Abstract
As part of our ongoing efforts to uncover the phenotypic consequences of genetic variability among clinical Mycobacterium tuberculosis isolates, we previously reported that isolates of the “East Asian” or “W/Beijing” lineage constitutively overexpress the coordinately regulated transcriptional program known as the DosR regulon under standard in vitro conditions. This phenotype distinguishes the W/Beijing lineage from all other M. tuberculosis lineages, which normally induce expression of this regulon only once exposed to low oxygen or nitric oxide, both of which result in inhibition of bacterial respiration and replication. Transcription of the DosR regulon is controlled through a two-component regulatory system comprising the transcription factor DosR and two possible cognate histidine sensor kinases, DosS and DosT. Through sequence analysis of a carefully selected set of isolates representing each of the major M. tuberculosis lineages, we describe herein a naturally occurring frameshift mutation in the gene encoding the DosT sensor kinase for isolates of the most recently evolved W/Beijing sublineages. Intriguingly, the occurrence of the frameshift mutation correlates precisely with the appearance of the constitutive DosR regulon phenotype displayed by the same “modern” W/Beijing strains. However, complementation studies have revealed that the mutation in dosT alone is not directly responsible for the constitutive DosR regulon phenotype. Our data serve to highlight the evolutionary pressure that exists among distinct M. tuberculosis lineages to maintain tight control over DosR regulon expression.
The World Health Organization (WHO) estimates that there are currently more than 2 billion individuals worldwide who are latently (asymptomatically) infected with the tuberculosis (TB) bacillus, Mycobacterium tuberculosis. From this vast global reservoir, which is fueled with an additional 100 million new infections annually, 9 million cases of active TB develop each year. In 2007, 1.8 million people died as a result of this devastating disease, including 456,000 deaths among HIV-positive people (72). Clearly, despite significant advances in our knowledge of the molecular events underlying M. tuberculosis metabolism and pathogenesis in the “postgenomic” era of the past decade (9, 21), any measurable improvement in terms of TB treatment and prevention has thus far remained elusive. The increasing number of reports highlighting that clinical M. tuberculosis isolates are not as genetically or phenotypically homogeneous as once thought only adds to the complexity of the challenge that we face in our efforts to combat this global disease (19).
Like other members of the M. tuberculosis complex (MTC; M. bovis, M. caprae, M. microti, etc.), M. tuberculosis exhibits a highly clonal population structure and, unlike other pathogenic bacteria, shows little evidence of being able to acquire exogenous DNA via horizontal gene transfer and recombination (59). Thus, the only way that M. tuberculosis can evolve new phenotypes (i.e., “gain of function”) or modify its existing virulence is through an alteration in the complement of genetic material that it already has available. To date, most of the phenotypic variation that has been described for M. tuberculosis is associated with single nucleotide polymorphisms (SNPs) and deletions (19, 43). To put this in context, however, M. tuberculosis strains display much less diversity on a genome-wide scale than do other well-known pathogenic bacteria, sharing ≥99.93% sequence identity at the nucleotide level (26).
Recent SNP- and deletion-based surveys of global strain collections have demonstrated that M. tuberculosis has evolved via clonal expansion into six major lineages, the majority of which show a strong association with a particular geographic region (19, 26, 50). The assumption here is that geographical restriction has led to coevolution of host and bacterial genotypes, resulting in a unique form of host-pathogen adaptation. Of the six major M. tuberculosis lineages, the “W/Beijing” lineage (also referred to as the East Asian lineage) has come under the most intense scrutiny in recent years. There are two main reasons for this. First, in several diverse regions of the world, including Russia, Bangladesh, South Africa, and Western Europe, the TB incidence due to W/Beijing strains is found to be increasing (16). For example, there is no evidence of W/Beijing strains in Cape Town (South Africa) prior to 1965, but these strains now account for 20% of all TB cases in this region (11). This has led to speculation that strains belonging to the W/Beijing lineage possess unique attributes that confer an increased ability to cause disease and to transmit within certain geographic settings or patient ethnicities (23, 24). Second, the importance of the W/Beijing family is further emphasized by the fact that their recent epidemic spread has frequently been associated with the appearance of drug resistance (7, 13, 16, 20).
As part of our ongoing efforts to uncover the molecular basis for the enhanced pathogenicity attributed to the W/Beijing lineage, we previously reported that W/Beijing strains constitutively overexpress members of the coordinately regulated transcriptional program known as the DosR (dormancy survival regulator) regulon under standard growth conditions in vitro (49). This phenotype sets the W/Beijing lineage apart from all other M. tuberculosis lineages, which normally induce expression of the regulon only once exposed to hypoxia or nitric oxide (NO), both of which result in an inhibition of M. tuberculosis respiration and replication (6, 46, 53, 57, 69). Oxygen limitation and NO exposure are also proposed to be among the most critical signals leading to M. tuberculosis dormancy and, possibly, the development of latent TB (70). The DosR regulon is “switched on” inside infected macrophages in an NO-dependent manner, and although data from animal models have never been entirely consistent, induction of the regulon has been proposed to be associated with adaptation and survival of M. tuberculosis during infection (10, 29).
Transcription of the 48-gene DosR regulon is controlled through a two-component regulatory system (2CS) comprising the DNA binding transcription factor DosR and one of two possible cognate histidine sensor kinases, DosS and DosT (46, 53). DosS and DosT have both been shown to bind heme, and the redox state of the heme iron controls their level of kinase activity (32, 61). In the presence of O2, the activity of DosS and DosT is strongly inhibited. Dissociation of O2 or binding of NO (or CO) results in autophosphorylation and activation of the sensor kinases, followed by phosphoryl transfer and activation of the response regulator, DosR. The DosR regulon consists of a number of genes that are involved in anaerobic respiration and metabolism, including genes involved in alternative electron transport pathways (fdxA), nitrate metabolism (narK2 and narX), and deoxynucleoside triphosphate (dNTP) synthesis under microaerophilic conditions (nrdZ) (57, 69). As such, the ability of W/Beijing strains to constitutively overexpress the DosR regulon prior to encountering the signals leading to inhibition of bacterial replication may represent a unique adaptive strategy in the face of changing environmental conditions encountered in the host.
In the present study, we set out to determine the underlying cause of the constitutive DosR phenotype within strains of the W/Beijing lineage. Through sequence analysis of a representative set of clinical isolates from each of the major M. tuberculosis lineages, we identified a naturally occurring frameshift mutation in the gene encoding the DosT sensor kinase that correlates precisely with constitutive overexpression of the DosR regulon by “modern” W/Beijing strains. Subsequent complementation studies revealed that while the dosT mutation is not the direct cause of the DosR regulon phenotype in these strains, the presence of nonfunctional DosT appears to serve a compensatory function that restricts further induction of the regulon upon exposure to NO. The work described herein gives additional insight into the complexity of the selection events that lead to constitutive overexpression of the DosR 2CS within the M. tuberculosis W/Beijing lineage.
MATERIALS AND METHODS
Bacterial strains and culture.
All M. tuberculosis strains were grown at 37°C in Middlebrook 7H9 broth (Difco) supplemented with 10% ADC (8.1 g/liter NaCl, 50 g/liter bovine serum albumin [BSA] fraction V [Calbiochem], 20 g/liter glucose), 0.2% glycerol, and 0.05% Tween 80 or on Middlebrook 7H11 agar (Difco) supplemented with 10% OADC enrichment (ADC plus 0.6 ml/liter oleic acid and 3.6 mM NaOH). Kanamycin (25 μg/ml) was included where indicated. All liquid cultures were maintained with constant rolling (2.0 rpm). For generation of the plasmid constructs described in this study, Escherichia coli strain NEB 10-beta (New England Biolabs) was grown in Luria-Bertani (LB) broth or on LB agar (Difco), and ampicillin (100 μg/ml) or kanamycin (50 μg/ml) was added as necessary. For treatment of M. tuberculosis cultures with NO, the NO donor diethylenetriamine-nitric oxide (DETA-NO) was added at a final concentration of 150 μM to early-log-phase cultures (optical density at 600 nm [OD600] = 0.15 to 0.2) at 37°C with constant mixing for 2 h prior to RNA extraction. Control cultures were grown under identical conditions in the absence of DETA-NO. All chemicals were supplied by Sigma-Aldrich, Inc., unless otherwise noted.
