Abstract
Despite the fact that the presence of multiple large plasmids is a defining feature of extraintestinal pathogenic Escherichia coli (ExPEC), such as avian pathogenic E. coli (APEC), and despite the fact that these bacteria pose a considerable threat to both human and animal health, characterization of these plasmids is still limited. In this study, after successfully curing APEC of its plasmids, we were able to investigate, for the first time, the contribution to virulence of three plasmids, pAPEC-1 (103 kb), pAPEC-2 (90 kb), and pAPEC-3 (60 kb), from APEC strain χ7122 individually as well as in all combinations in the wild-type background. Characterization of the different strains revealed unique features of APEC virulence. In vivo assays showed that curing the three plasmids resulted in severe attenuation of virulence. The presence of different plasmids and combinations of plasmids resulted in strains with different pathotypes and levels of virulence, reflecting the diversity of APEC strains associated with colibacillosis in chickens. Unexpectedly, our results associated the decrease in growth of some strains in some media with the virulence of APEC, and the mechanism was associated with some combinations of plasmids that included pAPEC-1. This study provided new insights into the roles of large plasmids in the virulence, growth, and evolution of APEC by showing for the first time that both the nature of plasmids and combinations of plasmids have an effect on these phenomena. It also provided a plausible explanation for some of the conflicting results related to the virulence of ExPEC strains. This study should help us understand the virulence of other ExPEC strains and design more efficient infection control strategies.
Escherichia coli strains are members of the normal intestinal microflora of most mammals and birds. They colonize their primary habitat, the lower intestinal tract of the host, within the first few hours of the host's life (37, 54). E. coli strains are very versatile organisms, and the environment is considered their secondary habitat; approximately one-half of all living E. coli cells are actually living outside their hosts. Even though most E. coli strains are commensals and their presence provides a benefit to the host, a subset of these bacteria has acquired the ability to cause intestinal and extraintestinal diseases. These bacteria can be distinguished from commensals by their virulence factors (29, 37).
Extraintestinal pathogenic E. coli (ExPEC), including avian pathogenic E. coli (APEC), pose a considerable threat to both human and animal health due to potential economic losses stemming from illness (30, 55, 62). ExPECs are responsible for a broad spectrum of infections in humans, including urinary tract infection (UTI), newborn meningitis (NBM), and septicemia. In addition, they are involved in animal diseases, such as avian colibacillosis, one of the most significant and widespread infectious diseases occurring in poultry and the cause of increased mortality, condemnations, and decreased production (3, 16). The most common disease syndromes associated with E. coli in birds are lower-respiratory-tract infections (air sacculitis), cellulites, meningitis, and septicemia (3).
The different groups of E. coli have evolved mainly by acquisition of genes via horizontal gene transfer, a common phenomenon in bacteria that occurs even between very distantly related species (12, 45). This mechanism contributes to the evolution of E. coli variants, resulting in the development of novel strains and pathotypes. Conjugative plasmids are known to mediate transfer of genes between bacteria in diverse environments (42, 67). Acquisition of plasmids by bacteria is one of the fastest ways for survival in and adaptation to one or multiple hosts, as plasmids can encode multiple traits, including antibiotic and heavy metal resistance, virulence, and persistence in different environments (21).
ExPEC strains (ExPECs) are differentiated from other pathotypes by the presence of specific virulence genes that allow them to spread systemically in hosts (62). ExPECs, particularly APEC isolates, carry multiple large plasmids (13, 32-35) belonging to different incompatibility groups (35), and the most prevalent plasmids in APEC strains (APECs) are the IncFIB, IncFIC, IncFIIA, IncI1, incP, incB/O, and IncN plasmids, some of which encode virulence factors. Additionally, plasmids encoding multiple drug resistance have been isolated from both APEC and uropathogenic E. coli (UPEC) strains. To date, few studies have undertaken sequencing and characterization of plasmids from avian isolates, particularly the ColV and ColBM plasmids from the IncFIB incompatibility group, which are considered common among ExPEC strains (22, 32, 33, 48, 66). Each of these plasmids has a conserved region harboring the FIB replicon, the ColV and/or ColBM operon, several known virulence genes, and iron acquisition and transport operons. According to recent studies the zoonotic risk seems to be related to the presence of large plasmids in APECs (48, 61).
A fuller understanding of ExPEC virulence mechanisms is needed to develop treatments and preventative measures for use against ExPEC infections (55). Reductionism has been used for many years as a critical and powerful tool for identification of key genes responsible for microbial pathogenesis. However, the limitations of this approach for understanding the pathogenicity of bacteria include the multifactorial nature of virulence and the complex cross-regulation of gene expression. The ExPECs that cause diseases in humans and animals are very diverse, and although serotype and virulence factors are related to this diversity, the exact molecular mechanism behind the extensive diversity has not been elucidated yet.
APEC strain χ7122 (O78:K80:H9) has been used for many years as a model strain to study the molecular mechanisms of APEC pathogenicity. The results of such studies have contributed greatly to increasing our understanding of the virulence of both human and animal ExPECs. This bacterium has three large plasmids, pAPEC-1 (103 kb), pAPEC-2 (90 kb), and pAPEC-3 (60 kb) (48). Most known virulence factors associated with APEC, including iron acquisition systems, tsh, and colicin V, are located on pAPEC-1, whereas the contents of pAPEC-2 and pAPEC-3 are completely unknown.
Despite the fact that the presence of multiple large plasmids is a defining feature of the APEC pathotype (13, 32-35), characterization of these plasmids is still very limited. The exact role of many of them, as well the epistatic interactions between them, are unknown. The study of these plasmids has been complicated by their diversity and by the difficulty of curing them from the wild type. The few previous studies dedicated to understanding the role of the large plasmids of APEC in virulence were done in either E. coli K-12 (15, 31, 63) or avian commensal E. coli backgrounds (61, 70), which did not necessarily show the true functions of these plasmids in the wild-type background host strain.
A plasmidless strain obtained from a wild-type APEC strain would provide a better background to evaluate the potential virulence of individual plasmids. In this study, after successfully curing APEC of its plasmids, we were able to investigate the contribution to virulence of each of the three large plasmids of APEC χ7122 by generating a plasmidless strain, strains with each plasmid individually, and strains with two plasmids in different combinations. We then determined the genetic locations of different virulence genes and compared the plasmid-containing derivative strains to the wild-type strain in terms of virulence, growth rate, serum resistance, iron uptake, and lipopolysaccharide (LPS) and iron-regulated outer membrane protein (IROMP) profiles. The results of this study provide new insights into the role of large plasmids in virulence, growth, and evolution of APEC by showing for the first time that both the nature of plasmids and combinations of plasmids have an effect on these factors. They also provide a plausible explanation for some conflicting results related to the virulence of ExPECs.
MATERIALS AND METHODS
Chemicals and reagents.
Bacterial growth media used in this study were purchased from Becton, Dickinson and Company. The antibiotics were obtained from Sigma (St. Louis, MO). Restriction and modification enzymes were obtained from Invitrogen (Carlsbad, CA) or New England Biolabs (Beverly, MA) and used as recommended by the manufacturers. PCR primers were purchased from IDT Inc. (Coralville, IA).
The different bacterial media used to evaluate the growth of bacteria were LB broth (containing [per liter] 10 g tryptone, 5 g yeast extract, and 10 g NaCl) and MM9 medium (containing [per liter] 12.8 g Na2HPO4·7H2O, 5 g KH2PO4, 0.5 g NaCl, 1 g NH4Cl, 0.2 ml 1 M MgSO4, 5 μl 1 M CaCl2, and 20 ml 20% glucose). The cell culture media used included Dulbecco's modified Eagle's medium (DMEM) (Gibco), RPMI-1640 (Gibco), and GTSF-2 medium consisting of triple-sugar minimal essential medium α-L-15 base supplemented with 2.2 g/liter NaHCO3 and 2.5 mg/liter insulin-transferrin-sodium selenite (40).
Bacterial strains and culture.
Table 1 lists the E. coli strains and plasmids used in this study. APEC strain χ7122 (O78:K80:H9) was originally isolated from the liver of a deceased turkey (8). A rough mutant strain (O78−) of APEC strain χ7122, χ7145, and two derivatives of χ7145, χ7167 and χ7193, which express O antigens different from the native O78 antigen (O111 and O1, respectively), have been described previously (46, 47).
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristicsa | Parent | Reference(s) |
|---|---|---|---|
| Strains | |||
| χ2934 | E. coli K-12, Lac− F− Nalr | This study | |
| χ6092 | E. coli K-12, Lac− F− Tcr | 48 | |
| χ7122 | APEC O78:K80:H9, gyrA Nalr Strr | 8 | |
| χ7145 | χ7122 (χ289:hisG-zee), rfb deleted by replacement with E. coli K-12 region at 45 min | χ7122 | 46, 47 |
| χ7167 | O111 LPS derivative strain of χ7122 | χ7122 | 47 |
| χ7193 | O1 LPS derivative strain of χ7122 | χ7122 | 46 |
| χ7273 | χ7122 tsh::tetAR(B), Nalr Tcr | χ7122 | 15 |
| χ7274 | χ7273 ΔpAPEC-1, Nalr Strr | χ7273 | 15 |
| χ7276 | E. coli K-12 MG1655 Tn10::kan, Kmr | 15, 48 | |
| χ7277 | χ7276/pAPEC-1-1/pAPEC-2, Kmr Tcr Strr | χ7276 | 15, 48 |
| χ7345 | χ2934/pAPEC-1-1, Nalr Tcr | χ2934 | 48 |
| χ7346 | pAPEC-1, Tcr | χ6092 | 48 |
| χ7347 | pAPEC-2, Tcr Strr | χ6092 | This study |
| χ7348 | pAPEC-3, Tcr | χ6092 | This study |
| χ7367 | pAPEC-3, ΔpAPEC-1, ΔpAPEC-2, Nalr | χ7274 | This study |
| χ7368 | ΔpAPEC-1, ΔpAPEC-2, ΔpAPEC-3, Nalr | χ7367 | This study |
| χ7394 | pAPEC-1, Nalr | χ7368 | This study |
| χ7392 | pAPEC-2, Nalr Strr | χ7368 | This study |
| χ7561 | pAPEC-1, pAPEC-2, Nalr Strr | χ7394 | This study |
| χ7562 | pAPEC-1, pAPEC-3, Nalr | χ7367 | This study |
| 39R681 | E. coli containing four plasmids (147 kb, 63 kb, 35.85 kb, and 6.9 kb) | 44 | |
| Plasmids | |||
| pAPEC-1 | 103-kb plasmid of APEC χ7122 | 15, 48 | |
| pAPEC-1-1 | pAPEC-1 tsh::tetAR | pAPEC-1 | 15, 48 |
| pAPEC-2 | 90-kb plasmid of APEC χ7122 | 48 | |
| pAPEC-3 | 60-kb plasmid of APEC χ7122 | 48 |
Nalr, nalidixic acid resistant; Tcr, tetracycline resistant; Strr, streptomycin resistant; Kmr, kanamycin resistant.
