Abstract
In the thermophile Geobacillus stearothermophilus, the uptake of basic amino acids is mediated by an ABC transporter composed of the substrate binding protein (receptor) ArtJ and a homodimer each of the pore-forming subunit, ArtM, and the nucleotide-binding subunit, ArtP. We recently identified two putative binding sites in ArtJ that might interact with the Art(MP)2 complex, thereby initiating the transport cycle (A. Vahedi-Faridi et al., J. Mol. Biol. 375:448-459, 2008). Here we investigated the contribution of charged amino acid residues in the second extracellular loop of ArtM to contact with ArtJ. Our results demonstrate a crucial role for residues K177, R185, and E188, since mutations to oppositely charged amino acids or glutamine led to a complete loss of ArtJ-stimulated ATPase activity of the complex variants in proteoliposomes. The defects could not be suppressed by ArtJ variants carrying mutations in site I (K39E and K152E) or II (E163K and D170K), suggesting a more complex interplay than that by a single salt bridge. These findings were supported by cross-linking assays demonstrating physical proximity between ArtJ(N166C) and ArtM(E182C). The importance of positively charged residues for receptor-transporter interaction was underscored by mutational analysis of the closely related transporter HisJ/LAO-HisQMP2 of Salmonella enterica serovar Typhimurium. While transporter variants with mutated positively charged residues in HisQ displayed residual ATPase activities, corresponding mutants of HisM could no longer be stimulated by HisJ/LAO. Interestingly, the ATPase activity of the HisQM(K187E)P2 variant was inhibited by l- and d-histidine in detergent, suggesting a role of the residue in preventing free histidine from gaining access to the substrate binding site within HisQM.
ATP-binding cassette (ABC) transporters are found in all three kingdoms of life and form one of the largest protein families. Members of the family catalyze the uptake or export across biological membranes of a large variety of substrates, ranging from small inorganic ions to proteins, at the expense of ATP (16).
ABC transporters display a modular architecture composed of two nucleotide-binding domains (NBDs) that contain all conserved sequence motifs, such as Walker A and B sites and the ABC signature (LSGGQ motif) (31), and of two transmembrane domains (TMDs), which can be arranged in any possible combination (7, 11).
Structural and biochemical data suggest that alternating access of the translocation pore to the intra- and extracellular spaces achieves a net transport of substrate. The key feature of this model is that the alternation of the TMDs between outward-facing and inward-facing conformations is energized by the NBDs' catalytic cycle. The latter comprises NBD dimer closure upon ATP binding, hydrolysis of ATP in the closed conformer, and reopening toward a semiclosed, ADP-bound state (reviewed in reference 11).
Canonical ABC import systems that are involved in diverse cellular functions and thus far confined to prokaryotes require an additional module, either an extracellular solute binding protein (SBP) or a receptor, for functionality (27, 37, 38). While in Gram-negative bacteria SBPs are found as soluble proteins in the periplasm, in Gram-positive bacteria the binding proteins are anchored to the outer leaflet of the cytoplasmic membrane by fatty acids that are covalently linked to the N-terminal cysteine residue (34). SBPs typically consist of two lobes that are connected by a linker region. The interface between the two lobes forms the substrate binding site. Upon binding of ligand, the proteins undergo a conformational change from an open toward a closed state (27, 38), which initiates the transport process by interaction with extracytoplasmic peptide regions of TMDs of the cognate ABC transporter (12). Receptor-transporter interactions are highly specific, but the molecular details are still poorly understood. Three X-ray structures of complete ABC importers cocrystallized with their cognate solute binding proteins and mutational analyses have shed some light on the molecular basis of these interactions (5, 17, 19, 26). Accordingly, charged residues were found to contribute to the interface between liganded binding proteins and extracellular loops of the transmembrane subunits. However, whether salt bridges are a general feature of receptor-transporter interfaces, as implied by these structures, remains to be established.
To address this question, we studied two well-characterized members of the PAAT (polar amino acid transporter) subfamily of ABC transporters (7) for which crystal structures are still elusive (1, 3, 30).
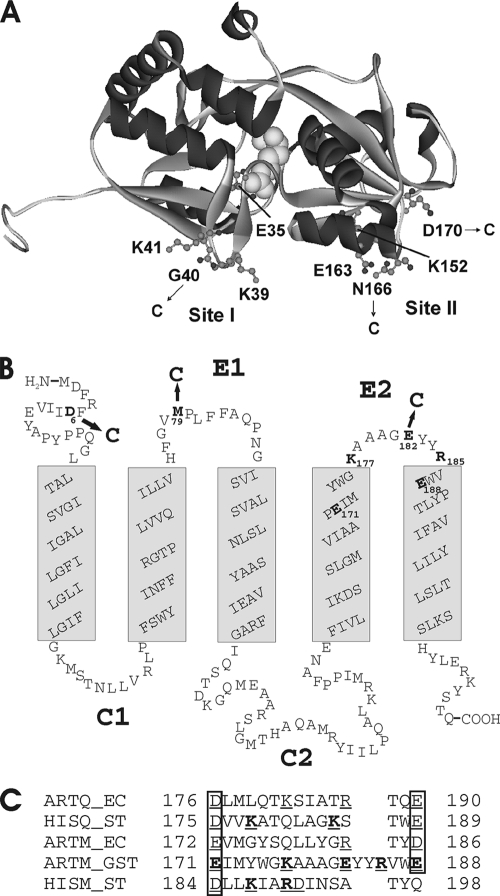
The ArtJ-Art(MP)2 transporter of Geobacillus stearothermophilus is composed of the solute receptor ArtJ, a homodimer of the membrane-integral subunit, ArtM, predicted to span the membrane five times, and a homodimer of the ATPase subunit, ArtP (14). Recently, we reported the crystal structures of ArtJ in complex with arginine, lysine, and histidine and identified residues in ArtJ that are crucial for interaction with the Art(MP)2 complex. These cluster in two putative binding sites, at the N- and C-terminal lobes of the protein (36) (Fig. 1A), as revealed by superimposition of ArtJ with the complex-bound receptor ModA of the molybdate/tungstate transporter (17). In particular, residue E163 from site II is of interest, as its replacement with glutamine completely abolished the capability of the variant to stimulate ATPase activity of Art(MP)2 in proteoliposomes. We speculated that E163 might form a salt bridge with a positively charged residue in ArtM. In fact, close inspection of the predicted extracytoplasmic loop regions of ArtM revealed two positively charged residues in loop E2, but there were also acidic residues present (Fig. 1B). Moreover, basic residues were also found in the corresponding peptide regions of close relatives of the ArtMP transporter with experimentally proven substrate specificities (Fig. 1C), suggesting common functions.
FIG. 1.
