Abstract
Cathelicidins are peptide components of the innate immune system of mammals. Apart from exerting a direct antibiotic activity, they can also trigger specific defense responses in the host. Their roles in various pathophysiological conditions have been studied, but there is a lack of published information on their expression and activities in the context of mastitis. The aims of this study were to investigate the expression of the bovine cathelicidins BMAP-27, BMAP-28, Bac5, and indolicidin in healthy and infected mammary tissue and in lipopolysaccharide (LPS)-treated cells, to determine their activities against bacteria isolated from bovine mastitis, and to examine their potentials to trigger defense responses in bovine mammary cells. The genes were found to be upregulated in LPS-stimulated neutrophils, but not in infected quarters or epithelial cells. All peptides showed a variably broad spectrum of activity against 28 bacterial isolates from bovine mastitis (MIC values, 0.5 to 32 μM), some of which were antibiotic resistant. The activity of each peptide was significantly enhanced when it was pairwise tested with the other peptides, reaching the synergy threshold when indolicidin was present. The bactericidal activity was sensitive to milk components; BMAP-27 and -28 were highly effective in mastitic bovine milk and inhibited in milk from healthy cows. Both peptides were also active in whey and in blood serum and triggered the expression of tumor necrosis factor alpha (TNF-α) in bovine mammary epithelial cells. Our results indicate multiple roles for the bovine cathelicidins in mastitis, with complementary and mutually enhanced antimicrobial activities against causative pathogens and the capacity to activate host cells.
Antimicrobial peptides (AMPs) are evolutionarily ancient peptide components of the innate immune system in vertebrates, insects, and plants (55). Several different AMP families have been identified in which peptides either derive from a common ancestor (i.e., they belong to a gene family) or have independently evolved similar primary or secondary structural features (8, 48). A distinctive trait of all these peptides is their ability to exert direct antimicrobial effects in vitro on an array of different microbial pathogens. This often derives from the fact that they have cationic and amphipathic structures capable of interfering with microbial membranes.
Cathelicidins (48, 53) and defensins (37) are among the largest and best-characterized AMP gene families in mammals. A growing body of evidence suggests that, in addition to providing a first line of defense against invading microbes, these AMPs also supply protection against infection by modulating other components of the innate or adaptive immune response (20, 51) and by promoting wound healing (39). In humans, cathelicidin and defensin members have been detected in both phagocytic and epithelial cells (37, 52) and are therefore thought to contribute substantially to host defense at sites that are in contact with the external environment. The sizes and tissue distributions of the two families, however, differ significantly among mammals. For instance, at least 7 distinct cathelicidin peptides have been described in the cow and have been detected in myeloid-derived cells (53) but with no published evidence to date of their presence in nonmyeloid cells. Most other mammals, including humans, instead seem to carry one cathelicidin gene, and in humans it is widely expressed in both myeloid and epithelial cells (52). With respect to defensins, three distinct defensin gene subfamilies, i.e., the α-, β- and θ-defensins, have been described in some primates, whereas only β-defensins have been described in the cow (11). Bovine β-defensins have been detected primarily in myeloid cells, as well as in respiratory (10), oral (35), intestinal (44), and mammary (34, 42) epithelia. Their expression in the last tissue has suggested a role for these molecules in protecting against infections, such as mastitis (34). In contrast, there is a lack of published information on the expression or activities of cathelicidins in the context of mastitis.
Mastitis is an inflammatory process of the mammary gland and is usually a consequence of microbial infection caused by pathogens that find their way into the lumen of the gland through the teat canal (57). It is a highly prevalent disease in dairy cattle and the most costly for the dairy industry worldwide, causing considerable economic losses due to decreased quality and quantity of milk production, increased cost of treatment and veterinary services, and loss of animals (36).
To evaluate the roles of cathelicidins in mastitis, the present study analyzed the expression and activities of representative cathelicidin members, i.e., the alpha-helical “bovine myeloid antimicrobial peptides” (BMAP-27 and BMAP-28), the proline-rich peptide Bac5, and the tryptophan-rich peptide indolicidin, in the context of this disease. Specifically, we examined their gene expression in healthy and infected bovine mammary tissue and in resting and lipopolysaccharide (LPS)-stimulated bovine mammary cells. Furthermore, we examined their in vitro antimicrobial activities against bacterial isolates obtained from cases of bovine mastitis under standard conditions and under conditions that mimicked the mammary gland environment, and addressed the mutual effect of multiple cathelicidins that would result from neutrophil degranulation at an inflammation site.
MATERIALS AND METHODS
Materials.
Derivatized PEG-PS resins, coupling reagents for peptide synthesis, and 9-fluorenylmethoxy carbonyl (Fmoc)-amino acids were purchased from Applied Biosystems (Foster City, CA), Novabiochem (Laufelfingel, Switzerland), and ChemImpex (Wood Dale, IL). Peptide synthesis grade N,N-dimethylformamide, dichloromethane, piperidine, and high-performance liquid chromatography (HPLC)-grade acetonitrile were from Biosolve (Valkenswaard, The Netherlands). Trifluoroacetic acid and N-methylmorpholine were from Acros Chimica (Beerse, Belgium). NCTC 135 medium was from Invitrogen (San Giuliano Milanese, Milan, Italy). Other media and reagents for cell cultures, including fetal bovine serum (FBS), casein, and commercial antibiotics, were from Sigma-Aldrich (St. Louis, MO). All other reagents were of analytical grade. Buffers were prepared in double-glass-distilled water.
Peptides.
The cathelicidin peptides BMAP-27, BMAP-28, Bac5, and indolicidin (see Table 2) were chemically synthesized according to standard methods (2). The β-defensin lingual antimicrobial peptide (LAP) (GVRNSQSCRRNKGICVPIRCPGSMRQIGTCLGAQVKCCRRK), which contains three disulfide bonds, was synthesized as a linear precursor and then oxidatively folded from high-quality crude peptide (1). The peptides were confirmed by mass spectrometry using a Q-STAR hybrid quadrupole time-of-flight mass spectrometer (Applied Biosystems/MDS Sciex, Concord, ON, Canada) equipped with an electrospray ion source. Peptide concentrations were determined in aqueous solution by measuring their absorbance at 257 or 280 nm, taking into account the extinction coefficients of 780.4 (Phe) at 257 nm for BMAP-27, 6,839 (Trp and Tyr) at 280 nm for BMAP-28, 1,280 (Tyr) at 280 nm for Bac5, and 27,795 (Trp) at 280 nm for indolicidin (2). The LAP concentration was measured using the Waddell method (50).
TABLE 2.
Sequences of bovine cathelicidin peptides
| Peptide designation | Sequence | Molecular wt |
|---|---|---|
| BMAP-27 | GRFKRFRKKFKKLFKKLSPVIPLLHL-NH2 | 3,225 |
| BMAP-28 | GGLRSLGRKILRAWKKYGPIIVPIIRI-NH2 | 3,074 |
| Bac5 | RFRPPIRRPPIRPPFYPPFRPPIRPPIFPPIRPPFRPPLGPFP-NH2 | 5,147 |
| Indolicidin | ILPWKWPWWPWRR-NH2 | 1,906 |
Bacterial strains.