Bacterial strains were routinely grown in LB broth and on MacConkey agar supplemented with 1% lactose at 37°C, except where indicated otherwise. Strains were stored as stock cultures at −80°C in peptone-glycerol medium. When required, antibiotics were added at the following concentrations: kanamycin, 30 μg/ml; nalidixic acid, 15 μg/ml; streptomycin, 50 μg/ml; and tetracycline, 10 μg/ml. Diaminopimelic acid (DAP) (50 μg/ml) was added for growth of Asd− strains.
Isolation of large plasmids.
The strains were cultured in LB broth at 37°C for 14 to 16 h, and plasmid DNA was isolated by the phenol-chloroform procedure (36) and by using a large-construct kit (Qiagen) according to the manufacturer's instructions.
Plasmid curing.
We previously cured χ7122 of its pAPEC-1 plasmid by insertion of a tetAR(B) cassette derived from Tn10 into the tsh gene to generate strain χ7273 (15) (Table 1). The pAPEC-1 plasmid-cured strain was designated χ7274 (Table 1).
We then used the Tnminitet insertion method (65) to cure χ7274 of its pAPEC-2 plasmid and generate strain χ7367 containing only pAPEC-3. Briefly, the pAPEC-2 plasmid was labeled with a Kmr insert in its parA region, which destabilized the plasmid and allowed isolation of plasmid-free derivatives by growing the strain in LB broth without antibiotics. Finally, to cure χ7367 of its pAPEC-3 plasmid, we used a two-step transposon-based method (26). Briefly, a Tn10-based transposable unit carrying a Kmr marker gene and the joined IS30 ends transposed from a replication-deficient conjugative plasmid into the pAPEC-3 replicon, and then the inducible IS30 transposase mediated loss of the whole virulence plasmid; the plasmidless strain was designated χ7368 (Table 1).
Reintroduction of plasmids into strains by bacterial mating.
Mating between different strains was done by mixing (1:1) overnight cultures of the donor and the recipient (Table 1). Mating was carried out overnight at 37°C. The mating mixtures were plated either on LB medium plates with tetracycline and colicin V produced as described previously (48), on LB medium plates with tetracycline and streptomycin, or on LB medium plates with tetracycline. The transconjugants were verified by using PCR and the plasmid profiles.
Because of the failure of colicin V to kill strains with the O78:K80 background (data not shown), we were not able to counterselect transconjugants with pAPEC-1 when the recipient strains had the χ7122 background. For practical reasons we used a tetracycline resistance marker plasmid, pAPEC-1-1. Once transferred into the recipient, the tsh gene disturbed by the tetA gene (15) was restored using a suicide vector containing the tsh gene as described below.
The stability of plasmids in different strains was evaluated after bacteria were subcultured in LB medium at 37°C for five consecutive days. One hundred isolated colonies from each culture were tested by PCR to determine their plasmid contents. The relative copy numbers of plasmids were evaluated by comparing the intensities of DNA plasmid bands on an agarose gel containing bacteria grown to the same optical density at 600 nm (OD600) using the AlphaEase FC software (Alpha Innotech Corp., CA).
Genetic techniques.
Standard molecular manipulations were performed as described by Sambrook et al. (58). Restoration of the tsh gene in pAPEC-1-1 transferred into the different recipient strains was performed by allelic exchange with a suicide vector using standard methods (49). The tsh region cloned into the suicide vector included the tsh gene (4,134 bp) and the left (616 bp) and right (530 bp) flanking regions (CP000836), and it was amplified from pAPEC-1 DNA by PCR using the high-fidelity polymerase Klentaq-LA (4) and primers Tsh F (5′-CGGGAATTCGTGACAGGCTATAGTACTTCC-3′) (EcoRI site underlined) and Tsh R (5′-CCCAAGCTTAGTGTTCCGTTCAGCCAGGTA-3′) (HindIII site underlined). The 5,280-bp PCR product was purified using a QIAquick gel extraction kit (Qiagen) and was sequenced at the sequencing facility at Arizona State University, using standard sequencing technology.
Detection of adhesin genes (fimH, tsh, csgA, ecpA, pilS and stgA), iron acquisition genes (iroN, iucC, sitA, eitA, feoB, fepA and mntH), and genes encoding other virulence factors (traT, etsA, cvaC, ompT, hylF, and iss) in the different strains with either the wild-type χ7122 or E. coli K-12 χ6092 background was performed by PCR amplification using primers specified in Table 2.
TABLE 2.
Primers used in this study
| Gene | Product and/or function | Primer typea | Primer sequence (5′-3′) | Amplicon size (bp) |
|---|---|---|---|---|
| fimH | Type 1 fimbria adhesin FimH, adhesion | F | TGCAGAACGGATAAGCCGTGG | 508 |
| R | GCAGTCACCTGCCCTCCGGTA | |||
| csgA | Cryptic curlin subunit CsgA, adhesion | F | ACTCTGACTTGACTATTACC | 200 |
| R | AGATGCAGTCTGGTCAAC | |||
| pilS | Type IV prepilin protein PilS, adhesion | F | CTTCTCTTTCTGCACACCGT | 327 |
| R | TGTGATTGTAACGGAGCC | |||
| ecpA | E. coli common pilus Ecp pilin, adhesion | F | GTAACGGTGTTTACCGGCAT | 345 |
| R | GATCATCACGGTATCGCCAG | |||
| stgA | Fimbrial structural protein subunit StgA, adhesion | F | ATATTATAGG CGGTGCATTC | 450 |
| R | CATCGATAGCGGTATAAGCA | |||
| tsh | Temperature-sensitive hemagglutinin, adhesion, heme binding protein | F | GTTCAGGTCTGGTTTTTG | 547 |
| R | TCGCCCTTAACACCATT | |||
| iroN | Enterobactin siderophore receptor protein, iron acquisition | F | ATTGACGCCAGGCATTTTAC | 202 |
| R | GCTCCTGGTTGGGTTGAATA | |||
| iucC | Aerobactin siderophore biosynthesis protein, iron acquisition | F | GACGGGCTTTCAGTAGTTGC | 200 |
| R | CTTCATCGCTGAACGTGGTA | |||
| sitA | Periplasmic binding protein, iron transport | F | ATCGGCATTACGTTGGTAGG | 196 |
| R | TCTCAATGGGGTTCCAGAAG | |||
| eitA | Periplasmic binding protein, iron transport | F | AACTGCGGCTATCAGGAGAC | 395 |
| R | CAGGTCATATCCCACAGCTT | |||
| feoB | Ferrous iron transport protein B | F | TTCGCATTGA AATTGATGCT | 580 |
| R | TGAATACCATGCACAAAGAG | |||
| fepA | Iron-enterobactin outer membrane transporter | F | AAGCTGAATTCGTCGCCCAG | 560 |
| R | CCGACCGATACTCCTGTTTC | |||
| mntH | Manganese transport protein | F | TAATCCCATC AGAATGACGA | |
| R | CTTACATTGTCGAGTTGATT | 530 | ||
| iss | Increased serum survival | F | CCGAACCACTTGATGTGCA | 651 |
| R | CTATGCAAAAACAACTGTAG | |||
| traT | TraT complement resistance protein precursor | F | GGTGTGGTGCGATGAGCACAG | 290 |
| R | CACGGTTCAGCCATCCCTGAG | |||
| cvaC | Colicin V synthesis protein | F | GGTATCCCTTCGGGTTTTTG | 204 |
| R | TGTTTCTGGTGGTGCTTCAG | |||
| hylF | Putative avian hemolysin | F | TTAGATCCCCAGGCAAGATG | 199 |
| R | GGTGCAACAGGATTTCTTGG | |||
| ompT | Outer membrane protein T | F | CCTCCACGACCAGCTAATGT | 196 |
| R | CGGAGATTGATTTTGGCACT | |||
| etsA | Macrolide-specific efflux protein EtsA | F | GGATGCGGAAAGAACAGGTA | 203 |
| R | TTCTTCACTGGCATGGACTG |
F, forward; R, reverse.
Colicin production.
Colicin production was detected using the chloroform overlay method as described previously (20).
LPS and OMP profiles.
Outer membrane proteins (OMPs) and LPS of the strains were prepared as previously described (25). LPS profiles were evaluated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and visualized by silver staining. OMPs were electrophoretically separated on 10% SDS-PAGE gels and visualized with Coomassie blue. OMPs were prepared from cultures of bacteria grown to an A600 of 1 under iron-restricted and iron-replete conditions by using LB broth with and without 2,2′-dipyridyl at a final concentration of 200 μM. LPS were prepared from overnight cultures of bacteria grown in LB broth.
Evaluation of bacterial growth in different media.
To compare the growth of bacteria in different media, five media (LB medium, MM9 medium, DMEM, RPMI-1640, and GTSF-2 medium) were inoculated (1:100) with overnight cultures of bacteria grown in LB broth and then incubated for 6 to 24 h with continuous rotation at 37°C. The OD600 of each culture was determined every hour. For iron-limited growth studies, 100 μM α,α′-dipyridyl was added to the LB medium.
Serum complement resistance assay.
The resistance to serum complement was determined by evaluating bacterial survival following incubation in 90% serum from guinea pigs (Innovative Research) and specific-pathogen-free chickens as previously described (46).
Siderophore detection.
Siderophore production was detected in an iron-limited medium, chrome azurol sulfonate (CAS) agar (60). Five microliters of an LB broth culture at an OD600 of 0.6 was spotted on agar plates and incubated overnight at 37°C. The presence of an orange halo around a colony indicated a culture positive for siderophore production, and the diameters of the orange haloes that formed on the CAS agar plates were measured after incubation at 28°C for different times.
Analysis of the DNA sequences of the enterobactin-encoding and feo regions in χ7122.
The sequences of the enterobactin-encoding and feo regions were derived from contig sequences of the whole genomic DNA of APEC χ7122, kindly provided by Suman Mukhopadhyay (National Institute of Allergy and Infectious Diseases, Bethesda, MD) and Steven Salzberg (University of Maryland, College Park, MD), using a BLAST comparison with fepA and feo sequences available in the public sequence database. The identified enterobactin-encoding and feo regions (23,700 bp and 2,300 bp) were characterized by DNA sequence analysis. Putative open reading frames (ORFs) were identified using vector NTI programs. BLAST programs (http://www.ncbi.nlm.nih.gov) were used to carefully review and confirm the annotation of every gene.
Infection of chickens.
Infection of chickens was performed in accordance with protocols approved by the Arizona State University Institutional Animal Care and Use Committee in dedicated facilities at the Biodesign Institute, Arizona State University. Specific-pathogen-free fertile White Leghorn chicken eggs were obtained from Charles River Labs (Wilmington, MA) and hatched at the animal facilities of the Biodesign Institute. During the study chickens were housed in isolators equipped with HEPA filters.