(A) Crystal structure of ArtJ complexed with l-arginine. The figure was drawn with DS ViewerPro 6.0 (Accelrys, Cambridge, United Kingdom), using the coordinates from protein sequence 2Q2A in the Brookhaven Protein Data Bank. l-Arginine is shown in space-filling representation. Residues mutated in this study are indicated. Site I and site II denote putative interaction sites with Art(MP)2 (36). (B) Topological model of ArtM. Membrane-spanning helices were predicted using the program TMHMM 2.0 (http://www.cbs.dtu.dk). Residues mutated in this study are indicated. E1 and E2, extracellular loop regions; C1 and C2, cytoplasmic loop regions. (C) Sequence alignment of the extended loop region E2 of ArtM and its close relatives. The proteins considered are as follows: ArtQ_EC and ArtM_EC, membrane-spanning subunits of the E. coli arginine transporter (GenBank accession no. CAA60103 and CAA60104); HisQ_ST and HisM_ST, membrane-spanning subunits of the S. enterica serovar Typhimurium histidine transporter (GenBank accession no. NP_461295 and NP_461294); and ArtM_GST, membrane-spanning subunit of the arginine transporter of G. stearothermophilus (currently within contig 298 of the incomplete genomic sequence of strain DSMZ 13240 [http://www.genome.ou.edu/bstearo.html]; the protein is 100% identical with the sequence ZP_03556510 of the sequenced genome from Geobacillus sp. strain Y412MC61). Charged residues in each sequence are underlined, and those that were mutated in this study are given in bold.
In this study, we investigated the putative role of the E2 loop in ArtM in transporter function by mutational analysis and site-specific cross-linking assays. In a comparative effort, we additionally studied the related but more complex histidine transporter of Salmonella enterica serovar Typhimurium. This system is composed of the membrane-spanning subunits HisM and HisQ, a homodimer of the ATPase subunit, HisP, and as a rare exception from the rule, two solute binding proteins, HisJ and LAO, which are ∼70% identical (1, 3). While HisJ displays its highest affinity for histidine (25), the LAO protein (product of the argT gene) prefers lysine, arginine, and ornithine over histidine (24).
Our results demonstrate that site II of ArtJ is presumably in close contact with loop E2 of ArtM and that positively charged residues of the latter are crucial for receptor-transporter interaction. These results were underscored by the finding that positively charged residues of the corresponding loops of HisQ and HisM are likewise required for histidine transporter function. Notably, a K187E mutation of HisM caused sensitivity of the transporter to inhibition by l- and d-histidine.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
All strains and plasmids used in this study are listed in Table 1. Escherichia coli strain JM109 served as a host for general cloning purposes. Bacteria were usually grown in LB (23) or TB (35) medium, supplemented with ampicillin (100 μg ml−1) and/or chloramphenicol (20 μg ml−1) if required.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant genotype or descriptionb | Reference or source |
|---|---|---|
| Escherichia coli strains | ||
| JM109 | e14− (mcrA) recA1 endA1 gyrA96 thi-1 hsdR17(rK− mK+) supE44 relA1 Δ(lac-proAB) F′[traD36 proAB+lacIqlacZΔM15] | Stratagene (La Jolla, CA) |
| Rosetta2 (DE3)pLysS | F−ompT hsdSB(rB− mB−) gal dcm (DE3) pLysS pRARE2 (Cmr) | Novagen (Bad Soden, Germany) |
| Rosetta2 (DE3)pLacI | F−ompT hsdSB(rB− mB−) gal dcm (DE3) pLacI pRARE2 (Cmr) | Novagen (Bad Soden, Germany) |
| Plasmids | ||
| Expression vectors | ||
| pQE60 | Apr, pT5, His6 coding sequence (3′) | Qiagen |
| pET15b | Apr, pT7lac, His6 coding sequence (5′), thrombin cleavage site | Novagen |
| pET22b | Apr, pT7lac, His6 coding sequence (3′) | Novagen |
| artJMP plasmids of G. stearothermophilus | ||
| pSN1 | artJGST on pET15b | Identical to pSS1 in reference 36 |
| pVE17 | artJ(N166C)GST on pSN1 | This study |
| pVE30 | artJ(E35K)GST on pSN1 | This study |
| pVE31 | artJ(G40A)GST on pSN1 | This study |
| pVE32 | artJ(K41E)GST on pSN1 | This study |
| pVE33 | artJ(E163K)GST on pSN1 | This study |
| pVE34 | artJ(D170K)GST on pSN1 | This study |
| pVE52 | artJ(G40C)GST on pSN1 | This study |
| pVE58 | artJ(K152E)GST on pSN1 | This study |
| pVE62 | artJ(K39E)GST on pSN1 | This study |
| pDW51 | artJ(D170C)GST on pSN1 | This study |
| pRF2 | artMPGST on pQE60 | 14 |
| pVE35 | artM(K177E)PGST on pRF2 | This study |
| pDW54 | artM(K177Q)PGST on pFR2 | This study |
| pVE36 | artM(R185E)PGST on pRF2 | This study |
| pDW55 | artM(R185Q)PGST on pRF2 | This study |
| pVE59 | artM(E171K)PGST on pRF2 | This study |
| pVE60 | artM(E188K)PGST on pRF2 | This study |
| pDW56 | artM(E188Q)PGST on pRF2 | This study |
| pVE56 | artMP(C46S)GST on pRF2 | This study |
| pVE18 | artM(D6C)P*GST on pVE56 | This study |
| pVE22 | artM(M79C)P*GST on pVE56 | This study |
| pVE23 | artM(E182C)P*GST on pVE56 | This study |
| argT-hisJQMP plasmids of S. enterica serovar Typhimuriuma | ||
| pSN2 | hisJST on pET15b | Lab collection |
| pSN3 | argTST on pET15b | Lab collection |
| pVE26 | hisQMPST on pET22 | This study |
| pVE37 | argT(D149K)ST on pSN3 | This study |
| pVE39 | hisJ(D149K)ST on pSN2 | This study |
| pVE41 | hisQM(K187E)PST on pVE26 | This study |
| pDW52 | hisQM(K187Q)PST on pVE26 | This study |
| pVE42 | hisQM(R190E)PST on pVE26 | This study |
| pDW53 | hisQM(R190Q)PST on pVE26 | This study |
| pVE43 | hisQ(K178E)MPST on pVE26 | This study |
| pVE44 | hisQ(K185E)MPST on pVE26 | This study |
argT encodes the LAO protein.
*, Cys-less variant.
Construction of plasmids and mutagenesis.
The hisQMP genes were amplified from chromosomal DNA of S. enterica serovar Typhimurium by PCR, thereby introducing NdeI and XhoI restriction sites at the 5′ and 3′ ends of the resulting DNA fragment, respectively. Subsequent ligation with expression vector pET22b, previously digested with the same enzymes, yielded plasmid pVE26. The hisJ and argT genes, the latter of which encodes the LAO protein, were amplified likewise and subsequently inserted into the expression vector pET15b, resulting in plasmids pSN2 and pSN3, respectively. Site-directed mutagenesis was carried out by using a Stratagene QuikChange kit according to the manufacturer's instructions.
Protein purification.
ArtJ (wild type and variants) was overproduced and purified as described in reference 36. Briefly, cells of E. coli strain Rosetta(pLysS) transformed with pSN1 or derivatives were grown in LB (Luria-Bertani) medium supplemented with ampicillin and chloramphenicol at 37°C. At an optical density at 650 nm (OD650) of 0.5, overexpression was induced by adding 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG), and growth was continued for 4 h. Cells were harvested, resuspended in 50 mM MOPS [3-(N-morpholino)propanesulfonic acid]-KOH, pH 7, 100 mM NaCl, 0.1 mM phenylmethylsulfonyl fluoride (PMSF), and disintegrated by passage through a French press. After ultracentrifugation, the supernatant containing His6-tagged ArtJ was subjected to metal-affinity chromatography using a Talon resin (Clontech).