The majority of the clinical M. tuberculosis isolates examined in this study were collected over a 6-year period (2001 to 2007) from mostly foreign-born TB patients resident on the island of Montreal, Quebec (Canada), as described previously (50). As part of a large molecular epidemiology study, each of these isolates was classified into one of six major lineages (East Asian, Indo-Oceanic, East African/Indian, Euro-American, West African-1, and West African-2) according to the lineage-defining large sequence polymorphisms (LSPs) described by Gagneux et al. (18). In a similar manner, all strains identified as belonging to the W/Beijing lineage were further classified into one of five discrete sublineages (groups 1 to 5) (18, 49, 66). Group 1 strains possess the RD105 (RD = region of difference) deletion only. Group 2 strains also possess the RD207 deletion. Group 3 strains contain the RD105, RD207, and RD181 deletions. Group 4 and 5 strains are independently derived from group 3 strains and contain the RD150 and RD142 deletions, respectively, in addition to the RD105, RD207, and RD181 deletions. All primer sets and reaction conditions used in the multiplex PCR assays to genotype these strains (or to confirm previously assigned genotypes) are published elsewhere (50). Due to previously noted variation in the structure of the RD207 deletion (50), two additional primers were used in the current study to verify the presence of this deletion: RD207rv2820c-F (5′-GCATGTCAGCGTATGTGCTCG-3′) and RD207sg-R (5′-CCCCGGCGAGGAACAGAA-3′). In addition to LSP typing, each isolate was typed by IS6110 restriction fragment length polymorphism (RFLP) analysis to exclude any isolates involved in chains of transmission from further analysis (18, 49, 50, 66).
Where a deficiency in a particular lineage (or sublineage) was noted for our Montreal strain database, we supplemented our collection with additional clinical isolates obtained from San Francisco TB patients, kindly provided by S. Gagneux (National Institute for Medical Research, Mill Hill, United Kingdom) and P. Small (Institute for Systems Biology, Seattle, WA). These strains had previously been typed as part of the whole-genome hybridization studies that formed the basis of the current LSP genotyping method (18). Strain CDC1551 was originally obtained from T. Shinnick (Centers for Disease Control, Atlanta, GA); strain H37Rv, M. bovis BCG, and M. canetti were obtained from the Pasteur Institute (Paris, France); strains HN878 and NHN5 were obtained from J. Musser (Methodist Hospital Research Institute, Houston, TX); and M. bovis Ravenel was purchased from the ATCC (Manassas, VA).
Sequence analysis.
Genomic DNAs were isolated from W/Beijing strains 95_1848 (group 1; called strain 1-3 in this study) and HN878 (group 5) according to the method of Pelicic et al. (47). Oligonucleotides used for both PCR amplification and sequencing of the regions containing the dosR/dosS and dosT genes are provided in Table S1 in the supplemental material. For the dosR/dosS-containing region, nucleotides 3497397 to 3501256 were sequenced (numbered according to the H37Rv sequence provided in TubercuList [http://genolist.pasteur.fr/TubercuList]), and for the dosT region, nucleotides 2271817 to 2275540 were sequenced. PCRs were carried out in 50-μl volumes that included 10 to 100 ng genomic DNA, a 1 μM concentration of each oligonucleotide, a 200 μM concentration of each dNTP, 1.5 mM MgCl2, 1× Taq buffer (plus KCl), and 1.25 U Taq polymerase (Fermentas). Where necessary, reactions were optimized through the addition of 5% to 10% dimethyl sulfoxide. A denaturation step was carried out at 94°C for 2 min, followed by 35 cycles of 94°C for 10 s, 56°C for 10 s, and 68°C for 60 s. Sequence analysis of the reaction products was carried out at McGill University and Genome Québec Innovation Centre. To screen for the presence of the dosT frameshift mutation, primers dosTseqF (5′-GACATCGGAACGCAGATGCT-3′) and dosTseqR (5′-CGTACGGTCGTGAAAGACTC-3′) were used to amplify and sequence a 249-bp region of dosT (nucleotides 655 to 903) from each of the clinical isolates included in this study. PCR and sequencing were carried out as described above.
Complementation studies.
Plasmids pPDM11, pdosTtrunc, and pdosTmut are all based on the pMV361 integrative vector, which contains the hsp60 promoter and a selectable kanamycin resistance marker. The presence of the phage-derived attP and int genes enables integration of the plasmid into the nonessential attB site of the M. tuberculosis genome (63). To construct the pPDM11 plasmid containing wild-type dosT from H37Rv, a 4.2-kb BamHI fragment from bacterial artificial chromosome (BAC) Rv175 (kindly provided by R. Brosch, Pasteur Institute) was initially cloned into pBluescript II KS(+). From this, a 1,676-bp EcoRV-ClaI fragment containing most of dosT and 274 bp of downstream sequence was cloned into the EcoRV site of pBluescript II KS(+) to generate 1.6dosT-pBluescript. A 399-bp fragment containing the 5′ portion of dosT was then amplified from H37Rv genomic DNA, using primers dosT-A (5′-CGCGGATCCGTGACACACCCTGACAGGC-3′; BamHI site is underlined) and dosT-B (5′-ATGCCGCGAGATATCGTCCAG-3′; EcoRV site is underlined), and was cloned into BamHI- and EcoRV-restricted 1.6dosT-pBluescript to generate pPDM5. Following sequence confirmation, a 2,086-bp BamHI-HindIII fragment from pPDM5 was cloned into BalI- and HindIII-restricted pMV261 to give pPDM8. In this manner, full-length dosT was fused in frame with the start codon of hsp60 and was transcribed under the control of the hsp60 promoter (63). Finally, a 2,471-bp XbaI-HindIII fragment from pPDM8 was inserted into the corresponding sites of the pMV306 vector to give pPDM11.
To construct pdosTmut, the primers dosTseqF and dosTSCrev (see Table S1 in the supplemental material) were used to amplify a 615-bp portion of dosT (including the frameshift mutation) from HN878 genomic DNA. The purified PCR fragment was subsequently digested with NcoI and SapI, ligated into the corresponding sites of pPDM11, and confirmed by sequencing. To generate pdosTtrunc, DosTtruncF (5′-CGCAAGCTTAGTACTTAGTGGCCGGAGAGATCTCC-3′; HindIII and ScaI restriction sites are underlined) and DosTtruncR (5′-GCGAAGCTTTCAGCGCAGCGGTGCAGA-3′; HindIII site is underlined) were used to amplify a 906-bp fragment of dosT located immediately downstream of the frameshift mutation from HN878 genomic DNA. The purified PCR product was ligated with HindIII-digested pBluescript II KS(+) and confirmed by sequencing. This pBS+CT plasmid was then restricted with HindIII and ScaI, and the recovered insert was ligated with BalI- and HindIII-digested pMV261 to yield pMV261-CT. Finally, pMV261-CT was treated with HindIII and XbaI, and the truncated dosT fragment was ligated into the corresponding sites of pMV306 to yield pdosTtrunc.
For transformation of M. tuberculosis, electrocompetent cells were prepared as previously described (60). All positive clones were screened for the presence of the cell wall lipid phthiocerol dimycocerosate (PDIM) prior to any further analysis, as described previously (12).
qRT-PCR.
Total RNA was purified from cultures grown to an OD600 of 0.15 to 0.2 by “bead beating” in the presence of TRIzol (Invitrogen) according to the method of Sherman et al. (49, 57). Turbo DNase (Ambion) was used per the manufacturer's recommendations to remove contaminating DNA both before and after the RNA was purified by use of an RNeasy Mini kit (Qiagen). SuperScript III (Invitrogen) reverse transcriptase was used to prepare random hexamer-primed first-strand cDNA from 300 ng of each RNA sample, and cDNA samples were diluted 1:200 prior to analysis by quantitative real-time PCR (qRT-PCR). For analysis of 16S rRNA expression, cDNA samples were diluted 1:106. RNA samples were prepared from at least two independent biological replicates for each strain under investigation.
All primers and TaqMan MGB probes (labeled with 6-carboxyfluorescein [FAM]; Applied Biosystems) used for qRT-PCR analysis were designed using Primer Express software, version 3.0 (Applied Biosystems), and are listed in Table S2 in the supplemental material. qRT-PCRs were carried out in triplicate, using a model 7300 real-time PCR system and either TaqMan universal PCR master mix or Power SYBR green PCR master mix according to the manufacturer's recommendations (Applied Biosystems). Primers and probes were used at concentrations of 300 nM and 200 nM, respectively. The relative standard curve method was used for quantification, and data were normalized against expression of the sigA housekeeping gene (and verified in some cases by normalization to 16S rRNA). The standard deviations (S) were calculated according to the Applied Biosystems formula S = (cv)(X), where X is the ratio of the mean values of the nominator (n) and denominator (d) and cv is the coefficient of variation (standard deviation normalized to the mean value for each gene) calculated from the formula cv = √[(cvn)2 + (cvd)2]. Hence, the full formula is as follows: S = √[(Sn/Xn)2 + (Sd/Xd)2] × (Xn/Xd) (3).
RESULTS
Sequencing the DosR 2CS in the W/Beijing background reveals the presence of a frameshift mutation within the DosT sensor kinase.