A total of six reference bacterial strains were used in this study: Escherichia coli ATCC 25922, Klebsiella pneumoniae ATCC 700603, Staphylococcus aureus ATCC 25923, Staphylococcus epidermidis ATCC 12228, Streptococcus agalactiae ATCC 13813, and Streptococcus uberis ATCC 19436. Additionally, 28 bacterial isolates were collected from cases of clinical and subclinical mastitis in dairy herds of Lombardia (Italy) and included 3 strains of S. agalactiae and 5 strains each of E. coli, K. pneumoniae, S. aureus, S. epidermidis, and S. uberis. The bacteria were maintained in Luria-Bertani (E. coli, K. pneumoniae, S. aureus, and S. epidermidis) or blood agar (S. uberis and S. agalactiae) plates. Bacterial isolates were tested against ampicillin, cefacetrile, cefoperazone, cefquinome, penicillin, rifaximin, norfloxacin, and tylosin using a broth microdilution assay in accordance with the guidelines and interpretative tables of the Clinical and Laboratory Standards Institute (CLSI) (6). The bacteria were cultured in Mueller-Hinton (MH) or brain heart infusion (BHI) broth (S. uberis) for 18 h, diluted 1:50 in fresh medium, and allowed to grow in a shaker at 37°C. Mid-log-phase bacteria were harvested after 10 min of centrifugation at 1,000 × g and resuspended in selected media. Bacterial density was assessed by turbidity at 600 nm, with reference to previously determined standards.
Antibacterial assays.
MIC values were determined using a broth microdilution assay in 96-well microtiter plates, according to the CLSI guidelines. The antimicrobial activity was measured in MH broth with logarithmic-phase microorganisms, as previously reported (2). S. uberis was assayed in BHI broth. Additive/synergistic effects against the reference strain, E. coli ATCC 25922, were evaluated in MH broth as described previously (29, 32). To detect MIC reduction for each peptide when used in pairwise combinations, 2-fold serial dilutions of one peptide were tested against 2-fold serial dilutions of each other peptide. The dilution ranges were 0.063 to 4, 0.063 to 8, 0.032 to 2, and 0.125 to 16 μM for BMAP-27, BMAP-28, Bac5, and indolicidin. The fractional inhibitory concentration (FIC) index for combinations of two peptides was calculated according to the following equation: FIC index = FICA + FICB = A/MICA + B/MICB, where A and B are the MICs of drug A and drug B in the combination, MICA and MICB are the MICs of drug A and drug B alone, and FICA and FICB are the FICs of drug A and drug B. The FIC indices were interpreted as follows: ≤0.5, synergy; 0.51 to 4.0, no interaction; >4.0, antagonism (27).
The bactericidal activity was assayed using E. coli ATCC 25922. Bacteria (4 × 104 to 7 × 104 CFU/ml) were incubated for 60 min at 37°C in the presence of each peptide at 1× MIC, 4× MIC, or 8× MIC, detected in MH broth, in each of the following media: heat-inactivated bovine serum, raw milk from healthy cows (healthy milk), milk from cows with clinical mastitis, bovine whey freshly prepared from healthy milk (5), and MH broth supplemented with casein (26 mg/ml) (3) or with milk fat obtained as reported previously (16). Milk and whey samples were pasteurized by heating them at 63°C for 30 min before use. After incubation in the presence of peptides, the samples were serially diluted in ice-cold physiological salt solution, and 50-μl aliquots were plated on MH agar. After overnight incubation at 37°C, the colonies were enumerated, and the CFU were calculated.
Mammary tissue sample collection.
Quarter milk samples were collected from the udders of 10 cows immediately after the organ was removed from the carcass. Thereafter, tissue samples (n = 17) were collected from each mammary gland quarter, immediately placed in RNAlater (Applied Biosystems), and stored at −20°C. The infection status was determined by bacteriological analysis and somatic cell counts (SCC) of quarter milk samples, following the National Mastitis Council guidelines (17). Briefly, an aliquot of 10 μl milk was spread on blood agar plates; after 24 to 48 h of incubation at 37°C, the bacterial colonies were isolated and identified by biochemical methods. Somatic cells were counted on a Bentley Somacount 150 (Bentley Instruments, Chaska, MN).
PMN isolation and in vitro stimulation with bacterial LPS.
Polymorphonuclear leukocytes (PMN) were purified from whole blood of freshly slaughtered cows by differential centrifugation, followed by hypotonic lysis of the supernatant. The PMN were plated at 7.5 × 106 cells/ml into 35-mm tissue culture petri dishes and maintained in RPMI 1640 supplemented with 100 units/ml of penicillin, 100 μg/ml streptomycin, and 2 mM l-glutamine in the presence of 10% heat-inactivated FBS at 37°C in a humidified atmosphere with 5% CO2. The cells were stimulated with 0.1 or 1 μg/ml purified LPS from E. coli serotype O111:B4 (Sigma-Aldrich). After 20 h at 37°C, cells were harvested with 350 μl of lysis buffer (GE Healthcare Europe, GmbH, Milan, Italy) and stored at −20°C for subsequent RNA isolation.
Bovine mammary epithelial cell cultures.
BME-UV1 cells were purchased from Istituto Zooprofilattico Sperimentale della Lombardia e dell'Emilia Romagna and cultured at 37°C with 5% CO2 in the following medium: 40% Ham's F-12, 30% RPMI 1640, 20% NCTC 135, 10% FBS containing 0.1% lactose, 0.1% lactalbumin hydrolysate, 1.2 mM glutathione, 10 μg/ml l-ascorbic acid, 1 μg/ml hydrocortisone, 1 μg/ml insulin, 5 μg/ml transferrin, and 0.5 μg/ml progesterone. The cells were seeded at a density of 40 × 104 cells/ml in 24-well plates and grown for 24 h in complete medium before stimulation. After 6 to 24 h at 37°C, cells were harvested with lysis buffer (GE Healthcare Europe) and processed for RNA isolation. An MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide] assay kit (Sigma-Aldrich) was used to assess cell viability according to the manufacturer's instructions.
Real-time PCR quantification.
RNA was isolated from tissue and cell samples using Illustra RNAspin (GE Healthcare Europe). One microgram of total RNA was retrotranscribed using 200 units of Superscript II reverse transcriptase (Invitrogen) and oligo(dT) primers. Quantitative real-time PCR (qPCR) was carried out for each sample in triplicate using the SYBR green fluorescent detection system and the iCycler iQ5 (Bio-Rad Laboratories, Segrate, Italy). Each reaction mixture (15 μl) contained 7.5 μl SYBR green Master Mix (Bio-Rad Laboratories), 0.45 μl each primer at 10 μM, 5.1 μl water, and 1.5 μl of a 5-fold-diluted cDNA product. The thermal-cycling program was 95°C for 3 min, followed by 40 cycles of 95°C for 10 s and 60°C for 30 s. Following qPCR cycles, dissociation curves were run to assess the presence of only one product and the absence of primer dimers. Primers for LAP and tumor necrosis factor alpha (TNF-α) (Table 1) were as previously reported (25, 40); primers for Bac5, BMAP-28, BMAP-27, indolicidin, and the reference glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene (Table 1) were designed using IDT Primerquest software. The abundance of specific gene transcripts was normalized in each sample relative to that of GAPDH, and the results were expressed as normalized fold expression relative to the control (uninfected).