Art(MP)2 (wild type and variants) was purified by making use of a His6 tag engineered into the carboxyl terminus of ArtP as described in reference 14. Briefly, E. coli strain JM109 transformed with pRF2 or derivatives was grown in LB-ampicillin to an OD650 of 0.5 prior to the addition of 0.5 mM IPTG for induction of gene expression. For purification of Art(MP)2, the membrane fraction obtained after cell disruption and ultracentrifugation (1 h at 200,000 × g) was resuspended in 50 mM Tris-HCl, pH 8, 5% (vol/vol) glycerol, 0.1 mM PMSF and solubilized with 1.2% decanoyl sucrose. After ultracentrifugation (30 min at 200,000 × g), the supernatant was adjusted to 10 mM ATP and 20 mM 2-mercaptoethanol and purified on a Talon matrix in the presence of 0.05% β-d-dodecylmaltoside.
HisJ and LAO were purified from the cytosolic fraction of E. coli strain Rosetta2(pLysS) harboring plasmid pSN2 (HisJ) or pSN3 (LAO) by metal-affinity chromatography on a Talon resin essentially as described for ArtJ (36). HisQMP2 was solubilized from the membrane fraction of E. coli strain Rosetta2(pLysS, pVE26) by use of decanoyl sucrose (1.2%) as described previously (3) and was purified on a Talon resin in the presence of β-d-dodecylmaltoside (0.05%), essentially as described for Art(MP)2.
Preparation of proteoliposomes.
Incorporation of Art(MP)2 into liposomes prepared from G. stearothermophilus total lipids in the presence or absence of arginine-loaded ArtJ was achieved as described in reference 36. Briefly, lipids (20 mg) were dried under a stream of nitrogen, slowly redissolved in 1 ml 50 mM MOPS-KOH, pH 7.5, containing 1% octyl-β-d-glucopyranoside, and sonicated for 15 min. Subsequently, Art(MP)2 variants (50 μg), ArtJ variants (230 μg), and l-arginine (1 mM final concentration) were added to 125 μl of the lipid-detergent mixture, resulting in a final volume of 300 μl. Proteoliposomes were formed by removal of detergent by adsorption to Biobeads (100 mg; Bio-Rad, Munich, Germany) at 4°C overnight. After replacing the beads with a new batch, incubation continued for 2 h. The mixture was then centrifuged for 1 min at 10,000 × g to pellet the beads, and subsequently, proteoliposomes were recovered by ultracentrifugation for 30 min at 220,000 × g, resuspended in 50 mM MOPS-KOH, pH 7.5, and assayed for ATPase activity. As controls, proteoliposomes were prepared by the same procedure, but omitting ArtJ variants and l-arginine. Proteoliposomes containing HisQMP2 were prepared in essentially the same way, but using E. coli total lipids (Avanti Polar Lipids) and 120 μg HisJ or LAO protein.
Cross-linking experiments.
Cross-linking experiments using Cu(1,10-phenanthroline)2SO4 (CuPhe) were performed essentially as described in reference 9. Complex (2.2 μM) and receptor variants (5 μM) were incubated in 50 mM MOPS-KOH, pH 7.5, 5% (vol/vol) glycerol, 0.3 M NaCl, 10 mM ATP, and 0.05% β-d-dodecylmaltoside with 3 μM CuSO4 and 9 mM 1,10-phenanthroline for 20 min at room temperature. Subsequently, the reaction was terminated by the addition of 5 mM N-ethylmaleimide, and aliquots of each sample were analyzed by SDS-PAGE (15% polyacrylamide) under nonreducing conditions unless stated otherwise.
Analytical procedures.
ATPase activity was assayed essentially as described previously (3). The assay temperature was 60°C for Art(MP)2 and 37°C in the case of HisQMP2. Reactions were started by adding detergent-solubilized or reconstituted complex to preheated buffer containing 50 mM MOPS (pH 7.5), 5% glycerol (vol/vol), 3 mM MgCl2 (10 mM in the case of soluble protein), and 2 mM ATP. Aliquots (25 μl containing 4 and 2 μg of protein, respectively) were taken in 4-min intervals and placed into wells of a microtiter plate containing 25 μl of a 12% sodium dodecyl sulfate solution. The amount of liberated phosphate was determined colorimetrically with ammonium molybdate complexes, using Na2HPO4 as the standard.
In-gel proteolysis for mass spectrometric characterization was carried out using the protocol of Shevchenko et al. (32), with minor modifications. For analysis of samples originating from organisms with unsequenced genomes, nano-high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry (nano-HPLC-ESI-MS/MS) was performed with an Agilent 1100 nano-HPLC system (Agilent, Waldbronn, Germany) coupled to a Finnigan LTQ-FT mass spectrometer (Thermo Scientific, Bremen, Germany) via a NanoMate 100 electrospray system (Advion, Ithaca, NY). The mass spectrometer recorded MS/MS spectra of interesting ions in the data-dependent acquisition mode. Raw spectra were submitted to the Sequest engine in the Bioworks software package (version 3.3.1; Thermo Scientific) for searches against custom databases containing expected sequences and sequences from related organisms, which were appended to a target/decoy database of E. coli K-12 sequences (28). The filter criterion was a peptide probability of <0.05, using at least two peptides with different sequences.
Protein determination, SDS-PAGE, and immunoblotting were carried out as described in reference 8. Binding assays with radiolabeled substrates were performed as described previously (36).
RESULTS
Mutational analysis of charged residues in extracellular loop E2 of ArtM.
In order to investigate the role of charged residues in the second extracellular loop region of ArtM in interaction with the receptor, we replaced E171, K177, R185, and E188 with oppositely charged residues (Fig. 1B and C). The variants were purified, incorporated into liposomes, and analyzed for functional consequences of the mutations by monitoring ArtJ/arginine-stimulated ATP hydrolysis. As shown previously (14), and in agreement with findings reported for other ABC importers (3, 14, 21, 34), the reconstituted Art(MP)2 complex displayed low intrinsic ATPase activity that was increased severalfold (in this case, 5-fold) by substrate-loaded ArtJ included in the lumen of the liposomes (Table 2). Receptor-stimulated ATPase activity is generally taken as a measure to demonstrate coupling of ATP hydrolysis to substrate translocation and, thus, functionality of the system (3, 12, 19, 20).
TABLE 2.