To date, there are three regulatory elements of the DosR 2CS that have been described, and these are localized in two distinct regions of the M. tuberculosis genome. The dosS and dosR genes (also known as devS and devR [53]) lie adjacent to one another in the chromosome and are cotranscribed along with Rv3134c (encoding a putative member of the universal stress protein [Usp] family). The gene encoding the alternate sensor histidine kinase in this system, dosT (Rv2027c), is located well away from dosS and dosR and appears to be transcribed independently of the neighboring genes, Rv2028c to Rv2031c (hspX), which are all regulated in a DosR-dependent manner upon exposure of M. tuberculosis to NO or hypoxia (69). Interestingly, the two genes either side of dosT (Rv2026c and Rv2028c) are, like Rv3134c, predicted to encode Usp-like proteins. While their specific functions remain largely unknown, the Usp superfamily—like bacterial 2CS—are involved in stress resistance, adaptation to energy deficiency, cell motility, and adhesion (42, 44). In M. tuberculosis, the Usp encoded by the Rv2623 gene is part of the DosR regulon and appears to regulate bacterial growth in an ATP-dependent manner (14). There is also at least one example in the E. coli literature where a Usp specifically modulates the activity of a 2CS (25).
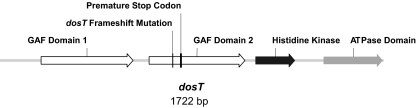
In order to identify polymorphisms that could potentially account for the constitutive overexpression of the DosR regulon in strains of the W/Beijing lineage, we sequenced the chromosomal regions containing dosR, dosS, and dosT from two distantly related W/Beijing strains, HN878 (group 5 [62]) and 95_1848 (group 1; kindly provided by S. Gagneux and referred to herein as strain 1-3), and compared these sequences to the available H37Rv (http://genolist.pasteur.fr/TubercuList) and W210 (http://www.tigr.org; kindly provided by B. Kreiswirth, Public Health Research Institute) genome databases. H37Rv is a non-W/Beijing strain of the Euro-American lineage, whereas W210 is a group 5 W/Beijing strain that, based on IS6110 fingerprinting, is closely related to HN878 (35). Due to the possibility that some of the surrounding genes could potentially influence the expression or function of the DosR 2CS, a total of 3,860 bp was sequenced for the region containing dosR and dosS, including the neighboring Rv3131 and Rv3134c genes and 1,341 bp of sequence upstream of dosR. Similarly, for the dosT region, a region of 3,723 bp was sequenced, encompassing Rv2025c to Rv2029c (pfkB) and 1,032 bp of sequence upstream of dosT. Three synonymous SNPs were identified in this manner, including two in Rv3134c (C507G [strains 1-3, HN878, and W210] and C601T [strains HN878 and W210]) and a single G1068A substitution in the dosS genes of strains HN878 and W210 (data not shown). More interestingly, an alignment of the dosT region revealed a frameshift mutation at position 775 in the sequences of the dosT genes obtained from the HN878 and W210 W/Beijing strains. This mutation corresponds to the loss of a single guanine residue and is expected to lead to premature termination of the resulting dosT transcript 36 bp downstream (Fig. 1 and 2). As indicated in Fig. 1, the mutation in dosT is located within the region encoding the second of the two N-terminal GAF domains (cGMP phosphodiesterase, adenylyl cyclase, and E. coli transcription factor FhlA) that are involved in heme binding and O2/NO sensing (55). Notably, this dosT frameshift mutation is absent from W/Beijing strain 1-3.
FIG. 1.
DosT sensor histidine kinase. A schematic representation of the dosT (Rv2027c) gene sequence is presented along with the relative position of each of its conserved functional domains (http://www.broadinstitute.org/annotation/genome/tbdb). Also indicated is the approximate location of the frameshift mutation identified within the dosT sequences from W/Beijing strains HN878 and W210 that is predicted to result in the introduction of an in-frame premature termination codon. The dosT frameshift mutation is localized to the second N-terminal GAF domain, which is involved in heme binding and O2/NO sensing. The putative C-terminal nucleotide binding and histidine kinase domains that form the kinase catalytic core of DosT are also highlighted.
FIG. 2.
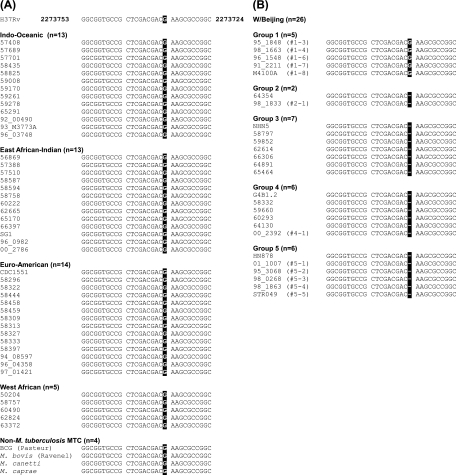
The dosT frameshift mutation is restricted to recently evolved W/Beijing strains. An alignment of the region of dosT that contains the frameshift mutation identified for recently evolved W/Beijing strains is shown (nucleotides 2273753 to 2273724 relative to the complete H37Rv genome sequence). (A) In total, 76 mycobacterial strains were screened for the presence of the dosT mutation, including 46 non-W/Beijing M. tuberculosis strains and 4 non-M. tuberculosis MTC strains. (B) Twenty-six W/Beijing strains representing each of the 5 sublineages described to date were also screened. The number of strains representing each lineage or sublineage is indicated in parentheses, and the position of the missing guanine residue that results in the frameshift mutation for the group 2 to 5 W/Beijing strains is highlighted. Note that the group 1 W/Beijing strains possess the wild-type dosT gene sequence.
Distribution of the dosT frameshift mutation across the M. tuberculosis W/Beijing lineage.
The preliminary data gathered above suggested that the dosT mutation may be restricted to recently evolved strains belonging to the W/Beijing lineage. To test this hypothesis, a broad sample of clinical isolates representing each of the major M. tuberculosis lineages (18) was examined for the presence of the aforementioned frameshift mutation through PCR-based sequence analysis. In total, 76 bacterial strains were screened for the presence of the dosT mutation, including 72 M. tuberculosis strains and 4 non-M. tuberculosis strains belonging to the MTC (M. canetti, M. bovis Ravenel, M. bovis bacillus Calmette-Guérin [Pasteur strain], and M. caprae). Of the non-W/Beijing M. tuberculosis strains that were screened, 13 belonged to the Indo-Oceanic lineage (RD239), 13 to the East African-Indian lineage (RD570), 15 to the Euro-American lineage (pks1-15Δ7bp), and 5 to the West African (M. africanum types I and II; RD9) lineages. The 26 W/Beijing strains that were screened represented each of the 5 sublineages of this diverse family (group 1 [n = 5], group 2 [n = 2], group 3 [n = 7], group 4 [n = 6], and group 5 [n = 6]).
In 100% of the non-W/Beijing strains that were screened, the dosT sequence was “wild type,” i.e., lacking the frameshift mutation (Fig. 2A). For the W/Beijing strains, a striking dichotomy was noted with regard to the appearance of the dosT mutation. Whereas all group 1 strains possess a wild-type dosT sequence, 100% of the more recently evolved strains, belonging to the group 2 to 5 sublineages, were found to contain the dosT frameshift (Fig. 2B). This clear-cut distinction between W/Beijing strains with the dosT mutation and those without the mutation suggested that there may also be a distinction between these strains in terms of their DosR regulon phenotype and their ability to respond to environmental signals such as NO via the DosR 2CS.
Constitutive overexpression of the DosR regulon is associated with the presence of the dosT frameshift mutation.
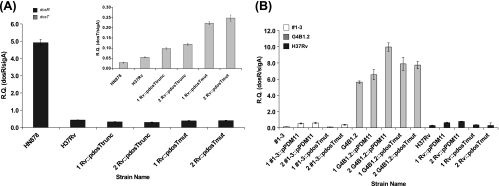
Since DosT is one of two M. tuberculosis histidine kinases capable of sensing environmental NO and O2 concentrations and integrating these signals via the DosR response regulator that controls expression of the DosR regulon (including expression of dosR itself), we decided to investigate the relationship between the presence of the dosT frameshift mutation in the W/Beijing lineage and the level of dosR transcription. To achieve this, dosR expression levels were analyzed by qRT-PCR for a series of W/Beijing and non-W/Beijing strains grown under standard in vitro culture conditions. To control for variable amounts of input cDNA in these assays, all quantification data were normalized to the expression of the housekeeping transcription factor sigA. In this manner, we once again observed a striking dichotomy between the group 1 and group 2 to 5 W/Beijing strains, possessing the wild-type and mutant forms of dosT, respectively. Indeed, all five group 1 W/Beijing strains possessing the wild-type dosT sequence expressed amounts of dosR that were equivalent to those in the non-W/Beijing strains H37Rv (Euro-American lineage) and 92_00490 (Indo-Oceanic lineage) (Fig. 3A). In contrast, the group 2 to 5 W/Beijing strains with dosT bearing the frameshift mutation were all found to overexpress dosR in a constitutive fashion (Fig. 3A). On average, the amounts of dosR expressed by the group 2 to 5 W/Beijing strains were 12-fold greater than the corresponding amounts expressed by group 1 W/Beijing strains.
FIG. 3.
