Abstract
Deletion of the taurine transporter gene (taut) results in lowered levels of taurine, the most abundant amino acid in mammals. Here, we show that taut−/− mice have lost their ability to self-heal blood-stage infections with Plasmodium chabaudi malaria. All taut−/− mice succumb to infections during crisis, while about 90% of the control taut+/+ mice survive. The latter retain unchanged taurine levels even at peak parasitemia. Deletion of taut, however, results in the lowering of circulating taurine levels from 540 to 264 μmol/liter, and infections cause additional lowering to 192 μmol/liter. Peak parasitemia levels in taut−/− mice are approximately 60% higher than those in taut+/+ mice, an elevation that is associated with increased systemic tumor necrosis factor alpha (TNF-α) and interleukin-1β (IL-1β) levels, as well as with liver injuries. The latter manifest as increased systemic ammonia levels, a perturbed capacity to entrap injected particles, and increased expression of genes encoding TNF-α, IL-1β, IL-6, inducible nitric oxide synthase (iNOS), NF-κB, and vitamin D receptor (VDR). Autopsy reveals multiorgan failure as the cause of death for malaria-infected taut−/− mice. Our data indicate that taut-controlled taurine homeostasis is essential for resistance to P. chabaudi malaria. Taurine deficiency due to taut deletion, however, impairs the eryptosis of P. chabaudi-parasitized erythrocytes and expedites increases in systemic TNF-α, IL-1β, and ammonia levels, presumably contributing to multiorgan failure in P. chabaudi-infected taut−/− mice.
Taurine, a nonprotein sulfur-containing amino acid, is the most abundant amino acid in mammals, occurring both in cells and in blood plasma (7, 22). It plays an important role in diverse biological processes, such as cell volume regulation, neuromodulation, antioxidant defense, protein stabilization, and stress responses (11, 16, 46, 48, 54). Taurine may protect cells against various types of injury (11, 16, 29, 38, 45, 48, 54, 56). In particular, taurine is considered a basic regulator of cell homeostasis, presumably as an osmolyte and chaperone (19). According to the current view, taurine is synthesized from cysteine primarily—if not exclusively—in hepatocytes, and it is then exported to the plasma and imported into other cells via the taurine transporter (22).
The disruption of the taurine transporter gene (taut) by homologous recombination causes reductions of 80 to 98% in taurine levels in plasma and blood cells, as well as in tissues such as the brain, the kidney, and skeletal and heart muscles (20, 50, 51). Moreover, taut−/− mice exhibit decreased resistance to osmotic shock and oxidation stress (31). Taurine levels in the liver are decreased by about 70% in adult taut−/− mice; in particular, they are decreased by more than 80% in Kupffer and sinusoidal endothelial cells and by only approximately 30% in liver parenchymal cells (50). Taurine availability is an important modulator of Kupffer cell functions, such as phagocytosis and eicosanoid synthesis (52). taut-deficient mice develop moderate unspecific hepatitis and liver fibrosis at older than 1 year (50, 51). Moreover, though our knowledge is still poor, some information is available on how the immune system is affected by taut depletion (17). For instance, taurine modulates basic functions of leukocytes, such as phagocytosis, prostanoid formation, and cytokine formation (22, 39, 43, 45, 52, 53). However, the consequences of lowered taurine levels for the outcomes of infectious diseases have not been investigated to date.
Malaria is one of the major infectious diseases worldwide, with about 1 million to 3 millions deaths per year (13). The liver plays a central role in malaria: it is the site where the preerythrocytic stages of the malaria parasites have to develop and multiply (in hepatocytes), but it is also the site where the intraerythrocytic stages of the parasites, which are responsible for disease and death, can be trapped and even destroyed (2, 3, 28). Predominantly the Kupffer cells, which constitute approximately 80 to 90% of total macrophages, contribute to the trapping capacity of the liver. Recent evidence obtained in experimental Plasmodium chabaudi malaria indicates that during the crisis phase of infection, when parasitemia drops from about 50% to below 1% within 3 to 4 days, the liver improves its trapping capacity, whereas the spleen is largely closed (27).
In the present study, we show that the lowering of taurine levels due to taut deletion results in a lethal outcome of otherwise self-healing blood-stage malaria caused by P. chabaudi in mice.
MATERIALS AND METHODS
Animals.
Mice with disrupted taurine transporters (taut−/−) have been generated previously by homologous recombination using embryonic stem (ES) cells of 129/SvJ origin in C57BL/6 mice (20). The same mixed genetic background is present in the taut+/+ mice. Mice were bred under specific-pathogen-free conditions in our central animal facilities. Experiments were performed with 10- to 14-week-old female mice. They were housed in plastic cages and received a standard diet (Wohrlin, Bad Salzuflen, Germany) and water ad libitum. The experiments were approved by the State authorities and followed German law on animal protection.
Blood-stage malaria.
We used a nonclonal line of P. chabaudi (59) exhibiting a restriction length polymorphism pattern very similar, but not identical, to that of Plasmodium chabaudi chabaudi AS (28). Erythrocytic stages of P. chabaudi were passaged weekly in NMRI mice. Blood was taken from these mice, and 106 P. chabaudi-infected erythrocytes were injected intraperitoneally (i.p.) into the taut+/+ and taut−/− mice. Parasitemia was evaluated in Giemsa-stained blood smears. The total number of erythrocytes was determined in a Neubauer chamber.
Liver histology.
Five taut+/+ mice and 5 taut−/− mice, all infected with P. chabaudi, were killed at peak parasitemia on day 8 postinfection (p.i.) by cervical dislocation. Livers were removed, cut into small pieces, fixed first with 2.5% glutaraldehyde buffered with 0.1 M sodium cacodylate (pH 7.2) at room temperature for 1 h and then with 2% OsO4 at 4°C for 2 to 3 h, dehydrated in graded solutions of ethanol, and embedded in Spurr's resin. Semithin sections were stained with toluidine blue-borax.
Determination of particle trapping.
