Abstract
The neisserial opacity (Opa) proteins are a family of antigenically distinct outer membrane proteins that undergo phase-variable expression. Opa+ variants of Neisseria gonorrhoeae strain FA1090 are selected in a cyclical pattern from the lower genital tract of estradiol-treated mice. Here we show that cyclical recovery of Opa+ gonococci does not occur in ovariectomized mice; therefore, the reproductive cycle plays a role in the selection kinetics in vivo. As predicted by the selection pattern shown by wild-type gonococci, we demonstrated that a constitutive Opa-expressing strain was more fit than an Opa-deficient mutant in the early and late phases of infection. We found no evidence that Opa-mediated colonization selects for Opa+ variants during murine infection based on adherence assays with cultured murine epithelial cells. We also tested the hypothesis that complement selects for Opa protein expression during infection. Although some Opa+ variants of a serum-sensitive derivative of strain FA1090 were more resistant to the bactericidal activity of normal human serum, selection for Opa expression was not abrogated in C3-depleted mice. Finally, as previously reported, Opa+ gonococci were more sensitive to serine proteases. Thus, proteases or protease inhibitors may contribute to the observed in vivo selection pattern. We concluded that Opa proteins promote persistence of N. gonorrhoeae in the female genital tract and that opa gene phase variation allows gonococci to evade or capitalize upon unidentified host factors of the mammalian reproductive cycle. This work revealed an intimate interaction between pathogen and host and provides evidence that hormonally related factors shape bacterial adaptation.
Neisseria gonorrhoeae is a Gram-negative pathogen that most commonly colonizes the urethra, cervix, pharynx, and rectum of infected individuals. The gonococcus frequently ascends to the upper reproductive tract of females, and ascension to the epididymis can also occur in males. Hematogenous dissemination to the skin and joints can occur in both genders (25, 26). The gonococcus has no reservoir outside humans and therefore has evolved many sophisticated mechanisms for ensuring its survival within humans, including variable expression of surface molecules via high-frequency, reversible frameshift mutations. This trait provides a mechanism for evading a specific immune response, as well as flexibility in colonizing different niches within the host due to functional differences among variant phenotypes. The neisserial opacity (Opa) proteins are a well-studied example of phase-variable surface structures. Gonococcal strains express 8 to 10 antigenically distinct Opa proteins that are encoded by separate chromosomal alleles (2, 11, 14). Each opa gene independently undergoes phase variation at a rate of 10−3/cell/generation (37) via frameshift mutations that cause changes in the number of pentameric repeats in the opa structural gene (39). Bacteria that express no Opa proteins, bacteria that express one Opa protein, and bacteria that express multiple Opa proteins simultaneously result from these reversible mutations.
The maintenance of 11 or 12 opa alleles by the gonococcus suggests that Opa proteins provide an advantage during infection. The best-characterized function for Opa proteins is adherence to and invasion of host cells via binding to members of the human carcinoembryonic antigen-related cellular adhesion molecule (CEACAM) family, namely, CEACAM1, CEACAM3, CEA, and CEACAM6. Some Opa proteins also mediate uptake through binding to heparin sulfate proteoglycans (HSPGs) (for reviews, see references 13 and 40]. Opa proteins may also mediate evasion of host defenses; recent in vitro studies suggested an immunosuppressive role for the CEACAM1-Opa interaction on lymphocytes (7, 44) and epithelial cells (54), and Bos et al. (5) showed that Opa protein expression causes increased resistance to complement-mediated bacteriolysis. The hypothesis that Opa proteins are important during infection is further supported by the demonstration that mostly Opa+ variants are recovered from the urethras of experimentally (29, 50, 58) and naturally (27) infected men. Opa expression also occurs in the female genital tract, although the recovery of different Opa phenotypes appears to be influenced by the hormonal state and anatomical site. In an analysis of 104 cervical isolates, predominantly opaque (Opa+) colonies were isolated from women prior to ovulation (high estrogen), while mostly transparent colonies were isolated prior to or during menses (27). Additionally, in one study Opa− variants were isolated from the fallopian tubes, regardless of the stage of the menstrual cycle (15).
Based on the analysis of gonococcal isolates from women, it has been hypothesized that certain Opa phenotypes are better adapted for different stages of the reproductive cycle (27). This hypothesis is supported by clinical evidence indicating that the gonococcus has evolved mechanisms for adapting to hormonally driven changes in the female genital tract. For example, there have been several reports that gonococci are either reduced in number or less accessible to cervical culture during the secretory stage of the menstrual cycle (high progesterone, low estrogen) (27, 32,35, 38), and the correlation between the onset of gonococcal pelvic inflammatory disease and menses is well established (1, 19). The factors responsible for the observed associations between Opa phenotype, culture rate, and the menstrual cycle are not known. Salit (49) showed that opaque colonies were more resistant to progesterone than transparent colonies; however, the resultant hypothesis that Opa proteins directly protect gonococci from progesterone was not supported when gonococci with defined Opa phenotypes were tested (52). It is possible that the expression of Opa protein colonization receptors is hormonally regulated. Fluctuations in complement and proteases over the course of the reproductive cycle could also select for Opa+ variants in vivo based on evidence that Opa proteins confer increased resistance to complement-mediated bacteriolysis (5) and serine proteases (27, 57).
The role of host factors in the recovery of certain Opa phenotypes has not been tested in a female infection model. Currently, the only female animal model of gonococcal genital tract infection is experimental infection of BALB/c mice that are treated with 17β-estradiol to promote an estrus-like state. While the capacity of female mice to reproduce human infection is limited due to several host restrictions, including the absence of human CEACAMS, several other aspects of human infection are mimicked by murine infection. Gonococci are associated with vaginal cells in infected mice (28) and are localized within vaginal and cervical tissue, including the lamina propria (56). Infected mice develop an inflammatory response that is characterized by an influx of neutrophils and the production of proinflammatory cytokines and chemokines (43). Also consistent with human infection, mice develop an unremarkable and transient antibody response to N. gonorrhoeae and are susceptible to reinfection with the same strain (56). Additionally, the in vivo phenotype of several defined gonococcal mutants when tested in mice was the phenotype predicted from studies with human neutrophils and antimicrobial peptides (55, 63-65). The recovery of Opa variants during infection of female mice is also similar to the selection dynamics that is predicted from analysis of human cervical isolates. Selection of subpopulations of Opa+ gonococci occurs early during infection of estradiol-treated female mice, and interestingly, the recovery of Opa+ variants over time is cyclical. Consistent with the hypothesis that Opa+ and Opa− populations of bacteria have distinct advantages in vivo, shifts in the predominant Opa phenotype of vaginal isolates are paralleled by fluctuations in the total number of gonococci recovered (52).
Here we tested the hypothesis that the cyclical recovery pattern is independent of estradiol administration and is due to endogenous factors related to the reproductive cycle. We also tested the hypothesis that Opa+ variants have an adherence advantage or survive better early in infection due to increased resistance to host complement. Our data demonstrated the importance of Opa protein expression for persistence of N. gonorrhoeae in the female genital tract and also provide a novel example of how the evolution of a bacterial species has been fine-tuned by the mammalian reproductive cycle.
MATERIALS AND METHODS
Bacterial strains.
N. gonorrhoeae strain FA1090 (porB1b, streptomycin resistant, serum resistant) was originally isolated from a female patient with disseminated gonococcal infection. Strain FA1090 has been extensively characterized using male volunteers (10, 29, 51). Strain FA1090F62por5-8 is a serum-sensitive derivative of strain FA1090 that does not bind human C4B-binding protein (C4BP) due to replacement of porin loops 5 to 8 with loops 5 to 8 of the serum-sensitive strain F62 (46) (kindly provided by Sanjay Ram, University of Massachusetts). Frozen stocks containing mostly piliated or nonpiliated colony variants of wild-type strain FA1090 or strain FA1090F62por5-8 were prepared by single-colony purification and colony suspension immunoblotting using antibodies specific to the Opa proteins of strain FA1090, as described previously (29, 52). Strains FA1090opaA-K and FA1090opaA-K(B+) were kindly provided by Janne Cannon, University of North Carolina. Strain FA1090opaA-K is an unmarked recombinant strain derived from wild-type strain FA1090 in which all 11 opa loci were insertionally inactivated via a selection-counterselection method (33) or single-step allelic exchange (17). Strain FA1090opaA-K(B+) is strain FA1090opaA-K carrying a phase-locked opaB gene. To create a locked-on opaB gene, we altered the opaB signal peptide-encoding region by replacing the coding repeats with nonrepeating nucleotides that encode the same amino acids. Plasmid pFLOB605, which is plasmid pBR322 carrying a cloned opaB gene from strain FA1090, was used as a template for site-directed mutagenesis (QuikChange kit; Stratagene). Mutagenesis using the RDELCR (5′-GCCTTCACTTGCCGCCTGCGCTGCACTAGTAGAGAAGGTTTTTT GCGGGCTGGATTC-3′) and FDELCR (5′-GAATCCAGCCCGCAAAAAACCTTCTCTACTAGTGCAGCGCAGGCGGCAAGTGAAGGC-3′) oligonucleotides resulted in deletion of 50 bp encompassing the coding repeats and creation of an SpeI restriction site (underlined in the sequences above). To create replacement nucleotides encoding a non-phase-variable signal peptide, the annealed oligonucleotide duplex linkers SIG1 (5′-TTATTCAGCAGCTTGCTGTTCAGCTCATTACTGTTCAGCTCACTGTC-3′) and SIG2 (5′-GACAGTGAGCTGAACAGTAATGAGCTGAACAGCAAGCTGCTGAATAA-3′) were ligated into the SpeI site following removal of the 5′ CTAG extension by digestion with mung bean nuclease. However, all of the plasmids that we recovered following transformation into Escherichia coli DH5α had frameshift mutations in the signal peptide-encoding region, suggesting that the opaB gene product was toxic. To eliminate expression from the native opaB promoter, we PCR amplified a region extending from the −10 region to the 3′ end of the opaB coding sequence using a clone with a frameshift deletion as the template and the PREco (5′-GAGAATTCGCCCTTCAACATCAGTG-3′) and PRBam (5′-GAGGATCCGACATCGTGCTTGCCGAC-3′) oligonucleotides. The resultant PCR product was digested with EcoRI and BamHI and ligated into pBR322. The correct reading frame was restored by insertion of a single thymine 78 bp downstream from the start of the opaB coding region. The resultant plasmid was used for allelic exchange of the insertionally inactivated opaB locus in FA1090opaA-K. The lack of Opa protein expression by the FA1090opaA-K mutant and the constitutive expression of OpaB by FA1090opaA-K(B+) bacteria were confirmed by colony suspension immunoblotting. The lipooligosaccharide (LOS) phenotypes were similar for all Opa variants of strains FA1090 and FA1090F62por5-8 tested and for strains FA1090opaA-K and FA1090opaA-K(B+) based on comparisons of the banding patterns of proteinase K-treated digests (24) fractionated on 4 to 12% bis-Tris gradient gels (Invitrogen), followed by silver staining.