The appearance of the dosT frameshift mutation is associated with constitutive overexpression of the DosR regulon. qRT-PCR analysis of dosR expression levels was performed with representative group 1 W/Beijing strains (white bars), group 2 to 5 W/Beijing strains (gray bars), and non-W/Beijing strains (H37Rv and the 92_00490 Indo-Oceanic strain [panel A only]; black bars) grown under standard in vitro conditions (A) or upon exposure to the NO donor DETA-NO (B). Expression levels of dosR in both DETA-NO-treated (hatched bars) and untreated samples are presented in panel B, while the relative induction ratios (DETA-NO-treated samples versus untreated controls) are given in panel C. For each panel, expression levels of dosR were normalized to the sigA housekeeping gene (RQ = quantity relative to sigA), and each sample was assayed in triplicate. At least two independent biological replicates were analyzed for each strain, and data from a single representative experiment are presented. Error bars represent standard deviations.
Since NO is both a ligand of DosT and a strong inducer of DosR regulon expression (51, 69), we next compared the expression levels of dosR among the same W/Beijing strains (and H37Rv) in the presence of the NO donor compound DETA-NO. As expected, treatment with DETA-NO resulted in a rapid increase in dosR expression for all strains tested (Fig. 3B). However, there was a very marked difference in the extent of dosR inducibility for the set of W/Beijing strains containing mutant dosT (Fig. 3C). On average, the dosR increase for the group 1 W/Beijing strains following NO exposure was 30-fold. In comparison, for the group 2 to 5 strains, the level of induction was 1 order of magnitude less (i.e., 3-fold). Nevertheless, following NO treatment, the relative dosR expression levels between strains with wild-type and mutant dosT did not differ to any significant degree (P > 0.05). It should be noted that to exclude the possibility that variable sigA levels between group 1 and group 2 to 5 W/Beijing strains upon exposure to NO were responsible for the observed differences in dosR induction, we compared the expression of sigA in each of the DETA-NO-treated samples following normalization with 16S rRNA. We did not observe any lineage-related differences in sigA expression levels following treatment with NO in these experiments (data not shown). Thus, the dosT frameshift mutation in the more recently evolved group 2 to 5 W/Beijing sublineages correlates precisely with the appearance of the constitutive dosR phenotype displayed by these strains. In addition, the presence of the mutant form of dosT also correlates with the relative lack of dosR induction in the same strains upon exposure to NO. Together, these results suggest that the evolution of the dosT mutation may be responsible for the DosR overexpression phenotype in modern W/Beijing lineage strains.
The dosT frameshift mutation is not directly responsible for the constitutive DosR regulon overexpression phenotype.
Since several previous studies have reported constitutively active mutations within sensor histidine kinases of bacterial 2CS systems (1, 15, 40, 54), we decided to explore the possibility that the frameshift mutation in dosT results in constitutive activation of the DosR regulon for the group 2 to 5 W/Beijing strains. In order to test this, we overexpressed two distinct versions of dosT under the control of the hsp60 promoter within the H37Rv strain background. H37Rv contains an endogenous wild-type copy of dosT and normally expresses low levels of dosR under standard growth conditions. The first plasmid construct (pdosTmut) contained the full-length, mutated dosT sequence cloned from the HN878 strain. The second construct (pdosTtrunc) was designed to express a C-terminal portion of DosT (amino acids 273 to 573), beginning with the first putative initiation codon downstream of the stop codon introduced into dosT as a result of the W/Beijing frameshift mutation. We speculated that expression of the C-terminal histidine kinase-containing region of DosT in the absence of the N-terminal heme-binding regulatory region may have resulted in a DosT derivative that displays constitutive kinase activity, in a manner analogous to that of the FixL protein of Rhizobium meliloti (40). As can be seen in Fig. 4A, qRT-PCR analysis of cDNAs prepared from two independent H37Rv clones representing each of the dosT constructs failed to reveal any effect of these mutant forms of dosT on the level of dosR expression in a non-W/Beijing strain background.
FIG. 4.
The dosT frameshift mutation is not directly responsible for constitutive overexpression of the DosR regulon in the W/Beijing lineage. (A) qRT-PCR analysis of dosR expression levels in H37Rv lines expressing either the C-terminal portion of DosT downstream of the W/Beijing frameshift mutation (Rv::pdosTtrunc) or the full-length, mutated dosT sequence (Rv::pdosTmut) under the control of the hsp60 promoter contained in the pMV361 vector. The parental H37Rv strain and HN878 (W/Beijing) were included as controls. The inset shows the corresponding dosT expression level in each of these strains. (B) qRT-PCR analysis of dosR expression levels in group 1 W/Beijing (1-3; white bars), group 4 W/Beijing (G4B1.2; gray bars), and H37Rv (black bars) parental and recombinant strains expressing either full-length wild-type (pPDM11) or full-length mutant (pdosTmut) dosT under the control of the constitutive hsp60 promoter. For each transformation in panels A and B, two independent clones were analyzed in triplicate, and the expression levels of dosR under standard in vitro growth conditions were normalized to the sigA housekeeping gene (RQ = quantity relative to sigA). Error bars represent standard deviations.
If expression of the mutant form of dosT was directly responsible for the constitutive overexpression of dosR and the DosR regulon in the modern W/Beijing background, we would expect to see reversion of this phenotype by expressing the wild-type dosT sequence within these strains. To explore this hypothesis, we transformed the pPDM11 plasmid that overexpresses the dosT sequence of H37Rv under the control of the hsp60 promoter into a group 4 W/Beijing strain (G4B1.2) as well as into the group 1 (1-3) and H37Rv strain backgrounds, as controls. As an additional control for the qRT-PCR experiments, we also included the same three strain backgrounds transformed with the pdosTmut construct (carrying the full-length, mutated dosT sequence) detailed above. The dosR expression patterns for two independent clones representing each of these transformations are presented in Fig. 4B. Clearly, expressing wild-type dosT within the G4B1.2 (group 4) strain did not reduce the expression of dosR to the levels seen in either the 1-3 or H37Rv strain. Likewise, expressing wild-type or mutant dosT also had no significant effect on dosR levels in the 1-3 and H37Rv strains, which normally generate only small amounts of this transcript under standard growth conditions in vitro. For all transformed strains, plasmid-driven dosT expression was confirmed through qRT-PCR analysis (Fig. 4A, inset, and 5C; data not shown). Thus, despite the fact that the presence of the dosT frameshift mutation is strongly associated with the appearance of the W/Beijing constitutive dosR phenotype, the data presented herein indicate that the mutant form of dosT alone is not responsible for overexpression of the DosR regulon.
Since the dosT mutation is not the cause of the constitutive DosR regulon overexpression phenotype in strains of the W/Beijing lineage, it is plausible that the frameshift mutation may have arisen as a direct consequence of this phenotype. As such, one can speculate that a possible explanation for the appearance of the dosT mutation is that it plays a compensatory role to limit further induction of dosR within strains displaying the constitutive DosR regulon phenotype. To look for evidence of a compensatory effect that is attributable to the frameshift mutation in dosT, we treated each of the G4B1.2, 1-3, and H37Rv recombinants expressing either wild-type or mutant dosT (full-length, mutated dosT sequence) with the NO donor DETA-NO (Fig. 5). In this manner, we were able to observe whether restoring expression of wild-type dosT in a strain whose endogenous copy of dosT is mutated (strain G4B1.2) could enhance the inducibility of DosR regulon expression. As anticipated, by comparing dosR expression levels among transformant lines within the same strain background, the data confirm that wild-type, not mutant, dosT is active in terms of being able to respond to the presence of NO (Fig. 5A). The data also indicate that the introduction of wild-type dosT into the G4B1.2 strain background is able to increase the level of dosR induction >2.5-fold beyond the level attained in the parental strain. This finding was subsequently confirmed in a member of an independent W/Beijing sublineage by transforming the group 5 HN878 strain with the pPDM11 plasmid expressing wild-type dosT. The results obtained in this case were virtually indistinguishable from those for the G4B1.2 strain (data not shown). The same trend was also observed for the 1-3 and H37Rv strains, possessing a wild-type endogenous copy of dosT, although the increase in dosR transcription resulting from dosT overexpression in H37Rv was less pronounced (1.5-fold).
FIG. 5.
The W/Beijing frameshift mutation renders DosT nonfunctional. qRT-PCR analysis of dosR (A), Rv2626c (B), and dosT (C) expression levels in group 1 W/Beijing (1-3; white bars), group 4 W/Beijing (G4B1.2; gray bars), and H37Rv (black bars) parental and recombinant strains expressing either full-length wild-type (pPDM11) or full-length mutant (pdosTmut) dosT under the control of the hsp60 promoter in the pMV361 vector. Expression levels for both DETA-NO-treated (hatched bars) and untreated (solid bars) samples are presented and are normalized to levels of the sigA housekeeping gene. Representative data for at least two independent biological replicates are shown, with each sample assayed in triplicate. Error bars represent standard deviations. Note that different scales were used for panels A, B, and C.