Mice were injected with 200 μl phosphate-buffered saline (PBS) containing 1.3 × 108 green fluorescent beads (diameter, 3 μm) by the method of Pinkerton and Webber (40), and uptake by the liver after 5 min was measured by a procedure detailed recently (27). In brief, mice were killed by cervical dislocation, and parts of liver lobes were removed and weighed. Then the tissue was dissolved in KOH and 0.5% Tween 80 in ethanol. Red beads were added as an internal control. The samples were then subjected to several extractions, and the purified beads were resuspended in distilled H2O. Their fluorescence intensity was measured at excitation and emission wavelengths of 450 and 480 nm for green beads and 520 and 590 nm for red beads, respectively (27).
Histopathology.
taut+/+ mice killed by cervical dislocation at peak parasitemia on day 8 p.i. and taut−/− mice that had succumbed to infection during crisis were ventrally opened by longitudinal cuts and were then immersed in 4% neutral formaldehyde. Organs were then prepared and embedded in paraffin. Five-micrometer-thick sections were cut, dewaxed with xylene, rehydrated, and stained with hematoxylin and eosin as well as with Giemsa stain for routine morphology. In addition, the sections were washed three times, for 5 min each time, in Tris buffer (pH 6.8), incubated at 4°C for 24 h with 3 μg/ml of a fluorescein isothiocyanate (FITC)-labeled isolectin IB4 antibody (Sigma, Deisenhofen, Germany), and examined by fluorescence/phase-contrast microscopy for brain microglial cells and stimulated murine macrophages and monocytes. Control sections were stained without IB4.
RNA isolation.
Approximately 250 mg frozen liver was homogenized with an Ultra-Turrax homogenizer in 5 ml Trizol (Peqlab Biotechnologie, Erlangen, Germany) for 1 min. After being mixed with 1 ml chloroform for 15 s, the suspension was incubated for 15 min at room temperature and was centrifuged at 3,000 × g for 45 min. After isopropanol precipitation of the supernatant, the pellet was washed twice with 80% ethanol, air dried, and dissolved in 200 μl RNase-free water. RNA concentrations were determined at 260 nm.
qRT-PCR.
All RNA samples were treated with DNase (Applied Biosystems, Darmstadt, Germany) for at least 1 h and were then converted into cDNA by following the manufacturer's protocol using the reverse transcription kit (Qiagen, Hilden, Germany). Quantitative real-time PCR (qRT-PCR) was performed using the ABI Prism 7500HT sequence detection system (Applied Biosystems, Darmstadt, Germany) with SYBR green PCR master mix from Qiagen (Hilden, Germany). We investigated the genes encoding the mRNAs for the following proteins: interleukin-1β (IL-1β), tumor necrosis factor alpha (TNF-α), IL-6, nuclear factor κB (NF-κB), inducible nitric oxide synthase (iNOS), vitamin D receptor (VDR), CYP3A11 (cytochrome P450, family 3, subfamily a, polypeptide 11), CYP7A1, SULT2B1 (sulfotransferase family 2B, member 1), UGT1A1 (UDP-glucuronosyltransferase family 1, polypeptide A1), multidrug resistance protein 4 (MRP4), and beta-actin (Actb). All primers used for qRT-PCR were obtained commercially from Qiagen. PCRs were conducted as follows: 2 min at 50°C to activate uracil-N-glycosylase (UNG); 95°C for 10 min to deactivate UNG; and 40 cycles at 94°C for 15 s, 60°C for 35 s, and 72°C for 30 s. Reaction specificity was checked by performing dissociation curves after PCR. For quantification, mRNA levels were normalized to those of 18S rRNA. The threshold cycle (CT) value is the cycle number, selected from the logarithmic phase of the PCR curve, in which an increase in fluorescence above background can be detected. ΔCT is determined by subtracting the CT of 18S rRNA from the CT of the target. The relative mRNA levels in noninfected mice are described as the ratio of the target mRNA copy number to the 18S rRNA copy number (2−ΔCT). The fold induction of mRNA expression on day 8 p.i. was determined using the 2−ΔΔCT method (−ΔΔCT = ΔCT at day 0 p.i. −ΔCT at day 8 p.i.).
Blood analysis.
Plasma and serum were prepared from blood and were then analyzed as follows. Plasma taurine levels were detected as described previously (20). Levels of ammonia, bilirubin, and bile acids (3α-hydroxysteroid dehydrogenase assay) in plasma, as well as activities of aspartate aminotransferase and alanine aminotransferase, were determined using the standard methods of the International Federation of Clinical Chemistry. In sera, IL-1β, IL-6, and TNF-α levels were measured using enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN) according to the manufacturer's protocols. Total NO was analyzed using a commercially available kit (R&D Systems).
Statistical analysis.
A two-tailed Student t test and a Fisher exact test were used for statistical analysis. Only P values of <0.01 were considered to be highly significant.
RESULTS
Course of infections.
Blood-stage infections with P. chabaudi take a self-healing course in female taut+/+ mice, i.e., approximately 90% of the challenged taut+/+ mice survive the infections (Fig. 1). The self-healing course is characterized by a precrisis phase with increasing parasitemia, culminating in peak parasitemia of approximately 40% on day 8 p.i., followed by the crisis phase, with parasitemia falling dramatically, below about 2%, within 5 days. Thereafter, a second peak with a parasitemia of approximately 25% occurs on day 18 p.i., followed by the chronic phase of persistent low-grade parasitemia, presumably controlled by protective immune mechanisms.
FIG. 1.
Course of P. chabaudi infections in female taut−/− and taut+/+ mice. Mice were infected with 106 P. chabaudi-parasitized erythrocytes. n stands for the number of mice infected. All values are given as means ± standard deviations. taut−/− mice differed significantly from taut+/+ mice with respect to survival (P < 0.001 by Fisher's exact test) and peak parasitemia (P < 0.01 by the t test).
In contrast to taut+/+ mice, however, all taut−/− mice succumb to infection. Peak parasitemia occurs on day 8 p.i. also but is significantly increased, to approximately 65% (Fig. 1), i.e., there are about 60% more parasitized erythrocytes in taut−/− mice than in taut+/+ mice at peak parasitemia. All taut−/− mice die during crisis, between days 8 and 13 p.i. Figure 1 indicates the days of death of the individual mice.
Liver structure and particle trapping.