Culture conditions.
All N. gonorrhoeae strains were cultured at 37°C under 7% CO2 on GC agar with Kellogg's supplement I and 12 μM Fe(NO3)3 or on GC agar supplemented with vancomycin, colistin, nystatin, trimethoprim sulfate, and streptomycin (VCNTS) for mouse experiments, as described previously (28). Growth curves were determined by growing bacteria at 37°C in supplemented GC broth (GCB) with 0.5 mM NaHCO3 with agitation. At hourly time points, aliquots were diluted in GCB with 0.05% saponin to break up aggregates and quantitatively cultured overnight on GC agar. Where indicated below, water-soluble 17β-estradiol (17β-estradiolws) (Sigma) was added to liquid cultures at a final concentration of 1,000, 100, or 10 pg/ml.
Tissue culture assay.
ME180 human cervical epithelial cells and HEC1B human endometrial cells were obtained from ATCC. ME180 cells were cultured in McCoy's 5A medium supplemented with 10% fetal bovine serum (FBS) and 2.2 g/liter sodium bicarbonate. HEC1B cells were cultured in Dulbecco modified Eagle medium (DMEM) with 10% FBS at 37°C with 5% CO2. Two mouse epithelial cell lines, IEC 4.1 (intestinal) and BM1.11 (oviduct) (generously provided by Harlan Caldwell [Rocky Mountain Laboratories, Hamilton, MT] and Raymond Johnson [Indiana University School of Medicine, Indianapolis, IN], respectively) were cultured as described previously (48). All tissue culture cells were maintained with 50 μg/ml gentamicin (Gm) during routine passage. For adherence and invasion assays, cells were seeded at a density of 1 × 105 cells per well in 24-well tissue culture plates in the absence of antibiotics and infected the following morning with FA1090opaA-K or FA1090opaA-K(B+) bacteria. The bacteria were suspended to an A600 of 0.07 in phosphate-buffered saline (PBS) and diluted 1:10 in RPMI with 10% FBS and 0.2 μM Fe(NO3)3. Five hundred microliters (3 × 106 to 4 × 106 CFU) was applied to each of the cell monolayers to obtain the desired multiplicity of infection (MOI) (30 to 40). For adherence assays, monolayers were incubated with bacteria for 2 h at 37°C under 7% CO2 and washed four times with PBS to remove nonadherent bacteria. Cells were lysed with 0.5% saponin, and the number of cell-associated bacteria was determined by serial dilution and culture. For invasion assays, monolayers were incubated with bacteria for 2 h and washed twice with PBS. Gm (50 μg/ml) was then added, and the cells were incubated for 1.5 h. Monolayers were washed five times with PBS and lysed with 0.5% saponin, and the number of internalized bacteria was determined after serial dilution and culture. The results are expressed below as the percentage of cell-associated bacteria relative to the inoculum (adherence) or the percentage of Gm-protected bacteria relative to the number of adherent bacteria (invasion). All conditions were analyzed in triplicate in an experiment, and each experiment was performed three times.
Bactericidal assays.
The bactericidal activity of normal human serum (NHS) against colony-purified Opa variants of strain FA1090F62por5-8 was tested using a microtiter plate assay essentially as described previously (18). Briefly, NHS (Quidel) or heat-inactivated NHS (HI-NHS) was serially diluted in minimal essential medium (MEM) to obtain a final serum concentration of 0.5 to 8%. Bacteria that were 18 to 22 h old were harvested from agar plates, suspended in PBS to an A600 of 0.07, and diluted 1:2,000 in MEM. Thirty microliters (∼1.5 × 103 CFU) of each suspension was added to each well, and the plates were incubated for 1 h at 37°C with 5% CO2. Fifty microliters of GC broth was then added to all wells, and a 30-μl aliquot from each well was cultured on GC agar. Colonies were enumerated after 24 h, and the results were expressed as the percent survival (100 × [number of CFU recovered from NHS-containing wells/number of CFU recovered from wells with the same concentration of HI-NHS]). The percent survival was plotted versus the dilution of NHS to determine the 50% bactericidal titer, which was defined as the dilution of NHS that resulted in survival of 50% of the bacteria. In other experiments, the percent survival values for different Opa variants following exposure to 3% NHS were determined based on a comparison with the results obtained with HI-NHS. No killing was observed with HI-NHS in any experiment.
Protease inhibition assay.
The sensitivities of wild-type Opa variants and strains FA1090opaA-K and FA1090opaA-K(B+) to bovine trypsin and α-chymotrypsin (Sigma) were measured by the disk diffusion assay using 6-mm disks and the method described previously (57), with the modification that 2-fold increasing concentrations of each protease (2.5 mg/ml to 40 mg/ml) were tested to determine the dose response. The average zone of inhibition for each concentration was calculated from the data for triplicate plates after 18 h of incubation.
Experimental murine infection.
Female intact and ovariectomized (Ov−) BALB/c mice that were 4 to 6 weeks old (National Cancer Institute, Frederick, MD) were housed with a regimen consisting of 12 h of light and 12 h of darkness and were given food and water ad libitum. The ovaries of Ov− mice were surgically removed at least 14 days prior to the start of the experiment, and all mice were allowed to acclimate to the Uniformed Services University of the Health Sciences (USUHS) animal facility for 10 to 14 days before the start of an experiment. There are four stages of the murine estrous cycle (proestrus, estrus, metestrus, and diestrus), which can be differentiated by cytological examination of stained vaginal smears. To promote long-term infection with N. gonorrhoeae, diestrus-stage mice were treated with 17β-estradiol via implantation of a 5-mg, 21-day slow-release pellet (Innovative Research of America) (28) for experiments designed to compare Ov− and intact mice, the recombinant strains FA109opaA-K and FA109opaA-K(B+), and the effects of complement component 3 (C3) depletion. In two experiments, estradiol was administered via subcutaneous injection of 17β-estradiolws (total dose, 1.5 mg) instead of pellets (56). Antibiotics were administered to mice under both treatment regimens as described previously (30). Mice were inoculated vaginally with bacteria 2 days after pellet implantation or after the second dose of 17β-estradiolws (28, 56). In some experiments, antibiotics but no estradiol were given, and transient colonization was achieved by inoculating mice in the proestrus stage with N. gonorrhoeae. In these experiments, soiled litter from a cage containing male mice was used to promote synchronization of the cycle (12).
For all mouse infection experiments, mice were inoculated vaginally with 20 μl (106 CFU) of a filtered bacterial suspension in PBS, and vaginal mucus was quantitatively cultured for N. gonorrhoeae on GC-VCNTS agar as described previously (28). In experiments with wild-type strain FA1090 bacteria, the inocula consisted of mostly nonpiliated colony-purified variants of Opa− and OpaB-expressing gonococci harvested from solid GC agar after 20 h of incubation. Nonpiliated variants were used to minimize the aggregation of Opa+ variants, which can interfere with accurate quantitation. Piliation is not required for experimental murine infection, and we previously showed that selection for Opa protein expression occurs during infection regardless of whether gonococci are piliated (28). Suspensions of variants were combined to obtain ratios of 10 to 20% OpaB-expressing variants versus 80 to 90% Opa− variants as described previously (52). The Opa phenotypes of 96 colonies isolated from each inoculum or of 36 colonies cultured from vaginal mucus from each mouse at each time point were determined by colony suspension immunoblotting (limits of detection for the Opa phenotypes, 1% and 3%, respectively) (28, 52). The results were expressed as the fold increases in the levels of the Opa+ variants relative to the inoculum. The OpaB and OpaD variants and the OpaE and OpaK variants were not distinguished and therefore are referred to below as the OpaB/D and OpaE/K variants, respectively. The multiple expression phenotype in which two phenotypes are expressed simultaneously is referred to as OpaB,I for OpaB and OpaI, etc., throughout.
C3 capture ELISA and complement depletion studies.
To measure vaginal C3 levels, vaginal washes were collected from uninfected BALB/c mice 3 days after implantation of a 5-mg 17β-estradiol pellet and then daily for the next 7 days. Antibiotics were also administered to mice per the infection protocol. Washes were obtained by pipetting 40 μl of PBS in and out of the vagina five times, and three washes were combined to obtain a total of 120 μl for each mouse. Vaginal washes were centrifuged at 13,000 rpm for 3 min to remove cellular debris, and the supernatant was frozen at −20°C. The concentration of C3 in vaginal washes was determined by a capture enzyme-linked immunosorbent assay (ELISA) (GenWay Biotech Inc.) performed according to the manufacturer's specifications. To study the effect of complement on the early phase of infection, cobra venom factor (CVF) (Quidel) was administered intraperitoneally to groups of estradiol-treated mice (20 μg CVF per 18- to 21-g mouse) to deplete C3 the day before bacterial inoculation. A PBS-treated control group was tested in parallel. To study later time points, estradiol-treated mice were colonized with bacteria for 4 days and then treated with CVF or PBS. For all C3 depletion studies, the numbers of bacteria recovered from CVF-treated mice and from PBS-treated mice each day for 3 days after treatment were compared. Serum was collected by centrifugation of whole blood obtained by retro-orbital bleeding at 18 and 72 h after each CVF dose, and the C3 concentration was determined by the capture ELISA as described above. The serum C3 levels were ∼1 mg/ml in normal mice and were 90 to 95% lower in CVF-treated mice. In pilot studies to determine the optimum dose and concentration of CVF, C3 levels decreased within 8 h after administration of 20 μg of CVF and remained low for at least 72 h. All experiments were performed at least twice to test reproducibility.