TABLE 1.
Primer pairs used in qPCR
| Target cDNA | Oligonucleotides (5′-3′)a | Product size (bp) | Accession no. (GenBank) |
|---|---|---|---|
| LAP | F: AATTCTCAAAGCTGCCGT | 164 | S76279.1 |
| R: CACAGTTTCTGACTCCGC | |||
| Bac5 | F: TGACTTCAAGGAGAATGGGCTG | 127 | L02650.1 |
| R: ACGACGGATTGGTGGACGAAAT | |||
| BMAP-28 | F: TCGGGAGTAACTTCGACATCACCT | 141 | X97609.1 |
| R: GGCCCACAATTCACCCAATTCTGA | |||
| BMAP-27 | F: ATGGGCTGGTGAAGCAATGTGTAG | 163 | X97608.1 |
| R: TGGAGTAGCGGAATGACTGGAGAA | |||
| Indolicidin | F: ACCCATCCAATGACCAGTTTGACC | 177 | X67340.1 |
| R: TTCACTGTCCAGAAGCCCGAATCT | |||
| TNF-α | F: CTGGTTCAGACACTCAGGTCCT | 183 | AF011926 |
| R: GAGGTAAAGCCCGTCAGCA | |||
| GAPDH | F: CCTGGAGAAACCTGCCAAGT | 214 | BC102589.1 |
| R: GCCAAATTCATTGTCGTACCA |
F, forward; R, reverse.
Statistical analysis.
Statistical differences among groups of data were analyzed by one-way analysis of variance, followed by the Bonferroni posttest, using GraphPad Prism version 5.0 (GraphPad Software, Inc., San Diego, CA). In all comparisons, a P of <0.05 was considered significant.
RESULTS
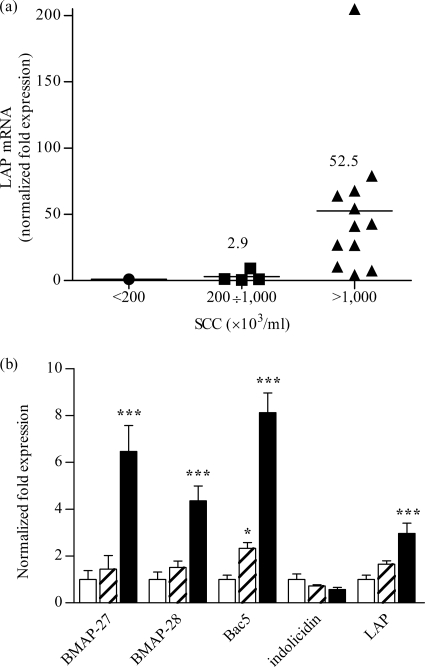
As an initial step to evaluate the roles of the bovine cathelicidin peptides in mammary gland infection, the expression of selected cathelicidin family genes, i.e., Bac5, BMAP-27, BMAP-28, and indolicidin, was analyzed by qPCR using RNA extracted from bovine mammary gland tissue, from the bovine mammary epithelial cell line BME-UV1, and from circulating neutrophils. Mammary gland tissue was classified as healthy or mastitic based on bacteriological analysis and SCC determination of quarter milk samples. The expression of a reference β-defensin, LAP, was also examined in parallel. Originally identified in the lingual tract (35), the LAP gene has since been described in other bovine tissues, as well, including mammary gland tissue (7), and has been found to be upregulated in mastitic glands (15, 40, 42). In agreement with previously reported data (40, 42), the expression of the LAP gene increased on average by 50-fold in diseased compared to healthy tissue samples (Fig. 1a) and by 15-fold in stimulated (24 h of incubation in the presence of 10 μg/ml bacterial LPS) over unstimulated mammary epithelial cells (data not shown). Cathelicidin transcript levels corresponding to Bac5, BMAP-27, and indolicidin were below the limit of detection in both mammary tissue and the epithelial cells. Basal level BMAP-28 gene transcription was detected in healthy and infected gland tissue and in resting or LPS-treated epithelial cells (data not shown). Low-level expression of all these genes was also observed in resting neutrophils isolated from peripheral blood. However, BMAP-27, BMAP-28, and Bac5, as well as LAP, were 4- to 8-fold upregulated in these cells after 20 h of stimulation with LPS (Fig. 1b), as revealed by a time course analysis of antimicrobial gene expression.
FIG. 1.
(a) Expression levels of the LAP gene in mammary glands. Tissue samples were grouped based on SCC in milk. The arithmetic mean for each group is indicated by a horizontal line. (b) Expression levels of antimicrobial peptide genes in bovine neutrophils incubated in vitro for 20 h in the absence (open bars) and presence of E. coli LPS at 0.1 μg/ml (hatched bars) or 1 μg/ml (solid bars). The gene expression levels were evaluated using qPCR and are reported as the normalized fold change compared to the expression level in healthy tissue (a) or in unstimulated cells (b). The data in panel b are the means plus standard deviations (SD) of three independent experiments. *, P < 0.05, and ***, P < 0.001 versus unstimulated cells, as assessed by one-way analysis of variance and the Bonferroni posthoc test.
To determine the efficacy of cathelicidins against bacterial pathogens commonly associated with mastitis, individual peptides (Table 2) were assayed in vitro against bacterial isolates collected from cases of bovine mastitis, including contagious (Staphylococcus aureus and Streptococcus agalactiae) and environmental (Streptococcus uberis, Staphylococcus epidermidis, Escherichia coli, and Klebsiella pneumoniae) species, and against the corresponding ATCC reference bacterial strains. Every bacterial isolate was first tested against ampicillin, cefacetrile, cefoperazone, cefquinome, penicillin, rifaximin, norfloxacin, and tylosin using a broth microdilution assay in accordance with the guidelines and interpretative tables of the CLSI (6). Of the 28 strains tested, 11 showed resistance to at least one antibiotic. Specifically, three E. coli isolates were resistant to rifaximin, one E. coli and all K. pneumoniae isolates were resistant to both rifaximin and ampicillin, and two S. epidermidis strains were resistant to tylosin.
When tested against bacterial isolates in standard bacterial growth media, cathelicidin peptides were effective in the low micromolar concentration range (Table 3). Indolicidin and BMAP-27 exhibited the broadest activity spectrum, with MIC values ranging from 0.5 to 8 μM. The activity of BMAP-28 was generally similar to that of BMAP-27, except for S. uberis, which was vulnerable to the latter but resistant (MIC > 32 μM in four out of five clinical isolates) to the former peptide. Bac5 has previously been reported to be primarily active against Gram-negative microorganisms (14). In the current study, Bac5 also showed MIC values in the low micromolar range against S. epidermidis among Gram-positive organisms. By comparison, LAP was ineffective against all bacteria under the medium conditions used (results not shown), in agreement with the recognized salt and medium sensitivities of β-defensins (13, 30). The MIC values obtained against the corresponding ATCC reference bacterial strains did not differ significantly from the range of values observed for clinical-isolate strains, except that S. uberis ATCC 19436 was somewhat more susceptible to BMAP-28 or Bac5 than S. uberis isolates and K. pneumoniae ATCC 700603 was more resistant to indolicidin than the corresponding K. pneumoniae isolates (Table 4).