To confirm that induction of dosR expression in the aforementioned strains translates to an increase in the amount of functional DosR protein being produced, we quantified two additional members of the DosR regulon whose expression is controlled by DosR. From Fig. 5B, it is evident that introducing the wild-type version of dosT into each strain background resulted in a corresponding rise in the level of Rv2626c gene expression in response to NO. This result serves to confirm that both dosT and dosR are functionally expressed within these strains. Although less pronounced, the same overall trend was observed when we looked at hspX expression (see Fig. S1 in the supplemental material). Finally, for all transformed lines, plasmid-driven dosT expression was also examined through qRT-PCR analysis, as we sought to confirm that both mutant and wild-type forms of dosT were expressed in equivalent amounts following DETA-NO treatment. Although some interstrain variability was observed in these experiments, the relative levels of wild-type and mutant dosT in each strain background were very similar (Fig. 5C). For the parental strains, dosT expression only marginally increased in response to NO treatment, confirming previous publications reporting that in contrast to dosS and dosR, dosT itself is not part of the DosR regulon (27, 69). One unanticipated side effect of the DETA-NO treatment was noted, however, in that it appeared to result in a substantial increase in dosT transcription from the plasmid-based copy. Given that this copy of dosT was under the control of the hsp60 promoter sequence, we measured endogenous hsp60 expression in these strains both before and after DETA-NO treatment (see Fig. S1 in the supplemental material). Consistent with previous reports indicating that hsp60 expression is upregulated under certain stress conditions (39), we found that for the G4B1.2 and H37Rv strains hsp60 was induced up to 6-fold following exposure to DETA-NO for 2 h. Thus, we feel that the most likely explanation for the enhanced dosT expression we observed within the transformed lines following NO treatment was an increase in transcription from the hsp60 promoter region.
Together, these data confirm that complementation of strains that lack functional DosT protein with the wild-type dosT gene enhances the inducibility of dosR and DosR regulon expression in these strains. The possible benefits of regulating DosR overexpression through evolution of the dosT mutation in the W/Beijing background are discussed below.
DISCUSSION
Until very recently, there was a widespread belief among TB researchers that clinical isolates of M. tuberculosis were highly restricted in terms of their genetic and antigenic variability (41, 62). However, technological advances in the form of whole-genome microarray and high-throughput sequencing approaches have firmly established that M. tuberculosis has evolved into several genetically diverse families of strains that are potentially variable in terms of the ability to influence the course of TB transmission and disease (2, 17, 18, 26, 71). In particular, the W/Beijing or East Asian strain lineage has gained considerable notoriety over the past decade as a result of published epidemiological studies describing a relative increase in overall TB incidence due to W/Beijing strains in geographically diverse regions of the world (11, 20, 23, 24, 67). Several additional studies have also described an increased risk of developing drug resistance or extrapulmonary forms of TB associated with W/Beijing strains (7, 13, 16, 30).
The potential reasons underlying the apparent global success of W/Beijing strains are not yet understood but could involve a combination of “host-related factors” (genetic or environmental) and/or “bacterial factors” inherent to the W/Beijing lineage. Intriguingly, a specific interaction between both host and bacterial genotypes was recently suggested for this lineage, whereby W/Beijing infection in Vietnamese patients bearing a particular Toll-like receptor 2 (TLR2) allele was associated with development of TB (8). Consistent with the epidemiological data indicative of enhanced transmission and/or pathogenicity of W/Beijing isolates, laboratory-based studies have reproducibly demonstrated that certain W/Beijing isolates are “hypervirulent,” causing a short-time-to-death phenotype in mouse models of infection (4, 35-37, 48). At least in some strains, this hypervirulent phenotype appears to be associated with the production of a complex phenolic glycolipid known as PGL-tb (35, 48, 64). Two additional animal studies have also reported that the live attenuated BCG vaccine is significantly less effective at protecting against subsequent challenge with W/Beijing than non-W/Beijing strains (22, 65). This finding is particularly interesting in light of speculation that the recent mass increase in BCG immunization programs may have inadvertently selected for the emergence of W/Beijing strains on a global basis (31, 34, 68). Lastly, several in vitro studies have indicated that W/Beijing strains replicate at a higher rate than non-W/Beijing strains within mouse and human macrophages (4, 33, 73, 74).
While searching for W/Beijing-specific bacterial factors that could potentially account for the diverse epidemiological and laboratory-based phenomena attributed to this lineage, we previously described the constitutive upregulation or overexpression of the DosR transcription factor and associated DosR-regulated genes (hspX, Rv3130c, fdxA, and narX) within a sample of W/Beijing isolates obtained from our collaborators (18, 49). For us, the most intriguing part of this phenotype was that it seemed to distinguish members of the W/Beijing lineage from other lineages included in the study (Euro-American and Indo-Oceanic) that expressed genes within the DosR regulon only at low (basal) levels under standard in vitro culture conditions. Non-W/Beijing strains such as H37Rv are known to upregulate the DosR-controlled regulon upon exposure to NO, hypoxia, or CO (51, 53, 58, 69). At the time, the DosR regulon was under investigation in a number of laboratories as a potential link to latent TB infection due to the fact that hypoxia and NO have been proposed to be among the most critical signals that trigger M. tuberculosis persistence or dormancy (56, 57, 69, 70). Although DosR appears to be important for survival of bacteria maintained under hypoxic conditions in vitro (6), considerable controversy still exists in the TB literature regarding exactly what role the DosR regulon plays during M. tuberculosis infection. Every conceivable in vivo phenotype has now been reported for dosR mutants, ranging from hypervirulence to attenuation to no effect at all (10, 45, 52). However, to the best of our knowledge, each of these in vivo studies has involved H37Rv—a ubiquitous laboratory strain of M. tuberculosis that has been in continuous in vitro culture since it was first isolated in 1905. It remains to be seen whether the DosR regulon plays more or less of a role in infection for other M. tuberculosis strains. In this regard, the W/Beijing strains that constitutively overexpress dosR are of particular interest.
Following on from our previous work (49), the present study was initiated in an attempt to identify the underlying cause of the W/Beijing DosR regulon phenotype. The fact that each of the environmental stimuli that trigger expression of the DosR regulon are known to signal via the dosS- and dosT-encoded sensor histidine kinases suggested to us that the constitutive dosR phenotype may be the result of an alteration in the functioning of either kinase or, possibly, of the dosR transcription factor itself. Sequence analysis of each of the components of the DosR 2CS led to the identification of a previously undescribed frameshift mutation in the dosT gene sequences of the HN878 and W210 W/Beijing strains that is the result of a missing guanine residue. Additional analysis of a carefully genotyped set of W/Beijing strains representing each of the five genetically distinct sublineages revealed a striking dichotomy in the distribution of the dosT frameshift mutation. While the group 1 W/Beijing strains and all non-W/Beijing strains that were tested contained the wild-type dosT sequence (with respect to H37Rv), 100% of W/Beijing strains belonging to groups 2 to 5 contained the mutated version. As such, the appearance of the dosT mutation appears to coincide with the presence of the RD207 deletion common to all group 2 to 5 W/Beijing strains. In turn, this suggests that these mutation events may have evolved around the same time and are possibly associated with some form of functional distinction between the group 1 and group 2 to 5 strains. The RD207 deletion is responsible for the characteristic spoligotyping profile that is frequently used in classifying W/Beijing strains (5, 18).
The fact that we failed to detect the dosT mutation within any group 1 strains and did not detect any group 2 strains that lack the mutation obviously does not preclude the possibility that such strains do exist. At present, our strain database is relatively poor in terms of isolates belonging to the group 1 and 2 W/Beijing sublineages (we currently have only two confirmed group 2 strains available). It is therefore possible that the dosT frameshift mutation arose in a group 1 strain whose progeny then became the common ancestor of the group 2 strains. Alternatively, there may be group 2 strains with the mutation and some without, with the former giving rise to the group 3 strains. However, because the W/Beijing sublineages are monophyletic, we think it highly unlikely that there are any group 3 to 5 strains in existence that possess a wild-type dosT sequence.
The W/Beijing dichotomy we report herein extends beyond the sequence of dosT and is also observed when one looks at dosR transcription levels displayed by these strains. All group 1 strains express dosR at basal (H37Rv) levels, while the other sublineages overexpress dosR in a constitutive fashion. In addition, the fact that the group 2 to 5 strains are committed to overexpressing dosR constitutively appears to severely restrict the relative inducibility of dosR upon exposure to NO. In effect, these strains naturally produce near-maximal amounts of dosR. Presumably, the dosR induction that we did observe was due to the presence of functional DosS protein. As previously reported, both DosS and DosT are required for maximal DosR induction in the H37Rv strain (27, 51). Although not tested as part of the present study, it is also possible that lower concentrations of NO are required to reach this maximal level in group 2 to 5 W/Beijing strains compared to other lineages, which may be relevant to the timing of DosR regulon expression events during phagocytosis (see below). As such, the more recently evolved W/Beijing strains may already be “primed” to achieve optimal DosR regulon induction as soon as possible upon host cell entry.