Animal use.
Animal experiments were conducted in the laboratory animal facility at USUHS, which is fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care, using a protocol approved by the USUHS Institutional Animal Care and Use Committee.
Statistical analysis.
For tissue culture experiments, bactericidal assays, protease inhibition assays, and comparisons of C3 levels in vaginal washes, an unpaired Student t test was used to assess statistical significance. A two-way analysis of variance (ANOVA) followed by an unpaired Student t test was used to determine if there was a difference in the numbers of gonococci recovered from infected mice or a difference in the fold changes in recovery of Opa+ variants. The log rank test was used to assess the difference in duration of infection between recombinant strains FA1090opaA-K and FA1090opaA-K(B+). For all analyses, a P value of <0.05 was considered significant.
RESULTS
The cyclical recovery pattern is not dependent on estradiol treatment.
N. gonorrhoeae strain FA1090 expresses 8 antigenically distinct Opa proteins (OpaA, -B, -C, -D, -E, -F, -K, and -I). We demonstrated previously that Opa+ variants of strain FA1090 were selected early during infection (days 1 to 3) of estradiol-treated BALB/c mice. In these experiments, mice were inoculated with a bacterial suspension containing predominantly Opa− variants that was spiked with a lower percentage of Opa+ variants. This early selection phase was followed by an increase in the proportion of Opa− variants on days 5 to 7 (middle phase) and subsequent isolation of predominantly Opa+ variants on days 9 to 11 (late phase). The changes in the total numbers of gonococci recovered from vaginal swabs mirrored the shifts in Opa phenotype, with higher numbers corresponding to time points at which Opa+ gonococci predominated and a period of decreased recovery corresponding to the middle phase. We described the observed changes in the percentage of Opa+ variants and the total number of CFU recovered as the cyclical recovery pattern (52).
The cyclical recovery pattern was first described in studies using mice that were under the influence of high concentrations of estradiol for at least 2 weeks due to the implantation of slow-release 17β-estradiol pellets to promote susceptibility to infection. Estradiolws also promotes susceptibility to N. gonorrhoeae, but the serum estradiol levels return to physiological concentrations within 24 h after administration (56). To determine whether the cyclical recovery pattern also occurs in mice that are not under the influence of sustained, nonphysiological concentrations of estradiol, we inoculated estradiolws-treated mice with a defined bacterial suspension that consisted of 89% Opa− variants, 11% OpaB variants, and <1% other Opa+ variants and determined the Opa phenotypes of colonies isolated from vaginal mucus over time. Three- and 7-fold increases in the proportion of Opa+ variants compared to the proportion of Opa+ variants in the inoculum were observed on days 1 and 2 postinoculation, respectively (Fig. 1A). Decreases in the proportion of Opa+ variants occurred on days 3 to 5 postinoculation (middle phase), and this was followed by an increase in the proportion of Opa+ variants compared to the proportion in the inoculum (late phase). As seen with pellet-treated mice, the changes in the relative proportion of Opa+ variants in the middle and late phases were accompanied by a decrease and an increase in the average number of gonococci recovered, respectively (data not shown).
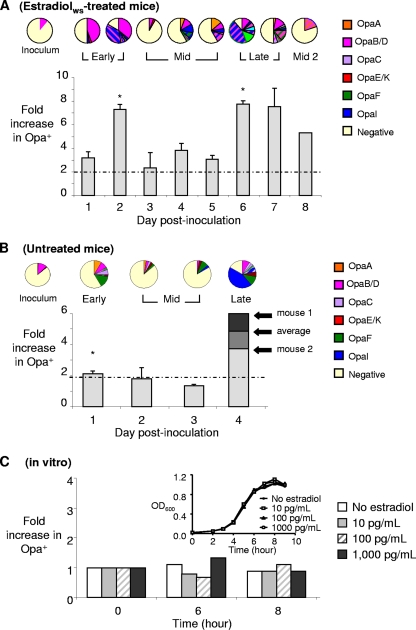
FIG. 1.
For both estradiol-treated and untreated mice there was cyclical recovery of Opa+ variants, and estradiol does not select for Opa protein expression in vitro. For mice treated with 17β-estradiolws (A) or not treated with estradiol (B) there was cyclical recovery of Opa+ variants after the mice were inoculated with a defined suspension containing predominantly Opa− variants. The average fold increases for Opa+ variants among vaginal isolates compared to the inoculum are indicated in the histograms, and selection was defined as a ≥2-fold increase (dotted line). The bars indicate standard errors of the means; the asterisks indicate that the P value is <0.03 for a comparison with the value for the middle phase, which was defined as days 3 to 5 for panel A and as day 3 for panel B. The results in panel A are the results of one experiment performed with 6 mice inoculated with 89% Opa− and 11% OpaB variants and are representative of two independent experiments (5 or 6 mice per experiment). The data in panel B are combined data from three experiments in which untreated mice were colonized for 1 to 4 days. The average fold increase was based on the results for 11 mice on day 1, for 5 mice on day 2, for 3 mice on day 3, and for 2 mice on day 1. In each panel the pie charts show the Opa phenotype of a representative mouse, and the colors indicate the different Opa proteins of strain FA1090. The striped patterns show the results for variants that express multiple Opa proteins. (C) Recovery of Opa+ isolates from a liquid culture at different stages of growth, expressed as fold increases compared to the inoculum. GCB with no estradiol or with 10, 100, or 1,000 pg/ml 17β-estradiolws was inoculated with a bacterial suspension that contained 75% Opa− variants. Concentrations were based on serum estradiol levels in untreated, pellet-treated, and estradiolws-treated mice (56). The Opa phenotypes of 96 colonies (zero time) and 36 colonies (6 and 8 h) isolated from each culture at different time points were determined, and the fold changes compared to the inoculum are indicated. No change in the Opa phenotype was observed under any conditions. OD600, optical density at 600 nm.
The distribution of Opa phenotypes among primary vaginal isolates from estradiolws-treated mice was also consistent with the previously observed recovery pattern. All mice selected for variants that expressed OpaB early in infection, and higher proportions of gonococci that expressed more than one Opa protein were recovered in the late phase. An example of the distribution of Opa variants in vaginal isolates from one representative mouse is shown in Fig. 1A (pie charts). In this example, OpaB (pink areas) and OpaB,I (pink and blue striped areas) variants predominated in vaginal isolates on days 1 and 2 postinoculation. This early phase was followed by a 3-day period in which the relative proportion of Opa+ variants recovered decreased (middle phase). Increased recovery of gonococci that express OpaB,I and other Opa+ phenotypes then occurred on days 6 and 7 (late phase), and in this mouse evidence of a second middle phase was observed on day 8.
Untreated mice can support transient colonization with N. gonorrhoeae when they are challenged in the proestrus stage of the estrous cycle (12, 31). We therefore also examined the selection kinetics for Opa+ variants in mice that were not treated with estradiol. While not as pronounced as the data obtained for estradiolws-treated mice, the combined data from three independent experiments with untreated mice showed that there was a 2-fold or greater increase in the proportion of Opa+ variants in vaginal isolates compared to the proportion in the inoculum at day 1 postinoculation. This increase occurred in 8 of 11 mice (Fig. 1B). The average percentage of Opa+ isolates declined to a value that was less than 2-fold greater than the value obtained for the inoculum on days 2 and 3 postinoculation, and there was clearly a middle phase in 3 of 5 mice. The middle phase was followed by dramatic increases in the percentage of Opa+ variants (∼3.5- and 6-fold) isolated from the two mice that remained colonized for 4 days.
The Opa phenotypes of vaginal isolates from one infected untreated mouse are shown in Fig. 1B (pie charts). Following inoculation with a population of bacteria that consisted of 87% Opa−, 11% OpaB, 1% OpaA, and 1% OpaE/K variants, 45% of the primary vaginal isolates from this mouse were Opa+ on the first day after inoculation, and several different Opa+ variants were represented. Following a decrease in the relative number of Opa+ variants on days 2 and 3, 83% of the isolates on day 4 expressed one Opa protein (solid colors) or several Opa proteins simultaneously (striped patterns), and the majority of the isolates expressed OpaI (dark blue area). A similar pattern was observed in a second mouse that was colonized through day 4, although the predominant Opa phenotype on day 4 was OpaE/K (69% of isolates). These results are consistent with the three phases of recovery seen in estradiol-treated mice and show that Opa-expressing variants have a clear advantage in vivo over time.
The timing of each phase of the recovery pattern was different in untreated, pelleted, and estradiolws-treated mice. Additionally, the early and middle phases observed in untreated mice were less dramatic than those observed in estradiolws-treated mice (Fig. 1A). The latter observation could have been due to the fact that the appearance and subsequent loss of the selective factor may be more rapid or less uniform in untreated mice than in estradiol-treated mice. Alternatively, estradiol itself may select for Opa expression, which could explain the differences in degree of selection and selection kinetics in these three different experimental models. We therefore tested whether estradiol directly selects for Opa+ variants in vitro. We found no change in the proportion of Opa+ variants relative to the proportion in the starting inoculum when a suspension of primarily Opa− gonococci was cultured in broth that contained 17β-estradiol at concentrations that are similar to the peak serum estradiol levels in pellet-treated, estradiolws-treated, and untreated mice (Fig. 1C). We concluded there is an endogenous host factor in the lower genital tract of female mice that selects for distinct subpopulations with different Opa phenotypes in a cyclical pattern. Estradiol itself is not directly responsible for the pattern observed, although the selective factor(s) appears to be modulated in vivo by the administration of 17β-estradiol.