Lethality for 1-day-old chicks.
The pathogenicities of different strains were assessed by subcutaneously inoculating groups of 14 1-day-old chicks with 0.1 ml of either phosphate-buffered saline (PBS) or a washed overnight broth culture of an E. coli strain (about 108 CFU) as previously described (18). Death was recorded for 6 days after inoculation.
Experimental infection of chickens via the air sacs.
The abilities of different strains to disseminate in the respiratory tract and internal organs of chickens were compared. Briefly, groups of 14 3.5-week-old chickens were inoculated with the appropriate strains in the right thoracic air sac as described previously (46). All birds were euthanized at 48 h postinfection by CO2 asphyxiation and then necropsied. Organs were aseptically removed, the presence and number of bacteria were determined, and macroscopic fibrinous lesions were scored using the scale described previously (46). Colonies were selected at random from MacConkey plates and tested for agglutination with the anti-O78 antisera and to determine their plasmid profiles. Lesion scores and bacterial counts in organs were compared for groups of chickens inoculated with the parent strain and with derivative(s) of it by using analysis of variance (ANOVA).
Statistical analysis.
Data were analyzed by one-way analysis of variance (ANOVA), followed by Bonferroni's multiple-comparison test (GraphPad Prism software, version 5.07). Differences between average values were also tested for significance by performing an unpaired, two-sided Student t test. The levels of significance (P values) are reported below.
Nucleotide sequence accession numbers.
The complete and annotated genome sequences of the enterobactin-encoding and feo regions have been deposited in the DDBJ/EMBL/GenBank database under accession numbers GU361605 and GU361604, respectively.
RESULTS
Generation of a plasmid-cured strain and strains containing different combinations of large plasmids.
Curing APEC of its plasmids by standard methods has proven to be problematic in the past (61). Chemical curing was avoided in this study because of the eventual mutational effect on the chromosomal DNA. APEC strain χ7122 has three large plasmids, pAPEC-1, pAPEC-2, and pAPEC-3 (Table 1 and Fig. 1A) (48), all of which we successfully cured to generate plasmidless strain χ7368, as described in Materials and Methods. We then reintroduced plasmids to generate strains containing either a single plasmid, including χ7394 (pAPEC-1), χ7392 (pAPEC-2), and χ7367 (pAPEC-3), or two plasmids, including χ7561 (pAPEC-1 and pAPEC-2), χ7562 (pAPEC-1 and pAPEC-3), and χ7274 (pAPEC-2 and pAPEC-3) (Table 1).
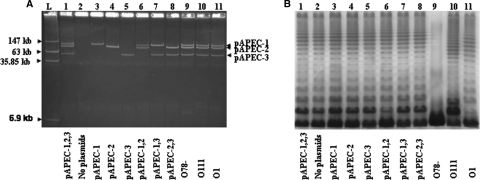
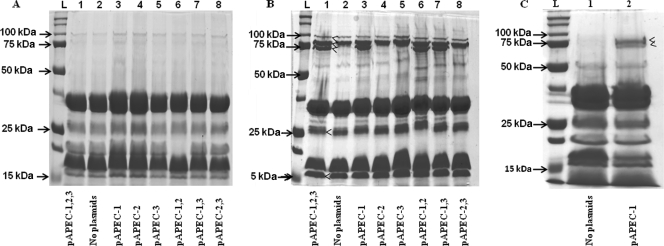
FIG. 1.
Plasmid and LPS profiles of different strains. (A) Plasmid profiles of strains in a 0.5% agarose gel stained with ethidium bromide. (B) LPS profiles of different strains in a silver-stained SDS-PAGE gel. Lane L, four plasmids (147 kb, 163 kb, 35.85 kb, and 6.9 kb) of strain 39R681 used as a ladder; lane 1, χ7122; lane 2, χ7368; lane 3, χ7394; lane 4, χ7392; lane 5, χ7367; lane 6, χ7561; lane 7, χ7562; lane 8, χ7274; lane 9, χ7145; lane 10, χ7167; lane 11, χ7193. Abbreviations: pAPEC-1,2,3, pAPEC-1, pAPEC-2, and pAPEC-3; pAPEC-1,2, pAPEC-1 and pAPEC-2; pAPEC-1,3, pAPEC-1 and pAPEC-3.
Plasmids pAPEC-1, pAPEC-2, and pAPEC-3 were also introduced individually into E. coli K-12 strain χ6092 to generate strains χ7346, χ7347, and χ7348, respectively (Table 1).
Plasmid profiles of strains.
The plasmid profiles of strains are shown in Fig. 1A. Wild-type strain χ7122 has three large plasmids, pAPEC-1, pAPEC-2, and pAPEC-3. No plasmid bands were detected for strain χ7368; strains χ7394, χ7392, and χ7367 contain a single plasmid corresponding to pAPEC-1, pAPEC-2, and pAPEC-3, respectively, and strains χ7561, χ7562, and χ7274 contain two plasmids (pAPEC-1 and pAPEC-2, pAPEC-1 and pAPEC-3, and pAPEC-2 and pAPEC-3, respectively). The plasmid stability in all strains was 100%, as determined under in vitro conditions, and the copy numbers of plasmids in the new strains that were generated were not altered, as determined by the method described in Materials and Methods.
Genotypic characterization of APEC strain χ7122.
Using PCR, we confirmed the presence of 16 virulence genes in APEC χ7122. These genes include genes encoding adhesins (fimH, tsh, csgA, ecpA, and stgA) and iron acquisition systems (iroN, iucC, sitA, feoB, and mntH), as well as other genes (traT, etsA, cvaC, ompT, hylF, and iss). We also detected three new virulence genes, fepA, eitA, and pilS, in χ7122 (Table 3). Six of the 19 genes, fimA, csgA, ecpA, feoB, fepA, mntH, were also found in E. coli K-12 χ6092 (Table 3), whereas the remaining 13 genes were not detected in the nonpathogenic strain E. coli K-12 χ6092.
TABLE 3.
Pathotype profiles of different strains
| Strain | Plasmid(s) | Pathotype genes |
|---|---|---|
| Wild-type background | ||
| χ7122a | pAPEC-1, pAPEC-2, pAPEC-3 | fimA, csgA, ecpA, stgA, feoB,fepA, mntH, tsh, iroN, iucC, sitA, iss, cvaC, hlyF, ompT, etsA, eitA,traT, pilS |
| χ7368 | None | fimA, csgA, ecpA, stgA, feoB, fepA, mntH |
| χ7394c | pAPEC-1 | fimA, csgA, ecpA, stgA, feoB, fepA, mntH, tsh, iroN, iucC, sitA, iss, cvaC, hlyF, ompT, etsA |
| χ7392 | pAPEC-2 | fimA, csgA, ecpA, stgA, feoB, fepA, mntH, eitA, traT |
| χ7367 | pAPEC-3 | fimA, csgA, ecpA, stgA, feoB, fepA, mntH, pilS |
| χ7561 | pAPEC-1, pAPEC-2 | fimA, csgA, ecpA, stgA, feoB, fepA, mntH, tsh, iroN, iucC, sitA, iss, cvaC, hlyF, ompT, etsA, eitA,traT |
| χ7562c | pAPEC-1, pAPEC-3 | fimA, csgA, ecpA, stgA, feoB, fepA, mntH, tsh, iroN, iucC, sitA, iss, cvaC, hlyF, ompT, etsA, pilS |
| χ7274 | pAPEC-2, pAPEC-3 | fimA, csgA, ecpA, stgA, feoB, fepA, mntH, eitA,traT, pilS |
| E. coli K-12 background | ||
| χ6092 | None | fimA, csgA, ecpA, feoB, fepA, mntH |
| χ7346 | pAPEC-1 | fimA, csgA, ecpA, feoB, fepA, mntH, tsh, iroN, iucC, sitA, iss, cvaC, hlyF, ompT, etsA |
| χ7347 | pAPEC-2 | fimA, csgA, ecpA, feoB, fepA, mntH, eitA,traT |
| χ7348 | pAPEC-3 | fimA, csgA, ecpA, feoB, fepA, mntH, pilS |
By testing different plasmid-containing clones derived from either wild-type strain χ7122 or E. coli K-12 χ6092 (Table 3) for the presence of the 19 virulence genes using PCR (Table 2), we determined that seven genes, fimA, csgA, ecpA, stgA, feoB, fepA, and mntH, are located on the chromosome of APEC χ7122, since regardless of their plasmid profiles all of the derivative strains were positive for these genes. With the exception of stgA, the same genes were detected in E. coli K-12 χ6092. Twelve of the 19 genes were considered plasmid genes (Table 3) due to their absence in the plasmidless strains χ7368 and χ6092 and their presence in strains that contain at least one of the three plasmids. Nine of the 12 genes, tsh, iroN, iucC, sitA, iss, cvaC, hlyF, ompT, and etsA, are located on pAPEC-1, 2 of the 12 genes, eitA and traT, are located on pAPEC-2, and pilS is located on pAPEC-3. We confirmed these results by performing the same PCRs with the corresponding purified plasmid DNAs (data not shown).
LPS profiles of strains.
The LPS profiles of all strains are shown in Fig. 1B. Strains derived from the O78 wild-type background possess full-length LPS, a long-chain LPS with a ladder pattern similar to the wild-type O78 LPS pattern. The smooth LPS of APEC was not affected by the presence of plasmids, since all isolates expressed long-chain LPS. The LPS profile of the O78 LPS rough mutant χ7145 lacks the ladder pattern, whereas the O111- and O1-substituted O antigens of strains χ7167 and χ7193, respectively, produced smooth LPS profiles with ladder patterns distinct from the ladder pattern of the native O78 LPS of the wild-type strain (Fig. 1B).
Colicin production.
Overall, all clones that have pAPEC-1 in their plasmid profiles, including wild-type strain χ7122 and its derivatives χ7394 (pAPEC-1), χ7561 (pAPEC-1 and pAPEC-2), and χ7562 (pAPEC-1 and pAPEC-3), were colicin positive (data not shown). Plasmidless strain χ7368 and strains χ7392 (pAPEC-2), χ7367 (pAPEC-3), and χ7274 (pAPEC-2 and pAPEC-3) were colicin negative (data not shown). The pAPEC-1-encoded colicin was previously identified as a colicin V (48).
CAS assay detected expression of pAPEC-1 siderophores and a new chromosomal siderophore system in APEC χ7122.