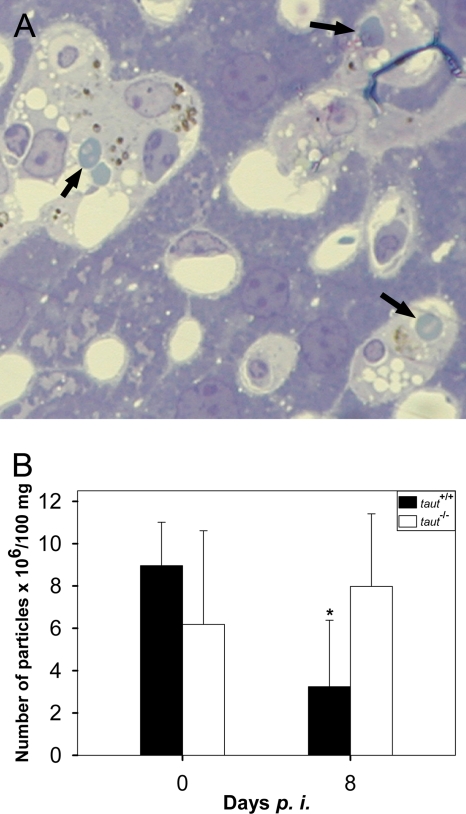
Deletion of the taut gene does not result in any visible changes in the structures of the livers of 2-month-old mice at the light microscopic level (50). Upon infection, however, the liver progressively experiences alterations. At peak parasitemia, the tissue reveals some necrotic areas and changes typical of inflammation. Kupffer cells of taut−/− mice are apparently more hypertrophic and more overloaded with hemozoin (Fig. 2A) than those of taut+/+ mice (see Fig. S1 in the supplemental material). It appears that Kupffer cells of taut−/− mice are more engaged in phagocytosis than those of taut+/+ mice, as suggested by an increased content of hemozoin and more-frequent occurrence of tightly associated Plasmodium-infected erythrocytes (Fig. 2A; see also Fig. S1 in the supplemental material).
FIG. 2.
Liver structure and particle trapping. (A) Light microscopy of liver from a taut−/− mouse infected with P. chabaudi for 8 days. Arrows indicate parasitized erythrocytes in tight association with Kupffer cells. (B) The trapping capacity of the liver was determined on days 0 and 8 after infection with P. chabaudi as described in Materials and Methods. Data are means ± standard deviations for at least 5 different mice.
In order to semiquantify possible differences in phagocytic activity between taut−/− and taut+/+ mice in vivo, we have determined the trapping capacity of the liver. Figure 2B shows that the livers of noninfected taut+/+ mice are able to entrap approximately 9 × 106 fluorescent, 3-μm-diameter polystyrene beads per 100 μg of liver. At peak parasitemia, however, this trapping capacity is significantly reduced, by about 60%. In contrast, there is no significant reduction in the specific trapping capacity of the liver in taut−/− mice at peak parasitemia (Fig. 2B).
Hepatic gene expression.
Quantitative real-time PCR was used to detect changes in the mRNA levels of different genes in the liver. Deletion of the taut gene did not affect the expression of the genes tested in noninfected mice. Upon infection, however, there was a significant increase in the mRNA expression of genes in taut−/− mice in comparison to that in taut+/+ mice: IL-1β, TNF-α, IL-6, and iNOS were more highly expressed in taut−/− mice. Also, expression of the genes encoding NF-κB and VDR was increased. In contrast, the mRNA expression patterns of CYP3A11, SULT2B1, UGT1A1, MRP4, and Actb in response to malaria did not differ significantly between taut−/− and taut+/+ mice (Fig. 3).
FIG. 3.
Hepatic gene expression. The mRNA levels and fold mRNA induction of malaria-relevant genes were determined by quantitative real-time PCR as described in Materials and Methods. Data are means ± standard deviations for 5 different mice. Symbols: *, significant difference between infected and noninfected mice; §, significant difference between taut+/+ and taut−/− mice.
Blood parameters.
Deletion of taut results in a significant reduction (about 50%) in taurine levels in the plasma (Table 1), in accordance with previous data (20). Also, aspartate transaminase (AST) levels are apparently diminished by about the same percentage (Table 1). However, the concentrations of alanine transaminase (ALT), bile acids, and bilirubin in plasma are not affected by the deletion of taut. Also, there is no influence on the levels of IL-1β, IL-6, TNF-α, and total NO in serum (Table 2).
TABLE 1.
Plasma parameters of P. chabaudi-infected taut+/+ and taut−/− mice on days 0 and 8 p.i.a
| Parameter | Day 0 p.i. |
Day 8 p.i. |
P (day 8 p.i. vs day 0 p.i.) |
|||||
|---|---|---|---|---|---|---|---|---|
| Mean value ± SEM (no. of mice) |
P (taut−/− vs taut+/+ mice) | Mean value ± SEM (no. of mice) |
P (taut−/− vs taut+/+ mice) | |||||
| taut+/+ | taut−/− | taut+/+ | taut−/− | taut+/+ mice | taut−/− mice | |||
| Taurine (μmol/liter) | 540 ± 47 (3) | 264 ± 25 (3) | <0.01 | 569 ± 16 (6) | 192 ± 19 (4) | <0.01 | 0.48 | 0.05 |
| AST (U/liter) | 93 ± 28 (6) | 42 ± 4 (6) | 0.14 | 574 ± 117 (9) | 589 ± 142 (7) | 0.88 | <0.01 | <0.01 |
| ALT (U/liter) | 19 + 2 (6) | 14 + 3 (6) | 0.41 | 150 + 32 (9) | 131 + 30 (7) | 0.68 | <0.01 | <0.01 |
| Bile acids (μmol/liter) | 20 ± 2 (3) | 22 ± 5 (5) | 0.79 | 40 ± 8 (9) | 51 ± 9 (6) | 0.39 | 0.2 | 0.02 |
| Bilirubin (ng/dl) | 0.13 ± 0.02 (6) | 0.17 ± 0.03 (6) | 0.19 | 1.18 ± 0.03 (6) | 0.80 ± 0.23 (6) | 0.33 | <0.01 | <0.01 |
| Ammonia (μg/dl) | 61 ± 6 (4) | 74 ± 9 (4) | 0.31 | 165 ± 16 (8) | 291 ± 23 (8) | <0.01 | <0.01 | 0.02 |
Blood was taken from mice, and plasma was prepared and analyzed, as described in Materials and Methods. Significance was evaluated with Student's t test; P values of <0.01 were considered significant. γ-Glutamyltransferase activity was lower than 3 U/liter in all samples.