The cyclical recovery pattern is dependent on the ovaries.
Cyclical fluctuations in the number of gonococci cultured from vaginal mucus and in the Opa phenotype of vaginal isolates suggests that hormonal factors challenge N. gonorrhoeae during murine infection. Ovariectomiced (Ov−) mice can be infected with N. gonorrhoeae, although 17β-estradiol and antibiotic treatment is required (30), and these mice can be used to study different aspects of gonococcal infection in the absence of the reproductive cycle. To test the role of the reproductive cycle in the cyclical recovery pattern, we inoculated estradiol-treated intact and Ov− mice with a defined suspension containing primarily Opa− variants and determined the numbers of CFU recovered and the Opa phenotypes of vaginal isolates over time. Selection for Opa+ variants occurred in both intact and Ov− mice on day 1 of infection. The cyclical recovery pattern was then observed in intact mice, as expected, with increased percentages of Opa+ variants in the early and late phases (Fig. 2A) corresponding to periods when there were increased numbers of recovered total CFU (Fig. 2B). In contrast, selection for Opa+ gonococci was maintained in Ov− mice (Fig. 2A), and the number of gonococci recovered was constant throughout the 15-day experiment (Fig. 2B). We concluded that changes in Opa protein selection and the colonization load, which together define the cyclical recovery pattern, are influenced directly or indirectly by ovarian factors.
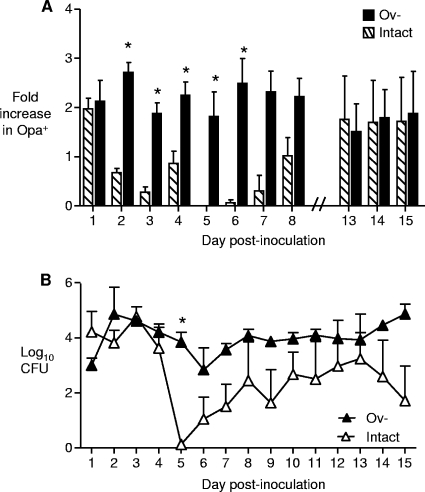
FIG. 2.
Normal (intact) mice, but not Ov− mice, show a cyclical recovery pattern. Five-milligram 17β-estradiol pellets were implanted in members of groups of intact or Ov− mice, and the mice were inoculated with 106 CFU of a suspension containing 70% Opa−, 23% OpaB, 3% OpaC, 2% OpaF, and 1% OpaF and OpaI variants. The Opa phenotype of vaginal isolates and the number of gonococci recovered were determined over time. (A) Opa phenotype of vaginal isolates. Selection for Opa expression, which was defined as a ≥2-fold increase in the percentage of Opa+ variants compared to the inoculum, occurred in both groups on day 1. However, the subsequent loss and reemergence of Opa+ variants that occurred in intact mice did not occur in Ov− mice. (B) Total number of CFU recovered over time. Fluctuations in the total number of CFU recovered from vaginal swab suspensions were observed in intact mice, as reported previously (47), and the dramatic decline that characterized the middle phase occurred on days 5 to 8. Ov− mice showed more uniform recovery of viable gonococci over time. An asterisk indicates a significant difference (P < 0.05) between the numbers of gonococci recovered from groups, and the error bars indicate the standard errors of the means. In the experiment whose results are shown, there were 3 mice per group, except for intact mice on days 11 to 15, for which results for 2 mice are shown. The third mouse was not colonized at these time points. The results were consistent in a second experiment performed with 5 mice per group.
Opa proteins provide a colonization advantage in both the early and late stages of infection.
Due to the strong selection for Opa+ gonococci in the early phase of infection, we hypothesized that Opa protein expression confers an advantage soon after bacteria enter the vagina and that Opa− gonococci would not colonize mice as well as Opa-expressing gonococci. To test this hypothesis, we utilized a genetically defined mutant of strain FA1090 in which all of the opa genes were inactivated (FA1090opaA-K) and a derivative of this Opa-deficient mutant into which a phase-locked opaB gene was reintroduced [FA1090opaA-K(B+)]. The growth kinetics of strains FA1090opaA-K and FA1090opaA-K(B+) were similar when these organisms were cultured in liquid media (Fig. 3A). Therefore, next we compared the capacities of these two strains to infect estradiol-treated mice. Mice were inoculated with 106 CFU of one of these strains, and vaginal mucus was cultured over time. Consistent with the hypothesis that Opa protein expression confers a colonization or survival advantage early during infection, a higher number of bacteria was recovered from mice inoculated with the OpaB-expressing strain on day 1 than from mice inoculated with the Opa-deficient strain (P < 0.05) (Fig. 3B). There was a decrease in the number of bacteria recovered in both groups on days 4 to 7, which is typical of the middle phase. A steady increase in the recovery of OpaB-expressing bacteria then began on day 8 and lasted through day 14 in 12/16 mice, which is temporally consistent with the late phase. The number of gonococci recovered was significantly higher for mice inoculated with the OpaB-expressing strain than for mice inoculated with the Opa-deficient mutant on days 9 to 12 and 14 (P < 0.05). Additionally, in contrast to the results for the mice infected with the OpaB-expressing strain, the increase in the number of CFU that typifies the late stage occurred in only 5/16 mice infected with the Opa-deficient strain following this period of reduced bacterial recovery. A comparison of the colonization curves further demonstrated that strain FA1090opaA-K(B+) was more fit than strain FA1090opaA-K in terms of establishing persistent infection; on day 14 69% and 31% of mice were infected with these strains, respectively (P = 0.037) (Fig. 3C).
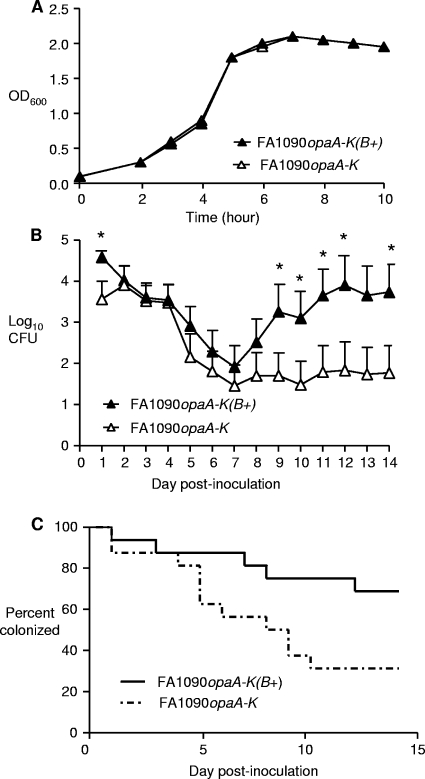
FIG. 3.
OpaB-expressing strain colonizes 17β-estradiol-treated mice better than Opa-deficient mutant. The Opa-deficient strain FA1090opaA-K and the OpaB-expressing strain FA1090opaA-K(B+) were compared to examine differences in growth in liquid culture and the capacity to colonize estradiol-treated mice. (A) Optical density at 600 nm (OD600) for GCB cultures inoculated with FA1090opaA-K or FA1090opaA-K(B+) bacteria over time. (B) Average number of CFU recovered by vaginal swabbing following inoculation of estradiol-treated mice with 106 CFU of Opa-deficient or OpaB-expressing bacteria. The error bars indicate the standard errors of the means, and the asterisks indicate significant differences between groups (P < 0.05, Student's t test). (C) Percentage of mice colonized on each day following inoculation with strain FA1090opaA-K and with strain FA1090opaA-K(B+). Significantly more mice were culture positive following inoculation with the constitutive OpaB-expressing strain than following inoculation with the Opa-deficient strain (P = 0.037, log rank test). The results in panels A and B are results from two combined experiments, and each symbol represents 16 mice.
These results are the first demonstration of the importance of Opa proteins during infection using defined mutants in an in vivo model. We concluded that (i) OpaB provides an early advantage during infection of female mice, (ii) the factors that are responsible for the decreased recovery of viable bacteria in the middle phase appear to be Opa independent based on the decreased recovery of both the Opa-deficient and Opa-expressing strains on days 5 to 8 postinoculation, and (iii) Opa-deficient gonococci are less able to recover from the middle phase, presumably due to a factor that appears later in infection, which gives Opa-expressing gonococci a selective advantage in vivo.
OpaB does not mediate invasion of murine tissue culture cells.
In addition to members of the human CEACAM family, other receptors for Opa-mediated adherence and uptake have been described (for reviews, see references 13 and 40). The presence of an Opa-specific colonization receptor in the murine lower genital tract could explain the in vivo selection for Opa-expressing bacteria. Immortalized murine epithelial cells of vaginal, cervical, or endometrial origin have not been described; therefore, to examine this hypothesis, we compared the capacities of the OpaB-expressing strain FA1090opaA-K(B+) and the Opa-deficient strain FA1090opaA-K to adhere to and invade murine IEC4.1 (intestinal) and BM1.11 (oviduct) cells. We also tested the ME180 cell line, which is a CEACAM-expressing human endocervical cell line, and the HEC1B cell line, which is a human CEACAM-negative endometrial cell line that supports Opa-dependent invasion (59), as controls. Both human cell lines supported Opa-dependent invasion, as shown by the fact that significantly more OpaB-expressing bacteria than Opa-deficient bacteria were recovered following Gm treatment (Fig. 4). Tenfold more internalized OpaB-expressing gonococci were recovered from ME180 cells than from HEC1B cells, a result that is consistent with Opa protein-CEACAM interactions mediating more efficient uptake. There was no difference in the numbers of gonococci recovered from IEC4.1 and BM1.11 murine cells following Gm treatment (Fig. 4). This result suggests that murine cells do not support Opa-dependent invasion. We also found no difference in the capacities of the Opa+ and Opa− strains to adhere to IEC4.1 or BM1.11 cells, and the numbers of cell-associated bacteria were similar for murine and human epithelial cells (data not shown). These results are inconsistent with the hypothesis that the in vivo advantage exhibited by strain FA1090opaA-K(B+) was due to OpaB-mediated adherence or invasion. However, we cannot rule out the possibility that Opa-specific colonization receptors may be expressed by epithelial cells in the murine genital tract during infection.
