Abstract
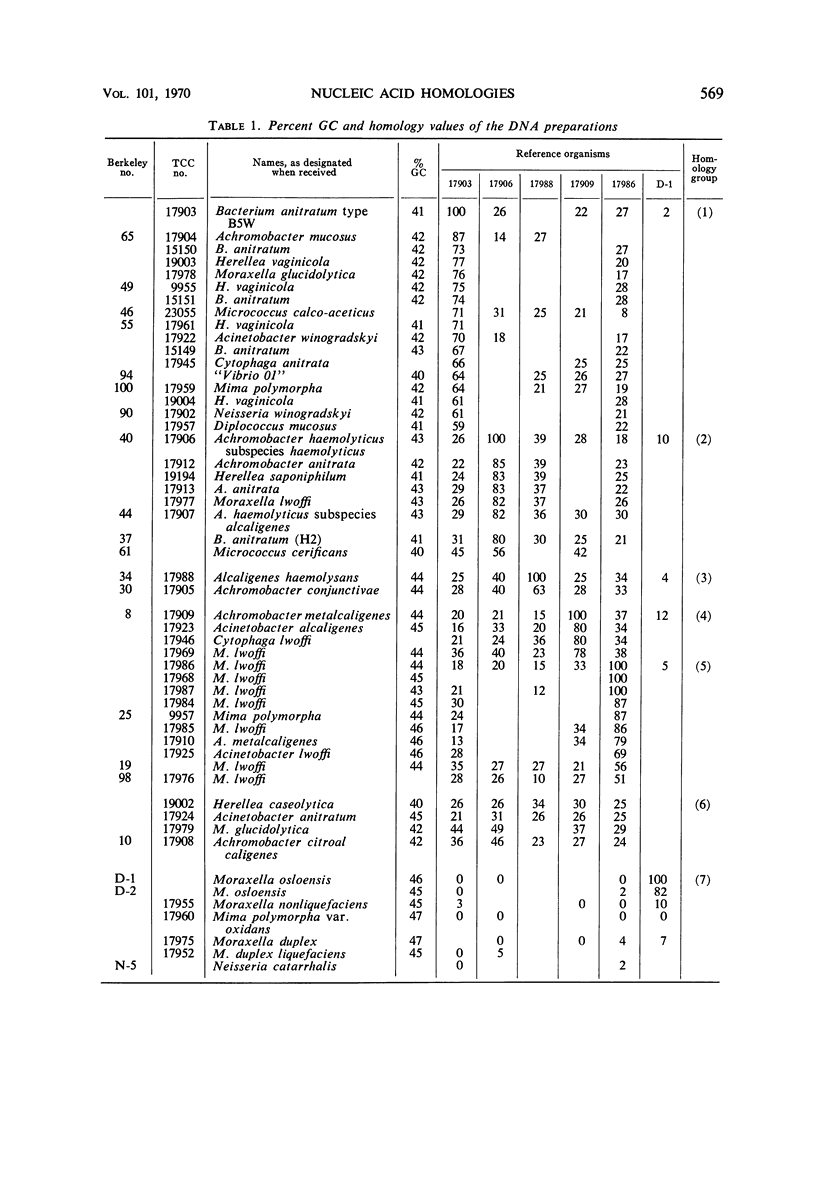
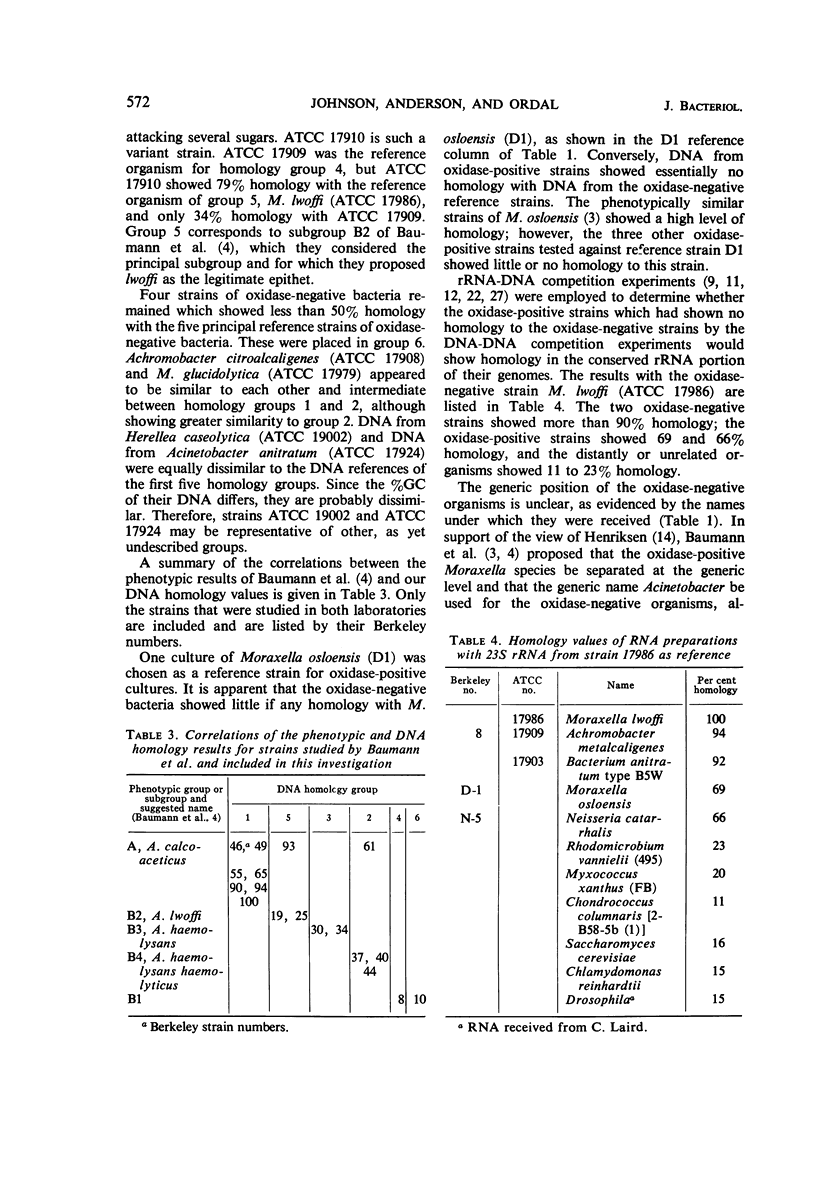
The deoxyribonucleic acid (DNA) base composition and DNA homologies of more than 40 strains of oxidase-negative Moraxella species were determined. These bacteria have also been identified as belonging to the Mima-Herellea-Acinetobacter group and the Bacterium anitratum group, as well as to several other genera including Achromobacter and Alcaligenes. The DNA base content of these strains ranged from 40 to 46% guanine plus cytosine. DNA–DNA competition experiments distinguished five groups whose members were determined by showing 50% or more homology to one of the reference strains: B. anitratum type B5W, Achromobacter haemolyticus var. haemolyticus, Alcaligenes haemolysans, Achromobacter metalcaligenes, and Moraxella lwoffi. A sixth group comprised those strains showing less than 50% homology to any of the reference strains. Negligible homology was found between strains of oxidase-negative and oxidase-positive Moraxella species in DNA–DNA competition experiments. However, evidence of a distant relationship between the two groups was obtained in competition experiments by using ribosomal ribonucleic acid.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BILLING E. Studies on a soap tolerant organism: a new variety of Bacterium anitratum. J Gen Microbiol. 1955 Oct;13(2):252–260. doi: 10.1099/00221287-13-2-252. [DOI] [PubMed] [Google Scholar]