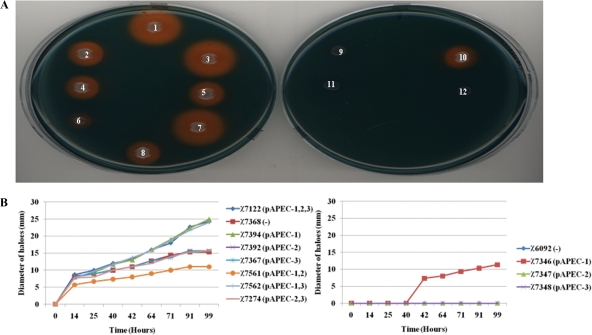
Siderophore production was detected in different strains using the CAS assay based on a change in the color of the CAS-iron complex from blue to orange after chelation of iron by siderophores. Although an orange halo was observed around each colony of wild-type strain χ7122 and its derivatives, the sizes of the haloes were different (Fig. 2). In fact, the haloes surrounding colonies of wild-type strain χ7122 and its derivatives χ7394 (pAPEC-1) and χ7562 (pAPEC-1 and pAPEC-3) were larger than the haloes surrounding colonies of plasmidless strain χ7368 and strains χ7392 (pAPEC-2), χ7367 (pAPEC-3), and χ7274 (pAPEC-2 and pAPEC-3) (Fig. 2). The decrease in iron uptake in the latter strains was related to the absence of pAPEC-1, which is known to encode two siderophores, salmochelin and aerobactin (48). The exception to these findings was χ7561 (pAPEC-1 and pAPEC-2), which had a growth defect in CAS medium which affected its ability to acquire iron, as shown by the small orange haloes surrounding its colonies (Fig. 2). The ability of plasmidless strain χ7368 to acquire iron (Fig. 2) demonstrates that a chromosome-encoded siderophore that was not detected previously was present.
FIG. 2.
Iron uptake by different strains. (A) Iron uptake on CAS agar. (B) Diameters of orange haloes around colonies on CAS agar incubated for different times. Colony 1, χ7122; colony 2, χ7368; colony 3, χ7394; colony 4, χ7392; colony 5, χ7367; colony 6, χ7561; colony 7, χ7562; colony 8, χ7274; colony 9, χ6092; colony 10, χ7346; colony 11, χ7347; colony 12, χ7348. Abbreviations: pAPEC-1,2,3, pAPEC-1, pAPEC-2, and pAPEC-3; pAPEC-1,2, pAPEC-1 and pAPEC-2; pAPEC-1,3, pAPEC-1 and pAPEC-3; −, no plasmids.
E. coli K-12 χ6092 was unable to acquire iron in CAS agar medium, whereas its plasmid derivative χ7346 (pAPEC-1) was positive for iron acquisition (Fig. 2). The presence of an orange halo surrounding a colony indicated that siderophores encoded by pAPEC-1 were expressed in E. coli K-12 (Fig. 2A). The fact that the results for χ7347 (pAPEC-2) and χ7348 (pAPEC-3) in this test were negative indicates that neither pAPEC-2 nor pAPEC-3 encodes a siderophore system.
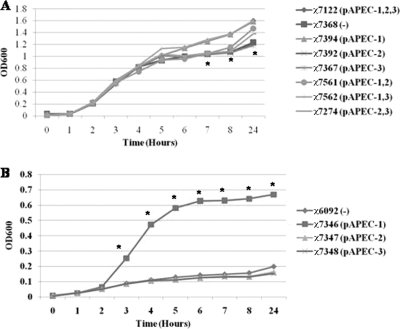
The presence of pAPEC-1 resulted in increased growth of bacteria in iron-restricted medium.
In LB medium supplemented with the ferrous iron chelator 2,2′-dipyridyl, plasmidless strain χ7368 and the strains containing pAPEC-2 and/or pAPEC-3 grew significantly (P < 0.001) slower than the wild-type strain between 7 and 24 h (Fig. 3). All of the strains containing pAPEC-1 except χ7561 (pAPEC-1 and pAPEC-2) grew as well as the wild-type strain (Fig. 3).
FIG. 3.
Growth of bacteria in iron-restricted media. Bacteria were grown in LB medium containing 2,2′-dipyridyl at 37°C for 24 h. (A) Strains with wild-type background. (B) Strains with E. coli K-12 background. The data were obtained from at least 3 independent experiments in which each strain was tested in triplicate. Abbreviations: pAPEC-1,2,3, pAPEC-1, pAPEC-2, and pAPEC-3; pAPEC-1,2, pAPEC-1 and pAPEC-2; pAPEC-1,3, pAPEC-1 and pAPEC-3; −, no plasmids.
Although E. coli K-12 χ6092 exhibits a growth defect in iron-restricted media, addition of pAPEC-1 resulted in a 6-fold increase in growth. However, addition of either pAPEC-2 or pAPEC-3 did not have any effect (Fig. 3B).
Sequence analysis of the two chromosomal iron uptake systems in APEC χ7122, the enterobactin and feo systems.
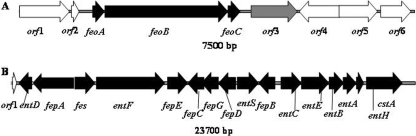
The availability of the rough genomic DNA sequence of χ7122, along with PCR results (Table 3), allowed us to detect and fully analyze the siderophore enterobactin and feo regions on the chromosome. The sequences of 23,700-bp and 7,500-bp regions located on the chromosome of APEC χ7122 contain the genes encoding the siderophore enterobactin and an ABC ferrous iron uptake feo system, respectively (Fig. 4 and Table 4). The regions or specific segments of them are highly homologous to sequences of other E. coli or Shigella strains (see Table S1 in the supplemental material).
FIG. 4.
Genetic organization of the ABC ferrous iron feo (A) and enterobactin (B) regions of APEC χ7122. The black arrows represent known genes, the white arrows represent hypothetical protein genes, and the gray arrows represent insertion sequence genes.
TABLE 4.
Characteristics of three large IROMPs associated with APEC χ7122
| IROMP | Gene | Gene length (bp) | No. of amino acids | Protein mol wt | Location | Accession no. | Reference |
|---|---|---|---|---|---|---|---|
| Ferrienterobactin receptor precursor FepA | fepA | 2,469 | 822 | 90,493.23 | Chromosome | GU361605 | This study |
| Ferric aerobactin receptor IutA | iutA | 2,199 | 731 | 80,594.27 | pAPEC-1 | ACM18305 | 48 |
| Iron-related siderophore receptor IroN | IroN | 2,178 | 724 | 78,981.37 | pAPEC-1 | ACM18227 | 48 |
Six IROMPs are associated with APEC χ7122, two of which are pAPEC-1 encoded and four of which are chromosomally encoded.
Iron limitation-induced IROMPs are not expressed in bacteria grown in normal LB medium. Five bands at different molecular weights were not present in the OMP profiles of wild-type strain χ7122 and its derivatives χ7394 (pAPEC-1), χ7561 (pAPEC-1 and pAPEC-2), and χ7562 (pAPEC-1 and pAPEC-3) grown in LB medium (Fig. 5A), but they were present in the OMP profiles of bacteria grown in LB medium without iron (Fig. 5B). Four of these IROMPs were also expressed in plasmidless strain χ7368 and strains χ7392 (pAPEC-2), χ7367 (pAPEC-3), and χ7274 (pAPEC-2 and pAPEC-3) (Fig. 5B). To distinguish between the chromosome- and plasmid-encoded IROMPs, we analyzed the IROMP profiles of E. coli K-12 χ6092 and its plasmid-containing derivatives. The OMP profiles of these strains confirmed that two large IROMPs are expressed by a strain containing pAPEC-1 (χ7346) (Fig. 5C) and no IROMPs are encoded by pAPEC-2 and pAPEC-3 (data not shown). Altogether, our results show that two pAPEC-1-encoded IROMPs are the aerobactin receptor IutA and the siderophore receptor IroN (Table 4) (48). Here, we identified one of the four chromosome-encoded IROMPs, the enterobactin receptor FepA (Table 4 and Fig. 4; see Table S1 in the supplemental material); the three others need to be identified in the future.
FIG. 5.
Coomassie brilliant blue-stained SDS-PAGE profiles of outer membranes proteins (OMPs) of strains. (A and B) Bacteria with the wild-type background grown in the presence (A) or absence (B) of iron. Lane L, standard molecular weight markers (Bio-Rad); lane 1, χ7122; lane 2, χ7368; lane 3, χ7394; lane 4, χ7392; lane 5, χ7367; lane 6, χ7561; lane 7, χ7562; lane 8, χ7274. (C) Bacteria with the E. coli K-12 background grown in the absence of iron. Lane 1, χ 6092; lane 2, χ7346. The arrowheads indicate the IROMP bands. Abbreviations: pAPEC-1,2,3, pAPEC-1, pAPEC-2, and pAPEC-3; pAPEC-1,2, pAPEC-1 and pAPEC-2; pAPEC-1,3, pAPEC-1 and pAPEC-3.
Large plasmids have a minor effect on the serum resistance of APEC χ7122; however, the presence and nature of O LPS affected the sensitivities of strains to serum complement differently.
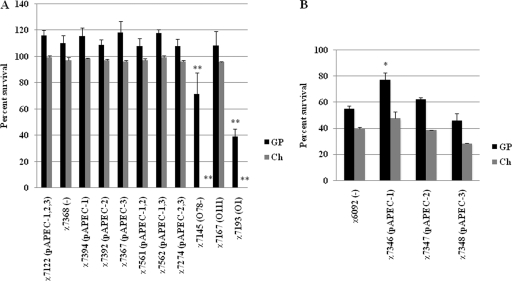
Bacterial strains were tested to determine their abilities to resist complement in both chicken and guinea pig sera. Overall, the results showed that the survival of strains was greater in guinea pig serum than in chicken serum (Fig. 6). Wild-type strain χ7122, as well its plasmid-containing derivatives, were all resistant to both guinea pig and chicken serum complement (Fig. 6A).
FIG. 6.
Serum complement resistance of strains. The survival percentage was determined for each strain following incubation in 90% guinea pig (GP) or chicken (Ch) serum. (A) Bacteria with the wild-type background. (B) Bacteria with the E. coli K-12 background. The data were obtained from at least 3 independent experiments in which each strain was tested in triplicate. The error bars indicate the standard errors of the means. Significant differences are indicated by asterisks (*, P < 0.05 compared to the parent strain; **, P < 0.005 compared to the parent strain). Abbreviations: pAPEC-1,2,3, pAPEC-1, pAPEC-2, and pAPEC-3; pAPEC-1,2, pAPEC-1 and pAPEC-2; pAPEC-1,3, pAPEC-1 and pAPEC-3; −, no plasmids.
Of the LPS-derived strains included in this study, both the χ7145 rough mutant and a derivative expressing O1 LPS, χ7193, were confirmed to be sensitive to complement (46), and χ7193 (O1) was the strain that was most sensitive, especially to chicken serum (Fig. 6A). In this study, we showed that the LPS derivative strain χ7167 (O111) was as resistant to complement as wild-type strain χ7122 (Fig. 6A).
pAPEC-1 increased the survival of E. coli K-12 in serum.