TABLE 3.
Antimicrobial activities of bovine cathelicidin peptides against bacterial isolates obtained from clinical cases of bovine mastitis
| Organism (no. of strains) | MIC range (μM)a |
|||
|---|---|---|---|---|
| BMAP-27 | BMAP-28 | Bac5 | Indolicidin | |
| E. coli (5) | 0.5-4 | 4-8 | 0.5-1 | 4 |
| K. pneumoniae (5) | 1 | 1-2 | 1-4 | 4-8 |
| S. aureus (5) | 4-8 | 2-4 | >32 | 2-8 |
| S. epidermidis (5) | 0.5-1 | 1-2 | 1-2 | 1-2 |
| S. uberis (5)b | 4 | 2->32 | 16->32 | 1-2 |
| S. agalactiae (3) | 4 | 2 | 4-16 | 1-2 |
Data are means of at least four independent experiments.
Determined in BHI broth.
TABLE 4.
Antimicrobial activities of bovine cathelicidin peptides against reference strains
| Organism | MIC (μM)a |
|||
|---|---|---|---|---|
| BMAP-27 | BMAP-28 | Bac5 | Indolicidin | |
| E. coli ATCC 25922 | 1 | 2 | 0.5 | 4 |
| K. pneumoniae ATCC 700603 | 1 | 2 | 2 | >32 |
| S. aureus ATCC 25923 | 2 | 2 | >32 | 4 |
| S. epidermidis ATCC 12228 | 2 | 1 | 8 | 4 |
| S. uberisb ATCC 19436 | 2 | 4 | 8 | 8 |
| S. agalactiae ATCC 13813 | 2 | 2 | 4 | 4 |
Data are means of at least four independent experiments.
Determined in BHI broth.
Cathelicidins are stored together as proforms, packed in the cytoplasmic granules of bovine neutrophils under resting conditions, and simultaneously released and processed into active AMPs upon degranulation (54). We took into consideration the possibility of mutual effects deriving from the presence of multiple cathelicidin peptides at inflammation sites and assayed the antimicrobial activity of each possible pairwise combination against the reference strain E. coli ATCC 25922. Using the checkerboard method, we determined the FIC index as a measure of synergy between peptides. The MIC values of individual peptides against E. coli ATCC 25922 in MH broth were 0.5, 1, 2, and 4 μM, respectively, for Bac5, BMAP-27, BMAP-28, and indolicidin. These values decreased at least 2-fold for every peptide when tested in pairwise combinations. The corresponding FIC indices were consistently <1 (Table 5). In particular, indolicidin showed a FIC index of 0.5, predictive of synergy, when combined with BMAP-28 and FIC indices of 0.56 and 0.62 when combined pairwise with Bac5 and BMAP-27. As a method control, each peptide was tested against itself in the checkerboard assay, and the FIC indices were consistently ≥1 (Table 5).
TABLE 5.
FIC indices of bovine cathelicidin peptides against E. coli ATCC 25922
| Peptide | FIC indexa |
|||
|---|---|---|---|---|
| BMAP-27 | BMAP-28 | Indolicidin | Bac5 | |
| BMAP-27 | 1.00 | 0.75 | 0.62 | 0.75 |
| BMAP-28 | 1.00 | 0.50 | 0.56 | |
| Indolicidin | 2.00 | 0.56 | ||
| Bac5 | 1.36 | |||
FIC indices are representative of three independent experiments.
We next determined the bactericidal activity of each individual peptide against E. coli ATCC 25922 in bovine serum and in healthy versus mastitic bovine milk to assess efficacy under conditions that mimicked the mammary gland environment. Bacterial cultures were maintained in the absence and presence of each peptide for 60 min. When tested in MH as a reference bacterial culture broth at concentrations corresponding to four times the MIC, all peptides showed CFU numbers decreased to undetectable levels (Table 6). However, only BMAP-27 and -28 retained substantial activity at these concentrations in bovine serum (Table 6), in line with the reported ability of helix-forming AMPs to function under a wide range of physiological conditions (45, 56).
TABLE 6.
Bactericidal activities of bovine cathelicidin peptides against E. coli ATCC 25922 in different biological fluids
| Peptide | Bacterial viability (103 CFU/ml)a |
|||
|---|---|---|---|---|
| MH brothb | Bovine serumb | Bovine milkc | Bovine wheyc | |
| Control | 125 ± 20 | 115 ± 17 | 148 ± 43 | 119 ± 8.6 |
| BMAP-27 | <0.2d | <0.2d | 107 ± 5.2 | 0.8 ± 0.6d |
| BMAP-28 | <0.2d | 1.5 ± 1.2d | 136 ± 21 | 38 ± 7.2d |
| Bac5 | <0.2d | 86.6 ± 22 | 146 ± 41 | 124 ± 17 |
| Indolicidin | <0.2d | 92 ± 6.4 | 119 ± 11 | 143 ± 3.8 |
The data are means ± standard deviations (SD) of at least four independent experiments.
Sixty-minute incubation at 4× MIC in MH broth (Table 4).
Sixty-minute incubation at 8× MIC.
Statistically significant difference from control (P < 0.001) as assessed by one-way analysis of variance and the Bonferroni posthoc test.
None of the peptides exhibited bactericidal activity against E. coli ATCC 25922 in raw milk from healthy animals, even at concentrations that corresponded to 8 times the MIC obtained in MH broth (Table 6), and although the association of all four peptides at these concentrations did affect bacterial viability, significant variations in CFU decreases (34% to 77% over control values) were observed under these conditions in different milk batches (data not shown).
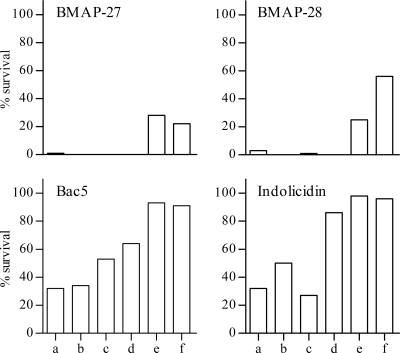
Quite different results were obtained when the bactericidal activity was assayed in milk collected from six distinct cases of acute clinical mastitis. Typically, these samples were flocculent and exhibited an abnormal appearance, ranging from watery and whey-like (samples a to d) to opaque and milky (samples e and f). As shown in Fig. 2, BMAP-27 and -28 produced virtually complete killing in the mastitic milk samples designated a to d and a significant CFU reduction in samples e and f, while Bac5 and indolicidin retained approximately 50% to 70% activity in samples a to c.
FIG. 2.
Killing of E. coli ATCC 25922 in milk collected from cases of clinical mastitis. Bacteria (4 × 104 to 7 × 104 CFU/ml) were incubated in mastitic milk samples (a to f) at 37°C for 60 min in the absence and presence of BMAP-27, BMAP-28, Bac5, or indolicidin at concentrations corresponding to 8× MIC detected in MH broth. The bacteria were diluted in ice-cold physiological salt solution and plated on MH agar. The petri dishes were incubated at 37°C for 16 to 18 h to allow colony counts. The results are reported as percent survival relative to untreated bacteria and are representative of two experiments with similar results.