ATPase activities of Art(MP)2 variants in proteoliposomes stimulated by wild-type ArtJ and mutant proteins
| Art(MP)2 variant (amino acid change in ArtM) | ArtJ variant | ATPase activitya (μmol Pi min−1 mg−1) | Stimulation by ArtJ variant (n-fold) |
|---|---|---|---|
| Wild type | None | 0.1 ± 0.02 | |
| Wild type | 0.5 ± 0.04 | 5 | |
| K39E | 0.22 ± 0.01 | 2.2 | |
| K152E | 0.25 ± 0.03 | 2.5 | |
| E171K | None | 0.125 ± 0.03 | |
| Wild type | 0.2 ± 0.04 | 1.6 | |
| K39E | 0.18 ± 0.02 | 1.4 | |
| K152E | 0.19 ± 0.03 | 1.5 | |
| E188K | None | 0.08 ± 0.03 | |
| Wild type | 0.09 ± 0.02 | ||
| K39E | 0.07 ± 0.03 | ||
| K152E | 0.09 ± 0.01 | ||
| E188Q | None | 0.08 ± 0.02 | |
| Wild type | 0.08 ± 0.02 | ||
| Wild type | None | 0.11 ± 0.03 | |
| Wild type | 0.56 ± 0.04 | 5 | |
| E163K | 0.09 ± 0.01 | ||
| D170K | 0.48 ± 0.02 | 4.4 | |
| K177E | None | 0.16 ± 0.04 | |
| Wild type | 0.16 ± 0.02 | ||
| E163K | 0.13 ± 0.04 | ||
| D170K | 0.15 ± 0.01 | ||
| K177Q | None | 0.16 ± 0.02 | |
| Wild type | 0.11 ± 0.03 | ||
| R185E | None | 0.092 ± 0.05 | |
| Wild type | 0.092 ± 0.03 | ||
| E163K | 0.09 ± 0.05 | ||
| D170K | 0.087 ± 0.04 | ||
| R185Q | None | 0.13 ± 0.01 | |
| Wild type | 0.16 ± 0.03 | 1.2 |
ATPase activities were measured as described in Materials and Methods. Data are means with standard errors (n > 3).
In analysis in the presence of arginine-loaded wild-type ArtJ, 1.6-fold stimulation of the intrinsic ATPase activity was observed for a complex containing ArtM(E171K). In contrast, ArtJ failed to stimulate the activity of any of the remaining variants. Moreover, replacing selected residues with glutamine (polar but uncharged) resulted in a similar loss of activity (Table 2).
To exclude the (unlikely) possibility that the mutations affected the catalytic activity of the ArtP dimer, we monitored ATPase activity of the variants in detergent solution. It is well established that detergent-solubilized ABC importers exhibit an uncoupled ATPase activity that in most, but not all, cases is insensitive to the presence of liganded receptor (3, 14, 29). Compared to wild-type Art(MP)2 (0.43 μmol Pi min−1 mg−1), activities ranging from 70 to 100% were found for all variants (not shown). Together, these results suggest that the mutations impair correct interactions of the ArtM dimer with ArtJ.
Next, we addressed the question of whether the defects of the ArtM variants could be suppressed by ArtJ mutants carrying oppositely charged residues in sites I and II from those in wild-type ArtJ (Fig. 1A). To this end, ArtJ K39E (site I), K152E, E163K, and D170K (all site II) variants were constructed and purified. Subsequently, the dissociation constant (Kd) for l-arginine was determined for each variant to exclude effects of the mutations on substrate binding. The mutants displayed Kd values only 2- to 3-fold higher than that of wild-type ArtJ (0.039 μM) (36; data not shown), indicating a largely intact substrate binding site.
We next monitored ATPase activity of reconstituted wild-type Art(MP)2 in the presence of the ArtJ variants. As summarized in Table 2, ArtJ(K39E) and ArtJ(K152E) stimulated ATP hydrolysis about half as much as wild-type ArtJ did, indicating no crucial role of a positively charged residue at this position. Furthermore, both ArtJ variants failed to restore activity of a complex containing ArtM(E171K) above the wild-type ArtJ level, suggesting that formation of single ion pairs between these residues is rather unlikely. The same holds for the ArtM(E188K) variant, whose defect was suppressed by neither ArtJ(K39E) nor ArtJ(K152E).
Concerning the ArtJ variants with a negative-to-positive switch of charges, the E163K variant, like an E163Q variant (36), had completely lost any stimulating effect on wild-type Art(MP)2. Likewise, the variant was incapable of restoring the activity of complexes containing ArtM(K177E) or ArtM(R185E). ArtJ(D170K) stimulated Art(MP)2 almost like wild-type ArtJ did, suggesting that a negative charge at this position is dispensable, and thus it also failed to suppress the defects of the ArtM variants tested (Table 2).
Cross-linking reveals physical proximity of ArtJ (site II) and loop E2 of ArtM.
Although these findings provided no direct clue for ion pair formation between the considered residues in ArtJ and ArtM, the mutated residues in ArtM are nonetheless crucial for transport, which underscores the role of loop E2 in ArtJ-ArtM interaction. Consequently, we set out to study the physical proximity of the putative binding sites I and II of ArtJ to extracellular peptide regions of ArtM by site-directed cross-linking. To this end, ArtM residues D6 (N-terminal region), M79 (loop E1), and E182 (loop E2) were replaced by cysteine (Fig. 1B), and the respective complex variants were purified according to the method used for the wild type. To reduce unspecific reactions, the only native cysteine residue of the transporter (ArtP C46) was replaced by serine. All mutants were analyzed for ATPase activity in proteoliposomes in the presence of wild-type ArtJ to demonstrate functionality of the proteins. As summarized in Table 3, all mutants displayed substantial activity compared to the wild type.
TABLE 3.
ArtJ-stimulated ATPase activities of monocysteine variants of Art(MP)2 in proteoliposomes
| Art(MP)2 variant | ArtJ variant | ATPase activitya (μmol Pi min−1 mg−1) | Stimulation by ArtJ variant (n-fold) |
|---|---|---|---|
| Wild type | None | 0.1 ± 0.02 | |
| Wild type | 0.5 ± 0.04 | 5 | |
| Art(M(D6C)P*)2 | None | 0.1 ± 0.03 | |
| Wild type | 0.3 ± 0.02 | 3 | |
| Art(M(M79C)P*)2 | None | 0.08 ± 0.01 | |
| Wild type | 0.26 ± 0.05 | 3.3 | |
| Art(M(E182C)P*)2 | None | 0.09 ± 0.02 | |
| Wild type | 0.27 ± 0.04 | 3 |
ATPase activities were measured as described in Materials and Methods. Data are means with standard errors (n > 4).