TABLE 2.
Serum parameters of P. chabaudi-infected taut+/+ and taut−/− mice on days 0 and 8 p.i.a
| Parameter | Day 0 p.i. |
Day 8 p.i. |
P (day 8 p.i. vs day 0 p.i.) |
|||||
|---|---|---|---|---|---|---|---|---|
| Mean value ± SEM (no. of mice) |
P (taut−/− vs taut+/+ mice) | Mean value ± SEM (no. of mice) |
P (taut−/− vs. taut+/+ mice) | |||||
| taut+/+ | taut−/− | taut+/+ | taut−/− | taut+/+ mice | taut−/− mice | |||
| IL-1β (ng/liter) | 1.27 ± 0.1 (3) | 2.1 ± 0.2 (3) | 0.15 | 4.7 ± 1.1 (3) | 14.5 ± 1.9 (3) | <0.01 | 0.05 | 0.02 |
| IL-6 (ng/liter) | 1.2 ± 0.1 (3) | 2.1 ± 0.5 (3) | 0.37 | 39.1 ± 12.3 (5) | 69.4 ± 22.6 (5) | 0.25 | <0.01 | <0.01 |
| TNF-α (ng/liter) | 23.8 ± 0.6 (4) | 27.3 ± 2.1 (4) | 0.22 | 29.4 ± 6.2 (5) | 97.2 ± 23.6 (5) | <0.01 | 0.46 | 0.02 |
| Total NO (μmol/liter) | 25 ± 2 (3) | 74 ± 41 (3) | 0.35 | 118 ± 9 (4) | 198 ± 35 (3) | 0.08 | <0.01 | 0.02 |
Blood was taken from mice, and serum was prepared and analyzed, as described in Materials and Methods. Significance was evaluated with Student's t test; P values of <0.01 were considered significant. γ-Glutamyltransferase activity was lower than 3 U/liter in all samples.
Upon infection, the taurine levels remained unaffected at peak parasitemia in taut+/+ mice, whereas taut−/− mice exhibited a further dropping of the circulating taurine level in comparison to that in noninfected taut−/− mice on day 8 p.i. (Table 1). Infection induced strong increases in AST, ALT, bile acid, and bilirubin levels on day 8 p.i., but these parameters were not significantly different between taut+/+ and taut−/− mice. The major significant difference between taut+/+ and taut−/− mice at peak parasitemia was the content of ammonia in plasma, which was increased by about 270% in taut+/+ mice and by about 400% in taut−/− mice (Table 1).
As expected, infections caused increases in the levels of the three cytokines IL-1β, IL-6, and TNF-α, and in total NO levels, in both taut+/+ and taut−/− mice at peak parasitemia (Table 2). Although IL-6 levels increased dramatically at peak parasitemia, the difference between taut+/+ and taut−/− mice was not significant (Table 2). The only significant differences at peak parasitemia were the higher levels of TNF-α and IL-1β in taut−/− mice (Table 2).
Histopathology.
P. chabaudi infections caused increased levels of ammonia in the blood of taut−/− mice at peak parasitemia (Table 1), suggesting undesired adverse effects on other organs. The brain is especially sensitive to ammonia intoxication, which eventually leads to hepatic encephalopathy (8, 9, 18, 19, 36). When we examined slices of cerebrum and cerebellum from taut−/− mice, which had succumbed to P. chabaudi infections during the crisis phase, destruction of the microvasculature was widespread (Fig. 4A). In particular, the vessels contained parasitized erythrocytes, as well as monocytes and macrophages with hemozoin deposits (Fig. 4A and B). The vessel walls were partially destroyed, allowing blood cells to invade adjacent brain tissue (Fig. 4C). Damage of the microvasculature also manifested as intravascular monocyte aggregation, vasodilatation, edema, endothelial cell activation, swelling of endothelial cell nuclei, increased numbers of activated microglial cells in perivascular species, plugging of small vessels with erythrocytes and mononuclear cells, and occasional rupture of vessel walls (37). In particular, small vessels were plugged with both parasitized and nonparasitized erythrocytes, causing focal microhemorrhages. Moreover, the lumens of vessels were occasionally occluded by monocytes that adhered tightly to the endothelium of the microvasculature.
FIG. 4.
Histopathology of tissues of taut−/− mice that succumbed to P. chabaudi malaria. (A, B, and C) Slices of the brain including the cortex cerebri. Histological changes of the brain (A and C) include the presence of several parasitized erythrocytes in the cerebral blood vessels, infiltration of malarial-pigment-containing hypertrophic monocytes (A, arrow), endothelial cell activation with enlarged nuclei (C, black arrows), adherence of mononuclear cells to endothelial cells of small cerebral vessels, and focal vessel disruption (C, white arrow) with perivascular hemorrhage (C, double-headed arrow). Arrows in panel B show isolectin IB4 antibody staining of stimulated murine macrophages in a blood vessel. (D) Slices of heart muscle show interstitial edema, differences in the sizes of cardiomyocytes, and abnormal, thinner myofibers with a wavy appearance. (E) Pulmonary edema of the lung. (F) Hemorrhagic infarct of the lung. (G) Isolectin staining showing interstitial stimulated murine pigment-containing macrophages/monocytes in the lung. (H) Arrows indicate chromoprotein cylinders in the kidney. (I) Double-headed arrow shows tubular necrosis in the kidney. (K) Small and large arrows indicate small and large necrotic areas in the liver. Some tissues were stained with hematoxylin and eosin (A, C, D, E, and F) or were subjected to immune staining with the FITC-labeled isolectin IB4 antibody (B and G). Original magnifications, ×400 (A, B, C, and D), ×100 (E, G, H, and K), and ×200 (F and I).