Overall, these results denoted more favorable killing conditions in mastitic than in healthy milk. We reasoned that the inhibitory effect of healthy milk could be connected with the known ability of hydrophobic and/or anionic molecules present in biological fluids to bind and sequester antimicrobial peptides (18, 19). Raw milk contains a high proportion of lipids and hydrophobic and anionic substances, such as casein. The bactericidal activities of the peptides were therefore determined in the presence of whey, which is the milk fraction depleted of both lipid and casein. At concentrations of BMAP-27 and -28 at which both peptides were virtually inactive in raw milk (eight-fold their MIC in MH broth), they resulted in 100% and 60% CFU reductions, respectively, in whey (Table 6), confirming a sequestering role for these milk components. In contrast, the activities of Bac5 and indolicidin did not demonstrate any improvement (Table 6), further indicating that these molecules exert their antimicrobial effects under only a limited range of conditions.
To assess the separate involvement of casein and fat in modulating antimicrobial activity, each peptide was next tested in MH broth supplemented with 26 mg/ml casein, according to the average casein concentration in milk, or with milk lipid (high-fat cream separated from a given volume of fresh milk and added to an equivalent volume of MH), at a concentration corresponding to the MIC value in MH broth alone. BMAP-27 and -28 caused almost complete suppression of bacterial viability in casein-supplemented MH broth and 70% and 32% CFU reductions, respectively, in milk fat-supplemented MH broth, whereas Bac5 and indolicidin were virtually ineffective under both conditions (Table 7).
TABLE 7.
Effects of milk casein and milk lipid on the bactericidal activities of bovine cathelicidins against E. coli ATCC 25922
| Broth | Bacterial viability (103 CFU/ml)a |
||||
|---|---|---|---|---|---|
| Control | BMAP-27 | BMAP-28 | Bac5 | Indolicidin | |
| MH | 91.3 ± 11.2 | 0d | 0d | 12.8 ± 5.6d | 0.8 ± 0.4d |
| MH+Cb | 143 ± 44 | 0.2 ± 0.08d | 14 ± 8.2d | 120 ± 21 | 123 ± 35 |
| MH+Lc | 186 ± 55 | 56 ± 7e | 126 ± 36 | 184 ± 50 | 169 ± 42 |
After 60 min of incubation at the MIC. The data are means ± SD of at least four independent experiments.
MH broth supplemented with casein.
MH broth supplemented with milk lipid.
P < 0.001 versus control as assessed by one-way analysis of variance and the Bonferroni posthoc test.
P < 0.05 versus control as assessed by one-way analysis of variance and the Bonferroni posthoc test.
Finally, we explored the possibility that, in addition to exerting a direct antibiotic activity, these peptides could contribute to innate host defense through modulation of mammary epithelial cell function. To this end, the capacities of cathelicidin peptides to promote TNF-α gene expression in BME-UV1 cells were evaluated as a paradigm of cytokine gene induction upon cell activation.
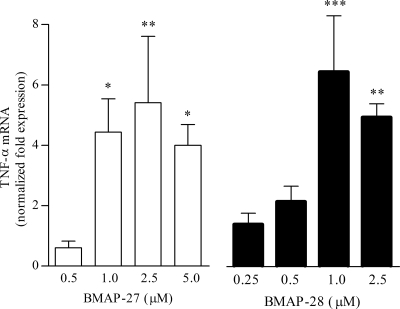
A dose-dependent increase in the expression of the TNF-α gene was observed by qPCR analysis of cells incubated with micromolar concentrations of either BMAP-27 or BMAP-28. The level of transcription of this gene was increased after 6 h and remained steady for up to 12 h of incubation (data not shown), with the highest induction achieved within this time interval using 2.5 μM BMAP-27 and 1 μM BMAP-28 (Fig. 3). In contrast, Bac5 and indolicidin failed to induce expression of TNF-α in these cells at concentrations up to 10 μM. MTT measurements performed in parallel revealed that the cell viability was never lower than 96%, indicating that none of the peptides was cytotoxic at the concentrations used for qPCR analysis.
FIG. 3.
Induction of the TNF-α gene in BME-UV1 cells. The cells were incubated for 8 h in the presence of increasing concentrations of BMAP-27 or BMAP-28. The TNF-α gene expression levels were evaluated using qPCR and are reported as the normalized fold change compared to the expression level in unstimulated cells. The data are the means ± SD of three independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001 versus unstimulated cells, as assessed by one-way analysis of variance and the Bonferroni posthoc test.
DISCUSSION
In this study, we investigated the regulated gene expression of selected bovine cathelicidins and their antimicrobial efficacies in mastitis.
A role for AMPs in the defense system of the mammary gland has previously been suggested by the finding that various β-defensins, including LAP, are expressed in mammary epithelial cells (34, 43) and are upregulated in naturally occurring and experimentally induced mastitis and in epithelial cells stimulated in vitro with bacterial membrane components (15, 40, 43). This is strong circumstantial evidence that β-defensins might play a role in defending the mammary epithelium from infection. We investigated an equivalent role for bovine cathelicidins in bacterial mastitis with a specific focus on their antimicrobial activities under conditions that mimicked the physiological milieu.
Our gene expression studies indicated that, at variance with the LAP gene, which is induced both in mammary gland tissue and in neutrophils following infectious stimuli, cathelicidin genes are induced in LPS-stimulated neutrophils, but not in infected quarters or LPS-stimulated mammary epithelial cells. These results confirm the role of neutrophils as a major source of cathelicidins both from granular stores and from de novo synthesis after granule depletion. Our observations are in line with prior data indicating that in inflamed bovine lung, the expression of the Bac5 gene is induced in activated neutrophils but not in airway epithelial cells (46).
Initial support for an active role of these peptides in mastitic infection is provided by their in vitro efficacy against different bacterial isolates from mastitis cases, including antibiotic-resistant strains. In particular, we found that the alpha-helical peptides BMAP-27 and -28, which act principally through disruption of bacterial membrane integrity (38), are effective in vitro, not only in standard bacterial growth media, but also in complex biological fluids, such as blood serum and whey. The ability of BMAPs to retain activity in serum is common to those helical AMPs that acquire this conformation only in the presence of an anisotropic environment, such as at the bacterial membrane surface, whereas they remain largely unstructured in aqueous medium at physiological salt concentrations (38, 49). We have previously termed this type of helical peptide F form, with reference to some helical primate cathelicidins that behave similarly (26, 45, 56). In rarer cases, such as the human LL-37, a helical conformation can also be induced by physiological salts in the absence of biological membranes (A-form helical peptides), and we have shown these to be considerably more susceptible to serum or medium components (45).