Our inability to identify strains displaying the constitutive dosR phenotype but lacking the dosT mutation (or vice versa) is fairly convincing evidence that the two phenotypes are linked somehow in evolutionary terms. Again, we have screened relatively few isolates from the group 1 and 2 sublineages, so such strains may exist, or alternatively, some form of negative selection may have taken place, resulting in their complete elimination from the population. Our complementation studies have ruled out any direct causal effect of the dosT frameshift mutation on expression of the DosR regulon, which is perhaps not surprising given that the mutation occurs partway through the second GAF sensing domain and is predicted to result in a severely truncated protein lacking both the kinase and ATPase domains. Therefore, we can think of three possible scenarios to account for the appearance of the dosT mutation. First, in response to the evolution of DosR regulon overexpression, the dosT mutation may serve a compensatory role that acts to limit further induction of the regulon, either because it is unnecessary or because it is detrimental to the bacterium in some way. Second, DosT may simply no longer be required in the group 2 to 5 W/Beijing strains and the frameshift mutation effectively renders it a pseudogene in this population. These bacteria already have a basal level of dosR and dosS (G. Kolly and P. Domenech, unpublished data) expression that is significantly elevated compared to those for other strain types, and they appear to respond to NO to the same extent as strains that possess functional copies of both DosS and DosT sensor kinases. From this perspective, our data are consistent with a recent report from the Voskuil lab suggesting that it is DosS rather than DosT which sustains the induction of DosR regulon genes (27). Third, it is also possible that the mutation event(s) resulting in the constitutive overexpression of dosR may have been selected for in response to the inactivation of the DosT sensor kinase, and as such, the dosT frameshift mutation may actually be the primary genetic event that led to the W/Beijing DosR regulon phenotype.
Finally, what could be the advantage of constitutively overexpressing members of the DosR regulon? As noted elsewhere, time-efficient regulatory reactions appear to be particularly important for the stress response (28, 38). Thus, our current view is that the ability of W/Beijing strains to constitutively overexpress dosR prior to encountering signals, such as NO and hypoxia, that lead to inhibition of bacterial replication represents a unique survival strategy in the face of changing environmental conditions encountered in the host. Although to the best of our knowledge a macrophage phenotype has not yet been reported for dosR mutant strains prepared in the H37Rv background, we believe that the W/Beijing strategy of overexpressing the DosR regulon prior to encountering host cells may aid the bacteria during the critical period immediately following macrophage entry or, alternatively, during the macrophage activation process, when significant amounts of NO begin to be released. Similarly, DosR regulon overexpression may also afford a selective advantage during encounters with previously stimulated macrophages, such as those of BCG-vaccinated individuals. If this is correct, it could help to explain previous reports suggesting that vaccination is less effective at protecting against W/Beijing strain infection (22, 65).
Supplementary Material
Acknowledgments
This work was supported by Canadian Institutes of Health Research (CIHR) grant MOP82931. M.B.R. is supported by a Peter Lougheed/CIHR New Investigator Award. P.D. is supported by a Montreal General Hospital 175th Anniversary Fellowship.
We are indebted to Fiona McIntosh and Victoria Pichler for their assistance in genotyping many of the TB strains included in the present study.
Footnotes
Published ahead of print on 12 February 2010.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Adhikari, R. P., and R. P. Novick. 2008. Regulatory organization of the staphylococcal sae locus. Microbiology 154:949-959. [DOI] [PubMed] [Google Scholar]
- 2.Alland, D., D. W. Lacher, M. H. Hazbon, A. S. Motiwala, W. Qi, R. D. Fleischmann, and T. S. Whittam. 2007. Role of large sequence polymorphisms (LSPs) in generating genomic diversity among clinical isolates of Mycobacterium tuberculosis and the utility of LSPs in phylogenetic analysis. J. Clin. Microbiol. 45:39-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Applied Biosystems. 2005. Real-time PCR systems: chemistry guide. Part number 4348358. Applied Biosystems, Foster City, CA.
- 4.Barczak, A. K., P. Domenech, H. I. Boshoff, M. B. Reed, C. Manca, G. Kaplan, and C. E. Barry III. 2005. In vivo phenotypic dominance in mouse mixed infections with Mycobacterium tuberculosis clinical isolates. J. Infect. Dis. 192:600-606. [DOI] [PubMed] [Google Scholar]
- 5.Bifani, P. J., B. Mathema, N. E. Kurepina, and B. N. Kreiswirth. 2002. Global dissemination of the Mycobacterium tuberculosis W-Beijing family strains. Trends Microbiol. 10:45-52. [DOI] [PubMed] [Google Scholar]
- 6.Boon, C., and T. Dick. 2002. Mycobacterium bovis BCG response regulator essential for hypoxic dormancy. J. Bacteriol. 184:6760-6767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buu, T. N., M. N. Huyen, N. T. Lan, H. T. Quy, N. V. Hen, M. Zignol, M. W. Borgdorff, F. G. Cobelens, and D. van Soolingen. 2009. The Beijing genotype is associated with young age and multidrug-resistant tuberculosis in rural Vietnam. Int. J. Tuberc. Lung. Dis. 13:900-906. [PubMed] [Google Scholar]
- 8.Caws, M., G. Thwaites, S. Dunstan, T. R. Hawn, N. T. N. Lan, N. T. T. Thuong, K. Stepniewska, M. N. T. Huyen, N. D. Bang, T. H. Loc, S. Gagneux, D. van Soolingen, K. Kremer, M. van der Sande, P. Small, P. T. H. Anh, N. T. Chinh, H. T. Quy, N. T. H. Duyen, D. Q. Tho, N. T. Hieu, E. Torok, T. T. Hien, N. H. Dung, N. T. Q. Nhu, P. M. Duy, N. van Vinh Chau, and J. Farrar. 2008. The influence of host and bacterial genotype on the development of disseminated disease with Mycobacterium tuberculosis. PLoS Pathog. 4:e1000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry III, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, A. Krogh, J. McLean, S. Moule, L. Murphy, K. Oliver, J. Osborne, M. A. Quail, M. A. Rajandream, J. Rogers, S. Rutter, K. Seeger, J. Skelton, R. Squares, S. Squares, J. E. Sulston, K. Taylor, S. Whitehead, and B. G. Barrell. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 10.Converse, P. J., P. C. Karakousis, L. G. Klinkenberg, A. K. Kesavan, L. H. Ly, S. S. Allen, J. H. Grosset, S. K. Jain, G. Lamichhane, Y. C. Manabe, D. N. McMurray, E. L. Nuermberger, and W. R. Bishai. 2009. Role of the dosR-dosS two-component regulatory system in Mycobacterium tuberculosis virulence in three animal models. Infect. Immun. 77:1230-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cowley, D., D. Govender, B. February, M. Wolfe, L. Steyn, J. Evans, R. J. Wilkinson, and M. P. Nicol. 2008. Recent and rapid emergence of W-Beijing strains of Mycobacterium tuberculosis in Cape Town, South Africa. Clin. Infect. Dis. 47:1252-1259. [DOI] [PubMed] [Google Scholar]
- 12.Domenech, P., and M. B. Reed. 2009. Rapid and spontaneous loss of phthiocerol dimycocerosate (PDIM) from Mycobacterium tuberculosis grown in vitro: implications for virulence studies. Microbiology 155:3532-3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drobniewski, F., Y. Balabanova, V. Nikolayevsky, M. Ruddy, S. Kuznetzov, S. Zakharova, A. Melentyev, and I. Fedorin. 2005. Drug-resistant tuberculosis, clinical virulence, and the dominance of the Beijing strain family in Russia. JAMA 293:2726-2731. [DOI] [PubMed] [Google Scholar]
- 14.Drumm, J. E., K. Mi, P. Bilder, M. Sun, J. Lim, H. Bielefeldt-Ohmann, R. Basaraba, M. So, G. Zhu, J. M. Tufariello, A. A. Izzo, I. M. Orme, S. C. Almo, T. S. Leyh, and J. Chan. 2009. Mycobacterium tuberculosis universal stress protein Rv2623 regulates bacillary growth by ATP-binding: requirement for establishing chronic persistent infection. PLoS Pathog. 5:e1000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eguchi, Y., T. Oshima, H. Mori, R. Aono, K. Yamamoto, A. Ishihama, and R. Utsumi. 2003. Transcriptional regulation of drug efflux genes by EvgAS, a two-component system in Escherichia coli. Microbiology 149:2819-2828. [DOI] [PubMed] [Google Scholar]