Moreover, the hearts of these dead taut−/− mice revealed a series of morphological alterations. There was interstitial edema with moderate mononuclear cell infiltration; the sizes of cardiomyocytes differed; and myofibers often appeared thinner than normal and wavy (Fig. 4D). Furthermore, severe changes were also detected in the lungs. These changes became evident as thickened alveolar septa, interstitial and/or intra-alveolar edema, increased numbers of macrophages, intensified adhesiveness of malaria pigment-containing monocytes, septal pneumonitis with monocyte infiltrates, and occasional hyperplasia of type II pneumocytes (Fig. 4E to G). Three out of 16 mice revealed hemorrhagic infarcts of the lung, and 1 mouse showed alveolar macrophages with hemosiderin pigment (Fig. 4G).
The deceased mice also suffered from acute renal failure, as indicated by acute tubular necrosis and chromoprotein cylinders (Fig. 4H). In acute tubular necrosis, necrosis occurs mainly in the proximal tubular epithelium and is characterized by cells with no nuclei and homogenous, intensely eosinophilic cytoplasm. Necrotic cells penetrate the lumens of tubules, which become obliterated, eventually resulting in acute renal failure (Fig. 4I). Finally, the liver revealed large necrotic areas (Fig. 4K).
In this context, however, it should also be mentioned that taut+/+ mice killed at peak parasitemia on day 8 p.i. did not reveal such dramatic pathological changes in the brain, lung, heart, kidney, and liver as taut−/− mice that succumbed to infection during crisis (see Fig. S1 in the supplemental material).
DISCUSSION
Previous studies of different mouse malaria models have revealed that blood-stage malaria is under complex control, involving both genes of the mouse major histocompatibility complex (MHC), i.e., the H-2 complex, and genes of the non-H-2 background (42, 57, 58), as well as soluble factors, such as testosterone (57, 58) and estrogen (5, 6). Different genes and/or loci of the non-H-2 background have already been reported to be critical for a fatal outcome of malaria (14, 15, 21, 30, 35, 41). Here we report another critical gene of the non-H-2 background, i.e., the taut gene, encoding the taurine transporter TAUT. Indeed, deletion of the taut gene causes loss of the mice's ability to self-heal blood-stage infections with P. chabaudi.
Our data, however, also indicate that it is not the loss of the taut gene or the gene product TAUT per se that causes the fatal outcome of malaria. Rather, it is the physiological consequence, namely, the breakdown of taurine homeostasis, evident as lowered levels of taurine in the cells and blood, since taurine is the major factor transported by TAUT. Indeed, deletion of taut results in a reduction of about 50% in taurine levels in the blood plasma, which, in turn, is associated with a lowering of intracellular taurine levels, as shown previously (20, 50, 51). Moreover, the taurine levels in taut−/− mice are lowered another 25% at peak parasitemia. This suggests that the fatal outcome of P. chabaudi infections during crisis is causally related to taurine deficiency. However, the taurine deficiency per se is not lethal; rather, it becomes lethal only when mice have to respond to P. chabaudi infection. This becomes evident at peak parasitemia, when taut−/− mice exhibit significant increases in parasitemia, systemic cytokine levels, and liver damage relative to those for taut+/+ mice, which exhibit normal taurine homeostasis even at peak parasitemia.
There is ample evidence that lowered taurine levels also critically affect the stability of cells (18). In accordance, our findings suggest altered stability of, e.g., macrophages and parasitized erythrocytes. Indeed, P. chabaudi infections in taurine-deficient nonhealer taut−/− mice result in a peak parasitemia that is approximately 60% higher, on average, than that for self-healer taut+/+ mice. But even noninfected erythrocytes exhibit a different level of stability, as indicated by previous data showing that erythrocytes of taut−/− mice are characterized by impaired eryptosis (apoptosis of erythrocytes) (31). Such impaired eryptosis may be even further delayed by intraerythrocytic P. chabaudi, as is known to occur for host cells infected by other intracellular parasites (1, 32, 49). Recent evidence indeed has shown impaired eryptosis of erythrocytes infected with Plasmodium berghei (26) or Plasmodium falciparum, presumably due to a parasite-maintained low Ca2+ concentration in the cytosol of the host erythrocyte (23). Moreover, the macrophages of taut−/− mice appear to change their stability in response to malaria, as circumstantially indicated by the increased systemic levels of TNF-α and IL-1β, which are produced primarily by macrophages. This accords with other data showing, conversely, that taurine is able to dampen the effect of proinflammatory cytokines, including IL-1β (12) and TNF-α (10, 24, 33, 34, 44, 47, 60).
About 80% to 90% of all macrophages reside as Kupffer cells in the liver, in particular in the periportal area, supervising the invasion of pathogens (18). It is therefore reasonable to assume that taurine deficiency also affects the stability of malaria-activated Kupffer cells in taut−/− mice, which presumably release more proinflammatory cytokines than taut+/+ mice, thus contributing to the increased levels of circulating TNF-α and IL-1β. The increased release of TNF-α and IL-1β may, in turn, induce local inflammatory responses, which may be associated with more-pronounced liver injuries in taut−/− mice than in taut+/+ mice (4). This view, that increased liver injuries are due predominantly to overwhelming host responses to infection, is also supported by our data showing increased ammonia levels in malaria-infected taut−/− mice and perturbed particle-entrapping activity of the liver at peak parasitemia. However, our analyses of hepatic gene expression indicate that the liver is obviously not uniformly damaged in response to malaria, since only some genes, such as those encoding IL-1β, TNF-α, IL-6, iNOS, NF-κB, and VDR, show increased expression; the expression of others, such as the CYP3A11, CYP7A1, SULT2B1, UGT1A1, and MRP4 genes, is the same in taut−/− mice as in taut+/+ mice.
The malaria-induced increase in the systemic ammonia levels of taut−/− mice ultimately contributes to injuries in other organs, which also suffer from the lowered taurine levels due to the breakdown of taurine homeostasis. In particular, it is known that increased ammonia levels lead to hepatic encephalopathy, characterized by astrocyte swelling and low-grade cerebral edema (19, 36, 55). Our data reveal that the brains of the taut−/− mice that succumbed to malaria were massively damaged. Incidentally, human P. falciparum malaria patients suffer from hepatic encephalopathy (25). Although brain damage, such as that which occurs in taut−/− mice, could be a sufficient and exclusive cause for death, our autopsy analysis also revealed massive injuries in other organs, such as the lung, kidney, and heart. Incidentally, taut−/− are prone to pressure overload and cardiac hypertrophy (50, 51). All these data indicate multiple organ failure as the cause of death for malaria-infected taut−/− mice. Obviously, taut−/− mice, due to their taurine deficiency, are not able to robustly activate those mechanisms that mediate self-healing from P. chabaudi malaria, as taut+/+ mice do.