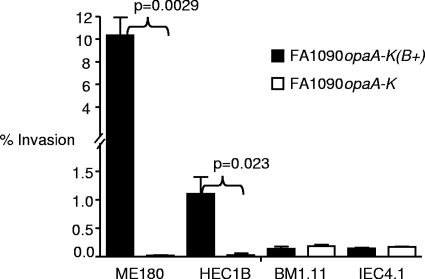
FIG. 4.
OpaB-mediated invasion occurs in immortalized human tissue culture cells but not in murine tissue culture cells. The capacities of strains FA1090opaA-K(B+) and FA1090opaA-K to invade human (ME180 and HEC1B) and mouse (IEC4.1 and BM1.11) cells were compared using the Gm protection assay. A higher number of FA1090opaA-K(B+) CFU than of FA1090opaA-K CFU was recovered from both human cell lines after Gm treatment, and there was 10-fold-greater internalization of the Opa-expressing strain by the CEACAM-expressing ME180 cells than by the CEACAM-negative HEC1B cells. No difference in invasion was detected in either mouse cell line for FA1090opaA-K(B+) or FA1090opaA-K. No difference in the number of cell-associated gonococci was observed between the two strains for any cell line (data not shown). The level of invasion was calculated by comparison with the number of adherent bacteria recovered before Gm treatment (data not shown). The error bars indicate the standard errors of the means for three independent wells. Similar results were obtained in three independent experiments.
C3 depletion does not affect the early selection phase.
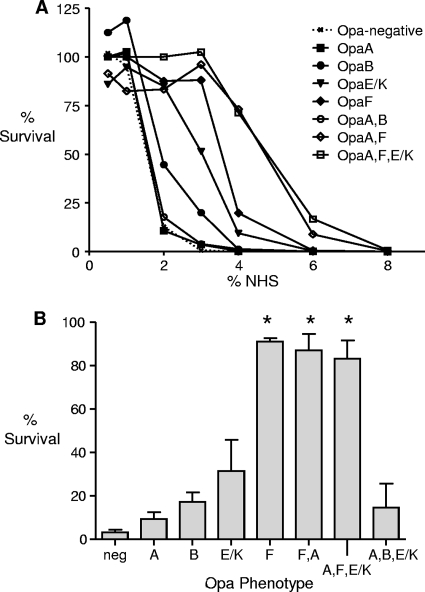
Hormonal regulation of complement levels in the female genital tracts of humans (21) and mice (36) is well established, and Bos et al. (5) reported that Opa+ variants of N. gonorrhoeae strain MS11 were more resistant to the bactericidal activity of low concentrations of NHS. Strain FA1090, in contrast to strain MS11, is highly resistant to NHS, and our attempts to demonstrate Opa-mediated increases in serum resistance in strain FA1090 were unsuccessful. The high level of serum resistance in strain FA1090 is due to porin-mediated binding of human C4b-binding protein (C4BP) (46). Because this form of serum resistance is host restricted (41) and does not occur during murine infection, it seemed prudent to further investigate the role of Opa proteins in evading host complement in a serum-sensitive strain. We therefore utilized strain FA1090F62por5-8, which is a serum-sensitive derivative of strain FA1090 that does not bind human C4BP due to an altered porB gene. Gonococci that express OpaA, OpaB, OpaE/K, OpaF, or OpaI are frequently isolated from mice (28, 52), and therefore OpaA, OpaB, OpaE/K, and OpaF variants were isolated from strain FA1090F62por5-8 for these studies. OpaI variants, which aggregate at a high level and thus form very opaque colonies, were not used due to the risk that clumping would interfere with the results. We also isolated variants that express two or three Opa proteins simultaneously to measure the effect of the multiple-expression phenotype on serum resistance. The 50% bactericidal titers of NHS ranged from 1.8% (Opa−, OpaA, and OpaA,B) to 5.1% (OpaA,F and OpaA,F,E/K) when the results of assays in which two to four different variants of strain FA1090F62por5-8 were tested (Fig. 5A). As titration of the bactericidal activity of serum is too labor-intensive to test all variants in the same experiment, we also tested a single concentration of NHS, 3%, which fell in the linear region of the bactericidal curves. A similar resistance gradient was observed, although only OpaF, OpaA,F, and OpaA,F,E/K variants were statistically more resistant to NHS than Opa− variants when combined results from at least three independent experiments were compared (P < 0.001) (Fig. 5B). A multiple-expression phenotype (OpaA,F and OpaA,F,E/K) did not increase serum resistance compared to expression of OpaF alone when 3% NHS was tested.
FIG. 5.
Expression of some FA1090 Opa proteins is associated with increased resistance to NHS. The bactericidal activity of NHS was tested using different Opa variants of the serum-sensitive derivative FA1090F62por5-8. (A) Plot of the level of survival of each variant tested versus the dilution of NHS tested. The representative data from several independent assays show that there was an increase in the 50% bactericidal titer compared to the 50% bactericidal titer for Opa− variants for gonococci that express OpaB, OpaE/K, OpaF, OpaA,F, and OpaA,F,E/K but not for variants that express OpaA or OpaA,B. The 50% bactericidal titers were 1.8% (Opa−, OpaA, and OpaA,B), 2.2% (OpaB), 3.4% (OpaE/K), 3.8% (OpaF), and ca. 5.1% (OpaA,F and OpaA,F,E/K). (B) Levels of survival of different Opa variants following incubation in 3% NHS. The results are expressed as the average levels of survival when data from at least three independent experiments were combined. The error bars indicate the standard errors of the means, and the asterisks indicate that there was a significant difference (P < 0.0001). The level of survival was calculated by dividing the number of CFU recovered from wells with NHS by the number of CFU recovered from wells with the same concentration of HI-NHS and multiplying by 100.
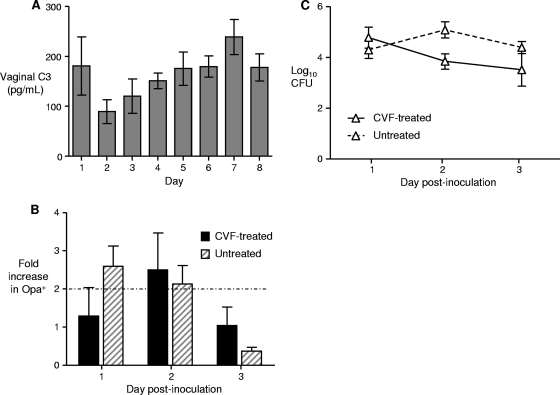
Murine complement is very labile, and thus mouse serum cannot be used to test the bactericidal activity of low concentrations of complement, such as the concentration which is present at mucosal surfaces. To further analyze the role of complement as a selective factor for Opa protein expression, we measured the level of C3 in vaginal washes from uninfected, estradiol-treated mice. In two experiments, the average vaginal C3 concentration appeared to decline at the time point that corresponded to day 2 of infection and then gradually increased through day 8, although there was great variability among mice and no statistically significant differences between time points were detected (Fig. 6A). This result suggests that the basal levels of complement are highest at the time points that correspond to the early and late phases of infection. To more directly test the potential of complement to select for Opa+ variants during infection, we next treated mice with cobra venom factor (CVF) on the day prior to inoculation to deplete C3 and then challenged them with a suspension of wild-type gonococci that contained 80% Opa− variants. A 2-fold increase in the level of Opa+ variants occurred on day 1 or 2 postinoculation in the CVF-treated group and the PBS-treated control group, a result that suggests that complement does not mediate the selection for Opa expression that occurs early in infection (Fig. 6B). We also inoculated mice treated with CVF or PBS as described above with the Opa-deficient strain, predicting that the level of recovery of the mutant would increase in the early phase of infection if Opa− gonococci were susceptible to complement in vivo. We found no difference in the numbers of CFU recovered from CVF-treated mice and from PBS-treated mice on days 1 to 3 (Fig. 6C). From these results, we concluded that complement does not select for Opa protein expression in the early phase of the cyclical recovery pattern.
FIG. 6.
Fluctuations in vaginal C3 levels occur in uninfected mice, but C3 depletion does not abrogate the early selection for Opa+ variants. (A) Concentration of C3 in vaginal washes from uninfected mice that were treated with 5-mg 17β-estradiol slow-release pellets. A significantly higher concentration of C3 was present in vaginal washes from days 7 and 8 (late phase) than in vaginal washes from days 3 to 5 (middle phase). The results of two independent experiments in which 10 to 13 mice per day were used were combined. (B) Recovery of Opa+ variants in mice treated with CVF to deplete C3 the day prior to inoculation with wild-type bacteria. Control mice were treated with PBS. Mice were challenged with a predominantly Opa− (80%) suspension of wild-type FA1090 gonococci, and the proportion of Opa+ variant colonies among colonies isolated from vaginal mucus was determined on days 1 to 3 postinoculation. Selection for Opa expression, as defined as a ≥2-fold increase compared to the inoculum (dotted line), occurred in both groups on day 1 or day 2 after bacterial challenge. The results of a single experiment in which there were 3 or 4 mice per group are shown. (C) Effect of C3 depletion on the recovery of strain FA1090opaA-K. There was no difference in the average numbers of Opa-deficient gonococci recovered from mice treated with CFU (solid line) and mice treated with PBS (dashed line) on days 1 to 3 of infection. The results are the results of a single experiment performed with 7 mice per group, and similar results were obtained in a second independent experiment. In all panels, the error bars indicate the standard errors of the means.
Wild-type Opa+ variants and the constitutive OpaB-expressing strain are more susceptible to growth inhibition by serine proteases.