- 16.European Concerted Action on New Generation Genetic Markers and Techniques for the Epidemiology and Control of Tuberculosis. 2006. Beijing/W genotype Mycobacterium tuberculosis and drug resistance. Emerg. Infect. Dis. 12:736-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Filliol, I., A. S. Motiwala, M. Cavatore, W. Qi, M. H. Hazbon, M. Bobadilla del Valle, J. Fyfe, L. Garcia-Garcia, N. Rastogi, C. Sola, T. Zozio, M. I. Guerrero, C. I. Leon, J. Crabtree, S. Angiuoli, K. D. Eisenach, R. Durmaz, M. L. Joloba, A. Rendon, J. Sifuentes-Osornio, A. Ponce de Leon, M. D. Cave, R. Fleischmann, T. S. Whittam, and D. Alland. 2006. Global phylogeny of Mycobacterium tuberculosis based on single nucleotide polymorphism (SNP) analysis: insights into tuberculosis evolution, phylogenetic accuracy of other DNA fingerprinting systems, and recommendations for a minimal standard SNP set. J. Bacteriol. 188:759-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gagneux, S., K. DeRiemer, T. Van, M. Kato-Maeda, B. C. de Jong, S. Narayanan, M. Nicol, S. Niemann, K. Kremer, M. C. Gutierrez, M. Hilty, P. C. Hopewell, and P. M. Small. 2006. Variable host-pathogen compatibility in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 103:2869-2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gagneux, S., and P. M. Small. 2007. Global phylogeography of Mycobacterium tuberculosis and implications for tuberculosis product development. Lancet Infect. Dis. 7:328-337. [DOI] [PubMed] [Google Scholar]
- 20.Glynn, J. R., J. Whiteley, P. J. Bifani, K. Kremer, and D. van Soolingen. 2002. Worldwide occurrence of Beijing/W strains of Mycobacterium tuberculosis: a systematic review. Emerg. Infect. Dis. 12:843-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gordon, S. V., D. Bottai, R. Simeone, T. P. Stinear, and R. Brosch. 2009. Pathogenicity in the tubercle bacillus: molecular and evolutionary determinants. Bioessays 31:378-388. [DOI] [PubMed] [Google Scholar]
- 22.Grode, L., P. Seiler, S. Baumann, J. Hess, V. Brinkmann, A. Nasser Eddine, P. Mann, C. Goosmann, S. Bandermann, D. Smith, G. J. Bancroft, J. M. Reyrat, D. van Soolingen, B. Raupach, and S. H. Kaufmann. 2005. Increased vaccine efficacy against tuberculosis of recombinant Mycobacterium bovis bacille Calmette-Guerin mutants that secrete listeriolysin. J. Clin. Invest. 115:2472-2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanekom, M., G. D. van der Spuy, N. C. Gey van Pittius, C. R. McEvoy, S. L. Ndabambi, T. C. Victor, E. G. Hoal, P. D. van Helden, and R. M. Warren. 2007. Evidence that the spread of Mycobacterium tuberculosis strains with the Beijing genotype is human population dependent. J. Clin. Microbiol. 45:2263-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanekom, M., G. D. van der Spuy, E. Streicher, S. L. Ndabambi, C. R. McEvoy, M. Kidd, N. Beyers, T. C. Victor, P. D. van Helden, and R. M. Warren. 2007. A recently evolved sublineage of the Mycobacterium tuberculosis Beijing strain family is associated with an increased ability to spread and cause disease. J. Clin. Microbiol. 45:1483-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heermann, R., A. Weber, B. Mayer, M. Ott, E. Hauser, G. Gabriel, T. Pirch, and K. Jung. 2009. The universal stress protein UspC scaffolds the KdpD/KdpE signaling cascade of Escherichia coli under salt stress. J. Mol. Biol. 386:134-148. [DOI] [PubMed] [Google Scholar]
- 26.Hershberg, R., M. Lipatov, P. M. Small, H. Sheffer, S. Niemann, S. Homolka, J. C. Roach, K. Kremer, D. A. Petrov, M. W. Feldman, and S. Gagneux. 2008. High functional diversity in Mycobacterium tuberculosis driven by genetic drift and human demography. PLoS Biol. 6:e311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Honaker, R. W., R. L. Leistikow, I. L. Bartek, and M. I. Voskuil. 2009. Unique roles of DosT and DosS in DosR regulon induction and Mycobacterium tuberculosis dormancy. Infect. Immun. 77:3258-3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jenal, U., and R. Hengge-Aronis. 2003. Regulation by proteolysis in bacterial cells. Curr. Opin. Microbiol. 6:163-172. [DOI] [PubMed] [Google Scholar]
- 29.Karakousis, P. C., T. Yoshimatsu, G. Lamichhane, S. C. Woolwine, E. L. Nuermberger, J. Grosset, and W. R. Bishai. 2004. Dormancy phenotype displayed by extracellular Mycobacterium tuberculosis within artificial granulomas in mice. J. Exp. Med. 200:647-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kong, Y., M. D. Cave, L. Zhang, B. Foxman, C. F. Marrs, J. H. Bates, and Z. H. Yang. 2007. Association between Mycobacterium tuberculosis Beijing/W lineage strain infection and extrathoracic tuberculosis: insights from epidemiologic and clinical characterization of the three principal genetic groups of M. tuberculosis clinical isolates. J. Clin. Microbiol. 45:409-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kremer, K., M. J. van-der-Werf, B. K. Au, D. D. Anh, K. M. Kam, H. R. van-Doorn, M. W. Borgdorff, and D. van-Soolingen. 2009. Vaccine-induced immunity circumvented by typical Mycobacterium tuberculosis Beijing strains. Emerg. Infect. Dis. 15:335-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar, A., J. C. Toledo, R. P. Patel, J. R. Lancaster, Jr., and A. J. Steyn. 2007. Mycobacterium tuberculosis DosS is a redox sensor and DosT is a hypoxia sensor. Proc. Natl. Acad. Sci. USA 104:11568-11573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li, Q., C. C. Whalen, J. M. Albert, R. Larkin, L. Zukowski, M. D. Cave, and R. F. Silver. 2002. Differences in rate and variability of intracellular growth of a panel of Mycobacterium tuberculosis clinical isolates within a human monocyte model. Infect. Immun. 70:6489-6493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lopez, B., D. Aguilar, H. Orozco, M. Burger, C. Espitia, V. Ritacco, L. Barrera, K. Kremer, R. Hernandez-Pando, K. Huygen, and D. van Soolingen. 2003. A marked difference in pathogenesis and immune response induced by different Mycobacterium tuberculosis genotypes. Clin. Exp. Immunol. 133:30-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manca, C., M. B. Reed, S. Freeman, B. Mathema, B. Kreiswirth, C. E. Barry III, and G. Kaplan. 2004. Differential monocyte activation underlies strain-specific Mycobacterium tuberculosis pathogenesis. Infect. Immun. 72:5511-5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manca, C., L. Tsenova, C. E. Barry III, A. Bergtold, S. Freeman, P. A. Haslett, J. M. Musser, V. H. Freedman, and G. Kaplan. 1999. Mycobacterium tuberculosis CDC1551 induces a more vigorous host response in vivo and in vitro, but is not more virulent than other clinical isolates. J. Immunol. 162:6740-6746. [PubMed] [Google Scholar]
- 37.Manca, C., L. Tsenova, A. Bergtold, S. Freeman, M. Tovey, J. M. Musser, C. E. Barry III, V. H. Freedman, and G. Kaplan. 2001. Virulence of a Mycobacterium tuberculosis clinical isolate in mice is determined by failure to induce Th1 type immunity and is associated with induction of IFN-alpha/beta. Proc. Natl. Acad. Sci. USA 98:5752-5757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitrophanov, A. Y., and E. A. Groisman. 2008. Signal integration in bacterial two-component regulatory systems. Genes Dev. 22:2601-2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Monahan, I. M., J. Betts, D. K. Banerjee, and P. D. Butcher. 2001. Differential expression of mycobacterial proteins following phagocytosis by macrophages. Microbiology 147:459-471. [DOI] [PubMed] [Google Scholar]
- 40.Monson, E. K., M. Weinstein, G. S. Ditta, and D. R. Helinski. 1992. The FixL protein of Rhizobium meliloti can be separated into a heme-binding oxygen-sensing domain and a functional C-terminal kinase domain. Proc. Natl. Acad. Sci. USA 89:4280-4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Musser, J. M., A. Amin, and S. Ramaswamy. 2000. Negligible genetic diversity of Mycobacterium tuberculosis host immune system protein targets: evidence of limited selective pressure. Genetics 155:7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nachin, L., U. Nannmark, and T. Nystrom. 2005. Differential roles of the universal stress proteins of Escherichia coli in oxidative stress resistance, adhesion, and motility. J. Bacteriol. 187:6265-6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nicol, M. P., and R. J. Wilkinson. 2008. The clinical consequences of strain diversity in Mycobacterium tuberculosis. Trans. R. Soc. Trop. Med. Hyg. 102:955-965. [DOI] [PubMed] [Google Scholar]