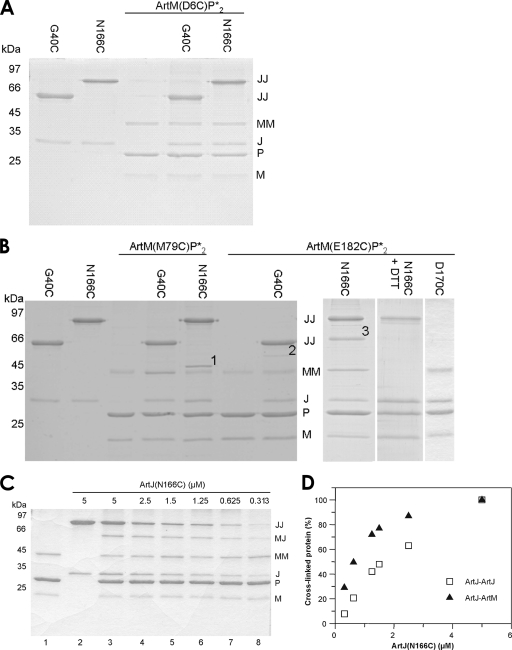
Monocysteine variants of ArtJ representing putative interaction sites I and II were constructed at positions G40 and N166, respectively (Fig. 1A). For both variants, a 3- to 4-fold higher dissociation constant (for the G40C variant, 0.095 μM; and for the N166C variant, 0.14 μM) (36) than that of the wild type was determined. Both ArtJ variants displayed less stimulation of the ATPase activity of Art(MP)2 when assayed under standard conditions. However, almost full activity could be restored by adding dithiothreitol (DTT), suggesting that the effect was due to spontaneous dimer formation of the monocysteine variants under nonreducing conditions (Fig. 2B). Initial cross-linking experiments were then performed with all possible combinations of complex and receptor variants in the presence of Cu(1,10-phenanthroline)2SO4 (CuPhe) in detergent solution. As shown in Fig. 2B, additional bands compared to those for samples containing either complex or receptor were observed for ArtJ(N166C) in combination with complexes containing ArtM(M79C) (band 1) and ArtM(E182C) (band 3). A faint band was also visible when ArtJ(G40C) was combined with Art(M(E182C)P)2 (band 2). No disulfide bond was formed between ArtM(D6C) and the ArtJ variants (Fig. 2A). Immunoblots of the same samples probed with an antibody directed against the N-terminal His6 tag of the ArtJ variants clearly revealed the presence of ArtJ in bands 1 and 3. A weak reaction was obtained with band 2, which could not, however, be intensified by longer exposures to the developing agent (not shown). Thus, band 2 was omitted from further analyses. Since an antiserum against ArtM is not available, we determined the chemical nature of bands 1 and 3 by mass spectrometry. The analysis identified peptides belonging to both ArtJ and ArtM for band 3, while in the case of band 1, only peptides aligning with ArtJ were found. Due to this ambiguous result, only band 3 was investigated further. Additional proof for band 3 being a product of cross-linking due to disulfide bond formation was obtained by subjecting a CuPhe-treated sample of Art(M(E182C)P)2-ArtJ(N166C) to SDS-PAGE in the presence of DTT. As expected, band 3 was clearly absent under reducing conditions (Fig. 2B). The apparent lack of interaction between ArtJ(N166C) and ArtM(D6C) already provided a first hint for the specificity of the reaction with ArtM(E182C) leading to band 3. This notion was further strengthened by the finding that ArtJ(D170C) failed to form a cross-linked product with Art(M(E182C)P)2 (Fig. 2B). Residue D170 is positioned at the periphery of site II (Fig. 1A) and displayed no phenotype when mutated to lysine (Table 2).
FIG. 2.
Site-specific CuPhe-induced cross-linking of ArtJ and ArtM variants in detergent solution. Experiments were carried out as described in Materials and Methods. JJ and MM denote dimers of ArtJ and ArtM variants, respectively. (A) Cross-linking of ArtJ(G40C) and ArtJ(N166C) with Art(M(D6C)P*)2. (B) Cross-linking of ArtJ(G40C), ArtJ(N166C), and ArtJ(D170C) with Art(M(M79C)P*)2 and Art(M(E182C)P*)2. Possible cross-links between ArtJ and ArtM are indicated by numbers. (C) Cross-linking of ArtJ(N166C) with Art(M(E182C)P*)2 as a function of ArtJ concentration. Reactions were performed as described above, using a constant complex concentration and various receptor concentrations, as indicated. Lanes: 1, Art(M(E182C)P*)2; 2, ArtJ(N166C); 3 to 8, Art(M(E182C)P*)2 cross-linked with ArtJ(N166C) at concentrations ranging from 5 to 0.313 μM. (D) Quantitation by densitometric scanning of cross-linked ArtJ-ArtJ and ArtJ-ArtM products from panel C. Amounts of cross-linked proteins relative to those determined with 5 μM ArtJ(N166C) (100%) were plotted against the ArtJ(N166C) concentration.
Additionally, we performed cross-link experiments with different ArtJ concentrations. Since cross-linking results from collisions between reactive cysteine pairs, accidental (nonspecific) reactions should be linearly dependent on the protein concentration, while cross-links due to specific protein-protein interactions should not. The results are shown in Fig. 2C. An ArtM-ArtJ cross-link was clearly visible even with the lowest ArtJ concentration (0.3 μM). Quantitation by densitometric scanning of the ArtM-ArtJ and ArtJ-ArtJ cross-links and plotting of the data as a function of ArtJ concentration revealed that in contrast to the case for ArtJ-ArtJ cross-links, formation of the ArtM-ArtJ band clearly occurred nonlinearly, thus being consistent with a specific interaction (Fig. 2D). [Note that the absolute number of ArtJ-ArtJ dimers was higher than that of the ArtM-ArtJ cross-links due to storage of ArtJ(N166C) under nonreducing conditions in order to ensure optimal oxidation conditions in the presence of CuPhe (Fig. 2C, lane 2).]
Next, we performed cross-linking experiments with the reconstituted system, since the interaction with the receptor might be different in a lipid environment. The results were perfectly in line with those obtained for detergent solution (not shown).
In the initial stage of this project, we noticed that an active transport complex could be purified only in the presence of ATP or ADP. Thus, the above cross-linking results were obtained with ATP-bound complex preparations. In order to investigate whether formation of the ArtJ(N166C)-ArtM(E182C) product was dependent on ATP, we carried out cross-linking with a complex preparation that was purified in the presence of ADP, thereby mimicking the posthydrolysis state. No difference in the amount of cross-linked product was observed. The same result was obtained with an ArtJ(N166C) preparation that was extensively dialyzed to minimize the amount of bound arginine. However, as found previously, the procedure does not yield a preparation completely free of substrate (36), which might explain the result. Together, these findings support an interaction of site II of ArtJ with the second loop of ArtM.
Comparative mutational analysis of the histidine transporter HisJ/LAO-HisQMP2 of S. enterica serovar Typhimurium.
Finally, we wished to investigate whether loop E2 of closely related amino acid transporters is similarly important for receptor-transporter interactions, as implied by sequence alignment (Fig. 1C). The experiments with the ArtJ-Art(MP)2 complex revealed a crucial role of positively charged residues in loop E2 in contacting the binding protein. Thus, we performed a mutational analysis of the histidine ABC transporter of S. enterica serovar Typhimurium.
We focused on investigating the roles of K178 and K185 of HisQ and of K187 and R190 of HisM by single replacement of these residues with glutamate. Purified variants were reconstituted into proteoliposomes and analyzed for stimulation of ATPase activity by wild-type HisJ and LAO. All transporter variants exhibited ATPase activities in detergent solution which were highly comparable to that of the wild type (0.15 μmol Pi min−1 mg−1), thereby indicating that the mutations did not affect ATP binding/hydrolysis at the HisP dimer.
Reconstituted wild-type HisQMP2 displayed a basal activity of 0.07 μmol phosphate released per min and mg, which was stimulated 10-fold by HisJ/histidine and 5.7-fold by LAO/arginine (Table 4), which are in the range of published values (2, 3). Investigating the ATPase activity of transporter variants carrying either HisQ(K178E) or HisQ(K185E) revealed a substantially lower level of stimulation by HisJ than that of the wild-type complex, with position 178 being affected more than position 185 (Table 4). Similar results were obtained with LAO. We concluded that a negative charge at position 178 or 185 impairs but does not abolish interaction with HisJ/LAO.
TABLE 4.