Indolicidin displayed a broad activity spectrum in standard bacterial growth media. This AMP exhibits a mechanism of action partly based on permeabilization of bacterial membranes, although there is evidence that it also acts on internal targets, such as bacterial DNA and RNA (12, 23, 41). Instead, Pro-rich antimicrobial peptides, such as Bac5 and Bac7, which generally show higher selectivity for Gram-negative than for Gram-positive organisms, have been reported to kill bacteria without significant membrane lysis (14). Their mode of action may rather involve internalization via specific transporters and recognition of cytoplasmic molecular targets (9). The SbmA transporter appears to be required for internalization of Pro-rich cathelicidin peptides in E. coli and Salmonella enterica serovar Typhimurium (24, 31). The finding that mutations in the sbmA gene lead to reduced bacterial susceptibility to Pro-rich peptides but do not affect the antimicrobial activity of indolicidin or alpha-helical peptides (24, 31) provides clear evidence for separate modes of action. Interestingly, no orthologues of the sbmA gene have been identified in Gram-positive species (24), which partly explains the generally lower susceptibility of Gram-positive organisms to Bac5. Our current observation that this peptide inhibits the growth of the Gram-positive strains S. epidermidis and S. agalactiae implies the presence of an alternative/additional and as yet unidentified molecular target(s)/transport system(s).
Given the occurrence of different mechanisms of action and distinct molecular targets, it is likely that the simultaneous presence of all these peptides at an inflammatory site results in mutual potentiation of the antimicrobial activity. This may be achieved, for instance, through faster cellular delivery of AMPs acting on intracellular targets as a result of perturbation of bacterial membranes by membrane-active AMPs. The occurrence of multiple targets would also decrease the likelihood of microorganisms acquiring resistance to the AMPs.
In support of mutual potentiation, we showed that the activity of each single peptide in bacterial growth medium was significantly enhanced when it was tested in pairwise combination with other peptides, reaching the synergy threshold when indolicidin was present. This cooperation may allow peptides to reach active concentrations in pathophysiologically relevant contexts. By extension, the overall antimicrobial activity is expected to increase dramatically when the entire arsenal of antimicrobial peptides, including cathelicidins, defensins, and other AMPs (22), is released from neutrophils, a circumstance that likely takes place in vivo in mastitic milk following neutrophil recruitment and activation at sites of udder infection.
Evidence for a significant role of cathelicidins in such a setting is provided by in vitro testing of the bactericidal activity against E. coli ATCC 25922 in milk collected from cases of acute mastitis. BMAPs were highly effective in all these samples, and Bac5 and indolicidin were active in some, suggesting that the mastitic milieu is compatible with cathelicidin activity.
Conversely, none of the peptides exhibited bactericidal effects at equivalent concentrations in healthy milk; a detectable but variable activity was observed in this fluid only when all four cathelicidins were present in combination. The suppressive effect of healthy milk may possibly serve to control untoward activities of these peptides under physiological conditions. Based on killing data in lipid or casein-supplemented MH broth, we showed that this inhibitory effect is in part related to the presence of milk lipid and casein, although our results also indicate a differentiated susceptibility to these milk components. Specifically, the lipid component inactivated Bac5 and indolicidin and significantly reduced the bactericidal activity of BMAP-28, but only slightly reduced that of BMAP-27, even though the last two peptides are structurally related. Casein inhibited Bac5 and indolicidin but neither of the helical BMAPs. It should be emphasized, however, that these assays were carried out in a test medium showing a relatively simple chemical composition. By comparison, milk is a complex biological fluid, and other chemical and/or physical factors in addition to lipid and casein likely also contribute to the inactivation of cathelicidins. As a case in point, we showed that BMAP-27, which was largely inhibited in milk and fully active in whey, remained highly effective in casein-supplemented MH and was only partly inhibited in lipid-supplemented MH, consistent with the presence of other inactivating milk components not analyzed in detail in this study.
The strong influence of the milk composition on the antimicrobial activities of cathelicidins becomes particularly relevant when the physical and chemical properties of healthy and mastitic milk are compared. Mastitis causes alterations that turn milk into a whey-like fluid with a reduced content of different milk components, including caseins and fat (18), and this provides a more suitable environment for efficient activity of AMPs, as confirmed by the finding that cathelicidins in general maintained efficient activity in mastitic milk.
In addition to exerting a broad antimicrobial activity, BMAPs were also able to effectively trigger expression of the proinflammatory mediator TNF-α in mammary epithelial cells at low micromolar concentrations, thus providing an important mechanism for the stimulation of other immune responses. The induction levels, compared to those caused by infectious stimuli, such as LPS, were moderate. This may be an indication that the stimulatory function of BMAPs is more along the lines of modulation of the immune response than induction of an acute response. The relevance of TNF-α to mastitis is suggested by its increased expression in milk somatic cells after E. coli intramammary infection (21) and by the finding that it improves phagocytosis and killing of S. aureus by neutrophils (33). Additionally, the cytokine promotes the recruitment of neutrophils to infected mammary glands by inducing expression of adhesion molecules in endothelial cells (28) and stimulates AMP production (37), setting up a positive loop.
Overall, our studies indicate differential roles for the bovine cathelicidins, regarding both their direct antimicrobial activities and their capacities to activate host cells. The alpha-helical cathelicidins BMAP-27 and -28, in particular, maintain potent antimicrobial activity under mammary inflammatory conditions and may also play roles in activation of the immune response by stimulating the expression of cytokines, such as TNF-α. Bac5 and indolicidin do not appear to modulate the expression of this gene but may trigger other protective responses, such as the reported induction of interleukin 8 (IL-8) by indolicidin (4) and stimulation of cell proliferation by Pro-rich peptides (47). Furthermore, the simultaneous presence of all the peptides as a result of infection may produce a far more robust response than that of the single molecules. These distinct and complementary functions may contribute to a thorough and sustained response to infection and point to a protective role of cathelicidins in bovine mastitis.
Acknowledgments
This work was supported by the Italian Ministry of Education, University and Research (Progetti di Ricerca di Interesse Nazionale 2007) and Regione Friuli Venezia Giulia (grant art. 23 L.R. 26/2005).
Editor: B. A. McCormick
Footnotes
Published ahead of print on 25 January 2010.
REFERENCES
- 1.Antcheva, N., F. Morgera, L. Creatti, L. Vaccari, U. Pag, S. Pacor, Y. Shai, H. G. Sahl, and A. Tossi. 2009. Artificial beta-defensin based on a minimal defensin template. Biochem. J. 421:435-447. [DOI] [PubMed] [Google Scholar]
- 2.Benincasa, M., B. Skerlavaj, R. Gennaro, A. Pellegrini, and M. Zanetti. 2003. In vitro and in vivo antimicrobial activity of two alpha-helical cathelicidin peptides and of their synthetic analogs. Peptides 24:1723-1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonizzi, I., J. N. Buffoni, and M. Feligini. 2009. Quantification of bovine casein fractions by direct chromatographic analysis of milk. Approaching the application to a real production context. J. Chromatogr. A 1216:165-168. [DOI] [PubMed] [Google Scholar]
- 4.Bowdish, D. M., D. J. Davidson, M. G. Scott, and R. E. Hancock. 2005. Immunomodulatory activities of small host defense peptides. Antimicrob. Agents Chemother. 49:1727-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chockalingam, A., D. S. Zarlenga, and D. D. Bannerman. 2007. Antimicrobial activity of bovine bactericidal permeability-increasing protein-derived peptides against gram-negative bacteria isolated from the milk of cows with clinical mastitis. Am. J. Vet. Res. 68:1151-1159. [DOI] [PubMed] [Google Scholar]
- 6.Clinical and Laboratory Standards Institute. 2009. Performance standards for antimicrobial disk susceptibility tests; approved standards, 10th ed., vol. 29. CLSI, Wayne, PA.