Collectively, our data show that the breakdown of taurine homeostasis, manifesting as lowered taurine levels as a consequence of taut deletion, is not lethal per se but becomes lethal in response to blood-stage malaria. Obviously, the taut gene and taurine homeostasis are essential for self-healing of P. chabaudi blood-stage malaria. It remains to be seen whether taurine deficiency also affects the outcome of human malarial infections.
Supplementary Material
Acknowledgments
We are grateful to C. Barthuber and I. Mönnighoff for help with blood analysis and to A. Grunwald and P. Marinovski for technical assistance.
This work was supported by the Deutsche Forschungsgemeinschaft through GRK 1427 and SFB 575 as well as by the Centre of Excellence for Biodiversity Research, College of Science, King Saud University, Riyadh, Saudi Arabia.
Editor: J. H. Adams
Footnotes
Published ahead of print on 25 January 2010.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Aga, E., D. M. Katschinski, G. van Zandbergen, H. Laufs, B. Hansen, K. Müller, W. Solbach, and T. Laskay. 2002. Inhibition of the spontaneous apoptosis of neutrophil granulocytes by the intracellular parasite Leishmania major. J. Immunol. 169:898-905. [DOI] [PubMed] [Google Scholar]
- 2.Aikawa, M., M. Suzuki, and Y. Gutierrez. 1980. Pathology of malaria, p. 47-102. In J. P. Kreier (ed.), Malaria, vol. 2. Academic Press, New York, NY. [Google Scholar]
- 3.Balmer, P., J. Alexander, and R. S. Phillips. 2000. Protective immunity to erythrocytic Plasmodium chabaudi AS infection involves IFN-γ-mediated responses and a cellular infiltrate to the liver. Parasitology 121:473-482. [DOI] [PubMed] [Google Scholar]
- 4.Barua, M., Y. Liu, and M. R. Quinn. 2001. Taurine chloramine inhibits inducible nitric oxide synthase and TNF-α gene expression in activated alveolar macrophages: decreased NF-κB activation and IκB kinase activity. J. Immunol. 167:2275-2281. [DOI] [PubMed] [Google Scholar]
- 5.Benten, W. P. M., F. Wunderlich, R. Herrmann, and W. N. Kühn-Velten. 1993. Testosterone-induced compared with oestradiol-induced immunosuppression against Plasmodium chabaudi malaria. J. Endocrinol. 139:487-494. [DOI] [PubMed] [Google Scholar]
- 6.Benten, W. P. M., F. Wunderlich, and H. Mossmann. 1992. Plasmodium chabaudi: estradiol suppresses acquiring, but not once-acquired immunity. Exp. Parasitol. 75:240-247. [DOI] [PubMed] [Google Scholar]
- 7.Bouckenooghe, T., C. Remacle, and B. Reusens. 2006. Is taurine a functional nutrient? Curr. Opin. Clin. Nutr. Metab. Care 9:728-733. [DOI] [PubMed] [Google Scholar]
- 8.Butterworth, R. F. 2002. Pathophysiology of hepatic encephalopathy: a new look at ammonia. Metab. Brain Dis. 17:221-227. [DOI] [PubMed] [Google Scholar]
- 9.Butterworth, R. F., J. F. Giguère, J. Michaud, J. Lavoie, and G. P. Layrargues. 1987. Ammonia: key factor in the pathogenesis of hepatic encephalopathy. Neurochem. Pathol. 6:1-12. [DOI] [PubMed] [Google Scholar]
- 10.Cetiner, M., G. Sener, A. O. Sehirli, E. Ekçsioğlu-Demiralp, F. Ercan, S. Sirvanci, N. Gedik, S. Akpulat, T. Tecimer, and B. C. Yeğen. 2005. Taurine protects against methotrexate-induced toxicity and inhibits leukocyte death. Toxicol. Appl. Pharmacol. 209:39-50. [DOI] [PubMed] [Google Scholar]
- 11.Chapman, R. A., M. S. Suleiman, and Y. E. Earm. 1993. Taurine and the heart. Cardiovasc. Res. 27:358-363. [DOI] [PubMed] [Google Scholar]
- 12.Chorazy, M., E. Kontny, J. Marcinkiewicz, and W. Maśliński. 2002. Taurine chloramine modulates cytokine production by human peripheral blood mononuclear cells. Amino Acids 23:407-413. [DOI] [PubMed] [Google Scholar]
- 13.Doolan, D. L., C. Dobano, and J. K. Baird. 2009. Acquired immunity to malaria. Clin. Microbiol. Rev. 22:13-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foote, S. J., R. A. Burt, T. M. Baldwin, A. Presente, A. W. Roberts, Y. L. Laural, A. M. Lew, and V. M. Marshall. 1997. Mouse loci for malaria-induced mortality and the control of parasitemia. Nat. Genet. 17:380-381.9398834 [Google Scholar]
- 15.Fortin, A., L. R. Cardon, M. Tam, E. Skamene, M. M. Stevenson, and P. Gros. 2001. Identification of a new malaria susceptibility locus (Char4) in recombinant congenic strains of mice. Proc. Natl. Acad. Sci. U. S. A. 98:10793-10798. (Erratum, 98:14744.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green, T. R., J. H. Fellman, A. L. Eicher, and K. L. Pratt. 1991. Antioxidant role and subcellular localization of hypotaurine and taurine in human neutrophils. Biochim. Biophys. Acta 1073:91-97. [DOI] [PubMed] [Google Scholar]
- 17.Grimble, R. F. 2006. The effects of sulphur amino acid intake on immune functions in humans. J. Nutr. 136:1660S-1665S. [DOI] [PubMed] [Google Scholar]
- 18.Häussinger, D., R. Kubitz, R. Reinehr, J. G. Bode, and F. Schliess. 2004. Molecular aspects of medicine: from experimental to clinical hepatology. Mol. Aspects Med. 25:221-360. [DOI] [PubMed] [Google Scholar]
