Campylobacter immunity and quantitative excretion rates in Thai children. J. Infect. Dis. 168:754-758. [DOI] [PubMed] [Google Scholar]
- 46.Tee, W., and A. Mijch. 1998. Campylobacter jejuni bacteremia in human immunodeficiency virus (HIV)-infected and non-HIV-infected patients: comparison of clinical features and review. Clin. Infect. Dis. 26:91-96. [DOI] [PubMed] [Google Scholar]
- 47.Teunis, P., W. Van den Brandhof, M. Nauta, J. Wagenaar, H. Van den Kerkhof, and W. Van Pelt. 2005. A reconsideration of the Campylobacter dose-response relation. Epidemiol. Infect. 133:583-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tribble, D. R., S. Baqar, L. W. Pang, C. Mason, H. S. Houng, C. Pitarangsi, C. Lebron, A. Armstrong, O. Sethabutr, and J. W. Sanders. 2008. Diagnostic approach to acute diarrheal illness in a military population on training exercises in Thailand, a region of campylobacter hyperendemicity. J. Clin. Microbiol. 46:1418-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Doorn, P. A., L. Ruts, and B. C. Jacobs. 2008. Clinical features, pathogenesis, and treatment of Guillain-Barré syndrome. Lancet Neurol. 7:939-950. [DOI] [PubMed] [Google Scholar]
- 50.Viret, J. F., D. Favre, B. Wegmuller, C. Herzog, J. U. Que, S. J. Cryz, Jr., and A. B. Lang. 1999. Mucosal and systemic immune responses in humans after primary and booster immunizations with orally administered invasive and noninvasive live attenuated bacteria. Infect. Immun. 67:3680-3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walz, S. E., S. Baqar, H. J. Beecham, P. Echeverria, C. Lebron, M. McCarthy, R. Kuschner, S. Bowling, A. L. Bourgeois, and D. A. Scott. 2001. Pre-exposure anti-Campylobacter jejuni immunoglobulin A levels associated with reduced risk of Campylobacter diarrhea in adults traveling to Thailand. Am. J. Trop. Med. Hyg. 65:652-656. [DOI] [PubMed] [Google Scholar]
- 52.Westrell, T., N. Ciampa, F. Boelaert, B. Helwigh, H. Korsgaard, M. Chriel, A. Ammon, and P. Makela. 2009. Zoonotic infections in Europe in 2007: a summary of the EFSA-ECDC annual report. Euro Surveill. 14:1-3. [PubMed] [Google Scholar]
- 53.Yu, R. K., S. Usuki, and T. Ariga. 2006. Ganglioside molecular mimicry and its pathological roles in Guillain-Barre syndrome and related diseases. Infect. Immun. 74:6517-6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yuki, N. 2005. Carbohydrate mimicry: a new paradigm of autoimmune diseases. Curr. Opin. Immunol. 17:577-582. [DOI] [PubMed] [Google Scholar]