- Baumann P., Doudoroff M., Stanier R. Y. A study of the Moraxella group. II. Oxidative-negative species (genus Acinetobacter). J Bacteriol. 1968 May;95(5):1520–1541. doi: 10.1128/jb.95.5.1520-1541.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann P., Doudoroff M., Stanier R. Y. Study of the Moraxella group. I. Genus Moraxella and the Neisseria catarrhalis group. J Bacteriol. 1968 Jan;95(1):58–73. doi: 10.1128/jb.95.1.58-73.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann P. Isolation of Acinetobacter from soil and water. J Bacteriol. 1968 Jul;96(1):39–42. doi: 10.1128/jb.96.1.39-42.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Click R. E., Hackett D. P. The isolation of ribonucleic acid from plant, bacterial or animal cells. Biochim Biophys Acta. 1966 Oct 24;129(1):74–84. doi: 10.1016/0005-2787(66)90010-4. [DOI] [PubMed] [Google Scholar]
- DOI R. H., IGARASHI R. T. CONSERVATION OF RIBOSOMAL AND MESSENGER RIBONUCLEIC ACID CISTRONS IN BACILLUS SPECIES. J Bacteriol. 1965 Aug;90:384–390. doi: 10.1128/jb.90.2.384-390.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Doi R. H., Igarashi R. T. Heterogeneity of the conserved ribosomal ribonucleic acid sequences of Bacillus subtilis. J Bacteriol. 1966 Jul;92(1):88–96. doi: 10.1128/jb.92.1.88-96.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau D., Smith I., Morell P., Marmur J. Gene conservation in Bacillus species. I. Conserved genetic and nucleic acid base sequence homologies. Proc Natl Acad Sci U S A. 1965 Aug;54(2):491–498. doi: 10.1073/pnas.54.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- HENRIKSEN S. D. Moraxella: classification and taxonomy. J Gen Microbiol. 1952 May;6(3-4):318–328. doi: 10.1099/00221287-6-3-4-318. [DOI] [PubMed] [Google Scholar]
- Henderson A. The Moraxella iwoffi group of bacteria; a review. Antonie Van Leeuwenhoek. 1965;31(4):395–413. doi: 10.1007/BF02045919. [DOI] [PubMed] [Google Scholar]