- 19.Häussinger, D., and F. Schliess. 2008. Pathogenetic mechanisms of hepatic encephalopathy. Gut 57:1156-1165. [DOI] [PubMed] [Google Scholar]
- 20.Heller-Stilb, B., C. van Royen, K. Rascher, H. G. Hartwig, A. Huth, M. W. Seeliger, U. Warskulat, and D. Häussinger. 2002. Disruption of the taurine transporter gene (taut) leads to retinal degeneration in mice. FASEB J. 16:231-233. [DOI] [PubMed] [Google Scholar]
- 21.Hernandez-Valladares, M., J. Naessens, J. P. Gibson, A. J. Musoke, S. Nagda, P. Rihet, O. K. Ole-Moi Yoi, and F. A. Iraqi. 2004. Confirmation and dissection of QTL controlling resistance to malaria in mice. Mamm. Genome 15:390-398. [DOI] [PubMed] [Google Scholar]
- 22.Huxtable, R. J. 1992. Physiological actions of taurine. Physiol. Rev. 72:101-163. [DOI] [PubMed] [Google Scholar]
- 23.Kasinathan, R. S., M. Föller, S. Koka, S. M. Huber, and F. Lang. 2007. Inhibition of eryptosis and intraerythrocytic growth of Plasmodium falciparum by flufenamic acid. Naunyn Schmiedebergs Arch. Pharmacol. 374:255-264. [DOI] [PubMed] [Google Scholar]
- 24.Kincius, M., R. Liang, A. Nickkholgh, K. Hoffmann, C. Flechtenmacher, E. Ryschich, C. N. Gutt, M. M. Gebhard, J. Schmidt, M. W. Büchler, and P. Schemmer. 2007. Taurine protects from liver injury after warm ischemia in rats: the role of Kupffer cells. Eur. Surg. Res. 39:275-283. [DOI] [PubMed] [Google Scholar]
- 25.Kochar, D. K., P. Agarwal, S. K. Kochar, R. Jain, N. Rawat, R. K. Pokharna, S. Kachhawa, and T. Srivastava. 2003. Hepatocyte dysfunction and hepatic encephalopathy in Plasmodium falciparum malaria. QJM 96:505-512. [DOI] [PubMed] [Google Scholar]
- 26.Koka, S., S. M. Huber, K. M. Boini, C. Lang, M. Föller, and F. Lang. 2007. Lead decreases parasitemia and enhances survival of Plasmodium berghei-infected mice. Biochem. Biophys. Res. Commun. 363:484-489. [DOI] [PubMed] [Google Scholar]
- 27.Kruecken, J., M. A. Dkhil, J. V. Braun, R. M. Schroetel, M. El-Khadragy, P. Carmeliet, H. Mossmann, and F. Wunderlich. 2005. Testosterone suppresses protective responses of the liver to blood-stage malaria. Infect. Immun. 73:436-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kruecken, J., L. I. Mehnert, M. A. Dkhil, M. El-Khadragy, W. P. M. Benten, H. Mossmann, and F. Wunderlich. 2005. Massive destruction of malaria-parasitized red blood cells despite spleen closure. Infect. Immun. 73:6390-6398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurz, A. K., F. Schliess, and D. Häussinger. 1998. Osmotic regulation of the heat shock response in primary hepatocytes. Hepatology 28:774-781. [DOI] [PubMed] [Google Scholar]
- 30.Kwiatkowski, D. 2000. Genetic susceptibility to malaria getting complex. Curr. Opin. Genet. Dev. 10:320-324. [DOI] [PubMed] [Google Scholar]
- 31.Lang, P. A., U. Warskulat, B. Heller-Stilb, D. Y. Huang, A. Grenz, S. Myssina, M. Duszenko, F. Lang, D. Häussinger, V. Vallon, and T. Wieder. 2003. Blunted apoptosis of erythrocytes from taurine transporter deficient mice. Cell. Physiol. Biochem. 13:337-346. [DOI] [PubMed] [Google Scholar]
- 32.Liu, L., W. P. M. Benten, L. Wang, X. Hao, Q. Li, H. Zhang, D. Guo, Y. Wang, F. Wunderlich, and Z. Qiao. 2005. Modulation of Leishmania donovani infection and cell viability by testosterone in bone marrow-derived macrophages: signaling via surface binding sites. Steroids 70:604-614. [DOI] [PubMed] [Google Scholar]
- 33.Marcinkiewicz, J., A. Grabowska, J. Bereta, K. Bryniarski, and B. Nowak. 1998. Taurine chloramine down-regulates the generation of murine neutrophil inflammatory mediators. Immunopharmacology 40:27-38. [DOI] [PubMed] [Google Scholar]
- 34.Marcinkiewicz, J., M. Mak, M. Bobek, R. Biedroń, A. Białecka, M. Koprowski, E. Kontny, and W. Maśliński. 2005. Is there a role of taurine bromamine in inflammation? Interactive effects with nitrite and hydrogen peroxide. Inflamm. Res. 54:42-49. [DOI] [PubMed] [Google Scholar]
- 35.Min-Oo, G., A. Fortin, G. Pitari, M. Tam, M. M. Stevenson, and P. Gros. 2007. Complex genetic control of susceptibility to malaria: positional cloning of the Char9 locus. J. Exp. Med. 204:511-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Munoz, S. J. 2008. Hepatic encephalopathy. Med. Clin. North Am. 92:795-812. [DOI] [PubMed] [Google Scholar]
- 37.Neill, A. L., T. Chan-Ling, and N. H. Hunt. 1993. Comparisons between microvascular changes in cerebral and non-cerebral malaria in mice, using the retinal whole-mount technique. Parasitology 107:477-487. [DOI] [PubMed] [Google Scholar]
- 38.Park, E., G. Schuller-Levis, and M. R. Quinn. 1995. Taurine chloramine inhibits production of nitric oxide and TNF-α in activated RAW 264.7 cells by mechanisms that involve transcriptional and translational events. J. Immunol. 154:4778-4784. [PubMed] [Google Scholar]
- 39.Peters-Regehr, T., J. G. Bode, R. Kubitz, and D. Häussinger. 1999. Organic osmolyte transport in quiescent and activated rat hepatic stellate cells (Ito cells). Hepatology 29:173-180. [DOI] [PubMed] [Google Scholar]