- 7.Cormican, P., K. G. Meade, S. Cahalane, F. Narciandi, A. Chapwanya, A. T. Lloyd, and C. O'Farrelly. 2008. Evolution, expression and effectiveness in a cluster of novel bovine beta-defensins. Immunogenetics 60:147-156. [DOI] [PubMed] [Google Scholar]
- 8.Crovella, S., N. Antcheva, I. Zelezetsky, M. Boniotto, S. Pacor, M. V. Verga Falzacappa, and A. Tossi. 2005. Primate beta-defensins—structure, function and evolution. Curr. Protein Pept. Sci. 6:7-21. [DOI] [PubMed] [Google Scholar]
- 9.Cudic, M., and L. Otvos, Jr. 2002. Intracellular targets of antibacterial peptides. Curr. Drug Targets 3:101-106. [DOI] [PubMed] [Google Scholar]
- 10.Diamond, G., M. Zasloff, H. Eck, M. Brasseur, W. L. Maloy, and C. L. Bevins. 1991. Tracheal antimicrobial peptide, a cysteine-rich peptide from mammalian tracheal mucosa: peptide isolation and cloning of a cDNA. Proc. Natl. Acad. Sci. U. S. A. 88:3952-3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fjell, C. D., H. Jenssen, P. Fries, P. Aich, P. Griebel, K. Hilpert, R. E. Hancock, and A. Cherkasov. 2008. Identification of novel host defense peptides and the absence of alpha-defensins in the bovine genome. Proteins 73:420-430. [DOI] [PubMed] [Google Scholar]
- 12.Friedrich, C. L., A. Rozek, A. Patrzykat, and R. E. Hancock. 2001. Structure and mechanism of action of an indolicidin peptide derivative with improved activity against gram-positive bacteria. J. Biol. Chem. 276:24015-24022. [DOI] [PubMed] [Google Scholar]
- 13.Ganz, T. 2005. Defensins and other antimicrobial peptides: a historical perspective and an update. Comb. Chem. High Throughput Screen. 8:209-217. [DOI] [PubMed] [Google Scholar]
- 14.Gennaro, R., M. Zanetti, M. Benincasa, E. Podda, and M. Miani. 2002. Pro-rich antimicrobial peptides from animals: structure, biological functions and mechanism of action. Curr. Pharm. Des. 8:763-778. [DOI] [PubMed] [Google Scholar]
- 15.Gunther, J., D. Koczan, W. Yang, G. Nurnberg, D. Repsilber, H. J. Schuberth, Z. Park, N. Maqbool, A. Molenaar, and H. M. Seyfert. 2009. Assessment of the immune capacity of mammary epithelial cells: comparison with mammary tissue after challenge with Escherichia coli. Vet. Res. 40:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hernandez, A., and F. M. Harte. 2009. Isolation of caseins from whey proteins by microfiltration modifying the mineral balance in skim milk. J. Dairy Sci. 92:5357-5362. [DOI] [PubMed] [Google Scholar]
- 17.Hogan, J. S., R. N. Gonzalez, R. J. Harmon, S. C. Nickerson, S. P. Oliver, J. W. Pankey, and K. L. Smith. 1999. Laboratory handbook on bovine mastitis. National Mastitis Council Inc., Madison, WI.
- 18.Kerr, D. E., and O. Wellnitz. 2003. Mammary expression of new genes to combat mastitis. J. Anim. Sci. 81(Suppl 3):38-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuang, Y., H. Jia, K. Miyanaga, and Y. Tanji. 2009. Effect of milk on antibacterial activity of tetracycline against Escherichia coli and Staphylococcus aureus isolated from bovine mastitis. Appl. Microbiol. Biotechnol. 84:135-142. [DOI] [PubMed] [Google Scholar]
- 20.Lai, Y., and R. L. Gallo. 2009. AMPed up immunity: how antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 30:131-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee, J. W., D. D. Bannerman, M. J. Paape, M. K. Huang, and X. Zhao. 2006. Characterization of cytokine expression in milk somatic cells during intramammary infections with Escherichia coli or Staphylococcus aureus by real-time PCR. Vet. Res. 37:219-229. [DOI] [PubMed] [Google Scholar]
- 22.Levy, O. 2004. Antimicrobial proteins and peptides: anti-infective molecules of mammalian leukocytes. J. Leukoc. Biol. 76:909-925. [DOI] [PubMed] [Google Scholar]
- 23.Marchand, C., K. Krajewski, H. F. Lee, S. Antony, A. A. Johnson, R. Amin, P. Roller, M. Kvaratskhelia, and Y. Pommier. 2006. Covalent binding of the natural antimicrobial peptide indolicidin to DNA abasic sites. Nucleic Acids Res. 34:5157-5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mattiuzzo, M., A. Bandiera, R. Gennaro, M. Benincasa, S. Pacor, N. Antcheva, and M. Scocchi. 2007. Role of the Escherichia coli SbmA in the antimicrobial activity of proline-rich peptides. Mol. Microbiol. 66:151-163. [DOI] [PubMed] [Google Scholar]
- 25.Mitchell, G. B., M. H. Al-Haddawi, M. E. Clark, J. D. Beveridge, and J. L. Caswell. 2007. Effect of corticosteroids and neuropeptides on the expression of defensins in bovine tracheal epithelial cells. Infect. Immun. 75:1325-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morgera, F., L. Vaccari, N. Antcheva, D. Scaini, S. Pacor, and A. Tossi. 2009. Primate cathelicidin orthologues display different structures and membrane interactions. Biochem. J. 417:727-735. [DOI] [PubMed] [Google Scholar]
- 27.Odds, F. C. 2003. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 52:1. [DOI] [PubMed] [Google Scholar]
- 28.Oviedo-Boyso, J., J. J. Valdez-Alarcon, M. Cajero-Juarez, A. Ochoa-Zarzosa, J. E. Lopez-Meza, A. Bravo-Patino, and V. M. Baizabal-Aguirre. 2007. Innate immune response of bovine mammary gland to pathogenic bacteria responsible for mastitis. J. Infect. 54:399-409. [DOI] [PubMed] [Google Scholar]
- 29.Patrzykat, A., L. Zhang, V. Mendoza, G. K. Iwama, and R. E. Hancock. 2001. Synergy of histone-derived peptides of coho salmon with lysozyme and flounder pleurocidin. Antimicrob. Agents Chemother. 45:1337-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pazgier, M., D. M. Hoover, D. Yang, W. Lu, and J. Lubkowski. 2006. Human beta-defensins. Cell Mol. Life Sci. 63:1294-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pranting, M., A. Negrea, M. Rhen, and D. I. Andersson. 2008. Mechanism and fitness costs of PR-39 resistance in Salmonella enterica serovar Typhimurium LT2. Antimicrob. Agents Chemother. 52:2734-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rabel, D., M. Charlet, L. Ehret-Sabatier, L. Cavicchioli, M. Cudic, L. Otvos, Jr., and P. Bulet. 2004. Primary structure and in vitro antibacterial properties of the Drosophila melanogaster attacin C Pro-domain. J. Biol. Chem. 279:14853-14859. [DOI] [PubMed] [Google Scholar]