HisJ/LAO-stimulated ATPase activities of HisQMP2 variants in proteoliposomes
| HisQMP2 variant | HisJ or LAO | ATPase activitya (μmol Pi min−1 mg−1) | Stimulation by HisJ/LAO (n-fold) |
|---|---|---|---|
| Wild type | None | 0.07 ± 0.02 | |
| HisJ | 0.73 ± 0.1 | 10 | |
| LAO | 0.4 ± 0.04 | 5.7 | |
| Q(K178E) | None | 0.25 ± 0.02 | |
| HisJ | 0.55 ± 0.07 | 2.2 | |
| LAO | 0.4 ± 0.03 | 1.6 | |
| Q(K185E) | None | 0.23 ± 0.01 | |
| HisJ | 1.3 ± 0.09 | 5.6 | |
| LAO | 0.9 ± 0.1 | 3.9 | |
| M(K187E) | None | 0.32 ± 0.08 | |
| HisJ | 0.31 ± 0.05 | ||
| LAO | 0.32 ± 0.04 | ||
| M(K187Q) | None | 0.64 ± 0.04 | |
| HisJ | 0.95 ± 0.04 | 1.5 | |
| LAO | 0.82 ± 0.04 | 1.3 | |
| M(R190E) | None | 0.39 ± 0.05 | |
| HisJ | 0.43 ± 0.03 | ||
| LAO | 0.39 ± 0.04 | ||
| M(R190Q) | None | 0.52 ± 0.03 | |
| HisJ | 0.67 ± 0.02 | 1.3 | |
| LAO | 0.36 ± 0.01 |
ATPase activities were measured as described in Materials and Methods. Data are means with standard errors (n = 6).
Table 4 also summarizes the effects of mutations in the second periplasmic loop of HisM on transporter activity. In contrast to the HisQ mutants, stimulation of ATPase activity above background levels by either HisJ or LAO was not observed with complexes containing HisM(K187E) or HisM(R190E). In addition, both residues were also replaced by glutamine, resulting in the same nonfunctional phenotype (Table 4).
It was routinely found that the mutations in HisQ or HisM caused a higher basal level of ATPase activity in proteoliposomes than that seen for the wild-type complex. This might indicate that a negative charge or a polar but uncharged residue at the respective positions in loop 2 affects the flexibility of HisQM in such a way that HisJ/LAO-independent (uncoupled) ATP hydrolysis is increased.
Previously, replacement of D149 in HisJ (corresponding to E163 of ArtJ) with asparagine or alanine was found to strongly impair the capability of the variants to stimulate ATPase activity of HisQMP2 (21). To study a possible participation of the residue in a salt bridge with HisQM, we monitored ATPase activity of the HisQM mutant complexes in the presence of HisJ(D149K)and LAO(D149K). Both proteins failed to raise ATPase activities above levels observed with wild-type HisJ/LAO (not shown).
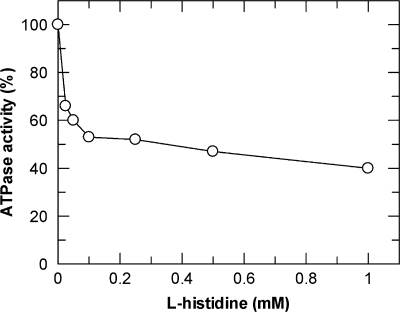
As noted above, all complex variants were routinely assayed for ATPase activity in detergent solution in the absence and presence of HisJ or LAO. Interestingly, we found that histidine-loaded HisJ seemed to inhibit the activity of the HisQM(K187E)P2 complex, while all other mutant complexes, like the wild type, were insensitive to the presence of the liganded binding protein. Exploring this unexpected finding further revealed an inhibitory effect of l-histidine on the transporter, independent of HisJ. As shown in Fig. 3, maximal inhibition of 60% was observed at 1 mM l-histidine (50% inhibitory concentration [I50], ∼0.2 mM). d-Histidine was similarly effective, while the transporter was insensitive to imidazole, lysine, or arginine, even at higher millimolar concentrations. Interestingly, the HisQMP(K187Q)P2 variant also did not exhibit this phenotype (not shown). Our findings suggest a special role of HisM K187 in transporter-receptor interactions, as discussed below.
FIG. 3.
Inhibition of ATPase activity of HisQM(K187E)P2 in detergent solution by l-histidine. Hydrolysis of ATP was monitored as described in Materials and Methods. Data represent the averages for two independent experiments.
DISCUSSION
In this communication, we report on experiments to identify residues that are involved in the interaction of binding proteins for basic amino acids with their cognate transport complexes. In particular, we have focused on the analysis of charged residues within the second extracellular (periplasmic) loop region of the pore-forming subunits ArtM of the arginine transporter of G. stearothermophilus and of the HisQM proteins of the histidine transporter of S. enterica serovar Typhimurium.
Our results demonstrate that charged residues within loop E2 are crucial for receptor-transporter interplay, as mutations to oppositely charged residues or to glutamine substantially reduced or completely abolished stimulation of ATPase activities of the variants by their respective wild-type binding proteins. While in the case of ArtM(E171K) residual ATPase activity could be monitored, complexes containing the ArtM(E188K) or ArtM(E188Q) variant failed to productively interact with ArtJ. The latter phenotype was also observed when both positively charged residues from loop E2, K177 and R185, were individually mutated to glutamate or glutamine.
Similar findings were obtained with the histidine transporter. However, while mutations in HisQ only reduced HisJ/LAO-stimulated ATPase activity, mutations affecting positively charged residues in HisM completely abolished productive interaction with the binding proteins. We found no significant difference in the interactions of the mutants with HisJ and LAO, which was not unexpected due to the high sequence identity of both proteins, which holds especially for site 2 residues.
In previous studies, structural and mutational analysis led to the proposal of two sites by which ArtJ and HisJ make contact with the pore-forming subunits of their cognate transporters (21, 36). While site I was considered a supporting site, the main interaction was assigned to residues within site II (Fig. 1A). In particular, ArtJ(E163K) and HisJ(D149K) fully lost the capability to stimulate ATPase activity (Table 2 and data not shown) and were thus considered candidates for interaction with positively charged residues of loop E2. However, none of the receptor variants analyzed suppressed the defects in transporter function caused by E2 loop mutations, which, at first glance, seems at least to question the participation of salt bridges in receptor-transporter interaction.
The crystal structure of the vitamin B12 transporter BtuF-Btu(CD)2 of E. coli revealed that salt bridges are formed between the R56 residues of both BtuC subunits and glutamate residues 72 and 202, from the N- and C-terminal lobes, respectively, of the cognate receptor, BtuF (19). Similarly, in the molybdate/tungstate transporter ModA-Mod(BC)2 of Archaeoglobus fulgidus, ion pairing is observed between D126 of both ModB monomers and K36 and R215 of the receptor, ModA (17). Both lobes of the unliganded receptor MalE of the maltose transporter, MalFGK2, also make extensive interactions with the MalFG subunits (26). Salt bridges are formed between residues in MalF and MalG and individual residues in MalE.
The arrangement seen in the Btu and Mod systems provides a plausible explanation for the experimental findings with the Art system. Taking into account that mutation of a single codon in artM introduces two identical exchanges in the ArtM homodimer, and assuming that identical positively charged residues in both copies of ArtM contact different residues in ArtJ, the switch in charge between receptor and transporter would restore only one interaction and might thus be insufficient to recover activity.