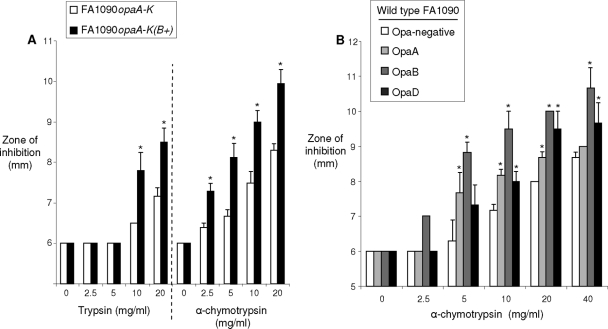
Several proteases are differentially expressed during the mammalian reproductive cycle (34). Proteases may challenge Opa+ gonococci more than Opa− gonococci during infection based on reports by Swanson and colleagues that gonococci in opaque colonies were more sensitive to the serine proteases than transparent variants (3, 27, 57). As several Opa proteins of strain FA1090 (OpaB, OpaE, and OpaK) do not confer detectable photoopacity to a colony, we reexamined this hypothesis using bacteria with known Opa phenotypes. Consistent with the previous report, the Opa-deficient strain FA1090opaA-K was less susceptible than the complemented OpaB-expressing strain to growth inhibition when a trypsin concentration of 10 mg/ml or 20 mg/ml was used (Fig. 7A). A broader dose response was observed when α-chymotrypsin was tested, and statistically significant differences were detected at concentrations of ≤2.5 mg/ml. We also found that OpaA, OpaB, and OpaD wild-type variants were more susceptible to α-chymotrypsin than Opa-negative gonococci (Fig. 7B). Because Opa+ gonococci are selected for in vivo, we hypothesize that protease inhibitors, which can also fluctuate over the course of the reproductive cycle (34), may protect Opa+ gonococci from proteases expressed early and late in infection. This hypothesis does not adequately explain the selection for Opa expression that was observed in vivo, however, since presumably Opa− gonococci would also be protected by protease inhibitors.
FIG. 7.
Opa+ gonococci are more susceptible to growth inhibition by serine proteases. A disk diffusion assay was used to measure the sensitivities of recombinant and wild-type gonococci to trypsin or α-chymotrypsin. Opa+ gonococci were more susceptible to growth inhibition when they were exposed to serine proteases. (A) Zones of inhibition for the Opa-deficient mutant FA1090opaA-K and the complemented OpaB-expressing mutant FA1090opaA-K(B+) with different amounts of trypsin and α-chymotrypsin. (B) Zones of inhibition for wild-type Opa-negative gonococci and OpaA, OpaB, and OpaD variants with different amounts of α-chymotrypsin. The average zones of inhibition (mm) are indicated by the bars, and the error bars indicate standard errors. The disk diameter was 6 mm. The asterisks indicate statistical significance (P ≤ 0.05).
DISCUSSION
The capacity to maintain reservoirs of bacteria that differ in expression of surface molecules is a major adaptation mechanism of N. gonorrhoeae. The neisserial Opa proteins exhibit phase-variable expression, and we previously reported cyclical recovery of Opa+ variants from the lower genital tract of female mice (52), a result that is consistent with Opa+ and Opa− phenotypes having distinct roles during infection. Here we showed that administration of estradiol, which is used to promote susceptibility to N. gonorrhoeae in mice, is not required for cyclical recovery of Opa+ gonococci. We also showed that early selection for Opa expression also occurs in mice that lack ovaries but is maintained at a constant level over 15 days. We concluded the reversible expression of Opa proteins allows N. gonorrhoeae to evade or capitalize on hormonally driven changes in the female reproductive tract. This finding is the first experimental evidence from a highly reproducible infection model that supports speculation based on data for human cervical isolates (27) concerning the importance of opa gene phase variation during infection of women.
The results of experiments with Ov− mice further illuminated the basis for the decrease in the total number of gonococci recovered that occurs during the middle phase of infection. We previously referred to the middle phase as the culture-negative “window” (28) since often no gonococci are recovered during this period. Here we showed that, in contrast to what occurs in intact mice, there was no decrease in the total number of gonococci during this time period when ovariectomized mice were used. This observation suggests that hormonally regulated host factors are responsible for the reduced recovery of gonococci during this phase. Hormonally regulated proteases could be expressed during this time period and effectively inhibit the growth of N. gonorrhoeae. A second hypothesis is that unidentified effectors of the innate response are responsible for the middle phase based on evidence that receptors of the innate response, including Toll-like receptors, are hormonally regulated (23, 47). This hypothesis is currently our favored hypothesis based on a recent report from our laboratory in collaboration with Robin Ingalls (Boston University Medical School) that signaling through Toll-like receptor 4 partially protects against N. gonorrhoeae (42). We also cannot rule out the possibility that bacterial invasion may contribute to the decrease in recoverable bacteria during the middle phase. Tissue remodeling in preparation for implantation or for sloughing when fertilization does not occur is orchestrated by hormonally regulated proteases. Such proteases may directly select for Opa− variants or open an avenue for bacterial invasion. Interestingly, Edwards recently reported that progesterone increases gonococcal survival within epithelial cells (16), a finding that is also consistent with the possibility that there are times when gonococci may be less accessible for culture. Gonococci are seen within vaginal and cervical tissue as early as 2 days postinfection in the mouse model (56), and whether bacterial invasion into tissue contributes to the cyclical recovery pattern is not known. We attempted to use polarized HEC1B cells to determine whether Opa proteins mediate invasion across an epithelial cell layer in the absence of CEACAMs. However, the HEC1B monolayers did not maintain their integrity for longer than 2 h, as measured by a horseradish peroxidase leakage assay (62), which was an inadequate time period to evaluate this possibility.
The decreased isolation of gonococci in intact mice that characterizes the middle phase is consistent with the reported association between the hormonal state and the culture rate for women with gonorrhea. Women with gonorrhea are more often culture positive in the proliferative stage of the menstrual cycle and culture negative in the middle secretory phase (27, 32, 35, 38). In one study, 4 infected women with normal menstrual cycles were not treated and hospitalized so the recovery of N. gonorrhoeae could be followed over the course of a full cycle. Positive cultures correlated strongly with the proliferative and ovulatory phases, and all 4 women had five or six consecutive negative cultures during the secretory phase. Cultures became positive again in the premenstrual phase or during menses (35). The factors responsible for these culture-negative periods are not known but are likely related to the reproductive cycle.
A second important finding was our demonstration that Opa proteins promote persistence of N. gonorrhoeae in the lower genital tract of female mice using genetically engineered bacterial strains. Mice infected with bacteria that constitutively express OpaB were colonized longer than mice infected with an isogenic Opa-deficient strain. The Opa+ strain also colonized mice at higher levels early in infection and after the period of reduced isolation during the middle phase. These results confirm the prediction based on the results of our experiments with wild-type N. gonorrhoeae that Opa protein expression is advantageous early and late during infection. Studies with wild-type gonococci also suggested that Opa− variants have an advantage during the middle phase. To our surprise, however, the middle phase appeared to be Opa independent when defined mutants were tested since the recovery of both Opa-deficient and Opa-expressing strains was reduced on days 5 to 8 after inoculation. Because other workers have reported an association between the isolation of opaque and transparent human cervical isolates and different stages of the menstrual cycle (27), we hypothesized that the forces selecting for the Opa− phenotype during the middle phase may be subtle and detectable only when the relative numbers of Opa− and Opa+ gonococci in the same mouse are compared.
The final outcome of this work was the generation of data that do not support two well-characterized Opa protein functions as the basis for the selection for Opa+ variants during murine infection. Opa proteins mediate adherence and invasion of cultured human cells via interactions with different receptors, and these interactions have been fully investigated at the molecular level (4, 6, 45, 61). Here, however, we found no difference in the capacities of Opa+ and Opa− gonococci to adhere to or invade cultured murine cells and thus have no evidence that Opa proteins act as colonization factors in the mouse model. We cannot rule out the possibility that there is an Opa-specific colonization receptor in the murine genital tract, which is not expressed by the mouse cell lines used but selects for Opa expression in vivo. The best-characterized Opa receptors are the human CEACAMs. Mice express only CEACAM1, which differs from human CEACAM1 in several amino acids shown to be critical for Opa-mediated binding (60, 61). It is therefore unlikely that Opa-mediated colonization via murine CEACAMs selects for Opa+ variants during murine infection. Some Opa proteins mediate uptake by epithelial cells via binding to heparin sulfate proteoglycans (HSPG) (40), and HSPG is present on genital tract tissues (22). However, the only Opa protein of strain FA1090 that binds HSPG is OpaI; OpaB, which was the main Opa phenotype selected in our study, binds human CEACAMs (17). We also found that although OpaI variants are selected in mice following inoculation with a suspension containing mostly Opa− variants (52), inoculation of mice with a suspension containing similar numbers of OpaI and OpaB variants did not result in preferential selection for OpaI during infection (J. G. Cole and A. E. Jerse, unpublished observation). Therefore, the importance of Opa protein-HSPG interactions during murine infection is questionable.
Whether Opa-CEACAM interactions are responsible for or contribute to the selection for Opa+ gonococci in the human cervix or the male urethra (27, 29, 58) is also not clear. The tissue distribution of the major class of Opa protein adherence receptors, the human CEACAMs, does not correlate with the predominant sites of gonococcal infection in humans. For example, limited or no CEACAM expression was detected on primary male urethral epithelial cells (20) and some normal cervical and fallopian tube cells (59).
A second hypothesis was that host complement is responsible for early selection for Opa+ variants early and late during infection based on the known hormonal regulation of complement in female mice and women of reproductive age (21, 36) and the demonstration of Opa-mediated serum resistance in another strain. Serum resistance is a complicated phenotype in N. gonorrhoeae due the existence of several mechanisms that are not expressed by all strains. We found that in the absence of porin-mediated resistance in strain FA1090, a highly serum-resistant strain, some Opa proteins, either alone or in conjunction with other Opa proteins, conferred increased resistance to concentrations of NHS that reasonably mimic the concentration of complement at mucosal surfaces. Whether the higher 50% bactericidal titers that we observed are biologically significant is not known; however, at one NHS concentration tested, 3%, the level of survival for OpaB and OpaE/K variants, which showed cyclical recovery here and in our previous studies (52), was not significantly greater than that for Opa− bacteria. In studies with strain MS11, expression of certain phase-variable species of lipooligosaccharide (LOS) was phenotypically dominant over expression of Opa proteins for mediating serum resistance (5). We saw no difference in the LOS species produced by the Opa variants tested here or the Opa-deficient and constitutive OpaB-expressing strains (data not shown), however, and thus our results are unlikely to be confounded by differences in LOS phenotype. We also examined whether complement selects for Opa+ gonococci early in infection using C3-depleted mice. Depletion of C3 to a level that was 90 to 95% of the level found in untreated mice did not abrogate the early selection for Opa+ gonococci. We concluded that while complement may contribute to the selection of Opa variants during murine infection, it does not mediate the early selection of Opa+ variants. The use of complement-deficient mice would be a more stringent test of our hypothesis; however, C3- and C5-deficient mice are available only in the C57/BL6 background, and we have not characterized Opa protein expression in C57/BL6 mice.