- 33.Rainard, P., C. Riollet, B. Poutrel, and M. J. Paape. 2000. Phagocytosis and killing of Staphylococcus aureus by bovine neutrophils after priming by tumor necrosis factor-alpha and the des-arginine derivative of C5a. Am. J. Vet. Res. 61:951-959. [DOI] [PubMed] [Google Scholar]
- 34.Roosen, S., K. Exner, S. Paul, J. M. Schroder, E. Kalm, and C. Looft. 2004. Bovine beta-defensins: identification and characterization of novel bovine beta-defensin genes and their expression in mammary gland tissue. Mamm. Genome 15:834-842. [DOI] [PubMed] [Google Scholar]
- 35.Schonwetter, B. S., E. D. Stolzenberg, and M. A. Zasloff. 1995. Epithelial antibiotics induced at sites of inflammation. Science 267:1645-1648. [DOI] [PubMed] [Google Scholar]
- 36.Seegers, H., C. Fourichon, and F. Beaudeau. 2003. Production effects related to mastitis and mastitis economics in dairy cattle herds. Vet. Res. 34:475-491. [DOI] [PubMed] [Google Scholar]
- 37.Selsted, M. E., and A. J. Ouellette. 2005. Mammalian defensins in the antimicrobial immune response. Nat. Immunol. 6:551-557. [DOI] [PubMed] [Google Scholar]
- 38.Skerlavaj, B., R. Gennaro, L. Bagella, L. Merluzzi, A. Risso, and M. Zanetti. 1996. Biological characterization of two novel cathelicidin-derived peptides and identification of structural requirements for their antimicrobial and cell lytic activities. J. Biol. Chem. 271:28375-28381. [DOI] [PubMed] [Google Scholar]
- 39.Steinstraesser, L., T. Koehler, F. Jacobsen, A. Daigeler, O. Goertz, S. Langer, M. Kesting, H. Steinau, E. Eriksson, and T. Hirsch. 2008. Host defense peptides in wound healing. Mol. Med. 14:528-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strandberg, Y., C. Gray, T. Vuocolo, L. Donaldson, M. Broadway, and R. Tellam. 2005. Lipopolysaccharide and lipoteichoic acid induce different innate immune responses in bovine mammary epithelial cells. Cytokine 31:72-86. [DOI] [PubMed] [Google Scholar]
- 41.Subbalakshmi, C., and N. Sitaram. 1998. Mechanism of antimicrobial action of indolicidin. FEMS Microbiol. Lett. 160:91-96. [DOI] [PubMed] [Google Scholar]
- 42.Swanson, K., S. Gorodetsky, L. Good, S. Davis, D. Musgrave, K. Stelwagen, V. Farr, and A. Molenaar. 2004. Expression of a beta-defensin mRNA, lingual antimicrobial peptide, in bovine mammary epithelial tissue is induced by mastitis. Infect. Immun. 72:7311-7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Swanson, K. M., K. Stelwagen, J. Dobson, H. V. Henderson, S. R. Davis, V. C. Farr, and K. Singh. 2009. Transcriptome profiling of Streptococcus uberis-induced mastitis reveals fundamental differences between immune gene expression in the mammary gland and in a primary cell culture model. J. Dairy Sci. 92:117-129. [DOI] [PubMed] [Google Scholar]
- 44.Tarver, A. P., D. P. Clark, G. Diamond, J. P. Russell, H. Erdjument-Bromage, P. Tempst, K. S. Cohen, D. E. Jones, R. W. Sweeney, M. Wines, S. Hwang, and C. L. Bevins. 1998. Enteric beta-defensin: molecular cloning and characterization of a gene with inducible intestinal epithelial cell expression associated with Cryptosporidium parvum infection. Infect. Immun. 66:1045-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tomasinsig, L., F. Morgera, N. Antcheva, S. Pacor, B. Skerlavaj, M. Zanetti, and A. Tossi. 2009. Structure dependence of biological activities for primate cathelicidins. J. Pept. Sci. 15:576-582. [DOI] [PubMed] [Google Scholar]
- 46.Tomasinsig, L., M. Scocchi, C. Di Loreto, D. Artico, and M. Zanetti. 2002. Inducible expression of an antimicrobial peptide of the innate immunity in polymorphonuclear leukocytes. J. Leukoc. Biol. 72:1003-1010. [PubMed] [Google Scholar]
- 47.Tomasinsig, L., B. Skerlavaj, N. Papo, B. Giabbai, Y. Shai, and M. Zanetti. 2006. Mechanistic and functional studies of the interaction of a proline-rich antimicrobial peptide with mammalian cells. J. Biol. Chem. 281:383-391. [DOI] [PubMed] [Google Scholar]
- 48.Tomasinsig, L., and M. Zanetti. 2005. The cathelicidins—structure, function and evolution. Curr. Protein Pept. Sci. 6:23-34. [DOI] [PubMed] [Google Scholar]
- 49.Tossi, A., L. Sandri, and A. Giangaspero. 2000. Amphipathic, alpha-helical antimicrobial peptides. Biopolymers 55:4-30. [DOI] [PubMed] [Google Scholar]
- 50.Waddell, W. J. 1956. A simple ultraviolet spectrophotometric method for the determination of protein. J. Lab. Clin. Med. 48:311-314. [PubMed] [Google Scholar]
- 51.Zanetti, M. 2004. Cathelicidins, multifunctional peptides of the innate immunity. J. Leukoc. Biol. 75:39-48. [DOI] [PubMed] [Google Scholar]
- 52.Zanetti, M. 2005. The role of cathelicidins in the innate host defenses of mammals. Curr. Issues Mol. Biol. 7:179-196. [PubMed] [Google Scholar]
- 53.Zanetti, M., R. Gennaro, and D. Romeo. 1995. Cathelicidins: a novel protein family with a common proregion and a variable C-terminal antimicrobial domain. FEBS Lett. 374:1-5. [DOI] [PubMed] [Google Scholar]
- 54.Zanetti, M., L. Litteri, G. Griffiths, R. Gennaro, and D. Romeo. 1991. Stimulus-induced maturation of probactenecins, precursors of neutrophil antimicrobial polypeptides. J. Immunol. 146:4295-4300. [PubMed] [Google Scholar]
- 55.Zasloff, M. 2002. Antimicrobial peptides of multicellular organisms. Nature 415:389-395. [DOI] [PubMed] [Google Scholar]
- 56.Zelezetsky, I., A. Pontillo, L. Puzzi, N. Antcheva, L. Segat, S. Pacor, S. Crovella, and A. Tossi. 2006. Evolution of the primate cathelicidin. Correlation between structural variations and antimicrobial activity. J. Biol. Chem. 281:19861-19871. [DOI] [PubMed] [Google Scholar]
- 57.Zhao, X., and P. Lacasse. 2008. Mammary tissue damage during bovine mastitis: causes and control. J. Anim. Sci. 86:57-65. [DOI] [PubMed] [Google Scholar]