- Johnson J. L., Ordal E. J. Deoxyribonucleic acid homology in bacterial taxonomy: effect of incubation temperature on reaction specificity. J Bacteriol. 1968 Mar;95(3):893–900. doi: 10.1128/jb.95.3.893-900.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEMOIGNE M., GIRARD H., JACOBELLI G. Bactérie du sol utilisant facilement le 2-3 butanediol. Ann Inst Pasteur (Paris) 1952 Apr;82(4):389–398. [PubMed] [Google Scholar]
- MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol. 1962 Jul;5:109–118. doi: 10.1016/s0022-2836(62)80066-7. [DOI] [PubMed] [Google Scholar]
- MARMUR J., DOTY P. Thermal renaturation of deoxyribonucleic acids. J Mol Biol. 1961 Oct;3:585–594. doi: 10.1016/s0022-2836(61)80023-5. [DOI] [PubMed] [Google Scholar]
- MCCARTHY B. J., BOLTON E. T. An approach to the measurement of genetic relatedness among organisms. Proc Natl Acad Sci U S A. 1963 Jul;50:156–164. doi: 10.1073/pnas.50.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R. L., McCarthy B. J. Comparative study of ribosomal ribonucleic acid cistrons in enterobacteria and myxobacteria. J Bacteriol. 1967 Oct;94(4):1066–1074. doi: 10.1128/jb.94.4.1066-1074.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIECHAUD M., SECOND L. Etude de 26 souches de Moraxella lwoffi. Ann Inst Pasteur (Paris) 1951 Jan;80(1):97–99. [PubMed] [Google Scholar]
- Schaub I. G., Hauber F. D. A Biochemical and Serological Study of a Group of Identical Unidentifiable Gram-negative Bacilli from Human Sources. J Bacteriol. 1948 Oct;56(4):379–385. doi: 10.1128/jb.56.4.379-385.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]