In conclusion, the host factors responsible for Opa selection during murine infection remain elusive. The female genital tract is a dynamic environment in which multiple effectors may play a selective role. Hormonally regulated changes in innate receptors (23, 47), the levels of antimicrobial peptides (34), lactoferrin (9), pH, the viscosity of mucus, and the number and composition of commensal organisms (8) also occur. One or more of these factors may differentially select for Opa+ or Opa− gonococci, or a combination of factors, such as the reported synergy between lactoferrin, lysozyme, and serine leukocyte protease inhibitor (SLPI) against E. coli (53), may be required. Continued investigation of the basis of the cyclical recovery pattern that we describe here should further elucidate how N. gonorrhoeae, a well-adapted pathogen, has evolved to evade or capitalize on hormonally driven factors within the female genital tract.
Acknowledgments
This work was supported by NIH/NIAID grant AI42053.
We thank Cara Olsen for assistance with statistical analysis and Janne Cannon for helpful reading of the manuscript.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 25 January 2010.
REFERENCES
- 1.Al-Suleiman, S. A., E. M. Grimes, and H. S. Jonas. 1983. Disseminated gonococcal infections. Obstet. Gynecol. 61:48-51. [PubMed] [Google Scholar]
- 2.Bhat, K. S., C. P. Gibbs, O. Barrera, S. G. Morrison, F. Jahnig, A. Stern, E. M. Kupsch, T. F. Meyer, and J. Swanson. 1991. The opacity proteins of Neisseria gonorrhoeae strain MS11 are encoded by a family of 11 complete genes. Mol. Microbiol. 5:1889-1901. [DOI] [PubMed] [Google Scholar]
- 3.Blake, M. S., E. C. Gotschlich, and J. Swanson. 1981. Effects of proteolytic enzymes on the outer membrane proteins of Neisseria gonorrhoeae. Infect. Immun. 33:212-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bos, M. P., F. Grunert, and R. J. Belland. 1997. Differential recognition of members of the carcinoembryonic antigen family by Opa variants of Neisseria gonorrhoeae. Infect. Immun. 65:2353-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bos, M. P., D. Hogan, and R. J. Belland. 1997. Selection of Opa+ Neisseria gonorrhoeae by limited availability of normal human serum. Infect. Immun. 65:645-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bos, M. P., M. Kuroki, A. Krop-Watorek, D. Hogan, and R. J. Belland. 1998. CD66 receptor specificity exhibited by neisserial Opa variants is controlled by protein determinants in CD66 N-domains. Proc. Natl. Acad. Sci. U. S. A. 95:9584-9589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boulton, I. C., and S. D. Gray-Owen. 2002. Neisserial binding to CEACAM1 arrests the activation and proliferation of CD4+ T lymphocytes. Nat. Immunol. 3:229-236. [DOI] [PubMed] [Google Scholar]
- 8.Braude, A. I. 1982. Maxwell Finland lecture. Resistance to infection with the gonococcus. J. Infect. Dis. 145:623-624. [DOI] [PubMed] [Google Scholar]
- 9.Cohen, M. S., B. E. Britigan, M. French, and K. Bean. 1987. Preliminary observations on lactoferrin secretion in human vaginal mucus: variation during the menstrual cycle, evidence of hormonal regulation, and implications for infection with Neisseria gonorrhoeae. Am. J. Obstet. Gynecol. 157:1122-1125. [DOI] [PubMed] [Google Scholar]
- 10.Cohen, M. S., J. G. Cannon, A. E. Jerse, L. M. Charniga, S. F. Isbey, and L. G. Whicker. 1994. Human experimentation with Neisseria gonorrhoeae: rationale, methods, and implications for the biology of infection and vaccine development. J. Infect. Dis. 169:532-537. [DOI] [PubMed] [Google Scholar]
- 11.Connell, T. D., D. Shaffer, and J. G. Cannon. 1990. Characterization of the repertoire of hypervariable regions in the protein II (opa) gene family of Neisseria gonorrhoeae. Mol. Microbiol. 4:439-449. [DOI] [PubMed] [Google Scholar]
- 12.Dalal, S. J., J. S. Estep, I. E. Valentin-Bon, and A. E. Jerse. 2001. Standardization of the Whitten effect to induce susceptibility to Neisseria gonorrhoeae in female mice. Contemp. Top. Lab. Anim. Sci. 40:13-17. [PubMed] [Google Scholar]
- 13.Dehio, C., S. D. Gray-Owen, and T. F. Meyer. 1998. The role of neisserial Opa proteins in interactions with host cells. Trends Microbiol. 6:489-495. [DOI] [PubMed] [Google Scholar]
- 14.Dempsey, J. A., W. Litaker, A. Madhure, T. L. Snodgrass, and J. G. Cannon. 1991. Physical map of the chromosome of Neisseria gonorrhoeae FA1090 with locations of genetic markers, including opa and pil genes. J. Bacteriol. 173:5476-5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Draper, D. L., J. F. James, G. F. Brooks, and R. L. Sweet. 1980. Comparison of virulence markers of peritoneal and fallopian tube isolates with endocervical Neisseria gonorrhoeae isolates from women with acute salpingitis. Infect. Immun. 27:882-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edwards, J. L. Neisseria gonorrhoeae survival during primary, human, cervical epithelial cell infection requires nitric oxide and is augmented by progesterone. Infect. Immun., in press. [DOI] [PMC free article] [PubMed]
- 17.Fulcher, N. B. 2004. The role of Neisseria gonorrhoeae opacity proteins in host cell interactions and pathogenesis. Ph.D. dissertation. University of North Carolina, Chapel Hill, NC.
- 18.Garvin, L. E., M. C. Bash, C. Keys, D. M. Warner, S. Ram, W. M. Shafer, and A. E. Jerse. 2008. Phenotypic and genotypic analyses of Neisseria gonorrhoeae isolates that express frequently recovered PorB PIA variable region types suggest that certain P1A porin sequences confer a selective advantage for urogenital tract infection. Infect. Immun. 76:3700-3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Handsfield, H. H. 1975. Disseminated gonococcal infection. Clin. Obstet. Gynecol. 18:131-142. [DOI] [PubMed] [Google Scholar]
- 20.Harvey, H. A., M. P. Jennings, C. A. Campbell, R. Williams, and M. A. Apicella. 2001. Receptor-mediated endocytosis of Neisseria gonorrhoeae into primary human urethral epithelial cells: the role of the asialoglycoprotein receptor. Mol. Microbiol. 42:659-672. [DOI] [PubMed] [Google Scholar]
- 21.Hasty, L. A., J. D. Lambris, B. A. Lessey, K. Pruksananonda, and C. R. Lyttle. 1994. Hormonal regulation of complement components and receptors throughout the menstrual cycle. Am. J. Obstet. Gynecol. 170:168-175. [DOI] [PubMed] [Google Scholar]
- 22.Hayashi, K., M. Hayashi, E. Boutin, G. R. Cunha, M. Bernfield, and R. L. Trelstad. 1988. Hormonal modification of epithelial differentiation and expression of cell surface heparan sulfate proteoglycan in the mouse vaginal epithelium. An immunohistochemical and electron microscopic study. Lab. Invest. 58:68-76. [PubMed] [Google Scholar]
- 23.Hirata, T., Y. Osuga, K. Hamasaki, Y. Hirota, E. Nose, C. Morimoto, M. Harada, Y. Takemura, K. Koga, O. Yoshino, T. Tajima, A. Hasegawa, T. Yano, and Y. Taketani. 2007. Expression of Toll-like receptors 2, 3, 4, and 9 genes in the human endometrium during the menstrual cycle. J. Reprod. Immunol. 74:53-60. [DOI] [PubMed] [Google Scholar]
- 24.Hitchcock, P. J. 1984. Analyses of gonococcal lipopolysaccharide in whole-cell lysates by sodium dodecyl sulfate-polyacrylamide gel electrophoresis: stable association of lipopolysaccharide with the major outer membrane protein (protein I) of Neisseria gonorrhoeae. Infect. Immun. 46:202-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holmes, K. K., G. W. Counts, and H. N. Beaty. 1971. Disseminated gonococcal infection. Ann. Intern. Med. 74:979-993. [DOI] [PubMed] [Google Scholar]
- 26.Hook, E. W., and H. H. Handsfield. 1999. Gonococcal infections in the adult, p. 451-466. In K. K. Holmes, P.-A. Mardh, P. F. Sparling, S. M. Lemon, W. E. Stamm, P. Piot, and J. N. Wasserheit (ed.), Sexually transmitted diseases, 3rd ed. McGraw-Hill, New York, NY.