To evaluate if plasmids could affect the sensitivity of E. coli K-12 to complement, we compared the sensitivity of E. coli K-12 to serum with the sensitivities of its plasmid-containing derivatives χ7346 (pAPEC-1), χ7347 (pAPEC-2), and χ7348 (pAPEC-3). As expected, E. coli K-12 strain χ6092 was sensitive to serum complement. Since no cells of either χ6092 or its derivatives were detected after 3 h of incubation in serum (data not shown), we reduced the time of incubation of bacteria in serum from 3 h to 1 h. After 1 h of incubation in guinea pig serum, the presence of pAPEC-1 in χ7346 significantly (P < 0.05) increased the survival of this strain compared to parent strain χ6092. Neither pAPEC-2 in χ7347 nor pAPEC-3 in χ7348 had any effect on survival (Fig. 6B).
The degree of virulence of APEC depends on the nature of its large plasmids and combinations of these plasmids.
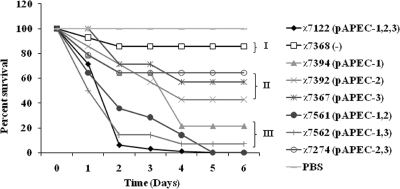
Based on lethality for 1-day-old chicks following subcutaneous inoculation, APEC isolates were classified in different lethality classes ranging from high to low (15, 53). Here, our results show that the plasmid-containing derivatives of wild-type strain χ7122 behaved differently in terms of lethality for 1-day-old chicks. The high-lethality class included wild-type strain χ7122 and strains χ7394 (pAPEC-1), χ7561 (pAPEC-1 and pAPEC-2), and χ7562 (pAPEC-1 and pAPEC-3), which killed 100%, 78.6%, 100%, and 92.9% of the chicks tested, respectively. The moderately virulent class included χ7392 (pAPEC-2), χ7367 (pAPEC-3), and χ7274 (pAPEC-2 and pAPEC-3), which killed 57.2%, 42.8%, and 35.7% of the chicks tested, respectively. The low-lethality class included plasmidless strain χ7368, which killed only 14.3% of the chicks tested. No death was observed in the group of chicks inoculated with PBS (Fig. 7).
FIG. 7.
Pathogenicities of different strains in 1-day-old chicks. The survival percentages were evaluated for groups of chicks inoculated subcutaneously at 6 days after inoculation with either wild-type strain χ7122 or its plasmid-containing derivatives. Strains were classified as low-virulence (I), moderately virulent (II), and highly virulent (III) strains. Abbreviations: pAPEC-1,2,3, pAPEC-1, pAPEC-2, and pAPEC-3; pAPEC-1,2, pAPEC-1 and pAPEC-2; pAPEC-1,3, pAPEC-1 and pAPEC-3; −, no plasmids.
pAPEC-1 has a major role in dissemination of bacteria in blood and internal organs of infected chickens, and pAPEC-3 has a cumulative effect on virulence.
The air sac infection model is able to differentiate between strains that are able to disseminate in internal organs, generate gross lesions, and cause systemic infection and attenuated strains whose capacity to colonize deeper tissues is impaired (15, 46). In this study, we confirmed that χ7122 was able to produce signs of colibacillosis in chickens inoculated via the air sacs (15, 46). Bacteria were able to colonize all internal organs and body fluids of the inoculated chickens (Table 5; data for blood not shown). Its derivatives exhibited different degrees of pathogenicity. Without the three large plasmids, χ7368 persisted less well than the parent strain, did not multiply in pericardial fluid and blood, and colonized the lungs, air sacs, spleen, or liver less well than the parent strain or did not colonize these organs. However, in the presence of pAPEC-1 alone or in combination with pAPEC-2, although there was no statistically significant difference, bacteria were able to persist and colonize internal organs at a level lower than the wild-type level (Table 5), whereas the strain with pAPEC-1 and pAPEC-3 showed greater colonization of internal organs and the difference was statistically significant in the lungs (P = 0.004). In the absence of pAPEC-1, strains with pAPEC-2 and/or pAPEC-3 colonized and persisted poorly in body fluids and internal organs of infected chickens (Table 5; data for blood not shown).
TABLE 5.
Abilities of strains to colonize respiratory organs and invade internal organs of chickens
| Strain or solution | Plasmid(s) | Size of inoculum (log CFU) | No. of chickens colonized/total no. |
Lung |
Spleen |
Liver |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| Air sacs | Pericardial fluid | No. of chickens colonized/total no. | Mean no. of bacteria (log10 CFU/g)a | No. of chickens colonized/total no. | Mean no. of bacteria (log10 CFU/g)a | No. of chickens colonized/total no. | Mean no. of bacteria (log10 CFU/g)a | |||
| χ7122 | pAPEC-1, pAPEC-2, pAPEC-3 | 7.2 | 11/12 | 12/12 | 12/12 | 3.10 ± 0.94 | 8/8 | 4.01 ± 0.57 | 7/8 | 3.06 ± 1.57 |
| χ7368 | None | 7.1 | 1/14 | 03/14 | 01/14 | 0.19 ± 0.72c | 2/10 | 0.74 ± 1.06c | 1/10 | 0.12 ± 0.45d |
| χ7394b | pAPEC-1 | 7.1 | 11/12 | 11/12 | 10/12 | 3.28 ± 2.03 | 7/8 | 3.65 ± 1.71 | 6/8 | 2.41 ± 2.04 |
| χ7392 | pAPEC-2 | 7.1 | 3/14 | 00/14 | 02/14 | 0.49 ± 1.25c | 2/10 | 0.62 ± 1.34c | 0/10 | 0.16 ± 0.59d |
| χ7367 | pAPEC-3 | 7.0 | 0/14 | 00/14 | 03/14 | 0.51 ± 1.12d | 2/10 | 0.59 ± 1.31c | 0/10 | 0.12 ± 0.45d |
| χ7561 | pAPEC-1, pAPEC-2 | 7.3 | 12/14 | 14/14 | 12/14 | 2.76 ± 1.84 | 9/10 | 2.98 ± 1.23 | 5/10 | 1.92 ± 1.59 |
| χ7562b | pAPEC-1, PAPEC-3 | 7.2 | 12/13 | 12/13 | 12/13 | 4.29 ± 1.87e | 8/8 | 4.28 ± 0.80 | 7/8 | 3.45 ± 1.70 |
| χ7274 | pAPEC-2, pAPEC-3 | 7.4 | 04/14 | 06/14 | 01/14 | 0.18 ± 0.68c | 2/10 | 0.80 ± 1.35c | 0/10 | 0.00 ± 0.00d |
| PBS | 0 | 0/8 | 0/8 | 0/8 | 0.00 ± 0.00 | 0/8 | 0.00 ± 0.00 | 0/8 | 0.00 ± 0.00 | |
The values are means ± standard deviations for 8 or 14 birds from each group. Counts were determined at 48 h postinoculation.
Two chickens died between 24 and 48 h after inoculation.
Significantly different from the wild type (P < 0.001).
Significantly different from the wild type (P < 0.0001).
Significantly different from the wild type (P = 0.004).
The gross colibacillosis lesions evaluated in the air sacs, livers, and pericardia of infected chickens were consistent with the bacterial levels observed in different tissues (data not shown). No bacteria or lesions were detected in chickens inoculated with PBS.
pAPEC-1 and pAPEC-2 of χ7122 regulate the growth of bacteria differently in different media.
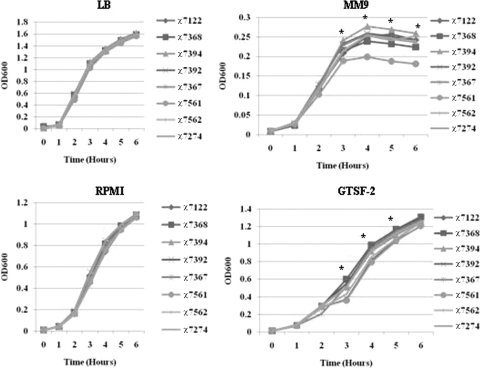
We compared the growth of APEC wild-type strain χ7122 and its plasmid-containing derivatives in different bacterial and cell culture media. Our results showed that all bacteria grew similarly in LB medium, DMEM, and RPMI-1640 (Fig. 8) (data for DMEM not shown). A fitness cost was associated with the presence of pAPEC-1 and pAPEC-2 when bacteria were grown in MM9 minimal medium, but not when bacteria were grown in LB medium. Compared to the wild type, strain χ7561 (pAPEC-1 and pAPEC-2) grew significantly (P < 0.001) slower in MM9 medium after 3 h of incubation (Fig. 8). In this medium, strain χ7394 (pAPEC-1) had the highest level of growth compared to both the wild-type strain and other plasmid-containing derivatives (Fig. 8).
FIG. 8.
Growth curves for different strains in different media. The amounts of growth of wild-type strain χ7122 and its plasmid-containing derivatives in different media (LB medium, MM9 medium, RPMI-1640, and GFTS-2 medium) at different times were compared. The data were obtained from at least 3 independent experiments in which each strain was tested in triplicate. Statistically significant differences compared with the wild-type strain are indicated by an asterisk (P < 0.001).
In the GTSF-2 cell culture medium the highly virulent clones, including wild-type strain χ7122, χ7561 (pAPEC-1 and pAPEC-2), and χ7562 (pAPEC-1 and pAPEC-3), grew significantly (P < 0.001) slower than the less virulent strains, including plasmidless strain χ7368, χ7392 (pAPEC-2), χ7367 (pAPEC-3), and χ7274 (pAPEC-2 and pAPEC-3) (Fig. 8).
DISCUSSION
Large plasmids are a source of pathodiversity in APEC.
Plasmids are extrachromosomal DNA that carry multiple genes expressed with complex cross-regulation involving both chromosomal and plasmid genes. Evaluating their function in the wild-type background individually or in combination with other coexisting plasmids would be the ideal scenario for understanding their contribution to virulence. In this study, we successfully cured APEC χ7122 of its three large plasmids, pAPEC-1 (103 kb), pAPEC-2 (90 kb), and pAPEC-3 (60 kb), and generated strains with different combinations of the three plasmids in the same χ7122 wild-type background. For comparison, we also generated clones of an E. coli K-12 strain with the same three plasmids.
PCR testing of plasmid-containing derivatives of both the wild-type and E. coli K-12 strains for different virulence factors associated with APEC χ7122 both defined different pathotypes for different strains and determined the genetic locations of 19 different virulence factors (Table 3). Except for ompT and hlyF, the implications of these virulence factors in χ7122 have been extensively studied (9, 14, 15, 43, 46, 47, 51, 52, 56, 57). In this study we identified three additional genes in APEC χ7122, fepA encoding enterobactin, a siderophore produced by numerous enteric bacteria (19), eitA of the eitABCD-encoded iron acquisition system, and pilS encoding the major components of the type IV fimbriae. The four eitABCD genes were identified first on a ColV plasmid of an APEC O2 strain (33) and later on a ColBM plasmid of an APEC O1 strain (32). The eitABCD genes were described as genes encoding a novel APEC ABC iron transport system because of the similarity of this system to a putative ABC iron transport system in the plant pathogen Pseudomonas syringae. In a previous study analysis of the sequence of pAPEC-1 did not reveal the presence of eitABCD on this ColV plasmid (48). In this study, however, we determined that the eitA gene was located on plasmid pAPEC-2 of APEC χ7122. This is the first time that eitABCD has been associated with a non-ColV/ColBM plasmid.