- 40.Pinkerton, W., and M. Webber. 1964. A method of injecting small laboratory animals by the ophthalmic plexus route. Proc. Soc. Exp. Biol. Med. 116:959-961. [DOI] [PubMed] [Google Scholar]
- 41.Roberts, C. W., W. Walker, and J. Alexander. 2001. Sex-associated hormones and immunity to protozoan parasites. Clin. Microbiol. Rev. 14:476-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sayles, P. C., and D. L. Wassom. 1988. Immunoregulation in murine malaria. Susceptibility of inbred mice to infection with Plasmodium yoelii depends on the dynamic interplay of host and parasite genes. J. Immunol. 141:241-248. [PubMed] [Google Scholar]
- 43.Schuller-Levis, G. B., and E. Park. 2004. Taurine and its chloramine: modulators of immunity. Neurochem. Res. 29:117-126. [DOI] [PubMed] [Google Scholar]
- 44.Seabra, V., R. F. Stachlewitz, and R. G. Thurman. 1998. Taurine blunts LPS-induced increases in intracellular calcium and TNF-α production by Kupffer cells. J. Leukoc. Biol. 64:615-621. [DOI] [PubMed] [Google Scholar]
- 45.Stapleton, P. P., L. O'Flaherty, H. P. Redmond, and D. J. Bouchier-Hayes. 1998. Host defense—a role for the amino acid taurine? J. Parenter. Enteral Nutr. 22:42-48. [DOI] [PubMed] [Google Scholar]
- 46.Suzuki, T., T. Suzuki, T. Wada, K. Saigo, and K. Watanabe. 2002. Taurine as a constituent of mitochondrial tRNAs: new insights into the functions of taurine and human mitochondrial diseases. EMBO J. 21:6581-6589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tan, B., D. J. Jiang, H. Huang, S. J. Jia, J. L. Jiang, C. P. Hu, and Y. J. Li. 2007. Taurine protects against low-density lipoprotein-induced endothelial dysfunction by the DDAH/ADMA pathway. Vascul. Pharmacol. 46:338-345. [DOI] [PubMed] [Google Scholar]
- 48.Timbrell, J. A., V. Seabra, and C. J. Waterfield. 1995. The in vivo and in vitro protective properties of taurine. Gen. Pharmacol. 26:453-462. [DOI] [PubMed] [Google Scholar]
- 49.Vutova, P., M. Wirth, D. Hippe, U. Gross, K. Schulze-Osthoff, I. Schmitz, and C. G. Lüder. 2007. Toxoplasma gondii inhibits Fas/CD95-triggered cell death by inducing aberrant processing and degradation of caspase 8. Cell. Microbiol. 9:1556-1570. [DOI] [PubMed] [Google Scholar]
- 50.Warskulat, U., E. Borsch, R. Reinehr, B. Heller-Stilb, I. Mönnighoff, D. Buchczyk, M. Donner, U. Flögel, G. Kappert, S. Soboll, S. Beer, K. Pfeffer, H. U. Marschall, M. Gabrielsen, M. Amiry-Moghaddam, O. P. Ottersen, H. P. Dienes, and D. Häussinger. 2006. Chronic liver disease is triggered by taurine transporter knockout in the mouse. FASEB J. 20:574-576. [DOI] [PubMed] [Google Scholar]
- 51.Warskulat, U., B. Heller-Stilb, E. Oermann, K. Zilles, H. Haas, F. Lang, and D. Häussinger. 2007. Phenotype of the taurine transporter knockout mouse. Methods Enzymol. 428:439-458. [DOI] [PubMed] [Google Scholar]
- 52.Warskulat, U., F. Zhang, and D. Häussinger. 1997. Taurine is an osmolyte in rat liver macrophages (Kupffer cells). J. Hepatol. 26:1340-1347. [DOI] [PubMed] [Google Scholar]
- 53.Weik, C., U. Warskulat, J. G. Bode, T. Peters-Regehr, and D. Häussinger. 1998. Compatible organic osmolytes in rat liver sinusoidal endothelial cells. Hepatology 27:569-575. [DOI] [PubMed] [Google Scholar]
- 54.Welch, W. J., and C. R. Brown. 1996. Influence of molecular and chemical chaperones on protein folding. Cell Stress Chaperones 1:109-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wendon, J., and W. Lee. 2008. Encephalopathy and cerebral edema in the setting of acute liver failure: pathogenesis and management. Neurocrit. Care 9:97-102. [DOI] [PubMed] [Google Scholar]
- 56.Wettstein, M., and D. Häussinger. 1997. Cytoprotection by the osmolytes betaine and taurine in ischemia-reoxygenation injury in the perfused rat liver. Hepatology 26:1560-1566. [DOI] [PubMed] [Google Scholar]
- 57.Wunderlich, F., P. Marinovski, W. P. M. Benten, H. P. Schmitt-Wrede, and H. Mossmann. 1991. Testosterone and other gonadal factor(s) restrict the efficacy of genes controlling resistance to Plasmodium chabaudi malaria. Parasite Immunol. 13:357-367. [DOI] [PubMed] [Google Scholar]
- 58.Wunderlich, F., H. Mossmann, M. Helwig, and G. Schillinger. 1988. Resistance to Plasmodium chabaudi in B10 mice: influence of the H-2 complex and testosterone. Infect. Immun. 56:2400-2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wunderlich, F., H. Stübig, and E. Königk. 1982. Development of Plasmodium chabaudi in mouse red blood cells: structural properties of the host and parasite membranes. J. Protozool. 29:60-66. [DOI] [PubMed] [Google Scholar]
- 60.Zhang, F., L. Tong, H. Qiao, X. Dong, G. Qiao, H. Jiang, and X. Sun. 2008. Taurine attenuates multiple organ injury induced by intestinal ischemia reperfusion in rats. J. Surg. Res. 149:101-109. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.