- 27.James, J. F., and J. Swanson. 1978. Studies on gonococcus infection. XIII. Occurrence of color/opacity colonial variants in clinical cultures. Infect. Immun. 19:332-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jerse, A. E. 1999. Experimental gonococcal genital tract infection and opacity protein expression in estradiol-treated mice. Infect. Immun. 67:5699-5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jerse, A. E., M. S. Cohen, P. M. Drown, L. G. Whicker, S. F. Isbey, H. S. Seifert, and J. G. Cannon. 1994. Multiple gonococcal opacity proteins are expressed during experimental urethral infection in the male. J. Exp. Med. 179:911-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jerse, A. E., N. D. Sharma, A. N. Simms, E. T. Crow, L. A. Snyder, and W. M. Shafer. 2003. A gonococcal efflux pump system enhances bacterial survival in a female mouse model of genital tract infection. Infect. Immun. 71:5576-5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson, A. P., M. Tuffrey, and D. Taylor-Robinson. 1989. Resistance of mice to genital infection with Neisseria gonorrhoeae. J. Med. Microbiol. 30:33-36. [DOI] [PubMed] [Google Scholar]
- 32.Johnson, D. W., K. K. Holmes, P. A. Kvale, C. W. Halverson, and W. P. Hirsch. 1969. An evaluation of gonorrhea case findings in the chronically infected female. Am. J. Epidemiol. 90:438-448. [DOI] [PubMed] [Google Scholar]
- 33.Johnston, D. M., and J. G. Cannon. 1999. Construction of mutant strains of Neisseria gonorrhoeae lacking new antibiotic resistance markers using a two gene cassette with positive and negative selection. Gene 236:179-184. [DOI] [PubMed] [Google Scholar]
- 34.King, A. E., H. O. Critchley, and R. W. Kelly. 2003. Innate immune defences in the human endometrium. Reprod. Biol. Endocrinol. 1:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koch, M. L. 1947. A study of cervical cultures take in cases of acute gonorrhea with special reference to the phases of the menstrual cycle. Am. J. Obstet. Gynecol. 54:861-866. [DOI] [PubMed] [Google Scholar]
- 36.Li, S. H., H. L. Huang, and Y. H. Chen. 2002. Ovarian steroid-regulated synthesis and secretion of complement C3 and factor B in mouse endometrium during the natural estrous cycle and pregnancy period. Biol. Reprod. 66:322-332. [DOI] [PubMed] [Google Scholar]
- 37.Mayer, L. W. 1982. Rates in vitro changes of gonococcal colony opacity phenotypes. Infect. Immun. 37:481-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCormack, W. M., and G. H. Reynolds. 1982. Effect of menstrual cycle and method of contraception on recovery of Neisseria gonorrhoeae. JAMA 247:1292-1294. [PubMed] [Google Scholar]
- 39.Murphy, G. L., T. D. Connell, D. S. Barritt, M. Koomey, and J. G. Cannon. 1989. Phase variation of gonococcal protein II: regulation of gene expression by slipped-strand mispairing of a repetitive DNA sequence. Cell 56:539-547. [DOI] [PubMed] [Google Scholar]
- 40.Nassif, X., C. Pujol, P. Morand, and E. Eugene. 1999. Interactions of pathogenic Neisseria with host cells. Is it possible to assemble the puzzle? Mol. Microbiol. 32:1124-1132. [DOI] [PubMed] [Google Scholar]
- 41.Ngampasutadol, J., S. Ram, A. M. Blom, H. Jarva, A. E. Jerse, E. Lien, J. Goguen, S. Gulati, and P. A. Rice. 2005. Human C4b-binding protein selectively interacts with Neisseria gonorrhoeae and results in species-specific infection. Proc. Natl. Acad. Sci. U. S. A. 102:17142-17147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Packiam, M., S. J. Veit, R. R. Ingalls, and A. E. Jerse. 2009. Protective role of Toll-like receptor 4 (TLR-4) in experimental gonococcal infection of mice, abstr. E-024. Abstr. 109th Am. Soc. Microbiol. Gen. Meet. [DOI] [PMC free article] [PubMed]
- 43.Packiam, M., S. J. Veit, D. J. Anderson, R. R. Ingalls, and A. E. Jerse. 2010. Mouse strain-dependent differences in susceptibility to Neisseria gonorrhoeae infection and induction of innate immune responses. Infect. Immun. 78:433-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pantelic, M., Y. J. Kim, S. Bolland, I. Chen, J. Shively, and T. Chen. 2005. Neisseria gonorrhoeae kills carcinoembryonic antigen-related cellular adhesion molecule 1 (CD66a)-expressing human B cells and inhibits antibody production. Infect. Immun. 73:4171-4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Popp, A., C. Dehio, F. Grunert, T. F. Meyer, and S. D. Gray-Owen. 1999. Molecular analysis of neisserial Opa protein interactions with the CEA family of receptors: identification of determinants contributing to the differential specificities of binding. Cell. Microbiol. 1:169-181. [DOI] [PubMed] [Google Scholar]
- 46.Ram, S., M. Cullinane, A. M. Blom, S. Gulati, D. P. McQuillen, B. G. Monks, C. O'Connell, R. Boden, C. Elkins, M. K. Pangburn, B. Dahlback, and P. A. Rice. 2001. Binding of C4b-binding protein to porin: a molecular mechanism of serum resistance of Neisseria gonorrhoeae. J. Exp. Med. 193:281-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rettew, J. A., Y. M. Huet, and I. Marriott. 2009. Estrogens augment cell surface TLR4 expression on murine macrophages and regulate sepsis susceptibility in vivo. Endocrinology 150:3877-3884. [DOI] [PubMed] [Google Scholar]
- 48.Roshick, C., H. Wood, H. D. Caldwell, and G. McClarty. 2006. Comparison of gamma interferon-mediated antichlamydial defense mechanisms in human and mouse cells. Infect. Immun. 74:225-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salit, I. E. 1982. The differential susceptibility of gonococcal opacity variants to sex hormones. Can. J. Microbiol. 28:301-306. [DOI] [PubMed] [Google Scholar]
- 50.Schmidt, K. A., C. D. Deal, M. Kwan, E. Thattassery, and H. Schneider. 2000. Neisseria gonorrhoeae MS11mkC opacity protein expression in vitro and during human volunteer infectivity studies. Sex. Transm. Dis. 27:278-283. [DOI] [PubMed] [Google Scholar]
- 51.Seifert, H. S., C. J. Wright, A. E. Jerse, M. S. Cohen, and J. G. Cannon. 1994. Multiple gonococcal pilin antigenic variants are produced during experimental human infections. J. Clin. Invest. 93:2744-2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simms, A. N., and A. E. Jerse. 2006. In vivo selection for Neisseria gonorrhoeae opacity protein expression in the absence of human carcinoembryonic antigen cell adhesion molecules. Infect. Immun. 74:2965-2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Singh, P. K., B. F. Tack, P. B. McCray, Jr., and M. J. Welsh. 2000. Synergistic and additive killing by antimicrobial factors found in human airway surface liquid. Am. J. Physiol. Lung Cell. Mol. Physiol. 279:L799-L805. [DOI] [PubMed] [Google Scholar]
- 54.Slevogt, H., S. Zabel, B. Opitz, A. Hocke, J. Eitel, D. N′Guessan, P., L. Lucka, K. Riesbeck, W. Zimmermann, J. Zweigner, B. Temmesfeld-Wollbrueck, N. Suttorp, and B. B. Singer. 2008. CEACAM1 inhibits Toll-like receptor 2-triggered antibacterial responses of human pulmonary epithelial cells. Nat. Immunol. 9:1270-1278. [DOI] [PubMed] [Google Scholar]
- 55.Soler-Garcia, A. A., and A. E. Jerse. 2007. Neisseria gonorrhoeae catalase is not required for experimental genital tract infection despite the induction of a localized neutrophil response. Infect. Immun. 75:2225-2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Song, W., S. Condron, B. T. Mocca, S. J. Veit, D. Hill, A. Abbas, and A. E. Jerse. 2008. Local and humoral immune responses against primary and repeat Neisseria gonorrhoeae genital tract infections of 17β-estradiol-treated mice. Vaccine 26:5741-5751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Swanson, J. 1978. Studies on gonococcus infection. XII. Colony color and opacity variants of gonococci. Infect. Immun. 19:320-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Swanson, J., O. Barrera, J. Sola, and J. Boslego. 1988. Expression of outer membrane protein II by gonococci in experimental gonorrhea. J. Exp. Med. 168:2121-2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Swanson, K. V., G. A. Jarvis, G. F. Brooks, B. J. Barham, M. D. Cooper, and J. M. Griffiss. 2001. CEACAM is not necessary for Neisseria gonorrhoeae to adhere to and invade female genital epithelial cells. Cell. Microbiol. 3:681-691. [DOI] [PubMed] [Google Scholar]
- 60.Villullas, S., D. J. Hill, R. B. Sessions, J. Rea, and M. Virji. 2007. Mutational analysis of human CEACAM1: the potential of receptor polymorphism in increasing host susceptibility to bacterial infection. Cell. Microbiol. 9:329-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Virji, M., D. Evans, A. Hadfield, F. Grunert, A. M. Teixeira, and S. M. Watt. 1999. Critical determinants of host receptor targeting by Neisseria meningitidis and Neisseria gonorrhoeae: identification of Opa adhesiotopes on the N-domain of CD66 molecules. Mol. Microbiol. 34:538-551. [DOI] [PubMed] [Google Scholar]
- 62.Wang, Y., D. F. Lewis, Y. Gu, Y. Zhang, J. S. Alexander, and D. N. Granger. 2004. Placental trophoblast-derived factors diminish endothelial barrier function. J. Clin. Endocrinol. Metab. 89:2421-2428. [DOI] [PubMed] [Google Scholar]
- 63.Warner, D. M., W. M. Shafer, and A. E. Jerse. 2008. Clinically relevant mutations that cause derepression of the Neisseria gonorrhoeae MtrC-MtrD-MtrE efflux pump system confer different levels of antimicrobial resistance and in vivo fitness. Mol. Microbiol. 70:462-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu, H., and A. E. Jerse. 2006. α-2,3-Sialyltransferase enhances Neisseria gonorrhoeae survival during experimental murine genital tract infection. Infect. Immun. 74:4094-4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu, H., A. A. Soler-Garcia, and A. E. Jerse. 2009. A strain-specific catalase mutation and mutation of the metal-binding transporter gene mntC attenuate Neisseria gonorrhoeae in vivo but not by increasing susceptibility to oxidative killing by phagocytes. Infect. Immun. 77:1091-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]