The second virulence gene identified in χ7122 was pilS, the gene that encodes the major components of the type IV fimbriae (64). These fimbriae have been described in various Gram-negative bacteria and are encoded mainly by plasmids, such as R64, ColIb-P9, pO113, and pHG1 (38, 39, 59, 71). Type IV fimbriae are considered key virulence factors of many pathogens as they are involved in different bacterial processes, including adhesion to host cells, microcolony and biofilm formation, bacterial aggregation, receptors for phages, immune evasion, twitching motility, DNA uptake, and cell signaling (10). We are in the process of determining the implications of this gene for the virulence of APEC χ7122.
The nature of plasmids and combinations of plasmids generate strains with different degrees of virulence: new insight into the virulence of plasmids pAPEC-2 and pAPEC-3.
A successful infection by a pathogen not only depends on its virulence factors but also on the host and the route of infection (27). In this study, we evaluated the virulence of different plasmid-containing derivatives in two widely used chicken models. We showed that APEC χ7122 infection is deadly for young chicks and confirmed that it causes systemic infection in older chickens within 2 days after inoculation (15, 46). Our results strongly suggest that the three large plasmids have a role in the virulence of APEC strain χ7122, since a strain without these plasmids was severely attenuated in 1-day-old chicks and was not able to colonize internal organs of older chickens infected via air sacs. Previously, we determined that in the absence of pAPEC-1 bacteria were attenuated, and we speculated that pAPEC-1 plays a role in the virulence of APEC (15). The full sequence of this plasmid revealed the presence of important virulence genes, such as genes encoding iron acquisition systems (48). The results of this study demonstrate that although pAPEC-1 genes are in fact required for effective colonization of the host, the presence of this plasmid alone does not restore virulence to wild-type levels and that combining pAPEC-1 with pAPEC-3 made the strain more virulent than the wild type. At this point it is difficult to explain this finding because the sequence of pAPEC-3 is still unknown, although the pilS gene present on this plasmid may contribute to virulence. We showed for the first time that plasmids pAPEC-2 and pAPEC-3 have virulence attributes that are apparent when these plasmids are used in the 1-day-old chick model, but not when they are used in the air sac infection model. The full sequences of the plasmids would provide more information concerning the mechanism of virulence. Together, the results suggest that both the nature of plasmids and combinations of plasmids contribute to the generation of strains with different degrees of virulence and that plasmids play a major role in the diversity of APECs.
Inhibition of growth as a new mechanism of virulence in APEC χ7122.
During the process of infection, a pathogen gains access privileged sites in a host by responding to specific nutritional cues in host microenvironments. At different steps of infection, the pathogen has to acquire nutrients that are necessary for its growth and survival. In this study, we evaluated the in vitro growth of bacteria in both bacterial culture media and cell culture media that are designed to maintain cells under the conditions found in the original tissue (40). The results of this study showed that plasmid-containing derivatives of APEC χ7122 grow differently in the different media tested. The growth of some strains, including wild-type strain χ7122, χ7561 (pAPEC-1 and pAPEC-2), and χ7562 (pAPEC-1 and pAPEC-3), was impaired in GTSF-2 medium, which suggests that some host environments would not be favorable for growth of these bacteria. The growth defect of the bacteria also suggests that at certain steps of infection bacteria can become dormant or less active in order to avoid host reactions that could be harmful, as observed in some pathogens that tolerate antibiotics (41), thus protecting themselves from different stresses at the cost of suspending growth. The mechanism of this phenomenon in χ7122 is related to the large plasmid pAPEC-1 combined with pAPEC-2 and/or pAPEC-3, since the growth of all clones containing these combinations of plasmids was impaired, whereas all other clones were able to grow normally in this medium. The ability of χ7122 to control its growth in some media could be an important virulence mechanism, since all clones that grew slowly in GTSF-2 medium were virulent in the chicken models mentioned above and the virulence was also related to the presence of pAPEC-1 combined with pAPEC-2, with pAPEC-3, or with both of these plasmids. As this is the first report to associate the inhibition of bacterial growth with the virulence of ExPECs, the exact mechanism of this phenomenon has yet to be determined.
On the other hand, bacteria that are able to persist in a host must have specific enzymes to synthesize metabolites that are present at limiting concentrations at some sites. We have shown that in APEC χ7122, a combination of pAPEC-1 and pAPEC-2 impairs the growth of bacteria in minimal media but not in other media. Since individually these two plasmids did not have the same effect, this finding may be related to a cross-regulation effect on some genes that occurs only when the two plasmids are together in the absence of pAPEC-3. This plasmid combination may inhibit the synthesis of some important factors that are not present in the media, as suggested by the high level of growth of bacteria containing pAPEC-1 alone in minimal media. The influence of the nutritional environment on tissue tropism has been reported previously for other pathogens, such UPEC, in which d-serine metabolism gives bacteria an advantage in urinary tract colonization and infection (50). In χ7122, plasmid-encoded factors could have a similar role in chickens.
The serum resistance of χ7122 is related mainly to the presence of LPS and the nature of LPS, and large plasmids provide partial protection.
Most Gram-negative bacteria that enter the bloodstream are rapidly killed by the innate immune defense that forms an important and early barrier to invading bacteria. Successful pathogens have developed strategies to evade these host defenses. Identification of bacterial virulence factors involved in immune escape could be an interesting target for immune interference. The complement system of a host forms a powerful immune barrier. Its activation upon entry of a foreign invader generates a very potent antimicrobial response, and many pathogens have evolved means to control or evade complement. Although many virulence factors are associated with the serum resistance of ExPECs, there is still controversy concerning the implication of some of them, including Iss and TraT, in this phenomenon (46, 69). This is probably due to the diversity of ExPEC serotypes, plasmid profiles, and the multifactorial status of the virulence.
This is the first study that evaluated the role of three large plasmids as well as different O LPS in the same wild-type background with regard to resistance of bacteria to serum. As expected, wild-type strain χ7122 was resistant to both serum complement, and in the absence of O78 LPS, the rough mutant survived less well than the wild type in the serum. Although the rough mutant was less resistant than the wild type, the survivability of this in serum was greater than that of E. coli K-12, which demonstrates that a mechanism of resistance other than LPS was able to provide partial protection against the lytic effect of complement. This mechanism is probably related to the large plasmids because in E. coli K-12 pAPEC-1 was able to increase the survivability of bacteria in serum. This may be related to Iss, a protein encoded by pAPEC-1 and previously shown to be involved in the complement resistance of bacteria (5). The effect of plasmid-related mechanisms in serum resistance was probably not apparent in the wild-type χ7122 background because it was masked by the resistance conferred by O78 LPS.
We have clearly demonstrated that some factors, such as O78 LPS and O111 LPS, are major factors in complement resistance and that plasmid-related mechanisms can provide partial protection to serum. Moreover, since the effect of plasmids was apparent in E. coli K-12 but not in the wild-type background, this could explain the controversy over the role of Iss and TraT in serum resistance, as their effect would not be apparent in different genetic backgrounds. In fact, in APEC χ7122 the O78 LPS that plays a major role in serum resistance masks the effects of both Iss and TraT. This should be considered in future interpretations of results to avoid confusion.
Interestingly, our results show that the presence of full-length O LPS is often not enough for protection of bacteria against the bactericidal effect of complement and that the nature of the O LPS seems to be a key factor in the resistance of bacteria to serum. For example, while an O111-substituted LPS clone was as resistant as the O78 LPS wild type, replacement of O78 LPS with O1 LPS made the strains more sensitive to serum than the rough mutant. This could suggest not only that some LPS are unable to protect bacteria against the complement complex but also that their presence could accelerate the lytic effect of the complement.
Iron uptake systems associated with APEC χ7122 are both pAPEC-1 and chromosomally encoded.
As part of the innate immune defense, the host limits iron availability via iron-binding proteins in order to reduce the levels of free iron to levels that are not sufficient for bacterial growth. A pathogen's ability to acquire iron in mammalian hosts during infection is crucial for successful pathogenesis. Iron is tightly bound by high-affinity iron-binding proteins, which limits the availability of free iron. To counter the iron restriction, some pathogens have evolved strategies involving iron-regulated OMPs (IROMPs), including chelators called siderophores and G protein-like transporters (2, 7). Several iron uptake systems have been associated with APEC χ7122 (Tables 3 and 4). The presence of multiple iron uptake systems is not unusual as bacteria may use different systems under different conditions, enabling them to survive in different environments (2, 6, 72). To date, only two siderophores have been associated with APEC χ7122, salmochelin (IroBCDE IroN) and the hydroxamate aerobactin (IucABCD IutA), both of which are encoded on pAPEC-1, as confirmed by our PCR and phenotype results. Here, we fully characterized the siderophore enterobactin and the ABC ferrous iron system encoded on the chromosome (17). Enterobactin, a siderophore produced by enteric bacteria, is not effective as an iron-scavenging agent for bacteria growing in animals because it is sequestered by the host siderocalin, a component of the innate immune system (19). However, pathogenic strains of E. coli and Salmonella possess the siderophore salmochelin, a modified form of enterochelin that can evade siderocalin because of the presence of two sugars at its scaffold periphery. In APEC χ7122, the salmochelin is pAPEC-1 encoded (48).
In the present study we also showed that variation in plasmid content affects the IROMP profiles of the strains, explaining considerable variations in IROMP production previously reported for APEC isolates (1, 23).
Large plasmids are important agents of the evolution of pathogenicity of APEC.
Genomic sequencing of both pathogenic and nonpathogenic bacteria has revealed that the transition between commensalism and pathogenicity may be due to gene acquisition and loss (68). In this study we clearly showed that in APEC χ7122 plasmids play a major role in this transition. Without its plasmids χ7122 was completely attenuated and was unable to cause disease in chickens.
ExPEC strains differ not only from commensal E. coli strains but also from each other with respect to genomic content and virulence gene repertoire (28). Previously, by using a genomic subtraction technique with strain χ7122 and an E. coli K-12 strain, Brown and Curtiss (8) determined the presence of 12 unique chromosomal regions associated with the virulence of χ7122. In this study, by curing the large plasmids of χ7122 and generating clones with different combinations of the three plasmids, we generated strains with the same background with different pathotypes and degrees of virulence, which reflected the diversity of APEC strains associated with colibacillosis in chickens (11, 16) and demonstrated the importance of plasmids in this diversity.
Bacterial species that live in diverse environments need to be able to adapt to different conditions. Acquisition of different mechanisms increases their chances of adaptation and survival in different niches. In the present work transfer of plasmids into E. coli K-12 conferred to this nonpathogenic bacterium new attributes of virulent strains, such as survivability in serum and iron uptake.