In ArtJ, the putative interaction site I contains only one negatively charged residue, E35, located at its N-terminal end. The crystal structure revealed that the residue is oriented toward the substrate binding cleft (Fig. 1A), which is consistent with the finding that a mutation to lysine caused a drop in affinity for arginine. Moreover, the mutation still allowed substantial stimulation of Art(MP)2 by the variant (36). Thus, a salt bridge between a positively charged residue in loop E2 and site I of ArtJ is rather unlikely, but a hydrogen bond cannot be ruled out. Such an interaction is found in the maltose transporter, in which R367 of MalE forms a salt bridge and a hydrogen bond with D453 and T457, respectively, of MalF.
In the case of the histidine transporter containing heterodimeric pore-forming subunits, our results also point to a mode of interaction that involves contact of a given residue in HisQM with more than one residue in the binding proteins. Otherwise, and along the above lines, a switch in charge between subunits should have recovered stimulation of ATPase activity of the transporter variant.
Together, we interpret our results to mean that a salt bridge between the conserved glutamate in site II of ArtJ, HisJ, and LAO and one of the positively charged residues in loop 2 of the corresponding membrane-spanning subunit cannot be excluded. If existing, the E2 loop residue is likely to be involved in an additional, still-to-be identified contact that is disturbed when changed to a glutamate or glutamine.
The unforeseen finding that the intrinsic ATPase activity of HisQM(K187E)P2, but not that of any of the other mutants, is inhibited in detergent by micromolar concentrations of l- or d-histidine supports the notion that loop E2 not only plays a crucial role in contacting HisJ but might be involved in guiding the released substrate to the binding site within the pore. In a previous report, the wild-type complex was shown to be inhibited by l-arginine to a similar extent, but half-maximal inhibition was obtained at a 40 times higher concentration (21). The authors interpreted their finding to mean that the postulated substrate binding site within the HisQM dimer is somewhat accessible to arginine in the absence of HisJ/LAO. Our result led us to speculate that HisM K187 might function specifically as a gatekeeper, preventing free histidine from leaking into the binding pocket. A negative charge at this position apparently eliminates this property. How a histidine molecule occupying the binding pocket interferes with the catalytic cycle is unknown. Possibly, closing and reopening of the HisP dimer upon ATP binding/hydrolysis are hampered.
The role of the second extracellular loop of ArtM in contacting ArtJ was underscored by cross-linking reactions in detergent solution and in proteoliposomes. A disulfide bond was formed between ArtJ(N166C) (site II variant) and ArtM(E182C) in the presence of ATP or ADP, mimicking pre- and posthydrolysis states of the transporter. It is well established that ATP binding causes the NBD dimer to close, while hydrolysis leads to reopening (6, 9, 15, 18, 22, 33). In the case of the maltose and histidine transporters, biochemical evidence supports the notion that the respective binding protein is associated with the complex in the absence of cofactors, including the substrate (2, 4, 10). In contrast, Doeven et al. (13) found that the oligopeptide transporter OppBCDF of the Gram-positive bacterium Lactococcus lactis, studied by fluorescence correlation spectroscopy of giant unilamellar vesicles, binds only the receptor, OppA, in its liganded form. Moreover, OppA dissociates from the transport complex upon addition of ATP. Since OppA, unlike MalE or HisJ, is a lipoprotein anchored to the outer leaflet of the lipid bilayer, it was speculated that different signaling pathways might exist between Gram-negative and Gram-positive organisms. The data presented here for the arginine transporter of the Gram-positive organism G. stearothermophilus are rather consistent with the findings on the maltose and histidine transporters. However, other than OppA, which can be overproduced in its native organism, L. lactis, synthesis of sufficient amounts of ArtJ as a lipoprotein in E. coli is not feasible. Thus, it remains to be unraveled whether membrane insertion of the binding protein would change its interaction with the transporter.
In summary, our data provide the first clue on intersubunit communication in members of the PAAT subfamily of ABC transporters in the absence of a crystal structure.
Acknowledgments
E.S. is grateful to G. F.-L. Ames (Children's Hospital Oakland Research Institute) for general support.
This work was supported by the Deutsche Forschungsgemeinschaft (grant SFB 449, B14).
Footnotes
Published ahead of print on 12 February 2010.
REFERENCES
- 1.Ames, G. F.-L. 1986. Bacterial periplasmic transport systems: structure, mechanism, and evolution. Annu. Rev. Biochem. 55:397-452. [DOI] [PubMed] [Google Scholar]
- 2.Ames, G. F.-L., C. E. Liu, A. K. Joshi, and K. Nikaido. 1996. Liganded and unliganded receptors interact with equal affinity with the membrane complex of periplasmic permeases, a subfamily of traffic ATPases. J. Biol. Chem. 272:14264-14270. [DOI] [PubMed] [Google Scholar]
- 3.Ames, G. F.-L., K. Nikaido, I. X. Wang, P.-Q. Liu, C. E. Liu, and C. Hu. 2001. Purification and characterization of the membrane-bound complex of an ABC transporter, the histidine permease. J. Bioenerg. Biomembr. 33:79-92. [DOI] [PubMed] [Google Scholar]
- 4.Bohl, E., H. A. Shuman, and W. Boos. 1995. Mathematical treatment of the kinetics of binding protein dependent transport systems reveals that both the substrate loaded and unloaded binding proteins interact with the membrane components. J. Theor. Biol. 172:83-94. [DOI] [PubMed] [Google Scholar]
- 5.Braun, V., and C. Herrmann. 2007. Docking of the periplasmic FecB binding protein to the FecCD transmembrane proteins in the ferric citrate transport system of Escherichia coli. J. Bacteriol. 189:6913-6918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, J., G. Lu, J. Lin, A. L. Davidson, and F. A. Quiocho. 2003. A tweezers-like motion of the ATP-binding cassette dimer in an ABC transport cycle. Mol. Cell 12:651-661. [DOI] [PubMed] [Google Scholar]
- 7.Dassa, E. 2003. Phylogenetic and functional classification of ABC (ATP-binding cassette) systems, p. 3-35. In I. B. Holland et al. (ed.), ABC proteins: from bacteria to man. Academic Press, New York, NY.
- 8.Daus, M. L., H. Landmesser, A. Schlosser, P. Müller, A. Herrmann, and E. Schneider. 2006. ATP induces conformational changes of periplasmic loop regions of the maltose ATP-binding cassette transporter. J. Biol. Chem. 281:3856-3865. [DOI] [PubMed] [Google Scholar]
- 9.Daus, M. L., M. Grote, P. Müller, M. Doebber, A. Herrmann, H.-J. Steinhoff, E. Dassa, and E. Schneider. 2007. ATP-driven MalK dimer closure and reopening and conformational changes of the “EAA” motifs are crucial for function of the maltose ATP-binding cassette transporter (MalFGK2). J. Biol. Chem. 282:22387-22396. [DOI] [PubMed] [Google Scholar]
- 10.Daus, M. L., S. Berendt, S. Wuttge, and E. Schneider. 2007. Maltose binding protein (MalE) interacts with periplasmic loops P2 and P1, respectively, of the MalFG subunits of the maltose ATP-binding cassette transporter (MalFGK2) from Escherichia coli/Salmonella during the transport cycle. Mol. Microbiol. 66:1107-1122. [DOI] [PubMed] [Google Scholar]
- 11.Davidson, A. L., E. Dassa, C. Orelle, and J. Chen. 2008. Structure, function, and evolution of bacterial ATP-binding cassette systems. Microbiol. Mol. Biol. Rev. 72:317-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davidson, A. L., H. A. Shuman, and H. Nikaido. 1992. Mechanism of maltose transport in Escherichia coli: transmembrane signaling by periplasmic binding proteins. Proc. Natl. Acad. Sci. USA 89:2360-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doeven, M. K., G. van den Bogaart, V. Krasnikov, and B. Poolman. 2008. Probing receptor-translocator interactions in the oligopeptide ABC transporter by fluorescence correlation spectroscopy. Biophys. J. 94:3956-3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleischer, R., A. Wengner, H. Landmesser, F. Scheffel, and E. Schneider. 2005. Functional reconstitution of an arginine-lysine-ornithine ATP-binding-cassette transporter from the thermophilic gram-positive bacterium Geobacillus stearothermophilus. Microbiology 151:835-840. [DOI] [PubMed] [Google Scholar]
- 15.Grote, M., E. Bordignon, Y. Polyhach, G. Jeschke, H.-J. Steinhoff, and E. Schneider. 2008. A comparative EPR study of the nucleotide-binding domains' catalytic cycle in the assembled maltose ABC-importer. Biophys. J. 95:2924-2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holland, E. B., S. Cole, K. Kuchler, and C. F. Higgins (ed.). 2003. ABC proteins: from bacteria to man. Academic Press, New York, NY.