- 44.O'Toole, R., and H. D. Williams. 2003. Universal stress proteins and Mycobacterium tuberculosis. Res. Microbiol. 154:387-392. [DOI] [PubMed] [Google Scholar]
- 45.Parish, T., D. A. Smith, S. Kendall, N. Casali, G. J. Bancroft, and N. G. Stoker. 2003. Deletion of two-component regulatory systems increases the virulence of Mycobacterium tuberculosis. Infect. Immun. 71:1134-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park, H. D., K. M. Guinn, M. I. Harrell, R. Liao, M. I. Voskuil, M. Tompa, G. K. Schoolnik, and D. R. Sherman. 2003. Rv3133c/dosR is a transcription factor that mediates the hypoxic response of Mycobacterium tuberculosis. Mol. Microbiol. 48:833-843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pelicic, V., M. Jackson, J. M. Reyrat, W. R. Jacobs, Jr., B. Gicquel, and C. Guilhot. 1997. Efficient allelic exchange and transposon mutagenesis in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 94:10955-10960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reed, M. B., P. Domenech, C. Manca, H. Su, A. K. Barczak, B. N. Kreiswirth, G. Kaplan, and C. E. Barry III. 2004. A glycolipid of hypervirulent tuberculosis strains that inhibits the innate immune response. Nature 431:84-87. [DOI] [PubMed] [Google Scholar]
- 49.Reed, M. B., S. Gagneux, K. Deriemer, P. M. Small, and C. E. Barry III. 2007. The W-Beijing lineage of Mycobacterium tuberculosis overproduces triglycerides and has the DosR dormancy regulon constitutively upregulated. J. Bacteriol. 189:2583-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reed, M. B., V. K. Pichler, F. McIntosh, A. Mattia, A. Fallow, S. Masala, P. Domenech, A. Zwerling, L. Thibert, D. Menzies, K. Schwartzman, and M. A. Behr. 2009. Major Mycobacterium tuberculosis lineages associate with patient country of origin. J. Clin. Microbiol. 47:1119-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roberts, D. M., R. P. Liao, G. Wisedchaisri, W. G. Hol, and D. R. Sherman. 2004. Two sensor kinases contribute to the hypoxic response of Mycobacterium tuberculosis. J. Biol. Chem. 279:23082-23087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rustad, T. R., M. I. Harrell, R. Liao, and D. R. Sherman. 2008. The enduring hypoxic response of Mycobacterium tuberculosis. PLoS One 3:e1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saini, D. K., V. Malhotra, D. Dey, N. Pant, T. K. Das, and J. S. Tyagi. 2004. DevR-DevS is a bona fide two-component system of Mycobacterium tuberculosis that is hypoxia-responsive in the absence of the DNA-binding domain of DevR. Microbiology 150:865-875. [DOI] [PubMed] [Google Scholar]
- 54.Sanowar, S., A. Martel, and H. L. Moual. 2003. Mutational analysis of the residue at position 48 in the Salmonella enterica serovar Typhimurium PhoQ sensor kinase. J. Bacteriol. 185:1935-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sardiwal, S., S. L. Kendall, F. Movahedzadeh, S. C. Rison, N. G. Stoker, and S. Djordjevic. 2005. A GAF domain in the hypoxia/NO-inducible Mycobacterium tuberculosis DosS protein binds haem. J. Mol. Biol. 353:929-936. [DOI] [PubMed] [Google Scholar]
- 56.Schnappinger, D., S. Ehrt, M. I. Voskuil, Y. Liu, J. A. Mangan, I. M. Monahan, G. Dolganov, B. Efron, P. D. Butcher, C. Nathan, and G. K. Schoolnik. 2003. Transcriptional adaptation of Mycobacterium tuberculosis within macrophages: insights into the phagosomal environment. J. Exp. Med. 198:693-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sherman, D. R., M. Voskuil, D. Schnappinger, R. Liao, M. I. Harrell, and G. K. Schoolnik. 2001. Regulation of the Mycobacterium tuberculosis hypoxic response gene encoding alpha-crystallin. Proc. Natl. Acad. Sci. USA 98:7534-7539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shiloh, M. U., P. Manzanillo, and J. S. Cox. 2008. Mycobacterium tuberculosis senses host-derived carbon monoxide during macrophage infection. Cell Host Microbe 3:323-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith, N. H., R. G. Hewinson, K. Kremer, R. Brosch, and S. V. Gordon. 2009. Myths and misconceptions: the origin and evolution of Mycobacterium tuberculosis. Nat. Rev. Microbiol. 7:537-544. [DOI] [PubMed] [Google Scholar]
- 60.Snapper, S. B., R. E. Melton, S. Mustafa, T. Kieser, and W. R. Jacobs, Jr. 1990. Isolation and characterization of efficient plasmid transformation mutants of Mycobacterium smegmatis. Mol. Microbiol. 4:1911-1919. [DOI] [PubMed] [Google Scholar]
- 61.Sousa, E. H., J. R. Tuckerman, G. Gonzalez, and M. A. Gilles-Gonzalez. 2007. DosT and DevS are oxygen-switched kinases in Mycobacterium tuberculosis. Protein Sci. 16:1708-1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sreevatsan, S., X. Pan, K. E. Stockbauer, N. D. Connell, B. N. Kreiswirth, T. S. Whittam, and J. M. Musser. 1997. Restricted structural gene polymorphism in the Mycobacterium tuberculosis complex indicates evolutionarily recent global dissemination. Proc. Natl. Acad. Sci. USA 94:9869-9874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stover, C. K., V. F. de la Cruz, T. R. Fuerst, J. E. Burlein, L. A. Benson, L. T. Bennett, G. P. Bansal, J. F. Young, M. H. Lee, G. F. Hatfull, S. B. Snapper, R. G. Barletta, W. R. Jacobs, and B. R. Bloom. 1991. New use of BCG for recombinant vaccines. Nature 351:456-460. [DOI] [PubMed] [Google Scholar]
- 64.Tsenova, L., E. Ellison, R. Harbacheuski, A. L. Moreira, N. Kurepina, M. B. Reed, B. Mathema, C. E. Barry III, and G. Kaplan. 2005. Virulence of selected Mycobacterium tuberculosis clinical isolates in the rabbit model of meningitis is dependent on phenolic glycolipid produced by the bacilli. J. Infect. Dis. 192:98-106. [DOI] [PubMed] [Google Scholar]
- 65.Tsenova, L., R. Harbacheuski, N. Sung, E. Ellison, D. Fallows, and G. Kaplan. 2007. BCG vaccination confers poor protection against M. tuberculosis HN878-induced central nervous system disease. Vaccine 25:5126-5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tsolaki, A. G., S. Gagneux, A. S. Pym, Y. O. Goguet de la Salmoniere, B. N. Kreiswirth, D. Van Soolingen, and P. M. Small. 2005. Genomic deletions classify the Beijing/W strains as a distinct genetic lineage of Mycobacterium tuberculosis. J. Clin. Microbiol. 43:3185-3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van der Spuy, G. D., K. Kremer, S. L. Ndabambi, N. Beyers, R. Dunbar, B. J. Marais, P. D. van Helden, and R. M. Warren. 2009. Changing Mycobacterium tuberculosis population highlights clade-specific pathogenic characteristics. Tuberculosis (Edinburgh) 89:120-125. [DOI] [PubMed] [Google Scholar]
- 68.van Soolingen, D., L. Qian, P. E. de Haas, J. T. Douglas, H. Traore, F. Portaels, H. Z. Qing, D. Enkhsaikan, P. Nymadawa, and J. D. van Embden. 1995. Predominance of a single genotype of Mycobacterium tuberculosis in countries of East Asia. J. Clin. Microbiol. 33:3234-3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Voskuil, M. I., D. Schnappinger, K. C. Visconti, M. I. Harrell, G. M. Dolganov, D. R. Sherman, and G. K. Schoolnik. 2003. Inhibition of respiration by nitric oxide induces a Mycobacterium tuberculosis dormancy program. J. Exp. Med. 198:705-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wayne, L. G., and C. D. Sohaskey. 2001. Nonreplicating persistence of Mycobacterium tuberculosis. Annu. Rev. Microbiol. 55:139-163. [DOI] [PubMed] [Google Scholar]
- 71.Wirth, T., F. Hildebrand, C. Allix-Beguec, F. Wolbeling, T. Kubica, K. Kremer, D. van Soolingen, S. Rusch-Gerdes, C. Locht, S. Brisse, A. Meyer, P. Supply, and S. Niemann. 2008. Origin, spread and demography of the Mycobacterium tuberculosis complex. PLoS Pathog. 4:e1000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.World Health Organization. 2009. Global tuberculosis control 2009—epidemiology, strategy, financing. World Health Organization, Geneva, Switzerland.
- 73.Wu, S., S. T. Howard, D. L. Lakey, A. Kipnis, B. Samten, H. Safi, V. Gruppo, B. Wizel, H. Shams, R. J. Basaraba, I. M. Orme, and P. F. Barnes. 2004. The principal sigma factor sigA mediates enhanced growth of Mycobacterium tuberculosis in vivo. Mol. Microbiol. 51:1551-1562. [DOI] [PubMed] [Google Scholar]
- 74.Zhang, M., J. Gong, Z. Yang, B. Samten, M. D. Cave, and P. F. Barnes. 1999. Enhanced capacity of a widespread strain of Mycobacterium tuberculosis to grow in human macrophages. J. Infect. Dis. 179:1213-1217. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.