None of the genes on plasmids pAPEC-1, pAPEC-2, and pAPEC-3 were detected in E. coli K-12, indicating that they were acquired during evolution and that acquisition of these plasmids was an integral part of the transition from commensalism to pathogenicity of this organism. The cohabitation of E. coli with different species of bacteria in the gut could promote the promiscuous exchange of genetic material that contributes to the continuing evolution of bacterial pathogens and generation of different E. coli pathotypes. Because of the great diversity of strains potentially generated in this transition, no single strain can be considered highly representative of the species. APEC strain χ7122 and E. coli K-12 still share some genes, including the chromosomal genes encoding adhesins, such as type 1 fimbriae, curli, and the ecp product, and metal acquisition, such as feoB, fepA, and mntH. This demonstrates that these genes were likely present in common ancestors and have a primary role in persistence, providing bacteria with the ability to survive in the intestines of hosts. Acquisition of other genes by these strains during evolution through plasmids or other mechanisms conferred the ability to cause diseases in one host or multiple hosts.
Plasmids are not the only source of evolution in APEC strains. Compared to APEC χ7122, E. coli K-12 lacks the stg gene and a siderophore gene in its chromosome. The stg gene was previously suggested to be a gene that was acquired late during the evolution of pathogenic E. coli because of its low G+C content and its similarity to its ortholog in Salmonella enterica serovar Typhi (43). The same study also showed that the four-gene stgABCD operon was located in the glmS-pstS intergenomic region of χ7122, a region known as a hot spot of DNA insertion in E. coli (24).
Conclusion and final remarks.
Together, the results of this study strongly support the hypothesis that the acquisition of large plasmids is important in the evolution of bacterial pathogens from nonpathogenic ancestors. We clearly defined the importance of the nature of plasmids, the diversity of plasmids, and combinations of different plasmids in generating strains with different pathotypes and levels of virulence. In APEC χ7122, plasmids are involved in different steps of infection and persistence, and without these plasmids the bacteria lose most of the features important in virulence. For the first time, our results implicated large plasmids in the control of bacterial growth under different conditions, a mechanism which could have a very important role in the pathogenicity of bacteria. This new insight into the virulence of APEC should help us understand the virulence of other ExPECs and design a more efficient strategy to control ExPEC infections in the future.
Supplementary Material
Acknowledgments
Funding for this research was provided by National Institutes of Health grant U01060557 and USDA NRI CSREES grant 2007-35201-18519.
We are grateful to Béla Nagy (Veterinary Medical Research Institute of the Hungarian Academy of Sciences, Budapest, Hungary), who kindly provided the plasmids used in the two-step transposon-based curing method used in this study, and Cheryl Nickerson (Biodesign Institute, Arizona State University) for providing the GTSF-2 medium. We thank George Vo and Jacob Maddux (Biodesign Institute, Arizona State University) for their technical help and Erika Arch (Biodesign Institute, Arizona State University) for editorial help.
Editor: A. J. Bäumler
Footnotes
Published ahead of print on 19 January 2010.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Allan, B. J., J. V. van den Hurk, and A. A. Potter. 1993. Characterization of Escherichia coli isolated from cases of avian colibacillosis. Can. J. Vet. Res. 57:146-151. [PMC free article] [PubMed] [Google Scholar]
- 2.Andrews, S. C., A. K. Robinson, and F. Rodriguez-Quinones. 2003. Bacterial iron homeostasis. FEMS Microbiol. Rev. 27:215-237. [DOI] [PubMed] [Google Scholar]
- 3.Barnes, H. J., J. Vaillancourt, and W. B. Gross. 2003. Colibacillosis, p. 631-652. In Y. M. Saif (ed.), Diseases of poultry. Iowa State University Press, Ames, IA.
- 4.Barnes, W. M. 1994. PCR amplification of up to 35-kb DNA with high fidelity and high yield from lambda bacteriophage templates. Proc. Natl. Acad. Sci. U. S. A. 91:2216-2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Binns, M. M., J. Mayden, and R. P. Levine. 1982. Further characterization of complement resistance conferred on Escherichia coli by the plasmid genes traT of R100 and iss of ColV, I-K94. Infect. Immun. 35:654-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyer, E., I. Bergevin, D. Malo, P. Gros, and M. F. Cellier. 2002. Acquisition of Mn(II) in addition to Fe(II) is required for full virulence of Salmonella enterica serovar Typhimurium. Infect. Immun. 70:6032-6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braun, V. 2005. Bacterial iron transport related to virulence. Contrib. Microbiol. 12:210-233. [DOI] [PubMed] [Google Scholar]
- 8.Brown, P. K., and R. Curtiss III. 1996. Unique chromosomal regions associated with virulence of an avian pathogenic Escherichia coli strain. Proc. Natl. Acad. Sci. U. S. A. 93:11149-11154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caza, M., F. Lepine, S. Milot, and C. M. Dozois. 2008. Specific roles of the iroBCDEN genes in virulence of an avian pathogenic Escherichia coli O78 strain and in production of salmochelins. Infect. Immun. 76:3539-3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Craig, L., M. E. Pique, and J. A. Tainer. 2004. Type IV pilus structure and bacterial pathogenicity. Nat. Rev. Microbiol. 2:363-378. [DOI] [PubMed] [Google Scholar]
- 11.Delicato, E. R., B. G. de Brito, L. C. Gaziri, and M. C. Vidotto. 2003. Virulence-associated genes in Escherichia coli isolates from poultry with colibacillosis. Vet. Microbiol. 94:97-103. [DOI] [PubMed] [Google Scholar]
- 12.Dionisio, F., I. Matic, M. Radman, O. R. Rodrigues, and F. Taddei. 2002. Plasmids spread very fast in heterogeneous bacterial communities. Genetics 162:1525-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doetkott, D. M., L. K. Nolan, C. W. Giddings, and D. L. Berryhill. 1996. Large plasmids of avian Escherichia coli isolates. Avian Dis. 40:927-930. [PubMed] [Google Scholar]
- 14.Dozois, C. M., F. Daigle, and R. Curtiss III. 2003. Identification of pathogen-specific and conserved genes expressed in vivo by an avian pathogenic Escherichia coli strain. Proc. Natl. Acad. Sci. U. S. A. 100:247-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dozois, C. M., M. Dho-Moulin, A. Bree, J. M. Fairbrother, C. Desautels, and R. Curtiss III. 2000. Relationship between the Tsh autotransporter and pathogenicity of avian Escherichia coli and localization and analysis of the Tsh genetic region. Infect. Immun. 68:4145-4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dziva, F., and M. P. Stevens. 2008. Colibacillosis in poultry: unravelling the molecular basis of virulence of avian pathogenic Escherichia coli in their natural hosts. Avian Pathol. 37:355-366. [DOI] [PubMed] [Google Scholar]
- 17.Eisenhauer, H. A., S. Shames, P. D. Pawelek, and J. W. Coulton. 2005. Siderophore transport through Escherichia coli outer membrane receptor FhuA with disulfide-tethered cork and barrel domains. J. Biol. Chem. 280:30574-30580. [DOI] [PubMed] [Google Scholar]
- 18.Fantinatti, F., W. D. Silveira, and A. F. Castro. 1994. Characteristics associated with pathogenicity of avian septicaemic Escherichia coli strains. Vet. Microbiol. 41:75-86. [DOI] [PubMed] [Google Scholar]
- 19.Fischbach, M. A., H. Lin, D. R. Liu, and C. T. Walsh. 2006. How pathogenic bacteria evade mammalian sabotage in the battle for iron. Nat. Chem. Biol. 2:132-138. [DOI] [PubMed] [Google Scholar]
- 20.Fredericq, P. 1964. Colicins and colicinogeny. Ann. Inst. Pasteur (Paris) 107(Suppl):7-17. [PubMed] [Google Scholar]
- 21.Frost, L. S., R. Leplae, A. O. Summers, and A. Toussaint. 2005. Mobile genetic elements: the agents of open source evolution. Nat. Rev. Microbiol. 3:722-732. [DOI] [PubMed] [Google Scholar]
- 22.Ginns, C. A., M. L. Benham, L. M. Adams, K. G. Whithear, K. A. Bettelheim, B. S. Crabb, and G. F. Browning. 2000. Colonization of the respiratory tract by a virulent strain of avian Escherichia coli requires carriage of a conjugative plasmid. Infect. Immun. 68:1535-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griffiths, E., H. Chart, and P. Stevenson. 1988. High-affinity iron uptake systems and bacterial virulence, p. 121-137. In J. Roth (ed.), Virulence mechanisms of bacterial pathogens. ASM Press, Washington, DC.
- 24.Gringauz, E., K. A. Orle, C. S. Waddell, and N. L. Craig. 1988. Recognition of Escherichia coli attTn7 by transposon Tn7: lack of specific sequence requirements at the point of Tn7 insertion. J. Bacteriol. 170:2832-2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hitchcock, P. J., and T. M. Brown. 1983. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J. Bacteriol. 154:269-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imre, A., F. Olasz, J. Kiss, and B. Nagy. 2006. A novel transposon-based method for elimination of large bacterial plasmids. Plasmid 55:235-241. [DOI] [PubMed] [Google Scholar]
- 27.Jaureguy, F., E. Carbonnelle, S. Bonacorsi, C. Clec'h, P. Casassus, E. Bingen, B. Picard, X. Nassif, and O. Lortholary. 2007. Host and bacterial determinants of initial severity and outcome of Escherichia coli sepsis. Clin. Microbiol. Infect. 13:854-862. [DOI] [PubMed] [Google Scholar]
- 28.Jaureguy, F., L. Landraud, V. Passet, L. Diancourt, E. Frapy, G. Guigon, E. Carbonnelle, O. Lortholary, O. Clermont, E. Denamur, B. Picard, X. Nassif, and S. Brisse. 2008. Phylogenetic and genomic diversity of human bacteremic Escherichia coli strains. BMC Genomics 9:560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson, J. 2002. Evolution of pathogenic Escherichia coli, p. 55-77. In M. Donnenberg (ed.), Escherichia coli virulence mechanisms of a versatile pathogen. Academic Press, San Diego, CA.