- 17.Hollenstein, K., D. C. Frei, and K. P. Locher. 2007. Structure of an ABC transporter in complex with its binding protein. Nature 446:213-216. [DOI] [PubMed] [Google Scholar]
- 18.Hunke, S., M. Mourez, M. Jèhanno, E. Dassa, and E. Schneider. 2000. ATP modulates subunit-subunit interactions in an ATP-binding cassette transporter (MalFGK2) determined by site-directed chemical cross-linking. J. Biol. Chem. 275:15526-15534. [DOI] [PubMed] [Google Scholar]
- 19.Hvorup, R. N., B. A. Goetz, M. Niederer, K. Hollenstein, E. Perozo, and K. P. Locher. 2007. Asymmetry in the structure of the ABC transporter-binding protein complex BtuCD-BtuF. Science 317:1387-1390. [DOI] [PubMed] [Google Scholar]
- 20.Landmesser, H., A. Stein, B. Blüschke, M. Brinkmann, S. Hunke, and E. Schneider. 2002. Large-scale purification, dissociation and functional reassembly of the maltose ATP-binding cassette transporter (MalFGK2) of Salmonella typhimurium. Biochim. Biophys. Acta 1565:64-72. [DOI] [PubMed] [Google Scholar]
- 21.Liu, C. E., P.-Q. Liu, A. Wolf, E. Lin, and G. F.-L. Ames. 1999. Both lobes of the soluble receptor of the periplasmic histidine permease, an ABC transporter (traffic ATPase), interact with the membrane-bound complex. J. Biol. Chem. 274:739-747. [DOI] [PubMed] [Google Scholar]
- 22.Lu, G., J. M. Westbrooks, A. L. Davidson, and J. Chen. 2005. ATP hydrolysis is required to reset the ATP-binding cassette dimer into the resting-state conformation. Proc. Natl. Acad. Sci. USA 102:17969-17974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 24.Nikaido, K., and G. F.-L. Ames. 1992. Purification and characterization of the periplasmic lysine-arginine-ornithine-binding protein (LAO) from Salmonella typhimurium. J. Biol. Chem. 267:20706-20712. [PubMed] [Google Scholar]
- 25.Oh, B.-H., C.-H. Kang, H. De Bondt, S.-H. Kim, K. Nikaido, A. K. Joshi, and G. F.-L. Ames. 1994. The bacterial periplasmic histidine-binding protein. Structure/function analysis of the ligand-binding site and comparison with related proteins. J. Biol. Chem. 269:4135-4143. [PubMed] [Google Scholar]
- 26.Oldham, M. L., D. Khare, F. A. Quiocho, A. L. Davidson, and J. Chen. 2007. Crystal structure of a catalytic intermediate of the maltose transporter. Nature 450:515-521. [DOI] [PubMed] [Google Scholar]
- 27.Quiocho, F. A. 1990. Atomic structures of periplasmic binding proteins and the high affinity active transport systems in bacteria. Philos. Trans. R. Soc. Lond. B 326:341-351. [DOI] [PubMed] [Google Scholar]
- 28.Reidegeld, K. A., M. Eisenacher, M. Kohl, D. Chamrad, G. Körting, M. Blüggel, H. E. Meyer, and C. Stephan. 2008. An easy-to-use Decoy Database Builder software tool, implementing different decoy strategies for false discovery rate calculation in automated MS/MS protein identifications. Proteomics 8:1129-1137. [DOI] [PubMed] [Google Scholar]
- 29.Scheffel, F., R. Fleischer, and E. Schneider. 2004. Functional reconstitution of a maltose ATP-binding cassette transporter from the thermoacidophilic gram-positive bacterium Alicyclobacillus acidocaldarius. Biochim. Biophys. Acta 1656:57-65. [DOI] [PubMed] [Google Scholar]
- 30.Schneider, E. 2003. Import of solutes by ABC transporters—the maltose and other systems, p. 157-185. In E. B. Holland, S. Cole, K. Kuchler, and C. Higgins (ed.), ABC proteins: from bacteria to man. Academic Press, New York, NY.
- 31.Schneider, E., and S. Hunke. 1998. ATP-binding-cassette (ABC) transport systems: functional and structural aspects of the ATP-hydrolyzing subunits/domains. FEMS Microbiol. Rev. 22:1-20. [DOI] [PubMed] [Google Scholar]
- 32.Shevchenko, A., H. Tomas, J. Havlis, J. V. Olsen, and M. Mann. 2006. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 1:2856-2860. [DOI] [PubMed] [Google Scholar]
- 33.Smith, P. C., N. Karpowich, L. Millen, J. E. Moody, J. Rosen, P. J. Thomas, and J. F. Hunt. 2002. ATP binding to the motor domain from an ABC transporter drives formation of a nucleotide sandwich dimer. Mol. Cell 10:139-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sutcliffe, I. C., and R. B. Russell. 1995. Lipoproteins of gram-positive bacteria. J. Bacteriol. 177:1123-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tartoff, K. D., and C. A. Hobbs. 1987. Improved media for growing plasmid and cosmid clones. Bethesda Res. Labs Focus 9:12. [Google Scholar]
- 36.Vahedi-Faridi, A., V. Eckey, F. Scheffel, C. Alings, H. Landmesser, E. Schneider, and W. Saenger. 2008. Crystal structures and mutational analysis of the arginine-, lysine-, histidine-binding protein ArtJ from Geobacillus stearothermophilus. Implications for interactions of ArtJ with its cognate ATP-binding cassette transporter, Art(MP)2. J. Mol. Biol. 375:448-459. [DOI] [PubMed] [Google Scholar]
- 37.van der Heide, T., and B. Poolman. 2002. ABC transporters: one, two or four extracytoplasmic substrate-binding sites? EMBO Rep. 3:938-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilkinson, A. J., and K. H. G. Verschueren. 2003. Crystal structures of periplasmic solute binding proteins in ABC-transport complexes illuminate their function, p. 187-208. In E. B. Holland, S. Cole, K. Kuchler, and C. Higgins (ed.), ABC proteins: from bacteria to man. Academic Press, New York, NY.