- 30.Johnson, J. R., and T. A. Russo. 2005. Molecular epidemiology of extraintestinal pathogenic (uropathogenic) Escherichia coli. Int. J. Med. Microbiol. 295:383-404. [DOI] [PubMed] [Google Scholar]
- 31.Johnson, T. J., C. W. Giddings, S. M. Horne, P. S. Gibbs, R. E. Wooley, J. Skyberg, P. Olah, R. Kercher, J. S. Sherwood, S. L. Foley, and L. K. Nolan. 2002. Location of increased serum survival gene and selected virulence traits on a conjugative R plasmid in an avian Escherichia coli isolate. Avian Dis. 46:342-352. [DOI] [PubMed] [Google Scholar]
- 32.Johnson, T. J., S. J. Johnson, and L. K. Nolan. 2006. Complete DNA sequence of a ColBM plasmid from avian pathogenic Escherichia coli suggests that it evolved from closely related ColV virulence plasmids. J. Bacteriol. 188:5975-5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson, T. J., K. E. Siek, S. J. Johnson, and L. K. Nolan. 2006. DNA sequence of a ColV plasmid and prevalence of selected plasmid-encoded virulence genes among avian Escherichia coli strains. J. Bacteriol. 188:745-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson, T. J., Y. M. Wannemeuhler, J. A. Scaccianoce, S. J. Johnson, and L. K. Nolan. 2006. Complete DNA sequence, comparative genomics, and prevalence of an IncHI2 plasmid occurring among extraintestinal pathogenic Escherichia coli isolates. Antimicrob. Agents Chemother. 50:3929-3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson, T. J., Y. M. Wannemuehler, S. J. Johnson, C. M. Logue, D. G. White, C. Doetkott, and L. K. Nolan. 2007. Plasmid replicon typing of commensal and pathogenic Escherichia coli isolates. Appl. Environ. Microbiol. 73:1976-1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kado, C. I., and S. T. Liu. 1981. Rapid procedure for detection and isolation of large and small plasmids. J. Bacteriol. 145:1365-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaper, J. B., J. P. Nataro, and H. L. Mobley. 2004. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2:123-140. [DOI] [PubMed] [Google Scholar]
- 38.Kim, S. R., and T. Komano. 1992. Nucleotide sequence of the R721 shufflon. J. Bacteriol. 174:7053-7058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim, S. R., and T. Komano. 1997. The plasmid R64 thin pilus identified as a type IV pilus. J. Bacteriol. 179:3594-3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lelkes, P. I., E. Ramos, V. V. Nikolaychik, D. M. Wankowski, B. R. Unsworth, and T. J. Goodwin. 1997. GTSF-2: a new, versatile cell culture medium for diverse normal and transformed mammalian cells. In Vitro Cell. Dev. Biol. Anim. 33:344-351. [DOI] [PubMed] [Google Scholar]
- 41.Levin, B. R., and D. E. Rozen. 2006. Non-inherited antibiotic resistance. Nat. Rev. Microbiol. 4:556-562. [DOI] [PubMed] [Google Scholar]
- 42.Licht, T. R., and A. Wilcks. 2006. Conjugative gene transfer in the gastrointestinal environment. Adv. Appl. Microbiol. 58:77-95. [PubMed] [Google Scholar]
- 43.Lymberopoulos, M. H., S. Houle, F. Daigle, S. Leveille, A. Bree, M. Moulin-Schouleur, J. R. Johnson, and C. M. Dozois. 2006. Characterization of Stg fimbriae from an avian pathogenic Escherichia coli O78:K80 strain and assessment of their contribution to colonization of the chicken respiratory tract. J. Bacteriol. 188:6449-6459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mainil, J. G., F. Bex, P. Dreze, A. Kaeckenbeeck, and M. Couturier. 1992. Replicon typing of virulence plasmids of enterotoxigenic Escherichia coli isolates from cattle. Infect. Immun. 60:3376-3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mazodier, P., and J. Davies. 1991. Gene transfer between distantly related bacteria. Annu. Rev. Genet. 25:147-171. [DOI] [PubMed] [Google Scholar]
- 46.Mellata, M., M. Dho-Moulin, C. M. Dozois, R. Curtiss III, P. K. Brown, P. Arne, A. Bree, C. Desautels, and J. M. Fairbrother. 2003. Role of virulence factors in resistance of avian pathogenic Escherichia coli to serum and in pathogenicity. Infect. Immun. 71:536-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mellata, M., M. Dho-Moulin, C. M. Dozois, R. Curtiss III, B. Lehoux, and J. M. Fairbrother. 2003. Role of avian pathogenic Escherichia coli virulence factors in bacterial interaction with chicken heterophils and macrophages. Infect. Immun. 71:494-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mellata, M., J. W. Touchman, and R. Curtiss III. 2009. Full sequence and comparative analysis of the plasmid pAPEC-1 of avian pathogenic E. coli χ7122 (O78:K80:H9). PLoS One 4:e4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miller, V. L., and J. J. Mekalanos. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 170:2575-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moritz, R. L., and R. A. Welch. 2006. The Escherichia coli argW-dsdCXA genetic island is highly variable, and E. coli K1 strains commonly possess two copies of dsdCXA. J. Clin. Microbiol. 44:4038-4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Provence, D. L., and R. Curtiss III. 1994. Isolation and characterization of a gene involved in hemagglutination by an avian pathogenic Escherichia coli strain. Infect. Immun. 62:1369-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Provence, D. L., and R. Curtiss III. 1992. Role of crl in avian pathogenic Escherichia coli: a knockout mutation of crl does not affect hemagglutination activity, fibronectin binding, or curli production. Infect. Immun. 60:4460-4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosenberger, J. K., P. A. Fries, S. S. Cloud, and R. A. Wilson. 1985. In vitro and in vivo characterization of avian Escherichia coli. II. Factors associated with pathogenicity. Avian Dis. 29:1094-1107. [PubMed] [Google Scholar]
- 54.Rotimi, V. O., and B. I. Duerden. 1981. The development of the bacterial flora in normal neonates. J. Med. Microbiol. 14:51-62. [DOI] [PubMed] [Google Scholar]
- 55.Russo, T. A., and J. R. Johnson. 2006. Extraintestinal isolates of Escherichia coli: identification and prospects for vaccine development. Expert Rev. Vaccines 5:45-54. [DOI] [PubMed] [Google Scholar]
- 56.Sabri, M., M. Caza, J. Proulx, M. H. Lymberopoulos, A. Bree, M. Moulin-Schouleur, R. Curtiss III, and C. M. Dozois. 2008. Contribution of the SitABCD, MntH, and FeoB metal transporters to the virulence of avian pathogenic Escherichia coli O78 strain χ7122. Infect. Immun. 76:601-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sabri, M., S. Leveille, and C. M. Dozois. 2006. A SitABCD homologue from an avian pathogenic Escherichia coli strain mediates transport of iron and manganese and resistance to hydrogen peroxide. Microbiology 152:745-758. [DOI] [PubMed] [Google Scholar]
- 58.Sambrook, J. E., F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 59.Schwartz, E., A. Henne, R. Cramm, T. Eitinger, B. Friedrich, and G. Gottschalk. 2003. Complete nucleotide sequence of pHG1: a Ralstonia eutropha H16 megaplasmid encoding key enzymes of H(2)-based ithoautotrophy and anaerobiosis. J. Mol. Biol. 332:369-383. [DOI] [PubMed] [Google Scholar]
- 60.Schwyn, B., and J. B. Neilands. 1987. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 160:47-56. [DOI] [PubMed] [Google Scholar]
- 61.Skyberg, J. A., T. J. Johnson, J. R. Johnson, C. Clabots, C. M. Logue, and L. K. Nolan. 2006. Acquisition of avian pathogenic Escherichia coli plasmids by a commensal E. coli isolate enhances its abilities to kill chicken embryos, grow in human urine, and colonize the murine kidney. Infect. Immun. 74:6287-6292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith, J. L., P. M. Fratamico, and N. W. Gunther. 2007. Extraintestinal pathogenic Escherichia coli. Foodborne Pathog. Dis. 4:134-163. [DOI] [PubMed] [Google Scholar]
- 63.Stehling, E. G., T. Yano, M. Brocchi, and W. D. da Silveira. 2003. Characterization of a plasmid-encoded adhesin of an avian pathogenic Escherichia coli (APEC) strain isolated from a case of swollen head syndrome (SHS). Vet. Microbiol. 95:111-120. [DOI] [PubMed] [Google Scholar]
- 64.Strom, M. S., and S. Lory. 1993. Structure-function and biogenesis of the type IV pili. Annu. Rev. Microbiol. 47:565-596. [DOI] [PubMed] [Google Scholar]
- 65.Tinge, S. A., and R. Curtiss III. 1990. Conservation of Salmonella typhimurium virulence plasmid maintenance regions among Salmonella serovars as a basis for plasmid curing. Infect. Immun. 58:3084-3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tivendale, K. A., A. H. Noormohammadi, J. L. Allen, and G. F. Browning. 2009. The conserved portion of the putative virulence region contributes to virulence of avian pathogenic Escherichia coli. Microbiology 155:450-460. [DOI] [PubMed] [Google Scholar]
- 67.Toomey, N., A. Monaghan, S. Fanning, and D. Bolton. 2009. Transfer of antibiotic resistance marker genes between lactic acid bacteria in model rumen and plant environments. Appl. Environ. Microbiol. 75:3146-3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Touchon, M., C. Hoede, O. Tenaillon, V. Barbe, S. Baeriswyl, P. Bidet, E. Bingen, S. Bonacorsi, C. Bouchier, O. Bouvet, A. Calteau, H. Chiapello, O. Clermont, S. Cruveiller, A. Danchin, M. Diard, C. Dossat, M. E. Karoui, E. Frapy, L. Garry, J. M. Ghigo, A. M. Gilles, J. Johnson, C. Le Bouguenec, M. Lescat, S. Mangenot, V. Martinez-Jehanne, I. Matic, X. Nassif, S. Oztas, M. A. Petit, C. Pichon, Z. Rouy, C. S. Ruf, D. Schneider, J. Tourret, B. Vacherie, D. Vallenet, C. Medigue, E. P. Rocha, and E. Denamur. 2009. Organised genome dynamics in the Escherichia coli species results in highly diverse adaptive paths. PLoS Genet. 5:e1000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vandekerchove, D., F. Vandemaele, C. Adriaensen, M. Zaleska, J. P. Hernalsteens, L. De Baets, P. Butaye, F. Van Immerseel, P. Wattiau, H. Laevens, J. Mast, B. Goddeeris, and F. Pasmans. 2005. Virulence-associated traits in avian Escherichia coli: comparison between isolates from colibacillosis-affected and clinically healthy layer flocks. Vet. Microbiol. 108:75-87. [DOI] [PubMed] [Google Scholar]
- 70.Wooley, R. E., P. S. Gibbs, T. P. Brown, J. R. Glisson, W. L. Steffens, and J. J. Maurer. 1998. Colonization of the chicken trachea by an avirulent avian Escherichia coli transformed with plasmid pHK11. Avian Dis. 42:194-198. [PubMed] [Google Scholar]
- 71.Yoshida, T., N. Furuya, M. Ishikura, T. Isobe, K. Haino-Fukushima, T. Ogawa, and T. Komano. 1998. Purification and characterization of thin pili of IncI1 plasmids ColIb-P9 and R64: formation of PilV-specific cell aggregates by type IV pili. J. Bacteriol. 180:2842-2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou, D., W. D. Hardt, and J. E. Galan. 1999. Salmonella typhimurium encodes a putative iron transport system within the centisome 63 pathogenicity island. Infect. Immun. 67:1974-1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.